Growth characteristics associated with biomass production in three varieties of Trichloris crinita (Poaceae), a forage grass native to the arid regions of Argentina
S. A. Greco A and J. B. Cavagnaro A BA Fac. Ciencias Agrarias. – Univ. Nacional de Cuyo. Alte Brown 500. Chacras de Coria. (5505) Mendoza, Argentina.
B Corresponding author. Email: bcavagnaro@fca.uncu.edu.ar
The Rangeland Journal 27(2) 135-142 https://doi.org/10.1071/RJ05011
Submitted: 15 September 2004 Accepted: 12 April 2005 Published: 21 November 2005
Abstract
Trichloris crinita (Lag.) Parodi is an important perennial native grass widespread in the range areas of the arid and semi-arid phytogeographical region of Monte, Argentina. Previous studies have shown great variability in forage biomass production per plant among different varieties of this species. The aim of this work was to assess which morphological and physiological traits are associated with differential productivity of T. crinita varieties. Three varieties: Pichi, of high productivity, Arroyito, of medium productivity, and Encon, of low productivity were tested in a field experiment. Dry matter (DM) produced by different organs, assimilates partitioning, and leaf area per plant were measured on three different dates for each variety, during an annual growth cycle, under watered conditions. Relative growth rate (RGR), net assimilation rate (NAR), leaf area ratio (LAR), specific leaf area (SLA), leaf weight ratio (LWR) and leaf area development rate (LADR) were calculated at 72, 128 and 172 days after transplanting. Significant differences among varieties were found for DM production of blades, sheaths + culms, panicles, roots and shoot/root ratio. Pichi and Arroyito produced more total plant biomass than Encon and this was associated with higher dry matter accumulation in aboveground organs and larger leaf area. LADR, LAR and one of its components SLA were the parameters that best explained differences in biomass production. DM partitioning to roots (considered as the percentage of total DM) was very high in Encon, the least productive variety. Differences in productivity seem to be directly associated with the mean annual rainfall and inversely associated with the mean annual temperature of the environment where plants were collected. Thus, the growth characteristics of each variety reflect differential adaptation to their areas of origin.
Additional keywords: intraspecific variability, partitioning, specific leaf area.
Introduction
Native grasses are important for range grazing in arid and semi-arid regions, as they are the main forage resources in these ecosystems. Species from arid and semiarid regions have special features that may confer advantages for higher productivity under stress conditions (Turner 1979). Varieties or ecotypes from the same grass species frequently show variation in morphological and physiological traits that may account for their differential performances under such environments. Therefore, in order to perform an efficient plant breeding selection, it is important to know which traits account for differences in productivity. In particular, it is important to focus on those processes directly involved in yield (Hall 1980; Boyer 1982).
Trichloris crinita (synonymous = Chloris crinita), which is a C4 species, is one of the most important perennial native grasses in the west arid region of Argentina known as ‘Monte’ (Cavagnaro 1988), mainly due to its forage quality and its wide area of distribution (Waistein and González 1969; Roig 1971). Previous work comparing 18 varieties of this species in three different environments within the Monte Region showed great differences in forage production (Cavagnaro et al. 1989; Passera et al. 1997). Here, annual dry matter production per plant varied 10-fold between the least (20 g) and most (205 g) productive varieties.
In some species, total dry matter production is not always associated with photosynthetic rate per unit of leaf area. Instead, it may depend on many factors including life-history characteristics, canopy structure, respiration rates, translocation and partitioning of assimilates and environmental conditions (Nasyrov 1978; Lambers 1987; Poorter et al. 1991; Poorter and Pothmann 1992; Reich 1998). Newly-developed varieties of most crop species may have the same total plant biomass as old ones but differ in the proportion of biomass allocated to each organ. Gifford and Evans (1981) and Gifford et al. (1984) demonstrated that increased yield in modern varieties of cereals, soybean, etc., is not due to higher photosynthetic rates or higher total biomass, but rather, to a larger assimilate partitioning to sink organs that are of interest for human consumption: i.e. they had a larger harvest index. Based on those papers, our first hypothesis was that the higher productivity shown by some varieties of T. crinita was due to a larger DM partitioning to shoots than to roots.
In addition to differences in DM partitioning, we demonstrated that those varieties of T. crinita with higher productivity showed a higher total dry matter production per plant (Greco and Cavagnaro 2003), which was not found by Gifford and Evans (1981) and Gifford et al. (1984) with some crops species. Thus, we also postulate that their higher biomass production could be due to an increased rate of leaf area development.
Plants species may differ greatly in their inherent growth rate, even when they are grown under optimal conditions. Genetic variations responsible for differences in growth rate arise from evolutionary selection under diverse environments. In general, species from rich environments present higher growth rate than those from poor sites (Grime and Hunt 1975; Lambers 1987; Poorter and Remkes 1990; Hunt and Cornelissen 1997; Reich 1998; Poorter and Nagel 2000). A higher dry matter production has been associated with certain morphological and physiological leaf characteristics such as net assimilation rate (NAR, increase in plant DM per unit of leaf area and unit of time), leaf area ratio (LAR, ratio total leaf area/total plant weight), specific leaf area (SLA, ratio leaf area/leaf weight), and leaf weight ratio (LWR, fraction of plant biomass allocated to leaves) (Lambers 1987; Poorter and Remkes 1990; Garnier 1992; Hunt and Cornelissen 1997; Poorter and Evans 1998; Poorter and Nagel 2000; Evans and Poorter 2001).
According to Schulze (1983), differences in partitioning among different life forms could have provided adaptive advantages to species under certain environmental conditions. This work deals with only one life form, perennial grasses, but with three varieties differing in productivity. We also are interested in determining whether the differences in productivity are associated with differences in temperature and rainfall in their native environments.
The objective of this work was to investigate which traits are associated with higher DM production in three varieties of Trichloris crinita contrasting in their biomass production. The relationships among these traits and the environment of each variety are also discussed.
Materials and methods
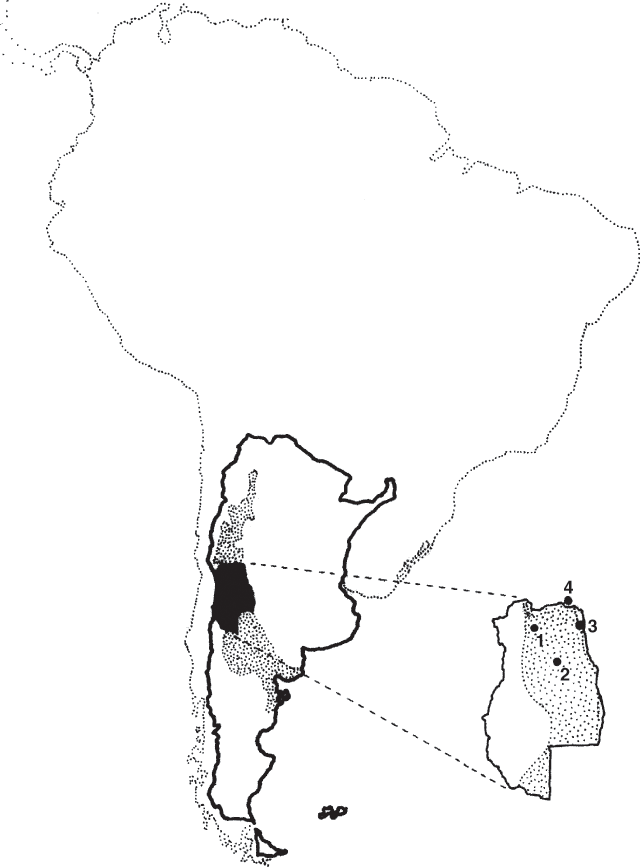
The phytogeographical province of ‘Monte’ is an extended north–south area located along the eastern base of the Andes Mountains in Argentina (Fig. 1). It is a shrubland dominated by species of the genus Larrea interspersed with grasses and other herbaceous species (Morello 1958; Cabrera 1976) as well as a few tree species, mostly of Prosopis genus. The latter is mainly found in areas where groundwater is present. A comprehensive description of the structure and function of the flora and fauna in this warm desert has been compiled by Orians and Solbrig (1977). Under natural conditions Trichloris crinita (Lag.) Parodi behaves as a typical aestival species growing whenever soil water is available and temperature is above 10°C (Seligman et al. 1992).
|
The trial was conducted in the experimental field of IADIZA (Instituto Argentino de Investigación de Zonas Aridas), Mendoza, Argentina (32°53′ S; 68°51′ W; 827 m altitude) (Fig. 1). Mean annual rainfall is 245 mm, occurring mainly in summer. Mean temperature is 8°C in July, and 24°C in January. South-easterly winds are predominant at a mean velocity of 9.5 km/h
Varieties used in this trial originated from seeds obtained from a single plant and propagated during five generations. We called these plant materials ‘varieties’ rather than ‘ecotypes’ since they may not represent the whole sampled population, but only a fraction of it (King and Standfield 1997). The mother plant was chosen because it was the most frequently observed phenotype of a certain population. These varieties did not segregate after 5 generations under open pollination conditions so we suppose they probably are autogamous or apomictic. Populations were collected in different environments of the Monte (Fig. 1).
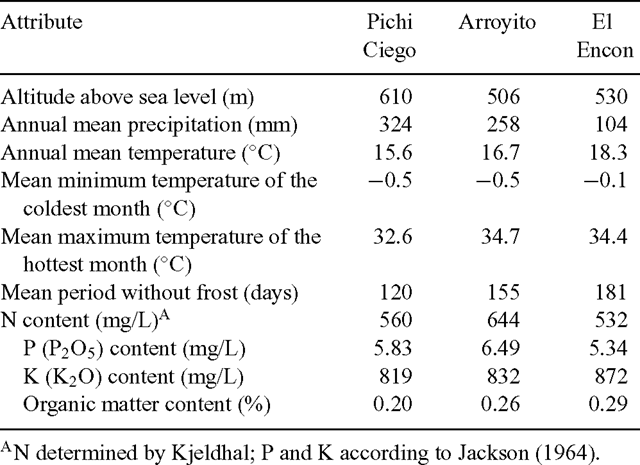
Three varieties of different productivities were assayed: (1) Pichi, of high productivity, (2) Arroyito, of medium productivity, and (3) Encon, of low productivity; from now on referred to as Pichi, Arroyito and Encon, respectively. The mean aboveground plant biomass evaluated in a 3-year trial performed in 3 sites of the Monte, was 205, 90 and 40 g DM/plant.year for Pichi, Arroyito and Encon, respectively (Cavagnaro et al. 1989). The name ‘Pichi’ derives from Pichi Ciego, Mendoza (33°50′ S, 68°12′ W); ‘Arroyito’ from Arroyito, Mendoza, (32°50′ S, 67°27′ W) and ‘Encon’ from El Encon, San Juan, (32°09′ S, 67°59′ W) (Fig. 1). The most important climatic variables and soil characteristics of these sites are described in Table 1. The nearest meteorological stations are located 52, 70 and 1 km away from Pichi Ciego, Arroyito and El Encon, respectively. In general, rainfall decreases and temperature increases from Pichi Ciego to Arroyito and from Arroyito to El Encon. The xeric conditions follow a similar trend increasing from Pichi Ciego to El Encon.
|
Seeds of each variety were sown in seedling boxes filled with sandy-loam soil previously sterilised with methyl bromide. When plants reached an height of 4–5 cm, they were transplanted to 330 cm3 plastic pots and filled with the same nursery soil. When plants had 4–5 tillers they were transplanted to the experimental field. Physico-chemical characteristics of the soil in the experimental field were: electrical conductivity 785 µS/cm; pH 7.11; N 441 mg/kg (determined by Kjeldhal); P (P2O5) 10.4 mg/kg (Jackson 1964); K (K2O) 1084 mg/kg (Jackson 1964); texture: sandy; field capacity: 0.143 g/g (at a water potential of –0.01 MPa); wilting point 0.07 g/g (at a water potential of –1.5 MPa). Sandy soil is the typical soil where the varieties were collected.
Treatment plots were distributed in a randomized block-design with 8 replications. Each plot had an area of 10.50 m2, with 14 plants, 1.00 × 0.75 m apart to prevent any competition among plants. Plants were irrigated weekly. Entire plants were harvested 75, 128 and 172 days after being transplanted (DAT). Eight plants from each variety (1 per plot) were harvested on the first and second sampling dates and 2 plants per plot on the last date. Aboveground material was divided into leaf blades, sheaths + culms, and panicles and oven-dried at 70°C until constant weight. Four plants per variety were used for leaf area measurement using a leaf area meter (Li-Cor Mod. 3000A, Li-Cor, Lincoln, NE) and determination of DM. A regression equation between the two variables was calculated. Subsequent leaf areas values were estimated using the varieties’ leaf DM values and the corresponding equation.
We calculated most of the parameters used in quantitative analysis of plant growth (Evans 1972; Hunt 1978) to investigate which of them explain best differences in DM production of the varieties.
The parameters were calculated for the periods corresponding to the three sampling dates, according to the classical approach (Hunt 1978).
-
RGR (relative growth rate) = increase in plant DM per unit DM and unit time (mg/g.day).
-
NAR (net assimilation rate) = increase in plant DM per unit leaf area and unit time (g/m.day).
-
LAR (leaf area ratio) = plant leaf area/plant total DM (m2/g).
-
SLA (specific leaf area) = leaf area/leaf DM (m2/g).
-
LWR (leaf weight ratio) = leaf DM/plant DM (g/g).
-
LARD (leaf area rate development) = increase in leaf area per unit time (mm2/day).
Roots were collected by washing the soil on top of a 0.6 mm screen. Subsequently the samples were oven-dried at 70°C. On the first sampling date, the total soil volume explored by the roots was used. On the second and third dates (128 and 172 DAT), soil samples were obtained with a core sampler at 0, 20 and 40 cm from the centre of each plant at 2 depths: 0.0–0.20 m and 0.20–0.40 m, according to Bohm (1979). Total root biomass was obtained by extrapolation of the root biomass from the sampled cores to the total soil volume explored by roots.
ANOVA and Tukey’s test at P ≤ 0.05 was used to compare means. Dry matter data were transformed by log (x + 1) (Poorter and Nagel 2000) and LADR by ln to fulfill ANOVA requirements.
Results
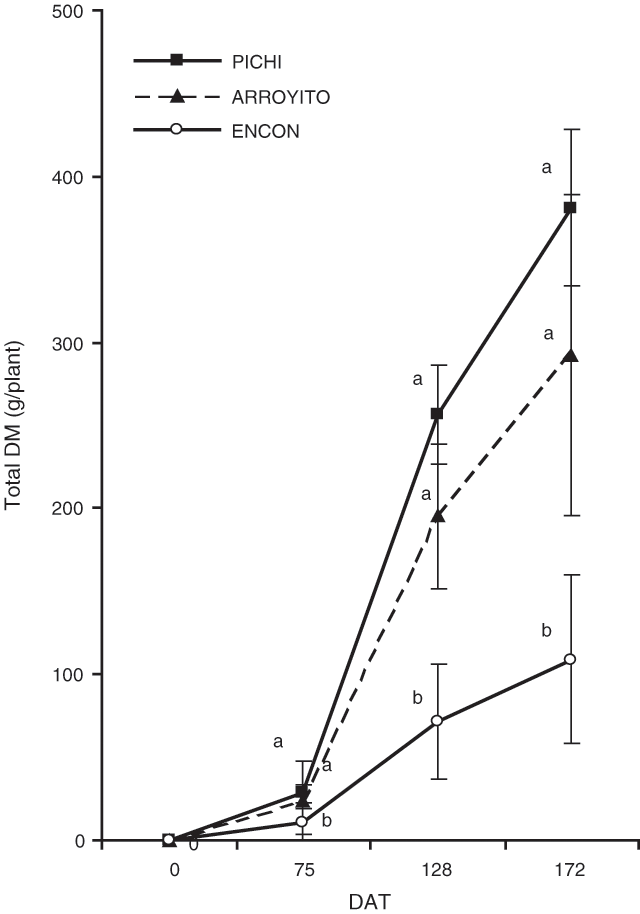
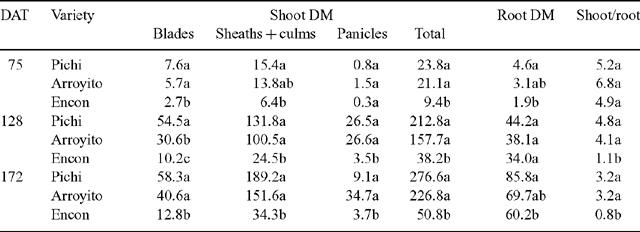
Total plant DM of Pichi and Arroyito (Fig. 2) was significantly higher than that of Encon for all sampling dates. Total plant biomass of Encon was only 39% on the first sampling date and 18% on the following sampling dates compared with the most productive variety Pichi (Table 2). Considering shoot DM components, Pichi was higher than Encon for all organs at each date, except for panicles at the first date. Arroyito presented a similar pattern to Pichi. These two varieties showed no differences between them except for blades on the second date (Table 2).
|
|
Shoot/root ratio of Encon was significantly lower than the other 2 varieties on the second and third dates (Table 2). This low ratio was associated with a low shoot DM of Encon, since it was only 18% of that observed for Pichi in the final harvest. In contrast, root DM of Encon on the same date (60.2 g/plant), although significantly lower than Pichi, was equivalent to 70% of the latter.
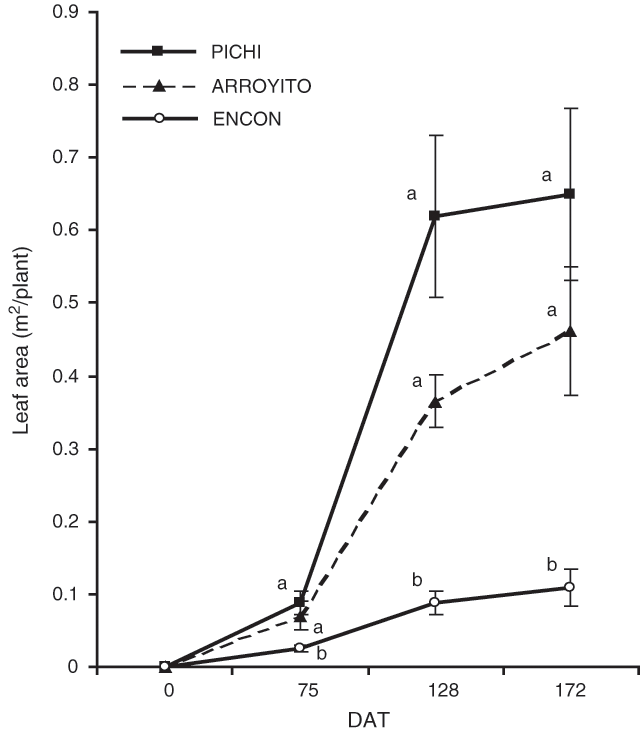
Leaf area values of Pichi and Arroyito (Fig. 3) were higher than Encon for all dates considered. Interestingly, a fast leaf area development was recorded for Pichi, as shown by the slope of the lines between dates and data corresponding to LADR (Table 3). The final leaf area was six times larger in Pichi than in Encon.
|
|
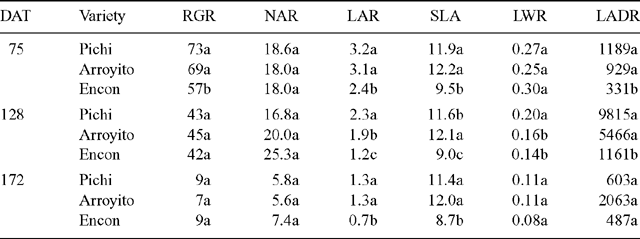
Despite the large differences among varieties in DM production on all sampling dates, the varieties’ RGRs differed only in the first period (0–75 DAT). Here, RGR was very low at the end of the annual cycle (third period) for all varieties. The varieties did not differ in NAR. Pichi and Arroyito showed significantly higher LAR than Encon for each of the three DAT (Table 3). Also SLA, one of the components of LAR, was higher in the more productive varieties (Pichi and Arroyito), but leaf biomass, relative to total plant biomass (LWR), was different only on the second date. Significant differences in LADR were found among varieties in the first and second period. The rate of leaf area development of Pichi was eight times higher than that of Encon for this latter period.
The lower biomass and smaller leaf area per plant as well as the lower specific leaf area of Encon, suggests that this variety possesses a less developed photosynthetic area per gram of biomass allocated in the leaves.
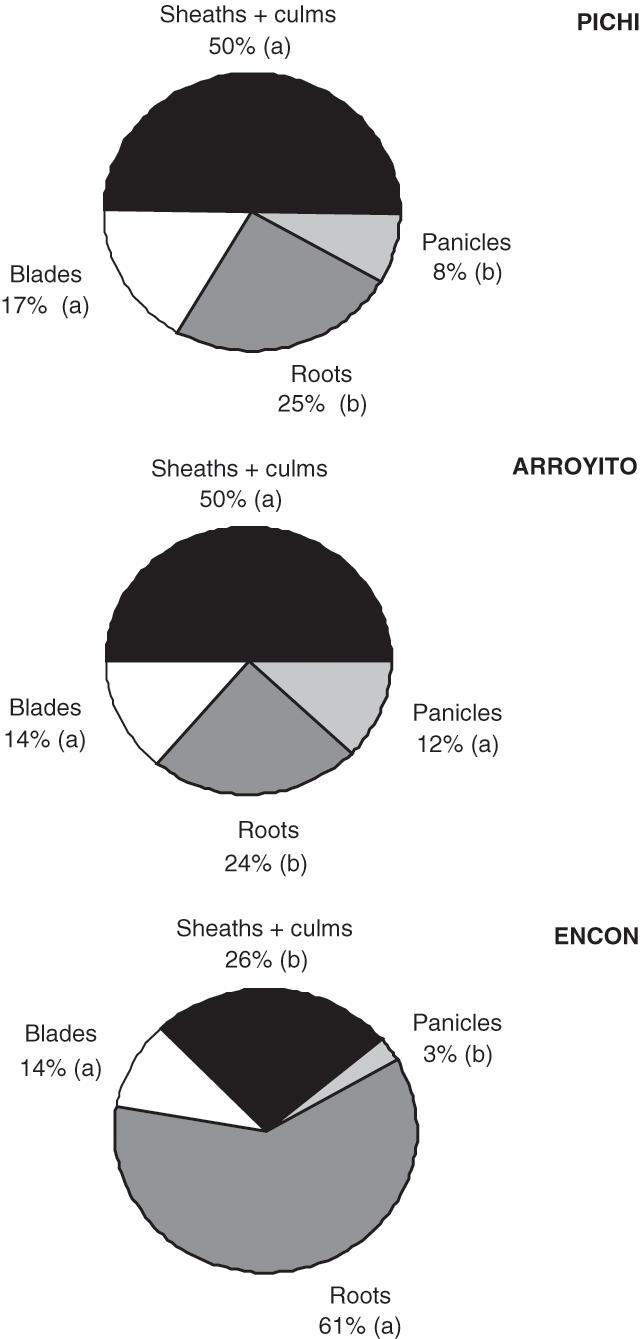
Considering DM partitioning to different organs, expressed as the percentage of plant total DM (Fig. 2), we observed differences in partitioning to sheaths + culms, and panicles and roots, but no differences among varieties in the proportion of biomass allocated to leaf blades, was noted. Compared with Encon, that sent more than 60% of the total biomass to roots, Pichi and Arroyito partitioned about 50% of DM to sheaths and culms. Arroyito partitioned the largest proportion to panicles.
Discussion
The results confirmed our hypothesis that the higher aboveground productivity of some varieties of T. crinita was due to a larger DM partitioning to shoots than to roots. Varieties Pichi and Arroyito, with high and medium productivity respectively, partitioned more DM to shoots relative to root, as compared with the less productive variety (Encon). We also have shown that the higher total plant biomass produced by Pichi and Arroyito can be explained by a larger leaf area and higher LADR (Fig. 3; Table 3) as postulated in the second hypothesis.
The low DM of Encon can be interpreted in various ways. A first approach is to consider total DM accumulation as a function of the radiation intercepted by the leaves through the conversion of that radiation to dry matter (Monteith 1977). The amount of light intercepted by leaves depends, to a great extent, on the total leaf area developed by a plant, the foliage duration and the morphology and spatial disposition of leaves. The varieties tested here did not differ in foliage persistence, which was 195 days, but they did differ in the production of total leaf area, being significantly lower for the least productive variety. Compared with the other varieties, the rate of leaf area development in Encon was also slower (Fig. 3). Thus, both factors could be contributing to a reduced light interception. Similar results were obtained by McNaughton (1974) using varieties of Typha latifolia.
The more productive varieties (Pichi and Arroyito) had a higher LAR indicating that they have a larger photosynthetic area per gram of total plant biomass. These results are in agreement with those reported by other authors (Poorter and Remkes 1990; Poorter and Pothmann 1992; Reich 1998). Since LAR = LWR × SLA (Hunt 1978), and since the varieties did not differ in LWR (except for the second date), the differences that we observed in photosynthetic areas do not mean that Pichi and Arroyito invested more biomass in producing leaf blades. Instead, they reflect a larger leaf area per gram of DM invested in leaves (SLA) (Table 3; Fig. 4). These results are in agreement with those of Poorter and Lambers (1991), who found that differences in growth rates among 24 herbaceous species were mainly associated with variations in SLA rather than to differences in LWR. Dijkstra and Lambers (1989), working with two lines of Plantago major, and Poorter and Remkes (1990), Garnier (1992), Poorter and Pothmann (1992) and Reich (1998) studying different groups of species came to similar conclusions. A strong correlation between LAR and SLA was also found in herbaceous monocotyledons (Hunt and Cornelissen 1997).
|
Varieties’ RGRs were different only on the first DAT, but this parameter cannot explain the great differences in biomass production during the second one. This may be due to its importance in the exponential phase of growth but later on RGR is not a good indicator for growth (Loomis and Connor 1992). NAR could not explain differences in DM production either.
The least productive variety invested 60% of its biomass in roots, while the other two invested mainly in sheaths + culms. The larger proportion of roots in Encon suggests less efficiency in the use of radiation because of greater respiratory losses by roots; contrarily, the large proportion of sheaths + culms in Pichi and Arroyito would mean an extra contribution of assimilates through photosynthesis by these organs. In an experiment with Agropyron, Caldwell et al. (1981) showed that sheaths + culms contributed more than 50% to the total photosynthesis.
The hypothesis concerning differential assimilate partitioning to organs in forages was based on differences in productivity found among old and modern cultivars (Gifford et al. 1984). In such crop species, productivity increased as harvest index increased. In the case of Trichloris crinita, the harvest index (considered as shoot DM) was also higher for those varieties with higher productivity. However, considering that this is a native forage species where no breeding programs have been carried out it is expected that varieties have evolved achieving an optimal shoot/root ratio, thus increasing their adaptation to the native environments (Gleeson and Tilman 1992).
Our results showed that the lower biomass production by Encon plants is associated with smaller leaf area, slower leaf area development, less developed photosynthetic area per gram of biomass produced, and probably higher respiratory cost due to a larger proportion of roots. All these traits can be interpreted as adaptations to the more xeric conditions found in its area.
Increased root/shoot ratio can be the consequence of nutrient deficiency, lack of water in the soil, or temperatures unfavourable for optimal root functioning (Brouwer and de Wit 1969; Poorter and Nagel 2000). Therefore, we can speculate that the larger investment in root biomass observed in Encon might represent a physiological mechanism adapting this genotype to its native area of scarce rainfall (104 mm/year) high temperatures (18.3°C mean annual temperature) and nutrient-deficient soils (see Tables 1 and 2). Under these conditions, a larger root system will assure a better exploitation of soil resources (water and nutrients), while a limited leaf area development would be advantageous for avoiding water losses through transpiration. Both types of adaptations could increase survival in such a dry and rather unfertile site (Grime 1979). Similarly, along an aridity gradient in Patagonia, Schulze et al. (1996) found that plant total biomass decreased as precipitation decreased, but belowground biomass decreased at a lower rate than aboveground biomass, resulting in increasing root/shoot ratios. Fernández and Reynolds (2000) studying growth parameters and drought tolerance in eight desert grasses did not find a trade-off between total biomass or root/shoot ratios and drought tolerance. Instead, in this experiment, the increases in the root/shoot ratio showed by Encon implied a trade-off in total dry matter production.
The lower SLA of Encon is another trait associated with an adaptation to arid conditions, according to Tsunoda (1978).
Congruently, Pichi, which has a larger leaf area and a smaller proportion of roots, comes from an area with higher rainfall and lower temperatures (324 mm/year and 15.6°C mean annual temperature). This feature allows Pichi plants to achieve a very high biomass production whenever appropriate temperatures and rainfall are available, as this occurs more often in the region where this variety belongs (Seligman et al. 1992).
These results will benefit future programs oriented to the re-vegetation of degraded areas of the Monte by providing an objective means for choosing varieties – based on productivity and adaptation - suitable for different environments within the Monte region.
In summary, the largest above ground productivity of PICHI is associated with higher shoot/root ratio and higher total DM production, which, in turn, is due to a larger leaf area production and higher rate of leaf area development. These traits have evolved in areas of the Monte with lower temperatures and higher rainfall. Instead, under more xeric conditions (higher temperature and lower rainfall) plants have evolved to have a lower leaf area and a higher root/shoot ratio allowing, thus, a more thorough soil exploration with a more extended root system. These seem to be among the best adaptations to couple with severe drought conditions, as observed in the variety Encon.
Acknowledgments
This research was supported by CONICET and Universidad Nacional de Cuyo, Argentina. We thank Dr A. H. Hall and Dr Kent Bradford for their comments on the manuscript. We are grateful to H. Morales for his valuable technical assistance, and to N. Horak, J. Lucero, M. Delugan and M. Paez for their kind collaboration.
Bohm, W. (1979).
Boyer J. S.
(1982) Plant productivity and environment. Science 218, 443–448.

Brouwer R., de Wit C. T.
(1969) A simulation model of plant growth with special attention to root growth and its consequences. : ‘Root growth. Proceedings 15th Eastern School Agricultural Science’. University of Nottingham, UK. (Ed. W. J. Whitington )
pp. 224–242. (Butterworths: London.)
Caldwell M. M.,
Richards J. H.,
Johnson D. A.,
Nowak R. S., Dzurec R. S.
(1981) Coping with herbivory: photosynthetic capacity and resource allocation in two semiarid Agropyron bunchgrasses. Oecologia 50, 14–24.
| Crossref | GoogleScholarGoogle Scholar |

Cavagnaro J. B.
(1988) Distribution of C3 and C4 grasses at different altitudes in a temperate arid region of Argentina. Oecologia 76, 273–277.
| Crossref | GoogleScholarGoogle Scholar |

Cavagnaro J. B., Lemes J., Ventura J. L., Passera C. B.
(1989) Variabilidad ecotípica y producción forrajera de Trichloris crinita. : ‘Resúmenes 14th Congreso de Produccion Animal. (supl.1)’. (Asociacion Argentina de Produccion Animal: Buenos Aires.)
Dijkstra P., Lambers H.
(1989) Analysis of specific leaf area and photosynthesis of two inbred lines of Plantago major differing in relative growth rate. New Phytologist 113, 283–290.

Evans, G. C. (1972).
Evans J. R., Poorter H.
(2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant, Cell and Environment 24, 755–767.
| Crossref | GoogleScholarGoogle Scholar |

Fernández R. G., Reynolds J. F.
(2000) Potential growth and drought tolerance of eight desert grasses: lack of trade off? Oecologia 123, 90–98.
| Crossref | GoogleScholarGoogle Scholar |

Garnier E.
(1992) Growth analysis of congeneric annual and perennial grass species. Journal of Ecology 80, 665–675.

Gifford R. M., Evans L. T.
(1981) Photosynthesis, carbon partitioning and yield. Annual Review of Plant Physiology 32, 485–489.
| Crossref | GoogleScholarGoogle Scholar |

Gifford R. M.,
Thorne J. H.,
Hitz W. D., Giaquinta R.
(1984) Crop productivity and photoassimilate partitioning. Science 225, 801–808.

Gleeson S., Tilman D.
(1992) Plant allocation and the multiple limitation hypothesis. American Naturalist 139, 1322–1343.
| Crossref | GoogleScholarGoogle Scholar |

Greco S. A., Cavagnaro J. B.
(2003) Effects of drought in biomass production and allocation in three varieties of Trichloris crinita P. (Poaceae) a forage grass from the arid Monte region of Argentina. Plant Ecology 164, 125–135.
| Crossref | GoogleScholarGoogle Scholar |

Grime, J. P. (1979).
Grime J. P., Hunt R.
(1975) Relative growth-rate: its range and adaptive significance in a local flora. Journal of Ecology 63, 393–422.

Hall A. J.
(1980) Los componentes fisiológicos del rendimiento de los cultivos. Revista de la Facultad de Agronomia de la Universidad de Buenos Aires 1, 73–86.

Hunt, R. (1978).
Hunt R., Cornelissen J. H. C.
(1997) Components of relative growth rate and their interrelations in 59 temperate plant species. New Phytologist 135, 395–417.
| Crossref | GoogleScholarGoogle Scholar |

Jackson, M. L. (1964).
King, R. C. ,
and
Standfield, W. C. (1997).
Lambers H.
(1987) Does variation in photosynthetic rate explain variation in growth rate? Netherland Journal of Agricultural Science 35, 505–519.

Loomis, R. S. ,
and
Connor, D. J. (1992).
McNaughton S. J.
(1974) Developmental control of net productivity in Typha latifolia varieties. Ecology 55, 864–869.

Monteith J.L.
(1977) Climate and the efficiency of crop production in Britain. Philosophical Transactions of the Royal Society London B 281, 277–294.

Morello J.
(1958) La provincia fitogeográfica del Monte. Opera Lilloana 2, 1–155.

Nasyrov Y.
(1978) Genetic control of photosynthesis and improving of crop productivity. Annual Review of Plant Physiology 29, 215–237.
| Crossref | GoogleScholarGoogle Scholar |

Orians G. H., Solbrig O. T.
(1977) An evolutionary approach to ecosystems. : ‘Convergent evolution in warm deserts’. (Eds G. H. Orians, O. T. Solbrig)
pp. 1–12. (Dowden, Hutchinson and Ross Inc.: Stroudsburg.)
Passera C.,
Cavagnaro J. B.,
Lemes J., Allegretti L.
(1997) Gramíneas nativas de zonas áridas, banco de germoplasma y selección de ecotipos en el Monte, Argentina. Actae Etnobotánica [Córdoba, España] 92, 181–189.

Poorter H., Remkes C.
(1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83, 553–559.
| Crossref | GoogleScholarGoogle Scholar |

Poorter H., Lambers H.
(1991) Is interspecific variation in relative growth rate positively correlated with biomass allocation to the leaves? American Naturalist 138, 1264–1268.
| Crossref | GoogleScholarGoogle Scholar |

Poorter H.,
van der Werf A.,
Atkin K., Lambers H.
(1991) Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiologia Plantarum 83, 469–475.
| Crossref | GoogleScholarGoogle Scholar |

Poorter H., Pothmann P.
(1992) Growth and carbon economy of fast-growing and slow-growing grass species as dependent on ontogeny. New Phytologist 120, 159–166.

Poorter H., Nagel O.
(2000) The role of biomass allocation in the growth responses of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology 27, 595–607.

Poorter H., Evans J. R.
(1998) Photosynthetic nitrogen use efficiency of species that differ inherently in specific leaf area. Oecologia 116, 26–37.
| Crossref | GoogleScholarGoogle Scholar |

Reich P. B.
(1998) Variation among plant species in leaf turnover rates and associated traits: implications for growth at all life stages. : ‘Inherent variation in plant growth. Physiological mechanisms and ecological consequences’. (Eds H. Lambers, H. Poorter, M. M. I. Van Vuren)
pp. 476–487. (Backhuys: Leiden.)
Roig F. A.
(1971) Flora y. vegetación de la Reserva Forestal de Ñacuñan. Deserta 1, 25–232.

Schulze E. D.
(1983) Root–shoot interactions and plant life forms. Netherland Journal Agricultural Science 4, 291–303.

Schulze E. D.,
Mooney H. A.,
Sala O. E.,
Jobbagy E., Buchmann N. , et al.
(1996) Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 108, 503–511.
| Crossref | GoogleScholarGoogle Scholar |

Seligman N.,
Cavagnaro J. B., Horno M.
(1992) Simulation of defoliation effects on primary production of a warm season semiarid perennial species grassland. Ecological Modelling 60, 45–61.
| Crossref | GoogleScholarGoogle Scholar |

Tsunoda S.
(1978) Adaptive differentiation in photosynthetic properties in wheat. : ‘Proceedings of 5th Internacional Wheat Genetics Symposium’. New Delhi, India
Turner N. C.
(1979) Drought resistance and adaptation to water deficits in crop plants. : ‘Stress physiology in crop plants’. (Eds H. Mussell, R. C. Staples)
pp. 343–372. (John Wiley and Sons: New York)
Waistein P., González S.
(1969) Valor nutritivo de forrajeras del Este de la provincia de Mendoza, Reserva Ecológica de Ñacuñán. I. Revista Facultad de Ciencias Agrarias, Universidad Nacional de Cuyo. XV(
), 133–142.



