Therapeutic equivalence program: continued economic benefits in the context of rising costs and increased demand
Tom Chynoweth A B and Ian Larmour AA Monash Medical Centre, Monash Health, 246 Clayton Road, Clayton, Vic. 3168, Australia. Email: ian.larmour@monash.edu
B Corresponding author. Email: tom.chynoweth@monashhealth.org
Australian Health Review 43(5) 585-590 https://doi.org/10.1071/AH17177
Submitted: 2 August 2017 Accepted: 25 June 2018 Published: 27 August 2018
Journal Compilation © AHHA 2019 Open Access CC BY-NC-ND
Abstract
Objective The aim of this study was to describe the effect of a therapeutic equivalence program (TEP) in achieving financial sustainability from 2010–11 to 2014–15.
Methods A TEP was introduced at Monash Health in 2006–07. Therapeutic medicine classes for inclusion were selected by stakeholder consensus and a preferred medicine for each class was chosen based upon therapeutic equivalence and cost considerations. New patients were commenced on a preferred medicine, but patients already prescribed another medicine from the same therapeutic class were not automatically switched to the preferred medicine. Data was obtained retrospectively from the pharmacy dispensing system, including the purchasing and issuing of all medicines from the preferred medicine classes. The prescribing patterns for preferred and comparator medicines were used as a measure of acceptance of the TEP, along with the savings produced by the program.
Results Over the 5-year evaluation period, 18 therapeutic classes were targeted, including seven new classes. Six therapeutic classes from the 11 included in the TEP before 2010–11 were removed throughout the evaluation period when the comparative economic benefits were no longer present. The use of all preferred medicines increased following implementation and a total of AU$7.38 million was saved from 2010–11 to 2014–15 and AU$10.54 million across 2006–07 to 2014–15.
Conclusions This paper provides an update on the progress of the TEP at Monash Health and outlines additional learnings gained. The market dynamics for pharmaceuticals means ongoing maintenance and review of the therapeutic medicine classes targeted is important to enable continued economic benefits.
What is known about the topic? There is continued and increasing focus on efficient, cost-effective and financially sustainable medication management. There is limited information available on strategies that can be implemented at a health service level.
What does this paper add? The TEP has resulted in sustained savings. The market dynamics for pharmaceuticals means ongoing maintenance and review of the therapeutic classes targeted is important to enable continued economic benefits.
What are the implications for practitioners? TEP is a process of genuine disinvestment. Identification and resolution of critical factors in the success of the program may assist implementation at other health services.
Additional keywords: health policy, health services management, hospitals, pharmaceuticals.
Introduction
There is continued and increasing focus on efficient, cost-effective and financially sustainable medication management at an organisational, professional and government level. This focus has led the Monash Health (MH) Pharmacy Department to explore a diverse range of opportunities for improving efficient medication management.
MH is Victoria’s largest metropolitan health service, located in Melbourne’s south-east and including five public hospitals, one day surgery centre, one private hospital and an extensive network of ambulatory care services, community health centres and residential aged care facilities. MH was previously called Southern Health until 2012.
A therapeutic equivalence program (TEP) was introduced at MH in the 2006–07 financial year to improve the cost-effective use of medicines,1 and this paper reports on the continuation of this work. Although the concept of therapeutic equivalence is not new in the promotion of the efficient use of medications,1–5 the implementation and application of the concept has not been common.6 Generally, when the concept has been used to reduce drug costs, it has been associated with mandatory substitution.4,7 An alternative strategy that has been used to encourage the use of less expensive therapeutically equivalent medicines, employed as part of the Pharmaceutical Benefits Scheme (PBS) Therapeutic Group Premium pricing structure, has been to only reimburse patients for the cost of the cheaper medicine alternative.3–5 The approach at MH was to develop an effective TEP through the voluntary collaboration of prescribers and support through extensive stakeholder engagement, especially with senior medical staff.
In this paper we provide an update on the progress of the TEP introduced at MH and outline some of the additional learnings we have obtained from our experience in this area.
Methods
This paper covers the evaluation of the TEP at MH over the financial years from 2010–11 to 2014–15 and builds on the initial evaluation of the TEP from 2006–07 to 2009–10. Potential therapeutic classes were selected by: (1) identifying therapeutic classes of medicines associated with significant costs; (2) engaging with key clinical stakeholders, including head of unit or other key specialists, to evaluate the acceptability of the potential therapeutic class for inclusion in the TEP, based upon safety and efficacy; (3) investigating the suitability of the potential therapeutic class for inclusion in the TEP, based upon published evidence; and (4) undertaking preliminary financial impact assessment of the potential therapeutic class.
When a therapeutic medicine class was selected for inclusion in the program, pharmaceutical companies were invited to submit an expression of interest in becoming the preferred medication for that class at MH. The preferred medicine was selected on the basis of therapeutic equivalence and cost considerations. New patients were recommended to be commenced on a preferred medicine, but patients already prescribed another medicine from the same therapeutic class were not automatically switched to the preferred medicine and with the other medications in the class still being available for prescribing. The PBS Therapeutic Group Premium Policy differs to the TEP in that the TEP is driven by prescribers rather than by patient costs. The PBS does list medicines interchangeable by class, but does not specify a preferred medicine. The medicines in each therapeutic class could change as market dynamics changed. Changes in the preferred medicine status of a therapeutic class were subject to continued agreement from key stakeholders.
Data was obtained retrospectively from the pharmacy dispensing system progressively throughout the evaluation period, including the purchasing and issuing of all medicines from the preferred medicine classes. When a lower price was negotiated through the preferred medicine process, the cost difference between the preferred medicine and the appropriate comparative medicine was multiplied by the total number of preferred medicine units used at MH over the relevant period.
Results
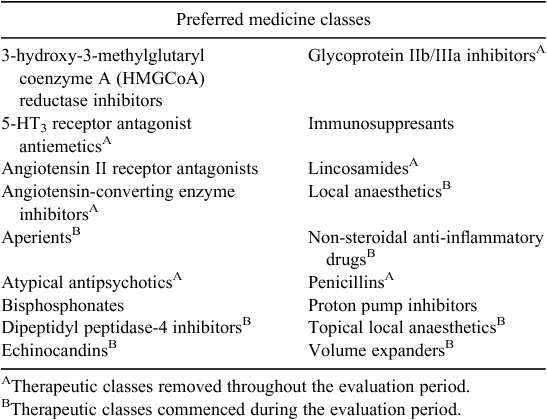
Over the 5-year study period, 18 therapeutic medicine classes were targeted (Table 1), including the introduction of seven new classes. Six therapeutic classes from the 11 included in the TEP before 2010–11 were removed when the comparative economic benefits were no longer present. The key reason for this was due to changes in the market dynamics created by significant generic competition within the class.
|
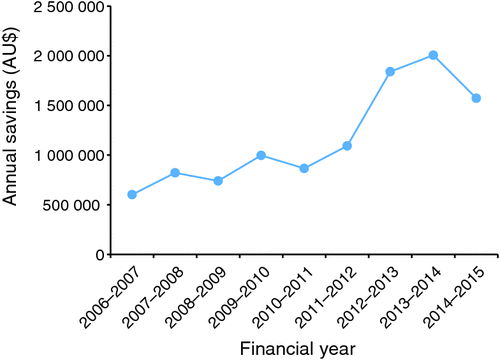
The TEP over the 5-year period from 2010–11 to 2014–15 provided a total of AU$7.38 million in savings to the health service (Fig. 1). Annual savings from the program increased by 81.6% (from AU$865 965 in 2010–11 to AU$1 572 995 in 2014–15), whereas patient separations increased by 23.2% (ranging from 193 824 in 2010–11 to 238 798 in 2014–15) over the evaluation period. A reduction in the annual savings from AU$2 006 172.85 in 2013–14 to $1 572 994.61 in 2014–15 is seen. This reduction was attributed to the removal of several medications from the TEP throughout 2014–15 because comparative economic benefits were no longer present. However, many of the withdrawn medications had significant ongoing saving resulting from the introduction of generic competition within the class, with these savings occurring as a result of tenders conducted on behalf of Victorian public hospitals by Health Purchasing Victoria, but were not considered to be a result of the TEP. The savings achieved by the TEP in 2014–15 were equivalent to 2.1% of the total expenditure on pharmaceuticals and 9.3% of net expenditure on pharmaceuticals.
|
The costs incurred were the salary for the TEP pharmacist and expenditure on promotional materials, which are not included in the calculations of annual savings above. With consideration of savings achieved between 2006–07 and 2009–10 of AU$3.16 million across 11 therapeutic medicine classes, the overall savings from the program have reached AU$10.54 million across 2006–07 to 2014–15 (Fig. 1).
Usage increased for nine of the 12 designated preferred medicines continuing throughout the study period, including the seven new preferred medicines added to the TEP. For the remaining three classes, although no increase occurred in the study period, the preferred medicine maintained its higher level of use compared with when the class was implemented and with the other medicines in the class. The patterns of use varied according to the proportion of medication prescribing that is exclusively commenced in a hospital setting, compared with those medicines that could be commenced in either the hospital or the ambulatory care setting. Several examples have been included in this paper to illustrate and discuss the reasons identified for some of the key variations found in use of the preferred medicines. For local anaesthetics, and similarly with echinocandins and volume expanders, where initiation is largely in the hospital in-patient setting, there was a substantial rise in the use of the preferred medicine following its inclusion in the TEP (Fig. 2a). In this setting, the use of the comparator local anaesthetic was reduced to inconsequential levels. For aperients implemented as part of the TEP, there was also a substantial rise in the use of the preferred medicine following implementation (Fig. 2b). In this case, the peak in use of the comparator medicine in July–September 2013 was associated with a shortage of the preferred medicine, with this continuing to have an effect on comparator medicine use in the following 6 months. For dipeptidyl peptidase (DPP)-4 inhibitors, there was ongoing background use of two alterative medicines owing to common use in the ambulatory care setting. Despite this, there was an increase in the cumulative use of the preferred medicine across the evaluation period (Fig. 2c). For topical local anaesthetics, and similarly with non-steroidal anti-inflammatory drugs, there was a rise in the use of the preferred medicine following implementation (Fig. 2d). The re-emergence of significant use of the comparator medicine, despite the majority of initiation occurring in the hospital setting, provides an example addressed in later discussion relating to diverse and unknown key stakeholders and influencers affecting preferred medicine use. For proton pump inhibitors, and similarly with angiotensin II receptor antagonists and bisphosphonates, which were implemented before the evaluation period, there was a decrease in the use of the preferred medicine across the evaluation period. Despite this decrease, the use of the preferred medicine was still greater than when the therapeutic class was included in the TEP, and there was still significantly greater use of the preferred medicine than the comparator medicine (Fig. 2e). For 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, and similarly with immunosuppressants, which were implemented before the evaluation period, there was an increase in the use of the preferred medicine across the evaluation period (Fig. 2f). This demonstrates the potential for ongoing benefit from the implementation of a preferred medicine.
Discussion
The TEP at MH commenced in the 2006–07 financial year and our experience over the subsequent 9 years has enabled a greater insight into the factors that have a significant effect on the effectiveness of the program.
Choice of therapeutic classes
A key component of the TEP is the choice of medications targeted. In our initial range of therapeutic groups, the following factors were considered: (1) the availability of significant evidence to support the concept of clinical equivalence in terms of safety and efficacy; (2) the opportunity for competition between medications in a therapeutic group; and (3) the potential extent of the cost savings to be gained.
Outstanding results were achieved with several of the therapeutic groups that were chosen, whereas in other cases only moderate financial benefit was achieved.
It is also critically important to recognise that market dynamics are in a constant state of change, hence the necessity to closely consider and monitor the effects of changes relating to the targeted therapeutic groups and to adjust the TEP targets and strategy as needed. Consequently, over the years, several of the original TEP-preferred medicines have been changed or removed from the program when the economic benefits were no longer present.
One of the key factors in such decisions is related to the stage in the life cycle of a medication. In particular, when the medication’s patent protection expires there is a marked reduction in the price of the product. The effect on the TEP of a marked reduction in price may: (1) change the dynamics on which medication in the therapeutic group offers the greatest benefit, which may lead to a change in the designated preferred medicine; (2) the price reduction (e.g. due to generic competition) may be so marked for all medications in the therapeutic group that including the group in the TEP is no longer required, because the benefits are automatically obtained; or (3) the preferred medicine may continue to provide benefit, but this may be lower than before the expiry of a medicine’s patent protection.
Conversely, these market dynamic changes may present new opportunities for the inclusion of new therapeutic groups in the TEP, including when new medications become available within a therapeutic class, such as the example of the DPP-4 inhibitors.
Factors affecting price
The greatest effect on price is the degree of competition, with the key factors limiting competition being patent protection and having only a single manufacturer for an off-patent medication.
It is also important to distinguish between benefits obtained from the availability of competition from generic products for a medication when it comes off patent and the benefits achieved by a TEP. In the case of generic medications, it is the availability of several brands of the same medication that produces reduced prices. However, in the case of a TEP, the aim is to promote competition between different, but similar, medications; this usually means different medications in the same pharmacological therapeutic class. It could also be related to different medication options for the same medical indication. That is, a TEP aims to promote and create competition between like products, regardless of the protection provided by patents or single supplier monopolies. However, to be effective, a TEP is dependent on the availability of comparative evidence and a belief of clinical equivalence by key stakeholders in terms of safety and efficacy. This is relatively straightforward when comparing different medications from a particular pharmacological class, especially for small molecule medications. However, in the case of biosimilars, a similar but more complex methodology is likely to be needed.
When the focus is on treating the same medical indication with medications from different medication classes, the process increases markedly in its complexity due to the availability of comparative data. Nevertheless, it is likely to become an increasingly important subject, especially with the ongoing growth in the number of biosimilars and increasing diversity of modes of action with regard to pharmacological activity. This highlights the increasing need for Phase 4 clinical drug trials to fully evaluate the comparative effectiveness, safety and duration of benefit of different medications for specific medical indications. Given the high cost and extensive pipeline of biological medication treatments entering the market, such studies will be critical to ensure pharmacological treatment options will provide the best value for money for the PBS.8 The potential benefits of using TEP to promote competition for these products is a matter that needs to be given high priority on an international basis in health policy.
Structural factors
The fundamental focus of the TEP run at MH is the voluntary collaboration and participation of prescribers, with a preferred medicine in a therapeutic group but other medications still being available. This depends on the leadership of the senior medical staff, and a preferred medicine only adopted once there is consensus within the relevant stakeholder groups, predominantly prescribers but possibly also clinical pharmacists and nursing staff as part of multidisciplinary care teams. This process has been a successful approach in most cases, but in some cases this approach has met issues unknown before implementation. Once these issues are identified, a modified strategy acceptable to stakeholders can be introduced to overcome these aspects.
We have identified several structural factors that may affect the effectiveness of the TEP in some areas and require additional education and engagement strategies. The factors identified are as follows: (1) when a diverse range of key multidisciplinary stakeholders are involved in medication prescribing and administration; (2) the presence of multiple unknown stakeholders or those who are not easily identified as key influencers when decision making occurs across medical, clinical pharmacy and nursing staff; (3) the degree of ownership of the strategy by stakeholders, for example different engagement of permanent and visiting staff across speciality units, which may lead to different viewpoints or understanding of the program; and (4) the opportunity and authority of a clear stakeholder leader or leadership group to influence policy and the practice of other prescribers and decision makers; this may include when there are differences in size, hierarchal structures and leadership styles used within stakeholder groups.
Therefore, it is important before commencement to identify all stakeholders and, in particular, every attempt should be made to draw out previously unknown or unidentified stakeholders. If the latter group is not addressed initially, the engagement of such stakeholders should occur immediately after identification.
In a small number of cases, it is more appropriate to only have one medication available within a therapeutic group. Such a strategy is most appropriate and has been most successful for medications used exclusively within the in-patient hospital setting, because patients are not commenced on an alternative to the preferred medicine before hospital admission.
Importantly, in addition to working closely with stakeholders, the TEP needs to work in conjunction with the hospital therapeutics committee or equivalent to provide the appropriate governance and support for prescribing and medication formulary procedures and guidelines.
The key components of a successful TEP are still closely linked with: (1) extensive and effective education that clearly and simply outlines the details of how the TEP functions, and the promotion of constructive awareness of the program and its objectives when attending medical staff unit meetings; (2) effective and collaborative negotiations with senior medical staff and all other key stakeholders, considering the diverse background of these stakeholders and their characteristics of practice; and (3) regular focused feedback on the results and benefits of the program, including a focus on reporting benefits, especially the benefits to patients, directly to the relevant unit.
Conclusion
The introduction of a collaborative TEP at MH has been extremely successful in producing significant and sustained savings over 9 years. The benefits found in the initial study1 have been ongoing. The underlying concept of this success is in the premise of cost savings without compromising the safety and clinical effectiveness of patient care in order to enable expenditure on other important areas of medicine use where the opportunity for such savings does not exist. Critical to the success of the TEP has been the collaborative environment and positive support of the MH medical staff.
This can also be described as a form of genuine disinvestment, where limited resources are diverted to their most productive use, a concept that is being promoted in many areas of healthcare.9,10
This work has enabled many critical factors in the success of a TEP to be identified and resolved, hopefully creating an easier path for others to follow.
Competing interests
The authors declare no conflicts of interest.
Acknowledgements
The support of the following people is gratefully acknowledged: Natalie Cincotta, Kerryn Griffett, Stav Mantas and Silvana Pignataro. This research did not receive any specific funding.
References
[1] Larmour I, Pignataro S, Barned KL, Mantas S, Korman MG. A therapeutic equivalence program: evidence-based promotion of more efficient use of medicines. Med J Aust 2011; 194 631–4.[2] Larmour I, Creber E. Cultural change and drug expenditure. Aust J Hosp Pharm 1995; 25 220–5.
[3] Schneeweiss S. Reference drug programs: effectiveness and policy implications. Health Policy 2007; 81 17–28.
| Reference drug programs: effectiveness and policy implications.Crossref | GoogleScholarGoogle Scholar |
[4] Gumbs PD, Verschuren WM, Souverein PC, Mantel‐Teeuwisse AK, De Wit GA, De Boer A, Klungel OH. Society already achieves economic benefits from generic substitution but fails to do the same for therapeutic substitution. Br J Clin Pharmacol 2007; 64 680–5.
| Society already achieves economic benefits from generic substitution but fails to do the same for therapeutic substitution.Crossref | GoogleScholarGoogle Scholar |
[5] Schneeweiss S, McClure M, Dormuth CR, Glynn RJ, Canning C, Avorn J. A therapeutic substitution policy for proton pump inhibitors: clinical and economic consequences. Clin Pharmacol Ther 2006; 79 379–88.
| A therapeutic substitution policy for proton pump inhibitors: clinical and economic consequences.Crossref | GoogleScholarGoogle Scholar |
[6] Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects of reform. JAMA 2016; 316 858–71.
| The high cost of prescription drugs in the United States: origins and prospects of reform.Crossref | GoogleScholarGoogle Scholar |
[7] Johansen ME, Richardson C. Estimation of potential savings through therapeutic substitution. JAMA Intern Med 2016; 176 769–75.
| Estimation of potential savings through therapeutic substitution.Crossref | GoogleScholarGoogle Scholar |
[8] Gleeson D, Townsend B, Lopert R, Lexchin J, Moir H. Financial costs associated with monopolies on biologic medicines in Australia. Aust Health Rev 2017;
| Financial costs associated with monopolies on biologic medicines in Australia.Crossref | GoogleScholarGoogle Scholar |
[9] Elshaug AG, Hiller JE, Moss JR. Exploring policy-makers’ perspectives on disinvestment from ineffective healthcare practices. Int J Technol Assess Health Care 2008; 24 1–9.
| Exploring policy-makers’ perspectives on disinvestment from ineffective healthcare practices.Crossref | GoogleScholarGoogle Scholar |
[10] Moynihan RN. A healthy dose of disinvestment. Med J Aust 2012; 196 158
| A healthy dose of disinvestment.Crossref | GoogleScholarGoogle Scholar |



