Cold-blooded indifference: a case study of the worsening status of threatened reptiles from Victoria, Australia
Nick ClemannArthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, PO Box 137, Heidelberg, Vic. 3084, Australia. Email: nick.clemann@depi.vic.gov.au
Pacific Conservation Biology 21(1) 15-26 https://doi.org/10.1071/PC14901
Submitted: 6 January 2014 Accepted: 19 June 2014 Published: 21 April 2015
Abstract
For the first time in the history of life, a biodiversity extinction crisis is being driven by a single species – humans. Humans also have unprecedented control over both the threats and conservation actions that influence this crisis. When prioritising conservation actions, innate human bias often favours endothermic vertebrates over other fauna. Reptiles are the least popular terrestrial vertebrate class, and consequently are particularly disadvantaged in terms of being listed as threatened and receiving conservation management. Despite 30 years of formally evaluating and listing threatened vertebrates in the Australian State of Victoria, there is a strong worsening trend in the conservation status of all faunal groups. The deteriorating status of Victorian reptiles mirrors worrying documented trends in reptile conservation status around the world. I review the history of listing threatened reptiles in Victoria, detail worsening trends in their conservation status, and suggest that, as in other parts of the world, the threats common to most listed taxa are climate change, habitat loss and degradation, and elevated rates of predation by exotic predators. I also identify poor advice and planning as a considerable threat to Victorian reptiles; this threat is rarely reported, but may be more pervasive than currently recognised. I argue that what is needed for most reptiles to have the greatest chance of persisting in the long term is prevention of habitat loss and degradation, research to underpin listing and management, improved policy so that unproven management strategies are not sanctioned, and vetting of consultant’s reports so that unproven ‘mitigation’ strategies and inadequate preimpact surveys do not mask the true cost of loss and degradation of habitat.
Additional keywords: threatening process, threat mitigation, threatened species.
Introduction
Each extant taxon represents an unbroken evolutionary trajectory that, collectively, comprises the Earth’s biodiversity. At any point in time a taxon’s status sits somewhere on a gradient ranging from abundant and secure to the brink of extinction. Extinction is a process that occurs naturally over deep time, although, rarely, the ‘background’ extinction rate spikes. The current Holocene extinction spike (Pimm and Raven 2000; Dirzo and Raven 2003; Pimm et al. 2014) represents the sixth and latest of these episodes of atypically high extinction rates in the Earth’s history (Leakey and Lewin 1996; Ceballos et al. 2010; Wagler 2011); the last extinction spike occurred 65 million years ago, at the Cretaceous–Paleogene boundary. A distinguishing feature of the Holocene extinction episode is that, for the first time in the history of life, the spike can be attributed to the actions of a single species – humans (Steffen et al. 2007). And just as we humans are driving the current biodiversity crisis, we also have unprecedented influence over the outcomes of the crisis – we can choose which habitats and species to influence positively or negatively, and also choose where to direct the resources that positively or negatively influence these habitats and species.
Many governments around the world reflect the value that society places on biodiversity by legislating for the preservation of threatened species. These listed threatened species are more likely to receive management and resource investment than those that are not listed (Walsh et al. 2013). However, prioritising typically scant conservation resources is subject to considerable human biases. A significant factor in the allocation of resources for biodiversity investigations or conservation is the popularity of individuals or groups of taxa (Bonnet et al. 2002; Farrier et al. 2007). Of the classes of vertebrate fauna, reptiles are typically least popular (Davies et al. 2004), and ‘charismatic’, usually endothermic, species tend to be favoured in conservation listing (Walsh et al. 2013) and investment (Metrick and Weitzman 1996; Farrier et al. 2007; Salt and Possingham 2013). ‘Visceral’ characteristics, such as physical size and the degree to which a species is considered a ‘higher life form’ (sensu Bennett 1978) (with endothermic fauna more highly valued), tend to play a much greater role than scientific characteristics in determining government decisions regarding threatened species (at least in the United States: Metrick and Weitzman 1996).
Traditionally considered a ‘lower’ class of vertebrate compared to endothermic classes (mammals and birds) (e.g. Bennett 1978), reptile popularity amongst humans is likely to be hampered by dissimilarity to us in features such as body shape (Metrick and Weitzman 1996) and thermoregulatory mode, as well as underappreciation of the complex social lives of some taxa (e.g. White et al. 2009). The potential of a minority of species, especially some serpents and crocodilians, to cause fatalities amongst humans undoubtedly also negatively affects human attitudes. For example, it is likely that most humans have a powerful innate tendency to fear snakes (DeLoache and LoBue 2009). This tendency is present even in young children (LoBue and DeLoache 2008), and this aversion arose early in the coevolution of snakes and mammals (Isbell 2006).
As a consequence of this deep-rooted unpopularity, generating support for the protection and conservation of reptiles is typically more challenging than it is for more popular classes of vertebrates (Bonnet et al. 2002), despite worrying trends in the conservation status of reptiles (Gibbons et al. 2000; Reading et al. 2010; Sinervo et al. 2010; Böhm et al. 2013). Such taxonomic bias has meant that reptiles are poorly represented compared to mammals, birds and amphibians on the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species (Böhm et al. 2013).
Recent studies have documented unmitigated worsening of the conservation status of the world’s reptiles (Böhm et al. 2013), and biases that disadvantage reptiles in threatened species listing processes and recovery planning (Walsh et al. 2013). The southern Australian State of Victoria has a range of terrestrial ecosystems (including deserts, wet and dry forests, woodlands, grasslands, alpine areas and heathlands), each of which contains threatened reptiles. These reptiles are subject to a range of threatening processes that exemplify such threats around the world. In this paper I use the reptile fauna of Victoria as a case study of these worsening conservation trends. I then consider the steps necessary to conserve Victorian reptiles, and the applicability of these conservation measures beyond Victoria.
Status of threatened Victorian reptiles
Approximately 120 taxa – species and well recognised subspecies – of reptiles occur naturally within Victoria (the precise figure depends on acceptance of the latest taxonomic research, and uncertainty about whether or not some species persist in the State). Reptiles are significant components of the State’s fauna for several reasons. They are the most abundant and conspicuous ground-dwelling vertebrates in many ecosystems, and as a consequence they are important elements of food webs and nutrient cycling. Some are partly or mostly herbivorous, and almost certainly play a role in seed dispersal and germination. Some, especially varanid lizards and the larger elapid snakes, are the pinnacle native predators remaining within their ecosystems in Victoria. Consequently, reptiles are (or at least should be) important components of broad conservation strategies (e.g. Cogger et al. 1993). Whilst understanding of the distribution of some (but certainly not all) Victorian reptiles is reasonable, knowledge of population trends is lacking for almost all species (DSE 2013; author’s obs.), a trend that mirrors knowledge of reptiles around the world (Böhm et al. 2013).
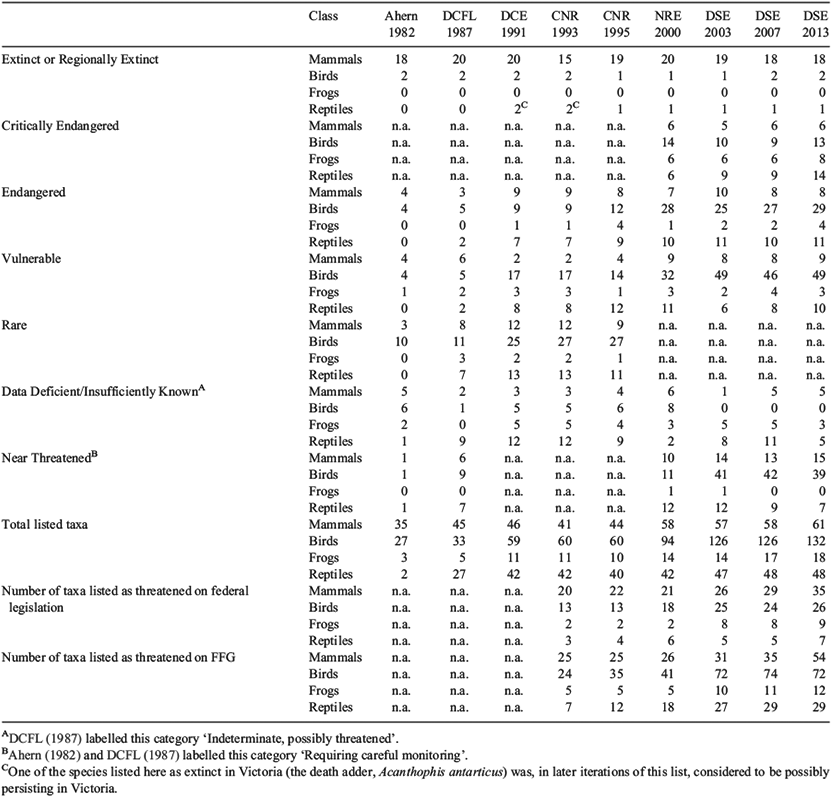
The gazettal of the Victorian Wildlife Act 1975 was the earliest formal listing of threatened species in the State, and resulted in a small number of taxa being listed as highly threatened. The first systematic attempt to formalise threatened species listing in Victoria was conducted by Ahern (1982); at that time two reptiles were amongst the 67 taxa listed as threatened or extinct in Victoria. Subsequent to this, Ahern et al. (1985a, 1985b) developed and applied a ‘taxon priority system’ for the State’s fauna, which was a ‘means of assessing current status of a taxon, its survival prospects, perceived values, and the need for and feasibility of further action to conserve that taxon’ (Ahern et al. 1985b, p. 2). In 1987 the (then) Department of Conservation, Forests & Lands published a list of threatened fauna for the State (Table 1; DCFL 1987). The advent of the Victorian Flora & Fauna Guarantee Act (FFG) in 1988 allowed for the listing of species and communities as threatened in Victoria (it also allowed for the listing of threatening processes). Listing under this legislation follows a nomination, consideration and subsequent recommendation by a Scientific Advisory Committee, public consultation, and approval by the relevant State minister(s). There are currently 29 Victorian reptiles listed as threatened under this legislation (Table 1), although this number is likely to increase in the near future as more nominations are prepared and processed (author’s obs.). The capacity for these legislative tools to protect Victorian wildlife has been questioned, resulting in early optimism (Mansergh et al. 1993) that seems to have been unfounded (Victorian Auditor-General 2009).
A parallel process allows for a taxon’s listing as nationally threatened under the federal Environment Protection & Biodiversity Conservation Act 1999 (EPBC). Currently, seven Victorian reptiles are listed as nationally threatened under this Act (each of these species are also listed as threatened under the FFG), although more Victorian species almost certainly qualify, but have not yet been nominated. Compared to other vertebrate classes, reptiles have fared poorly in terms of being listed as threatened under the EPBC Act and having national recovery plans prepared under that Act (Walsh et al. 2013). Walsh et al. (2013) suggest that this federal list ‘is inherently biased as species that are charismatic, large in body size, well studied and easily accessible to the general public and special interest groups are more likely to be nominated and therefore listed as threatened than less well known, lower order species’ (p. 140).
As well as these legislated lists, the Victorian Department of Environment, Land, Water and Planning publishes an ‘advisory’ list of threatened vertebrate Victorian fauna, which is revised and updated every few years (Table 1). First prepared in 1991 (DCE 1991), revised lists have been published in 1993 (CNR 1993), 1995 (CNR 1995), 2000 (NRE 2000), 2003 (DSE 2003), 2007 (DSE 2007) and 2013 (DSE 2013). These lists are based on assessments within each vertebrate class conducted by Victorian experts, and the more recent lists apply criteria dictated by the IUCN (http://www.iucnredlist.org/technical-documents/categories-and-criteria/2001-categories-criteria accessed 30 March 2015). Each list is intended to provide a more comprehensive coverage of Victoria’s vertebrate fauna than that so far achieved under the FFG Act by using taxon experts to rank species known to be threatened, as well as those that are thought to be on a trajectory that will eventually result in them being listed (‘Near Threatened’ taxa), and those suspected to be in trouble, but for which there is not enough information to make an informed assessment (‘Data Deficient’ taxa). A disadvantage of these lists is that they do not receive the independent vetting of a Scientific Advisory Committee. Conversely, these lists have the advantage of being particularly relevant at a State-only level, and typically being updated to reflect new information or changes in a taxon’s status or changes in threatening processes more quickly than the other forms of threatened species’ listing. Another advantage of these lists is that they are created by taxon experts, so they avoid the bias that might arise from nominations by members of the public. Currently, 48 reptiles are listed as threatened in Victoria on this list.
Whilst it is not possible to directly compare numbers of threatened reptile taxa between iterations of these State-level lists because more species have been discovered in Victoria since listing began (e.g. Clemann et al. 2007), or recognised taxonomically (and listed) over time (e.g. Donnellan et al. 2002), some trends are evident. Although better information on some species’ conservation or taxonomic status has resulted in their being removed from the lists or having their status eased (e.g. eastern water skink, Eulamprus quoyii, and tessellated gecko, Diplodactylus tessellatus) (all nomenclature follows Wilson and Swan 2013), no threatened reptile has been delisted due to successful recovery. Unfortunately, at a national level the same applies to all nationally threatened species in Australia – not just reptiles – on the federal EPBC list (Walsh et al. 2013). The status of some Victorian reptiles (e.g. alpine she-oak skink, Cyclodomorphus praealtus, and lace monitor, Varanus varius) has worsened, and further taxa have been progressively added to the Victorian advisory lists over time (Table 1) due to increasing concerns about their status, or to worsening threats, especially deleterious changes to the extent or condition of their habitat.
The Victorian Environment Defenders Office has been critical of the application of environmental legislation in this State, and the conservation outcomes that have (or have not) been achieved for Victorian wildlife (Environment Defenders Office 2012). In the last 20 years, the number of Victorian reptiles listed as threatened under the FFG Act has increased 4-fold, and in the 30 years between Ahern’s (1982) classification and the most recently published advisory list (DSE 2013) the total number of listed reptiles has increased 24-fold (Table 1). In comparison, mammals and frogs listed under the FFG Act have slightly more than doubled, and birds have tripled; and total numbers of listed threatened species in Victoria for these classes of vertebrates have nearly doubled for mammals, and increased by nearly five and six times for birds and frogs respectively. Other trends for Victorian vertebrate fauna that are obvious from this advisory list include significant losses of mammals from Victoria early in the European occupation of the State, and greater certainty around the conservation status of birds than for other classes (e.g. despite the large number of bird species that occur in Victoria, relatively few are listed in the ‘Data Deficient/Insufficiently Known’ category), probably due to the efforts of large numbers of knowledgeable professional and amateur ornithologists who regularly collect data on species’ occurrences throughout the state. Conversely, there is a strong global trend for ectotherms to be over-represented in the ‘Data Deficient’ category compared to the more ‘charismatic’ or conspicuous birds and mammals (Böhm et al. 2013), reflecting a lack of basic data collection on ectothermic fauna.
At a State-only level, listing bias against Victorian reptiles has now been addressed to a degree via the use of taxon experts with the Advisory List. However, bias remains for Victorian reptiles at the national level (Walsh et al. 2013), and funding for research and conservation programs for threatened Victorian reptiles is grossly inadequate. Even for (arguably) the most threatened reptile in the State, the grassland earless dragon, Tympanocryptis pinguicolla, which has not been verifiably recorded in Victoria for 45 years, there is a severe lack of financial investment for even basic survey, despite ongoing destruction of potential habitat for this species (Clemann et al. 2013a).
For a small proportion of threatened reptile taxa significant progress has been made in terms of learning more about their distribution, biology and status (e.g. alpine she-oak skink; Corangamite water skink, Eulamprus tympanum marniae; striped legless lizard, Delma impar; hooded scaly-foot, Pygopus schraderi; pink-tailed worm lizard, Aprasia parapulchella; broad-shelled turtle, Chelodina expansa), or high-quality management prescriptions have resulted from detailed investigations of particular threatened species (e.g. Robertson and Canessa 2012). However, few significant, positive changes have occurred in terms of limiting or slowing the threatening processes that are threatening reptiles in Victoria.
Threats to Victorian reptiles
Specialised habitat preferences or biological features predispose some reptiles to rarity or susceptibility to threats (e.g. Reed and Shine 2002). Victorian examples include the striped legless lizard, which is restricted to specific grassland habitats that have been systematically degraded or removed since European occupation; and the lace monitor, which is not only dependent on treed landscapes, but has specialised nesting requirements (it usually lays its eggs in termitaria: Vincent and Wilson 1999). Consequently, this apex predator is vulnerable to loss and degradation of habitat in general, and loss of nesting resources in particular.
For some taxa, rarity and vulnerability may be exacerbated by compounding specialisation. For example, the diet of the elapid snake bandy bandy, Vermicella annulata, consists solely of blind snakes, Ramphotyphlops spp. (Shine 1980); in turn, the diet of blind snakes consists entirely of larvae and pupae of ants (Webb and Shine 1993). Consequently, not only is the bandy bandy reliant on species that are themselves dietary specialists, but both the bandy bandy and its prey have suffered from large-scale habitat loss and degradation across northern Victoria.
The following overview is not an exhaustive list of all threats to every Victorian reptile, but discusses the threats likely to be affecting most threatened taxa. These are threats common to reptiles around the world.
Climate change
Climate change, and the raft of impacts that are likely to accompany a changing climate, looms as the most difficult-to-manage threat to reptiles (e.g. Sinervo et al. 2010). This threat is especially and immediately concerning for species that are restricted to narrow climatic regimes (Pincheira-Donoso et al. 2013). The frequency and severity of both droughts (Dai 2011; Kirono et al. 2011) and fires (Flannigan et al. 2009) is predicted to increase with a changing climate, with resulting profound impacts on biodiversity and ecosystem function. South-eastern Australia has recently experienced one of the most severe and prolonged droughts in the region’s recorded history (the ‘millennium drought’: Bond et al. 2008; Murphy and Timbal 2008; Clemann et al. 2013b). Concurrently, the region is experiencing a trend towards conditions that favour more frequent and severe fires (Hennessy et al. 2005), and parts of north-eastern Victoria have experienced four severe wildfires between 2003 and 2013.
It is likely that some ectothermic species will benefit from a changing climate, just as some will suffer (Penman et al. 2010). Those most likely to suffer include specialised taxa, or those with narrow thermal niches, such as species that are adapted to particularly cold climates because they occur at or near the tops of mountains and plateaux (e.g. Pincheira-Donoso et al. 2013). For example, the alpine bioregion of south-eastern Australia has several reptile taxa that are restricted to disjunct, high-elevation peaks and plains in this region (e.g. alpine she-oak skink and guthega skink, Liopholis guthega: Koumoundouros et al. 2009). Because they occur only at the highest elevations, reptiles restricted to the alpine and subalpine zone in Victoria, all of which are listed as threatened, have little or no scope for uphill migration in response to a warming climate. A changing climate is also likely to affect the vegetation structure that is vital to these species, and facilitate the ingression of competitors (Green and Osborne 2012). For species with temperature-dependent sex determination, changing climate without concomitant behavioural, elevational or latitudinal changes may result in unsustainable biases to population sex ratios (Doody et al. 2006). In short, large-scale, difficult-to-manage threats such as climate change are likely to exacerbate other, more proximate threats, such as deleterious changes to habitat.
Habitat loss and degradation
Broad-scale loss and degradation of native vegetation across much of Victoria has occurred since European occupation of the State began in 1835. It is the most pervasive immediate threatening process to Victorian reptiles (and most threatened reptiles around the world: Böhm et al. 2013), is common to most listed taxa, and is exacerbating most other threats. A focus on preserving the habitat, rather than individuals, of Victorian reptiles was called for more than three decades ago (Rawlinson 1981), but has been largely ignored. Ironically, because habitat damage is most often and most devastatingly caused directly by humans, we have the greatest ability to mitigate this process by ceasing or reducing destructive activities. Frequently, individual parcels of land that are known or suspected to contain threatened reptiles in Victoria are considered expendable because, in isolation, they form a fraction of the species’ historic range (author’s obs.). However, collective development of this land amounts to a ‘death by a thousand cuts’. The effects on reptiles of such habitat changes are understudied (Gardner et al. 2007).
Threatened reptiles occur in most Victorian ecosystems, and continuing land clearing for agriculture, timber harvesting, recreational and residential development (and related infrastructure) and industrial estates means that remaining habitat is shrinking and becoming increasingly isolated. Degradation and fragmentation of key habitat for threatened reptiles also occurs during development and maintenance for recreational facilities such as ski runs (Sato et al. 2014a). Fragmentation of threatened reptile habitat is exemplified by the draining and removal of swamps, wet heath, riparian vegetation and saltmarsh that forms critical habitat for the swamp skink, Lissolepis coventryi, and glossy grass skink, Pseudemoia rawlinsoni, in southern Victoria. These two species’ occurrence is now severely disjunct (author’s obs.), and these now-fragmented populations are especially vulnerable to deleterious deterministic and stochastic processes.
Diminishing quality, extent and connectivity of habitat will frequently cause populations of threatened reptiles to be particularly susceptible to stochastic processes and/or ‘mopping up’ by exotic predators.
Elevated rates of predation
Predation is a natural regulatory process, but predation rates can be elevated by habitat modification (Sato et al. 2014b), and predation by exotic predators can be catastrophic for some populations of threatened species, especially those rendered particularly vulnerable due to the impacts of other factors, such as habitat loss and fragmentation, or being physically exposed due to short-term loss of vegetative cover after fire. Prey species typically develop defences against coevolved predators over evolutionary timeframes (e.g. Downes and Shine 1998); however, they are often naïve to recently introduced predators (e.g. Gillespie 2001). For example, predation of eggs by the red fox, Vulpes vulpes, is considered the most significant threat to the broad-shelled turtle in Victoria (Thompson 1983; Spencer 2002; Spencer and Thompson 2005), and cats, Felis catus, are known to kill many small reptiles (e.g. Read and Bowen 2001). The impact of invasive species on threatened reptiles in southern Australia has been specifically highlighted in a global assessment of the conservation status of reptiles (Böhm et al. 2013).
Poor advice and planning
Some very good ecological consultants operate in Victoria, and they play key roles in directing the listing (e.g. DSE 2013) and management (e.g. Robertson and Canessa 2012) of threatened Victorian reptiles. However, some others expedite and hasten the loss of critical habitat for threatened reptiles in Victoria by advocating unproven ‘mitigation’ strategies or biodiversity trading in order to facilitate development whilst appearing to allow for the needs of threatened species, in effect appeasing their clients at the expense of populations of threatened species. Most Environmental Impact Statements and client reports prepared by ecological consultants are not subject to peer review or other forms of effective scientific vetting.
Management strategies for threatened fauna that have not been proven to adequately mitigate threats, such as relocation or fauna underpasses, are frequently recommended by some consultants and agency planners (e.g. Biosis 2010; Hamer and Organ 2012; Ecology and Heritage Partners 2014). Eagerness to develop habitat occupied by threatened species is facilitated and justified under the notion that populations are relocatable, newly created barriers can be surmounted with underpasses and the like, and habitat can be traded or created. Measures such as relocation can provide a misleading impression that biodiversity is ‘transferable’ from a location slated for development to a safer place. However, studies that have explicitly examined the results of relocations of threatened reptiles from areas to be developed suggest that the outcomes are usually very poor, or unknown, for relocated individuals (e.g. Dodd and Seigel 1991; Reinert 1991, Platenberg and Griffiths 1999; Sullivan et al. 2015). Similarly, it is common for proponents of relocation to cite well planned, careful ‘recovery’ translocations (e.g. Towns and Ferreira 2001) to erroneously suggest that ad hoc predevelopment relocations will be similarly successful (author’s obs.).
Clients of ecological consultants, such as land developers and some government agencies, frequently lack the biological and ecological expertise to objectively evaluate the information presented to them in these reports. Consequently, advocacy of unproven strategies that are supposed to reduce the impact of development can lead to unqualified acceptance that such measures provide certainty regarding the postdevelopment, long-term persistence of the ‘managed’ population (Beebee 2013).
Misidentification of an organism by inexperienced or poorly trained consultants offering contract identification is a problem (Wheeler 2003), and when planning the management of threatened species some consultants and agency planners fail to consult with relevant taxon experts, which can result in consultants recommending unsound management practices that may be detrimental to individuals and populations. Detection of some reptiles can be difficult and, as a consequence, ecological consultants sometimes draw misguided conclusions about the likelihood of such species occupying some habitat types. For example, swamp skinks are notoriously challenging to detect in some habitats (Clemann 2000).
Management strategies for swamp skinks suggested by consultants include relocation (DEPI 2013); however, swamp skinks react violently to intraspecific intrusions into their territory (author’s obs.), and will kill conspecifics in captivity (P. Robertson, pers. comm.). Consequently, relocation into habitat already occupied by conspecifics is likely to incite aggression, and relocated lizards may not be able to access sufficient resources to survive. Alternatively, release of this species into unoccupied habitat likely represents an introduction beyond its natural range, or release into areas that, for some reason, are not currently suitable for the species. Although neither outcome is desirable, relocation of this species has been recently recommended (Biosis 2010; Ecology Partners 2011, Ecology and Heritage Partners 2014), and swamp skinks have been relocated during development works (D. Gilmore pers. comm.). A growing literature across diverse phylogenetic lineages (e.g. Dodd and Seigel 1991; Nowack 1998; Platenberg and Griffiths 1999; Fischer and Lindenmayer 2000; Plummer and Mills 2000; Sullivan et al. 2004; Butler et al. 2005; Sullivan et al. 2015) confirms that ad hoc relocation of reptiles is problematic, outcomes are often not assessed, and, of those that are assessed, many are unsuccessful.
Many reptiles, particularly rare and/or declining species, can be difficult to detect (e.g. Clemann 2000; Roughton and Seddon 2006). Due to limited budgets and tight timelines (author’s obs.), many, if not most, predevelopment surveys conducted by ecological consultants in and near areas to be modified are too brief and fail to incorporate detection probabilities (e.g. MacKenzie et al. 2002) for key taxa. Sometimes assessments employ inappropriate methods or too little effort, some involve staff who are inexperienced with specific cryptic fauna, and some are even conducted at times of the year or during weather conditions when detection of target species is suboptimal (Fraser et al. 2003; author’s obs.). Some authors even admit that suboptimal timing or spatial scales are applied during preimpact fauna surveys (Powell and Sedunary 2013). The habitat of threatened species can be subject to timber harvesting if preharvest surveys fail to detect the species (Powell and Sedunary 2013), despite the obvious risk of simply failing to detect rare or cryptic species that may persist in the area (MacKenzie et al. 2005). Also, a reliance on detection-based protection of habitat can remove currently unoccupied habitat that is necessary for species’ recovery once threats are mitigated (Scheele et al. 2014).
A commonly used form of trading biodiversity is ‘offsetting’. Whilst there is a dearth of studies specifically examining the use of offsets for reptiles, conclusions in the literature on this form of biodiversity trading are unanimous – as currently practised, offsetting is resulting in net losses of biodiversity. During trading programs, the immediate loss of habitat occupied by threatened species is obvious, but the benefits of the associated offset(s) can be far from certain (Moilanen et al. 2009), the criteria defining success are often vague (Matthews and Endress 2008), and the practical application of offsetting is proving problematic, both spatially (Pickett et al. 2013) and temporally (Curran et al. 2013). Offsetting proffers a development-facilitating compromise that can be attractive to both development and conservation interests (and therefore facilitates rapid development of habitat: Maron et al. 2012), but many authors note that the standards applied, resources needed, timelines necessary and amounts of land sufficient to genuinely provide the putative offsets are rarely adequate (e.g. Gibbons and Lindenmayer 2007; Moilanen et al. 2009; Walker et al. 2009b; Maron et al. 2012; Pickett et al. 2013). The time lag between habitat removal and realising that an offset has failed or is inadequate means it is almost always too late to reverse these losses (Moreno-Mateos et al. 2012). In other words, strategies such as habitat offsets, relocation and underpasses require robust and repeated assessment before they are accepted as mitigations for loss of habitat.
Conservation management of threatened reptiles
Management of certain large-scale pervasive threats, such as climate change, are beyond the scope of specific management programs for threatened reptiles, although reptiles and other ectothermic fauna may be of immense value in terms of assessing and understanding the implications of climate change (e.g. Janzen 1994; Massot et al. 2008). However, precisely because such threats cannot be mitigated by local conservation programs, the need to manage threats that can be influenced by these programs is especially pressing.
Although there is a trend away from resourcing programs for individual threatened species, and towards shortcuts (such as the use of ‘umbrella’ or ‘flagship’ species for valuing and protecting habitat) for maintaining biodiversity (sensu Roberge and Angelstam 2004), most threatened reptiles have specific ecological or habitat requirements (e.g. Towns and Ferreira 2001; Reed and Shine 2002), and therefore require specific attention. Using iconic (usually endothermic) vertebrates as ‘umbrella’ species in order to protect ecosystems and their constituent fauna (Roberge and Angelstam 2004) works only when the protection extends to the habitat and processes relevant to the threatened fauna (Lindenmayer and Burgman 2005). Whilst there are a couple of examples of threatened reptile populations in Victoria benefiting from habitat protection resulting from co-occurrence with a threatened iconic endotherm (e.g. swamp skink habitat protected by habitat management for sympatric helmeted honeyeaters, Lichenostomus melanops cassidix, at Yellingbo), reptile richness in a given area tends to differ from that of other classes of vertebrates (Powney et al. 2010), and few threatened reptiles can rely for protection on measures afforded to other species. Additionally, reptiles (and amphibians) have tended to be overlooked during conservation strategies that are based on coarse-scale biodiversity surrogate metrics (Araújo et al. 2001).
The following comments outline actions that are within the scope of local and regional conservation programs.
Resourcing
Australia is a relatively wealthy nation, at least theoretically capable of exemplifying high standards of biodiversity conservation (Rodrigues et al. 2014). Conservation of threatened fauna requires significant financial inputs, but rarely generates money (exceptions exist, but they rarely involve reptiles). In order to effectively manage and conserve threatened Victorian reptiles, adequate, specific financial and human resourcing is essential. Walsh et al. (2013, p. 141) note that ‘funding for conservation is being distributed inefficiently because the listing and planning processes for threatened species in Australia appear to be driven by charisma, body size and level of knowledge or appeal’ – all categories where reptiles tend to fare poorly in the minds of many people. In a review of the FFG Act, the Victorian Auditor-General (2009) criticised the level of resourcing for threatened species planning in the State. Collaborative efforts between multiple people and agencies can bring synergies to research and management of threatened species that maximise the benefits attained from resource investment; recent examples that have benefited threatened reptiles in Victoria include works by Koumoundouros et al. (2009) and Maldonado et al. (2012).
Acquisition and/or appropriate management of habitat is one of the most effective ways to conserve taxa (e.g. Czech 2002), but requires significant resources. Once adequate resources for conservation actions are secured, threat management can commence, and the highest priority for the most species is preserving habitat.
Preventing habitat loss and degradation
Reptiles can be particularly sensitive to habitat loss and degradation due to their often poor dispersal ability across barriers and the landscape (Driscoll 2004), ecomorphological specialisation that is frequently related to substrate type (e.g. Clemann et al. 2008), relatively small home ranges, and thermoregulatory constraints (Kearney et al. 2009). Preventing further loss and degradation of habitat is the single most achievable, urgent and pressing strategy for conserving reptiles in Victoria and elsewhere (Böhm et al. 2013). Unfortunately, even in relatively wealthy regions such as Victoria, the drivers of habitat loss (e.g. urban, agricultural, industrial and recreational development, timber harvesting) are stronger than measures to conserve habitat, and public and political imperatives to conserve reptiles are weak. ‘Compromises’ between habitat retention and development in Victoria are typically weighted heavily in favour of the latter (author’s obs.), and there are many reports that the habitat retained for wildlife during biodiversity trading is almost never based on the amount deemed necessary for long-term persistence by taxon experts or indicated by quantitative data (e.g. Morris et al. 2006; Moilanen et al. 2009; Maron et al. 2012; Pickett et al. 2013). Where concessions to biodiversity are made during development projects, they are reported as often minor, probably inadequate, not reflecting the needs of particular taxa, and are not subject to adequate (or any) postdevelopment assessment (Fraser et al. 2003). The current manner of offsetting habitat loss provides little certainty that there is no net loss of habitat for each taxon (e.g. Gibbons and Lindenmayer 2007; Bekessy et al. 2010).
Similarly, ongoing degradation of habitats renders them increasingly less suitable for occupation by reptiles (e.g. Gibbons et al. 2000; Webb and Shine 2000; Sato et al. 2014b), and species with more specialised requirements will be the first to be eliminated as habitat quality degrades (e.g. Reed and Shine 2002). Many processes degrade reptile habitat, such as removal of rocks (including exfoliating slabs: Webb and Shine 2000) and fallen timber, overgrazing and trampling, creation and maintenance of recreational infrastructure (e.g. Sato et al. 2014a), inappropriate fire regimes, and weed invasion (Gibbons et al. 2000) (but, notably, some threatened reptiles persist, and even thrive, in weedy environments, such as the swamp skink, which can persist in areas with an understorey dominated by weeds, provided that the vegetation structure remains suitable: author’s obs.). Populations persisting in degraded habitat may be particularly susceptible to stochastic events or systematic pressure from other threats, such as predation by exotic (Gibbons et al. 2000) or even native species (Sato et al. 2014b). And because total population loss in degraded habitats is rarely instantaneous, apparent persistence can mask gradual, inexorable loss (Kuussaari et al. 2009).
Research to underpin management, and monitoring of population status and management effectiveness
Walsh et al. (2013) noted that Australian birds, amphibians and mammals have been more thoroughly studied than reptiles, and that this has contributed to bias in the relative contribution of these vertebrate classes to federal threatened species listing. The Victorian Auditor-General (2009, p. 3) recommended that the Department of Environment, Land, Water and Planning should ‘continue to build its knowledge-base on threatened species, causes of their decline and how best to mitigate threats to them’. In the absence of robust, quantitative and appropriate information, management of reptiles is, at best, educated guesswork, and at worst a waste of scarce resources. Effective management requires basic biological and ecological data, and, crucially, monitoring of the effectiveness of management actions followed by adaptive responses to the monitored results of management (Lindenmayer et al. 2013). Gaining robust understanding of the biology and ecology of reptiles, and their response to threats and management activities, must be viewed as long-term activities; it is unreasonable and frequently misleading to draw conclusions from short-term investigations (Byron et al. 2000). The most extreme example of short-term investigations are predevelopment surveys (or preharvest surveys in logging coupes); consequently, unless great care is taken to convey the limitations of these brief surveys, conclusions drawn from predevelopment investigations run the highest risk of misleading conservation management. If quantitative data or robust models are not available, taxon experts may provide valuable insights into a taxon’s biology and ecology (e.g. Murray et al. 2009), and likely response to both threats and management (and perhaps to caution against poorly informed decisions).
Vetting of consultant reports and Environmental Impact Assessments
Reporting and advice from consultants often forms the basis for decisions on human impacts on the habitat of threatened species. Conservation of reptiles would benefit from independent, effective and critical peer review of consultant reports and advice, followed by remedial action or intervention if reports are not sound or biodiversity values are unduly downplayed, underestimated or overlooked. In particular, advice promoting supposed mitigations such as relocation, underpasses and biodiversity trading should be examined very cautiously. Until management measures have been proven to work well enough to maintain populations in the landscape (verified by robust long-term monitoring), advocacy of such measures by consultants, developers, and agency planners should be subject to careful scrutiny, and these measures should not be permitted to stand alone as assurances that the population will persist after the development or after impacts such as timber harvesting. Genuine mitigation successes are rare, and can be due to very specific habits of certain species, such as the relocation of an entire den of communally sheltering snakes in the USA (Walker et al. 2009a). It is misleading to assume that such measures will work for different species. Habitat procurement or creation sufficient to maintain wetland species in an offset may need to be many times the size of the habitat that is destroyed (Pickett et al. 2013), and it is likely that the amounts of land sufficient to retain or create appropriate terrestrial habitats will be much larger again (Morris et al. 2006). Information generated during studies, surveys and monitoring of threatened species should be readily available, preferably via peer-reviewed publication. The methods and results of Environmental Impact Assessments should also be broadly available, and archived to enable them to be consulted years later.
Despite being the most important taxa to detect during preimpact assessments, rare or declining taxa are those most likely to remain undetected, even when present (MacKenzie et al. 2005). Surveys should be tailored to the species of interest. They should be conducted during the season(s) when detection probability is maximised (MacKenzie et al. 2006), and under the right weather conditions (Blomberg and Shine 1996). Surveys of sufficient duration and intensity should be employed, ensuring that the best methods are used by experienced staff. Too often habitat destruction is, at least in principle, permitted to occur simply because a brief survey failed to detect a species (e.g. Powell and Sedunary 2013), or there is not an historic record at the precise point of impact. Incorporation and refinement of detection probabilities during preimpact surveys is important (MacKenzie et al. 2002), but, if a particular threatened species is not detected during a survey, presence should be assumed where there is a reasonable expectation that occurrence is possible (i.e. historical records occur in the general vicinity, potential habitat occurs on or near the site, expert opinion or species’ distribution modelling indicates possible occurrence). Preferably a combination of these factors would feature in determinations of the likely presence of taxa, and the Precautionary Principle would apply (i.e. the onus should be on the development proponent to show with a high degree of confidence that certain fauna could not or do not occur in the area, or that development or other impacts would have no particular negative effect on threatened taxa, rather than being on conservation-interested parties to prove that threatened taxa do occur in the area). If declining species are to have any chance of recovery, it is crucial that habitat is protected beyond their currently reduced range so that range (re-)expansion is possible (Scheele et al. 2014).
Conclusion
In the 30 years of formally assessing the conservation status and management needs of Victorian reptiles, the number of taxa formally listed as threatened has burgeoned. While some of these increases are due to the way that conservation status is assessed or because of greater understanding of the status of some taxa, it is clear that the impact of threatening processes has increased over this time, the status of many species has worsened, and the need to mitigate threats has grown ever urgent. Whilst knowledge of a select few taxa is growing, many remain poorly known, and documented recovery for any threatened taxon is non-existent. Proactive conservation measures are crucial, but evidently remain a low priority for the Victorian public and their elected representatives.
The deteriorating status of Victorian reptiles mirrors worrying trends documented for reptiles around the world (Böhm et al. 2013). Similarly, the processes that continue to threaten Victorian reptiles exemplify the threats around the world. An often overlooked contribution to losses of populations of threatened species is poor advice from consultants, and uncritical acceptance of this advice by agency staff. This issue is also a threat in the neighbouring state of New South Wales (D. Hunter, pers. comm.), and it remains to be seen whether this threat is problematic elsewhere, and will be recognised by other authors. The variety of reptile taxa and habitats in Victoria makes this State an ideal model for examining these global threats and trends in the status of threatened reptiles, and there is enormous potential for environmental management agencies in this relatively wealthy region to demonstrate effective and proactive conservation measures that would afford its reptiles the greatest chance to persist in spite of a changing climate (Rodrigues et al. 2014).
Due to a poor image in the public consciousness, reptiles tend to fare poorly in terms of public sympathy relative to endothermic vertebrates. Entire organisations are devoted to the conservation of birds (e.g. Birdlife Australia), and even single species of non-threatened mammals (the Australian Platypus Conservancy), but these measures are presently inconceivable for reptiles. To date, not a single organisation is devoted solely to the conservation of Australian reptiles.
If we wish to maximise biodiversity conservation efforts, allocation of resources for research and conservation must be based on utilitarian and objective criteria, rather than being unduly influenced by emotion (Metrick and Weitzman 1996; Walsh et al. 2013). This will only be achieved via education of the public who drive wildlife sentiment, and agency staff who determine the allocation of resources. Ahern (1982, p. 31) stated that ‘there are several aspects of wildlife communities in Victoria into which the Division [i.e. the former Victorian Fisheries and Wildlife Division] has created few, if any, inroads for research or management’. Unfortunately, 30 years later this statement remains accurate, and as a result the status of Victorian reptiles matches the worrying global trends documented by Böhm et al. (2013).
Acknowledgements
I thank the following people for refining my thinking on threatened species: Peter Robertson, the late A. John Coventry, the late Leigh Ahern, the late John Seebeck, Peter Brown, Peter Menkhorst, Mike Smith and David Hunter. I thank those who provided threatened species lists, including Vikki Cail, Peter Menkhorst, Jerry Alexander, Peter Robertson and Martin O’Brien. I thank Giuliana McAlpin for thoughtful discussions on this topic, and Peter Menkhorst, Lindy Lumsden, Kim Lowe, Michael Calver, Michael Craig and an anonymous referee for providing critiques and comments that improved drafts of this paper.
References
Ahern, L. D. (1982). Threatened wildlife in Victoria and issues related to its conservation. Fisheries and Wildlife Paper, Victoria No. 27. Fisheries and Wildlife Division, Ministry for Conservation, Victoria.Ahern, L. D., Brown, P. R., Robertson, P., Seebeck, J. H., Brown, A. M., and Begg, R. J. (1985a). A proposed taxon priority system for Victorian vertebrate fauna. Technical Report Series No. 30. Arthur Rylah Institute for Environmental Research, Department of Conservation Forests and Lands, Melbourne, Victoria.
Ahern, L. D., Brown, P. R., Robertson, P., and Seebeck, J. H. (1985b). Application of a taxon priority system to some Victorian vertebrate fauna. Technical Report Series No. 32. Arthur Rylah Institute for Environmental Research, Department of Conservation Forests and Lands, Melbourne, Victoria.
Araújo, M. B., Humphries, C. J., Densham, P. J., Lampinen, R., Hagemeijer, W. J. M., Mitchell-Jones, A. J., and Gasc, J. P. (2001). Would environmental diversity be a good surrogate for species diversity? Ecography 24, 103–110.
| Would environmental diversity be a good surrogate for species diversity?Crossref | GoogleScholarGoogle Scholar |
Beebee, T. J. C. (2013). Effects of road mortality and mitigation measures on amphibian populations. Conservation Biology 27, 657–668.
| Effects of road mortality and mitigation measures on amphibian populations.Crossref | GoogleScholarGoogle Scholar |
Bekessy, A. A., Wintle, B. A., Lindenmayer, D. B., McCarthy, M. A., Colyvan, M., Burgman, M. A., and Possingham, H. P. (2010). The biodiversity bank cannot be a lending bank. Conservation Letters 3, 151–158.
| The biodiversity bank cannot be a lending bank.Crossref | GoogleScholarGoogle Scholar |
Bennett, A. F. (1978). Activity metabolism of the lower vertebrates. Annual Review of Physiology 40, 447–469.
| Activity metabolism of the lower vertebrates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXhvVKgsbo%3D&md5=9600d6e532b5b958db805bf91c9092e4CAS | 345952PubMed |
Biosis (2010). Peninsula Link Swamp Skink Translocation Plan, The Pines Flora and Fauna Reserve, Frankston. Biosis Research, Port Melbourne, Victoria.
Blomberg, S., and Shine, R. (1996). Reptiles. In ‘Ecological Census Techniques: A Handbook’. (Ed. W. J. Sutherland.) pp. 218–226. (Cambridge University Press: Cambridge.)
Böhm, M., et al. (2013). The conservation status of the world’s reptiles. Biological Conservation 157, 372–385.
| The conservation status of the world’s reptiles.Crossref | GoogleScholarGoogle Scholar |
Bond, N. R., Lake, P. S., and Arthington, A. H. (2008). The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia 600, 3–16.
| The impacts of drought on freshwater ecosystems: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Bonnet, X., Shine, R., and Lourdais, O. (2002). Taxonomic chauvinism. Trends in Ecology & Evolution 17, 1–3.
| Taxonomic chauvinism.Crossref | GoogleScholarGoogle Scholar |
Butler, H., Malone, B., and Clemann, N. (2005). The effects of translocation on the spatial ecology of tiger snakes (Notechis scutatus) in a suburban landscape. Wildlife Research 32, 165–171.
| The effects of translocation on the spatial ecology of tiger snakes (Notechis scutatus) in a suburban landscape.Crossref | GoogleScholarGoogle Scholar |
Byron, H. J., Treweek, J. R., Sheate, W. R., and Thompson, S. (2000). Road developments in the UK: an analysis of ecological assessment in Environmental Impact Statements produced between 1993 and 1997. Journal of Environmental Planning and Management 43, 71–97.
| Road developments in the UK: an analysis of ecological assessment in Environmental Impact Statements produced between 1993 and 1997.Crossref | GoogleScholarGoogle Scholar |
Ceballos, G., García, A., and Ehrlich, P. R. (2010). The sixth extinction crisis: loss of animal populations and species. Journal of Cosmology 8, 1821–1831.
Clemann, N. (2000). Survival in the suburbs! The (re)discovery of the swamp skink Egernia coventryi east of Melbourne, with comments on the failure of Elliott traps in survey of this species. The Victorian Naturalist 117, 180–183.
Clemann, N., Robertson, P., Gibbons, D., Heard, G., Steane, D., Coventry, A. J., and Chick, R. (2007). An addition to the snake fauna of Victoria: De Vis’ banded snake Denisonia devisi (Serpentes: Elapidae) Waite and Longman, 1920. The Victorian Naturalist 124, 33–38.
Clemann, N., Melville, J., Ananjeva, N. B., Scroggie, M., Milto, K., and Kruezberg, E. (2008). Microhabitat occupation and functional morphology of four sympatric species of agamid lizards in the Kyzylkum Desert, central Uzbekistan. Animal Biodiversity and Conservation 31, 51–62.
Clemann, N., Howard, K., and Lindeman, M. (2013a). Survey for the grassland earless dragon Tympanocryptis pinguicolla between Melbourne and Geelong. Arthur Rylah Institute for Environmental Research, Department of Environment and Primary Industries, Victoria.
Clemann, N., Scroggie, M. P., Smith, M. J., Peterson, G. N., and Hunter, D. (2013b). Characteristics of refugia used by the threatened Australian growling grass frog (Litoria raniformis) during a prolonged drought. Wildlife Research 40, 385–392.
CNR (1993). Threatened fauna in Victoria – 1993. Victorian Department of Conservation and Natural Resources, East Melbourne.
CNR (1995). Threatened fauna in Victoria – 1995. Victorian Department of Conservation and Natural Resources, East Melbourne.
Cogger, H. G., Cameron, E. E., Sadlier, R. A., and Eggler, P. (1993). The action plan for Australian reptiles. Australian Nature Conservation Agency, Endangered Species Program. Project No. 124.
Curran, M., Hellweg, S., and Beck, J. (2013). Is there any empirical support for biodiversity offset policy? Ecological Applications , .
| Is there any empirical support for biodiversity offset policy?Crossref | GoogleScholarGoogle Scholar |
Czech, B. (2002). A transdisciplinary approach to conservation land acquisition. Conservation Biology 16, 1488–1497.
| A transdisciplinary approach to conservation land acquisition.Crossref | GoogleScholarGoogle Scholar |
Dai, A. (2011). Drought under global warming; a review. Wiley Interdisciplinary Reviews: Climate Change 2, 45–65.
| Drought under global warming; a review.Crossref | GoogleScholarGoogle Scholar |
Davies, R. G., Webber, L. M., and Barnes, G. S. (2004). Urban wildlife management – it’s as much about people! In ‘Urban Wildlife: More Than Meets the Eye’. (Eds D. Lunney, and S. Burgin.) pp. 38–43. (Royal Zoological Society of New South Wales: Sydney.)
DCE (1991). List of threatened fauna in Victoria in 1991. Department of Conservation and Environment, East Melbourne.
DCFL (1987). Conservation in Victoria. Department of Conservation, Forests & Lands, East Melbourne.
DeLoache, J. S., and LoBue, V. (2009). The narrow fellow in the grass: human infants associate snakes and fear. Developmental Science 12, 201–207.
| The narrow fellow in the grass: human infants associate snakes and fear.Crossref | GoogleScholarGoogle Scholar | 19120429PubMed |
DEPI (2013). Sub-regional species strategy for the growling grass frog. Department of Environment and Primary Industries, East Melbourne, Victoria.
Dirzo, R., and Raven, P. H. (2003). Global state of biodiversity and loss. Annual Review of Environment and Resources 28, 137–167.
| Global state of biodiversity and loss.Crossref | GoogleScholarGoogle Scholar |
Dodd, C. K., and Seigel, R. A. (1991). Relocation, repatriation and translocation of amphibians and reptiles: are they conservation strategies that work? Herpetologica 47, 336–350.
Donnellan, S. C., Hutchinson, M. N., Dempsey, P., and Osborne, W. S. (2002). Systematics of the Egernia whitii species group (Lacertilia: Scincidae) in south-eastern Australia. Australian Journal of Zoology 50, 439–459.
| Systematics of the Egernia whitii species group (Lacertilia: Scincidae) in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Doody, J. S., Guarino, E., Georges, A., Corey, B., Murray, G., and Ewert, M. (2006). Nest site choice compensates for climate effects on sex ratios in a lizard with environmental sex determination. Evolutionary Ecology 20, 307–330.
| Nest site choice compensates for climate effects on sex ratios in a lizard with environmental sex determination.Crossref | GoogleScholarGoogle Scholar |
Downes, S., and Shine, R. (1998). Sedentary snakes and gullible geckos: predator–prey coevolution in nocturnal rock-dwelling reptiles. Animal Behaviour 55, 1373–1385.
| Sedentary snakes and gullible geckos: predator–prey coevolution in nocturnal rock-dwelling reptiles.Crossref | GoogleScholarGoogle Scholar | 9632520PubMed |
Driscoll, D. A. (2004). Extinction and outbreaks accompany fragmentation of a reptile community. Ecological Applications 14, 220–240.
| Extinction and outbreaks accompany fragmentation of a reptile community.Crossref | GoogleScholarGoogle Scholar |
DSE (2003). Advisory list of threatened vertebrate fauna in Victoria – 2003. Victorian Department of Sustainability and Environment, East Melbourne.
DSE (2007). Advisory list of threatened vertebrate fauna in Victoria – 2007. Victorian Department of Sustainability and Environment, East Melbourne.
DSE (2013). Advisory list of threatened vertebrate fauna in Victoria – 2013. Victorian Department of Sustainability and Environment, East Melbourne.
Ecology Partners (2011). Officer precinct structure plan: conservation management plan (excluding Cardinia Creek). Unpublished report to the Growth Areas Authority. Ecology Partners Pty Ltd, Brunswick, Victoria.
Ecology and Heritage Partners (2014). Threatened Species Conservation Management Plan for the Peninsula Link Freeway Service Centres, Baxter, Victoria. Report for Hansen Planning Services by Ecology and Heritage Partners Pty Ltd, Ascot Vale, Victoria.
Environment Defenders Office (2012). Where’s the guarantee? Implementation and enforcement of the Flora and Fauna Guarantee Act 1988 and Wildlife Act 1975. Environment Defender’s Office (Victoria) Ltd. Carlton, Victoria.
Farrier, D., Whelan, R., and Mooney, C. (2007). Threatened species listing as a trigger for conservation action. Environmental Science & Policy 10, 219–229.
| Threatened species listing as a trigger for conservation action.Crossref | GoogleScholarGoogle Scholar |
Fischer, J., and Lindenmayer, D. B. (2000). An assessment of the published results of animal relocations. Biological Conservation 96, 1–11.
| An assessment of the published results of animal relocations.Crossref | GoogleScholarGoogle Scholar |
Flannigan, M., Stocks, B., Turetsky, M., and Wotton, M. (2009). Impacts of climate change on fire activity and fire management in the circumboreal forest. Global Change Biology 15, 549–560.
| Impacts of climate change on fire activity and fire management in the circumboreal forest.Crossref | GoogleScholarGoogle Scholar |
Fraser, J. L., Thompson, G. G., and Moro, D. (2003). Adequacy of terrestrial fauna surveys for the preparation of Environmental Impact Assessments in the mining industry of Western Australia. Ecological Management & Restoration 4, 187–192.
| Adequacy of terrestrial fauna surveys for the preparation of Environmental Impact Assessments in the mining industry of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Gardner, T. A., Barlow, J., and Peres, C. A. (2007). Paradox, presumption and pitfalls in conservation biology: the importance of habitat change for amphibians and reptiles. Biological Conservation 138, 166–179.
| Paradox, presumption and pitfalls in conservation biology: the importance of habitat change for amphibians and reptiles.Crossref | GoogleScholarGoogle Scholar |
Gibbons, J. W., Scott, D. E., Ryan, T. J., Buhlmann, K. A., Tuberville, T. D., Metts, B. S., Greene, J. L., Mills, T., Leiden, Y., Poppy, S., and Winne, C. T. (2000). The global decline of reptiles, déjà vu amphibians. Bioscience 50, 653–666.
| The global decline of reptiles, déjà vu amphibians.Crossref | GoogleScholarGoogle Scholar |
Gibbons, P., and Lindenmayer, D. B. (2007). Offsets for land clearing: no net loss or the tail wagging the dog? Ecological Management & Restoration 8, 26–31.
| Offsets for land clearing: no net loss or the tail wagging the dog?Crossref | GoogleScholarGoogle Scholar |
Gillespie, G. R. (2001). The role of introduced trout in the decline of the spotted tree frog (Litoria spenceri) in south-eastern Australia. Biological Conservation 100, 187–198.
| The role of introduced trout in the decline of the spotted tree frog (Litoria spenceri) in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Green, K., and Osborne, W. (2012). ‘Field Guide to Wildlife of the Australian Snow-Country.’ (Reed New Holland: Sydney.)
Hamer, A., and Organ, A. (2012). Integrating road underpasses for the threatened growling grass frog (Litoria raniformis) into a broadscale residential development in south-east Melbourne. In ‘Reducing the Impact of Development on Wildlife’. (Eds J. Gleeson, and D. Gleeson.) pp. 102–104. (CSIRO Publishing: Melbourne.)
Hennessy, K., Lucas, C., Nicholls, N., Bathols, J., Suppiah, R., and Ricketts, J. (2005). Climate change impacts on fire-weather in south-east Australia. CSIRO Marine and Atmospheric Research, Bushfire CRC and Australian Bureau of Meteorology, Aspendale, Victoria.
Isbell, L. A. (2006). Snakes as agents of evolutionary change in primate brains. Journal of Human Evolution 51, 1–35.
| Snakes as agents of evolutionary change in primate brains.Crossref | GoogleScholarGoogle Scholar | 16545427PubMed |
Janzen, F. J. (1994). Climate change and temperature-dependent sex determination in reptiles. Proceedings of the National Academy of Sciences of the United States of America 91, 7487–7490.
| Climate change and temperature-dependent sex determination in reptiles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2czivFGnsg%3D%3D&md5=f8abbbfce96eec647bb978238c656ef0CAS | 8052608PubMed |
Kearney, M., Shine, R., and Porter, W. P. (2009). The potential for behavioural thermoregulation to buffer “cold-blooded” animals against climate warming. Proceedings of the National Academy of Sciences of the United States of America 106, 3835–3840.
| The potential for behavioural thermoregulation to buffer “cold-blooded” animals against climate warming.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt1GksLc%3D&md5=a794597f03313b97b68e2fbb5d5336b3CAS | 19234117PubMed |
Kirono, D. G. C., Kent, D. M., Hennessy, K. J., and Mpelasoka, F. (2011). Characteristics of Australian droughts under enhanced greenhouse conditions: results from 14 global climate models. Journal of Arid Environments 75, 566–575.
| Characteristics of Australian droughts under enhanced greenhouse conditions: results from 14 global climate models.Crossref | GoogleScholarGoogle Scholar |
Koumoundouros, T., Sumner, J., Clemann, N., and Stuart-Fox, D. (2009). Current genetic isolation and fragmentation contrasts with historical connectivity in an alpine lizard (Cyclodomorphus praealtus) threatened by climate change. Biological Conservation 142, 992–1002.
| Current genetic isolation and fragmentation contrasts with historical connectivity in an alpine lizard (Cyclodomorphus praealtus) threatened by climate change.Crossref | GoogleScholarGoogle Scholar |
Kuussaari, M., Bommarco, R., Heikkenen, R. K., Helm, A., Krauss, J., et al. (2009). Extinction debt: a challenge for biodiversity conservation. Trends in Ecology & Evolution 24, 564–571.
| Extinction debt: a challenge for biodiversity conservation.Crossref | GoogleScholarGoogle Scholar |
Leakey, R., and Lewin, R. (1996). ‘The Sixth Extinction: Biodiversity and its Survival.’ (Phoenix: London.)
Lindenmayer, D., and Burgman, M. (2005). ‘Practical Conservation Biology.’ (CSIRO Publishing: Melbourne.)
Lindenmayer, D. B., Piggott, M. P., and Wintle, B. A. (2013). Counting the books while the library burns: why conservation monitoring programs need a plan for action. Frontiers in Ecology and the Environment 11, 549–555.
| Counting the books while the library burns: why conservation monitoring programs need a plan for action.Crossref | GoogleScholarGoogle Scholar |
LoBue, V., and DeLoache, J. S. (2008). Detecting the snake in the grass: attention to fear-relevant stimuli by adults and young children. Psychological Science 19, 284–289.
| Detecting the snake in the grass: attention to fear-relevant stimuli by adults and young children.Crossref | GoogleScholarGoogle Scholar | 18315802PubMed |
MacKenzie, D. I., Nichols, J. D., Lachman, G. B., Droege, S., Royle, J. A., and Langtimm, C. A. (2002). Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255.
| Estimating site occupancy rates when detection probabilities are less than one.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D. I., Nichols, J. D., Sutton, N., Kawanishi, K., and Bailey, L. L. (2005). Improving inferences in population studies of rare species that are detected imperfectly. Ecology 86, 1101–1113.
| Improving inferences in population studies of rare species that are detected imperfectly.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D. I., Nichols, J. D., Royle, J. A., Pollock, K. H., Bailey, L. L., and Hines, J. E. (2006). ‘Occupancy Estimation and Modeling.’ (Academic Press: USA.)
Maldonado, S. P., Melville, J., Peterson, G. N. L., and Sumner, J. (2012). Human-induced versus historical habitat shifts: identifying the processes that shaped the genetic structure of the threatened grassland legless lizard, Delma impar. Conservation Genetics 13, 1329–1342.
| Human-induced versus historical habitat shifts: identifying the processes that shaped the genetic structure of the threatened grassland legless lizard, Delma impar.Crossref | GoogleScholarGoogle Scholar |
Mansergh, I., Davey, G., and Robertson, P. (1993). Reptiles and amphibians of Victoria – legislation. In ‘Herpetology in Australia: A Diverse Discipline’. (Eds. D. Lunney and D. Ayers.) pp. 373–376 (Royal Zoological Society of New South Wales: Sydney.)
Maron, M., Hobbs, R. J., Moilanen, A., Matthews, J. W., Christie, K., Gardner, T. A., Keith, D. A., Lindenmayer, D. B., and McAlpine, C. A. (2012). Faustian bargains? Restoration realities in the context of biodiversity offset policies. Biological Conservation 155, 141–148.
| Faustian bargains? Restoration realities in the context of biodiversity offset policies.Crossref | GoogleScholarGoogle Scholar |
Massot, M., Clobert, J., and Ferriere, R. (2008). Climate warming, dispersal inhibition and extinction risk. Global Change Biology 14, 461–469.
| Climate warming, dispersal inhibition and extinction risk.Crossref | GoogleScholarGoogle Scholar |
Matthews, J. W., and Endress, A. G. (2008). Performance criteria, compliance success, and vegetation development in compensatory mitigation wetlands. Environmental Management 41, 130–141.
| Performance criteria, compliance success, and vegetation development in compensatory mitigation wetlands.Crossref | GoogleScholarGoogle Scholar | 17676406PubMed |
Metrick, A., and Weitzman, M. L. (1996). Patterns of behaviour in endangered species preservation. Land Economics 72, 1–16.
| Patterns of behaviour in endangered species preservation.Crossref | GoogleScholarGoogle Scholar |
Moilanen, A., van Teeffelen, A. J. A., Ben-Haim, Y., and Ferrier, S. (2009). How much compensation is enough? A framework for incorporating uncertainty and time discounting when calculating offset ratios for impacted habitat. Restoration Ecology 17, 470–478.
| How much compensation is enough? A framework for incorporating uncertainty and time discounting when calculating offset ratios for impacted habitat.Crossref | GoogleScholarGoogle Scholar |
Moreno-Mateos, D., Power, M. E., Comin, F. A., and Yockteng, R. (2012). Structural and functional loss in restored wetland ecosystems. PLoS Biology 10, 1–8.
| Structural and functional loss in restored wetland ecosystems.Crossref | GoogleScholarGoogle Scholar |
Morris, R. K. A., Alonso, I., Jefferson, R. G., and Kirby, K. J. (2006). The creation of compensatory habitat – can it secure sustainable development? Journal for Nature Conservation 14, 106–116.
| The creation of compensatory habitat – can it secure sustainable development?Crossref | GoogleScholarGoogle Scholar |
Murphy, B. F., and Timbal, B. (2008). A review of recent climate variability and climate change in southeastern Australia. International Journal of Climatology 28, 859–879.
| A review of recent climate variability and climate change in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Murray, J. V., Goldizen, A. W., O’Leary, R. A., McAlpine, C. A., Possingham, H. P., and Low Choy, S. (2009). How useful is expert opinion for predicting the distribution of a species within and beyond the region of expertise? A case study using brush-tailed rock wallabies Petrogale penicillata. Journal of Applied Ecology 46, 842–851.
| How useful is expert opinion for predicting the distribution of a species within and beyond the region of expertise? A case study using brush-tailed rock wallabies Petrogale penicillata.Crossref | GoogleScholarGoogle Scholar |
Nowack, E. M. (1998). Implications of nuisance rattlesnake relocation at Montezuma Castle National Monument. Sonoran Herpetologist 11, 2–5.
NRE (2000). Threatened vertebrate fauna in Victoria – 2000. Department of Natural Resources and Environment, East Melbourne.
Penman, T. D., Pike, D. A., Webb, J. K., and Shine, R. (2010). Predicting the impact of climate change on Australia’s most endangered snake, Hoplocephalus bungaroides. Diversity & Distributions 16, 109–118.
| Predicting the impact of climate change on Australia’s most endangered snake, Hoplocephalus bungaroides.Crossref | GoogleScholarGoogle Scholar |
Pickett, E. J., Stockwell, M. P., Bower, D. S., Garnham, J. I., Pollard, C. J., Clulow, J., and Mahoney, M. J. (2013). Achieving no net loss in habitat offsets of a threatened frog required high offset ratio and intensive monitoring. Biological Conservation 157, 156–162.
| Achieving no net loss in habitat offsets of a threatened frog required high offset ratio and intensive monitoring.Crossref | GoogleScholarGoogle Scholar |
Pimm, S. L., and Raven, P. (2000). Extinction by numbers. Nature 403, 843–845.
| Extinction by numbers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhs1Omt7k%3D&md5=2dbe7d46c85cb2a75d7956d1e78855c6CAS | 10706267PubMed |
Pimm, S. L., Jenkins, C. N., Abell, R., Brooks, T. M., Gittleman, J. L., Joppa, L. N., Raven, P. H., Roberts, C. M., and Sexton, J. O. (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 987.
| The biodiversity of species and their rates of extinction, distribution, and protection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXosFSmu7Y%3D&md5=4b968371cdd59430998c8edb81951cb0CAS |
Pincheira-Donoso, D., Treganza, T., Witt, M. J., and Hodgson, D. J. (2013). The evolution of viviparity opens opportunities for lizard radiation but drives it into a climatic cul-de-sac. Global Ecology and Biogeography , .
| The evolution of viviparity opens opportunities for lizard radiation but drives it into a climatic cul-de-sac.Crossref | GoogleScholarGoogle Scholar |
Platenberg, R. J., and Griffiths, R. A. (1999). Translocation of slow-worms (Anguis frangilis) as a mitigation strategy: a case study from south-eastern England. Biological Conservation 90, 125–132.
| Translocation of slow-worms (Anguis frangilis) as a mitigation strategy: a case study from south-eastern England.Crossref | GoogleScholarGoogle Scholar |
Plummer, M. V., and Mills, N. E. (2000). Spatial ecology and survivorship of resident and translocated hognose snakes (Heterodon platyrhinos). Journal of Herpetology 34, 565–575.
| Spatial ecology and survivorship of resident and translocated hognose snakes (Heterodon platyrhinos).Crossref | GoogleScholarGoogle Scholar |
Powell, C. J., and Sedunary, D. N. (2013). Development of a threatened fauna management framework across Victoria’ state forests. Australian Forestry 76, 10–15.
| Development of a threatened fauna management framework across Victoria’ state forests.Crossref | GoogleScholarGoogle Scholar |
Powney, G. D., Grenyer, R., Orme, C. D. L., Owens, I. P. F., and Meiri, S. (2010). Hot, dry and different: Australian lizard richness is unlike that of mammals, amphibians and birds. Global Ecology and Biogeography 19, 386–396.
| Hot, dry and different: Australian lizard richness is unlike that of mammals, amphibians and birds.Crossref | GoogleScholarGoogle Scholar |
Rawlinson, P. A. (1981). Conservation of Australian amphibian and reptile communities. In ‘Proceedings of the Melbourne Herpetological Symposium’. (Eds C.B. Banks and A. A. Martin.) pp. 127–138. Available from the library of the Arthur Rylah Institute for Environmental Research.
Read, J., and Bowen, Z. (2001). Population dynamics, diet and aspects of the biology of feral cats and foxes in arid South Australia. Wildlife Research 28, 195–203.
| Population dynamics, diet and aspects of the biology of feral cats and foxes in arid South Australia.Crossref | GoogleScholarGoogle Scholar |
Reading, C. J., Luiselli, L. M., Akani, G. C., Bonnet, X., Amori, G., Ballouard, J. M., Filippi, E., Naulleau, G., Pearson, D., and Rugiero, L. (2010). Are snake populations in widespread decline? Biology Letters 6, 777–780.
| Are snake populations in widespread decline?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cbmtlertw%3D%3D&md5=4f0edceb5182b97d711bdf918ae5412eCAS | 20534600PubMed |
Reed, R. N., and Shine, R. (2002). Lying in wait for extinction: ecological correlates of conservation status among Australian elapid snakes. Conservation Biology 16, 451–461.
| Lying in wait for extinction: ecological correlates of conservation status among Australian elapid snakes.Crossref | GoogleScholarGoogle Scholar |
Reinert, H. K. (1991). Translocation as a conservation strategy for amphibians and reptiles: some comments, concerns and observations. Herpetologica 47, 357–363.
Roberge, J.-M., and Angelstam, P. (2004). Usefulness of the umbrella species concept as a conservation tool. Conservation Biology 18, 76–85.
| Usefulness of the umbrella species concept as a conservation tool.Crossref | GoogleScholarGoogle Scholar |
Robertson, P., and Canessa, S. (2012). Review of monitoring of the hooded scaly-foot (Pygopus schraderi) at Lake Ranfurly, Mildura, 2002 to 2011. Report to Lower Murray Water by Wildlife Profiles Pty Ltd, Hurstbridge, Victoria.
Rodrigues, A. S., Brooks, T. M., Butchart, S. H. M., Chanson, J., Cox, N., Hoffmann, M, and Stuart, S. N (2014). Spatially explicit trends in the global conservation status of vertebrates. PLOS ONE , .
| Spatially explicit trends in the global conservation status of vertebrates.Crossref | GoogleScholarGoogle Scholar | 25426636PubMed |
Roughton, C. M., and Seddon, P. J. (2006). Estimating site occupancy and detectability of an endangered New Zealand lizard, the Otago skink (Oligosoma otagense). Wildlife Research 33, 193–198.
| Estimating site occupancy and detectability of an endangered New Zealand lizard, the Otago skink (Oligosoma otagense).Crossref | GoogleScholarGoogle Scholar |
Salt, D., and Possingham, H. (2013). Clash of the icons: who’s to choose? Decision Point 69, 4–5.
Sato, C. F., Schroder, M., Green, K., Michael, D. R., Osborne, W. S., and Lindenmayer, D. B. (2014a). Managing ski resorts to improve biodiversity conservation: Australian reptiles as a case study. Ecological Management & Restoration 15, 147–154.
| Managing ski resorts to improve biodiversity conservation: Australian reptiles as a case study.Crossref | GoogleScholarGoogle Scholar |
Sato, C. F., Wood, J. T., Schroder, M., Green, K., Osborne, W. S., Michael, D. R., and Lindenmayer, D. B. (2014b). An experiment to test key hypotheses of the drivers of reptile distribution in subalpine ski resorts. Journal of Applied Ecology 51, 13–22.
| An experiment to test key hypotheses of the drivers of reptile distribution in subalpine ski resorts.Crossref | GoogleScholarGoogle Scholar |
Scheele, B. C., Guarino, F., Osborne, W., Hunter, D. A., Skerratt, L. F., and Driscoll, D. A. (2014). Decline and re-expansion of an amphibian with high prevalence of chytrid fungus. Biological Conservation 170, 86–91.
| Decline and re-expansion of an amphibian with high prevalence of chytrid fungus.Crossref | GoogleScholarGoogle Scholar |
Shine, R. (1980). Reproduction, feeding and growth in the Australian burrowing snake Vermicella annulata. Journal of Herpetology 14, 71–77.
| Reproduction, feeding and growth in the Australian burrowing snake Vermicella annulata.Crossref | GoogleScholarGoogle Scholar |
Sinervo, B., Mendez-de-la-Cruz, F., Miles, D. B., Heulin, B., Bastiaans, E., et al. (2010). Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899.
| 1:CAS:528:DC%2BC3cXlvVeltrY%3D&md5=d25156dc56accb54bf0fd1ab767d26daCAS | 20466932PubMed |
Spencer, R.-J. (2002). Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles. Ecology 83, 2136–2144.
| Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., and Thompson, M. B. (2005). Experimental analysis of the impact of foxes on freshwater turtle populations. Conservation Biology 19, 845–854.
| Experimental analysis of the impact of foxes on freshwater turtle populations.Crossref | GoogleScholarGoogle Scholar |
Steffen, W., Crutzen, P. J., and McNeill, J. R. (2007). The Anthropocene: are humans now overwhelming the great forces of nature? AMBIO: A Journal of the Human Environment 36, 614–621.
| 1:CAS:528:DC%2BD1cXksFSqsrs%3D&md5=728a859ef3a8f666a58275c06502467dCAS |
Sullivan, B. K., Kwiatkowski, M. A., and Schuett, G. W. (2004). Translocation of urban gila monsters: a problematic conservation tool. Biological Conservation 117, 235–242.
| Translocation of urban gila monsters: a problematic conservation tool.Crossref | GoogleScholarGoogle Scholar |
Sullivan, B. K., Nowak, E. M., and Kwiatkowski, M. A. (2015). Problems with mitigation translocation of herpetofauna. Conservation Biology 29, 12–18.
| Problems with mitigation translocation of herpetofauna.Crossref | GoogleScholarGoogle Scholar | 25040040PubMed |
Thompson, M. B. (1983). Populations of the Murray River tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes. Australian Wildlife Research 10, 363–371.
| Populations of the Murray River tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
Towns, D. R., and Ferreira, S. M. (2001). Conservation of New Zealand lizards (Lacertilia: Scincidae) by translocation of small populations. Biological Conservation 98, 211–222.
| Conservation of New Zealand lizards (Lacertilia: Scincidae) by translocation of small populations.Crossref | GoogleScholarGoogle Scholar |
Victorian Auditor-General (2009). Administration of the Flora and Fauna Guarantee Act 1988. Victorian Government Printer.
Vincent, M., and Wilson, S. (1999). ‘Australian Goannas.’ (New Holland Publishers: Sydney.)
Wagler, R. (2011). The Anthropocene mass extinction: an emerging curriculum theme for science educators. The American Biology Teacher 73, 78–83.
| The Anthropocene mass extinction: an emerging curriculum theme for science educators.Crossref | GoogleScholarGoogle Scholar |
Walker, M. L., Dorr, J. A., Benjamin, R. J., and Pisani, G. R. (2009a). Successful relocation of a threatened suburban population of timber rattlesnakes (Crotalus horridus): combining snake ecology, politics and education. IRCF Reptiles & Amphibians 16, 210–221.
Walker, S., Brower, A. L., Theo Stephens, R. T., and Lee, W. G. (2009b). Why bartering biodiversity fails. Conservation Letters 2, 149–157.
| Why bartering biodiversity fails.Crossref | GoogleScholarGoogle Scholar |
Walsh, J. C., Watson, J. E. M., Bottrill, M. C., Joseph, L. N., and Possingham, H. (2013). Trends and biases in the listing and recovery planning for threatened species: an Australian case study. Oryx 47, 134–143.
| Trends and biases in the listing and recovery planning for threatened species: an Australian case study.Crossref | GoogleScholarGoogle Scholar |
Webb, J. K., and Shine, R. (1993). Dietary habits of Australian blindsnakes (Typhlopidae). Copeia 1993, 762–770.
| Dietary habits of Australian blindsnakes (Typhlopidae).Crossref | GoogleScholarGoogle Scholar |
Webb, J. K., and Shine, R. (2000). Paving the way for habitat restoration: can artificial rocks restore degraded habitats of endangered reptiles? Biological Conservation 92, 93–99.
| Paving the way for habitat restoration: can artificial rocks restore degraded habitats of endangered reptiles?Crossref | GoogleScholarGoogle Scholar |
Wheeler, T. A. (2003). The role of voucher specimens in validating faunistic and ecological research. Document series no. 9. Biological Survey of Canada (Terrestrial Arthropods). Ottawa, Canada.
While, G. M., Uller, T., and Wapstra, E. (2009). Family conflict and the evolution of sociality in reptiles. Behavioural Ecology 20, 245–250.
| Family conflict and the evolution of sociality in reptiles.Crossref | GoogleScholarGoogle Scholar |
Wilson, S., and Swan, G. (2013). ‘A Complete Guide to Reptiles of Australia.’ 4th edn. (New Holland: Sydney.)