Lovers in strange places: amphibian calling and amplexus detected in tidal mangrove creeks after rainfall
John Gould A * , Chad Beranek A B , Kate Schmahl A , Rachael Donelly A , Lynne Matthews A , Alex Callen A and Colin McHenry A
A * , Chad Beranek A B , Kate Schmahl A , Rachael Donelly A , Lynne Matthews A , Alex Callen A and Colin McHenry A
A
B
Abstract
Temporary freshwater lenses can form in saline environments after rainfall, providing essential resources for species including drinking water and dispersal routes. However, there is limited indication that these lenses can also be used for breeding. Herein, we provide evidence of the green and golden bell frog, Litoria aurea, performing breeding activities, including calling and amplexus, in tidal mangrove creeks on Kooragang Island, NSW, Australia. Our findings suggest that these creeks intermittently phase into a low salinity state after the influx of fresh water from rainfall, forming temporary freshwater lenses that can be exploited before the creeks revert to a saline state. These lenses had salinities (1.4 ppt and 4.5 ppt) within the tolerance limit of L. aurea tadpoles, although we are unsure whether oviposition took place and offspring survival to metamorphosis was achieved. It is possible that anthropogenic disturbances to hydrology on Kooragang Island have benefited L. aurea by restricting tidal influences in mangrove creeks, prolonging the duration of freshwater lenses.
Keywords: amphibian, calling, ephemeral waterbody, freshwater lens, Litoria aurea, mangrove forest, oviposition site selection, salt tolerance.
Introduction
Temporary freshwater lenses that form in saltwater environments after rainfall provide unique ecological opportunities for species. For example, they can provide access to drinking water for species that have adapted to saline environments (Lillywhite et al. 2014, 2019), as well as a dispersal pathway for freshwater species (Nifong and Silliman 2017). However, there is limited indication that such lenses are used for breeding purposes, despite the potential advantages that come with exploiting what are essentially ephemeral waterbodies, including a reduction in the colonising capacity of some predators (Skelly 1996). There is a need for greater exploration of the benefits of freshwater lenses as supplementary or alternative breeding sites for species that exploit fresh water in proximity to saltwater systems.
Amphibians are closely associated with freshwater systems as desiccation is a potential threat to adults, which possess permeable skin and eggs that are laid externally without a protective shell (Shoemaker et al. 1992; Gould et al. 2022). These aquatic systems are used for shelter, foraging, and depositing eggs, ranging from permanent ponds to ephemeral pools and constructed burrows/nests connected to groundwater supplies (Anstis 2017). A reliance predominantly on fresh water would suggest that amphibians are excluded from habitats impacted by salt water. However, it has become increasingly apparent that salt tolerance is widespread in this group, the most extreme being the crab-eating frog, Fejervarya (=Rana) cancrivora, which is adapted to salt water at all stages (Gordon and Tucker 1965; Dunson 1977). Salt water exposure may occur inadvertently, particularly at embryo and tadpole life stages if freshwater systems are exposed to tidal influences, flooding or ocean spray, while post-metamorphic life stages may actively move into saltwater habitats for resource acquisition or temporarily during dispersal (Hopkins and Brodie 2015; Pyke and White 2022). A small number of amphibians (26 species) have also been reported to occur in the terrestrial, non-water component of mangrove forests (Rog et al. 2017), indicating that typically high salinity environments can be exploited under certain conditions without direct exposure to the potentially dangerous effects of salt water emersion.
The green and golden bell frog, Litoria aurea, is an Australian anuran that has suffered a precipitous decline due to habitat modification, predation by the invasive eastern mosquito fish, Gambusia holbrooki, and disease caused by the amphibian chytrid fungus (Penman et al. 2008; White and Pyke 2008; Mahony et al. 2013; Pollard et al. 2017). Populations are now primarily confined to coastal environments, which is hypothesised to be linked to the protective effect of salinity against chytrid (White 2006; Stockwell et al. 2012; Clulow et al. 2018). Indeed, several key populations exist in freshwater habitats on islands that are near oceans and estuaries that allow for temporary interactions with salt water and/or salt-laden substrates (Hamer et al. 2002; Pyke and White 2022). Although this species has been recorded breeding in rain-fed intertidal rock pools (Pyke et al. 2013; Pyke and White 2022), with tadpoles showing no adverse effect with exposure to salinity up to 6 ppt (Callen et al. 2023), they have not been demonstrated to use freshwater lenses in saline environments. In this study, we provide evidence of L. aurea breeding activity, including the formation of a male calling chorus and amplexus, in tidal mangrove creeks when temporary freshwater lenses form after rainfall. Although we were not able to determine breeding outcomes from our observations, we make suggestions on the potential benefits and risks of exploiting freshwater lenses as alternative breeding habitats, with particular emphasis on the effects of salt water exposure on the developing young.
Materials and methods
All observations were made on Kooragang Island, NSW, Australia, which contains one of the largest remaining L. aurea populations (Pyke and White 2001). This population has been intensely studied, with the first instance of ecological research occurring in 1999 (Hamer et al. 2002). Since then, a yearly long-term monitoring plan was established in 2011 and is on-going. This monitoring includes visual encounter surveys to detect L. aurea, which involves searching the banks and interior of wetlands at night with headtorches. During each pond survey, the number of frogs, calling males, and amplexus events were recorded.
During the on-going long-term monitoring programme, we detected L. aurea breeding activity within two separate tidal mangrove systems in the 2022–2023 breeding season (September–March) (Fig. 1). This was during a La Nina weather cycle for the region where the annual rainfall for 2022 was 1472 mm, which is within ~20 mm of the 90th% quantile for this region (Bureau of Meteorology 2022; weather station: Williamtown). Our first observation occurred in Dead Mangrove Creek (32.85837°S, 151.72480°E), which is east of two roads that dissect the creek line and a culvert restriction 1.5 km further west. The second site is a creek line 800 m north-west of the first observation site (32.85248°S, 151.71888°E, and does not appear to have any man-made restrictions. Mangrove species present on Kooragang Island include the grey mangrove, Avicennia marina, and the river mangrove, Aegiceras corniculatum (Streever and Genders 1997). Water quality measurements were taken at both sites using a PC60 multiparameter pocket tester (Instrument Choice). The outcome of breeding activities we observed, including whether eggs were deposited and offspring survival to metamorphosis was possible, could not be verified due to logistical constraints.
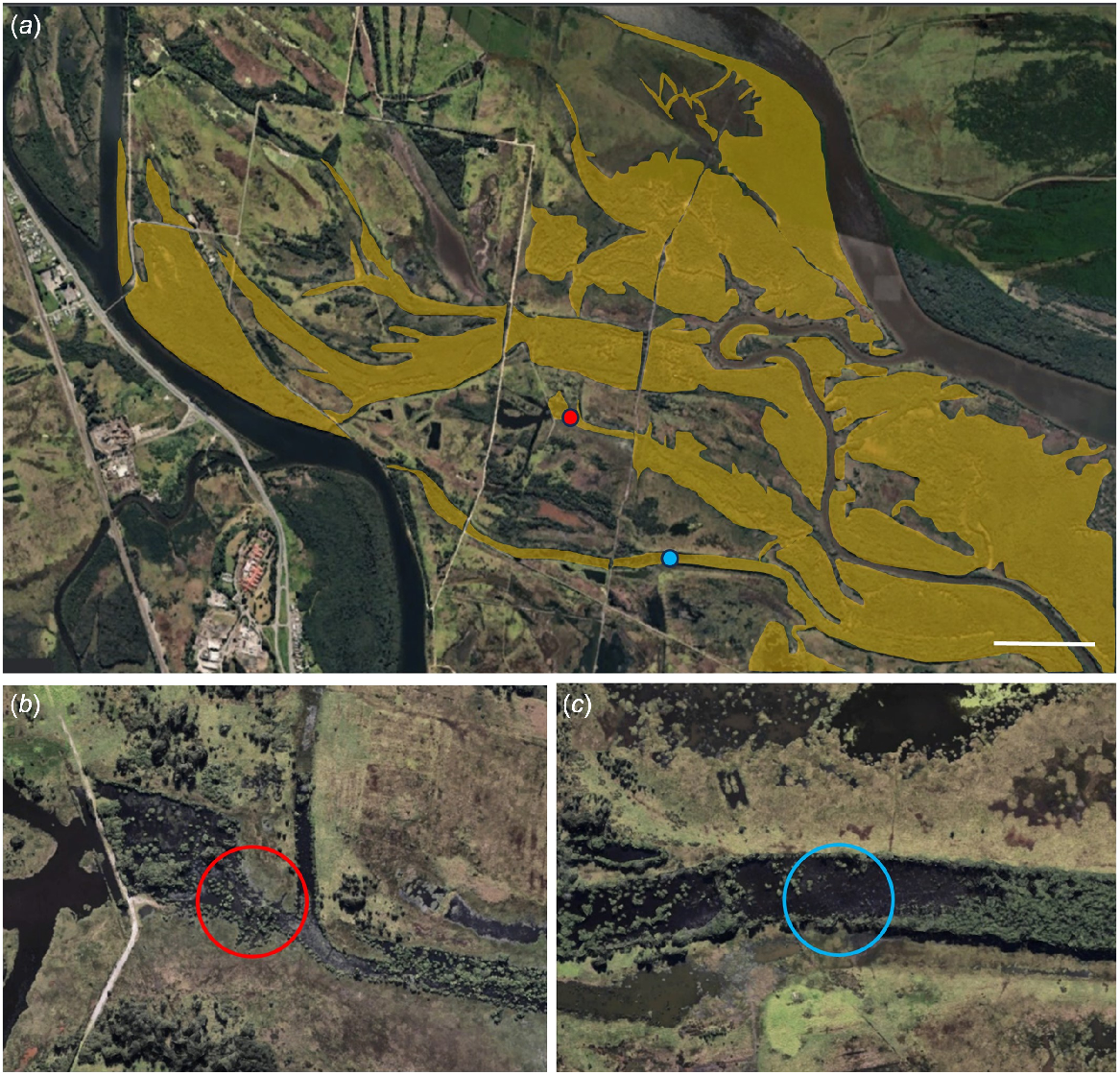
Location of tidal mangrove creek lines where Litoria aurea breeding activity was detected on Kooragang Island, NSW, Australia during the 2022–2023 breeding season. Images depict the extent of mangrove forests on Kooragang Island (highlighted in yellow), in comparison to the first (blue dot) and second (red dot) observation sites (a), and closeups of the two observational sites (b, c). Maps were obtained from Nearmap™ (http://www.nearmap.com/). Scale bar represents 500 m.
Results
Site 1
The first observation occurred at night on 27 October 2022 within Dead Mangrove Creek, after 20.6 mm of rainfall that spanned 7 days before and 2 days after a new moon during a spring tide event (NSW Tides 2022–2023). We heard 10–15 adult males calling as part of a chorus in the middle portion of the creek over an area spanning 0.67 ha (Appendix S1). The section of creek where L. aurea were detected was sparsely populated by mangrove trees compared to adjacent sections (Fig. 2a, b). We visually detected five adult individuals within the system; an adult male 20–30 cm from the bank among mangrove pneumatophores (Fig. 3a), a calling adult male at the water’s surface floating on an algal matt 4 m from the bank (Fig. 3b), an amplecting male/female pair partially covered by an algal matt at the surface of the water 5 m from the bank (Fig. 3c), and an adult male on a mangrove tree branch 30 cm above the water and 10 m from the bank (Fig. 3d).
Mangrove creek lines where Litoria aurea breeding activity was detected on Kooragang Island, NSW, Australia during the 2022–2023 breeding season. Images depict the first (a, b) and second observation (c) sites.

Litoria aurea detected in mangrove creek lines on Kooragang Island, NSW, Australia during the 2022–2023 breeding season. Images depict (a) adult male among mangrove pneumatophores near the bank, (b) adult male calling while perched on an algal matt at the water’s surface, (c) amplecting male/female pair submerged in water, and (d) adult male perched on a mangrove branch.

The water was approximately 40–50 cm deep, with large green algae matts covering more than 50% of the water’s surface and G. holbrooki present. Water quality was measured 5 m from the bank at the water’s surface (temperature = 20.6°C, conductivity = 2.79 mS, pH = 7.76, salinity = 1.4 ppt, TDS = 1.98 ppt) and near the bottom of the water column (temperature = 21.0°C, conductivity = 3.01 mS, pH = 7.37, salinity = 1.5 ppt, TDS = 2.14 ppt). Salinity was re-checked at the site on 28 November and had risen to 9.72 ppt, and no tadpoles were observed.
We also detected five calling males in a neighbouring ephemeral wetland approximately 80 m from the site of mangrove calling which also contained G. holbrooki and had comparatively higher salinity (temperature = 22.3°C, conductivity = 7.96 mS, pH = 7.65, salinity = 3.98 ppt, TDS = 5.66 ppt). Additionally, calling of two males was detected at a permanent wetland approximately 280 m from the site of mangrove calling that is a known breeding site in the area and free of G. holbrooki (temperature = 23.1°C, conductivity = 1.47 mS, pH = 7.13, salinity = 0.73 ppt, TDS = 1.04 ppt). The calling aggregation at Dead Mangrove Creek was the largest for the 2022–2023 breeding season on Kooragang Island; there were 107 instances of pond calling detected, including 32 occasions where calling aggregations consisted of five or more males.
Site 2
The second observation occurred at night on 25 November 2022 in a separate mangrove creek, 11 days after a rainfall event (27 mm), and 1 day after a new moon during a spring tide event (NSW Tides 2022–2023). The creek ended in a large opening of water dotted with mangrove trees (Fig. 2c) and was more than 400 m away from any freshwater wetlands where L. aurea have historically been recorded on the island. We heard four males calling over an area of approximately 0.4 ha and detected one calling male perched on a mangrove tree branch 20–30 cm over the water’s surface 5 m from the bank. We also heard one calling male in flooded samphire, Sarcocornia quinqueflora, saltmarsh adjacent to the creek. The water was approximately 20 cm deep and G. holbrooki was present. Water quality measurements were taken at the surface near to where calling was detected in the mangroves (temperature = 24.3°C, conductivity = 11.01 mS, pH = 6.47, salinity = 5.49 ppt, TDS = 7.8 ppt). No calling was detected in any other ponds nearby. Subsequent water quality measurements and surveys for tadpole presence were not made.
Discussion
Our findings show that tidal mangrove creeks on Kooragang Island can intermittently phase into a low salinity state after rainfall, with temporary freshwater lenses becoming available for L. aurea as novel breeding sites before the system reverts to a predominantly saline state. A similar observation has been made for another Australian frog, Litoria bicolor, which has been recorded in mangroves containing fresh water (Hutchings and Recher 1981), although not specifically for breeding. A limitation of our study was the lack of detection of eggs/tadpoles and/or metamorphs, which would have indicated that site selection did indeed result in breeding and that successful offspring development was possible. Nevertheless, the recording of several activities related to breeding, including obvious precursors to oviposition (Pyke and White 2022), suggests that the creeks had been selected by both males and females as potential breeding sites despite the presence of freshwater sites nearby.
Salinity patterns at observation sites
As our first observation occurred immediately after 7 days of rainfall, we hypothesise that fresh water had flooded into the creek, causing lower than usual salinity well within the tolerance for L. aurea tadpoles (Pyke and White 2022; Callen et al. 2023). This is supported by the increased salinity a month later that was at levels typical for mangrove systems on Kooragang Island (10–35 ppt) (Streever and Genders 1997). Yet, this was also a period only a few days after a spring tide, which would have led to greater than usual influx of salt water. Although we are unsure of the precise hydrological regime of the creek, it is possible that tidal flow in combination with rainwater influx led to lower salinity. The spring tide resulted in a higher than usual tide prior to our observation, which may have reduced salinity if it flushed hypersaline water from the creek; hypersaline conditions can result from less regular inundation which is influenced by tidal range, barriers to tidal flow, and evaporation (Benfer et al. 2007; Herbert 2007). The influx of rainwater may have (1) diluted tidal water that was delivered at this flood tide and remained after the ebb tide or (2) fed a buoyant, freshwater lens at the surface that was preserved during the flood tide and came to dominate the creek following the ebb tide, with the area subsequently experiencing limited further inundation of tidal water after the spring tide event. What remains undetermined is the effect of freshwater input on the river channels surrounding the island, which have been reported to drop to 1.2 ppt at the surface after significant rainfall (Brereton and Taylor-Wood 2010), and how this influences the salinity of water entering tidal creeks.
The second observation occurred at a site with higher salinity (5–6 ppt), which has been shown to have no impact on L. aurea tadpole survival or development (Callen et al. 2023), but is below typical salinity for tidal creeks on Kooragang Island (Streever and Genders 1997). This observation also occurred soon after a spring tide but several weeks after a rain event and so it is possible that the freshwater lens had already begun to lose its integrity and experience mixing with tidal water to cause higher salinity. This may have provided a unique scenario where conditions were still optimal for offspring while providing a level of salt exposure for adults that has been shown to be beneficial against chytrid, reducing the risk of chytrid transmission for breeding adults that physically touch (Clulow et al. 2018).
Site selection
Our observations suggest that reproductively active males were able to detect the presence of fresh or low salinity water within mangrove creeks after rainfall. Although we are unsure of the initial trigger for movement to these sites, it could be a result of the species’ preference for recently refilled wetlands for breeding purposes (Beranek et al. 2021), with adults perceiving freshwater lenses as such. Although this sensory capacity requires further investigation, L. aurea is known to synchronise spawning at wetlands with rainfall, including those that have been previously dry (Pyke and White 2001; Beranek et al. 2022). Yet, only one previous record has been made of calling activity in mangrove creeks across the species’ range (Hamer 2002), suggesting conditions are rarely suitable to trigger movement into these systems.
Our observation at Dead Mangrove Creek in particular shows that reproductively active adults congregated within the creek line instead of neighbouring freshwater sites that were also available, some of which are used for breeding. We did detect low levels of calling activity in these ponds, suggesting that males were not forced to call from the creeks as sub-optimal sites due to density-dependent constraints on space.
Potential benefits of using freshwater lenses
There are potential benefits of selecting creeks as oviposition sites when they have converted to fresh water. As freshwater lenses are effectively newly created waterbodies, they have not yet been colonised by predators that would be found in more permanent freshwater systems (e.g. dragonfly larvae: Beranek et al. 2022). Although G. holbrooki was present at both sites it appeared to be at low densities, which may allow for successful breeding outcomes (van de Mortel and Goldingay 1998; Goldingay 2008), despite the fish being a voracious predator of L. aurea offspring (Pyke and White 2000). If timed correctly, it is also possible that a gradual increase in salinity at the very end of the offspring developmental period could provide early protection against chytrid, as metamorphs show an increase in keratin production which the fungus feeds on (Marantelli et al. 2004).
Brackish creeks as breeding traps
Exploiting tidal systems for reproduction is a risky strategy if freshwater lenses do not remain available long enough for offspring to complete development, becoming reproductive traps. Akin to the risk of using ephemeral wetlands that lose fresh water as they begin to dry out (Gould et al. 2022), tidal creeks will lose their freshwater lenses as the rain subsides and there is an influx of salt via tidal influences, which may result in mass mortality of tadpoles. Although L. aurea adults select breeding sites based on cues that allow an assessment of current breeding conditions (e.g. Pollard et al. 2017), this may not guarantee breeding success if those conditions are not retained throughout offspring development. The sustained presence of fresh water may not be quantifiable based on the conditions that parents experience during site selection and egg deposition (e.g. Gould et al. 2022), and total clutch failure due to freshwater ephemerality has been recorded for this species (Beranek et al. 2020). Species developmental rate will also influence the probability of offspring being able to successfully metamorphose prior to the loss of fresh water, which for L. aurea ranges from 5 to 12 weeks (Pyke and White 2001).
Habitat modifications potentially benefiting Litoria aurea
Anthropogenic disturbances to hydrology on Kooragang Island have benefited L. aurea, including the installation of bunds around ponds to prevent flooding from estuaries (Beranek et al. 2021). We suggest that L. aurea has unintentionally benefited from restrictions to tidal influences in mangrove creeks via the addition of infrastructure such as culverts and roads (Streever and Genders 1997), which may allow freshwater input from rainfall to remain present for extended periods. It remains to be determined how L. aurea will be affected by the removal of culverts from several creeks on the island to increase wetland productivity, as this has led to improvements in tidal penetration (Streever et al. 1996).
Conclusion
These observations provide evidence that L. aurea exploits generally high salt systems for breeding when fresh/brackish or low-salinity water becomes temporarily available after rainfall. The population dynamics of amphibians in environments with a salt water–fresh water interface is important to investigate as they may reveal novel ideas that can be applied to species conservation (Hettyey et al. 2019), or reveal ecoevolutionary pathways that amphibians may gain to withstand chytridiomycosis (Fisher et al. 2021).
References
Benfer NP, King BA, Lemckert CJ (2007) Salinity observations in a subtropical estuarine system on the Gold Coast, Australia. Journal of Coastal Research 50, 646-651.
| Google Scholar |
Beranek CT, Clulow J, Mahony M (2020) A simple design feature to increase hydro-period in constructed ephemeral wetlands to avoid tadpole desiccation-induced mortality. Ecological Management & Restoration 21, 250-253.
| Crossref | Google Scholar |
Beranek CT, Maynard C, McHenry C, Clulow J, Mahony M (2021) Rapid population increase of the threatened Australian amphibian Litoria aurea in response to wetlands constructed as a refuge from chytrid-induced disease and introduced fish. Journal of Environmental Management 291, 112638.
| Crossref | Google Scholar |
Beranek CT, Sanders S, Clulow J, Mahony M (2022) Predator-free short-hydroperiod wetlands enhance metamorph output in a threatened amphibian: insights into frog breeding behaviour evolution and conservation management. Wildlife Research 49, 360-371.
| Crossref | Google Scholar |
Bureau of Meteorology (2022) Climate Data Online. Commonwealth of Australia, Canberra. Available at http://www.bom.gov.au/climate/data/
Callen A, Pizzatto L, Stockwell MP, Clulow S, Clulow J, Mahony MJ (2023) The effect of salt dosing for chytrid mitigation on tadpoles of a threatened frog, Litoria aurea. Journal of Comparative Physiology B 193, 239-247.
| Crossref | Google Scholar |
Clulow S, Gould J, James H, Stockwell M, Clulow J, Mahony M (2018) Elevated salinity blocks pathogen transmission and improves host survival from the global amphibian chytrid pandemic: implications for translocations. Journal of Applied Ecology 55, 830-840.
| Crossref | Google Scholar |
Dunson WA (1977) Tolerance to high temperature and salinity by tadpoles of the Philippine frog, Rana cancrivora. Copeia 1977, 375-378.
| Crossref | Google Scholar |
Fisher MC, Pasmans F, Martel A (2021) Virulence and pathogenicity of chytrid fungi causing amphibian extinctions. Annual Review of Microbiology 75, 673-693.
| Crossref | Google Scholar |
Goldingay R (2008) Conservation of the endangered green and golden bell frog: what contribution has ecological research made since 1996? Australian Zoologist 34, 334-349.
| Crossref | Google Scholar |
Gordon MS, Tucker VA (1965) Osmotic regulation in the tadpoles of the crab-eating frog (Rana cancrivora). Journal of Experimental Biology 42, 437-445.
| Crossref | Google Scholar |
Gould J, Clulow J, Clulow S (2022) High clutch failure rate due to unpredictable rainfall for an ephemeral pool-breeding frog. Oecologia 198, 699-710.
| Crossref | Google Scholar |
Hamer AJ, Lane SJ, Mahony MJ (2002) Management of freshwater wetlands for the endangered green and golden bell frog (Litoria aurea): roles of habitat determinants and space. Biological Conservation 106, 413-424.
| Crossref | Google Scholar |
Herbert C (2007) Mangrove proliferation and saltmarsh loss in the Hunter Estuary. The Whistler 1, 1-9.
| Google Scholar |
Hettyey A, Ujszegi J, Herczeg D, Holly D, Vörös J, Schmidt BR, Bosch J (2019) Mitigating disease impacts in amphibian populations: capitalizing on the thermal optimum mismatch between a pathogen and its host. Frontiers in Ecology and Evolution 7, 254.
| Crossref | Google Scholar |
Hopkins GR, Brodie ED, Jr (2015) Occurrence of amphibians in saline habitats: a review and evolutionary perspective. Herpetological Monographs 29(1), 1-27.
| Crossref | Google Scholar |
Hutchings PA, Recher HF (1981) The fauna of Australian mangroves. Proceedings of the Linnean Society of New South Wales 106, 83-121.
| Google Scholar |
Lillywhite HB, Sheehy CM, III, Brischoux F, Grech A (2014) Pelagic sea snakes dehydrate at sea. Proceedings of the Royal Society B: Biological Sciences 281, 20140119.
| Crossref | Google Scholar |
Lillywhite HB, Sheehy CM, III, Sandfoss MR, Crowe-Riddell J, Grech A (2019) Drinking by sea snakes from oceanic freshwater lenses at first rainfall ending seasonal drought. PLoS ONE 14, e0212099.
| Crossref | Google Scholar |
Mahony MJ, Hamer AJ, Pickett EJ, McKenzie DJ, Stockwell MP, Garnham JI, Keely CC, Deboo ML, O’Meara J, Pollard CJ, Clulow S, Lemckert FL, Bower DS, Clulow J (2013) Identifying conservation and research priorities in the face of uncertainty: a review of the threatened bell frog complex in eastern Australia. Herpetological Conservation and Biology 8, 519-538.
| Google Scholar |
Marantelli G, Berger L, Speare R, Keegan L (2004) Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pacific Conservation Biology 10, 173-179.
| Crossref | Google Scholar |
Nifong JC, Silliman B (2017) Abiotic factors influence the dynamics of marine habitat use by a highly mobile “freshwater” top predator. Hydrobiologia 802, 155-174.
| Crossref | Google Scholar |
Penman T, Muir G, Magarey E, Burns E (2008) Impact of a chytrid-related mortality event on a population of the green and golden bell frog Litoria aurea. Australian Zoologist 34, 314-318.
| Crossref | Google Scholar |
Pollard CJ, Stockwell MP, Bower DS, Garnham JI, Pickett EJ, Darcovich K, O’meara J, Clulow J, Mahony MJ (2017) Removal of an exotic fish influences amphibian breeding site selection. The Journal of Wildlife Management 81, 720-727.
| Crossref | Google Scholar |
Pyke G, White A (2000) Factors influencing predation on eggs and tadpoles of the endangered green and golden bell frog Litoria aurea by the introduced plague minnow Gambusia holbrooki. Australian Zoologist 31, 496-505.
| Crossref | Google Scholar |
Pyke G, White A (2001) A review of the biology of the green and golden bell frog Litoria aurea. Australian Zoologist 31, 563-598.
| Crossref | Google Scholar |
Pyke GH, White AW (2022) Frog reproduction and community structure in relation to water attributes: setting the stage to understand effects of climatic variables and climate change. Australian Zoologist 42(3), 667-689.
| Crossref | Google Scholar |
Pyke GH, Ahyong ST, Fuessel A, Callaghan S (2013) Marine crabs eating freshwater frogs: why are such observations so rare. Herpetology Notes 6, 195-199.
| Google Scholar |
Rog SM, Clarke RH, Cook CN (2017) More than marine: revealing the critical importance of mangrove ecosystems for terrestrial vertebrates. Diversity and Distributions 23, 221-230.
| Crossref | Google Scholar |
Skelly DK (1996) Pond drying, predators, and the distribution of Pseudacris tadpoles. Copeia 1996, 599-605.
| Crossref | Google Scholar |
Stockwell MP, Clulow J, Mahony MJ (2012) Sodium chloride inhibits the growth and infective capacity of the amphibian chytrid fungus and increases host survival rates. PLoS ONE 7, e36942.
| Crossref | Google Scholar |
Streever WJ, Genders AJ (1997) Effect of improved tidal flushing and competitive interactions at the boundary between salt marsh and pasture. Estuaries 20, 807-818.
| Crossref | Google Scholar |
Streever WJ, Wiseman L, Turner P, Nelson P (1996) Short term changes in flushing of tidal creeks following culvert removal. Wetlands Australia 15, 22-30.
| Crossref | Google Scholar |
van de Mortel T, Goldingay R (1998) Population assessment of the endangered green and golden bell frog Litoria aurea at Port Kembla, New South Wales. Australian Zoologist 30, 398-404.
| Crossref | Google Scholar |
White A (2006) A trial using salt to protect green and golden bell frogs from chytrid infection. Herpetofauna 36, 93-96.
| Google Scholar |
White A, Pyke G (2008) Green and golden bell frogs in New South Wales: current status and future prospects. Australian Zoologist 34, 319-333.
| Crossref | Google Scholar |


