Relationship between frailty and discharge outcomes in subacute care
Melanie N. Haley A B , Yvonne D. Wells C D and Anne E. Holland A BA Alfred Health, Alfred Hospital, Commercial Road, Prahran, Vic. 3181, Australia.
B La Trobe University, Bundoora, Vic. 3083, Australia.
C Australian Institute for Primary Care and Ageing, La Trobe University, Bundoora, Vic. 3083, Australia. Email: a.holland@alfred.org.au
D Corresponding author. Email: y.wells@latrobe.edu.au
Australian Health Review 38(1) 25-29 https://doi.org/10.1071/AH13067
Submitted: 7 April 2013 Accepted: 18 September 2013 Published: 22 November 2013
Abstract
Aims To determine whether level of frailty can predict length of stay, discharge destination, level of participation in physiotherapy, and degree of physical improvement with physiotherapy in older, subacute hospital patients.
Method The Edmonton Frail Scale (EFS) was administered to 75 older people in a subacute hospital setting. Relationships between EFS score and a range of other measures, including participation in physiotherapy, Elderly Mobility Scale, discharge destination and length of stay, were examined.
Results Level of frailty did not predict length of stay (rho = –0.13, P = 0.24), discharge destination (t = –1.32, P = 0.19), raw change on the Elderly Mobility Scale (rho = 0.06, P = 0.61) or rate of change on the Elderly Mobility Scale (r = –0.001, P = 0.98). In addition, participants with a high level of frailty were more likely to achieve a satisfactory level of participation in physiotherapy sessions than those with low frailty (OR 1.43, P = 0.02).
Conclusion Level of frailty measured with the EFS was not a useful predictor of rehabilitation and discharge outcomes for older people in subacute care. These results do not support the routine use of the EFS to measure frailty in subacute care.
What is known about this topic? In a community-dwelling population, level of frailty has been found to predict poor outcomes from surgery, falls, fractures, disability, need for residential care and mortality. However, little is known about the impacts of frailty in a subacute setting, nor how frailty could best be measured in this setting.
What does this paper add? The use of the EFS as a predictive tool was not supported by the results of this exploratory study.
What are the implications for practitioners? Alternative frailty measures may be more suitable than the EFS for patients in a subacute setting.
Introduction
Frailty has been defined as a clinical syndrome of apparent vulnerability or inability to withstand illness without loss of function.1–3 The majority of frailty studies have been conducted on community-dwelling older adults, and commonly investigated dependent variables include risk of hospitalisation or residential care placement.4–6
Quantifying frailty and its influence on functional status is important, as it may assist policy makers and program planners, and may help predict patient outcomes.7,8 Degree of frailty could also be an indicator of rehabilitation potential, allowing service providers to target those most at risk and to assist in advocacy for additional hospital staff and resources.3,9 In a community-dwelling population, level of frailty has been found to predict poor outcomes from surgery, falls, fractures, disability, need for residential care and mortality.4,5,10–12 Little is known, however, about the impacts of frailty among patients in the subacute hospital setting.
The Edmonton Frail Scale (EFS) is a 17-point scale that was developed as a screen in 2006 and tested for validity and reliability in a sample comprising both inpatients and outpatients.2 It covers the important domains of mood, cognition and social support.2 The EFS has been found to have good inter-rater reliability, good construct validity and acceptable internal consistency.2 Unlike other scales designed for use by geriatricians, the EFS can be used by any health professional, making it more widely applicable than many of the alternative measures. It delivers a wide range of scores (0–17), which is particularly important when investigating an older inpatient population due to the risk of floor effects. High scores on the EFS (indicating higher levels of frailty) predict poor discharge outcomes and post-operative complications in older adults in acute care,11 but its usefulness as a predictor of outcomes in subacute care has not been evaluated.
The aim of the present study was to investigate the usefulness of the EFS as a measure of frailty for use with aged care inpatients. The researchers hypothesised that high frailty would be associated with longer duration of subacute hospital length of stay (LOS), greater likelihood of discharge to residential care, smaller improvement in mobility between admission and discharge, and poorer attendance at inpatient physiotherapy sessions.
Methods
Ethics approval
Ethics approval was obtained from the Alfred Health Ethics Committee (273/10) and the La Trobe University Faculty of Health Sciences Human Ethics Committee (FHEC10/178).
Participants
Participants were 86 patients admitted consecutively to two subacute aged care wards of a large Melbourne rehabilitation hospital. Recruitment occurred between February and July 2011. Potential participants (or next of kin) provided informed consent before their inclusion in the study, according to the ethical requirements of the project.
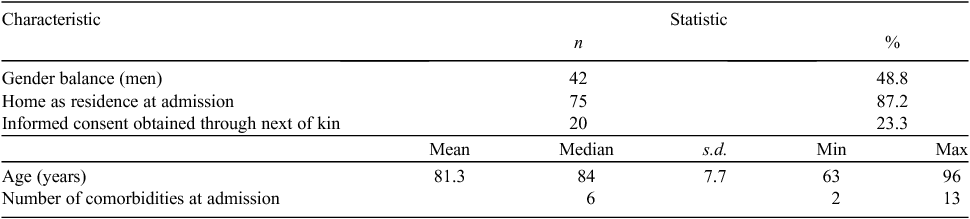
A summary of participants’ baseline characteristics is presented in Table 1. Participants were 60 years or older and slightly more than half were women. The majority of participants lived at home before admission, rather than in supported accommodation or residential care. The most common reason for participant hospital admission was fracture (33.7%), followed by presence of a cardiac condition (14.0%). The median number of co-morbidities per participant was six, with a maximum of 13.
|
Most participants provided consent for the project independently and assistance to answer questions was requested by only two participants. The remaining participants were able to answer questions independently, or the research coordinator was able to access the required information from the participant’s medical record without assistance from the participant’s next of kin.
Potential participants were excluded due to inadequate cognition (Mini Mental State Examination score less than 23)13 combined with lack of availability of next of kin to provide consent; inability to answer simple questions; admission to the subacute ward for less than 48 h duration; palliation; and culturally and linguistically diverse (CALD) background requiring written material to be translated.
Power
To detect a significant relationship between frailty, rehabilitation and discharge outcomes with medium effect size, a power of 0.80 and an α of 0.05, it was estimated that 85 participants should be recruited.14
Procedure
Of 250 potential participants, 164 were not recruited to the study. The most common reasons for exclusion were CALD background (n = 42), incomplete Mini Mental State Examination after 1 week of admission (n = 39) and declining to participate (n = 20). Of the 86 participants recruited, complete data were obtained for 75 participants; three participants died, five were transferred to another facility before discharge and three were unable to complete a component of the EFS.
Patients who consented to participate in the study were assessed on the EFS within 1 week of admission by the research coordinator using a medical record review and participant or next of kin interview. One item on the EFS requires the participant to complete a Timed Up and Go test: this item was completed by the treating therapists as part of routine physical assessment. Treating therapists also completed a record for each participant indicating whether the participant attended physiotherapy for each weekday of admission.
Outcome measures and independent variables
The outcome measures of interest were LOS (days), discharge destination, raw change and rate of change between admission and discharge of Elderly Mobility Scale (EMS) scores (indicators of improvement in mobility), and proportion of physiotherapy sessions attended.
Discharge destination was dichotomised as community or residential care. Participants who were admitted from a residential care facility were excluded from the analysis of the relationship between EFS and discharge destination, given the low probability of them being discharged anywhere but back to residential care.
The EMS is a 20-point mobility scale,15 chosen as a mobility measure for use in the current study because it has been found to have excellent intra-rater reliability, inter-rater reliability and concurrent validity in an acute hospital setting.16 The scale has also proved to be more sensitive to change in mobility than the Functional Independence Measure or the Barthel Index in day hospital patients.17 Unlike some alternative mobility scales, the EMS caters specifically for the older, inpatient population as it includes items that assess a low level of function (e.g. bed mobility). Difference between EMS at admission and discharge was used as the measure of improvement in mobility. Rate of change between admission and discharge was calculated by dividing difference scores by LOS in days.
Percentage of sessions attended was converted to a dichotomous variable because as a continuous variable it was substantially negatively skewed and untransformable. Attendance at 75% or more sessions was categorised as satisfactory by the researchers, a figure based on clinical judgement in the absence of precedence in the literature.
Score on the EFS was the primary independent variable of interest. Other independent variables explored in the study included age, gender, EMS score on admission, admission residential status (community or residential care), medical reason for hospital admission, number of comorbid conditions, and ability to complete the Timed Up and Go test on admission. These other independent variables were included because of their potential to confound the relationships between EFS and the outcome measures of interest.
Statistical analyses
Statistical analysis was undertaken using SPSS, Version 17.0.
Prior to investigating the relationships between all variables, normality of each continuous variable was assessed by analysing distribution histograms. Preliminary exploration of data involved testing bivariate relationships between all independent variables, including possible confounding variables, and the outcome measures of interest. Relationships between categorical variables were explored using the Chi-square statistic. Relationships between categorical variables and continuous variables were explored using t-tests, the Kruskal–Wallis test or analysis of variance as appropriate. Relationships between continuous variables were assessed using Spearman’s rho. Potential confounding variables that were found to relate to the outcome measures of interest at P < 0.5 were included in subsequent multivariate analyses.
Prior to hypothesis testing, continuous outcome measures that were not normally distributed were first transformed using either a natural log or log base 10. The capacity of the EFS to predict LOS and improvement in the EMS was tested using multivariate linear regression analyses. The capacity of the EFS to predict discharge destination (i.e. discharge to residential care or to the community) and level of participation in rehabilitation was tested using multivariate logistic regression analyses.
Results
Participant outcomes
Median LOS was 19 days (IQR 13–34 days). Forty-seven (54.7%) participants were discharged to residential care rather than to the community. The mean EFS score was 8.65 (s.d. 2.12), the mean EMS rate of change was 0.29 per day (s.d. 0.52), and the median change between admission and discharge EMS scores was 4 points (IQR 1–11 points). Sixty-five (75.6%) participants attended at least 75% of possible physiotherapy sessions.
Relationships between frailty and other measures
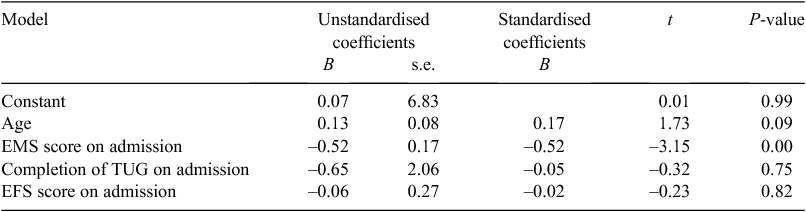
Frailty was not significantly related to LOS in bivariate analysis (rho = –0.13, P = 0.24), nor did it predict LOS in multivariate regression analysis (Table 2).
Frailty did not significantly increase the risk of discharge to residential care for this cohort. The mean EFS score for those participants discharged to the community was 8.95 (s.d. 1.99), whereas the mean EFS score for those participants admitted from the community but discharged to residential care was 8.31 (s.d. 2.25); the difference was not significant (t = –1.32, P = 0.19).
Frailty was not significantly related to degree of physical improvement (i.e. change in EMS score) with physiotherapy (rho = 0.06, P = 0.61), nor did it predict degree of physical improvement when possible confounding variables were taken into account (Table 3). In addition, frailty was not significantly related to rate of change in the physical outcome measure (r = 0.00, P = 0.98), and rate of change in EMS scores was not explored further.
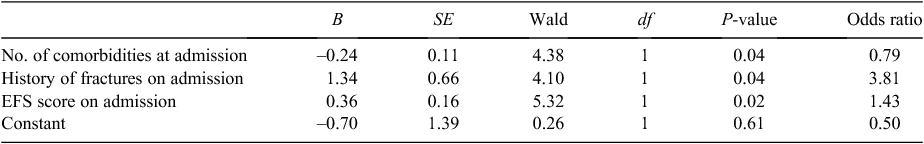
Contrary to the hypothesis, the proportion of physiotherapy sessions attended was most likely to be satisfactory when level of frailty was high rather than low (Table 4). Participants who attended a satisfactory proportion of physiotherapy sessions tended to have higher EFS scores than those who attended fewer sessions (mean EFS score 8.86 (s.d. 2.16) versus 7.95 (s.d. 1.84): t = –1.83, P = 0.08).
Discussion
The purpose of the analysis was to explore the usefulness of a frailty screen for older people in inpatient subacute care. Contrary to the investigators’ hypotheses, none of the major outcomes − LOS, discharge designation or either raw change or rate of change in mobility – were associated with the measure of frailty. Further, patients who attended a satisfactory percentage of physiotherapy sessions had higher levels rather than lower levels of frailty, as measured by the EFS, than those who did not. However, the usefulness of a measure of frailty in subacute settings warrants further investigation because both the measure of frailty used and hospital processes experienced by participants may have impacted significantly on the findings obtained.
EFS may not be an effective measure of frailty in this setting
The EFS may not have adequately captured the level of frailty for this inpatient cohort. The range of frailty scores obtained in the current study was restricted (the highest score being 13 out of a possible 17, where high scores indicate greater frailty). Similar findings were seen in a study of 125 elderly people (mean age of 77 years) who were awaiting surgery, whose highest EFS score was 11.11 It is surprising that maximum EFS scores were relatively low for both of these cohorts, given that the patient groups investigated would usually be considered as considerably frailer than the older population in general.
The EFS may be more appropriately applied to a community-dwelling population than to older people in subacute care. Two items require participants to recall their pre-admission status rather than their status at the time of questioning. For some participants, the reason for their hospital admission (e.g. stroke) had greatly altered their function and level of vulnerability, making an accurate response to this item challenging.
The EFS is not easy to use in a cohort with a high prevalence of moderate to severe cognitive impairment, such as subacute aged care. Some items on the EFS can only be answered by the participant, not by a proxy (e.g. In general, how would you describe your health?). Current literature suggests that cognition is likely to have some impact on level of frailty.4,5,18 Future studies in this area should ensure that people with cognitive impairment are included by utilising scales appropriate to this patient group.
Measurement was impacted by hospital decision making and process
Therapist clinical reasoning, not patient capacity or compliance, may have the most bearing on patient attendance at therapy. In the present study, failure to attend rehabilitation sessions was generally not related to medical reasons or because the participant declined to participate. Rather, participants more frequently did not receive therapy because they were a low priority for physiotherapy on that particular day or because of staffing constraints (e.g. the physiotherapist was on sick leave). Therefore, a possible explanation for the unexpected positive relationship between frailty and attendance at physiotherapy is that participants with high frailty were considered a higher priority by the treating therapist than those with low frailty.
LOS and discharge destination have limitations as outcome measures that are well recognised. Some patients have extended hospital stays because they are awaiting resolution of social issues, not because of illness or a physical need for longer rehabilitation. In contrast, some patients have short subacute hospital stays because they are swiftly transferred back to the acute hospital, not because they have achieved their rehabilitation goals. In relation to discharge destination, possible discharge to transition or interim care is not satisfactorily taken into account when the discharge destination is dichotomised into community or residential care.
Strengths of the study
The construct of frailty has been the focus of much research attention but rarely in the inpatient setting. The present study explored the usefulness of a measure of frailty in the hospital setting by applying a reliable and validated frailty screen to a subacute inpatient cohort. The challenges and discoveries of the current study may help to guide further studies. The wide range of variables investigated in the study accounted strongly for the potential impacts of confounding variables, and the identification of limitations to the EFS in an inpatient setting could prove invaluable in future research.
Limitations of the study
Of the 250 potential participants of the study, 164 were omitted and a further 10 did not complete testing. The high number of omitted potential participants is a limitation of the study, reducing the representativeness of the sample of patients admitted to a subacute aged care ward. The representativeness of the sample is also limited by the relatively small sample size.
The most common reason for omission from the study was inability to respond to the research interview in English (i.e. coming from a CALD background). Although the prevalence of high frailty among patients who are from CALD backgrounds in Australia is not known, a study by Santos-Eggimann et al.19 suggests that the prevalence of high frailty may be different among different ethnic groups. We did not have the resources to provide translators for CALD participants and this is a limitation of the study. Future well-resourced research is required to enhance our understanding of frailty in this important patient group.
Conclusion
The results of this preliminary research do not support the usefulness of the EFS as a measure of frailty in a subacute aged care setting. Both the outcome tool used to measure frailty and hospital processes may have had a significant impact on the study’s findings. Therapists and administrators should consider alternative measures of frailty and alternative measures of successful hospital discharge for inpatients in subacute aged care.
Competing interests
The authors declare there are no competing interests.
Acknowledgements
This research was supported by an Alfred Health Research Grant and was completed as part of the first author’s Masters of Gerontology degree, La Trobe University.
References
[1] Rockwood K, Mogilner A, Mitnitski A. Changes with age in the distribution of a frailty index. Mech Ageing Dev 2004; 125 517–9.| Changes with age in the distribution of a frailty index.Crossref | GoogleScholarGoogle Scholar | 15246748PubMed |
[2] Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton frail scale. Age Ageing 2006; 35 526–9.
| Validity and reliability of the Edmonton frail scale.Crossref | GoogleScholarGoogle Scholar | 16757522PubMed |
[3] Wells JL, Seabrook JA, Stolee P, Borrie MJ, Knoefel F. State of the art in geriatric rehabilitation. Part 1: review of frailty and comprehensive geriatric assessment. Arch Phys Med Rehabil 2003; 84 890–7.
| State of the art in geriatric rehabilitation. Part 1: review of frailty and comprehensive geriatric assessment.Crossref | GoogleScholarGoogle Scholar | 12808544PubMed |
[4] Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 2004; 52 1929–33.
| Operationalizing a frailty index from a standardized comprehensive geriatric assessment.Crossref | GoogleScholarGoogle Scholar | 15507074PubMed |
[5] Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010; 58 681–7.
| Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation.Crossref | GoogleScholarGoogle Scholar | 20345864PubMed |
[6] Woo J, Goggins W, Sham A, Ho SC. Public health significance of the frailty index. Disabil Rehabil 2006; 28 515–21.
| Public health significance of the frailty index.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD287is1WmtA%3D%3D&md5=b6195743fc1cab9ac627e32f3866958fCAS | 16513584PubMed |
[7] Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173 489–95.
| A global clinical measure of fitness and frailty in elderly people.Crossref | GoogleScholarGoogle Scholar | 16129869PubMed |
[8] Abellan Van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The IANA task force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008; 12 29–37.
| The IANA task force on frailty assessment of older people in clinical practice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c%2Fgt1Gisw%3D%3D&md5=5b390b8974ce7c6b50855f3568a3f42cCAS | 18165842PubMed |
[9] Fairhall N, Aggar C, Kurrle SE, Sherrington C, Lord S, Lockwood K, et al Frailty intervention trial (FIT). BMC Geriatr 2008; 8 27
| Frailty intervention trial (FIT).Crossref | GoogleScholarGoogle Scholar | 18851754PubMed |
[10] Ensrud KE, Ewing SK, Taylor BC, Howard AF, Cawthorn PM, Stone KL, et al Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008; 168 382–9.
| Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women.Crossref | GoogleScholarGoogle Scholar | 18299493PubMed |
[11] Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr 2009; 48 78–83.
| Frailty is associated with postoperative complications in older adults with medical problems.Crossref | GoogleScholarGoogle Scholar | 18068828PubMed |
[12] Garcia-Gonzalez JJ, Garcia-Pena C, Franco-Marina F, Gutierrez-Robledo LM. A frailty index to predict mortality risk in a population of senior Mexican adults. BMC Geriatr 2009; 9 47
| A frailty index to predict mortality risk in a population of senior Mexican adults.Crossref | GoogleScholarGoogle Scholar | 19887005PubMed |
[13] Folstein MF, Folstein SE. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12 189–98.
| ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE28%2FntFKjtw%3D%3D&md5=4d2a5c81f30c8088722b13e045c082a0CAS | 1202204PubMed |
[14] Cohen J. Quantitative methods in psychology. A power primer. Psychol Bull 1992; 112 155–9.
| Quantitative methods in psychology. A power primer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MvlsV2mug%3D%3D&md5=7e429fe6979edb8dbc4b370c822941ceCAS | 19565683PubMed |
[15] Smith R. Validation and reliability of the elderly mobility scale. Physiotherapy 1994; 80 744–7.
| Validation and reliability of the elderly mobility scale.Crossref | GoogleScholarGoogle Scholar |
[16] Nolan JS, Remilton LE, Green MM. The reliability and validity of the elderly mobility scale in the acute hospital setting. Internet J Allied Health Sci Prac 2008; 6 1–7.
[17] Spilg EG, Martin BJ, Mitchell SL. A comparison of mobility assessments in a geriatric day hospital. Clin Rehabil 2001; 15 296–300.
| A comparison of mobility assessments in a geriatric day hospital.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FhtFCmuw%3D%3D&md5=350ce708eb4674819a612f6daaae8be7CAS | 11386400PubMed |
[18] Raphael D, Cava M, Brown I, Renwick R, Heathcote K, Weir N, et al Frailty: a public health perspective. Can J Public Health 1995; 86 224–7.
| 1:STN:280:DyaK28%2FkvVWjsw%3D%3D&md5=0dc4429b8c9ab63fd4a067d9aba79b49CAS | 7497405PubMed |
[19] Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci 2009; 64A 675–81.
| Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries.Crossref | GoogleScholarGoogle Scholar |