Healthcare-associated infections in Australia: time for national surveillance
Philip L. Russo A E , Allen C. Cheng B C , Michael Richards D , Nicholas Graves A and Lisa Hall AA Institute of Health & Biomedical Innovation, School of Public Health and Social Work, Queensland University of Technology, 60 Musk Avenue, Kelvin Grove, Qld 4059, Australia. Email: l11.hall@qut.edu.au; n.graves@qut.edu.au
B Infectious Diseases Epidemiology Unit, Department of Epidemiology and Preventive Medicine, Monash University, The Alfred Centre, 99 Commercial Road, Prahran, Vic. 3181, Australia. Email: acscheng@gmail.com
C Infection Prevention and Healthcare Epidemiology Unit, Alfred Health, Commercial Road, Prahran, Vic. 3181, Australia.
D Faculty of Medicine, Dentistry and Health, University of Melbourne, Vic. 3010, Australia. Email: Michael.Richards@mh.org.au
E Corresponding author. Email: philip.russo@qut.edu.au
Australian Health Review 39(1) 37-43 https://doi.org/10.1071/AH14037
Submitted: 20 February 2014 Accepted: 22 August 2014 Published: 3 November 2014
Journal Compilation © AHHA 2015
Abstract
Objective Healthcare-associated infection (HAI) surveillance programs are critical for infection prevention. Australia does not have a comprehensive national HAI surveillance program. The purpose of this paper is to provide an overview of established international and Australian statewide HAI surveillance programs and recommend a pathway for the development of a national HAI surveillance program in Australia.
Methods This study examined existing HAI surveillance programs through a literature review, a review of HAI surveillance program documentation, such as websites, surveillance manuals and data reports and direct contact with program representatives.
Results Evidence from international programs demonstrates national HAI surveillance reduces the incidence of HAIs. However, the current status of HAI surveillance activity in Australian states is disparate, variation between programs is not well understood, and the quality of data currently used to compose national HAI rates is uncertain.
Conclusions There is a need to develop a well-structured, evidence-based national HAI program in Australia to meet the increasing demand for validated reliable national HAI data. Such a program could be leveraged off the work of existing Australian and international programs.
What is known about the topic? There is a large volume of literature demonstrating the effectiveness of national HAI surveillance programs in reducing the incidence of HAIs. Although some of the larger states of Australia have individual programs, a formalised national program does not exist. A well structured national HAI program in Australia would improve the understanding of the epidemiology of HAIs in Australia and provide high quality data for performance monitoring and ensuring that HAI prevention interventions are targeted appropriately.
What does this paper add? This paper reviews well established international HAI surveillance programs and highlights the benefits and limitations of these programs, and identifies the gaps that currently exist in Australia. The paper then maps out a pathway towards the development of a national program.
What are the implications for practitioners? This paper will act as a guide for future research and policy activities required for the establishment of a national HAI surveillance program in Australia.
Introduction
A healthcare-associated infection (HAI) is an infection that occurs as a result of a health care intervention.1 Historically called a ‘nosocomial’ infection, meaning ‘hospital acquired’, the term ‘healthcare’ is now used in recognition that today much health care occurs outside a hospital. Examples of HAIs are bloodstream infections commonly caused by the presence of an intravenous device, or an infected surgical wound following a surgical procedure. Many HAIs result in significant morbidity and mortality.2 It is estimated that in Europe and North America, 12–32% of HAI bloodstream infections result in death.3 In Australia, it has been suggested that 175 000 HAIs occur annually,4 but the exact figure is unknown.
Surveillance is defined as ‘the ongoing, systematic collection, analysis and interpretation of health data essential to the planning, implementation, and evaluation of public health practice, closely integrated with the timely dissemination of these data to those who need to know’.5 It is a fundamental component of modern health care, demonstrated by the recently released national safety and quality health service standards for Australian hospitals that include 19 criteria on the prevention and control of HAIs, and specifically mandate HAI surveillance.6
By its very existence, infection prevention implies that HAIs are preventable. Although it is challenging to quantify the preventable proportion of HAIs, there is agreement that a significant proportion and probably the majority of HAIs are preventable.7,8
The purpose of HAI surveillance is to provide quality data that can act as an effective monitoring and alert system.9 The aim is to reduce the incidence of preventable HAIs. A successful HAI surveillance program must be epidemiologically robust, valid, accurate, timely, useful, consistent and practical.5
Effective surveillance will deliver information to key stakeholders at all levels to inform decisions. The simple act of collecting HAI data will not reduce HAI;10 rather, data must stimulate action and drive improvement. HAI surveillance systems establish a baseline rate of infection which can then be used to detect clusters or outbreaks, identify problems, evaluate prevention and control measures, generate hypotheses concerning risk factors, guide treatment and prevention strategies, make comparisons with other facilities, inform planning and ultimately reduce the incidence of HAIs.11–14
Australia is one of the few developed countries without a national HAI surveillance program. Unlike the US, UK and many European countries, who have supported and maintained national HAI surveillance programs for decades, Australia lacks well structured processes to produce high quality national HAI data. In the UK and some states in the US, reporting of some HAIs has been mandated by law.15,16 Such international programs enable research on the epidemiology of HAIs, which also leads to enhanced and refined surveillance processes improving the quality of the HAI data now commonly reported in the public domain.17,18 In the US, hospitals are financially penalised on the occurrence of events, many of them HAIs, which are deemed preventable.19
Recent activity in Australia to develop national guides for the implementation of surveillance on Staphylococcus aureus bloodstream (SAB) infection, Clostridium difficile infection (CDI) and central line-associated bloodstream infection20 is positive but there is still much work to be done to improve our knowledge on the epidemiology of HAIs across Australia.
The purpose of this paper is to review well established international HAI surveillance programs and their impact on HAI rates, provide an overview of current Australian HAI surveillance programs and recommend a way forward to develop a national HAI surveillance program. This review focuses on the surveillance of infections in large acute public healthcare facilities, where the risk and consequences of infection are higher due to the nature of the care that takes place.
Methods
A review of current literature on national HAI surveillance programs was undertaken to identify existing national programs. The MEDLINE database from 1966 to 2013 was used by searching these key terms: cross-infection, nosocomial infection, nosocomial infection rates, healthcare-associated infection, healthcare-associated infection rates, surveillance, infection prevention and infection control. Australian jurisdictional programs and national programs from overseas that were the best described in the literature were then selected for review. To gain further information on international programs, a review of program websites, surveillance manuals, annual reports and data reports (where available) was performed, and program representatives from Germany, UK, Spain, Scotland and the Netherlands were contacted directly for clarification. For Australian surveillance activities, information was sourced from program websites and manuals, and representatives from each program were contacted for confirmation and clarification.
Results
International HAI surveillance programs and impact
The longest running national HAI surveillance program is the Centers for Disease Control’s National Healthcare Safety Network in the US.21 Originally called the National Nosocomial Infection Surveillance (NNIS) system, it commenced in 1970 with 62 hospitals voluntarily participating.21 In 2005, the program expanded to include coexisting healthcare worker exposure and renal dialysis surveillance programs to create the National Healthcare Safety Network.22 The definitions and methodology developed by the initial NNIS program have been largely adopted by many programs internationally.18
In the US, a review of HAI rates in hospitals participating in NNIS between 1990 and 1999 demonstrated decreases in urinary tract, respiratory tract and bloodstream infections monitored in intensive care units.23 Reductions in bloodstream infection rates varied from 31% to 44%. The authors acknowledge that other explanations, such as a national effort to reduce HAIs, may have also influenced these results.24
Other well described national HAI surveillance programs include the Krankenhaus Infektions Surveillance System (KISS) in Germany,25 the UK,26 Spain,27,28 France,29 Scotland,30 and the Netherlands.31
In Germany, Gastmeier demonstrated significant reductions in HAI of 20–30% over a 3-year period in hospitals participating in the Krankenhaus Infektions Surveillance System program. Significant reductions of 24–57% in surgical site infections (SSI) have been demonstrated in the Netherlands and Denmark following the introduction of national surveillance.32 A review of SSI in France over 6 years following the introduction of surveillance demonstrated a 30% reduction in the first 3 years, with an ongoing decrease in infection rates over the next 3 years.29 In the Netherlands, SSI surveillance commenced in 1996 as a component of the new national HAI surveillance program ‘PREZIES’. Geubbels et al. claim that surveillance led to a decrease in the risk of SSI of 31% when measured 4 years from the introduction of the program and a decrease of 57% in its fifth year.33
Current issues with international programs
A recent review of international surveillance programs noted that despite being similarly structured and following international recommendations and standardised definitions, widespread variation existed between programs.34 Grammatico-Guillon et al. identified variation in data collection methods and quality due to differences in the category of staff performing surveillance, variable data sources, prospective and retrospective data collection, and the presence of routine post-discharge surveillance.34 It was also noted that validation of data did not occur on a regular basis.34
Traditional surveillance methods are time-consuming, application of definitions is subject to interpretation and identification of cases is dependent on effort.35 Infection prevention staff spend up to 45% of their time undertaking surveillance.36 As Perl and Chaiwarth note, the integration of rapidly developing surveillance technologies is essential to the future of HAI surveillance. Electronic HAI surveillance systems can reduce the time spent by up to 65% compared with traditional surveillance methods, and improved sensitivity or specificity can be demonstrated.13 Recent studies have highlighted the advantages of using modern technology, such as the increased accuracy of hospital rankings when computer algorithms are used.37,38
Attempts have been made to use administrative code data to identify HAIs, but a recent systematic review found the use of administrative code data continues to demonstrate only moderate sensitivity.3 Goto et al. recommend that administrative code data may be useful as a factor within an algorithm but should not be used as the primary case-finding method.3
The use of automated technology and electronic data as an aid to traditional HAI surveillance methods has been well described.39 Automated systems ensure consistent application of surveillance definitions, significantly reduce the burden of data management, and provide improved sensitivity and specificity.39
Current situation in Australia
Of Australia’s eight states and territories, several States implemented HAI surveillance programs during the 1990s and 2000s, using infection definitions based on those developed by NNIS.40–43
In December 2008, the Australian Health Ministers’ Conference endorsed jurisdictional level surveillance of SAB and CDI. This was followed in 2009 by further endorsement of the Australian Commission on Safety and Quality in Health Care (ASQHC) recommendation that hospitals routinely monitor SAB and CDI.
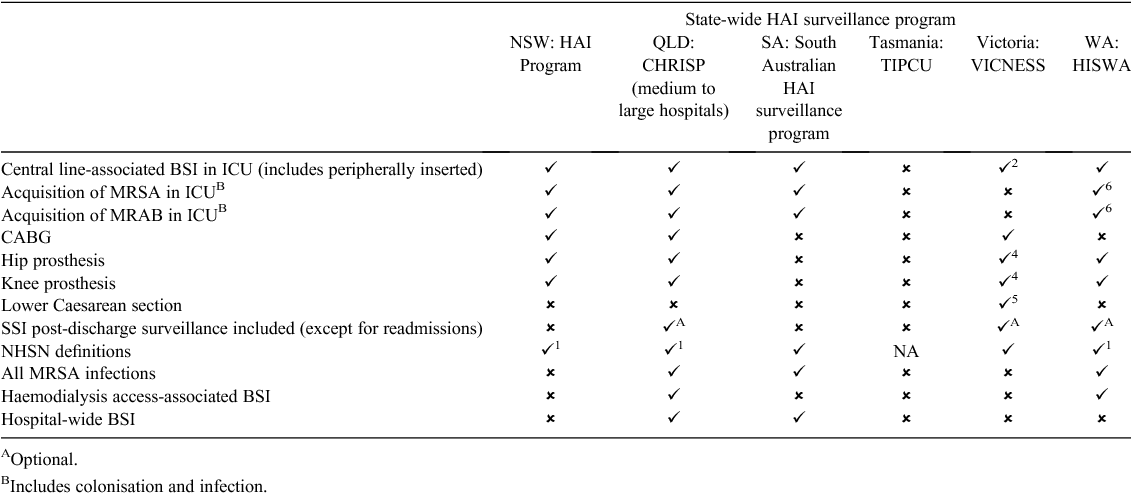
A comparison of surveillance components considered mandatory in existing statewide programs is demonstrated in Table 1. There is consistency in intensive care unit central line-associated bloodstream infection and SSI surveillance of knee and hip replacement surgery across the larger states. However, there is inconsistency in mandatory surveillance components, definitions, and post-discharge surveillance. Not included in the table due to the large degree of variation is inconsistency identified with regards to multiresistant or significant organism surveillance. Although some states report multiresistant or significant organism surveillance programs, others do not. The intensity of surveillance undertaken is peculiar to each jurisdiction with respect to the type of organism, infection versus colonisation, site, healthcare-associated versus hospital-onset infection, and requirements for the data to be notified at a state level. In Tasmania and Western Australia, notification of SAB is mandated.
Anecdotally, it is reported that many hospitals, networks and regions undertake HAI surveillance above and beyond the mandatory requirements of their jurisdiction. Examples include individual hospitals performing targeted surveillance in unique high-risk populations or in response to perceived problems. The extent of this activity and the quality of the data is unknown.
Discussion
This review has identified well established international HAI surveillance programs with evidence of a reduction of HAI rates, whilst highlighting some of the major gaps in HAI surveillance activities undertaken across Australia.
The evolution of HAI surveillance programs in Australia has been fragmented. Although some of the jurisdictional programs are now well established and embedded into routine health care safety and quality processes, it could be argued that without clear national direction, the programs have evolved in a competitive environment. This has resulted in variation among methods,44 duplication of effort and a limited ability to collate and analyse data at a national level. Potential differences between programs deserving of further research include the level of training of those involved in HAI surveillance, data analysis and reporting.
Unlike international programs, there is a lack of evidence demonstrating the effect of these statewide programs on HAI rates over time, although two of these programs have published validation studies.43,45–47
Current ASQHC strategies such as the National Surveillance Initiative20 have promoted and supported increased jurisdictional collaboration. The development of national definitions for SAB and CDI have been followed by identifiable hospital SAB data regularly published on the MyHospitals website.48 Although concerns regarding the validity and lack of risk adjustment49–51 need to be addressed, the work of the ACSQHC HAI program continues to provide direction for further national HAI surveillance activity. The recently completed ACSQHC report on antimicrobial resistance and antibiotic usage adds to the drive for better national HAI surveillance processes.52
Benefits of an Australian HAI surveillance program
As key stakeholders, consumers, healthcare workers and policy makers will all benefit from a well constructed national HAI surveillance program. Consumers clearly stand to gain from improved quality of care, resulting in reduced risk of acquiring a HAI. Healthcare workers will benefit from improved efficiency in surveillance processes that could relieve the current burden of data collection. The development of national education programs for those undertaking HAI surveillance will be uniformly accessible across Australia. The ready availability of benchmarking data will assist hospitals to allocate resources for infection prevention activities appropriately. Meaningful national comparisons of HAI rates by hospital size, type, speciality and, potentially, by specific patient risk factors will provide important contextual data across Australia. A comprehensive HAI surveillance program will provide analysis and interpretation of data, and drive investigation into unusual findings. This will lead to a sharing of information and, through informed policy making, will ultimately benefit patient care.
The ability to describe the epidemiology of HAIs will improve our understanding of the difference between populations. Detailed data will enable the identification of problem areas that may require more infection prevention resources and similarly highlight successful interventions that could act as role models and inform policy on state and national infection prevention initiatives. It will provide the foundation for local research initiatives to improve the safety and quality of health care to patients.
Where to from here?
In 2010, major infection prevention bodies including the Association for Professionals in Infection Control and Epidemiology, the Society for Healthcare Epidemiology of America, the Infectious Diseases Society of America, and the Centers for Disease Control and Prevention proposed four pillars for the elimination of HAIs, the fourth of which was ‘data to target prevention efforts and measure progress’.53 To deliver timely high-quality data, they recommended’1 reshaping standard definitions and surveillance methods to fit the new, emerging information system paradigms (e.g. electronic health information records and data mining);2 creating national and global data standards for key HAI prevention metrics; and3 creating or refining the data analysis and presentation tools available to prevention experts, clinicians and policy makers at the local, state, national and international levels.’53 These will provide valuable direction for a national HAI program in Australia.
There is much to be done in identifying a framework for a national surveillance program and the potential is exciting. First, we must take stock of the current situation in Australia to understand precisely the what, how and why of HAI surveillance currently being undertaken. To clearly identify, measure and describe exactly how much variation exists between hospitals and states, and how this influences outcomes is necessary to inform future endeavours. Information requirements need to be balanced against available resources and it is possible that current processes already exist that may be suitable to be extended into the national arena, and that better use of current data may be achievable. Although SAB data are currently being reported publicly, it is important that the data are validated and appropriately risk adjusted for meaningful comparisons to be made. Further, a meaningful way to report national CDI data that is currently collected needs to be identified.
Second, the resources, skill level and experience of those involved in current HAI surveillance will influence the quality of the program. An understanding of the ideal mix of these characteristics is essential.
Third, we must explore the use of technology as an aid to efficient HAI surveillance processes. Efficient data collection processes remain elusive. Current manual data collection methods are unsustainable and impede wider surveillance activity, so it is essential that the inclusion of automated electronic surveillance systems is considered. Existing data that are readily accessible may inform efforts to identify an agreed minimum level dataset for some HAIs.
Fourth, we must identify the key components of successful programs. No program will be perfect, but there are decades of lessons to be learnt from our colleagues across the world. Similarly, we must also draw upon the experience of our local experts and engage all key stakeholders to identify the barriers and enablers for national HAI surveillance. For example, a model mapping out the influences on reliable and valid HAI data has recently been developed by Australian researchers.54
Conclusion
Evidence clearly demonstrates that national HAI surveillance programs provide meaningful, reliable and valid data that ultimately reduce the incidence of HAIs. Although Australian jurisdictions continue to conduct disparate HAI surveillance programs, the utility of data at a national level remains limited. Centrally coordinated international HAI surveillance programs may act as a model for an Australian system, which could be further enhanced through the use of technology. The lack of a national program in Australia presents a unique opportunity to construct a HAI surveillance program based on the best available evidence.
Competing interests
PR is a member of the Australian Commission for Safety and Quality in Health Care’s (ACSQHC) Healthcare Associated Infection Advisory Committee and was previously Operations Director at the Victorian Healthcare Associated Infection Surveillance System (VICNISS) Coordinating Coordinating Centre. MR is the Director of the VICNISS Coordinating Centre, which established and runs the state healthcare infection surveillance program in Victoria. MR is a member of the ACSQHC’s Healthcare Associated Infection Advisory Committee. NG provides advice to the Centre for Healthcare Related Infection Surveillance and Prevention (CHRISP), Qld Health, and is a member of the ACSQHC’s Healthcare Associated Infection Advisory Committee. LH was previously the manager of epidemiology and research at CHRISP, and is a member of the ACSQHC’s Healthcare Associated Infection Technical Working Group.
Acknowledgements
The authors are grateful for the assistance from HAI surveillance program representatives. PR receives funding from the National Health and Medical Research Council-funded Centre of Research Excellence in reducing HAI (Grant 1030103) and the Rosemary Norman Foundation and the Nurses’ Memorial Centre through the award of the ‘Babe’ Norman Scholarship. AC is supported by an NHMRC Career Development Fellowship. NG is funded by an NHMRC Practitioner Fellowship (Grant 1059565). LH receives funding from the NHMRC Centre of Research Excellence in reducing HAI (Grant 1030103).
References
[1] National Health and Medical Research Council. Australian guidelines for the prevention and control of infection in healthcare. Canberra: Commonwealth of Australia; 2010.[2] Burke JP. Infection control: a problem for patient safety. N Engl J Med 2003; 348 651–6.
| Infection control: a problem for patient safety.Crossref | GoogleScholarGoogle Scholar | 12584377PubMed |
[3] Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19 501–9.
| Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3svksV2ksw%3D%3D&md5=be8b8e8f774623e45c91e8318f82d3f0CAS | 23473333PubMed |
[4] Graves N, Halton K, Paterson D, Whitby M. Economic rationale for infection control in Australian hospitals. Healthc Infect 2009; 14 81–8.
| Economic rationale for infection control in Australian hospitals.Crossref | GoogleScholarGoogle Scholar |
[5] Allen-Bridson K, Morrell GC, Horan TC. Surveillance of healthcare-associated infections. In: Mayhall CG, editor. Hospital epidemiology and infection control. 4th edn. Philadelphia: Wolters Kluwer; 2012. pp. 1329–43.
[6] Australian Commission on Safety and Quality in Health Care. Standard 3. Preventing and controlling hospital acquired infection. Sydney: Commonwealth of Australia; 2012.
[7] Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 2011; 32 101–14.
| Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs.Crossref | GoogleScholarGoogle Scholar | 21460463PubMed |
[8] Harbarth S, Sax H, Gastmeier P. The preventable proportion of nosocomial infections: an overview of published reports. J Hosp Infect 2003; 54 258–66.
| The preventable proportion of nosocomial infections: an overview of published reports.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3szotlOmsw%3D%3D&md5=5583ca981045560870b3d484fb22c322CAS | 12919755PubMed |
[9] Wilson J, Wloch C, Saei A, McDougall C, Harrington P, Charlett A, Lamagni T, Elgohari S, Sheridan E. Inter-hospital comparison of rates of surgical site infection following caesarean section delivery: evaluation of a multicentre surveillance study. J Hosp Infect 2013; 84 44–51.
| Inter-hospital comparison of rates of surgical site infection following caesarean section delivery: evaluation of a multicentre surveillance study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3svnsValsw%3D%3D&md5=99bc096e7fa5df19a58cceeb431aa695CAS | 23507051PubMed |
[10] Haley RW. The scientific basis for using surveillance and risk factor data to reduce nosocomial infection rates. J Hosp Infect 1995; 30 3–14.
| The scientific basis for using surveillance and risk factor data to reduce nosocomial infection rates.Crossref | GoogleScholarGoogle Scholar | 7560965PubMed |
[11] Edmond MB. National and international surveillance systems for nosocomial infections. In: Wenzel RP, editor. Prevention and control of nosocomial infections. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 109–19.
[12] Garcia-Abren A, Halperin W, Danel I. Public health surveillance toolkit. Washington, DC: The World Bank; 2002.
[13] Perl TM, Chaiwarth R. Surveillance: an overview. In: Lautenbach E, Woeltje KF, Malani PN, editors. Practical healthcare epidemiology. 3rd edn. London: The University of Chicago Press; 2012. p. 111–42.
[14] Reilly J, Stewart S, Allardice G, Cairns S, Ritchie L, Bruce J. Evidence-based infection control planning based on national healthcare-associated infection prevalence data. Infect Control Hosp Epidemiol 2009; 30 187–9.
| Evidence-based infection control planning based on national healthcare-associated infection prevalence data.Crossref | GoogleScholarGoogle Scholar | 19140744PubMed |
[15] Centers for Disease Conrtol and Prevention. Facilities in these states are required by law to report HAI data to NHSN 2014. Available at: http://www.cdc.gov/hai/stateplans/required-to-report-hai-NHSN.html [verified 11 July 2014].
[16] Public Health England. Mandatory surveillance of Staphypococcus aureus bacteraemia. 2014. Available at: http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/StaphylococcusAureus/EpidemiologicalData/MandatorySurveillance/ [verified 11 July 2014].
[17] Berríios-Torres SI, Mu Y, Edwards JR, Horan TC, Fridkin SK. Improved risk adjustment in public reporting: coronary artery bypass graft surgical site infections. Infect Control Hosp Epidemiol 2012; 33 463–9.
| Improved risk adjustment in public reporting: coronary artery bypass graft surgical site infections.Crossref | GoogleScholarGoogle Scholar |
[18] Haustein T, Gastmeier P, Holmes A, Lucet J-C, Shannon RP, Pittet D, Harbarth S. Use of benchmarking and public reporting for infection control in four high-income countries. Lancet Infect Dis 2011; 11 471–81.
| Use of benchmarking and public reporting for infection control in four high-income countries.Crossref | GoogleScholarGoogle Scholar | 21616457PubMed |
[19] Rosenthal VD, Ramachandran B, Villamil-Gomez W, Armas-Ruiz A, Navoa-Ng JA, Matta-Cortes L, Pawar M, Nevzat-Yalcin A, Rodríguiez-Ferrer M, Yildizdaş RD, Menco A, Campuzano R, Villanueva VD, Rendon-Campo LF, Gupta A, Turhan O, Barahona-Guzmán N, Horoz OO, Arrieta P, Brito JM, Tolentino MCV, Astudillo Y, Saini N, Gunay N, Sarmiento-Villa G, Gumus E, Lagares-Guzmán A, Dursun O. Impact of a multidimensional infection control strategy on central line-associated bloodstream infection rates in pediatric intensive care units of five developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection 2012; 40 415–23.
| Impact of a multidimensional infection control strategy on central line-associated bloodstream infection rates in pediatric intensive care units of five developing countries: findings of the International Nosocomial Infection Control Consortium (INICC).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38vksFeqsg%3D%3D&md5=c5c2389f3a363f2b35529f3efc9f6ecaCAS | 22371234PubMed |
[20] Australian Commission on Safety and Quality in Health Care. National surveillance initiative. Sydney: National Surveillance Initiative; 2013.
[21] Richards C, Emori TG, Edwards J, Fridkin S, Tolson J, Gaynes R. Characteristics of hospitals and infection control professionals participating in the National Nosocomial Infections Surveillance System 1999. Am J Infect Control 2001; 29 400–3.
| Characteristics of hospitals and infection control professionals participating in the National Nosocomial Infections Surveillance System 1999.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnpsVKntw%3D%3D&md5=3b8f8fc3640aad95d664168f136bfbf7CAS | 11743488PubMed |
[22] Edwards JR, Pollock DA, Kupronis BA, Li W, Tolson JS, Peterson KD, Mincey RB, Horan TC. Making use of electronic data: the National Healthcare Safety Network eSurveillance initiative. Am J Infect Control 2008; 36 S21–6.
| Making use of electronic data: the National Healthcare Safety Network eSurveillance initiative.Crossref | GoogleScholarGoogle Scholar | 18374208PubMed |
[23] Centers for Disease Control and Prevention Monitoring hospital-acquired infections to promote patient safety: United States, 1990–1999. MMWR Morb Mortal Wkly Rep 2000; 49 149–53.
| 10737441PubMed |
[24] Gaynes R, Richards C, Edwards J, Emori TG, Horan T, Alonso-Echanove J, Fridkin S, Lawton R, Peavy G, Tolson J, National Nosocomial Infections Surveillance System Hospitals Feeding back surveillance data to prevent hospital-acquired infections. Emerg Infect Dis 2001; 7 295–8.
| Feeding back surveillance data to prevent hospital-acquired infections.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzpsFSmsQ%3D%3D&md5=dd51753845d5f21a423bc02656e03735CAS | 11294727PubMed |
[25] Gastmeier P, Sohr D, Schwab F, Behnke M, Zuschneid I, Brandt C, Dettenkofer M, Chaberny IF, Rüden H, Geffers C. Ten years of KISS: the most important requirements for success. J Hosp Infect 2008; 70 11–6.
| Ten years of KISS: the most important requirements for success.Crossref | GoogleScholarGoogle Scholar | 18994676PubMed |
[26] Cooke EM, Coello R, Sedgwick J, Ward V, Wilson J, Charlett A, Ward B, Pearson A. A national surveillance scheme for hospital associated infections in England. J Hosp Infect 2000; 46 1–3.
| A national surveillance scheme for hospital associated infections in England.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvnvVeksQ%3D%3D&md5=38fb091b1e8716f2156f7d06fd9f0922CAS | 11023716PubMed |
[27] Gudiol F, Limón E, Fondevilla E, Argimon JM, Almirante B, Pujol M. The development and successful implementation of the VINCat Program. Enferm Infecc Microbiol Clin 2012; 30 3–6.
| The development and successful implementation of the VINCat Program.Crossref | GoogleScholarGoogle Scholar | 22776147PubMed |
[28] Pérez CD, Rodela AR, Monge Jodrá V, Quality Control Indicator Working Group The Spanish national health care-associated infection surveillance network (INCLIMECC): data summary January 1997 through December 2006 adapted to the new National Healthcare Safety Network Procedure-associated module codes. Am J Infect Control 2009; 37 806–12.
| The Spanish national health care-associated infection surveillance network (INCLIMECC): data summary January 1997 through December 2006 adapted to the new National Healthcare Safety Network Procedure-associated module codes.Crossref | GoogleScholarGoogle Scholar | 19560231PubMed |
[29] Rioux C, Grandbastien B, Astagneau P. Impact of a six-year control programme on surgical site infections in France: results of the INCISO surveillance. J Hosp Infect 2007; 66 217–23.
| Impact of a six-year control programme on surgical site infections in France: results of the INCISO surveillance.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2szoslOgtA%3D%3D&md5=a12eaebf96fa185c0533d7edb2469b7bCAS | 17540477PubMed |
[30] Reilly J, Cairns S, Fleming S, Hewitt D, Lawder R, Robertson C, et al Results from the second Scottish national prevalence survey: the changing epidemiology of healthcare-associated infection in Scotland. J Hosp Infect 2012; 82 170–4.
| Results from the second Scottish national prevalence survey: the changing epidemiology of healthcare-associated infection in Scotland.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3s%2FhtFeksA%3D%3D&md5=cacb2aeb42a1b1df949bd4c5286d38f9CAS | 23022370PubMed |
[31] Coello R, Gastmeier P, de Boer A. Surveillance of hospital‐acquired infection in England, Germany, and the Netherlands: will international comparison of rates be possible? Infect Control Hosp Epidemiol 2001; 22 393–7.
| Surveillance of hospital‐acquired infection in England, Germany, and the Netherlands: will international comparison of rates be possible?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mvns1CntA%3D%3D&md5=fbf75a18dbe8e03d672532c7057736eeCAS | 11519923PubMed |
[32] Gastmeier P. European perspective on surveillance. J Hosp Infect 2007; 65 159–64.
| European perspective on surveillance.Crossref | GoogleScholarGoogle Scholar | 17540263PubMed |
[33] Geubbels ELPE, Bakker HG, Houtman P, van Noort-Klaassen MA, Pelk MSJ, Sassen TM, Wille JC. Promoting quality through surveillance of surgical site infections: five prevention success stories. Am J Infect Control 2004; 32 424–30.
| Promoting quality through surveillance of surgical site infections: five prevention success stories.Crossref | GoogleScholarGoogle Scholar |
[34] Grammatico-Guillon L, Rusch E, Astagneau P. Surveillance of prosthetic joint infections: international overview and new insights for hospital databases. J Hosp Infect 2013;
| Surveillance of prosthetic joint infections: international overview and new insights for hospital databases.Crossref | GoogleScholarGoogle Scholar |
[35] van Mourik MS, Troelstra A, van Solinge WW, Moons KG, Bonten MJ. Automated surveillance for healthcare-associated infections: opportunities for improvement. Clin Infect Dis 2013; 57 85–93.
| Automated surveillance for healthcare-associated infections: opportunities for improvement.Crossref | GoogleScholarGoogle Scholar | 23532476PubMed |
[36] Gaynes RP. Surveillance of nosocomial infections: a fundamental ingredient for quality. Infect Control Hosp Epidemiol 1997; 18 475–8.
| Surveillance of nosocomial infections: a fundamental ingredient for quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szptV2qtg%3D%3D&md5=da5e1b6e47c5d24d4ce3cd6f56e3de84CAS | 9247829PubMed |
[37] Hota B, Lin M, Doherty JA, Borlawsky T, Woeltje K, Stevenson K, Khan Y, Young J, Weinstein RA, Trick W, CDC Prevention Epicenter Program Formulation of a model for automating infection surveillance: algorithmic detection of central-line associated bloodstream infection. J Am Med Inform Assoc 2010; 17 42–8.
| Formulation of a model for automating infection surveillance: algorithmic detection of central-line associated bloodstream infection.Crossref | GoogleScholarGoogle Scholar | 20064800PubMed |
[38] Lin MY, Hota B, Khan YM, Woeltje KF, Borlawsky TB, Doherty JA, Stevenson KB, Weinstein RA, Trick WE, CDC Prevention Epicenter Program Quality of traditional surveillance for public reporting of nosocomial bloodstream infection rates. JAMA 2010; 304 2035–41.
| Quality of traditional surveillance for public reporting of nosocomial bloodstream infection rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVWjurzM&md5=3cdcb87e656ceee347b26fdb434dcbb6CAS | 21063013PubMed |
[39] Freeman R, Moore LS, Garcia Alvarez L, Charlett A, Holmes A. Advances in electronic surveillance for healthcare-associated infections in the 21st century: a systematic review. J Hosp Infect 2013; 84 106–19.
| Advances in electronic surveillance for healthcare-associated infections in the 21st century: a systematic review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3snhtFygtw%3D%3D&md5=f67126fb5e0bc672407f5632e587d958CAS | 23648216PubMed |
[40] McLaws ML, Taylor PC. The Hospital Infection Standardised Surveillance (HISS) programme: analysis of a two-year pilot. J Hosp Infect 2003; 53 259–67.
| The Hospital Infection Standardised Surveillance (HISS) programme: analysis of a two-year pilot.Crossref | GoogleScholarGoogle Scholar | 12660122PubMed |
[41] Morton AP, Clements ACA, Doidge SR, Stackelroth J, Curits M, Whitby M. Surveillance of healthcare-acquired infections in Queensland, Australia: data and lessons from the first 5 years. Infect Control Hosp Epidemiol 2008; 29 695–701.
| Surveillance of healthcare-acquired infections in Queensland, Australia: data and lessons from the first 5 years.Crossref | GoogleScholarGoogle Scholar | 18690786PubMed |
[42] Russo PL, Bull A, Bennett N, Boardman C, Burrell S, Motley J, Berry K, Friedman ND, Richards M. The establishment of a statewide surveillance program for hospital-acquired infections in large Victorian public hospitals: a report from the VICNISS Coordinating Centre. Am J Infect Control 2006; 34 430–6.
| The establishment of a statewide surveillance program for hospital-acquired infections in large Victorian public hospitals: a report from the VICNISS Coordinating Centre.Crossref | GoogleScholarGoogle Scholar | 16945689PubMed |
[43] Van Gessel H, McCann RL, Peterson AM, Goggin LS. Validation of healthcare associated Staphylococcus aureus bloodstream infection surveillance in Western Australia. Healthc Infect 2010; 15 21–5.
| Validation of healthcare associated Staphylococcus aureus bloodstream infection surveillance in Western Australia.Crossref | GoogleScholarGoogle Scholar |
[44] Richards MJ, Russo PL. Surveillance of hospital-acquired infections in Australia: one nation, many states. J Hosp Infect 2007; 65 174–81.
| Surveillance of hospital-acquired infections in Australia: one nation, many states.Crossref | GoogleScholarGoogle Scholar | 17540266PubMed |
[45] Friedman ND, Russo PL, Bull AL, Richards MJ, Kelly H. Validation of coronary artery bypass graft surgical site infection surveillance data from a statewide surveillance system in Australia. Infect Control Hosp Epidemiol 2007; 28 812–7.
| Validation of coronary artery bypass graft surgical site infection surveillance data from a statewide surveillance system in Australia.Crossref | GoogleScholarGoogle Scholar | 17564983PubMed |
[46] Goggin LS, van Gessel H, McCann RL, Peterson AM, Van Buynder PG. Validation of surgical site infection surveillance in Perth, Western Australia. Healthc Infect 2009; 14 101–7.
| Validation of surgical site infection surveillance in Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
[47] McBryde ES, Brett J, Russo PL, Worth LJ, Bull AL, Richards MJ. Validation of statewide surveillance system data on central line-associated bloodstream infection in intensive care units in Australia. Infect Control Hosp Epidemiol 2009; 30 1045–9.
| Validation of statewide surveillance system data on central line-associated bloodstream infection in intensive care units in Australia.Crossref | GoogleScholarGoogle Scholar | 19803720PubMed |
[48] Australian Institute of Health and Welfare. MyHospitals. Available at: http://www.myhospitals.gov.au [verified 15 March 2013].
[49] Cheng AC, Woolnough E, Worth LJ, Pilcher DV. How should we interpret hospital infection statistics? Med J Aust 2013; 199 735–6.
| How should we interpret hospital infection statistics?Crossref | GoogleScholarGoogle Scholar | 24329623PubMed |
[50] Worth LJ, Thursky KA, Slavin MA. Public disclosure of health care-associated infections in Australia: quality improvement or parody? Med J Aust 2012; 197 29
| Public disclosure of health care-associated infections in Australia: quality improvement or parody?Crossref | GoogleScholarGoogle Scholar | 22762226PubMed |
[51] Worth LJ, Bull AL, Richards MK. Public reporting of health care-associated infection data in Australia: time to refine. Med J Aust 2013; 198 252–3.
| Public reporting of health care-associated infection data in Australia: time to refine.Crossref | GoogleScholarGoogle Scholar | 23496389PubMed |
[52] Shaban RZ, Cruickshank M, Christiansen K and the Antimicrobial Resistance Standing Committee. National surveillance and reporting of antimicrobial resistance and antibiotic usage for human health in Australia. Antimicrobial Resistance Standing Committee, Australian Heath Protection Principal Committee: Canberra; 2013.
[53] Cardo D, Dennehy PH, Halverson P, Fishman N, Kohn M, Murphy CL, Whitley RJ, HAI Elimination White Paper Writing Group Moving toward elimination of healthcare-associated infections: a call to action. Infect Control Hosp Epidemiol 2010; 31 1101–5.
| Moving toward elimination of healthcare-associated infections: a call to action.Crossref | GoogleScholarGoogle Scholar | 20929300PubMed |
[54] Mitchell BG, Gardner A. A model for influences on reliable and valid health care-associated infection data. Am J Infect Control 2014; 42 190–2.
| A model for influences on reliable and valid health care-associated infection data.Crossref | GoogleScholarGoogle Scholar | 24296274PubMed |