Going digital: a narrative overview of the effects, quality and utility of mobile apps in chronic disease self-management
Ian A. Scott A B H , Paul Scuffham C , Deepali Gupta A D , Tanya M. Harch E , John Borchi E and Brent Richards F GA Princess Alexandra Hospital, 199 Ipswich Road, Woolloongabba, Brisbane 4102, Australia. Email: deepali.gupta@health.qld.gov.au
B School of Clinical Medicine, University of Queensland, 37 Trent Street, Woolloongabba, Brisbane 4102, Australia.
C Menzies Health Institute Queensland, Griffith University (Nathan campus), 170 Kessels Road, Nathan, Brisbane 4111, Australia. Email: p.scuffham@griffith.edu.au
D Queen Elizabeth II Hospital, Troughton Rd and Kessels Rd, Coopers Plains, Brisbane, 4108, Australia.
E eHealth Queensland, 2/315 Brunswick St, Fortitude Valley, Brisbane 4006, Australia. Email: tanya.harch@health.qld.gov.au; john.borchi@health.qld.gov.au
F Gold Coast University Hospital, 1 Hospital Boulevard, Southport 4215, Australia. Email: brent.richards@health.qld.gov.au
G Griffith University (Gold Coast campus), Parklands Drive, Southport 4215, Australia.
H Corresponding author. Email: ian.scott@health.qld.gov.au
Australian Health Review 44(1) 62-82 https://doi.org/10.1071/AH18064
Submitted: 6 April 2018 Accepted: 4 September 2018 Published: 13 November 2018
Journal Compilation © AHHA 2020 Open Access CC BY-NC-ND
Abstract
Objective Smartphone health applications (apps) are being increasingly used to assist patients in chronic disease self-management. The effects of such apps on patient outcomes are uncertain, as are design features that maximise usability and efficacy, and the best methods for evaluating app quality and utility.
Methods In assessing efficacy, PubMed, Cochrane Library and EMBASE were searched for systematic reviews (and single studies if no systematic review was available) published between January 2007 and January 2018 using search terms (and synonyms) of ‘smartphone’ and ‘mobile applications’, and terms for each of 11 chronic diseases: asthma, chronic obstructive lung disease (COPD), diabetes, chronic pain, serious mental health disorders, alcohol and substance addiction, heart failure, ischaemic heart disease, cancer, cognitive impairment, chronic kidney disease (CKD). With regard to design features and evaluation methods, additional reviews were sought using search terms ‘design’, ‘quality,’ ‘usability’, ‘functionality,’ ‘adherence’, ‘evaluation’ and related synonyms.
Results Of 13 reviews and six single studies assessing efficacy, consistent evidence of benefit was seen only with apps for diabetes, as measured by decreased glycosylated haemoglobin levels (HbA1c). Some, but not all, studies showed benefit in asthma, low back pain, alcohol addiction, heart failure, ischaemic heart disease and cancer. There was no evidence of benefit in COPD, cognitive impairment or CKD. In all studies, benefits were clinically marginal and none related to morbid events or hospitalisation. Twelve design features were identified as enhancing usability. An evaluation framework comprising 32 items was formulated.
Conclusion Evidence of clinical benefit of most available apps is very limited. Design features that enhance usability and maximise efficacy were identified. A provisional ‘first-pass’ evaluation framework is proposed that can help decide which apps should be endorsed by government agencies following more detailed technical assessments and which could then be recommended with confidence by clinicians to their patients.
What is known about the topic? Smartphone health apps have attracted considerable interest from patients and health managers as a means of promoting more effective self-management of chronic diseases, which leads to better health outcomes. However, most commercially available apps have never been evaluated for benefits or harms in clinical trials, and there are currently no agreed quality criteria, standards or regulations to ensure health apps are user-friendly, accurate in content, evidence based or efficacious.
What does this paper add? This paper presents a comprehensive review of evidence relating to the efficacy, usability and evaluation of apps for 11 common diseases aimed at assisting patients in self-management. Consistent evidence of benefit was only seen for diabetes apps; there was absent or conflicting evidence of benefit for apps for the remaining 10 diseases. Benefits that were detected were of marginal clinical importance, with no reporting of hard clinical end-points, such as mortality or hospitalisations. Only a minority of studies explicitly reported using behaviour change theories to underpin the app intervention. Many apps lacked design features that the literature identified as enhancing usability and potential to confer benefit. Despite a plethora of published evaluation tools, there is no universal framework that covers all relevant clinical and technical attributes. An inclusive list of evaluation criteria is proposed that may overcome this shortcoming.
What are the implications for practitioners? The number of smartphone apps will continue to grow, as will the appetite for patients and clinicians to use them in chronic disease self-management. However, the evidence to date of clinical benefit of most apps already available is very limited. Design features that enhance usability and clinical efficacy need to be considered. In making decisions about which apps should be endorsed by government agencies and recommended with confidence by clinicians to their patients, a comprehensive but workable evaluation framework needs to be used by bodies assuming the roles of setting and applying standards.
Introduction
With the increasing prevalence of chronic multimorbidity, enhanced patient self-management is a priority in improving outcomes and reducing healthcare costs. With the advent of smartphones and tablets in 2007, now used by more than 80% of Australians,1 downloadable mobile health applications (apps) have become popular as a means for enabling patients with chronic disease to participate in more effective self-management. Funders and managers of healthcare systems are increasingly interested in the potential for apps to improve chronic disease self-management and reduce hospitalisations and healthcare costs.
In 2017, the number of health apps released from iTunes and Google Play exceeded 300 000,2 with nearly 25% dealing with disease self-management.3 One-third of adults in the US with smartphones or tablets use health apps to achieve health behaviour goals and help with medical decision making.4 However, although international standards exist with regard to software engineering, privacy, security and usability of mobile apps in general (e.g. International Organization for Standardization (ISO) and International Electrotechnical Commission (IEC) standards), there are currently few widely accepted criteria to ensure health apps are accurate in content, evidence based or efficacious.5 The efficacy of most commercially available apps in improving self-management and clinical outcomes related to chronic diseases has never been evaluated in clinical trials6 and although quality assessment tools exist for health-related websites,7,8 no universally agreed approach exists for evaluating app clinical efficacy and safety. Involvement of users and clinical experts in development is highly variable, and adherence of apps to available clinical evidence ranges from 0% to 87%.9 Most apps omit components that motivate patients to make lifestyle changes, instead providing information already available in paper form.10,11 Concerns also exist about interface design, interactivity, connectivity,12 the privacy and security of stored or transferred personal health information13,14 and risks to patient safety from inaccurate or poorly designed apps.15 In contrast with drugs or implantable devices, the US Food and Drug Administration (FDA)16 and the Australian Therapeutic Goods Administration (TGA)17 have no mandated regulatory oversight of health apps unless they are directly connected to regulated medical equipment. However, both agencies,18,19 together with the Australian Digital Health Agency (ADHA),20 have recently signalled their intention to focus attention on any software device, including apps, used to diagnose, prevent or manage disease.
In January 2018, the Queensland Policy and Advisory Committee on new Technology (QPACT), in partnership with Healthcare Evaluation and Assessment of Technology (HEAT) Team of Queensland Health (QH) and eHealth Queensland, established the mHealth Applications Evaluation Program (mHealth Apps Program) as a pilot initiative. The program welcomes submissions from clinicians who require funds to subject apps of their choosing to a rigorous field evaluation of quality, safety and benefit in order to receive official QH endorsement as an app that QH clinicians can recommend or ‘prescribe’ to their patients with confidence. The program was in rapid need of an evaluative framework and a better understanding of the efficacy and optimal design characteristics of mobile apps. The aims of this study were to: (1) assess the efficacy of currently available apps on chronic disease self-management; (2) identify design attributes that influence app usability and potential to confer benefit; and (3) review methods for evaluating app quality and utility and develop a provisional evaluative framework.
Methods
Objective no. 1: efficacy of apps in optimising chronic disease self-management
Criteria chosen by the mHealth program for app submissions (Table 1) determined the scope of literature reviews. PubMed, Cochrane Library and EMBASE were searched between January 2007 and January 2018 for systematic reviews (as defined using formal criteria21) of studies using search terms (and synonyms) of ‘smartphone’, ‘mobile applications’, ‘mobile health’ and terms for each of 11 chronic diseases, chosen for their burden of hospital utilisation,22 namely asthma, chronic obstructive lung disease (COPD), diabetes, chronic pain, serious mental health disorders, alcohol and substance addiction, heart failure (HF), ischaemic heart disease, cancer, cognitive impairment and chronic kidney disease (CKD).

|
Inclusion criteria comprised systematic reviews of randomised controlled trials (RCTs) or observational studies that evaluated patient-facing interactive apps related to disease self-management, reported at least one measure of efficacy and were conducted in developed countries. Reference lists of included reviews were checked for other relevant studies. Reviews not written in English, those that analysed digital interventions not meeting our definition of a mobile app (i.e. exclusive use of text messaging, interactive voice response, desktop applications, websites) or those that examined apps used for diagnosis, screening, risk assessment, primary prevention or health promotion were excluded from the analysis.
In instances where no reviews for a particular condition could be found, individual studies were searched using the same search strategy and selection criteria, but excluding conference abstracts or case reports. Where multiple reviews dealing with the same topic were retrieved, the most recently published reviews were selected; other reviews were included if they provided additional informative data derived from thematic or subgroup analyses.
Data extracted from each review or individual study comprised: date of publication, number and type of studies, sample size, assessment of risk of bias (as defined by authors), app description, measures of efficacy, including meta-analyses of pooled data, assessment of patient adherence, usability and reference to behavioural change theory23,24 underpinning the app, and the authors’ interpretive comments on study strengths and limitations and overall results. In assessing efficacy, we made no attempt to prespecify what constituted clinically meaningful benefits in terms of absolute changes in effect measures, because this will vary according to subjective interpretations of clinicians and users and the outcomes and conditions of interest.
Objective no. 2: design attributes that affect app usability and efficacy
Information about app design attributes affecting adherence, usability and potential to confer benefit were sought from the reviews retrieved, with additional reviews retrieved using app search terms that included ‘quality’, ‘content’, ‘usability’, ‘feasibility’, ‘acceptability’, ‘functionality’, ‘adherence’ and ‘design.’ In the absence of any validated usability score or taxonomy for mobile health apps, we considered all app design features that other researchers had proposed as being important on the basis of prima facie evidence.
Objective no. 3: methods for evaluating app quality and utility
Additional reviews were also sought using app search terms combined with ‘quality assessment’, ‘evaluation’ and related synonyms. Findings were used to construct a comprehensive but practical evaluation framework.
Results
Objective no. 1: efficacy of apps on chronic disease self-management
In total, 49 studies were retrieved,11,25–72 comprising 16 reviews11,25,29,31–39,42,51,60,64 and 33 individual studies.26–28,30,40,41,43–50,52–59,61–63,65–72 Key findings are presented below; more detailed evidence tables are provided in Appendix 1.
Asthma
In a review of 12 RCTs of digital aids,25 three app trials were reported; one saw a 45% increase in the number of patients with well-controlled asthma,26 another saw no effects on several outcomes27 and the third reported improvements in asthma-related quality of life and an 80% decrease in emergency department visits.28
Chronic obstructive pulmonary disease
In a review of three RCTs of digital interventions,29 a single app trial reported no difference in COPD-related quality of life; no other outcomes were reported.30
Diabetes
Four reviews (one with 16 RCTs involving apps,31 another with 12 RCTs,32 a third with 10 RCTs33 and a fourth with six RCTs34) all reported reductions in glycosylated haemoglobin levels (HbA1c) for all diabetics (mean reductions ranging from 0.48% to 0.51%) and those with type 2 diabetes (0.49–0.83%). Other reviews35,36 yielded mixed results, with only one-third of included studies reporting significant reductions in HbA1c.
Chronic pain
Although four reviews were retrieved assessing more than 300 apps,11,37–39 none contained any trials of efficacy. A single app RCT showed an improvement in pain control, functionality and quality of life of patients with low back pain,40 whereas another app involving women with widespread pain that included diaries and therapist feedback showed no effects at 11 months.41
Serious mental health disorders
In a review of 18 studies,42 14 app studies included four RCTs. Of two RCTs of depression apps, one showed no effect,43 whereas another RCT reported improved mental health scores and reduced lost or unproductive days.44 An RCT of an app for psychotic disorders reported ‘positive effects’,45 whereas another RCT for bipolar disorder reported no effects.46 A single-arm study of an app for schizophrenia and schizoaffective disorder was associated with significant reduction in symptoms.47 A review of 24 app studies in children and adolescents with various mental disorders48 included two RCTs, both showing no effects.49,50
Alcohol and substance addiction
In a review of five studies with three app RCTs,51 one RCT showed increased frequency of drinking in men but not women compared with controls.52 The second saw 1.37 fewer risky drinking days among app patients over 12 months,53 whereas the third noted less drinking over 14 days but no effect on heavy episodic drinking.54
Heart failure
No reviews were found, but, of two RCTs, one using a tablet computer reported 2.2 fewer HF-related days in hospital per patient and improved HF-related quality of life and physical function over 3 months.56 The second RCT using home telemonitoring reported no difference in hospital days and, instead, an increase in health care utilisation with more nurse visits and telephone contacts.56
Ischaemic heart disease
No reviews were found but, of three RCTs of apps targeting patients following acute coronary events, one reported improved medication adherence but no effect on smoking rates, physical activity or quality of life at 6 months.57 Another showed slight weight reduction and improved quality of life at 6 weeks.58 The third RCT showed weight loss and improved blood pressure and blood sugar control, but no change in medication adherence, smoking rates, physical activity or quality of life.59
Cancer
In a review of five studies60 that reported three app trials, a controlled trial involving prostate cancer patients receiving radiotherapy reported lower levels of fatigue, nausea, insomnia, urinary symptoms and emotional dysfunction.61 An RCT involving breast cancer patients showed no effect on physical function or quality of life,62 whereas a pre-post study involving survivors of breast and endometrial cancer noted weight reduction but no change in physical activity or quality of life.63
Cognitive impairment
In a review of 24 studies,64 two RCTs65,66 and one controlled trial67 of apps focused on cognitive training showed improved cognition, whereas one RCT did not.68 Three other RCTs, one of serious games coupled with cognitive training,69 one with a multicomponent program70 and another of engagement,71 showed no effects on various outcomes.
Chronic kidney disease
No reviews were found. Only one small pre-post cohort study of an app reported mean reductions in home blood pressure readings from baseline values.72
Objective no. 2: design attributes that affect app usability and efficacy
Eight reviews were considered relevant.35,64,73–78 In the review of diabetes apps by Fu et al.35 seven usability studies reported poor (38%) to average (80%) rates of use, due primarily to product design flaws (e.g. screen layout, system capability and reliability, and general characteristics), need for manual data entry, patients having to take more time and making more errors than expected when exporting and correcting blood sugar levels, difficulty encountered in system navigation whenever tasks required multiple steps and the absence of personalised feedback functions or social networking functions. No study considered confounders such as a patient’s history of, and levels of motivation in, using digital technologies. Only 51 of 295 (17.3%) cancer apps,73 12 of 117 (10.3%) depression apps74 and a minority of chronic pain apps75 provide skill-building tools to assist in self-management. On the basis of these and other reviews68,76–78 we identified several design features for enhancing usability (Table 2).

|
Objective no. 3: methods for evaluating app quality and utility
Methods for evaluating apps fall into three broad categories, as summarised in Table 3,79 each providing a different type of evidence but with limitations. For example, some lists of criteria focus on user ratings (ease of use, reliability, aesthetics) but may exclude reference to independent expert clinician involvement or assessment.80 Others cover development, implementation and integration, but omit evaluation of key usability attributes or privacy and security.81 Evaluation guidelines from the Health Care Information and Management Systems Society consider efficiency, effectiveness, user satisfaction and platform optimisation, but exclude accuracy and appropriateness of app health information.82 Such omissions pose risks to patient safety and explicit app risk assessment has been proposed.15 Various rating scales,83,84 scoring systems,85,86 checklists,87–91 toolkits,92,93 questionnaires,94 development guides95 and reporting standards for app studies96,97 all promote more transparent and objective reporting and evaluation. The existence of so many instruments suggests no single one meets all needs. Several government (https://www.vichealth.vic.gov.au/media-and-resources/vichealth-apps; https://www.healthnavigator.org.nz/app-library), private (http://myhealthapps.net/; https://imedicalapps.com/#; https://practicalapps.ca/) and developer (https://www.ourmobilehealth.com/; https://www.medappcare.com/en/) websites (all accessed 13 September 2018) also exist that post apps that have been assessed using various in-house rating methods.
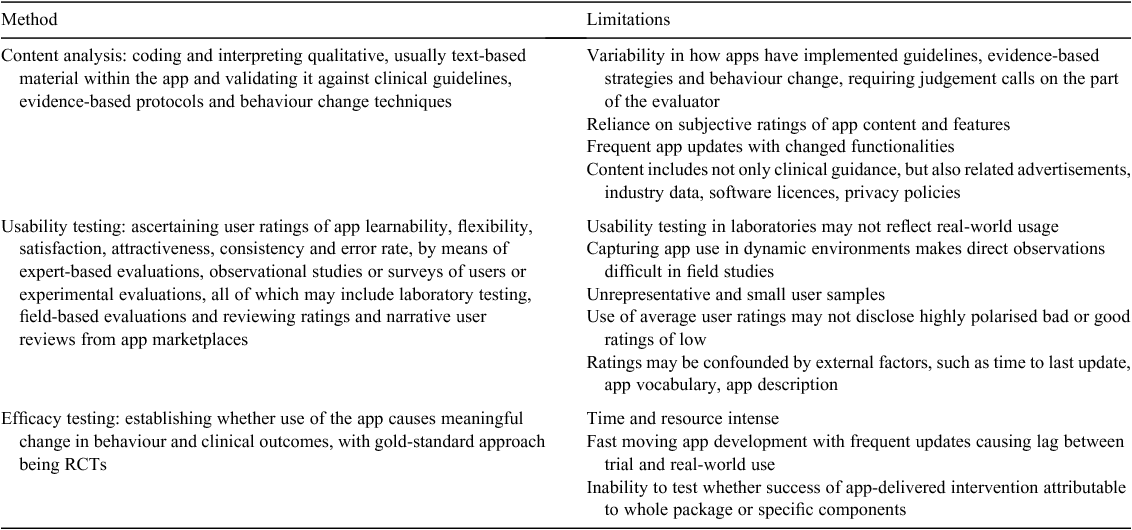
|
We contend that any evaluation method should consider clinical and technical attributes of an app that we have identified from this review as being important, while not being too general or complex or time consuming with need for training, or too specific to a particular health condition or outcome. After analysing the above reports and recent reviews,98–101 we derived a list of criteria (Table 4) to which yes/no or scalar (Likert) responses could gauge the extent to which an app meets each criterion. Obtaining informed responses to each criterion requires retrieving relevant data using strategies listed in Table 5.102 We suggest these criteria may suffice as a ‘first-pass’ assessment by panels involving clinicians in deciding whether the app justifies more detailed technical assessments under a second-phase due-diligence process.

|
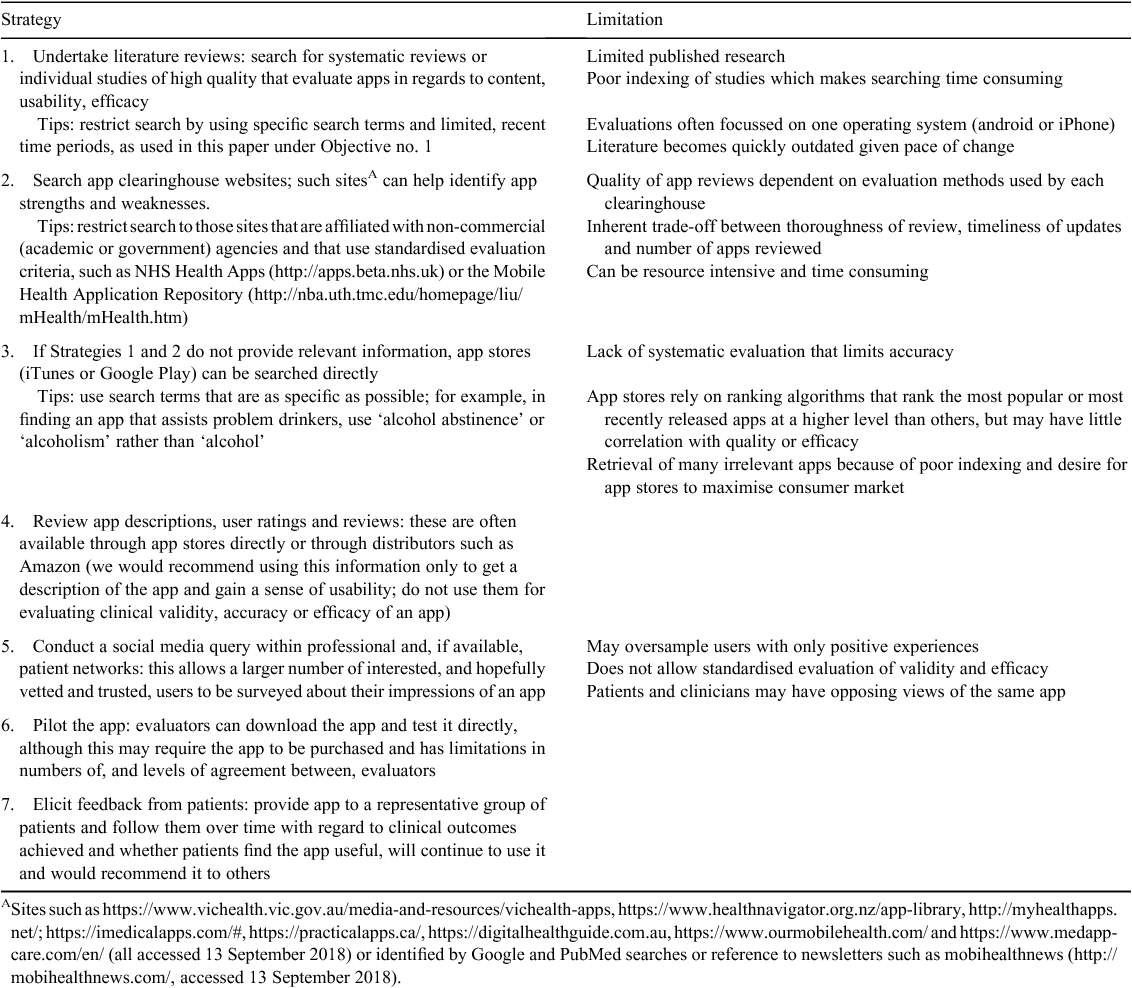
|
Discussion
To the best of our knowledge, this is the first review of evidence relating to the efficacy, usability and evaluation of apps that assist patients to self-manage chronic diseases. Limitations of this review are that, due to time and resource constraints, we did not search for and analyse every individual study of every app, but instead relied on systematic reviews, when these were available, some of which differed in inclusion criteria and conclusions. However, we do not feel any important study was overlooked and, in particular, RCTs with less risk of bias were diligently sought and given emphasis. Space limitations did not allow us to provide full descriptions of each app, some having multiple components, but readers can refer to the references provided for more detail. We view our framework as being a provisional ‘first-pass’ framework that may help select app submissions that warrant second-phase due-diligence reviews to which more detailed assessments of technical attributes relating to interoperability, security and privacy may be added.
Improving the evidence base of app efficacy
The rapid pace of the technological evolution of apps and the slowness and expense of gold-standard research designs, such as RCTs, make assessments of efficacy problematic. Evaluating apps is complex given the combination of content, user, platform, links and interface attributes, and determining whether benefits are attributable to a total app package or specific components. Apps can be released, updated, modified or removed by commercial developers as studies with fixed protocols evaluating a specific app (or type of app) are underway, rendering the results potentially obsolete by the time of public release. Control groups in comparative trials may be contaminated by being exposed to similar or changing digital interventions during the course of a study. In addition, results of new clinical studies may change the evidence on which the core content and behaviour change functions contained in an app are based, such that the app itself, despite having been studied, becomes invalid. Finally, apps investigated in research settings are often not the same as those that are disseminated commercially.
Alternative study designs have been suggested that aim to overcome some of these issues, albeit with limitations. These include multiphase optimisation (MOST) strategies (factorial or fractional designs and adaptive trials),103 n-of-1 studies that involve repeated measurement of many individuals over time in understanding within-person behaviour before and then after using an app, or using an app and then having it withdrawn,104 or triangulation of ‘fit-for-purpose’ studies that investigate sociocultural, organisational, cognitive and other contextual determinants of effects using surveys, focus group discussions and ethnographic studies.105
Relationship between app use and efficacy
In many reviews, more focus is given to assessing usability than to testing efficacy. We are concerned that apps rated by many users as highly usable and that become popular may be viewed by new users as apps that predictably confer health benefits. This is akin to ‘I really like and use the app so therefore it must be doing me good’. Design attributes that discourage people from using an app in the way intended will certainly compromise efficacy, but the reverse is not true. Usability is a multifaceted construct that requires app design to be informed by behaviour change theory, because information alone is insufficient to change behaviour.106 In this review, only a minority of studies explicitly reported using behaviour change theories to underpin app design, and adherence and retention rates were frequently <80%. Although many apps make reference to behavioural theories, self-monitoring alone is often the dominant function rather than incorporating proactive skills development in self-management, which may substantially enhance efficacy.107 Using apps pertaining to mental health and addiction problems as examples, apps need to incorporate a behavioural conditioning plan and interactive framework that include rapid access to expert help at times of crisis and that mitigate limited attention span and curtail time spent on devices by emphasising non-app-based activities.108 Personalised information, real-time feedback and access to expert consultation when required are features highly valued by users. There is a need for a checklist of usability and functionality attributes that apps should aspire to in maximising user engagement, which this review has helped create.
Implications for app evaluation and endorsement
Lack of evidence of app efficacy, poor descriptions of processes and data sources used to develop the app and discrepancies between information generated on apps and evidence-based guidelines are key issues in evaluation.109 Of note, apps requiring purchase are not necessarily more evidence based than free apps.110 With regard to efficacy testing, our overview highlights several shortcomings in the current evidence base: a paucity of rigorous efficacy trials with adequate sample sizes for most conditions; inconsistency of measured outcomes, with many based on subjective patient self-report, with relatively few hard end-points (e.g. mortality, clinical events, hospitalisations); insufficient duration for assessing long-term effects; minimal reference to adherence rates and underlying behavioural change techniques; questionable fidelity of patient compliance with data input and use of algorithms; and limited generalisability given heterogeneity in populations, interventions, outcome measures and conflicting estimates of effect. The exceptions to many of these limitations are diabetes apps, for which sufficient and consistent data confirm efficacy in optimising blood sugar control, although effects on other outcomes await assessment. For all conditions, there was little focus on the needs and competencies of older patients111 and culturally diverse groups,112 or on cost-effectiveness, including costs of misinformation transmitted, of addressing resulting problems or of the diverse workforce required for app implementation and monitoring.113
Methods for evaluating the quality and utility of an app must be capable of assessing all relevant attributes of technical integrity, usability and efficacy in an explicit and transparent manner. This review suggests an evaluation framework that could be refined and tested for construct validity and internal consistency. Potential users of the framework may include the ADHA, TGA, state-based government digital health agencies and networks, app developers and any other stakeholder group who has responsibilities in health app evaluation and endorsement. In Queensland, the mHealth Apps Program, under the auspices of QPACT, HEAT, and eHealth Queensland, is being resourced to further develop the framework and its application to all future submissions from developers and interested clinicians for QPACT funding of field trials of mobile health apps within QH facilities.
Conclusion
The numbers of smartphone apps will continue to grow as will the appetite for patients and clinicians to use them in chronic disease self-management. However, the evidence to date of clinical benefit of most of the apps already available is very limited. Design features that enhance users’ desire to use the app, and thereby derive most benefit, need to be considered in maximising efficacy. In making decisions about which apps should be endorsed by government agencies and recommended with confidence by clinicians to their patients, a workable evaluation framework needs to be used by those agencies that assume the role of formulating and applying app standards. The provisional framework presented in this report serves as a starting point for such work.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
There was no funding source for this research. The authors are all members of the Queensland Health mHealth Apps Working Group. The views expressed in this article should not be regarded as official policy of Queensland Health or any of its divisions, Queensland Policy and Advisory Committee on new Technology, Healthcare Evaluation and Assessment of Technology Team or eHealth Queensland. The views expressed are solely those of the authors.
References
[1] Mackay M. Australian mobile phone lifestyle index. 2014. Available at: https://www.dtcollective.org.au/LiteratureRetrieve.aspx?ID=180479 [verified 22 September 2018].[2] Jahns RG. The 8 drivers and barriers that will shape the mHealth app market in the next 5 years. r2g Mobile Health Economics. 2014. Available at: https://research2guidance.com/8-drivers-and-barriers-which-will-shape-mhealth-market-in-the-next-5-years/ [verified 22 September 2018].
[3] Aitken M, Lyle J. Patient adoption of mHealth: use, evidence and remaining barriers to mainstream acceptance. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2015.
[4] Bhuyan SS, Lu N, Chandak A, Kim H, Wyant D, Bhatt J, Kedia S, Chang CF. Use of mobile health applications for health seeking behaviour among US adults. J Med Syst 2016; 40 153
| Use of mobile health applications for health seeking behaviour among US adults.Crossref | GoogleScholarGoogle Scholar |
[5] Powell AC, Landman AB, Bates DW. In search of a few good apps. JAMA 2014; 311 1851–2.
| In search of a few good apps.Crossref | GoogleScholarGoogle Scholar |
[6] Boulos MN, Brewer AC, Karimkhani C, Buller DB, Dellavalle RP. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform 2014; 5 229
[7] Risk A, Dzenowagis J. Review of Internet health information quality initiatives. J Med Internet Res 2001; 3 E28
| Review of Internet health information quality initiatives.Crossref | GoogleScholarGoogle Scholar |
[8] Gagliardi A, Jadad AR. Examination of instruments used to rate quality of health information on the internet: chronicle of a voyage with an unclear destination. BMJ 2002; 324 569–73.
| Examination of instruments used to rate quality of health information on the internet: chronicle of a voyage with an unclear destination.Crossref | GoogleScholarGoogle Scholar |
[9] Subhi Y, Bube SH, Bojsen SR, Thomsen ASS, Konge L. Expert involvement and adherence to medical evidence in medical mobile phone apps: a systematic review. JMIR Mhealth Uhealth 2015; 3 e79
| Expert involvement and adherence to medical evidence in medical mobile phone apps: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[10] Goyal S, Cafazzo JA. Mobile phone apps for diabetes management: current evidence and future developments. QJM 2013; 106 1067–9.
| Mobile phone apps for diabetes management: current evidence and future developments.Crossref | GoogleScholarGoogle Scholar |
[11] Wallace LS, Dhingra LK. A systematic review of smartphone applications for chronic pain available for download in the United States. J Opioid Manag 2014; 10 63–8.
| A systematic review of smartphone applications for chronic pain available for download in the United States.Crossref | GoogleScholarGoogle Scholar |
[12] Reynoldson C, Stones C, Allsop M, Gardner P, Bennett MI, José Closs S, Jones R, Knapp P. Assessing the quality and usability of smartphone apps for pain self-management. Pain Med 2014; 15 898–909.
| Assessing the quality and usability of smartphone apps for pain self-management.Crossref | GoogleScholarGoogle Scholar |
[13] Martínez-Pérez B, De La Torre-Diez I, Lopez-Coronado M. Privacy and security in mobile phone apps: a review and recommendations. J Med Syst 2015; 39 181
| Privacy and security in mobile phone apps: a review and recommendations.Crossref | GoogleScholarGoogle Scholar |
[14] Pustozerov E, von Jan U, Albrecht UV. Evaluation of mHealth applications security based on application permissions. Stud Health Technol Inform 2016; 226 241–4.
[15] Lewis TL, Wyatt JC. mHealth and mobile medical apps: a framework to assess risk and promote safer use. J Med Internet Res 2014; 16 e210
| mHealth and mobile medical apps: a framework to assess risk and promote safer use.Crossref | GoogleScholarGoogle Scholar |
[16] U.S. Department of Health and Human Services, Food and Drug Administration. Mobile medical applications: guidance for industry and Food and Drug Administration staff. 2015. Available at: http://www.fda.gov/downloads/MedicalDevices/./UCM263366.pdf [verified 13 January 2018]
[17] Australian Government Department of Health. Therapeutic goods administration. n. Regulation of medical software and mobile medical ‘apps’. 2013. Available at: https://www.tga.gov.au/regulation-medical-software-and-mobile-medical-apps [verified 13 January 2018]
[18] U.S. Department of Health and Human Services, Food and Drug Administration. Software as a medical device (SAMD): Clinical evaluation. Guidance for industry and Food and Drug Administration Staff. 2017. Available at: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM524904.pdf [verified 21 September 2018].
[19] Hau J. Update on software as a medical device (SaMD). The TGA and IMDRF perspectives. 2017. Available at: https://www.tga.gov.au/sites/default/files/update-on-software-as-a-medical-device-samd.pdf [verified 20 January 2018].
[20] Australian Digital Health Agency (ADHA). Safe, seamless and secure: evolving health and care to meet the needs of modern Australia. Australia’s national digital health strategy. Canberra: ADHA; 2017.
[21] Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6 e1000097
| Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Crossref | GoogleScholarGoogle Scholar |
[22] Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380 2095–128. [published erratum appears in Lancet 2013; 381:628]
| Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010.Crossref | GoogleScholarGoogle Scholar |
[23] Abraham C, Mitchie S. A taxonomy of behaviour change techniques used in interventions. Health Psychol 2008; 27 379–87.
| A taxonomy of behaviour change techniques used in interventions.Crossref | GoogleScholarGoogle Scholar |
[24] Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011; 26 1479–98.
| A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy.Crossref | GoogleScholarGoogle Scholar |
[25] Hui CY, Walton R, McKinstry B, Jackson T, Parker R, Pinnock H. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc 2017; 24 619–32.
[26] Cingi C, Yorgancioglu A, Cingi CC, Oguzulgen K, Muluk NB, Ulusoy S, Orhon N, Yumru C, Gokdag D, Karakaya G, Çelebi S, Çobanoglu HB, Unlu H, Aksoy MA. The ‘physician on call patient engagement trial’ (POPET): measuring the impact of a mobile patient engagement application on health outcomes and quality of life in allergic rhinitis and asthma patients. Int Forum Allergy Rhinol 2015; 5 487–97.
| The ‘physician on call patient engagement trial’ (POPET): measuring the impact of a mobile patient engagement application on health outcomes and quality of life in allergic rhinitis and asthma patients.Crossref | GoogleScholarGoogle Scholar |
[27] Ryan D, Price D, Musgrave SD, Malhotra S, Lee AJ, Ayansina D, Sheikh A, Tarassenko L, Pagliari C, Pinnock H. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. BMJ 2012; 344 e1756
| Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[28] Liu WT, Huang CD, Wang CH, Lee KY, Lin SM, Kuo HP. A mobile telephone-based interactive self-care system improves asthma control. Eur Respir J 2011; 37 310–7.
| A mobile telephone-based interactive self-care system improves asthma control.Crossref | GoogleScholarGoogle Scholar |
[29] McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 5 CD011425
[30] Tabak M, Vollenbroek-Hutten MM, van der Valk PD, van der Palen J, Hermens HJ. A telerehabilitation intervention for patients with chronic obstructive pulmonary disease: a randomized controlled pilot trial. Clin Rehabil 2014; 28 582–91.
| A telerehabilitation intervention for patients with chronic obstructive pulmonary disease: a randomized controlled pilot trial.Crossref | GoogleScholarGoogle Scholar |
[31] Wu IXY, Kee JCY, Threapleton DE, Ma R, Lam VCK, Lee EKP, Wong SYS, Chung VCH. Effectiveness of smartphone technologies on glycaemic control in patients with type 2 diabetes: systematic review with meta-analysis of 17 trials. Obes Rev 2018; 19 825–38.
| Effectiveness of smartphone technologies on glycaemic control in patients with type 2 diabetes: systematic review with meta-analysis of 17 trials.Crossref | GoogleScholarGoogle Scholar |
[32] Wu Y, Yao X, Vespasiani G, Nicolucci A, Dong Y, Kwong J, Li L, Sun X, Tian H, Li S. Mobile app-based interventions to support diabetes self-management: a systematic review of randomized controlled trials to identify functions associated with glycemic efficacy. JMIR Mhealth Uhealth 2017; 5 e35
| Mobile app-based interventions to support diabetes self-management: a systematic review of randomized controlled trials to identify functions associated with glycemic efficacy.Crossref | GoogleScholarGoogle Scholar |
[33] Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care 2016; 39 2089–95.
| Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials.Crossref | GoogleScholarGoogle Scholar |
[34] Cui M, Wu X, Mao J, Wang X, Nie M. T2DM self-management via smartphone applications: a systematic review and meta-analysis. PLoS One 2016; 11 e0166718
| T2DM self-management via smartphone applications: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[35] Fu H, McMahon SK, Gross CR, Adam TJ, Wyman JF. Usability and clinical efficacy of diabetes mobile applications for adults with type 2 diabetes: a systematic review. Diabetes Res Clin Pract 2017; 131 70–81.
| Usability and clinical efficacy of diabetes mobile applications for adults with type 2 diabetes: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[36] Holmen H, Wahl AK, Cvancarova Småstuen M, Ribu L. Tailored communication within mobile apps for diabetes self-management: a systematic review. J Med Internet Res 2017; 19 e227
| Tailored communication within mobile apps for diabetes self-management: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[37] Lalloo C, Jibb LA, Rivera J, Agarwal A, Stinson JN. ‘There’s a pain app for that’: review of patient-targeted smartphone applications for pain management. Clin J Pain 2015; 31 557–63.
| ‘There’s a pain app for that’: review of patient-targeted smartphone applications for pain management.Crossref | GoogleScholarGoogle Scholar |
[38] Machado GC, Pinheiro MB, Lee H, Ahmed OH, Hendrick P, Williams C, Kamper SJ. Smartphone apps for the self-management of low back pain: a systematic review. Best Pract Res Clin Rheumatol 2016; 30 1098–109.
| Smartphone apps for the self-management of low back pain: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[39] Nicholl BI, Sandal LF, Stochkendahl MJ, McCallum M, Suresh N, Vasseljen O. Digital support interventions for the self-management of low back pain: a systematic review. J Med Internet Res 2017; 19 e179
| Digital support interventions for the self-management of low back pain: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[40] Irvine AB, Russell H, Manocchia M, Mino DE, Cox GT, Morgan R, Gau JM, Birney AJ, Ary DV. Mobile-web app to self-manage low back pain: randomized controlled trial. J Med Internet Res 2015; 17 e1
| Mobile-web app to self-manage low back pain: randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[41] Kristjánsdóttir ÓB, Fors EA, Eide E, Finset A, Stensrud TL, van Dulmen S, Wigers SH, Eide H. A smartphone-based intervention with diaries and therapist feedback to reduce catastrophizing and increase functioning in women with chronic widespread pain. Part 2: 11-month follow-up results of a randomized trial. J Med Internet Res 2013; 15 e72
| A smartphone-based intervention with diaries and therapist feedback to reduce catastrophizing and increase functioning in women with chronic widespread pain. Part 2: 11-month follow-up results of a randomized trial.Crossref | GoogleScholarGoogle Scholar |
[42] Batra S, Baker RA, Wang T, Forma F, DiBiasi F, Peters-Strickland T. Digital health technology for use in patients with serious mental illness: a systematic review of the literature. Med Devices (Auckl) 2017; 10 237–51.
| Digital health technology for use in patients with serious mental illness: a systematic review of the literature.Crossref | GoogleScholarGoogle Scholar |
[43] Ly KH, Trüschel A, Jarl L, Magnusson S, Windahl T, Johansson R, Carlbring P, Andersson G. Behavioural activation versus mindfulness-based guided self-help treatment administered through a smartphone application: a randomised controlled trial. BMJ Open 2014; 4 e003440
| Behavioural activation versus mindfulness-based guided self-help treatment administered through a smartphone application: a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[44] Watts S, Mackenzie A, Thomas C, Griskaitis A, Mewton L, Williams A, Andrews G. CBT for depression: a pilot RCT comparing mobile phone vs. computer. BMC Psychiatry 2013; 13 49
| CBT for depression: a pilot RCT comparing mobile phone vs. computer.Crossref | GoogleScholarGoogle Scholar |
[45] Forchuk C, Reiss JP, O’Regan T, Ethridge P, Donelle L, Rudnick A. Client perceptions of the Mental Health Engagement Network: a qualitative analysis of an electronic personal health record. BMC Psychiatry 2015; 15 250
| Client perceptions of the Mental Health Engagement Network: a qualitative analysis of an electronic personal health record.Crossref | GoogleScholarGoogle Scholar |
[46] Faurholt-Jepsen M, Ritz C, Frost M, Mikkelsen RL, Christensen EM, Bardram J, Vinberg M, Kessing LV. Mood instability in bipolar disorder type I versus type II: continuous daily electronic self-monitoring of illness activity using smartphones. J Affect Disord 2015; 186 342–9.
| Mood instability in bipolar disorder type I versus type II: continuous daily electronic self-monitoring of illness activity using smartphones.Crossref | GoogleScholarGoogle Scholar |
[47] Ben-Zeev D, Brenner CJ, Begale M, Duffecy J, Mohr DC, Mueser KT. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull 2014; 40 1244–53.
| Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia.Crossref | GoogleScholarGoogle Scholar |
[48] Grist R, Porter J, Stallard P. Mental health mobile apps for preadolescents and adolescents: a systematic review. J Med Internet Res 2017; 19 e176
| Mental health mobile apps for preadolescents and adolescents: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[49] Reid SC, Kauer SD, Hearps SJ, Crooke AH, Khor AS, Sanci LA, Patton GC. A mobile phone application for the assessment and management of youth mental health problems in primary care: health service outcomes from a randomised controlled trial of mobile type. BMC Fam Pract 2013; 14 84
| A mobile phone application for the assessment and management of youth mental health problems in primary care: health service outcomes from a randomised controlled trial of mobile type.Crossref | GoogleScholarGoogle Scholar |
[50] Veldhuis J. Media models matter in context: negotiated media effects of idealized body images. PhD Thesis, Vrije Universiteit Amsterdam Faculteit der Social Wetenschappen; 2014. Available at: https://research.vu.nl/en/publications/media-models-matter-in-context-negotiated-media-effects-of-ideali [verified 21 September 2018].
[51] Meredith SE, Alessi SM, Petry NM. Smartphone applications to reduce alcohol consumption and help patients with alcohol use disorder: a state-of-the-art review. Adv Health Care Technol 2015; 1 47–54.
[52] Gajecki M, Berman AH, Sinadinovic K, Rosendahl I, Andersson C. Mobile phone brief intervention applications for risky alcohol use among university students: a randomized controlled study. Addict Sci Clin Pract 2014; 9 11
| Mobile phone brief intervention applications for risky alcohol use among university students: a randomized controlled study.Crossref | GoogleScholarGoogle Scholar |
[53] Gustafson DH, McTavish FM, Chih MY, Atwood AK, Johnson RA, Boyle MG. A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA Psychiatry 2014; 71 566–72.
| A smartphone application to support recovery from alcoholism: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar |
[54] Witkiewitz K, Desai SA, Bowen S, Leigh BC, Kirouac M, Larimer ME. Development and evaluation of a mobile intervention for heavy drinking and smoking among college students. Psychol Addict Behav 2014; 28 639–50.
| Development and evaluation of a mobile intervention for heavy drinking and smoking among college students.Crossref | GoogleScholarGoogle Scholar |
[55] Hägglund E, Lyngå P, Frie F, Ullman B, Persson H, Melin M, Hagerman I. Patient-centred home-based management of heart failure. Findings from a randomised clinical trial evaluating a tablet computer for self-care, quality of life and effects on knowledge. Scand Cardiovasc J 2015; 49 193–9.
| Patient-centred home-based management of heart failure. Findings from a randomised clinical trial evaluating a tablet computer for self-care, quality of life and effects on knowledge.Crossref | GoogleScholarGoogle Scholar |
[56] Vuorinen AL, Leppänen J, Kaijanranta H, Kulju M, Heliö T, van Gils M, Lähteenmäki J. Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: randomized controlled trial. J Med Internet Res 2014; 16 e282
| Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[57] Johnston N, Bodegard J, Jerström S, Åkesson J, Brorsson H, Alfredsson J. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: a randomized study. Am Heart J 2016; 178 85–94.
| Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: a randomized study.Crossref | GoogleScholarGoogle Scholar |
[58] Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, Smith C, Walters DL. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014; 100 1770–9.
| Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[59] Blasco A, Carmona M, Fernández-Lozano I, Salvador CH, Pascual M, Sagredo PG, Somolinos R, Muñoz A, García-López F, Escudier JM, Mingo S, Toquero J, Moñivas V, González MA, Fragua JA, López-Rodríguez F, Monteagudo JL, Alonso-Pulpón L. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. J Cardiopulm Rehabil Prev 2012; 32 25–31.
| Evaluation of a telemedicine service for the secondary prevention of coronary artery disease.Crossref | GoogleScholarGoogle Scholar |
[60] Rincon E, Monteiro-Guerra F, Rivera-Romero O, Dorronzoro-Zubiete E, Sanchez-Bocanegra CL, Gabarron E. Mobile phone apps for quality of life and well-being assessment in breast and prostate cancer patients: systematic review. JMIR Mhealth Uhealth 2017; 5 e187
| Mobile phone apps for quality of life and well-being assessment in breast and prostate cancer patients: systematic review.Crossref | GoogleScholarGoogle Scholar |
[61] Sundberg K, Wengström Y, Blomberg K, Hälleberg-Nyman M, Frank C, Langius-Eklöf A. Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer. Support Care Cancer 2017; 25 2195–204.
| Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer.Crossref | GoogleScholarGoogle Scholar |
[62] Uhm KE, Yoo JS, Chung SH, Lee JD, Lee I, Kim JI, Lee SK, Nam SJ, Park YH, Lee JY, Hwang JH. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat 2017; 161 443–52.
| Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure?Crossref | GoogleScholarGoogle Scholar |
[63] McCarroll ML, Armbruster S, Pohle-Krauza RJ, Lyzen AM, Min S, Nash DW, Roulette GD, Andrews SJ, von Gruenigen VE. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol Oncol 2015; 137 508–15.
| Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application.Crossref | GoogleScholarGoogle Scholar |
[64] Bateman DR, Srinivas B, Emmett TW, Schleyer TK, Holden RJ, Hendrie HC, Callahan CM. Categorizing health outcomes and efficacy of mHealth apps for persons with cognitive impairment: a systematic review. J Med Internet Res 2017; 19 e301
| Categorizing health outcomes and efficacy of mHealth apps for persons with cognitive impairment: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[65] Chan SC, Chan CC, Derbie AY, Hui I, Tan DG, Pang MY, Lau SCL, Fong KNK. Chinese calligraphy writing for augmenting attentional control and working memory of older adults at risk of mild cognitive impairment: a randomized controlled trial. J Alzheimers Dis 2017; 58 735–46.
| Chinese calligraphy writing for augmenting attentional control and working memory of older adults at risk of mild cognitive impairment: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[66] Mansbach WE, Mace RA, Clark KM. The efficacy of a computer-assisted cognitive rehabilitation program for patients with mild cognitive deficits: a pilot study. Exp Aging Res 2017; 43 94–104.
| The efficacy of a computer-assisted cognitive rehabilitation program for patients with mild cognitive deficits: a pilot study.Crossref | GoogleScholarGoogle Scholar |
[67] Han JW, Oh K, Yoo S, Kim E, Ahn K-H, Son Y-J, Kim TH, Chi YK, Kim KW. Development of the ubiquitous spaced retrieval-based memory advancement and rehabilitation training program. Psychiatry Investig 2014; 11 52–8.
| Development of the ubiquitous spaced retrieval-based memory advancement and rehabilitation training program.Crossref | GoogleScholarGoogle Scholar |
[68] Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, Kramer JH. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord 2009; 23 205–10.
| Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial.Crossref | GoogleScholarGoogle Scholar |
[69] Finn M, McDonald S. Computerised cognitive training for older persons with mild cognitive impairment: a pilot study using a randomised controlled trial design. Brain Impair 2011; 12 187–99.
| Computerised cognitive training for older persons with mild cognitive impairment: a pilot study using a randomised controlled trial design.Crossref | GoogleScholarGoogle Scholar |
[70] Hattink BJ, Meiland FJ, Overmars-Marx T, de Boer M, Ebben PW, van Blanken M, Verhaeghe S, Stalpers-Croeze I, Jedlitschka A, Flick SE, van den Leeuw J, Karkowski I, Dröes RM. The electronic, personalizable Rosetta system for dementia care: exploring the user-friendliness, usefulness and impact. Disabil Rehabil Assist Technol 2016; 11 61–71.
| The electronic, personalizable Rosetta system for dementia care: exploring the user-friendliness, usefulness and impact.Crossref | GoogleScholarGoogle Scholar |
[71] Van der Ploeg ES, Eppingstall B, O’Connor DW. Internet video chat (Skype) family conversations as a treatment of agitation in nursing home residents with dementia. Int Psychogeriatr 2016; 28 697–8.
| Internet video chat (Skype) family conversations as a treatment of agitation in nursing home residents with dementia.Crossref | GoogleScholarGoogle Scholar |
[72] Ong SW, Jassal SV, Miller JA, Porter EC, Cafazzo JA, Seto E. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol 2016; 11 1054–62.
| Integrating a smartphone-based self-management system into usual care of advanced CKD.Crossref | GoogleScholarGoogle Scholar |
[73] Bender JL, Yue RY, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res 2013; 15 e287
| A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer.Crossref | GoogleScholarGoogle Scholar |
[74] Huguet A, Rao S, McGrath PJ, Wozney L, Wheaton M, Conrod J, Rozario S. A systematic review of cognitive behavioral therapy and behavioral activation apps for depression. PLoS One 2016; 11 e0154248
| A systematic review of cognitive behavioral therapy and behavioral activation apps for depression.Crossref | GoogleScholarGoogle Scholar |
[75] Rosser BA, Eccleston C. Smartphone applications for pain management. J Telemed Telecare 2011; 17 308–12.
| Smartphone applications for pain management.Crossref | GoogleScholarGoogle Scholar |
[76] Brzan PP, Rotman E, Pajnkihar M, Klanjsek P. Mobile applications for control and self-management of diabetes: a systematic review. J Med Syst 2016; 40 210
| Mobile applications for control and self-management of diabetes: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[77] Zhao J, Freeman B, Li M. Can mobile phone apps influence people’s health behavior change? An evidence review. J Med Internet Res 2016; 18 e287
| Can mobile phone apps influence people’s health behavior change? An evidence review.Crossref | GoogleScholarGoogle Scholar |
[78] Spring B, Gotsis M, Paiva A, Spruijt-Metz D. Healthy apps: mobile devices for continuous monitoring and intervention. IEEE Pulse 2013; 4 34–40.
| Healthy apps: mobile devices for continuous monitoring and intervention.Crossref | GoogleScholarGoogle Scholar |
[79] Jake-Schoffman DE, Silfee VJ, Waring ME, Boudreaux ED, Sadasivam RS, Mullen SP, Carey JL, Hayes RB, Ding EY, Bennett GG, Pagoto SL. Methods for evaluating the content, usability, and efficacy of commercial mobile health apps. JMIR Mhealth Uhealth 2017; 5 e190
| Methods for evaluating the content, usability, and efficacy of commercial mobile health apps.Crossref | GoogleScholarGoogle Scholar |
[80] Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore (NY) 2011; 7 256–61.
| mHealth (mobile health)-using apps for health and wellness.Crossref | GoogleScholarGoogle Scholar |
[81] Khoja S, Durrani H, Scott RE, Sajwani A, Piryani U. Conceptual framework for development of comprehensive e-health evaluation tool. Telemed J E Health 2013; 19 48–53.
| Conceptual framework for development of comprehensive e-health evaluation tool.Crossref | GoogleScholarGoogle Scholar |
[82] mHIMSS App Usability Work Group. Selecting a mobile app: evaluating the usability of medical applications. Chicago, IL: Health Care Information Management Systems Society; 2012. Available at: https://www.himss.org/selecting-mobile-app-evaluating-usability-medical-applications-0 [verified 21 September 2018]
[83] Stoyanov SR, Hides L, Kavanagh DJ, Zelenko O, Tjondronegoro D, Mani M. Mobile apps rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth 2015; 3 e27
| Mobile apps rating scale: a new tool for assessing the quality of health mobile apps.Crossref | GoogleScholarGoogle Scholar |
[84] Jin M, Kim J. Development and evaluation of an evaluation tool for healthcare smartphone applications. Telemed J E Health 2015; 21 831–7.
| Development and evaluation of an evaluation tool for healthcare smartphone applications.Crossref | GoogleScholarGoogle Scholar |
[85] Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol 2015; 125 1478–83.
| Rating pregnancy wheel applications using the APPLICATIONS scoring system.Crossref | GoogleScholarGoogle Scholar |
[86] Baumel A, Faber K, Mathur N, Kane JM, Muench F. Enlight: a comprehensive quality and therapeutic potential evaluation tool for mobile and web-based ehealth interventions. J Med Internet Res 2017; 19 e82
| Enlight: a comprehensive quality and therapeutic potential evaluation tool for mobile and web-based ehealth interventions.Crossref | GoogleScholarGoogle Scholar |
[87] McNiel P, McAthtur EC. Evaluating health mobile apps: information literacy in undergraduate and graduate nursing courses. J Nurs Educ 2016; 55 480–2.
| Evaluating health mobile apps: information literacy in undergraduate and graduate nursing courses.Crossref | GoogleScholarGoogle Scholar |
[88] Meurk C, Leung J, Hall W, Head BW, Whiteford H. Establishing and governing e-mental health care in Australia: a systematic review of challenges and a call for policy-focused research. J Med Internet Res 2016; 18 e10
| Establishing and governing e-mental health care in Australia: a systematic review of challenges and a call for policy-focused research.Crossref | GoogleScholarGoogle Scholar |
[89] Maheu MM, Pulier ML, Roy S. Finding, evaluating, and using smartphone applications. In: Koocher GP, Norcross JC, Breene BA, editors. Psychologists’ desk reference. 3rd edn. New York: Oxford University Press; 2013. pp. 704–8.
[90] Wyatt JC, Thimbleby H, Rastall P, Hoogewerf J, Wooldridge D, Williams J. What makes a good clinical app? Introducing the RCP Health Informatics Unit checklist. Clin Med (Lond) 2015; 15 519–21.
| What makes a good clinical app? Introducing the RCP Health Informatics Unit checklist.Crossref | GoogleScholarGoogle Scholar |
[91] Anderson K, Burford O, Emmerton L. App Chronic disease checklist: protocol to evaluate mobile apps for chronic disease self-management. JMIR Res Protoc 2016; 5 e204
| App Chronic disease checklist: protocol to evaluate mobile apps for chronic disease self-management.Crossref | GoogleScholarGoogle Scholar |
[92] Maheu MM, Nicolucci V, Pulier ML, Wall KM, Frye TJ, Hudlick E. The Interactive Mobile App Review Toolkit (IMART): a clinical practice-oriented system. J Technol Behav Sci 2017; 1 3–15. [published erratum appears in: J Technol Behav Sci 2017; 2 (3-4):211]
| The Interactive Mobile App Review Toolkit (IMART): a clinical practice-oriented system.Crossref | GoogleScholarGoogle Scholar |
[93] The Royal Australian College of General Practitioners (RACGP). mHealth in general practice: a toolkit for effective and secure use of mobile technology. Melbourne: RACGP; 2016.
[94] Yasini M, Beranger J, Desmarais P, Perez L, Marchand G. mHealth quality: aA process to seal the qualified mobile health apps. Stud Health Technol Inform 2016; 228 205–9.
[95] Dialogue Consulting. Guidelines for developing healthy living apps. Melbourne: VicHealth; 2015.
[96] Eysenbach G. CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions. J Med Internet Res 2011; 13 e126
| CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions.Crossref | GoogleScholarGoogle Scholar |
[97] Agarwal S, LeFevre AE, Lee J, L’Engle K, Mehl G, Sinha C, Labrique A. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ 2016; 352 i1174
| Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist.Crossref | GoogleScholarGoogle Scholar |
[98] BinDhim NF, Hawkey A, Trevena L. A systematic review of quality assessment methods for smartphone health apps. Telemed J E Health 2015; 21 97–104.
| A systematic review of quality assessment methods for smartphone health apps.Crossref | GoogleScholarGoogle Scholar |
[99] Chan S, Torous J, Hinton L, Yellowlees P. Towards a framework for evaluating mobile mental health apps. Telemed J E Health 2015; 21 1038–41.
| Towards a framework for evaluating mobile mental health apps.Crossref | GoogleScholarGoogle Scholar |
[100] Grundy QH, Wang Z, Bero LA. Challenges in assessing mobile health app quality. A systematic review of prevalent and innovative methods. Am J Prev Med 2016; 51 1051–9.
| Challenges in assessing mobile health app quality. A systematic review of prevalent and innovative methods.Crossref | GoogleScholarGoogle Scholar |
[101] McKay FH, Cheng C, Wright A, Shill J, Stephens H, Uccellini M. Evaluating mobile phone applications for health behaviour change: a systematic review. J Telemed Telecare 2018; 24 22–30.
| Evaluating mobile phone applications for health behaviour change: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[102] Boudreaux ED, Waring ME, Hayes RB, Sadasivam RS, Mullen S, Pagoto S. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med 2014; 4 363–71.
| Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations.Crossref | GoogleScholarGoogle Scholar |
[103] Collins LM, Murphy SA, Strecher V. The multiphase optimisation strategy (MOST) and the sequential multiple assignment randomiised trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med 2007; 32 S112–8.
| The multiphase optimisation strategy (MOST) and the sequential multiple assignment randomiised trial (SMART): new methods for more potent eHealth interventions.Crossref | GoogleScholarGoogle Scholar |
[104] McDonald S, Quinn F, Vieira R, O’Brien N, White M, Johnston DW, Sniehotta FF. The state of the art and future opportunities for using longitudinal n-of-1 methods in health behaviour research: a systematic literature overview. Health Psychol Rev 2017; 11 307–23.
| The state of the art and future opportunities for using longitudinal n-of-1 methods in health behaviour research: a systematic literature overview.Crossref | GoogleScholarGoogle Scholar |
[105] Kaplan B. Evaluating informatics applications – some alternative approaches: theory, social interactionism, and call for methodological pluralism. Int J Med Inform 2001; 64 39–56.
| Evaluating informatics applications – some alternative approaches: theory, social interactionism, and call for methodological pluralism.Crossref | GoogleScholarGoogle Scholar |
[106] Michie S, Johnston M. Theories and techniques of behaviour change: developing a cumulative science of behaviour change. Health Psychol Rev 2012; 6 1–6.
| Theories and techniques of behaviour change: developing a cumulative science of behaviour change.Crossref | GoogleScholarGoogle Scholar |
[107] Payne HE, Lister C, West JH, Bernhardt JM. Behavioral functionality of mobile apps in health interventions: a systematic review of the literature. JMIR Mhealth Uhealth 2015; 3 e20
| Behavioral functionality of mobile apps in health interventions: a systematic review of the literature.Crossref | GoogleScholarGoogle Scholar |
[108] Bakker D, Kazantzis N, Rickwood D, Rickard N. Mental health smartphone apps: review and evidence-based recommendations for future developments. JMIR Ment Health 2016; 3 e7
| Mental health smartphone apps: review and evidence-based recommendations for future developments.Crossref | GoogleScholarGoogle Scholar |
[109] Chomutare T, Fernandez-Luque L, Arsand E, Hartvigsen G. Features of mobile diabetes applications: review of the literature and analysis of current applications compared against evidence-based guidelines. J Med Internet Res 2011; 13 e65
| Features of mobile diabetes applications: review of the literature and analysis of current applications compared against evidence-based guidelines.Crossref | GoogleScholarGoogle Scholar |
[110] Pagoto S, Schneider K, Jojic M, DeBiasse M, Mann D. Evidence-based strategies in weight-loss mobile apps. Am J Prev Med 2013; 45 576–82.
| Evidence-based strategies in weight-loss mobile apps.Crossref | GoogleScholarGoogle Scholar |
[111] Arnhold M, Quade M, Kirch W. Mobile applications for diabetics: a systematic review and expert-based usability evaluation considering the special requirements of diabetes patients age 50 years or older. J Med Internet Res 2014; 16 e104
| Mobile applications for diabetics: a systematic review and expert-based usability evaluation considering the special requirements of diabetes patients age 50 years or older.Crossref | GoogleScholarGoogle Scholar |
[112] Sarkar U, Gourley GI, Lyles CR, Tieu L, Clarity C, Newmark L, Singh K, Bates DW. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med 2016; 31 1417–26.
| Usability of commercially available mobile applications for diverse patients.Crossref | GoogleScholarGoogle Scholar |
[113] Roess A. The promise, growth, and reality of mobile health – another data-free zone. N Engl J Med 2017; 377 2010–1.
| The promise, growth, and reality of mobile health – another data-free zone.Crossref | GoogleScholarGoogle Scholar |
Appendix 1. Evidence table for systematic reviews and individual trials of apps for chronic disease management
3D, three dimensional; A-CHESS, Addiction-Comprehensive Health Enhancement Support System; ACS, acute coronary syndrome; BDI, Beck’s Depression Inventory; BMI, body mass index; BP, blood pressure; BSL, blood sugar level; CAP-CR, Care Assessment Platform of cardiac rehabilitation; CCQ, Clinical COPD Questionnaire; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; EBAC, estimated blood alcohol concentration; ED, emergency department; EMAs, ecological momentary assessments; EQ5D, EuroQol 5D version; ESRD, end-stage renal disease; FACT-G, Functional Assessment of Cancer G version; GP, general practitioner; HADS, Hospital Anxiety and Depression Scale; HF, heart failure; HIS, home intervention system; HRQoL, health-related quality of life; IQR, interquartile range; IRR, incidence rate ratio; KDS, Kessler Psychological Distress Scale; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; m app, mobile health application; MD, mean difference; MI, myocardial infarction; NYHA, New York Heart Association; PANSS, positive and negative symptom scale; PEFR, peak expiratory flow rate; PHQ, Patient Health Questionnaire; QoL, quality of life; RCT, randomised controlled trial; RR, relative risk; SBP, systolic blood pressure; SDS, self-directed support; SF, Short Form; SGRQ, St George Respiratory Questionnaire; SMD, standardised mean difference; SR, systematic review; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TCR, traditional, centre-based cardiac rehabilitation; TMG, telemonitoring group; WEL, Weight Efficacy Lifestyle Questionnaire

|


