Informing best practice for conducting morbidity and mortality reviews: a literature review*
Corey W. Joseph A B , Marie L. Garrubba A and Angela M. Melder AA Centre for Clinical Effectiveness, Monash Health, 246 Clayton Road, Clayton, Vic. 3168, Australia. Email: Marie.Garrubba@monashhealth.org; angela.melder@monashhealth.org
B Corresponding author. Email: corey.joseph@monashhealth.org
Australian Health Review 42(3) 248-257 https://doi.org/10.1071/AH16193
Submitted: 2 September 2016 Accepted: 24 February 2017 Published: 20 April 2017
Journal compilation © AHHA 2018 Open Access CC BY-NC-ND
Abstract
Objective Preventable hospital mortality is a critical public health issue, particularly when mortalities are associated with events that are preventable. Mortality and morbidity reviews (MMRs) provide a rigorous, systematic, open, collaborative and transparent review process for clinicians to examine areas of improvement. The aim of the present review was to explore the evidence for best practice when conducting MMRs.
Methods Searches of published and grey literature from 2009 to February 2016 were conducted. This period was selected to update a previous review. Inclusion and exclusion criteria was established a priori and based on the Population-Intervention-Comparison-Outcome (PICO) framework. Specific search terms were generated and used to identify relevant articles, with reference lists and citing articles also screened for inclusions. Titles and abstracts were screened and duplicates removed. Study details regarding setting, study design, reported outcomes, tool type, clinicians present and the timing of MMRs were extracted and summarised.
Results After screening, 31 documents were included in the present review: 20 peer-reviewed articles and 11 items from the grey literature. Specific outcomes reported included mortality rates, satisfaction, education, cost and quality of care. The most common features of MMRs included timing, leadership, attendees, case presentation format, terms of reference, agenda and governance.
Conclusions MMRs decrease gross mortality rates and are effective in identifying and engaging clinicians in system improvements. MMRs should not focus on the actions of individuals, rather on education and/or quality improvement. MMRs should consist of a multidisciplinary team following a structured presentation format with an analysis of error process including actions to be followed-up. Further, it is possible for a single standardised MMR to be implemented hospital wide.
What is known about the topic? MMRs are conducted in a variety of clinical settings to educate clinicians and improve patient care.
What does this paper add? This review updates a previous review published in 2009 and summarises current evidence around morbidity and mortality reviews. This review also provides a framework for a standardised MMR to be implemented hospital wide.
What are the implications for practitioners? This summary of the evidence can be used to guide the development, formation or conduct of MMRs in any healthcare setting.
Introduction
Preventable hospital mortality is a serious public health issue, particularly when mortalities are associated with events that are preventable. As a result, there is a clear need to have rigorous, systematic and effective processes in place to enable the assessment of quality of care in a manner that is timely. A mortality and morbidity review (MMR) is a peer-review process that aims to provide medical education, improve patient care and increase clinical performance in cases where morbidities and mortalities occur. MMRs provide an open, collaborative and transparent review process for clinicians to examine practice and identify areas of improvement, such as patient outcomes and adverse events, without fear of blame or individual focus.1–3 Terms such as ‘morbidity and mortality conference’, ‘morbidity and mortality meeting’ and ‘patient safety and morbidity and mortality conferences’ are also used interchangeably throughout the literature.
Historically, MMRs are commonly conducted in surgical departments as a mode of clinical education and a way of reviewing and improving practice.4–9 However, MMRs have been conducted in a variety of settings, including acute care units,10,11 community medical centres,12 emergency departments,13 general medical units,1 intensive care units14 and palliative care units.15 In fact, standardised MMRs have been deployed and evaluated across entire hospitals or hospital networks.16–19 However, the declared purpose of MMRs varies, with the most commonly reported goals being teaching,5,7 medical management or quality improvement.4–9 Some have suggested that an effective MMR should contain certain elements, such as: identification of events resulting in adverse patient outcomes; fostering discussion of those events; identification and dissemination of information and insights about patient care that are drawn from experience; reinforcing accountability for providing high-quality care; and creating a forum in which physicians acknowledge and address reasons for mistakes.20
In Australia, health care providers independently organise and operate MMRs. There is no mandatory process or procedure that must occur when cases meet the requirements of a review. Instead, organisations conduct MMRs internally under their own direction. As a result, there is an absence of a consistent, standardised approach and it is likely that MMRs differ from healthcare network sites, departments and units. Although MMRs may be commonplace in a range of healthcare settings, the characteristics of how they are conducted, in terms of structure and format, vary considerably.1,8,21 A strength of the MMR process is that a structured methodology can be applied to each and every case with the view of systematically, and transparently, examining cases without bias or predisposition and with a view to system and process improvement.18 After exploring the information regarding preventable death in our network (Monash Health), it became apparent that there was a lack of consistency in how MMRs were being conducted. Therefore, we were interested in exploring the literature to inform the development of a gold standard or best practice. We were also interested in describing the outcomes and common features of MMRs.
Methods
Search strategy
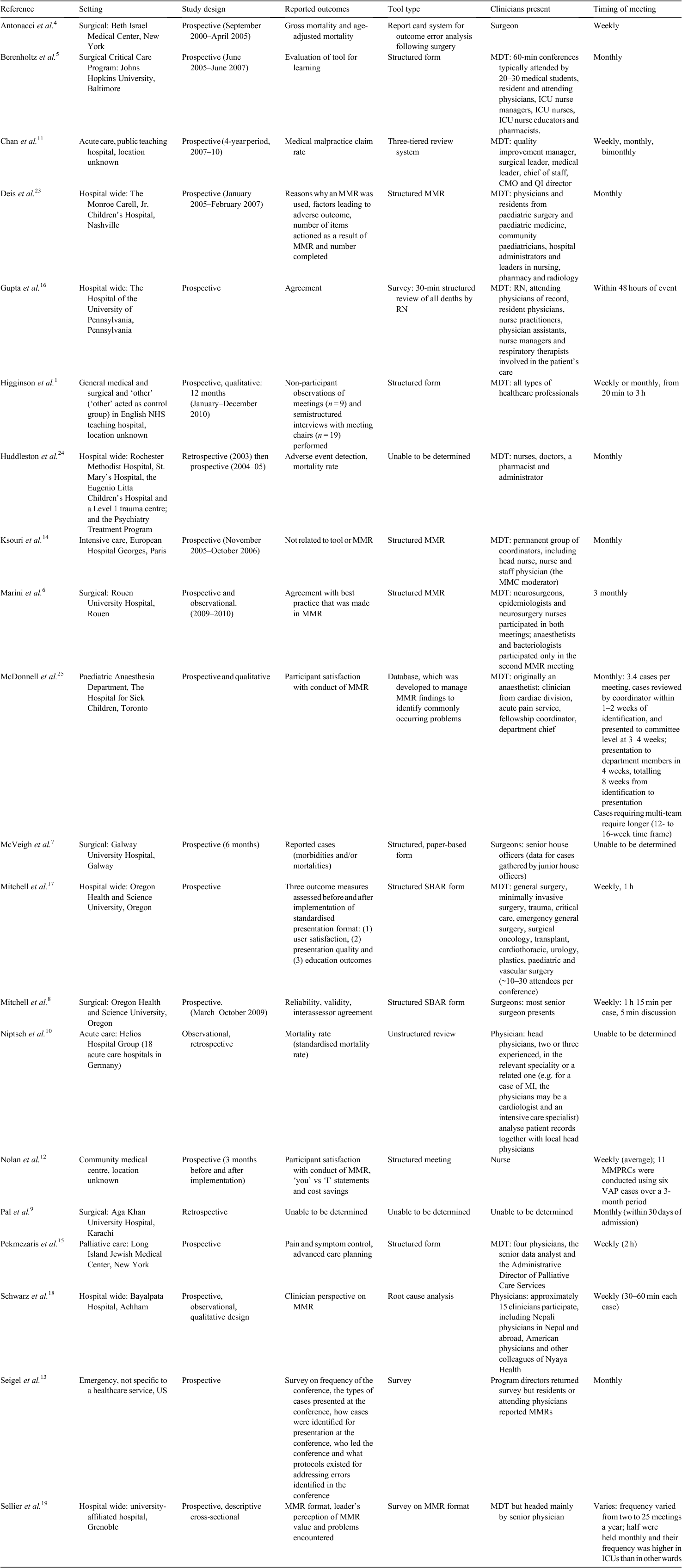
A search for peer-reviewed and grey literature published from 2009 to February 2016 was conducted in PubMed and Google. This time period was selected because the present review updated a previous review conducted on MMRs from inception until 2009.20 Documents identified were screened using inclusion and exclusion criteria established a priori and based on the Population-Intervention-Comparison-Outcome (PICO) framework (Table 1).21 Only documents that covered the mortality review process in a hospital setting were included, but items that included ‘mortality’ and ‘morbidity’ were not excluded.
Specific search terms selected were generated from a combination of those used in previous MMR reviews20 and searches of appropriate MeSH terms (Table 2). Reference lists and citing articles were also screened for potential inclusions.
|
There are several terms that refer to the process of formally discussing cases of morbidity and mortality, including ‘morbidity and mortality review’, ‘morbidity and mortality conference’ and ‘morbidity and mortality meeting’. To simplify, we refer to these clinical meetings as MMRs from this point onwards.
Review process
PubMed search results were exported into Endnote (X7; Thomson Reuters), where titles and abstracts were screened and duplicates were removed by one reviewer (CWJ). All potential papers were searched for inclusion and the full text was obtained where required. Following the Google search for grey literature, filters for English language and date were applied, and all entries were screened for inclusion by two reviewers (CWJ, MG). Those links that were relevant were then exported to a Microsoft Word document.
Data extraction
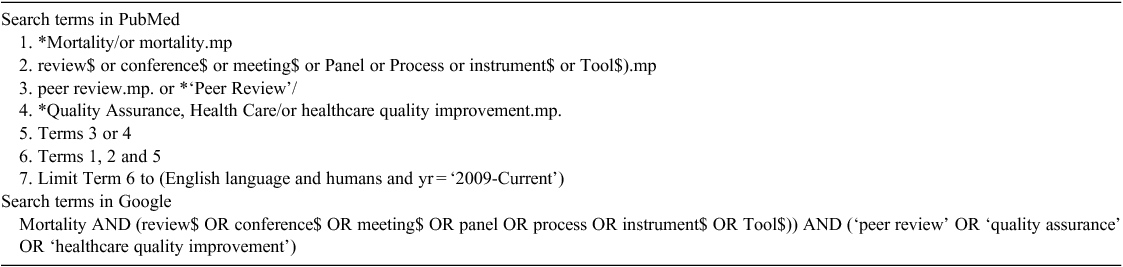
For each study identified from the PubMed search, details regarding setting, study design, reported outcomes, tool type, clinicians present and the timing of MMRs were extracted (Table 3). Similarly, data regarding setting, tool type, clinicians present and timing were also extracted for the grey literature items (Table 4).
Results
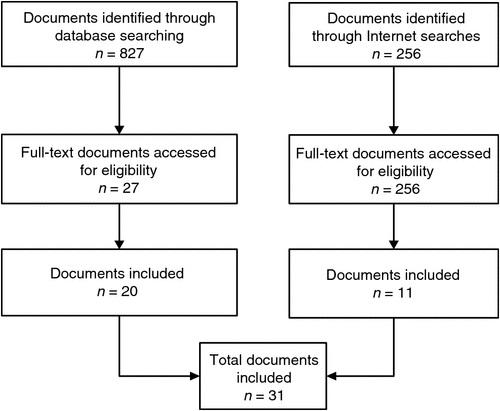
The PubMed search identified 827 publications, of which 27 full-text articles were retrieved (Table 3). The search of the grey literature identified 256 results. After screening, 31 documents were included in the present review (20 peer reviewed articles and 11 items of grey literature) (Fig. 1).1–19,22–33 After extracting and summarising the relevant information, the evidence presented herein relates to specific outcomes examined and reported in the literature, as well as common features of MMRs.
|
Outcomes of MMRs
Five studies explored mortality rates as an outcome.4,7,10,11,23 One study evaluated the effect of MMR on learning.5 Four studies explored satisfaction levels of how MMRs were conducted by participants.12,17,18,24 Six studies described or evaluated the use of an MMR tool.1,6,8,9,14,16 One study explored cost,12 two studies explored the format of MMRs13,19 and four studies explored quality of care or care delivery.1,15,22,23 The validity and reliability of a structured MMR tool has also been reported.9
Most common features for MMRs
Six studies used MMRs in a surgical setting,4–9 with other settings including acute care,10,11 community,12 emergency care,13 intensive care,14 palliative care,15 general medical1 and paediatric anaesthesia.24 Nearly all items of evidence reported information about the timing of their MMRs.1–6,8,9,11–15,17–19,24,25 Fifteen items of evidence used multidisciplinary teams in their MMRs;1–3,5,6,11,14–17,19,22–25,28 others included physicians,10,13,18 surgeons4,7,8 and nurses.12
Discussion
The present systematic review was conducted to update a previous review,20 and identified and synthesised recent evidence about MMRs. There is limited evidence in the literature evaluating outcomes or measurements of patient-centred care as a result of MMRs. A previous review has described other literature on the effectiveness of specific MMR approaches and identified MMRs to be effective in identifying and engaging clinicians in system improvements, reducing deaths from cholera and creating a safe forum for discussion of errors for junior medical staff, including removing fear of incrimination.20 Mortality rates, satisfaction, cost, quality of care and patient safety were reported in the studies included in the present review.
Outcomes of MMRs
Mortality rate
MMRs provide a quality management approach that can improve mortality rates in hospitals with sub-optimal performance.10 It should be noted that the finding of mortality rate improvement is based on observational, non-randomised data. Gross mortality and age-adjusted mortality are reduced following the implementation of MMRs.4,23 Structured MMR proforma capture more mortalities, morbidities and adverse events than standard MMRs.7 In addition, MMRs decrease medical malpractice claim rates.11
Satisfaction
MMRs lead to higher levels of clinician satisfaction because they provide rigorous case discussion, collaboration, understanding of operations of non-clinical staff and communication between all staff.18 The provision of a clear framework means that meetings are well organised, and the educational tone leads to a better appreciation of the topic.24 However, time restrictions mean that the discussion of cases and topics is limited.24 Neurosurgeons, anaesthetists and head nurses are satisfied with MMRs because they provide useful solutions to clinical practice problems.6 MMRs have a positive effect on teamwork, offering opportunities to discuss deficiencies and to decrease tensions.19 It is also suggested that MMRs promote a safety culture through the identification, analysis and correction of deficiencies.19 Alternately, there is some dissatisfaction in MMRs that contain root cause analysis because there are issues in training staff in this technique.18
Cost
Cost savings are evident following the implementation of an MMR.12 In patients with ventilator-associated pneumonia, MMRs have been shown to reduce ventilator-associated pneumonia cases leading to a cost saving of US$13 333 per month.12
Quality of care and patient safety
MMRs identify quality issues more effectively.4 Patient care is improved in palliative care environments with pain, dyspnoea, nausea and agitation all addressed at a higher rate.15 MMRs lead to improvements in patient care via enhanced governance and clinical management follow-up.1
In addition, those that lead MMRs believe patient care is enhanced through improved teamwork and an improved platform to discuss deficiencies and decrease tensions.19 MMRs are a powerful driver of the safety culture, with increases in motivation, resulting in improvements in harm minimisation practice and improved promotion of organisational learning.23 Information and data from MMRs are positively implemented into clinical practice via educational programs to allow for standardisation of best practice.23 The ability of MMRs to engage multiple members of the healthcare team in a discussion of adverse outcomes while collaboratively focusing on solving problems also leads to improvements in patient safety.22 This is brought about by the identification of potential system failures, empowering workgroups to address specific systems-based problems and making transparent accountability for regular follow-up.22
Most common features of MMRs
To build a picture of what the ideal MMR looks like, the most common features were identified from the literature, as summarised below.
Timing
There is large variability and no clear consensus on the ideal timing of when an MMR should occur. The number of reported cases can often determine the frequency of meetings.19 Options used throughout the literature are within 1 week of an event, whenever practical within days of the event, within 24 h of an event, weekly,1,4,11,12,15,17,18 monthly,1,5,8,9,11,13,14,22,24 bimonthly11 and 3 monthly.6 There has not been empirical evaluation of timing.
Length
Evidence regarding meeting duration varies, with MMRs going from 20 min up to 4 h.1,20 However, a 15-min presentation with 5 min for questions has been used previously.8,17 In addition, 30 min per case has been used.18
Leadership
Leaders should have high skills and expertise in the area of morbidity and mortality cases.2 Leaders should be trained or have skills in auditing, the ability to understand and interpret the clinical information accurately, the ability to access senior medical advice and have an understanding of the clinical environment.2 Leaders may include consultants, physicians or senior doctors or nurses; however, this role could be performed by any suitably trained staff member with access to senior medical advice as required.
Attendees
Most of the literature reports the use of a multidisciplinary team.1,5,6,11,14–17,19,22,24 Participants may include any of the providers involved in the care of the patient, selected experts and others who can contribute to the analysis of the event and to the development of practical recommendations to improve patient safety.25 The vast array of staff involved in MMRs is listed in Tables 3 and 4.
Case presentation
Case presentations vary in structure throughout the literature. The Situation, Background, Assessment and Analysis, Recommendation (SBAR) format is perhaps the most structured and best evaluated format.17 Some have suggested that in the month leading up to the MMR a core team should meet to gather, review and summarise information from the patient’s hospitalisation in a time series flow diagram.22 A brief literature review of the disease or illness specific to the case is presented.22 Other reviews are formally prepared in presentation format and shared with those attending the MMR 2 days before the meeting.4 Cause-and-effect diagrams have been used to identify factors that may have been related or contributed to the adverse outcome,22 as well as root cause analysis to identify quality issues and causes of error.4,17 In addition, a seven-step process has been used outlining case identification, case classification, case preparation and review, case analysis, case discussion, recommendations and closure or follow-up.14
Terms of reference
Clear and defined terminology when describing MMRs is important.1 Only factual information in incident or occurrence reports is provided, and restraint needs to be applied to statements of blame, speculation, opinion or other commentary as to the reasons for what happened.9,17,19,25 Emphasis must be placed on MMRs being a safe, supportive and blame-free forum to facilitate improvement and accountability.
Agenda
The present review found limited evidence regarding agenda. The following outline has been used previously:22 (1) a reminder of the systems-based approach and confidentiality by the MMR leader (5 min); (2) a review of task force progress from prior conferences by the MMR team (10 min); (3) a case presentation in a timeline format by resident leaders (10 min); (4) a brief literature review relevant to the case in question by resident leaders (5 min); (5) identification of key issues leading to the undesired outcome by all attending the MMR (25 min); (6) identification of workgroups to address the key issues by the MMR team (10 min); (7) a reminder of confidentiality by the MMR leader (5 min); and (8) evaluation of the conference by those who attended as administered by the MMR leader (5 min).
Governance and follow-up
There is varied evidence for positions responsible for follow-up within an organisation. Leadership can be involved in the findings of MMRs.2,25,29 In addition, risk or quality teams are involved in the process because findings may be the result of issues with system processes.1,2,25 Feedback functions have also been built in to online systems that automatically administer MMR forms where necessary and track the process of each MMR case.11 To assist with evidence translation and process improvement, improvement measures determined by the MMRs should also be communicated to frontline staff through ward-based speciality governance and clinical management positions or staff.1
A previous review identified that:
Follow up tends to be limited to either case summary reports and or designating individuals to follow up actions…some studies have reported more comprehensive approaches including documentation of outcomes, evaluations, development of action plans, verbal updates at subsequent MMRs, written reports and tracking of actions.20
The present review is not without limitations. Only one database was searched for relevant literature and one author reviewed the references retrieved. To overcome these limitations, the authors used secondary searching of citations and bibliographies to maximise the capture of studies that did not show up in the database search or were inappropriately excluded.
The result of the present review has since informed and steered a large organisational change. As such, the evidence in the present review has been used to frame a gap analysis between best practice and the current organisational procedures for MMR. Subsequently, items of best practice have been prioritised and reporting structures improved with terms of reference, procedures and implementation tools developed. These changes are serving as a benchmark that the organisation has selected to measure, monitor and gain feedback at the executive level in order to inform policy development and improve the quality of patient care in the future. More widely, the present review informs clinical practice by providing a current summary of evidence around MMRs.
Conclusion
There is very limited peer-reviewed literature exploring patient-centred outcomes as a result of the MMR process. MMRs have resulted in a decrease in gross mortality and are effective in identifying and engaging clinicians in system improvements, reducing deaths from cholera and creating a safe forum for the discussion of errors for junior medical staff, including removing fear of incrimination. Based on the best available evidence we are able to inform best practice in conducting MMRs. This includes an MMR that should not focus on the actions of individuals, rather on education and/or quality improvement. MMRs should include an agenda, a structured presentation format (i.e. SBAR), an analysis of error process and should conclude with actions to be performed, and these followed-up at the beginning of subsequent MMRs. MMRs should consist of a multidisciplinary team, including those who had the most contact with the patient. Finally, given the features are general in nature, it is possible for a single standardised MMR to be implemented hospital wide.
Competing interests
None declared.
Acknowledgements
The authors acknowledge the Royal Australasian College of Medical Administrators. In addition, the authors acknowledge Professor Erwin Loh for his support.
References
[1] Higginson J, Walters R, Fulop N. Mortality and morbidity meetings: an untapped resource for improving the governance of patient safety? BMJ Qual Saf 2012; 21 576–85.| Mortality and morbidity meetings: an untapped resource for improving the governance of patient safety?Crossref | GoogleScholarGoogle Scholar |
[2] Emergency Care Institute New South Wales. ED quality framework. Available at: http://www.ecinsw.com.au/sites/default/files/field/file/Standardised%20Death%20Reviews%20in%20ED%20resources_1.pdf [verified 29 July 2015].
[3] Robinson G, Davidge M, Davies J, et al. Providing assurance, driving improvement. Learning from mortality and harm reviews in NHS Wales. Cardiff: NHS Wales; 2013.
[4] Antonacci AC, Lam S, Lavarias V, et al A report card system using error profile analysis and concurrent morbidity and mortality review: surgical outcome analysis, part II. J Surg Res 2009; 153 95–104.
| A report card system using error profile analysis and concurrent morbidity and mortality review: surgical outcome analysis, part II.Crossref | GoogleScholarGoogle Scholar |
[5] Berenholtz SM, Hartsell TL, Pronovost PJ. Learning from defects to enhance morbidity and mortality conferences. Am Coll Med Qual 2009; 24 192–5.
| Learning from defects to enhance morbidity and mortality conferences.Crossref | GoogleScholarGoogle Scholar |
[6] Marini H, Merle V, Derrey S, et al Surveillance of unplanned return to the operating theatre in neurosurgery combined with a mortality–morbidity conference: results of a pilot survey. BMJ Qual Saf 2012; 21 432–8.
| Surveillance of unplanned return to the operating theatre in neurosurgery combined with a mortality–morbidity conference: results of a pilot survey.Crossref | GoogleScholarGoogle Scholar |
[7] McVeigh TP, Waters PS, Murphy R, et al Increasing reporting of adverse events to improve the educational value of the morbidity and mortality conference. J Am Coll Surg 2013; 216 50–6.
| Increasing reporting of adverse events to improve the educational value of the morbidity and mortality conference.Crossref | GoogleScholarGoogle Scholar |
[8] Mitchell EL, Lee DY, Arora S, et al SBAR M&M: a feasible, reliable, and valid tool to assess the quality of, surgical morbidity and mortality conference presentations. Am J Surg 2012; 203 26–31.
| SBAR M&M: a feasible, reliable, and valid tool to assess the quality of, surgical morbidity and mortality conference presentations.Crossref | GoogleScholarGoogle Scholar |
[9] Pal KMI, Pardhan A, Mazahir S, et al Morbidity meetings: what makes it to; what stays out of the forum. J Pak Med Assoc 2013; 63 161–4.
[10] Nimptsch U, Mansky T. Quality measurement combined with peer review improved German in-hospital mortality rates for four diseases. Health Aff 2013; 32 1616–23.
| Quality measurement combined with peer review improved German in-hospital mortality rates for four diseases.Crossref | GoogleScholarGoogle Scholar |
[11] Chan LS, Elabiad M, Zheng L, et al A medical staff peer review system in a public teaching hospital – an internal quality improvement tool. J Healthc Qual 2014; 36 37–44.
| A medical staff peer review system in a public teaching hospital – an internal quality improvement tool.Crossref | GoogleScholarGoogle Scholar |
[12] Nolan SW, Burkard JF, Clark MJ, et al Effect of morbidity and mortality peer review on nurse accountability and ventilator-associated pneumonia rates. J Nurs Adm 2010; 40 374–83.
| Effect of morbidity and mortality peer review on nurse accountability and ventilator-associated pneumonia rates.Crossref | GoogleScholarGoogle Scholar |
[13] Seigel TA, McGillicuddy DC, Barkin AZ, et al Morbidity and mortality conference in emergency medicine. J Emerg Med 2010; 38 507–11.
| Morbidity and mortality conference in emergency medicine.Crossref | GoogleScholarGoogle Scholar |
[14] Ksouri H, Balanant P-Y, Tadie J-M, et al Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice. Am J Crit Care 2010; 19 135–45.
| Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice.Crossref | GoogleScholarGoogle Scholar |
[15] Pekmezaris R, Cooper L, Efferen L, et al Transforming the mortality review conference to assess palliative care in the acute care setting: a feasibility study. Palliat Support Care 2010; 8 421–6.
| Transforming the mortality review conference to assess palliative care in the acute care setting: a feasibility study.Crossref | GoogleScholarGoogle Scholar |
[16] Gupta M, Fuchs B, Cutilli C, et al Preventable mortality: does the perspective matter when determining preventability? J Surg Res 2013; 184 54–60.
| Preventable mortality: does the perspective matter when determining preventability?Crossref | GoogleScholarGoogle Scholar |
[17] Mitchell EL, Lee DY, Arora S, et al Improving the quality of the surgical morbidity and mortality conference: a prospective intervention study. Acad Med 2013; 88 824–30.
| Improving the quality of the surgical morbidity and mortality conference: a prospective intervention study.Crossref | GoogleScholarGoogle Scholar |
[18] Schwarz D, Schwarz R, Gauchan B, et al Implementing a systems-oriented morbidity and mortality conference in remote rural Nepal for quality improvement. BMJ Qual Saf 2011; 20 1082–8.
| Implementing a systems-oriented morbidity and mortality conference in remote rural Nepal for quality improvement.Crossref | GoogleScholarGoogle Scholar |
[19] Sellier E, David-Tchouda S, Bal G, et al Morbidity and mortality conferences: their place in quality assessments. Int J Health Care Qual Assur 2012; 25 189–96.
| Morbidity and mortality conferences: their place in quality assessments.Crossref | GoogleScholarGoogle Scholar |
[20] Travaglia J, Debonoj D. Mortality and morbidity reviews: a comprehensive review of the literature. Centre for Clinical Governance Research in Health, Faculty of Medicine, University of New South Wales; 2009.
[21] Moher D, Liberati A, Tetzlaff J, et al Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151 264–69.
[22] Murayama K, Derossis A, DaRosa D, et al A critical evaluation of the morbidity and mortality conference. Am J Surg 2002; 183 246–50.
| A critical evaluation of the morbidity and mortality conference.Crossref | GoogleScholarGoogle Scholar |
[23] Deis J, Smith K, Warren M, et al. Transforming the morbidity and mortality conference into an instrument for systemwide improvement. In Henriksen K, Battles J, Keyes M, Grady M, editors. Advances in patient safety: new directions and alternative approaches, Vol. 2: culture and redesign. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
[24] Huddleston J, Diedrich D, Kinsey G, et al Learning from every death. J Patient Saf 2014; 10 6–12.
| Learning from every death.Crossref | GoogleScholarGoogle Scholar |
[25] McDonnell C, Laxer RM, Roy WL. Redesigning a morbidity and mortality program in a university-affiliated pediatric anesthesia department. Joint Comm J Qual Patient Saf 2010; 36 117–25.
| Redesigning a morbidity and mortality program in a university-affiliated pediatric anesthesia department.Crossref | GoogleScholarGoogle Scholar |
[26] Canadian Medical Protective Association. Learning from adverse events: fostering a just culture of safety in Canadian hospitals and health care institutions. Ottowa: Canadian Medical Protective Association; 2009.
[27] Dargon P, Mitchell E, Sevdalis N. Mortality and morbidity conference manual v.1.1. London: Oregon Health and Science University & Imperial College London; 2012.
[28] The Department of Health & Human Services State Government of Victoria. Understanding clinical practice toolkit. Melbourne: State Government of Victoria.
[29] Mills S. Maternal death audit as a tool: reducing maternal mortality. HNPNotes 2011; 1–10.
[30] National Office of Clinical Audit. Irish audit of surgical mortality. 2012. Available at: M&M%20Review%20Tools\National%20Office%20of%20Clinical%20Audiit%20Ireland%20Process_Flow_Chart.pdf [verified 8 March 2017].
[31] MedPro Group. Peer review in group practices. Patient safety & risk solutions. Fort Wayne: MedPro; 2014.
[32] Ferguson K, Arora S, Mitchell E, Sevdalis N. Anaesthesia morbidity and mortality meetings: a practical toolkit for improvement. London: The Royal College of Anaesthetists; 2013.
[33] The Royal Children’s Hospital Melbourne. Departmental morbidity & mortality review. Melbourne: The Royal Children’s Hospital; 2013.
[34] Yale-New Haven Hospital. Peer review & ongoing professional practice evaluation: Yale–New Haven Hospital, Department of Physician Services. 2013. Available at: http://extranet.acsysweb.com/vSiteManager/YNHH/Public/Upload/Images/Professionals/OPPE.pdf [verified 8 March 2017].
* *A condensed version of this article first appeared in The Quarterly newsletter in September 2015 (see http://racma.edu.au/?option=com_content&id=775&Itemid=451, accessed 8 March 2017).