Understanding and measuring uptake and coverage of oral pre-exposure prophylaxis delivery among adolescent girls and young women in sub-Saharan Africa
Megan S. Dunbar A M * , Katharine Kripke B * , Jessica Haberer C , Delivette Castor D , Shona Dalal E , Wanjiru Mukoma F , Saiqa Mullick G , Pragna Patel H , Jason Reed I , Hasina Subedar J , Daniel Were K , Mitchell Warren L and Kristine Torjesen AA FHI 360, 359 Blackwell Street, Suite 200, Durham, NC 27701, USA.
B Avenir Health, 7064 Eastern Avenue, NW, Washington, DC 20012, USA.
C Massachusetts General Hospital Center for Global Health, 125 Nashua Street, Suite 722, Boston, MA 02114, USA.
D Office of HIV/AIDS, Bureau for Global Health, USAID, 1300 Pennsylvania Avenue, Washington, DC, 20004, USA.
E World Health Organization, Avenue Appia 20, Geneva 1211, Switzerland.
F LVCT Health, Argwings Kodhek Road, PO Box 19835-00202 KNH, Nairobi, Kenya.
G Wits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, 22 Esselen Street, Hillbrow Health Precinct, Johannesburg 2001, South Africa.
H Centers for Disease Control and Prevention, Center for Global Health, Division of Global HIV and TB, 1600 Clifton Road, MS E-04, Atlanta, GA 30333, USA.
I Jhpiego, 1615 Thames Street, Baltimore, MD 21231, USA.
J South Africa National Department of Health, Civitas Building, 222 Thabo Sehume Street, CBD, Pretoria, 0001, South Africa.
K Jhpiego, 14 Riverside, off Riverside Drive, 2nd Floor, Arlington Block, PO Box 66119-00800, Nairobi, Kenya.
L AVAC, 423 West 127th Street, 4th floor, New York, NY 10027, USA.
M Corresponding author. Email: megansdunbar@gmail.com
Sexual Health 15(6) 513-521 https://doi.org/10.1071/SH18061
Submitted: 10 April 2018 Accepted: 25 September 2018 Published: 9 November 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
In response to World Health Organization (WHO) guidance recommending oral pre-exposure prophylaxis (PrEP) for all individuals at substantial risk for HIV infection, significant investments are being made to expand access to oral PrEP globally, particularly in sub-Saharan Africa. Some have interpreted early monitoring reports from new programs delivering oral PrEP to adolescent girls and young women (AGYW) as suggestive of low uptake. However, a lack of common definitions complicates interpretation of oral PrEP uptake and coverage measures, because various indicators with different meanings and uses are used interchangeably. Furthermore, operationalising these measures in real-world settings is challenged by the difficulties in defining the denominator for measuring uptake and coverage among AGYW, due to the lack of data and experience required to identify the subset of AGYW at substantial risk of HIV infection. This paper proposes an intervention-centric cascade as a framework for developing a common lexicon of metrics for uptake and coverage of oral PrEP among AGYW. In codifying these indicators, approaches to clearly define metrics for uptake and coverage are outlined, and the discussion on ‘low’ uptake is reframed to focus on achieving the highest possible proportion of AGYW using oral PrEP when they need and want it Recommendations are also provided for making increased investments in implementation research to better quantify the sub-group of AGYW in potential need of oral PrEP.and for improving monitoring systems to more efficiently address bottlenecks in the service delivery of oral PrEP to AGYW so that implementation can be taken to scale.
Introduction
Oral pre-exposure prophylaxis (PrEP) is the use of antiretroviral (ARV) drugs by people who do not have HIV infection to prevent the acquisition of HIV. In 2015, based on strong evidence of the efficacy and acceptability of oral PrEP, the World Health Organization (WHO) recommended that people at substantial risk of HIV (provisionally defined in WHO’s recommendation as HIV incidence of 3 per 100 person-years or higher in the absence of PrEPA) should be offered tenofovir-based oral PrEP as an additional prevention choice within a comprehensive HIV prevention packageB.1
In response to this guidance, and in an effort to make oral PrEP broadly accessible to all who might benefit from it, funders, global agencies and national governments have made significant investments towards expanding access to oral PrEP globally, particularly within sub-Saharan Africa, where it is estimated that ~50% of new HIV infections occur among women under the age of 25 years.2 Early implementation of oral PrEP in this region has focussed on expanding access through open-label extensions of clinical trials, demonstration projects and early scale-up programs. These have been conducted through the USA President’s Emergency Plan for AIDS Relief (PEPFAR), alone and in public–private partnerships through the DREAMS (Determined, Resilient, Empowered, AIDS-free, Mentored, and Safe) initiative;3 philanthropic organisations like the Bill and Melinda Gates Foundation and The Global Fund for AIDS, TB and Malaria (GFATM); as well as national programs, most notably in South Africa and Kenya and more recently Zimbabwe. Many of these programs have focussed on offering oral PrEP to adolescent girls and young women (AGYW) in high-HIV-prevalence locations,2,4 and to key population groups [e.g. men who have sex with men (MSM), female sex workers (FSWs)] and serodiscordant couples.
Reports from the early monitoring of programs delivering oral PrEP to AGYW have often been interpreted to suggest that uptake levels and pace of oral PrEP has been lower and slower than expected. Understanding the barriers to and facilitators of oral PrEP uptake among AGYW is an area of active research, with much to be learned in the near future. However, current understanding of PrEP uptake is limited, in part due to a lack of common definitions and varying interpretations of uptake and coverage measures. Moreover, the terms are often used interchangeably, although the intended meanings and uses differ.
Many factors likely contribute to an individual girl’s or woman’s access to and decision to use oral PrEP, and to programmatic coverage of oral PrEP delivery. The focus of this paper is on discussing inherent challenges in measuring oral PrEP uptake and coverage among AGYW. We propose potential indicators to address these challenges, and to work towards a better understanding of how the scientific and program communities could re-conceptualise ‘low’ uptake specifically. The need for greater clarity in terminology is not unique to oral PrEP delivery with AGYW. However, we focus attention on AGYW because the heterogeneity of risk within this large demographic group creates unique implementation challenges that underscore a need for precision in defined terms and subsequent data collection and interpretation. The proposed lexicon and the measurement principles proposed for oral PrEP delivery to AGYW may have wider application to other populations.
With over five decades of experience in developing and rolling out new contraceptive technologies, the reproductive health field has much experience to offer in conceptualising how to monitor the uptake of oral PrEP. Although the contraceptive field has a larger and ever-expanding set of options to offer individuals with various reproductive intentions and needs,5 both oral PrEP and contraceptive methods have to consider eligibility, contraindications, client choice and time period of need. Family planning programs have learned that monitoring actionable client-level data is critical, particularly during the first few years of introducing a new intervention. Addressing barriers early may affect long-term success in product uptake, while delays in detecting and rectifying problems may result in methods being discarded prematurely.6
A significant challenge for the delivery of oral PrEP to AGYW lies in precisely defining the numerator and denominator for uptake and coverage levels. Eligibility criteria for oral PrEP, and what constitutes the pool of AGYW ‘at substantial risk’ (the denominator in question), are defined heterogeneously across programs and settings. These differences are based not only on actual differences in individual- and population-level factors such as age-specific HIV incidence, individual sexual risk behaviour and exposure to violence, but also in how these component causes are defined. A blanket provision of oral PrEP for all AGYW, as has been considered for other key population groups, in which elevated risk is less heterogeneous compared with AGYW (such as among MSM or FSWs), is neither feasible nor likely to be cost-effective. However, when operationalised in real-world high-incidence settings, denominators have sometimes been defined as ‘all AGYW testing HIV negative,’ in large part due to a paucity of data on the risk characteristics or markers of HIV risk that would allow more precision in defining the denominator. Donor target requirements and programmatic considerations, such as budgetary or logistical constraints of the program platform where PrEP is delivered, also influence decisions. This lack of consistency in defining the denominator can make it difficult to compare indicators across settings.
Measurement challenges are not unique to the delivery of oral PrEP. Monitoring and tracking progress against UNAIDS 90–90–90 targets have been plagued by a lack of common indicators and reporting, making international comparisons difficult.7 Both WHO and UNAIDS have been working together to develop standardised programmatic indicators, in particular through the 90–90–90 cascade.8,9 WHO has also developed a cascade data use manual to identify gaps in HIV and health services for program improvement,10 which provides guidance on standard ways to track linkage to HIV prevention services for those at-risk individuals who test negative for HIV. Not unlike the treatment cascade, this prevention cascade suggests monitoring the proportion of those HIV-negative individuals at risk for HIV who are linked to prevention services, the proportion of those who accept or initiate the service offered and the proportion of those who continue to utilise prevention services (such as PrEP) over time. For those who continue to get retested for HIV, subsequent journeys through the cascade are monitored, rather than stopping reporting after the first HIV test. Although not yet standardised, the prevention cascade approach ultimately may enable priority setting to identify and address gaps in HIV prevention services.
The development of HIV prevention cascades is a complex exercise, given the large number of prevention interventions that an individual could access or use at any given time, the fact that individuals move in and out of risk (and thus in and out of periods when they may or may not benefit from the intervention(s)) and that risk and appropriate interventions are highly dependent on epidemiological context, individual behaviours and preferences and population group.11 Garnett et al. provide a conceptual framework for prevention cascades that are described as taking client-centred or intervention-centric perspectives.11,12 Cascades with client-centred perspectives, like the UNAIDS/WHO prevention cascade work described above, aim to optimise prevention through ensuring adequate supply of, demand for and adherence to a variety of prevention options. Cascades with intervention-centric perspectives narrow the focus to understanding how to optimise the impact of one specific prevention option.11,12
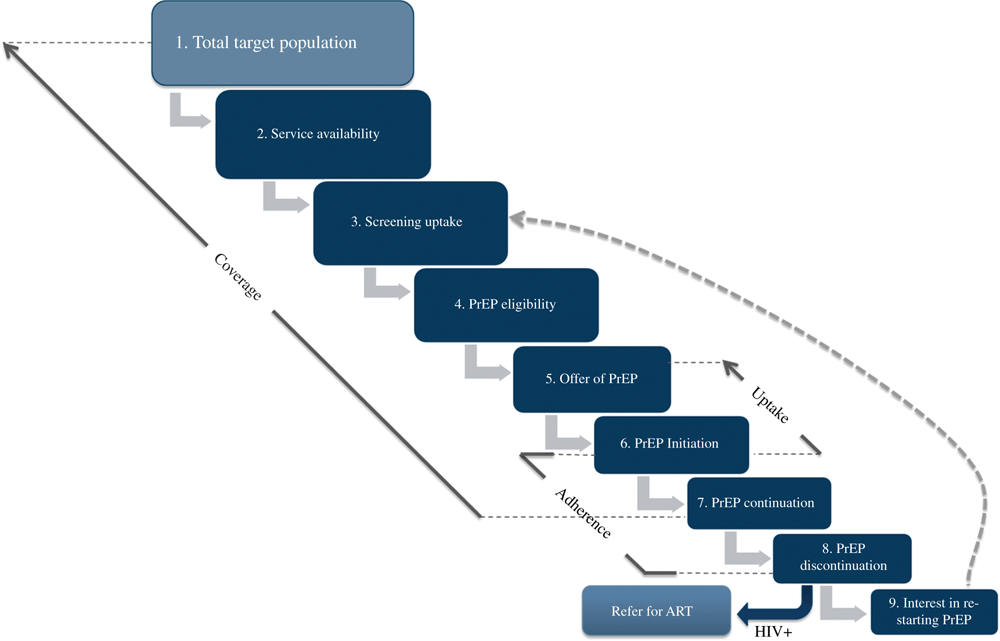
Through conceptualising an intervention-centric cascade (as depicted in Fig. 1), this paper explores a common lexicon for uptake and coverage in oral PrEP delivery to AGYW as a framework for exploring terminology and defining indicators for oral PrEP service delivery. We refer to this cascade and the definitions provided (Table 1) in the discussion below.

|
In codifying measurement for these indicators, we outline approaches for clearly defining metrics for uptake and coverage to reduce confusion, improve consistency and reframe the discussion on ‘low’ uptake to focus on achieving the highest possible proportion of AGYW using oral PrEP when they need and want it. Greater investment should be considered now in data collection to accurately measure and track these metrics, so that bottlenecks can be efficiently addressed, and oral PrEP delivery taken to scale.
Methods
The authors of this paper convened as an expert working group inclusive of policymakers, country-level program implementers, mathematical modellers, academics and experts in sexual and reproductive health and family planning, to examine emerging issues related to the definition, measurement and monitoring of oral PrEP uptake among AGYW in sub-Saharan Africa. Policymakers included representatives from those working within groups convened by WHO, UNAIDS and PEPFAR, focussed on developing guidance for HIV prevention and the monitoring and evaluation of oral PrEP implementation.
We conducted a targeted desk and literature review of existing documents, including WHO guidelines on ART and oral PrEP,1 the WHO implementation guidance for oral PrEP delivery,13 monitoring and evaluation1 (M&E), literature on treatment and prevention cascades,7–12 literature on indicators of uptake for family planning5,6,14–16 and available programmatic and country-level data on oral PrEP service delivery among AGYW in Kenya and South Africa, and other DREAMS countries.17,18 We discussed as a group the variety of ways uptake and coverage are being defined and the key challenges to measuring these indicators.
As an outcome of these collective efforts, the objectives of this paper are to:
-
Examine current approaches to measuring uptake and coverage among AGYW in oral PrEP programs in sub-Saharan Africa with a focus on articulating specific challenges to defining the denominator for these measures within the AGYW population;
-
Provide recommendations for defining the denominator for AGYW within the common lexicon proposed for uptake and coverage;
-
Make recommendations for improving the measuring and monitoring of uptake and coverage within oral PrEP programs for AGYW moving forward.
Measurement issues and challenges
Uptake and coverage as described in ongoing program implementation
To date, uptake of oral PrEP among AGYW has been defined in multiple ways. Approaches include: the percentage of all those testing HIV negative who initiate oral PrEP; the percentage of those deemed eligible based on clinical and behavioural factors who initiate oral PrEP; the percentage of the total estimated number of HIV-negative AGYW who initiate oral PrEP; progress towards program-defined targets; and sometimes even simply the total number of AGYW who are ‘on PrEP.’ These differences in definitions lead to confusion in expectations and in comparing uptake across programs. We begin by reviewing the different ways uptake and coverage have been defined and used in ongoing implementation in DREAMS, and in Kenya and South Africa.
Oral PrEP within DREAMS
Oral PrEP is being offered to AGYW as part of the DREAMS initiative,3 which has expanded from 10 countries to an additional five countries in 2018 (Botswana, Lesotho, Malawi, Namibia and Zambia). Each country defines a total target number (Step 1 in the cascade in Fig. 1) for oral PrEP service delivery based on a variety of factors including available funding, estimated program capacity, existing service delivery platform and epidemiological context. The DREAMS initiative incorporated the tracking of only one indicator at the outset of the program: the number of new initiates on oral PrEP through a PEPFAR indicator called ‘PrEP_New.’ New initiation is a one-time-ever event for any individual and is not contingent upon duration of PrEP use and not reflective of episodic use, including future restarts. DREAMS has been reporting progress against these targets at the country level as a percentage of the target for that fiscal year. Progress against targets is interpreted as a measure of uptake. The program has been through a process of refining programmatic reporting, and in fiscal year 19, PEPFAR-supported programs delivering PrEP will begin reporting on an additional indicator that tracks the cumulative number of current PrEP users, ‘PrEP_Curr.’ PrEP_Curr will be reported semi-annually and disaggregated by sex, age and key population group. While at the highest level, PEPFAR continues to be parsimonious in reported indicators, countries and programs are encouraged to develop custom indicators to improve tracking and remediation of PrEP services to ensure the program reaches its optimal effective scale at high quality. Ideally, custom indicators should align with national or global reporting guidance for cross-program and country comparisons. As such, this paper, and the learning that comes from standardised approaches, will support and inform PEPFAR’s ongoing work in identifying additional indicators to include in future program years. Additionally, PEPFAR is supporting an inter-US government agency process to develop a tool to support countries in creating clearly defined targets and to monitor progress in implementation throughout the cascade, which this paper could help inform.
Oral PrEP within Kenyan and South African national programs
With support from DREAMS, the Bill and Melinda Gates Foundation, The Global Fund to Fight AIDS, TB and Malaria, Unitaid and other donor agencies, as well as through national-level investments, Kenya and South Africa are two countries that have led the way in launching national oral PrEP programs. We briefly review here how these countries have defined uptake and coverage in the early phases of their national oral PrEP roll-out.
Kenya is rolling out oral PrEP to ‘all in need,’ with demand-creation activities focussed on priority populations including AGYW living in high-HIV-prevalence settings. Kenya has scaled up rapidly from a launch in May 2017. Oral PrEP monitoring and evaluation indicators have been defined and included in the national health management information system (HMIS), with tools developed, printed and distributed. The national program, overseen by the National AIDS & STI Control Program, tracks the number of oral PrEP initiations and uses the terms uptake and coverage interchangeably to refer to the proportion of individuals initiating oral PrEP among program-defined scale-up targets.17 According to this measure, ‘coverage’ was at ~10% by the end of 2017.17
South Africa began rolling out PrEP to sex workers in June 2016. South Africa also started delivering oral PrEP in selected sites to MSM as of April 2017, and more recently to students (both female and male) at selected university campus clinics. The South Africa program defines uptake as the proportion of those who initiate oral PrEP among those within a given program who test negative for HIV and are offered oral PrEP. Uptake according to this definition averages 12% in sex worker sites and 43% across MSM sites.18 Uptake data are not yet available from the university campus clinic sites.
Creating a common lexicon for uptake and coverage
Total target population
Step 1 in the cascade (Fig. 1 and Table 1) is defining the total target population. The total target population is the denominator for coverage. These are individuals at substantial risk of HIV infection (defined by the WHO as an incidence of >3 per 100 person-years) for a specified time period in any given country or program, who could potentially benefit from oral PrEP, and who are included in estimations for oral PrEP service delivery targets.
The WHO has recommended a multistage approach for estimating the target population for oral PrEP in the WHO PrEP Implementation Tool (module 9).13 This approach includes: (1) conduct a review of the most recent epidemiological data on HIV at national, regional and municipal levels, including data from specific subpopulations, by age and gender, and geographical locations; and (2) within high-risk populations and locations, engage in a risk assessment process for identifying individuals at substantial risk of HIV: (a) use a risk calculator or score; or (b) assess sexual and drug using behaviour; or (c) consider people who recognise their own HIV risk and request oral PrEP. Programs have used a variety of tools for assessing individual risk,19 and the WHO implementation tool also includes an aid to help clinicians take a brief history of clients’ sexual behaviour and drug use to assess their individual risk (module 1).13
Often, people who proactively ask for PrEP already consider themselves to be at risk and may have determined that oral PrEP is an appropriate prevention option. As such, they may be motivated to take PrEP and adhere to their regimen. However, many AGYW who are at risk for HIV do not perceive themselves to be at risk, so increasing accurate risk perception is a critical component of identifying AGYW who might benefit from oral PrEP.19,20 To make the issue even more complex, some AGYW may vary in time periods of risk; for example, sex workers who only work part of the year, women whose partners migrate for work or AGYW who simply are not in sexual relationships for an established period of time. These AGYW may appropriately discontinue PrEP use in consultation with their provider during periods when they anticipate they are unlikely to be HIV-exposed and later re-initiate during periods of higher exposure risk, a concept known as ‘prevention effective adherence’.20–22 Target population size would ideally be adjusted for these variable term or seasonal fluctuations in HIV risk.
Opinions differ about whether effective use of other HIV prevention strategies (e.g. condoms) should be an exclusion criterion in defining the target population for PrEP. Whichever approach a program or country chooses to use, criteria need to be clearly defined and documented to facilitate measurement and cross-program or cross-country comparisons. Another key challenge in some settings is age restriction of only offering PrEP to persons >18 years. While AGYW at substantial risk may include those aged 15‒24 years, those under age 18 years in some settings may be unable to access PrEP due to legal or policy restrictions.23 (Note: this is not the case in either South Africa or Kenya.).
Once the criteria for the target population have been determined based on individual risk factors, or living in a high-HIV-transmission setting, or both, defining the target population for specific PrEP programs requires estimating the size of the population that meets the stated criteria. For AGYW, especially because the process for identifying those at substantial risk of HIV is still poorly understood, estimating the size of the population who meet the defined criteria is particularly difficult. More research is warranted in this area. A particular program or country may want to define the target population to exclude those who are already using effective HIV prevention strategies such as condom use, viral suppression of positive partner, etc. This would require estimating the proportion of the population that meets these additional criteria. This proportion could change over time, especially if AGYW increasingly choose oral PrEP over their existing method as demand for and access to PrEP increases.
Programs may be tempted to limit the size of the target population based on resource constraints and/or service delivery capacity. However, to estimate epidemiological impact, it is important to clearly distinguish the population in need of services (item number 1 in Fig. 1) from realistic program targets based on service availability (item number 2 in Fig. 1), if these are different. In the case of resource or capacity constraints, programs would ideally devise targets that aim to scale up delivery to meet the total estimated need over time, with prioritisation given first to availing services to those that stand to benefit most from PrEP.
Oral PrEP uptake and coverage
A proportion of AGYW from within the total target population will be screened for interest and potential eligibility for oral PrEP, determined eligible for, and offered oral PrEP (items 3, 4, and 5 in Fig. 1). In this paper, we define uptake as the proportion of AGYW who initiate oral PrEP among those who are screened, eligible and offered PrEP (number who initiate oral PrEP/number offered oral PrEP) during a given period of time (e.g. within the last 12 months). It is important to note that oral PrEP may not be the right choice for all eligible AGYW for any number of reasons, such as lack of willingness to use oral PrEP, concern about side-effects, issues related to disclosing to partners and parents or guardians or because other HIV prevention methods are working for them. While this paper focuses on defining a common lexicon for terms and approaches to measurement, it will always be the case that programs, countries and donors will have varying programmatic objectives and priorities – especially during the early phase of rollout when we are still working to understand what the most optimal approaches are. For example, some programs may include criteria relating to ‘willingness/ability to take PrEP’ in determining whether to offer oral PrEP, whereas others may not. In fact, understanding the relative effectiveness of these different approaches towards identifying AGYW at substantial risk, and who take up and stay on PrEP when they need it, is part of the critical learning process. However, it is important to clearly document the criterion used, to ensure that comparisons across programs are indeed comparable and that we understand the heterogenous approaches when interpreting differing rates of uptake and coverage in different settings.
Coverage, in contrast, is a macro-level measure, defined as the proportion of the total target population using oral PrEP (e.g. those who have screened for and initiated PrEP and are still using PrEP) at a given time point or over a period of time divided by the target population (total number using PrEP during defined time period/total target population). Coverage, while difficult to estimate precisely due to difficulties in identifying target population size, is the metric that relates directly to the epidemiologic impact of the intervention. It is also used in modelling studies of PrEP impact. As depicted in Figure 1, coverage can be limited not just by uptake, but also by every step of the PrEP service delivery cascade — service availability, screening, eligibility, offer, initiation and continuation. Rather than focus purely on maximising uptake, programs should be concerned with coverage and identifying the specific steps in the cascade that are leading to low intervention coverage. Some of these limitations, such as eligibility, may be unavoidable, whereas others, such as service availability and screening, may be improved by focusing increased resources and attention on these steps of the cascade.
Given the heterogeneity across countries and programs in epidemiological context, eligibility criteria for oral PrEP and approaches to identifying programmatic targets, being clear about what measures are used in defining these terms, will help stakeholders accurately compare data across programs and countries and better understand factors leading to program success or failure.
The numerator – how many people are ‘on PrEP’?
Determining the numerator for coverage is not straightforward, either. Service statistics from several programs indicated that among those who initiate PrEP, a proportion will return for the next visit, and a proportion of those will return for the following visit, etc. In addition, some clients who discontinue deliberately or who are lost to follow up may later wish to reinitiate (item number 9, Fig. 1). Given the complexity of client participation in the cascade, the numerator for coverage could, for example, be reported as an average number of people attending any type of visit (including both initiation and continuation visits) over a defined period of time, or it could be reported as an average number of people attending a specified visit over a defined period of time (e.g. the average number of clients who completed their 3- or 6-month follow-up visit over the course of a year). It is important to note the need for similar efforts to clarify terms and measurements across the oral PrEP cascade for AGYW, in particular for continuation and adherence. However, the authors do not feel we can do justice to these topics in this paper given length constraints and a diversity of opinions that would require more space to fully articulate. For additional information, a taxonomy of initiation, adherence and persistence of medication use is extensively discussed in Vrijens et al.24 These concepts should be carefully considered when determining how to report the number of clients ‘on PrEP’ for the purpose of program management and evaluation, as all of these elements are essential to a program’s success and for maximising the epidemiological impact of the intervention.
Reframing the discussion about ‘low’ uptake
We have demonstrated how poorly defined metrics could lead to an inaccurate perception of low uptake, which would imply low acceptability of oral PrEP within the target population, and also how the terms uptake and coverage are often used interchangeably, causing confusion. For example, coverage could look artificially low if the denominator includes AGYW who are not truly at substantial risk for HIV infection, and uptake may look artificially low if the denominator includes those not actually screened for PrEP, or those who are eligible but not offered PrEP.14 Because data increasingly drive decisions about where and how to invest HIV prevention resources, clear and accurate measuring and reporting on oral PrEP uptake and coverage is essential to ensure that appropriate investments are made. Importantly, while low uptake may lead to low service delivery numbers, each step in the service delivery cascade must be considered when explaining program achievements that are lower than expected, rather than assuming that the entire problem is caused by low demand for the product within the population.
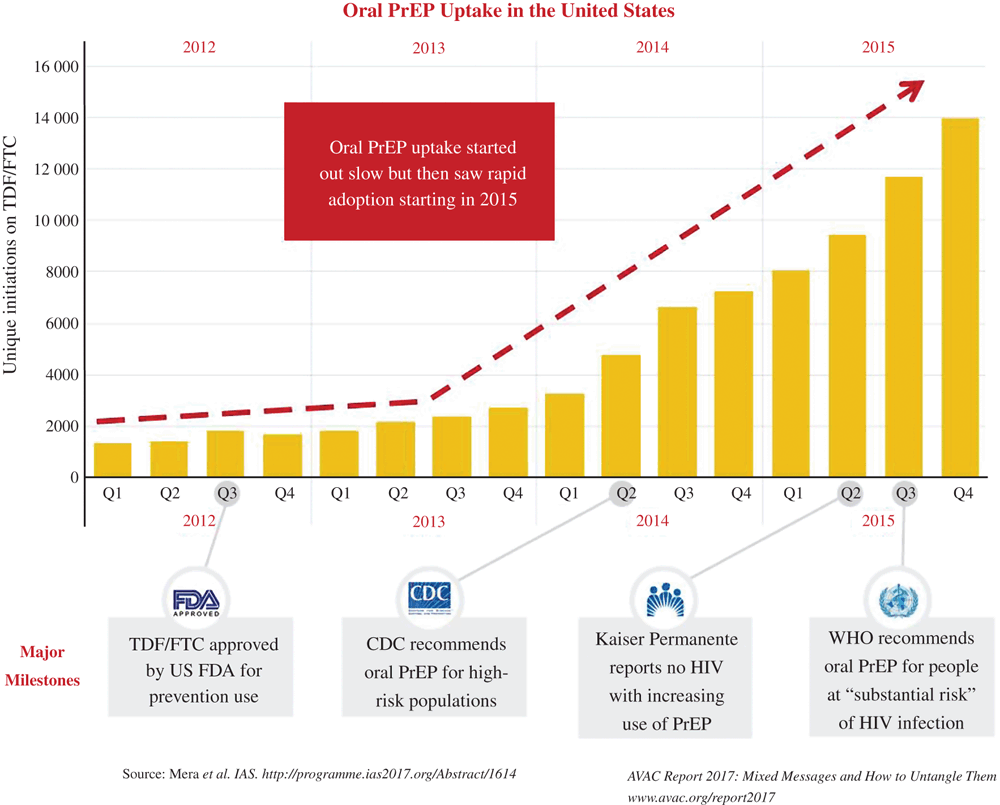
Another issue to consider is the appropriate management of expectations. Introduction of any new product takes time, and this is particularly true when products like oral PrEP are publicly funded. The implementation of oral PrEP among AGYW (and other populations) in sub-Saharan Africa is following familiar patterns to that in the United States, where there is strong evidence now of high uptake and use of oral PrEP among MSM (Fig. 2, as presented in the AVAC annual report14), even though it is still lower among certain subgroups, like young African–American MSM. However, when oral PrEP was introduced in the United States, its initial uptake was described as slow and even as a failure.
Furthermore, historically, new methods often have turbulent uptake trajectories. For example, several contraceptive methods have experienced the ‘boom-and-bust’ phenomenon – referring to initial optimism for a new product, followed by a rapid downturn due to unmet expectations.15 Some have highlighted similarities between oral PrEP and the introduction and initial slow uptake of the oral contraceptive pill. When the oral contraceptive pill was introduced in the 1950s, it was initially marketed for cycle control only in married women in an environment where contraception was taboo and choices were limited, and amidst fears that the pill would promote ‘sexual anarchy’ and promiscuity.16 Thus, use was highly constrained. However, now the contraceptive pill is one of the most widely used public health products globally.25 We must continue to interpret uptake of oral PrEP among AGYW in Africa with the larger understanding of product introduction timelines in mind.
Success in implementing oral PrEP is not necessarily defined by rapidly achieving large numbers of AGYW on oral PrEP, but by achieving the highest possible proportion of AGYW who are at substantial HIV risk who are using oral PrEP when they need and want it. As such, we should aim for high levels of uptake and coverage, as defined in this paper, with precisely defined and estimated denominators.
Conclusions and recommendations
We have outlined the oral PrEP cascade (Fig. 1), highlighted the variation in how metrics are defined, proposed a common lexicon for uptake and coverage (Table 1) and clarified why clear and accurate measurement is essential for appropriate investments to be made in oral PrEP delivery to AGYW. We have emphasised that uptake is only one step in the oral PrEP service delivery cascade that may limit coverage — the metric that ultimately determines epidemiological impact. As such, we have further noted that measures of ‘low’ uptake and coverage may not necessarily reflect ‘low’ demand exclusively. To improve program coverage, it is necessary to examine not just uptake, but each step of the cascade to identify bottlenecks and opportunities for improvement.
Specific recommendations
We recognise that operationalising data collection across the cascade may require significant investments in resources and time. We posit that it is important for this be done now and to use available data to improve implementation. Once oral PrEP programs are operating at scale, the need for intensive monitoring, with its consequent burden for facilities and programs, will likely be reduced. However, greater investments are needed now to:
-
Further refine the oral PrEP cascade and promote global guidance on standardised definitions and measures across the cascade;
-
Conduct size estimations to define the denominator for AGYW at substantial risk of HIV infection in priority settings to better estimate targets and coverage. No matter how we define the metrics, the absolute numbers of AGYW currently accessing oral PrEP is lower than optimal. One of the greatest barriers to estimating coverage among AGYW is the lack of quantification of those who are at substantial risk and likely to benefit from oral PrEP. We need better ways to understand and identify substantial risk that combine both spatial/environmental approaches with individual risk assessments, while at the same time appreciating that AGYW may be compelled to use PrEP for many reasons that require ongoing work to understand. Once these populations of AGYW at substantial risk are identified and quantified, we will be able to better understand the gap between those who could benefit from PrEP and those who are accessing it – an area of research that is currently underdeveloped;
-
Conduct operational and implementation research to identify bottlenecks and their drivers along the oral PrEP cascade, which can be addressed as oral PrEP delivery is taken to scale.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work reflects the insight and experience of the authors, who convened as an expert working group inclusive of modellers/academic experts, policymakers, program implementers and those engaged in country-level service delivery. The work was led by the OPTIONS Consortium, a program made possible by the generous assistance from the American people through the United States Agency for International Development (USAID), and the United States President’s Emergency Plan for AIDS Relief (PEPFAR). Financial assistance was provided by USAID to FHI 360, Wits RHI, and AVAC under the terms of Cooperative Agreement No. AID-OAA-A-15–00035. The contents do not necessarily reflect the the views of USAID or the United States Government.
References
[1] World Health Organization (WHO). Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: WHO; 2015.[2] United Nations Joint Programme on HIV/AIDS (UNAIDS). UNAIDS global HIV/AIDS fact sheet. Geneva: UNAIDS; 2017. Available online at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf [verified 11 October 2018].
[3] US Agency for International Development (USAID). Determined, Resilient, Empowered, AIDS-free, Mentored, and Safe (DREAMS) Program. DREAMS Partnership to reduce HIV/AIDS in adolescent girls and young women. Washington, DC: USAID; 2018. Available online at: https://www.usaid.gov/what-we-do/global-health/hiv-and-aids/technical-areas/dreams [verified 11 October 2018].
[4] Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc 2015; 18 19408
| Adolescent girls and young women: key populations for HIV epidemic control.Crossref | GoogleScholarGoogle Scholar |
[5] Delany-Moretlwe S, Mullick S, Eakle R, Rees H. Planning for HIV pre-exposure prophylaxis introduction: lessons learned from contraception. Curr Opin HIV AIDS 2016; 11 87–93.
| Planning for HIV pre-exposure prophylaxis introduction: lessons learned from contraception.Crossref | GoogleScholarGoogle Scholar |
[6] Mullick S, Chersich MF, Pillay Y, Rees H. Introduction of the contraceptive implant in South Africa: successes, challenges and the way forward. SAMJ: South African Medical Journal 2017; 107 812–4.
| Introduction of the contraceptive implant in South Africa: successes, challenges and the way forward.Crossref | GoogleScholarGoogle Scholar |
[7] Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90–90–90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Health 2016; 1 e000010
| Can the UNAIDS 90–90–90 target be achieved? A systematic analysis of national HIV treatment cascades.Crossref | GoogleScholarGoogle Scholar |
[8] World Health Organization (WHO). Consolidated strategic information guidelines for HIV in the Health Sector. Geneva: WHO; 2015.
[9] United Nations Joint Programme on HIV/AIDS (UNAIDS). Global AIDS Monitoring. Geneva: UNAIDS; 2018. Available online at: http://www.unaids.org/en/dataanalysis/knowyourresponse/globalaidsprogressreporting [verified 12 October 2018].
[10] World Health Organization (WHO). Cascade data use manual to identify gaps in HIV and health services for programme improvement. June 2018. Geneva: WHO; 2018.
[11] Hargreaves JR, Delany-Moretlwe S, Hallett TB, Johnson S, Kapiga S, Bhattacharjee P, Dallabetta G, Garnett GP. The HIV prevention cascade: integrating theories of epidemiological, behavioral, and social science into programme design and monitoring. Lancet HIV 2016; 3 e318–22.
| The HIV prevention cascade: integrating theories of epidemiological, behavioral, and social science into programme design and monitoring.Crossref | GoogleScholarGoogle Scholar |
[12] Garnett GP, Hallett TB, Takaruza A, Hargreaves J, Rhead R, Warren M, Nyamukapa C, Gregson S. Providing a conceptual framework for HIV prevention cascades and assessing feasibility of empirical measurement with data from east Zimbabwe: a case study. Lancet HIV 2016; 3 e297–306.
| Providing a conceptual framework for HIV prevention cascades and assessing feasibility of empirical measurement with data from east Zimbabwe: a case study.Crossref | GoogleScholarGoogle Scholar |
[13] World Health Organization (WHO). Implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection. Geneva: WHO; 2017.
[14] AVAC. Mixed messages and how to untangle them. New York: AVAC; 2017.
[15] Pleaner M, Morroni C, Smit J, Lince-Deroche N, Chersich MF, Mullick S, Pillay D, Makua M, Rees H. Lessons learnt from the introduction of the contraceptive implant in South Africa. S Afr Med J 2017; 107 933–8.
| Lessons learnt from the introduction of the contraceptive implant in South Africa.Crossref | GoogleScholarGoogle Scholar |
[16] Liao PV, Dollin J. Half a century of the oral contraceptive pill: historical review and view to the future. Can Fam Physician 2012; 58 e757–60.
[17] Kenya National AIDS & STI Control Programme (NASCOP). Implementation of PrEP in Kenya. Presented at the 19th Annual International Conference on AIDS and STIs in Africa (ICASA); 4–9 December 2017; Abidjan; Cote D’Ivoire. Abidjan: ICASA; 2017. Available online at: www.prepwatch.org/wp-content/uploads/2017/12/National_Implementation_Kenya.pdf [verified 31 October 2018].
[18] South African National Department of Health (NDoH). Pre-exposure prophylaxis implementation in South Africa. Presented at the 19th Annual International Conference on AIDS and STIs in Africa (ICASA); 4–9 December 2017; Abidjan; Cote D’Ivoire. Abidjan: ICASA; 2017. Available online at: www.prepwatch.org/wp-content/uploads/2017/12/PrEP_SA_ICASA2017.pdf [verified 31 October 2018].
[19] AVAC. Risk assessment tools and the identification of individuals at high-risk of HIV infection in the delivery of oral PrEP (Working Paper). New York: AVAC; 2017.
[20] Price JT, Rosenberg NE, Vansia D, Phanga T, Bhushan NL, Maseko B, Brar SK, Hosseinipour MC, Tang JH, Bekker L-G, Pettifor A. Predictors of HIV, HIV risk perception, and HIV worry among adolescent girls and young women in Lilongwe, Malawi. J Acquir Immune Defic Syndr 2018; 77 53–63.
[21] Haberer JE, Bangsberg DR, Baeten JM, Curran K, Koechlin F, Amico KR, Anderson P, Mugo N, Venter F, Goicochea P, Caceres C, O’Reilly K. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. AIDS 2015; 29 1277–85.
| Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm.Crossref | GoogleScholarGoogle Scholar |
[22] Haberer JE, Kidoguchi L, Heffron R, Mugo N, Bukusi E, Katabira E, Asiimwe S, Thomas KK, Celum C, Baeten JM. Alignment of adherence and risk for HIV acquisition in a demonstration project of pre-exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention-effective adherence. J Int AIDS Soc 2017; 20 21842
| Alignment of adherence and risk for HIV acquisition in a demonstration project of pre-exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention-effective adherence.Crossref | GoogleScholarGoogle Scholar |
[23] Hosek S, Celum C, Wilson CM, Kapogiannis B, Delany-Moretlwe S, Bekker L-G. Preventing HIV among adolescents with oral PrEP: observations and challenges in the United States and South Africa. J Int AIDS Soc 2016; 19 21107
| Preventing HIV among adolescents with oral PrEP: observations and challenges in the United States and South Africa.Crossref | GoogleScholarGoogle Scholar |
[24] Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, Matyjaszczyk M, Mshelia C, Clyne W, Aronson JK, Urquhart J. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012; 73 691–705.
| A new taxonomy for describing and defining adherence to medications.Crossref | GoogleScholarGoogle Scholar |
[25] Myers JE, Sepkowitz KA. A pill for HIV prevention: déjà vu all over again? Clin Infect Dis 2013; 56 1604–12.
| A pill for HIV prevention: déjà vu all over again?Crossref | GoogleScholarGoogle Scholar |
* These authors contributed equally to the conceptualisation and writing of the manuscript.
A Substantial risk of HIV infection is defined by the World Health Organization (WHO) as an incidence that is sufficiently high (>3% incidence) to make offering pre-exposure prophylaxis (PrEP) potentially cost-saving (or cost-effective).
B According to the World Health Organization (WHO), combination prevention includes the provision of HIV testing and counselling, and of male and female condoms, lubricants, antiretroviral treatment for partners with HIV infection, voluntary medical male circumcision and harm-reduction interventions for people who use drugs. 1