Declining spring usage of core habitat by endangered fish-eating killer whales reflects decreased availability of their primary prey
Monika W. Shields A C , Jimmie Lindell A B and Julie Woodruff AA Orca Behavior Institute, c/o 573 Westcott Drive, Friday Harbor, WA 98250, USA.
B University of Gothenburg, 405 30 Gothenburg, Sweden.
C Corresponding author. Email: monika@orcabehaviorinstitute.org
Pacific Conservation Biology 24(2) 189-193 https://doi.org/10.1071/PC17041
Submitted: 29 October 2017 Accepted: 9 April 2018 Published: 15 May 2018
Abstract
The salmon-eating Southern Resident killer whales (Orcinus orca) of the north-eastern Pacific Ocean are listed as endangered both in the United States and Canada. Their critical habitat has been defined as the region of the inland waters of Washington State and British Columbia known as the Salish Sea, where they have traditionally spent much of their time from spring through fall. Using reports from experienced observers to sightings networks, we tracked the daily presence of the Southern Residents in these waters from 1 April to 30 June from 1994 through 2016. We found that the escapement estimates of spring Chinook salmon (Oncorhynchus tshawytscha) on the Fraser River in British Columbia were a significant predictor of the cumulative presence/absence of the whales throughout the spring season. There was also a difference in both whale presence and salmon abundance before and after 2005, suggesting that the crash in Chinook salmon numbers has fallen below threshold where it is worthwhile for the whales to spend as much time in the Salish Sea. The use of the Salish Sea by the Southern Residents has declined in the spring months as they are either foraging for Chinook salmon elsewhere or are shifting to another prey species. In order to continue providing necessary protections to this endangered species, critical habitat designations must be re-evaluated as this population of killer whales shifts its range in response to prey availability.
Additional keywords: Chinook salmon, critical habitat, Orcinus orca, prey availability, Salish Sea
Introduction
Sustainable wild animal populations rely on having a large enough core habitat made up of the ecological features necessary for survival, including areas and resources to forage, breed, and rest without excessive disturbance. Exploitation and habitat loss are the most common causes of local or global extinction amongst marine organisms (Dulvy et al. 2003). When a population or species is listed as endangered, the term ‘critical habitat’ is used to refer to the geographic area containing features essential to the survival, protection, and recovery of the listed species (Salzman 1990). Policy decisions on critical habitat boundaries are informed by biologists and their research on the species in question. If the resources required by a species move or change, however, populations may shift their core habitat, for example in response to prey availability (Cimino and Lovari 2003; Worm and Tittensor 2011; Hazen et al. 2013). In these cases where core habitat shifts, critical habitat designations may also need to be reassessed.
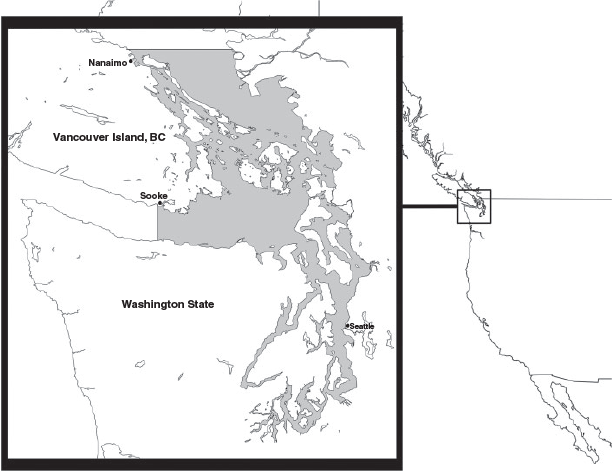
The north-eastern Pacific Ocean is inhabited by several sympatric populations of killer whales (Orcinus orca), which comprise three ecotypes known to specialise on different prey types (Ford et al. 2000). One of these populations is the fish-eating Southern Resident killer whales, made up of ~80 whales in three extended family groups, or pods: J-, K-, and L- Pods (Ford et al. 2000). The Southern Residents were listed as endangered under Canada’s Species at Risk Act in 2003 and under the Endangered Species Act in the United States in 2005, with key risk factors being identified as lack of prey, toxins, and vessel disturbances (National Marine Fisheries Service 2008). The Southern Resident killer whales have a known range from south-east Alaska to central California; however, historically, the inland waters of Washington State and British Columbia, known as the Salish Sea, have been the core habitat for the Southern Residents, particularly during the summer months but also in the spring and autumn (Osborne 1999; McCluskey 2006; Hauser et al. 2007). A main reason for the presence of the Southern Residents in the Salish Sea is the opportunity to feed on the Chinook salmon (Oncorhynchus tshawytscha), their primary prey species (Ford and Ellis 2006), particularly those returning to the Fraser River of British Columbia (Hanson et al. 2010). As part of the endangered listing process, each country designated a critical habitat for the Southern Resident population. In the United States, the critical habitat was designated as ~2580 square miles of the inland waters of Washington, with a few small exclusion zones due to national security concerns (National Marine Fisheries Service 2008). In Canada, critical habitat for Southern Residents was similarly designated as most of the Canadian waters of the Salish Sea (Fisheries and Oceans Canada 2011) (see Fig. 1).
|
Pacific salmon stocks have been severely depleted over the last century due to a combination of many factors, including overfishing, habitat loss and degradation, and impacts of hatcheries and fish farms on wild stocks (Waples and Teel 1990; Gustafson et al. 2007; Ford 2011). On the Fraser River, spring and summer Chinook runs declined dramatically before 1980, which led to the closing of targeted Chinook fishing (Bailey et al. 2001). This likely contributed to an increase in Chinook abundance for Fraser runs until the early 2000s, but these runs declined again beginning in 2005 (Pacific Salmon Commission 2017). Low Chinook salmon abundance has been found to negatively impact survival (Ford et al. 2010), fecundity (Ward et al. 2009) and population growth (Wasser et al. 2017) of Southern Resident killer whales. While additional work has examined patterns of distribution (Hauser et al. 2007) and behaviour (Noren and Hauser 2016) within core Southern Resident habitat, thus far no study has characterised the relationships between salmon abundance and Southern Resident visits to core habitat.
Published data on the spring diet for the Southern Residents is limited, with no samples in April and only a small number of samples in May, which were made up of one-third Fraser River Chinook (though this estimate has a large standard error). By June, prey samples from the Salish Sea were dominated by Chinook salmon that originated from the upper Fraser River watershed (Hanson et al. 2010). When not in the Salish Sea, the Southern Residents are presumably also feeding on salmon from a wide variety of other coastal watersheds, including the Columbia River.
From April 1976, when dedicated Southern Resident surveys began, until April 2009, at least one of the three pods was detected in the inland waters of the Salish Sea in every month of every year. In April 2009, for the first time on record, Southern Residents were not documented anywhere in inland waters. Since then, spring reports of the Southern Residents in the Salish Sea have become much more sporadic. We hypothesise that as spring Fraser River Chinook salmon runs have continued to decline, the Southern Residents have started spending fewer days in April through June in what has been considered their core habitat for the spring through autumn seasons. We also hypothesize that there is a threshold of salmon abundance, below which the whales will reduce visits to the Salish Sea.
Methods
Numerous sightings networks have recorded killer whale presence within the Salish Sea in detail since the 1990s. Due to their regular visitation, the Southern Residents are well known in the region and can be visually identified using identification catalogues produced by The Center for Whale Research. Many naturalists, whale watch captains, and members of the general public are adept at identifying the difference between Southern Resident killer whales and other ecotypes of killer whales, providing a reliable record of Southern Resident occurrence in the region (Hauser et al. 2006). Using The Whale Museum’s Orca Master dataset, sightings archives from Orca Network, and sightings reports from the Pacific Whale Watch Association, we noted the daily presence or absence of any Southern Residents within the Salish Sea from 1 April through 30 June, 1994–2016. For the purpose of this study, the Salish Sea was defined by the region of consistent sighting effort, and thus included all the inland waters of Washington and British Columbia east of Sooke and south of Nanaimo, including the southern Strait of Georgia, the Canadian Gulf Islands the US San Juan Islands, the eastern Strait of Juan de Fuca, and Puget Sound. Only reports where killer whales were confirmed as Southern Residents were used; rare cases where killer whales were not identified to ecotype were excluded.
Fraser River Chinook salmon runs have been tracked in detail by the Pacific Salmon Commission since 1975 (Pacific Salmon Commission 2017). The Pacific Salmon Commission divides Fraser River Chinook salmon into five stock groups with three run timings: spring, summer, and late. For the purpose of this study, we used escapement estimates (i.e. fish that return to the river to spawn, ‘escaping’ fisheries and natural predators) as a proxy for the number of fish available to whales in spring months. Escapement estimates are the primary statistic upon which many Pacific Coast fisheries rely to determine Chinook salmon abundance for management purposes (Pacific Salmon Commission 2017). Escapement estimates are conducted from visual surveys that are known to be biased low, but the methods used on the Fraser River include a correction factor and have been shown to be repeatable, precise, and accurate indicators of relative abundance over time (Bailey et al. 2000; Tompkins and Baxter 2015). We did not include commercial harvest numbers in estimating Chinook salmon abundance due to the lack of seasonal and run-specific data. However, focusing on two specific Fraser River spring runs, we could better examine the impact of the Chinook salmon upon which the Southern Residents primarily feed (Hanson et al. 2010). These two runs are used as indicator stocks by the Pacific Salmon Commission, requiring a rigorous survey each year. Spring Run 1.2 includes fish that return to the Lower Thompson River tributaries and Spring Run 1.3 comprises spring spawners to the rest of the Fraser River watershed. Spring runs are defined as all fish that return to their spawning grounds before 15 July, and are considered primarily available to coastal fisheries (and hence also the Southern Residents) in May and June (Bailey et al. 2001).
To compare the indicator variable of Fraser River spring Chinook salmon escapement with the response variable of a count of number of days that Southern Resident killer whales were in the Salish Sea in the spring, we initially used a Poisson model, but the data showed overdispersion, so we also compared quasi-Poisson and negative binomial regression models. Additionally, we look at descriptive statistics for both escapement and spring whale days for the period before and after the crash of the Fraser River spring Chinook salmon runs that started in 2005.
Results
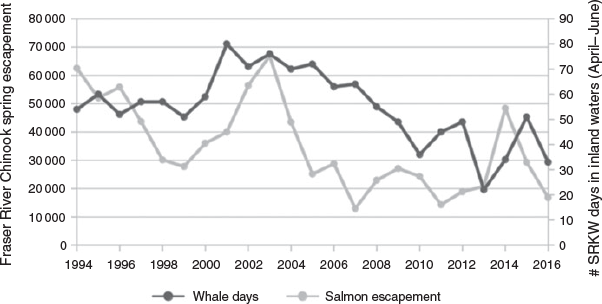
Between 1994 and 2016, both spring Fraser River Chinook salmon escapement numbers for the 1.2 and 1.3 runs and the number of spring days that Southern Resident killer whales spent in the Salish Sea declined (Fig. 2). A Poisson model showed a significant effect of Fraser River spring Chinook salmon escapement numbers on the number of whale days in the Salish Sea during April–June (β = 6.544E–6, χ2 = 33.9, P < 0.001); however, the scale factor was much greater than 1 (3.48), indicating overdispersion of the data. In a Poisson model, the dispersion parameter is forced to equal 1, so next we ran a quasi-Poisson where the dispersion parameter can be estimated; however, this did not change the residual deviance, so was not a better fit. A negative binomial regression model also indicated a significant effect of Fraser River spring Chinook salmon escapement numbers on number of whale days in the Salish Sea during April–June (β = 6.637E–6, χ2 = 33.9, P < 0.05) and had a scale factor of 1.08, indicating a good fit to the data.
|
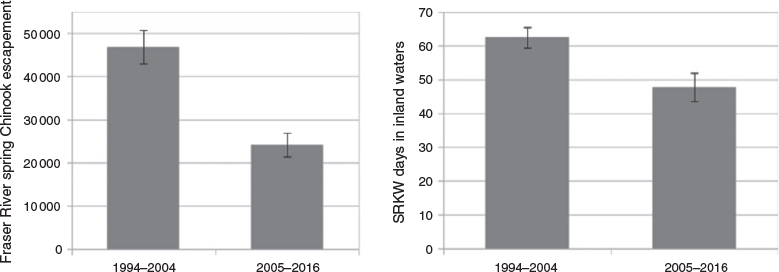
Since there was a sharp decline in spring Chinook salmon numbers in 2005, we also conducted an informal comparison of salmon numbers and whale presence for the two periods before and after this drop-off. Average escapement for the 1994–2004 period was 46 796 (s.d. = 12 942) compared with an average of 24 137 (s.d. = 9495) for 2005–16. The average number of days that the Southern Resident killer whales spent in the Salish Sea from 1 April to 30 June also dropped between these two periods, from an average of 62.45 days (s.d. = 10.1) for 1994–2004 to an average of 47.75 (s.d. = 14.6) days for 2005–16 (Fig. 3).
Discussion
We found an overall trend between the number of days that Southern Residents spend in the Salish Sea during April–June and Fraser River spring Chinook salmon escapement values, with both salmon run size and the whales’ usage of their core habitat declining over the last two decades. We also found evidence for a threshold effect as spring Fraser Chinook salmon runs crashed around 2005. As spring salmon run size reached new lows, the energetic cost of foraging in the Salish Sea may have been too high. While the whales do still visit inland waters during these months, their visits tend to be both shorter and more infrequent.
A predator’s presence in its habitat is likely to be causally related to the presence of its prey, so it is not surprising to see the Southern Residents reducing usage of their core habitat in response to declining salmon runs. While other conditions in the Salish Sea may have changed over this period, any abiotic factors such as changes in ocean temperatures or pH due to climate change would be more likely to affect salmon and thus indirectly affect the whales (Stachura et al. 2014).
Until spring Fraser River Chinook salmon runs recover, the whales must switch to an alternative prey during the spring. While killer whales are considered generalist predators, particular ecotypes tend to be very limited in their prey types (Felleman et al. 1991; Ford et al. 1998; Ford and Ellis 2006). For the Southern Residents, Chinook salmon make up most of their year-round diet (between 50 and 90% depending on season), though they are also known to regularly consume other salmonid species such as chum and steelhead (Ford and Ellis 2005, 2006). Hanson et al. (2010) argue that steelhead may be an important food source for the Southern Residents, especially before the summer runs of Chinook salmon. With the spring Fraser River Chinook salmon runs in such decline, it will be key to determine whether the Southern Residents are finding another run of Chinook salmon or if chum and steelhead are playing an increased role in their diet. Any of these alternatives will likely result in a shift in habitat.
There are likely additional factors determining whether or not the Southern Residents visit the Salish Sea. While low prey numbers in the Salish Sea could drive reduced whale visits to the area, it is also possible that elevated prey numbers somewhere else, which we did not integrate into the analysis, could drive this pattern. Because there are limited sighting data for the Southern Residents outside the Salish Sea, it is difficult to directly test this hypothesis. However, the Columbia River, the largest salmon-producing river within the known range of the Southern Residents, is the most likely candidate for a major alternative prey source. Data from satellite tagging has shown that the whales spend time in the winter and spring near the mouth of the Columbia River. Moreover, average minimum run size for Columbia River spring Chinook salmon using Washington Department of Fish and Wildlife estimates have not changed dramatically in the 2005–15 period (274 857, s.d. = 181 330) compared with 1994–2004 (247 775, s.d. = 99 162). Thus, future work should investigate whether the Southern Residents have increased usage of the Columbia River spring runs.
The Southern Resident killer whales are a dynamic, behaviourally complex endangered species living in an ever-changing habitat. As such, their protection is not simple, as both the prey sources and habitats they depend on must be continually re-evaluated as conditions change. We have demonstrated that the geographic area important to the Southern Residents is shifting, and thus it is key that designated critical habitat in the US and Canada is reconsidered accordingly.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
A study like this would be impossible without the sighting information reported by dozens of knowledgeable members of the public who have contributed to the Orca Network, The Whale Museum’s Orca Master, and Pacific Whale Watch Association sightings networks. J. Shields helped with mining these databases. This manuscript was also improved by the input from T. Hammond, B. Zeiger, A. Jones, and the comments from two anonymous reviewers and the associate editor. Map provided courtesy of S. Shimazu.
References
Bailey, R. E., Parken, C. K., Irvine, J. R., Rosenberger, B., and Farwell, M. K. (2000). Evaluation of utility of aerial overflight based estimates versus mark–recapture estimates of chinook salmon escapement to Nicola River, BC. Canadian Stock Assessment Secretariat 2000/152.Bailey, R. E., Irvine, J. R., Candy J. R., Parken, C. K., Lemke, S. L., Sullivan, M., and Wetklo, M. (2001). Summary of stock assessment information for selected early returning Chinook salmon populations of the Fraser River watershed. Canadian Science Advisory Secretariat 2001/134.
Cimino, L., and Lovari, S. (2003). The effects of food or cover removal on spacing patterns and habitat use in roe deer (Capreolus capreolus). Journal of Zoology 261, 299–305.
| The effects of food or cover removal on spacing patterns and habitat use in roe deer (Capreolus capreolus).Crossref | GoogleScholarGoogle Scholar |
Dulvy, N. K., Sadovy, Y., and Reynolds, J. D. (2003). Extinction vulnerability in marine populations. Fish and Fisheries 4, 25–64.
| Extinction vulnerability in marine populations.Crossref | GoogleScholarGoogle Scholar |
Felleman, F. L., Heimlich-Boran, J. R., and Osborne, R. W. (1991). The feeding ecology of killer whales (Orcinus orca) in the Pacific Northwest. In ‘Dolphin Societies: Discoveries and Puzzles’. (Eds K. Pryor and K. S. Norris.) Chapter 3, pp. 113–147. (University of California Press: Berkeley.)
Fisheries and Oceans Canada (2011). Recovery strategy for the northern and southern resident killer whales (Orcinus orca) in Canada. Species at Risk Act Recovery Strategy Series, Fisheries and Oceans Canada, Ottawa.
Ford, M. J. (Ed.) (2011). Status review update for Pacific salmon and steelhead listed under the Endangered Species Act: Pacific Northwest. US Department of Commerce, NOAA Technical Memorandum NMFS-NWFSC-113.
Ford, J. K., and Ellis, G. M. (2005). Prey selection and food sharing by fish-eating ‘resident’ killer whales (Orcinus orca) in British Columbia. Fisheries and Oceans Canada Research Document 2005/041.
Ford, J. K., and Ellis, G. M. (2006). Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Marine Ecology Progress Series 316, 185–199.
| Selective foraging by fish-eating killer whales Orcinus orca in British Columbia.Crossref | GoogleScholarGoogle Scholar |
Ford, J. K., Ellis, G. M., Barrett-Lennard, L. G., Morton, A. B., Palm, R. S., and Balcomb, K. C. (1998). Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Canadian Journal of Zoology 76, 1456–1471.
| Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters.Crossref | GoogleScholarGoogle Scholar |
Ford, J. K., Ellis, G. M., and Balcomb, K. C. (2000). ‘Killer Whales: The Natural History and Genealogy of Orcinus orca in British Columbia and Washington.’ (UBC Press.)
Ford, J. K., Ellis, G. M., Olesiuk, P. F., and Balcomb, K. C. (2010). Linking killer whale survival and prey abundance: food limitation in the oceans’ apex predator? Biology Letters 6, 139–142.
| Linking killer whale survival and prey abundance: food limitation in the oceans’ apex predator?Crossref | GoogleScholarGoogle Scholar |
Gustafson, R. G., Waples, R. S., Myers, J. M., Weitkamp, L. A., Bryant, G. J., Johnson, O. W., and Hard, J. J. (2007). Pacific salmon extinctions: quantifying lost and remaining diversity. Conservation Biology 21, 1009–1020.
| Pacific salmon extinctions: quantifying lost and remaining diversity.Crossref | GoogleScholarGoogle Scholar |
Hanson, M. B., Baird, R. W., Ford, J. K., and Balcomb, K. C. (2010). Species and stock identification of prey consumed by endangered southern resident killer whales in their summer range. Endangered Species Research 11, 69–82.
| Species and stock identification of prey consumed by endangered southern resident killer whales in their summer range.Crossref | GoogleScholarGoogle Scholar |
Hauser, D. D., VanBlaricom, G. R., Holmes, E. E., and Osborne, R. W. (2006). Evaluating the use of whalewatch data in determining killer whale (Orcinus orca) distribution patterns. The Journal of Cetacean Research and Management 8, 273–281.
Hauser, D. D., Logsdon, M. G., Holmes, E. E., VanBlaricom, G. R., and Osborne, R. W. (2007). Summer distribution patterns of southern resident killer whales Orcinus orca: core areas and spatial segregation of social groups. Marine Ecology Progress Series 351, 301–310.
| Summer distribution patterns of southern resident killer whales Orcinus orca: core areas and spatial segregation of social groups.Crossref | GoogleScholarGoogle Scholar |
Hazen, E. L., Jorgensen, S., Rykaczewski, R. R., Bograd, S. J., Foley, D. G., Jonsen, I. D., Shaffer, S. A., Dunne, J. P., Costa, D. P., and Crowder, L. B. (2013). Predicted habitat shifts of Pacific top predators in a changing climate. Nature Climate Change 3, 234–238.
| Predicted habitat shifts of Pacific top predators in a changing climate.Crossref | GoogleScholarGoogle Scholar |
McCluskey, S. (2006). Population trends and movement complexity patterns of southern resident killer whales (Orcinus orca) in relation to Pacific salmon (Oncorhynchus spp.) in the inland waters of Washington State and British Columbia. M.S. Thesis, University of Washington, Seattle.
National Marine Fisheries Service (2008). Recovery plan for southern resident killer whales (Orcinus orca). National Marine Fisheries Service, Northwest Region, Seattle.
Noren, D. P., and Hauser, D. D. W. (2016). Surface-based observations can be used to assess behavior and fine-scale habitat use by an endangered killer whale (Orcinus orca) population. Aquatic Mammals 42, 168–183.
| Surface-based observations can be used to assess behavior and fine-scale habitat use by an endangered killer whale (Orcinus orca) population.Crossref | GoogleScholarGoogle Scholar |
Osborne, R. W. (1999). A historical ecology of Salish Sea resident killer whales (Orcinus orca): with implications for management. Ph.D. Thesis, University of Victoria, Victoria, BC.
Pacific Salmon Commission (2017). Annual report of catch and escapement for 2016 report TCChinook (17)-2. Pacific Salmon Commission Joint Chinook Technical Committee.
Salzman, J. (1990). Evolution and application of critical habitat under the Endangered Species Act. The Harvard Environmental Law Review 14, 311–342.
Stachura, M. M., Mantua, N. J., and Scheuerell, M. D. (2014). Oceanographic influences on patterns in North Pacific salmon abundance. Canadian Journal of Fisheries and Aquatic Sciences 71, 226–235.
| Oceanographic influences on patterns in North Pacific salmon abundance.Crossref | GoogleScholarGoogle Scholar |
Tompkins, A., and Baxter, B. (2015). Pacific salmon escapement estimation methods for Canada. NPAFC Doc. 1604. Fisheries and Oceans Canada.
Waples, R. S., and Teel, D. J. (1990). Conservation genetics of Pacific salmon I. Temporal changes in allele frequency. Conservation Biology 4, 144–156.
| Conservation genetics of Pacific salmon I. Temporal changes in allele frequency.Crossref | GoogleScholarGoogle Scholar |
Ward, E. J., Holmes, E. E., and Balcomb, K. C. (2009). Quantifying the effects of prey abundance on killer whale reproduction. Journal of Applied Ecology 46, 632–640.
| Quantifying the effects of prey abundance on killer whale reproduction.Crossref | GoogleScholarGoogle Scholar |
Wasser, S. K., Lundin, J. I., Ayres, K., Seely, E., Giles, D., Balcomb, K., Hempelmann, J., Parsons, K., and Booth, R. (2017). Population growth is limited by nutritional impacts on pregnancy success in endangered southern resident killer whales (Orcinus orca). PLoS One 12, e0179824.
| Population growth is limited by nutritional impacts on pregnancy success in endangered southern resident killer whales (Orcinus orca).Crossref | GoogleScholarGoogle Scholar |
Worm, B., and Tittensor, D. P. (2011). Range contraction in large pelagic predators. Proceedings of the National Academy of Sciences of the United States of America 108, 11942–11947.
| Range contraction in large pelagic predators.Crossref | GoogleScholarGoogle Scholar |