Death model for tussock perennial grasses: a rainfall threshold for survival and evidence for landscape control of death in drought
K. C. Hodgkinson A C and W. J. Muller BA CSIRO Sustainable Ecosystems, GPO Box 284, Canberra, ACT 2601, Australia.
B CSIRO Mathematical and Information Sciences, GPO Box 664, Canberra, ACT, 2601, Australia.
C Corresponding author. Email: ken.hodgkinson@csiro.au
The Rangeland Journal 27(2) 105-115 https://doi.org/10.1071/RJ05009
Submitted: 12 October 2004 Accepted: 24 August 2005 Published: 21 November 2005
Abstract
We investigated relationships between rainfall (and landscape, zonation and nearby grazing disturbance) and the death rates of four perennial grass species in a highly functional semi-arid wooded grassland in eastern Australia. Two grasses were palatable C3 species (Monachather paradoxa Steud. and Thyridolepis mitchelliana (Nees) S. T. Blake) and two were unpalatable C4 species (Aristida jerichoensis (Domin) Henr. var. subspinulifera Henr. and Eragrostis eriopoda Benth.). During the 10-year study the grasses were protected from large herbivore grazing within paddocks continuously grazed by sheep. Death occurred only during droughts and rates of death were species-dependent. When plotted against several water availability indices, rainfall and rainfall/evaporation during the preceding 3 months provided best predictions of death. Longer preceding periods gave inferior predictions. A 3-month rainfall total of 75 mm and a 3-month rainfall/evaporation ratio of 0.15 were survival critical thresholds below which deaths began. The 3-month rainfall totals, rainfall/evaporation and estimated water status of plants were equally reasonable predictors of deaths, but were inconsistent in their effectiveness. Rainfall was adopted for the grass death model; death begins when 3-month rainfall total declines below a threshold of 75 mm and the death rate rises with lower rainfall. Position of plants in the gently undulating landscapes influenced water status and, hence, death rates. Water status of grasses on the two water-shedding zones and the ‘flat’ zone were similar at each assessment, but higher on ‘ridge run-on’ and ‘toe-of-slope’ zones. Foliage height and diameter also influenced death rate but were species dependent. Basal diameter did not influence death rate. Survivorship of several perennial grass species at widely spaced sites in south-eastern Australia provided equivocal support for generality of the grass death model.
Additional keywords: arid, critical threshold, grassland, rangeland, temperate.
Introduction
Most ecological and physiological processes occurring in the landscapes of mid-latitude arid regions are event driven (Noy-Meir 1973; Stafford Smith and Morton 1990). Germination, growth and reproduction in plant populations in these regions are driven by rainfall, with each functional type requiring different amounts and durations of rainfall to trigger successive stages in phenology (Westoby 1979/80; Sala and Lauenroth 1982; Schwinning et al. 2003). Event-driven processes in arid regions underpin the dynamics of the trigger-transfer-reserve-pulse landscape model (Ludwig et al. 1997) that evolved from the earlier ‘pulse-and-reserve’ model of Noy-Meir (1973). If the level of disturbance from grazing of herbivores is too high in arid regions then these event-driven ecosystems may easily change to a less productive but stable system as conceived in the state-and-transition vegetation model developed by Westoby et al. (1989). These authors suggest that opportunistic managements, such as prescribed fire and reduction in grazing pressure, may be required to prevent transition to other ‘states’. Both conceptual models focus on changes in temporal and spatial aspects of plant recruitment, growth and reproduction.
In contrast to plant recruitment, which has received considerable attention (e.g. Orr et al. 2004a, 2004b, 2004c), death of plants established in arid landscapes has rarely been studied, and knowledge of relationships between rainfall and death are inadequate for prediction and simulation. The death process usually occurs when the effectiveness of rainfall events declines and plants, especially perennials, come to rely on small and diminishing ‘pockets’ of water in the soil for survival. Death is an ecologically important process since it can be widespread, suddenly claiming high proportions of populations, threatening survival of other biota and contributing to landscape dysfunction (Ludwig et al. 1997). Grazing accelerates the death of grass plants during drought (Hodgkinson 1995) and excessive deaths may predispose the plant community to shifts in botanical composition (Harrington 1991; Walker et al. 1997) when different species, not necessarily endemic, capture unexploited soil resources after successful seedling establishment. Such shifts may change ecosystem function by a variety of flow-on effects. Grass death is important to managers of commercial grazing enterprises in event-driven systems; they strive by tactical grazing and vertebrate pest management to avoid crossing critical thresholds into less productive ecological states (Westoby et al. 1989; Hodgkinson 1994).
In the arid region of North America, survivorship of same-age cohorts of 6 perennial grass species was determined at annual intervals (Canfield 1957). Death rate was constant for some species while others exhibited low risk of death until middle age when death rate accelerated (Sarukhan and Harper 1973). In semi-arid temperate/sub-tropical (Williams 1970; Williams and Roe 1975; Michalk and Herbert 1978) and semi-arid tropical (Andrew 1986; Ash et al. 1997) regions, long-term annual observations have been made on different perennial grass species. Typically, death rates displayed by survivorship curves were constant but sometimes the curves became negatively skewed. Deevey (1947) had earlier categorised constant death rate curves as Type II and positively or negatively skewed curves as Type I and Type III, respectively.
In arid regions most perennial grasses are caespitose or tussocky in habit. Grazing intensity during evolutionary periods was probably always low and non-persistent; tight packing of tillers could have evolved to protect growing points at the base of tillers from elevated temperatures and low grazing pressure (Hodgkinson and Williams 1983). In general, grasses theoretically have indefinite life spans because of synchronous or asynchronous tillering patterns (Mott et al. 1992) and it would be scarcity of one or more resources, particularly soil water, which would induce plant death. Gardiner (1986) found evidence that rainfall amounts and site (landscape) features determine survival of arid perennial plant populations with different life forms, including grasses. There are no published studies, to our knowledge, where relationships between rainfall, landscape and disturbance factors and death rates of grasses have been sought. In a 5-year study of grass population dynamics, O’Connor (1994) found that plant death was more strongly influenced by differences in rainfall between years than by grazing pressure but no relationships emerged from the study. More frequent measurements of plant status would be required to relate death to rainfall. In a strongly seasonal environment where water was not limiting, Sarukhan and Harper (1973) found the annual death rate to be remarkably constant between years for mature plants of 3 species of Ranunculus (as shown by most survivorship curves), but within the year there was a seasonal cycle in which death was predictably high before and after a relatively death-free flowering period.
Rainfall triggers several plant physiological/ecological processes leading to production pulses in arid regions (Hodgkinson and Freudenberger 1997). In the mid-latitude semi-arid regions both grass growth and reproduction are switched on by rainfall events with temperature and daylength exercising little or no control (Hodgkinson and Quinn 1976, 1978). However, spatial redistribution of water across landscapes may occur during intense rainfall events. Such redistribution together with local variation in evaporation rates and soil type would lower predictability of plant death based on rainfall alone.
In this study, we gathered serial data on many individual grass plants from across landscapes and periodically determined the water stress level of each and when each died. Data on deaths, rainfall, evaporation and zone location of plants in landscapes allowed us to develop relationships between rainfall and plant death for 4 species.
The questions addressed in this paper are as follows.
-
Are there relationships between death rates of each species and rainfall?
-
Is there a critical threshold of rainfall below which death occurs and if so, is the relationship a general one?
-
Are there relationships between death rates and location of plants in the landscape?
-
Are there relationships between death rates and water status of individual plants?
Materials and methods
Study site
The main study took place at the CSIRO Lake Mere Research Facility (30°16′S, 144°54′E), located 35 km north-west of the village of Louth, New South Wales, and within the semi-arid woodlands of eastern Australia. The location site lies within the latitudinal belt of 20 and 35°S, identified by Gentilli (1972) as the most drought-prone area of Australia. Globally, rainfall events in Australia, and this region in particular, are the most variable of any landmass (McMahon et al. 1992). Rainfall at the site is non-seasonal (Hodgkinson and Freudenberger 1997) and averages 308 mm per annum.
The landscape is characterised by low and undulating stony ridges of mainly sedimentary rock. Sheet flow from these low ridges drains into weakly defined dendritic drainage lines (Tongway and Ludwig 1990). Soil at the site is a massive red earth Gn2.12 (Northcote 1979) or using USA terminology, a xerollic haplargid (Soil Survey Staff 1975).
The vegetation types at the site are typical of the Mulga (Acacia aneura F.Muell.) woodlands of semi-arid Australia occurring in a broad band across central Australia (Harrington et al. 1984; Johnson and Burrows 1994). Mulga typically occurs in a patterned manner throughout the Lake Mere landscape as island groves following contours across slopes (Anderson and Hodgkinson 1997) and as continuous stands following drainage lines. Tongway and Ludwig (1990) identified three distinct vegetation types within the site. These are associated with, and dependent on, the sequence of geomorphic zones in the landscape. The vegetation zones determine the flow and infiltration of water along catenary sequences from the highest to the lowest point in the landscape. The zones are (a) woollybutt (Eragrostis eriopoda Benth.) dominated grassland on the shallow soils of the long, low ridges and slopes (0.5% average slope) termed ‘ridge/slope’, (b) bandicoot grass (Monachather paradoxa Steud.) dominated grassland on deeper soils at the toe of these run-off slopes termed ‘toe-of-slope’ and (c) mulga woodland occurring on run-on areas (the drainage lines and the island-groves across slopes) termed ‘flat’. These three zones occupied 57, 12 and 31% of the study site, respectively. Mulga grass [Thyridolepis mitchelliana (Nees) S.T. Blake] and number 9 wiregrass [Aristida jerichoensis (Domin) Henr. var. subspinulifera Henr.] were also abundant throughout the 3 zones, as were a large number of annual forbs following cool-season rainfall.
The 204-ha site was fenced into 13 paddocks ranging in size from 4 to 30 ha (each paddock was 810 m long but varied in width from 93 to 360 m). The long axis of the paddocks was oriented across the landscape pattern so that each paddock contained a similar proportion of the 3 vegetation types. In 7 of the 13 paddocks, 6 young hogget sheep grazed at nominal sheep densities of 0.3, 0.4, 0.5, 0.6, 0.7, 0.8 and 1.0 sheep/ha.
Experimental design
In each paddock, five zones of the landscape were recognised and delineated (‘ridge run-off’, ‘ridge run-on’, ‘slope’, ‘toe-of-slope’ and ‘flat’) as described by Tongway and Ludwig (1990), except that the ‘ridge/slope’ zone was divided into ‘ridge’ and ‘slope’ zones. Furthermore, the ‘ridge’ zone was divided into the smaller areas that locally shed water (‘ridge run-off’) or received extra water by local run-on (‘ridge run-on’). When the zones were being located there had been a recent intense but short rainfall event and the wetter patches were clearly seen. A diagrammatic cross-section of the contiguous last three zones is provided elsewhere (Anderson and Hodgkinson 1997).
The grasses monitored during the study were Monachather paradoxa and Thyridolepis mitchelliana (both cool-season C3 and palatable to herbivores) and Aristida jerichoensis and Eragrostis eriopoda (both warm-season C4 and often unpalatable to herbivores). All species were common in the area and region.
In each zone, 10–15 locations were established by stepping along parallel lines about 5 m apart and at each 5th pace the point became the corner of a 1-m2 quadrat. The quadrat was selected if it contained plants of 2 or more of the 4 grass species; bare areas were ignored. One of these locations was randomly selected to become a ‘control’ (ungrazed) within each zone of each paddock. Other locations were open to sheep grazing. In all there were 35 ‘control’ locations (7 paddocks × 5 zones). At each ‘control’ location, 1 m high weldmesh panels were erected to exclude grazing from a 2.9 × 2.9-m area. Wooden blocks, 10–15 cm high, were placed under each of the four corners to prevent litter carried in overland flow from being trapped against the mesh panels. Data from the many ‘grazed’ quadrats will be presented in a subsequent paper about the effects of grazing, with and without drought, on survival of perennial grasses.
Field measurements
Within each exclosure, two metal pegs were driven into the ground 2 m apart, with one carrying a numbered tag, as permanent markers. At each assessment, a 2 m long wooden pole, graduated in centimetres, was laid between the pegs. Individual grass plants were located by their right-angled distance from the pole and the distance from this intercept on the pole to the tagged peg. These distances enabled repeated assessments on each plant.
Plants were assessed on 29 occasions between January 1987 and June 1996. These were usually done at 3-monthly intervals but occasionally there were 4 (thrice), 6 (once), 9 (once), and 13 (twice) months between assessments. At each assessment the basal diameter, foliage height, foliage diameter, number of panicles, and water stress status of each plant was measured or estimated. The water status was estimated on a 5-point scale where 5 signified an actively growing plant; 4, a plant with ‘dull’ non-growing leaves; 3, a plant with non-growing leaves and stems; 2, a plant with ‘brown’ dead leaves but some ‘green’ stems and 1, a senescent plant with dead leaves and stems. If the plant was given a stage-1 ranking on two successive occasions it was deemed to be dead on the first occasion. There was always sufficient rainfall between two successive occasions to promote leaf growth of the 4 grass species and the determination of the dead status of plants was straightforward. If there was any doubt about plant status the plant was deemed to be alive and reassessed later. The forage biomass (g/m2) in the surrounding grazed paddock was estimated every 3 months from 100 fixed quadrats (1 m2) in each paddock by using a double sampling technique (described by Wilson 1991).
Rainfall was automatically and continuously recorded at the study site. Daily evaporation was measured at the Cobar Meteorological Station, 164 km to the south-east of the study site.
Validation
The relationship between rainfall and grass plant death at the CSIRO Lake Mere Research Facility was independently assessed for a wide range of species at widely spaced locations in New South Wales west of the main divide. Survival of well established grass plants of common C3 and C4 species in naturally occurring populations on selected pastoral properties were determined. Populations were sampled in both semi-arid and temperate woodlands during 1998, 2000 or 2001. Cooperating pastoralists measured the rainfall at the locations during the assessment periods. Twenty established plants of one or more grass species were marked at each location (either on slopes or on level ground) along transects with a wire stake from which was suspended a numbered aluminium tag. Plants were selected by walking metre-wide transects and each plant encountered of the selected species was marked until a set of 20 was obtained. At 3–7 month intervals individual plants were examined three times for viability. If a plant was given a stage-1 ranking on two successive occasions it was deemed to be dead on the first occasion as at CSIRO Lake Mere Research Facility. All plants were open to paddock grazing.
The grass species and the latitude/longitude of sites on pastoral properties where measurement took place were; Austrodanthonia monticola (Vickery) H.P. Linder (34°58′S, 149°10′E), Austrodanthonia caespitosa (Gaudich.) H.P. Linder (34°52′S, 146°15′E; 35°02′S, 145°05′E; 35°15′S, 148°07′E; 35°37′S, 144°55′E), Austrodanthonia pilosa (R.Br.) H.P. Linder (34°18′S, 148°58′E), Austrodanthonia procera (Vickery) S.W.L. Jacobs (32°34′S, 147°53′E), Austrodanthonia racemosa (R.Br.) H.P. Linder (33°34′S, 149°09′E), Austrodanthonia setacea (R.Br.) H.P. Linder (32°18′S, 146°39′E), Chloris truncata (R.Br.) (34°52′S, 146°15′E; 35°02′S, 145°05′E), Enteropogon acicularis (Lindl.) Lazarides (35° 02′S, 145°05′E; 35°37′S, 144°55′E), Monachather paradoxa (26°53′S, 143°55′E; 27°34′S, 145°46′E; 27°56′S, 146°00′E; 30°59′S, 145°58′E) Microleana stipoides (Labill.) R.Br. (34°58′S, 149°10′E; 35°15′S, 148°07′E) and Sporobolis caroli Mez (35°02′S, 145°05′E). Species were identified using descriptions given by Wheeler et al. (2002).
Statistical analyses
Relationships between percentage death and predictor variables for all assessments were modelled by simple linear regressions after log-transforming both percentage death and predictor. Death of individual plants between selected assessments was modelled as a binary response (a given plant was either alive or not) using generalized linear models with binomial error and logit link (Dobson 2002).
The relationships between pairs of landscape zones in mean water stress estimates were derived by fitting linear regressions and then testing whether a quadratic component significantly improved the fit. All analyses were performed using GenStat for Windows (VSN International Ltd, Hemel Hempstead, UK).
Results
Survivorship of perennial grasses
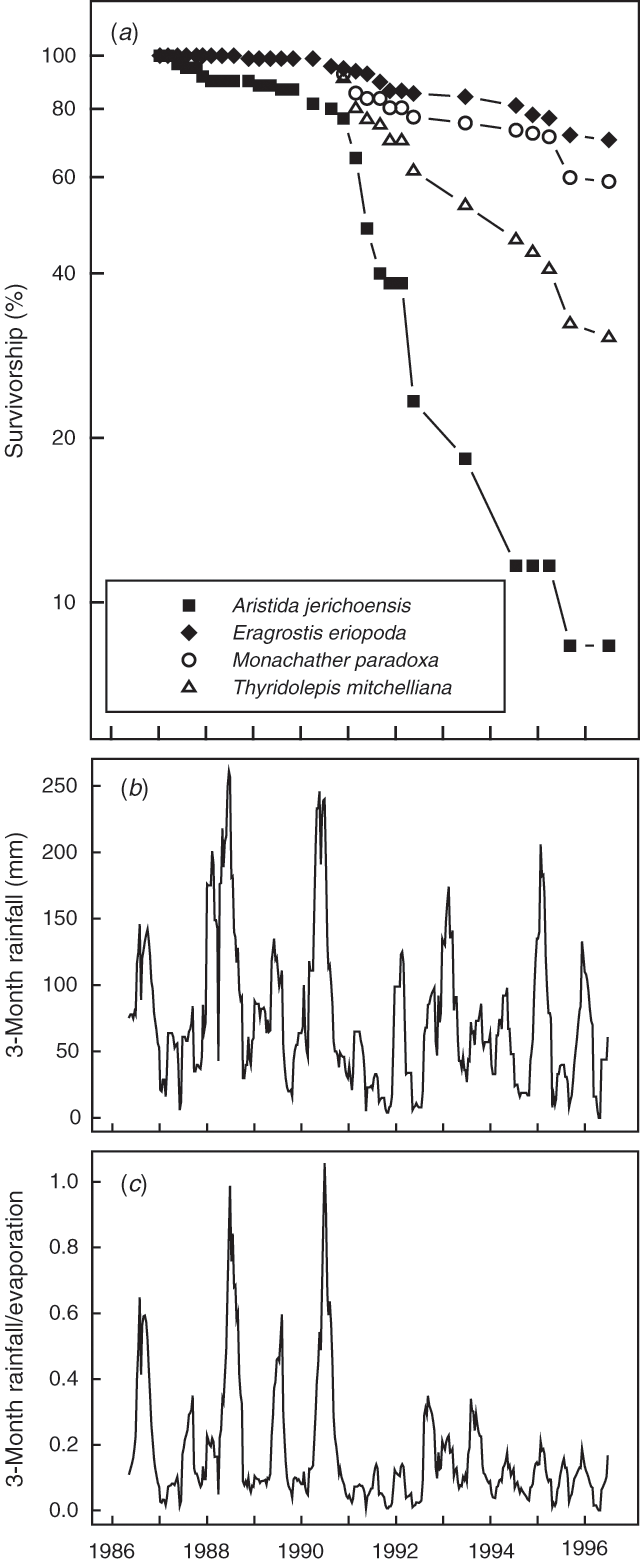
In late 1986, 60 individual Aristida jerichoensis, 95 Eragrostis eriopoda, 97 Monachather paradoxa, and 167 Thyridolepis mitchelliana plants were located and described within the 35 ‘control’ quadrats. Survival of these mixed-age populations of sexually mature plants over the ensuing 10 years was both species- and time-dependent (Fig. 1a); for Eragrostis eriopoda and Monachather paradoxa populations mean survival by 1996 was high (70 and 59%, respectively), for Thyridolepis mitchelliana survival was intermediate (30%) and for Aristida jerichoensis survival was low (8%). Species differences in survivorship emerged strongly only after 1990.
|
In general, the shapes of the survivorship curves conformed to Deevey Type I (Deevey 1947); risk of death was low during the 4-year period to 1990, but after 1990 there was an accelerating death risk. The onset of higher death rates coincided with a phase shift in both the amount and season of rainfall as shown in Fig. 1b, c. The running 3-month rainfall total after mid 1990 declined and despite long-term non-seasonality of rainfall at the site, there was a pronounced switch from winter to summer rainfall (Fig. 1b). When evaporation was taken into account, by dividing rainfall by evaporation losses, the shift from winter to summer rainfall approximately halved the effectiveness of the rainfall for plant growth as shown in Fig. 1c.
Death rates changed over the 10-year period as seen by the many shifts in the slope of the survivorship curves (Fig. 1a). Nearly all plants of all species survived during the first 4 years but then death periods occurred episodically. Death was a punctuated process rather than a continuous one as suggested by Deevey type relationships. Change in survivorship for each of the four species generally occurred in unison but the death rates differed amongst species. Changes in survivorship were related to smaller amounts (Fig. 1b) and lower effectiveness (Fig. 1c) of rainfall expressed as a running 3-month total.
Death rates during droughts
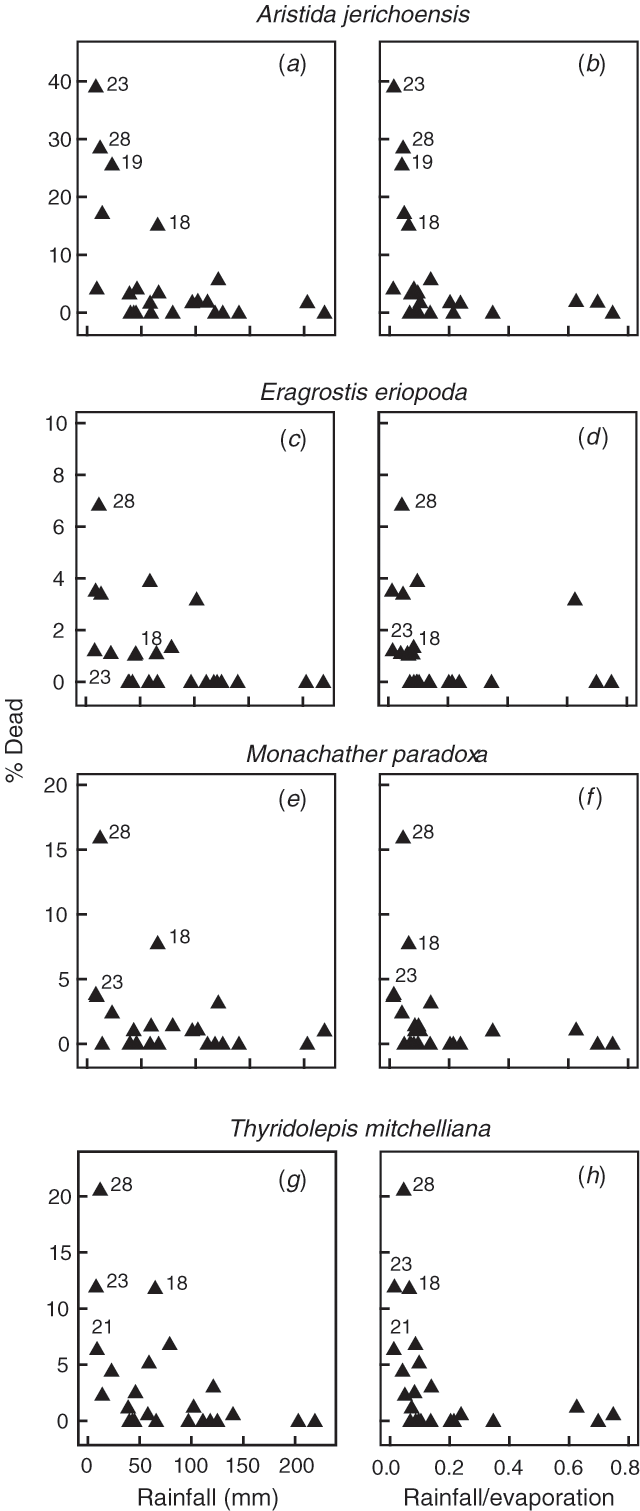
Death rate of plants among the 29 sampling occasions was related to the preceding 3-month rainfall (Fig. 2a, c, e, g) as an exponential increase below a critical threshold of rainfall. Where the interval between samplings exceeded 5 months the data were not included to avoid inflated deaths. Preceding rainfall over longer periods (6, 9 and 12 months) was more poorly related to death rates than the preceding 3 months. When evaporation rates were used to modify the rainfall for seasonal changes in temperature and radiation, the scatter of points was further reduced for the 3-month period (Fig. 2b, d, f, h) but not for the antecedent 6-, 9- and 12-month periods. Other drought indices, based on the ratio of rainfall (or rainfall/evaporation) in 3-, 6-, 9- or 12-month periods to mean rainfall (or rainfall/evaporation) for the equivalent period over the whole study, were examined but all were markedly inferior to the 3-month rainfall total and 3-month rainfall/evaporation indices.
|
There were consistent differences among the four grass species in death rate during any drought period (A. jerichoensis > T. mitchelliana > M. paradoxa > E. eriopoda), as seen by the y-axis scales in Fig. 2. However, the shape of the relationships between 3-monthly rainfall and rainfall/evaporation remained similar. For each species the critical threshold below which death began was visually appraised from the graphs at about 75 mm of rainfall or a rainfall/evaporation ratio of 0.15 for the preceding 3 months. Above these thresholds, grass plants survived.
A feature of the plots shown in Fig. 2 is the large scatter in percentage deaths when 3-month rainfall was below the survival thresholds; for most periods few or no deaths occurred, while other periods with apparently similar low rainfall experienced significant numbers of deaths. Despite this variation, significant linear relationships on the logarithmic scale were found between percentage deaths and 3-month rainfall and rainfall/evaporation for the sub-75 mm rainfall and the sub-0.15 rainfall/evaporation periods (Table 1). In addition, mean water status of plants at the previous assessment was similarly related to % deaths (Table 1). Multiple regressions involving these three variables were also fitted, but the addition of extra terms after the first variable failed to improve the relationships. Other variables (rainfall and rainfall/evaporation for 0–6, 0–9 and 0–12 months preceding periods, rainfall for 3–6 months, numbers of sequential days when rainfall was 20 mm or less, zone of the landscape, and water stress index) were added to the analysis but none, on their own or in combination, significantly improved the relationships for any species.
|
No single variable was consistently the best predictor of death for all four species. The percentages of variation explained were low and there were no other measurements made which could be used in the analyses.
Individual plant deaths related to landscape, plant factors and surrounding grazing pressure
When several measured variables (landscape zone, plant basal diameter, foliage height, foliage diameter, water status, and level of surrounding paddock forage at the assessment immediately before the assessment when plant viability was determined) were examined in stepwise regressions on occasions of high death rate (assessment 18 for all species; assessments 19, 21, 23 and 28 for some species), significant amounts of the variance in % deaths could be attributed to these measured variables on some occasions (Table 2). Because the landscape zone was nearly always the most significant single variable accounting for variance, and was seen to play a major role in redistribution of water during storm events, it was made the first variable in all stepwise analyses. The significance of the remaining variables was determined by forward stepwise regression. Each variable, except for the basal area of the grass plants, was sometimes found to be significant in the best model. Since basal area, foliage height and foliage diameter were highly positively correlated with each other, no more than one of these appeared in the best model.
|
Plant deaths in each of the 5 landscape zones were examined in detail for three assessments that had the highest overall death rates (18, 23 and 28). Percentages of plants dying varied widely between species and between zones, with percentages ranging from 0 to 75. No zone had consistently higher death rates for all species (Table 3) when these 3 assessments were made. The highest percentage of deaths over the 10-year period occurred in the ‘ridge run-on’ zone for A. jerichoensis and E. eriopoda; the ‘slope’ zone for T. mitchelliana and the ‘flat’ zone for M. paradoxa (Table 4).
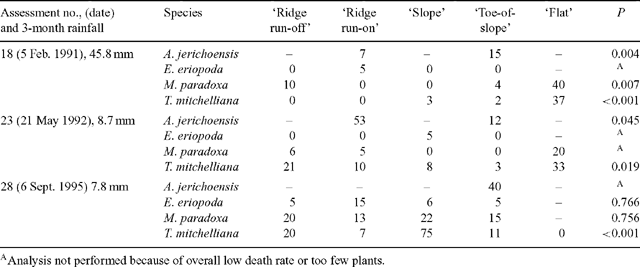
|
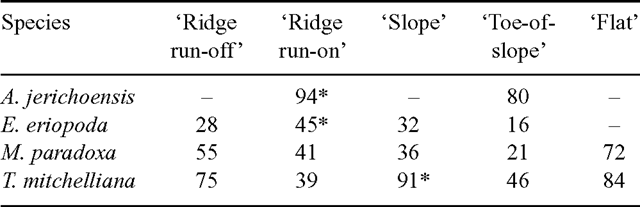
|
Inter- and intra-landscape zone water stress levels
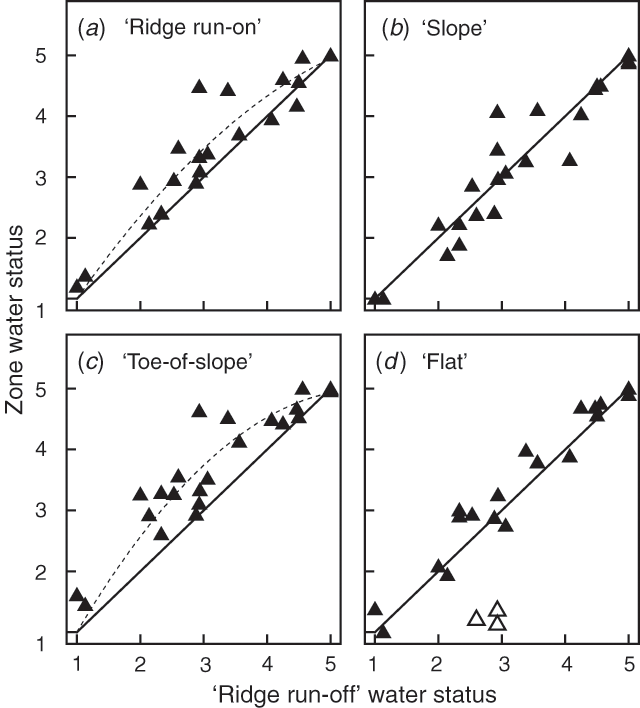
Inter-landscape zone water stress levels for T. mitchelliana are displayed in Fig. 3 by plotting the mean water stress values of measured plants in the ‘ridge run-off’ zone against the values from comparable plants in each of the other zones at the same assessment. The ‘ridge run-off’ zone was chosen as the archetypal driest zone because there was no ‘run-on’ water here. The shape of the relationships differed amongst zones. When ‘slope’ and ‘flat’ were plotted against ‘ridge run-off’ (Fig. 3b, d) the relationships were linear and not significantly different from a 1:1 slope. In contrast, the relationship was significantly curvilinear (P < 0.001) when the ‘ridge run-on’ and ‘toe-of-slope’ were plotted against ‘ridge run-off’ (Fig. 3a, c) indicating that plants were less water stressed here than in other zones of the landscape. Similar relationships were found for Monachather paradoxa. Insufficient numbers of plants in some zones prevented analysis of the remaining two species. In Fig. 3d three points ( ) were strongly displaced below the 1:1 line indicating that on these occasions the grass plants had recently begun leaf growth on the ‘ridge run-off’ and in other zones of the landscape, but not in the ‘flat’ zone. Perhaps water from recent rainfalls on these three occasions was ‘captured’ by the cone shaped mulga branches and channelled down stems to deprive grass plants of sufficient water to start leaf growth in the ‘flat’ zones.
) were strongly displaced below the 1:1 line indicating that on these occasions the grass plants had recently begun leaf growth on the ‘ridge run-off’ and in other zones of the landscape, but not in the ‘flat’ zone. Perhaps water from recent rainfalls on these three occasions was ‘captured’ by the cone shaped mulga branches and channelled down stems to deprive grass plants of sufficient water to start leaf growth in the ‘flat’ zones.
|
Intra-landscape zone variation in water stress was of interest because the first plants that become highly water stressed would be expected to die earlier than those that more slowly develop water stress. If the variation in water stress differs between zones then this could reveal why zones differ in progress towards plant death. This was examined by statistical comparison of the standard deviations of the plants in each zone at each assessment (excluding assessments with a 3-month rainfall/evaporation index of > 0.2) for Monachather paradoxa and Thyridolepis mitchelliana. The standard deviations of water status were found to be more variable within certain zones at some assessments but there were no consistent differences between variability of zones, nor were there trends in variability related to rainfall or rainfall/evaporation.
Validation of the drought index
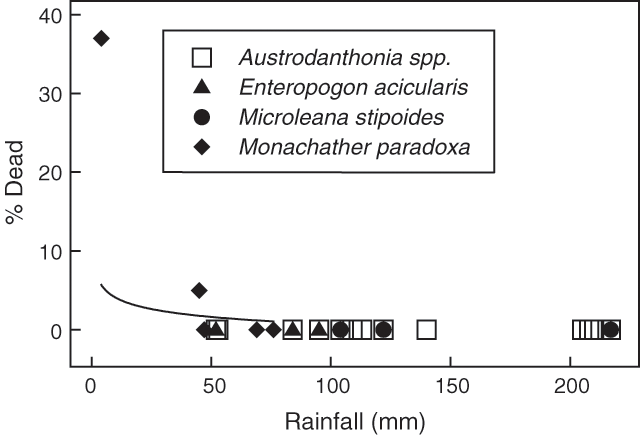
Death of grass plants during summer at each of the widely spaced locations on pastoral properties is shown in Fig. 4 along with the model line for Monachather paradoxa; the only species of the four at the main study site that was studied elsewhere. Only at four locations did the 3-monthly rainfall total fall below the survival threshold of 75 mm and at two of these locations (where Monachather paradoxa occurred) grass plant death was recorded. No death of grass plants occurred at other locations.
|
Discussion
Rainfall threshold for survival
Death of perennial grasses occurred mostly during the recurring droughts and was independent of season. No other causes of death, such as insect or fungal damage, were observed. Deaths began when rainfall events became smaller and less frequent and grass plants became water stressed beyond some critical but unknown physiological threshold. Generally, 75 mm of rain, measured over a 3-month period, appears to be a reasonable indicator of this threshold for both C3 and C4 perennial grass species (Fig. 2). Dividing rainfall by evaporation reduced variation (Fig. 2) and gave an index of rainfall effectiveness for the maintenance of plant processes. The rainfall/evaporation index of 0.15 was another useful expression of this critical threshold for grass death and the most precise.
Rainfall or rainfall/evaporation below the two thresholds did not necessarily mean death would occur (see Figs 2 and 4). Topography, soil type, rainfall pattern and environmental differences, singly and in combination, would be expected to shift the threshold up or down within space and time, through their influence on soil water availability to grass plants. Although various combinations of these physical differences would exist between the validation sites we expected that scarcity of rainfall would be the primary driver of plant death at all validation sites and at Lake Mere. However, we obtained only an equivocal validation of the relationship developed from the Lake Mere site because moderate to high rainfall prevailed at most of the other sites.
Individual grass plants access soil water differently in space and time influencing the threshold at a specific site. All deaths would be preceded by increase in resistance to water movement in the soil–plant system, and a slowing and eventual stoppage of gas exchange processes in leaves, and finally, death of leaves and stems (Doley and Trivett 1974; Ng et al. 1975; Sheriff and Ludlow 1984). The water status stages used in this study broadly indicated when these sequential physiological stages had been reached. For example, stage 4 indicated a build-up of resistance to water movement in the plant and a slowing of gas exchange processes. Death of leaves (stage 2) and then finally death of stems (stage 1) must precede plant death but plants that reached these highly water-stressed stages (Fig. 3) often survived when rain fell (Table 3) and rapidly regrew leaves from quiescent basal tillers (Busso et al. 1989). It seems that these native perennial grass species die only slowly, and somewhat erratically (Fig. 1), presumably because spatial variation in small, but critical, amounts of water in the soil profile determines the water available to individual plants.
Death rate control in drought
The patchiness of residual water in a drying soil and its accessibility by roots of individual grass plants would control plant death rate as a drought lengthens. Grass plants (Christie 1978) and neighbouring shrubs (Anderson and Hodgkinson 1997) experience similar soil water environments in these semi-arid landscapes presumably because grass and shrub roots occupy the same space. Observers of foliage of nearby plants would most likely perceive them to be the same or similar in water stress status at any one time. Within an individual landscape zone, however, high variability in water stress status would be expected because of the apparent patchiness of residual water, and this was observed in the field and expressed by high standard deviations. Interestingly, the high variation on some occasions was similar for each zone. When the landscape dried at the start of drought, irrespective of when each zone began drying, the suite of grass plants present varied widely in water stress status (Fig. 3). Only when the soil water was exhausted by transpiration and evaporation loss, did inter-plant variation become negligible. Zone differences in death rates (Tables 2–4) are therefore attributable to differences in soil-water storage following redistribution of surface water (Greene et al. 1994) and in extraction rates of the vegetation of each zone, especially when trees and shrubs are present (Fig. 3d).
Zone differences in the water stress of grasses at any one time (Fig. 3) indicated the extent to which the landscape significantly modifies the supply of water to grasses during drought by earlier surface redistribution of water during high intensity rainfall events. These zonal differences in water availability account for spatial differences in plant deaths amongst populations of the four grass species during drought (Tables 3 and 4). Survival of each C3 species was higher (Table 4) on run-on zones (‘ridge run-on’ and ‘toe-of-slope’) than adjacent run-off zones (‘ridge run-off’ and ‘slope’). C4 species were less common in run-off zones so survival of these could not be adequately documented except for Eragrostis eriopoda where survival was greater in ‘toe-of-slope’ than the ‘slope’. Survival of C3 species was low in the ‘flat’ zone, where tall shrubs of Acacia aneura were dense and continuous. Although the ‘flat’ zone periodically received extra water by runoff, the high shrub biomass extracted much of this water by transpiration and so returned the zone to the same grass water stress dynamics as the drier ‘ridge run-off’ and ‘slope’ zones (Fig. 3). The effect of shrubs (and trees) on grass water stress may apply to other zones should shrubs thicken in density, as they have done in all landscape zones of a vast area surrounding the study site (Daly and Hodgkinson 1996). The presence of widespread, dense shrubs (and trees) may reduce survival of grasses by hastening the onset of water stress during drought and if grazed, the simultaneous stresses may rapidly kill most grass plants.
Redistribution of water in these landscapes appears to broadly operate at two scales. At the large scale there is redistribution of water in wet years e.g. 1988–89 and when sudden heavy and localised storms occur. This large redistribution induces abundant grass recruitment and sustained plant growth in the mulga groves or ‘flats’ and to a lesser extent in other zones. Small-scale redistribution during moderate rainfall events in the more prevalent dry years (Table 3), benefits grass plants in run-on zones (‘ridge run-on’ and ‘toe-of-slope’) more than in run-off zones and is shown by lower death rates.
Death rate was also affected by some plant factors after variation attributable to landscape zone was taken into account (Table 2). On some occasions (assessments 23 and 28) the taller plants of some populations died at a slower rate. This may be explained by observations that the taller plants had greater leaf area and larger and probably more effective root systems, thereby enabling those plants to tolerate higher water-stress values in drought. A similar explanation can be offered for the lower death rate of wider-foliaged Eragrostis eriopoda plants at assessment 18. Water status 3 months earlier emerged only as a significant factor for survival of Aristida jerichoensis at assessment 19 probably because variance for landscape zone accounted largely for correlated water stress values. The amount of biomass surrounding exclosures (a measure of grazing intensity as well as plant growing conditions) significantly influenced death rate of only one species on one occasion. The higher plant biomass on this occasion may have lowered runoff so that less redistributed water reached the ungrazed plants.
Overall, the further the 3-month rainfall total was below the survival threshold of 75 mm, the higher was the death rate of each species (Fig. 2). Other measures of plant water availability were equally effective in explaining variation. The shape of the relationship between plant death and 3-monthly antecedent rainfalls was concave, indicating that there was no sudden rush of deaths as drought intensified. Slow death would be expected because the widely-spaced grass plants appear to have exclusive access to different sized pockets of water in the almost dry soil of the root zone (Nobel 1981) and therefore water-stress would be non-sychronous. Additionally, there are inter- and intra-species differences in drought tolerance amongst pot-grown grasses (Harradine and Whalley 1979) and in the field (Fig. 1) suggesting that death of plants in a local grass population should be a continuous rather than a punctuated process during drought.
Climate change and perennial grass death
It is predicted that by 2070, climate in the region of Lake Mere will be 6–7°C warmer in all seasons and wetter in summer/autumn and drier in winter/spring than at present (CSIRO 2004). Climatic events are predicted to be more extreme. These changes would both raise the evaporation rate and lower soil water balance in general. On the basis of our study, such climate change will significantly increase the risk of death in grass populations. The chance of successful seedling recruitment is difficult to predict but more recruitment may compensate for higher death rates.
Rising concentration of CO2 in the air may modify both death and recruitment responses because grass plants may be 44 and 33% higher in plant biomass on average than at present, for C3 and C4 types, respectively (Wand et al. 1999). Root biomass of C3 and C4 grasses could be in the order of 44 and 15% higher, respectively, suggesting that C3 grasses may be able to much more effectively ‘forage’ for limited pockets of water in the soil than C4 grasses because their root systems will be able to explore greater volumes of soil through higher allocation of photosynthate to roots. However, the larger plants may simply extract soil water at a faster rate than at present and become water-stressed sooner than if plants were smaller.
Towards a grass death model
The relationships between rainfall and death rate (Figs 2 and 4; Table 1) show a rainfall threshold for survival of 75 mm (measured over a 3-month period) and increasing death rate thereafter in the absence of effective rain. The relationships for different species, both at Lake Mere and elsewhere, should be adequate for broad prediction and mapping of drought-induced death of perennial grasses. Refinement of the model by wider testing would be worthwhile but the present model is adequate for initial modelling. Death from drought will be initially patchy in the landscape (Tables 3 and 4) but eventually death will be widespread at landscape and region scales while the 3-monthly running rainfall total remains below 75 mm.
The effect of grazing needs to be added to the death model. Data on the grazing effect is contradictory (e.g. Austin et al. 1981; Mott et al. 1992; Eneboe et al. 2002) and this needs resolution. The conceptual death trap model was earlier proposed (Hodgkinson 1995) to account for the death response from the combination of grazing and drought stresses. Clearly, death of grass plants in grazed landscapes is complex because low rainfall, landscape zones and grazing components probably strongly interact. This present study of ungrazed perennial grass plants establishes a base on which the effects of grazing on the slopes of the species-dependent rainfall/death relationships can be more fully determined and the death trap model evaluated.
Acknowledgments
Thanks to John Barber, Sylvia Brunner, Anthony Clancy, Jaimie Cook, Brian Dixon, Alex Drew, Martin Driver, Rod Edmundson, Alex Gartmann, David Jensen, Ivan McManus, Steve Marsden, Roger Oxley and Jacqui Stol for assisting with grass measurements on one or more occasions. Special thanks to Brian Dixon for setting up the sampling, locations and assessment procedures at the CSIRO Lake Mere Research Facility and for other critical input into technical aspects of the project. John Ludwig and David Tongway suggested sampling be stratified on the zones they identified in their baseline survey of the Research Facility. Steve Marsden computed the rainfall and evaporation data. Many people generously allowed access to their pastoral properties and rainfall records and provided logistic support; Dick and Diedre Beshman of ‘Glenvue’, Michael and Anna Couglan of ‘Tarabah’, Peter Dowling of the SGSKP National Site at Carcoar, Martin Driver of ‘Barrabool’, Richard and Kathryne Harley of ‘Pretty View’, Jarvis and Margaret Hayes of ‘Wacilaly’, John and Robyn Ive of ‘Talaheni’, Kent and Noel Keith of ‘Ballanda Park’, Tony and Jacqui Mills of ‘Woodlands’, Brian and Kylie Rutledge of ‘Moble’, Andrew and Kathy Schmidt of ‘Wallen’, Jim and Annabelle Strahan of ‘Lake Mere’, Mike and Kylie Sutherland of ‘Genaren’, Roderick and Adriana Taylor of ‘Adgingbong’ and Allan and Christine Wilson of ‘Cal Col’. Comments by Sarah Bruce, Ron Hacker, Richard Morton, Jim Noble and two anonymous referees improved the paper. From 1989 to 1991, the Wool Research and Development Corporation (now Australian Wool Innovations) provided partial financial support for this long-term study.
Anderson V. J., Hodgkinson K. C.
(1997) Grass-mediated capture of resource flows and the maintenance of banded mulga in semi-arid woodland. Australian Journal of Botany 45, 331–342.
| Crossref | GoogleScholarGoogle Scholar |
(accessed 8 June 2005)
Daly R. L., Hodgkinson K. C.
(1996) Relationships between grass, shrub and tree cover on four landforms of semi-arid eastern Australia, and prospects for change by burning. The Rangeland Journal 18, 104–117.

Deevey E. S.
(1947) Life tables for natural populations of animals. Quarterly Review Biology 22, 283–314.
| Crossref | GoogleScholarGoogle Scholar |

Dobson, A. J. (2002).
Doley D., Trivett N. B. A.
(1974) Effects of low water potentials on transpiration and photosynthesis in Mitchell grass (Astrebla lappacea). Australian Journal of Plant Physiology 1, 539–550.

Eneboe E. J.,
Sowell B. F.,
Heitschmidt R. K.,
Karl M. G., Haferkamp M. R.
(2002) Drought and grazing: IV. Blue grama and western wheatgrass. Journal of Range Management 55, 197–203.

Gardiner H. G.
(1986) Dynamics of perennial plants in the mulga (Acacia aneura F.Muell.) zone of Western Australia II. Survival of perennial shrubs and grasses. Australian Rangeland Journal 8, 28–35.

Gentilli, J. (1972).
Greene R. S. B.,
Kinnell P. I. A., Wood J. T.
(1994) Role of plant cover and stock trampling on run-off and soil erosion from semi-arid wooded rangelands. Australian Journal of Soil Research 32, 953–973.
| Crossref | GoogleScholarGoogle Scholar |

Harradine A. R., Whalley R. D. B.
(1979) Relative drought tolerance of three grass species from the northwestern slopes of New South Wales. Australian Rangeland Journal 1, 244–247.

Harrington G. N.
(1991) Effects of soil moisture on shrub seedling survival in a semi-arid grassland. Ecology 72, 1138–1149.

Harrington, G. N. ,
Wilson, A. D. ,
and
Young, M. D. (1984).
Hodgkinson K. C.
(1994) Tactical grazing can help maintain stability of semi-arid wooded grasslands. : ‘Proceedings XVIIth International Grassland Congress’. (New Zealand Grassland Society: Palmerston North.)
Hodgkinson K. C.
(1995) A model for perennial grass mortality under grazing. : ‘Rangelands in a sustainable biosphere. Proceedings Vth International Rangeland Congress. Vol. I’. (Ed. N. West )
pp. 240–241. (Society for Range Management: Denver.)
Hodgkinson K. C., Freudenberger D. O.
(1997) Production pulses and flow-ons in rangeland landscapes. : ‘Landscape ecology, function and management: principles from Australia’s rangelands’. (Eds J. Ludwig, D. Tongway, D. Freudenberger, J. Noble, K. Hodgkinson)
pp. 23–34. (CSIRO Publishing: Melbourne.)
Hodgkinson K. C., Quinn J. A.
(1976) Adaptive variability in growth of Danthonia caespitosa Gaud. populations at different temperatures. Australian Journal of Botany 24, 381–396.

Hodgkinson K. C., Quinn J. A.
(1978) Environmental and genetic control of reproduction in Danthonia caespitosa populations. Australian Journal of Botany 26, 351–364.

Hodgkinson K. C., Williams O. B.
(1983) Adaptation to grazing in forage plants. : ‘Genetic resources of forage plants’. (Eds J.G. McIvor, R.A. Bray)
pp. 85–100. (CSIRO Publishing: Melbourne.)
Johnson R. W., Burrows W. H.
(1994) Acacia open-forests, woodlands and shrublands. : ‘Australian vegetation’. 2nd edn(Ed. R. Groves)
pp. 257–290. (Cambridge University Press: Cambridge.)
Ludwig, J. A. ,
Tongway, D. J. ,
Freudenberger, D. O. ,
Noble, J. C. ,
and
Hodgkinson, K. C. (1997).
McMahon, T. A. ,
Finlayson, B. L. ,
Haines, A. T. ,
and
Srikanthan, R. (1992).
Michalk D. L., Herbert P. K.
(1978) The effects of grazing and season on the stability of Chloris spp. (windmill grasses) in natural pasture at Trangie, New South Wales. Australian Rangeland Journal 1, 106–111.

Mott J. J.,
Ludlow M. M.,
Richards J. H., Parsons A. D.
(1992) Effects of moisture supply in the dry season and subsequent defoliation on persistence of the savanna grasses Themeda triandra, Heteropogon contortus and Panicum maximum. Australian Journal of Agricultural Research 43, 241–260.
| Crossref | GoogleScholarGoogle Scholar |

Ng T. T.,
Wilson J. R., Ludlow M. M.
(1975) Influence of water stress on water relations and growth of a tropical (C4) grass, Panicum maximum var. trichoglume. Australian Journal of Plant Physiology 2, 581–595.

Nobel P.S.
(1981) Spacing and transpiration of various sized clumps of a desert grass, Hilaria rigida. Journal of Ecology 69, 735–742.

Northcote, K. H. (1979).
Noy-Meir I.
(1973) Desert ecosystems: environment and producers. Annual Review of Ecological Systematics 4, 25–51.
| Crossref | GoogleScholarGoogle Scholar |

O’Connor T. G.
(1994) Composition and population responses of an African savanna grassland to rainfall and grazing. Journal of Applied Ecology 31, 155–171.

Orr D. M.,
Paton C. J., Reid D. J.
(2004a) Dynamics of plant populations in Heteropogon contortus (black speargrass) pastures on a granite landscape in southern Queensland. 1. Dynamics of H. contortus populations. Tropical Grasslands 38, 17–30.

Orr D. M.,
Paton C. J., Playford C.
(2004b) Dynamics of plant populations in Heteropogon contortus (black speargrass) pastures on a granite landscape in southern Queensland. 2. Seed production and soil seed banks of H. contortus. Tropical Grasslands 38, 31–41.

Orr D. M.,
Paton C. J., Playford C.
(2004c) Dynamics of plant populations in Heteropogon contortus (black speargrass) pastures on a granite landscape in southern Queensland. 3. Dynamics of Aristida spp. populations. Tropical Grasslands 38, 65–76.

Sala O., Lauenroth W.
(1982) Small rainfall events: an ecological role in semiarid regions. Ecology 53, 301–304.

Sarukhan J., Harper J. L.
(1973) Studies on plant demography: Ranunculus repens L., R. bulbosus L. and R. acris L. Journal of Ecology 61, 675–716.

Schwinning S.,
Starr B. I., Ehleringer J. R.
(2003) Dominant cold desert plants do not partition warm season precipitation by event size. Oecologia 136, 252–260.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sheriff D. W., Ludlow M. M.
(1984) Physiological reactions to an imposed drought by Macroptilium atropurpureum and Cenchrus ciliaris in a mixed sward. Australian Journal of Plant Physiology 11, 23–34.

Soil Survey Staff (1975).
Stafford Smith D. M., Morton S. R.
(1990) A framework for the ecology of arid Australia. Journal of Arid Environments 18, 255–278.

Tongway D. J., Ludwig J. A.
(1990) Vegetation and soil patterning in semi-arid mulga lands of eastern Australia. Australian Journal of Ecology 15, 23–34.

Walker B. H.,
Langridge J. L., McFarlane F.
(1997) Resilience of an Australian savanna grassland to selective and non-selective perturbations. Australian Journal of Ecology 22, 125–135.

Wand S. J. E.,
Midgley G. F.,
Jones M. H., Curtis P. S.
(1999) Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Global Change Biology 5, 723–741.
| Crossref | GoogleScholarGoogle Scholar |

Westoby M.
(1979/80) Elements of a theory of vegetation dynamics in arid rangelands. Israel Journal of Botany 28, 169–194.

Westoby M.,
Walker B., Noy-Meir I.
(1989) Opportunistic management for rangelands not at equilibrium. Journal of Range Management 42, 266–274.

Wheeler, D. J. B. ,
Jacobs, S. W. L. ,
and
Whalley, R. D. B. (2002).
Williams O. B.
(1970) Population dynamics of two grasses in Australian semi-arid grassland. Journal of Ecology 58, 869–875.

Williams O. B., Roe R.
(1975) Management of arid grasslands for sheep: plant demography of six grasses in relation to climate and grazing. Proceedings of the Ecological Society of Australia 9, 142–156.

Wilson A. D.
(1991) The influence of kangaroos and forage supply on sheep productivity in the semi-arid woodlands. The Rangeland Journal 13, 69–80.



