A southern range extension for Sminthopsis macroura in Western Australia, at Eucla
Linette S. Umbrello A B * , Nathan Beerkens
A B * , Nathan Beerkens  C , Joshua Keen
C , Joshua Keen  C , Sylvie Schmidt
C , Sylvie Schmidt  C , Roy J. Teale
C , Roy J. Teale  B C , Kenny J. Travouillon
B C , Kenny J. Travouillon  B , Michael Westerman
B , Michael Westerman  D and Andrew M. Baker
D and Andrew M. Baker  A E
A E
A
B
C
D
E
Abstract
The stripe-faced dunnart (Sminthopsis macroura) is one of the most widespread dasyurids in Australia, occurring mostly in semiarid and arid habitats. It is not known to inhabit coastal regions of southern Australia, and no records have previously been recorded from latitudes greater than 28.5°S in Western Australia. Following the capture of an individual south of the known species range provisionally identified based on external morphology as S. macroura, we used DNA and craniodental morphology to corroborate the specimen’s identification, providing a record of the species at Eucla, Western Australia. This represents a large range extension for S. macroura of 630 km from the nearest confirmed records of the species in Western Australia and South Australia.
Keywords: biodiversity survey, Dasyuridae, marsupial, morphology, museum, phylogenetics, range extension, Sminthopsis.
Introduction
Biodiversity surveys are essential to our understanding of species distributions, yet large areas of Australia remain under surveyed (How and Cowan 2006). In the past, government agencies significantly advanced biodiversity knowledge through surveys to remote areas, but they are increasingly being conducted by private companies as a requirement for development proposals. In Australia, surveys by environmental consultants have contributed greatly to our understanding of species distributions and have led to species discovery (Doughty et al. 2007, 2014; Ellis et al. 2015). A sound understanding of local fauna and their distributions is essential for field biologists to correctly identify unusual species or records.
In this paper, we report on a range extension and novel habitat for the stripe-faced dunnart (Sminthopsis macroura) resulting from an individual captured during a biodiversity survey. This widespread species occurs throughout the arid zone and northern savannahs of Australia (Dickman and Greenville 2023) It has been documented to prefer clay-loam soils in the Pilbara (Gibson and McKenzie 2009) and Sturt National Park, New South Wales, stony soils at Sandringham Station, Queensland (Morton et al. 1983), and a range of substrates in the Northern Territory (Cole and Gibson 1991). Molecular studies have revealed genetic structure between key areas of the distribution: animals in the Pilbara and mid-west regions in Western Australia are genetically distinct from those in South Australia and eastern Australia (Blacket et al. 2001; Umbrello et al. 2020). This genetic distinction also corresponds to an apparent gap of over 900 km in the distribution of S. macroura, from Yamarna Station in Western Australia to the Gawler Ranges in south-central South Australia (Owens and Graham 2009; Atlas of Living Australia 2024). This gap may represent a true break in the range, as fauna surveys in the Great Victoria Desert and Nullarbor regions that span this area have recorded other Sminthopsis species (Burbidge et al. 1976; McKenzie and Robinson 1987; How et al. 1988; Brennan et al. 2012), but not S. macroura. Our new record represents a significant southern extension of the known S. macroura distribution in Western Australia and highlights the importance of using genetic and morphological evidence to verify unusual species records.
Materials and methods
Field methods and site description
The specimen was captured on 7 April 2022, in a 20 L dry pitfall trap set on a coastal foredune 25 km south-west of Eucla, Western Australia (31.825°S, 128.679°E). The habitat at the site consisted of long unburnt, scattered tall shrubs (Nitraria billardierei, Acacia spp. and Alyxia spp.) over very open low shrubs (Rhagodia, Tecticornia and Suaeda spp.) and scattered herbs (including Frankenia, Sonchus and Carpobrotus spp.) on white sand.
Molecular methods
The specimen was deposited at the Western Australian Museum for vouchering (registered as WAM M65257), and a liver sample was taken for genetic analysis. Total genomic DNA was extracted using a Qiagen DNeasy® tissue and blood kit according to manufacturer’s instructions with an elution volume of 100 μL. We amplified the mitochondrial Control Region (CR) gene and 12S rRNA, as these loci are commonly used in barcoding studies to corroborate the identification of species, following the protocol described in Umbrello et al. (2017) (for further details, see Supplementary Information). DNA was Sanger sequenced at the Australian Genome Research Facility (Perth, Western Australia), and the resulting sequence reads were assembled and processed in Geneious Prime (Kearse et al. 2012), as shown in Umbrello et al. (2017).
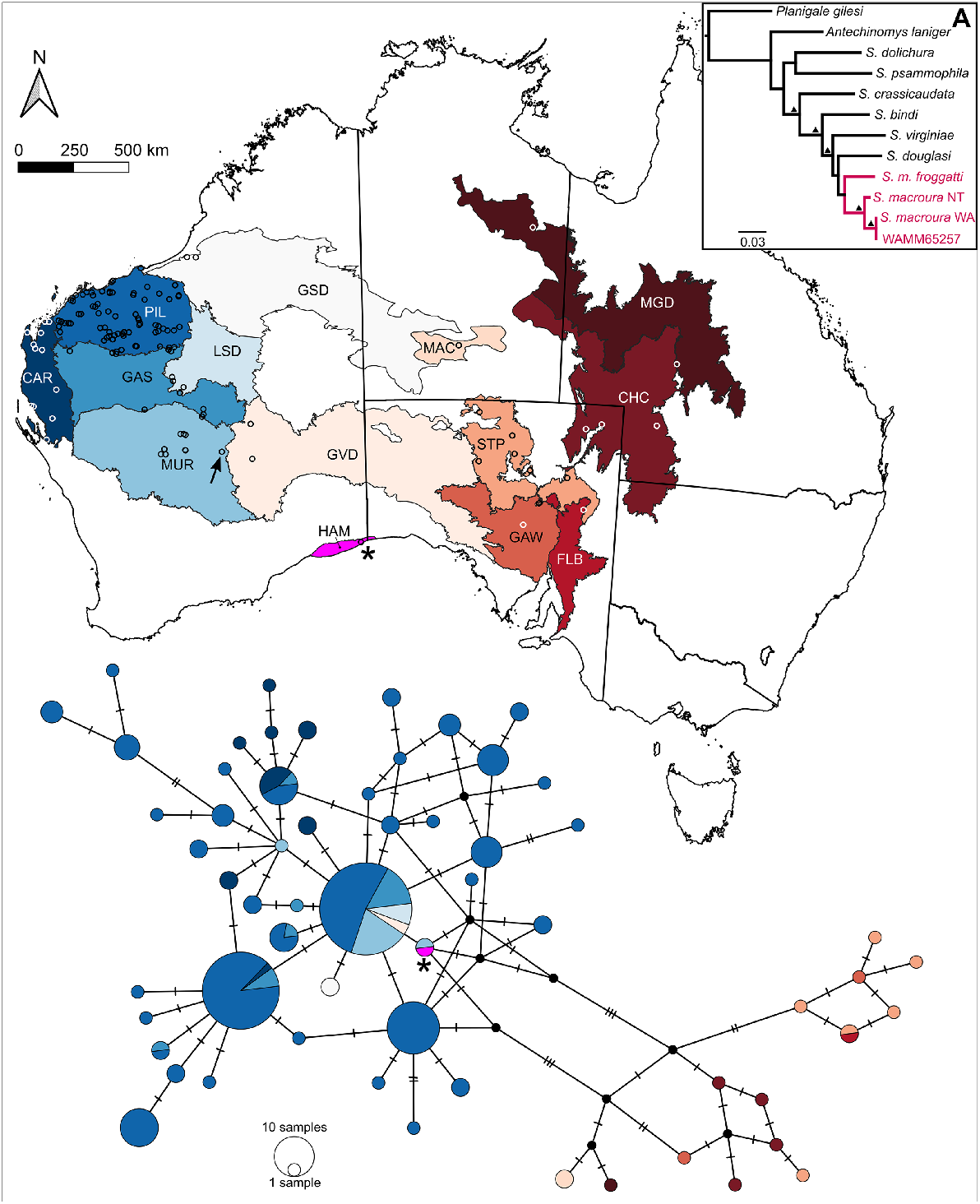
To corroborate the identity of WAM M65257 a Maximum Likelihood phylogeny, partitioned by loci, using a GTR nucleotide model and 1000 bootstrap replicates, was built in the RAxML (Stamatakis 2006) plug-in in Geneious with the new sequences aligned to exemplars from the published literature (Blacket et al. 1999; Blacket et al. 2001). To investigate the relationships between WAM M65257 and other S. macroura individuals, a Maximum Parsimony TCS haplotype network (Clement et al. 2000) was constructed for the CR sequence data including sequences from Umbrello et al. (2020) in the software program PopART (Leigh and Bryant 2015) with haplotypes grouped by Australian bioregion (Department of Climate Change, Energy, the Environment and Water 2020).
Morphological methods
The skull and dentary of WAM M65257 was removed and cleaned with fine forceps. The skull was identified to species level by comparison with other Sminthopsis skull specimens held at the Western Australian Museum and by keying out craniodental features using the identification key outlined in Archer (1981).
Results
The specimen was provisionally distinguished from other Sminthopsis occurring near the Eucla region (S. crassicaudata, S. dolichura, S. fuliginosa and S. gilberti) using external morphology, due to the combination of a prominent facial stripe, incrassated (fattened) tail and the enlarged, smooth granules on the interdigital pads on the underside of the foot (Fig. 1).
(a) Photograph of WAM M65257 in life. Note the distinct facial stripe running from between the ears down to between the eyes and the incrassated (fattened) tail base. Photo copyright Joshua Keen/Biota. (b) Hind-foot morphology of M65257 showing the underside of the hindfoot with enlarged apical granules (see enlarged inset) on the interdigital footpads (Photo copyright Western Australian Museum).

The skull was identified as that of S. macroura following the key provided in Archer (1981) and differs from the aforementioned dunnart species by having large entoconids on M1–3, short premaxillary vacuities and the hypocristid separate from the entoconid on M3. Archer’s (1981) revision precedes the splitting of S. murina into S. dolichura, S. griseoventer (note: now referred to as S. fuliginosa) and S. gilberti (Kitchener et al. 1984), but Kitchener et al. (1984) considered these three species to differ from all other Sminthopsis based on the same characters outlined in Archer (1981) for S. murina.
The CR genetic sequence was found to be identical to that of an individual (WAM M50650; Genbank Accession MT366297) captured at Duketon, Western Australia (27.79°S, 122.27°E), some 765 km north-west of our WAM M65257 S. macroura specimen (Fig. 2). The 12S sequence of our specimen was identical to published sequences AF339114 (Mardie Station, Pilbara) and AF339117 (Mileura, Murchison), which are over 1700 and 1200 km north-west of Eucla, respectively. Taken together, this places our WAM M65257 as part of the Western Australia genetic clade and not closely related to the S. macroura population in South Australia, the closest individuals of which are ~630 km east of Eucla. To our knowledge, there have previously been no records of S. macroura from the Hampton bioregion, where WAM M65257 was collected, or the adjacent bioregion (Nullarbor).
Mitochondrial haplotype network (Control Region) of Sminthopsis macroura samples coloured by bioregion (Department of Climate Change, Energy, the Environment and Water 2020) shown on a corresponding map of Australia. Circle size indicates the number of individuals sharing each unique haplotype, small black circles indicate unsampled nodes and hatch marks indicate number of nucleotide changes. Samples used in the haplotype network are shown as open circles on the map. The location of WAM M65257 is denoted by an asterisk on both the map and network, and WAM M50650 is indicated on the map by a black arrow. Inset (A) Maximum Likelihood phylogeny from combined mitochondrial loci (12S + CR) placing WAM M65257 with other S. macroura reference sequences from Blacket et al. (1999, 2001), black triangles indicate bootstrap support values greater than 70%.
Discussion
Based on total evidence (morphological features and mtDNA) we report a large range extension of the stripe-faced dunnart (Sminthopsis macroura) from coastal dune habitat near Eucla along the south-east coast of Western Australia. This location falls within the Roe Plains, a narrow coastal strip of sandy and clay soils bounded by the limestone escarpment of the Hampton range to the north (Greer et al. 1991). Prior to the surveys in which WAM M65257 was captured, there had been little systematic survey effort on the Roe Plains apart from targeted reptile surveys in which only two mammal species were captured: Cercartetus concinnus and Mus musculus (Porter 2019). WAM M65257 shares mitochondrial haplotypes with other specimens from Western Australia, rather than those in South Australia, which are found at similar latitudes (Fig. 2). There is a large gap in the records of S. macroura between mid-Western Australia and Eucla, mostly encompassing the Nullarbor region, and eastwards to the Gawler Ranges in South Australia (Atlas of Living Australia 2024). Sminthopsis macroura is also absent from subfossil material from Nullarbor caves, some 100–270 km west of Eucla (Lundelius 1983; Newman-Martin 2020), despite various other Sminthopsis species being found in these deposits, including S. crassicaudata, S. fuliginosa and S. gilberti, which suggests S. macroura is rare in the region. Further systematic survey work in the Eucla area and adjacent Nullarbor may yield more records of S. macroura. This would help us to understand the habitat requirements of the species and better assess whether it is absent from the Nullarbor.
Mitochondrial haplotype sharing over vast distances is also present in the most commonly occurring haplotype in our dataset (shown as the largest circle in Fig. 2), which is shared by individuals from the south-eastern Pilbara to the central Goldfields region, a distance of over 837 km. Given the internal position of this widely shared ancestral haplotype in the network, it could be evidence for population expansion from historical refugia, with the Pilbara having been an important refugium for many taxa (Kingman 1982; Pepper et al. 2013; Umbrello et al. 2020). The apparent rarity of the CR haplotype that is shared between WAM M65257 and WAM M50650 (see black arrow and asterisk, Fig. 2) likely reflects the paucity of sampling in this area, and more genetic samples from this region may increase the frequency of this haplotype.
The new record reported here highlights the value of conducting biodiversity surveys in poorly studied regions and the substantial value of voucher specimens to verify identifications using a total evidence approach (Kemper et al. 2011). It also highlights the contribution that biologists working in the private sector make to our understanding of species distributions. Having a sound understanding of the local fauna is crucial for identifying when specimens warrant further investigation and can lead to improved knowledge of species distributions (Umbrello et al. 2022). New records such as that reported here provide valuable information that deepens our understanding of how small mammals are distributed across vast Australian landscapes. As mammal species continue to undergo population declines throughout Australia (Legge et al. 2023), building on and expanding existing knowledge assists with conservation assessments and management of poorly understood taxa and is a fundamental service provided by scientists working in museums, government and the private sector.
Data availability
New sequence data generated in this study are available from the NCBI Genbank database with the accession numbers Control Region: OR778374 and 12S: OR789447.
Declaration of funding
This research was supported by the Australian Biological Resources Study National Taxonomy Research Grant Program Postdoctoral Research Grant scheme (grant no.: 4-G046WUZ) and research funding from the Queensland University of Technology. The specimen was captured during a field survey conducted by Biota Environmental Sciences, which was funded by the Western Green Energy Hub.
Acknowledgements
The specimen was captured during a field survey conducted by Biota Environmental Sciences, in accordance with Fauna Taking (Biological Assessment) Licence BA27000553 issued by the Western Australian Department of Biodiversity Conservation and Attractions. Botanical description of the site was provided by Ayesha Lapinski and Jason Teuber, both of Biota Environmental Sciences. Linette Umbrello was supported by the Australian Biological Resources Study National Taxonomy Research Grant Program Postdoctoral Research Grant scheme and the Queensland University of Technology.
References
Archer M (1981) Results of the Archbold Expeditions No. 104. Systematic revision of the dasyurid marsupial genus Sminthopsis Thomas. Bulletin of the American Museum of Natural History 168, 61-224.
| Google Scholar |
Atlas of Living Australia (2024) Atlas of living Australia Sminthopsis macroura occurrence download. Available at https://doi.org/10.26197/ala.b8c56fad-6c83-477d-8383-b02267755598 [Accessed 15 March 2024]
Blacket MJ, Krajewski C, Labrinidis A, Cambron B, Cooper S, Westerman M (1999) Systematic relationships within the dasyurid marsupial tribe Sminthopsini – a multigene approach. Molecular Phylogenetics and Evolution 12, 140-155.
| Crossref | Google Scholar | PubMed |
Blacket MJ, Adams M, Cooper SJB, Krajewski C, Westerman M (2001) Systematics and evolution of the dasyurid marsupial genus Sminthopsis: I. the Macroura species group. Journal of Mammalian Evolution 8, 149-170.
| Crossref | Google Scholar |
Brennan KEC, Twigg PJ, Watson A, Pennington A, Sumner J, Davis R, Jackson J, Brooks B, Grant F, Underwood R (2012) Cross-cultural systematic biological surveys in Australia’s Western Desert. Ecological Management & Restoration 13, 72-80.
| Crossref | Google Scholar |
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Molecular Ecology 9, 1657-1659.
| Crossref | Google Scholar | PubMed |
Cole JR, Gibson DF (1991) Distribution of stripe-faced dunnarts Sminthopsis macroura and desert dunnarts S. youngsoni (Marsupialia: Dasyuridae) in the Northern Territory. Australian Mammalogy 14, 129-131.
| Crossref | Google Scholar |
Doughty P, Maryan B, Melville J, Austin J (2007) A new species of Ctenophorus (Lacertilia: Agamidae) from Lake Disappointment, Western Australia. Herpetologica 63, 72-86.
| Crossref | Google Scholar |
Doughty P, Kealley L, Fitch A, Donnellan SC (2014) A new diminutive species of Varanus from the Dampier Peninsula, western Kimberley region, Western Australia. Records of the Western Australian Museum 29, 128-140.
| Crossref | Google Scholar |
Ellis RJ, Spencer PBS, Doody JS, Parkin T (2015) A significant south-western range extension for the desert mouse (Pseudomys desertor) in Western Australia. Australian Mammalogy 38, 120-123.
| Crossref | Google Scholar |
Gibson LA, McKenzie NL (2009) Environmental associations of small ground-dwelling mammals in the Pilbara region, Western Australia. Records of the Western Australian Museum, Supplement 78, 91-122.
| Crossref | Google Scholar |
Greer AE, Thorpe R, Malhotra A (1991) Natural history notes on lizards from the Roe Plain, Western Australia. The Western Australian Naturalist 18, 178-184.
| Google Scholar |
How RA, Cowan MA (2006) Collections in space and time: geographical patterning of native frogs, mammals and reptiles through a continental gradient. Pacific Conservation Biology 12, 111-133.
| Crossref | Google Scholar |
How RA, Dell J, Muir BG (1988) The biological survey of the Eastern Goldfields of Western Australia: Vertebrate Fauna. Records of the Western Australian Museum, Supplement 30, 44-83.
| Google Scholar |
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics (Oxford, England) 28, 1647-1649.
| Crossref | Google Scholar | PubMed |
Kemper CM, Cooper SJB, Medlin GC, Adams M, Stemmer D, Saint KM, McDowell MC, Austin JJ (2011) Cryptic grey-bellied dunnart (Sminthopsis griseoventer) discovered in South Australia: genetic, morphological and subfossil analyses show the value of collecting voucher material. Australian Journal of Zoology 59, 127-144.
| Crossref | Google Scholar |
Kingman JFC (1982) The coalescent. Stochastic Processes and their Applications 13, 235-248.
| Crossref | Google Scholar |
Kitchener D, Stoddartt J, Henry J (1984) A taxonomic revision of the Sminthopsis murina complex (Marsupialia, Dasyuridae) in Australia, including descriptions of four new species. Records of the Western Australian Museum 11, 201-284.
| Google Scholar |
Legge S, Rumpff L, Garnett ST, Woinarski JCZ (2023) Loss of terrestrial biodiversity in Australia: magnitude, causation, and response. Science 381, 622-631.
| Crossref | Google Scholar | PubMed |
Leigh JW, Bryant D (2015) PopART: full-feature software for haplotype network construction. Methods in Ecology and Evolution 6, 1110-1116.
| Crossref | Google Scholar |
Lundelius EL, Jr (1983) Climatic implications of Late Pleistocene and Holocene faunal associations in Australia. Alcheringa: An Australasian Journal of Palaeontology 7, 125-149.
| Crossref | Google Scholar |
Morton SR, Denny MJS, Read DG (1983) Habitat preferences and diets of sympatric Sminthopsis crassicaudata and S. macroura (Marsupialia: Dasyuridae). Australian Mammalogy 6, 29-34.
| Crossref | Google Scholar |
Owens H, Graham A (2009) ‘Census of South Australian vertebrates’. (Department of Environment and Natural Resources, South Australia and South Australian Museum) Available at https://www.environment.sa.gov.au/topics/science/information-and-data/census-of-sa-vertebrates [Accessed 12 March 2024]
Pepper M, Doughty P, Keogh JS (2013) Geodiversity and endemism in the iconic Australian Pilbara region: a review of landscape evolution and biotic response in an ancient refugium. Journal of Biogeography 40, 1225-1239.
| Crossref | Google Scholar |
Porter J (2019) Reptile studies report 2019: unpublished report on the reptiles of the Eyre Bird Observatory. (Birdlife Australia) Available at https://direct.birdlife.org.au/documents/EBO-Reptile_Course_Report-2019.pdf
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688-2690.
| Crossref | Google Scholar | PubMed |
Umbrello LS, Woolley PA, Westerman M (2017) Species relationships in the dasyurid marsupial genus Pseudantechinus (Marsupialia: Dasyuridae): a re-examination of the taxonomic status of Pseudantechinus roryi. Australian Journal of Zoology 65, 240-247.
| Crossref | Google Scholar |
Umbrello LS, Didham RK, How RA, Huey JA (2020) Multi-species phylogeography of arid-zone Sminthopsinae (Marsupialia: Dasyuridae) reveals evidence of refugia and population expansion in response to Quaternary change. Genes 11, 963.
| Crossref | Google Scholar | PubMed |
Umbrello LS, Potter LC, Westerman M, Woolley PA (2022) First record of Pseudantechinus macdonnellensis (Marsupialia: Dasyuridae) in the Kimberley region of Western Australia. Australian Mammalogy 44, 404-406.
| Crossref | Google Scholar |


