Reduced sensitivity from pooled urine, pharyngeal and rectal specimens when using a molecular assay for the detection of chlamydia and gonorrhoea near the point of care
Steven G. Badman A H * , Sara F. E. Bell B * , Judith A. Dean B , Jime Lemoire C , Luke Coffey C , Joseph Debattista D , Andrew M. Redmond C E , Owain D. Williams B , Charles F. Gilks B † and David M. Whiley F G †A The Kirby Institute, Level 6, Wallace Wurth Building, High Street, UNSW Sydney, Randwick, NSW 2032, Australia.
B School of Public Health, The University of Queensland, 288 Herston Road, Herston, Qld 4006, Australia.
C RAPID, Queensland Positive People, 21 Manilla Street, East Brisbane, Qld 4169, Australia.
D Metro North Public Health Unit, Bryden Street, Windsor, Qld 4030, Australia.
E Infectious Diseases Services, Royal Brisbane and Women’s Hospital, Herston, Qld 4029, Australia.
F Centre for Clinical Research, The University of Queensland, Building 71/918, Royal Brisbane and Women’s Hospital Campus, Herston, Qld 4029, Australia.
G Pathology Queensland, Level 4, Block 7, Royal Brisbane and Women’s Hospital, Herston, Qld 4006, Australia.
H Corresponding author. Email: sbadman@kirby.unsw.edu.au
Sexual Health 17(1) 15-21 https://doi.org/10.1071/SH19028
Submitted: 11 February 2019 Accepted: 12 September 2019 Published: 17 January 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Background: The aim of this study was to compare the performance of pooled self-collected urogenital, pharyngeal and anorectal specimens to that of individual specimen results for the molecular detection of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) near the point of care (POC) for diagnostic sensitivity. Methods: Clients (mostly men who have sex with men) attending an urban community testing service and three sex-on-premises venues in Brisbane, Australia, were offered CT and NG testing by trained lay providers. Participants provided three self-collected specimens (urine, pharyngeal and rectal) for testing by GeneXpert (Cepheid, Sunnyvale, CA, USA). If any of the individual specimens from a participant were positive, all three specimens were pooled and retested. Results: Of the 388 participants who provided three individual anatomical specimens, 76 (19.6%) were found to be positive for CT and/or NG at one or more sites. The pooling approach failed to detect five CT rectal and four NG pharyngeal infections. The overall performance (sensitivity) of the pooling approach compared with individual specimen testing and Cohen’s κ were 90.0% and 0.86 respectively for CT and 89.7% and 0.89 respectively for NG. Conclusions: Reduced sensitivity was observed when using pooled specimens for the detection of CT and NG using GeneXpert near the POC, similar to results reported in laboratory-based CT and NG pooling studies. These data suggest specimen pooling is feasible near to the POC, potentially saving time and costs when screening at-risk populations for CT and NG. Our data also suggest a reduction in pooled urine could improve overall test sensitivity.
Additional keywords: community, diagnostics, GeneXpert, men who have sex with men (MSM), peer, sexually transmissible infection (STI).
Introduction
Globally, it is estimated there are 131 million incident cases of Chlamydia trachomatis (CT) and 78 million incident cases of Neisseria gonorrhoeae (NG) infection each year.1 Both CT and NG can infect multiple anatomical sites, including urogenital, pharyngeal and anorectal sites in males and females.2–7 Infections are transmissible and often asymptomatic,8 so early detection and treatment of CT and NG rely on regular, comprehensive and effective testing of multiple anatomical sites for those at risk.4 For men who have sex with men (MSM), it is recognised that individuals may present with rectal and pharyngeal infections in the absence of genital infection.5,9 The Australian sexually transmissible infection (STI) management guidelines8 recommend that urethral, anorectal and pharyngeal testing is conducted up to four times a year for asymptomatic MSM depending on sexual practices. More recently, several studies have indicated the need to screen for rectal CT and NG among women,3,4,10–12 even in the absence of specific risk factors.7,13
However, testing multiple anatomical sites from the same person can increase costs and workload, and can be particularly onerous if trying to implement screening at or near the point of care (POC). To minimise testing costs and workload, three studies (n = 1064,14 n = 10015 and n = 10716) have investigated whether individual specimens from multiple anatomical sites can be pooled into one combined specimen for molecular testing. Two of these laboratory-based studies, conducted by experienced laboratory scientists and using the Aptima Combo 2 assay (Hologic, San Diego, CA, USA) and Abbott Real-time CT/NG test (Abbott, Chicago, IL, USA), identified a reduced sensitivity in the detection of both CT (92% and 90% respectively) and NG (90% and 91.7% respectively) using pooled urine, pharyngeal and rectal specimens.14,15 However, a third study using the GeneXpert (Xpert) CT/NG assay (Cepheid, Sunnyvale, CA, USA) observed 94% sensitivity for CT and 100% sensitivity for NG.16
The Xpert CT/NG assay is US Food and Drug Administration (FDA) approved for use in detecting urogenital CT and NG infections. Notably, the Xpert system is regarded as being suitable for POC testing,17 and studies conducted in Australia indicate it is well accepted as a POC method by clinical staff.18 Previous studies found the performance of the Xpert CT/NG assay also compares favourably with established laboratory-based assays,16–18 including for use in testing anorectal specimens at the POC.19 The Xpert CT/NG assay uses internal quality controls (sample adequacy and sample processing controls) in conjunction with predefined specimen cycling (maximum of 45) to determine the presence of DNA and establish specified test cut-off points and result types.
In this study, the performance of pooled urine, pharyngeal and rectal specimens compared with individual anatomical specimens in the Xpert CT/NG assay was further evaluated. Here, testing was conducted near the POC by trained lay providers who identify with the key population being targeted. These trained lay providers were existing service staff who identify as MSM and had already been trained in the provision of rapid HIV and syphilis POC testing for clients. Further training was provided in the use of the Xpert CT/NG assay, and standard operating procedures were established to ensure continuity and quality of testing. Day-to-day results and referral for treatment were supervised by a clinic physician.
Methods
Study design
This study was conducted in an established urban community clinic in Brisbane, Australia, providing free STI testing services. The clinic commenced trained lay provider-facilitated HIV and syphilis rapid POC testing predominately for MSM in August 2014. Acknowledging a gap in service, molecular POC testing using the Xpert CT/NG assay was introduced in March 2017.
This study undertook prospective consecutive sampling and recruitment of participants presenting at four testing locations (a community clinic and three sex-on-premises venues) from March 2017 to March 2018. Individuals aged ≥16 years were offered CT and NG testing in addition to HIV and/or syphilis rapid tests. Written consent was obtained before specimen collection.
Trained lay providers assisted each participant in making informed choices regarding anatomical sites to be tested based on individual risk factors. Participants who provided three individual specimens (urine, pharyngeal and rectal swabs) were included in the pooling comparison.
Participants at the four testing locations were guided through a self-collection instruction sheet (RAPID standard operating procedure; Queensland Positive People, Brisbane, Qld, Australia, pers. comm.) and asked to provide a first-pass urine specimen in a sterile container and further pharyngeal and rectal specimens using a different flocked swab for each collection. Participants were directed to the bathroom and, after collection, each swab was placed in a specimen transport tube by the participant with 2.3 mL Cepheid-manufactured universal transport medium (UTM). All three specimens were immediately returned to the trained lay providers, and neat urine was added to the specimen collection tube within 2 h to preserve DNA, in accordance with the manufacturer’s instructions.20
All specimens collected at the sex-on-premises venues were transferred back to the main clinic at the end of each period of testing in insulated containers for CT and NG POC testing. Because individual pharyngeal and rectal specimen testing occurred using an off-label method, the technique for specimen preparation and processing followed the manufacturer’s instructions for vaginal specimens.21
Specimen processing and pooling methods
For individual specimen testing on Xpert, 1 mL of UTM from the swab tubes or urine container was transferred by disposable pipette into an Xpert CT/NG assay cartridge specimen chamber before being tested in a 16-module Xpert instrument at the clinic. Time to result for all specimen types was approximately 90 min. If one or more anatomical specimens per participant provided a ‘detected’ result, then all three individual specimens were pooled and retested. The pooling method used in the present study has been described previously.16 Briefly, 1 mL of UTM from each of the pharyngeal and rectal swab specimen tubes was placed into an empty Cepheid urine collection tube. A further 7 mL of urine was added, providing a total volume of 9 mL for the pooled specimen. The pooled specimen was then inverted 10 times before a 1-mL aliquot of the pooled specimen was tested as described above.
Where possible, pooled specimens were tested on the same day as the individual specimens. However, this was not always possible due to clinic operating hours. Consequently, 51 of 76 of pooled specimens (67.1%) were tested on the same day as the individual specimens, 22 of 76 (28.9%) were tested the following day and three (3.9%) were tested within 3 days. Neat urine specimens were stored at room temperature for up to 3 days and in accordance with the manufacturer’s instructions.22 All samples were subjected to a predefined 45 cycles of testing for the detection of DNA, and samples produced either a ‘detected’ or ‘not detected’ result. Quality control was managed by internal sample adequacy and sample processing measures, plus monthly external CT and NG controls. All test results were recorded by a barcode number on a laptop computer connected to the Xpert system and transferred manually by trained lay providers to the clinic database.
We also conducted a laboratory experiment to determine if a reduction in urine volume from 7 to 1 mL (to minimise dilution of the swab samples) may improve detection in specimens. This experiment was conducted using a CT-positive clinical specimen and diluted in Cepheid UTM to provide a ‘base dilution’ sample with a Ct value of ~37.5 cycles (i.e. equivalent to the median Ct value observed for the five CT-positive rectal specimens not detected by pooling).
The base dilution sample was prepared and tested in three different ways (15 replicates for each): (1) 2 mL base dilution was added to 15 empty Cepheid transport tubes to emulate the same volume as 1 mL from an individual pharyngeal sample and 1 mL from a rectal sample before the addition of urine (Approach A); (2) 2 mL base dilution and 1 mL neat urine (known to be negative for both CT and NG) were added to a further 15 tubes (Approach B); and (3) 2 mL base dilution and 7 mL of neat urine were added to another 15 tubes (Approach C). All 45 specimens were tested on the Xpert system as outlined above, on the same day and in batches of 15.
Ethical considerations
The study was granted ethics approval by The University of Queensland Human Ethics Research Committee (UQHREC 2016001764, Therapeutic Goods Administration Clinical Trial Notification (CTN) Scheme Clinical Trial Number 00812-1).
Statistical analysis
Individual anatomical site results were compared with the corresponding pooled specimen results to determine relative sensitivity, by infection type and anatomical site, in conjunction with 95% confidence intervals (CI) using standard methods.23 Test result agreement was assessed using Cohen’s κ statistic.23 Cycle threshold (Ct) values (a semiquantitative marker of DNA concentration) of individual and pooled specimens were compared using paired-sample t-tests,23 Mann–Whitney tests23 and Wilcoxon signed-rank tests.23 Statistical analyses were performed using IBM SPSS Statistics for Windows, version 24.0. (IBM Corp., Armonk, NY, USA). An estimated percentage of pooled sensitivity was used to inform sample size calculation.
Data access
As per the approved study protocol, access to these data is limited to named investigators and trained lay providers and remains the property of the participating service providers. Access to these data may be considered by contacting the corresponding author. Access to the full study protocol is available on request.
Results
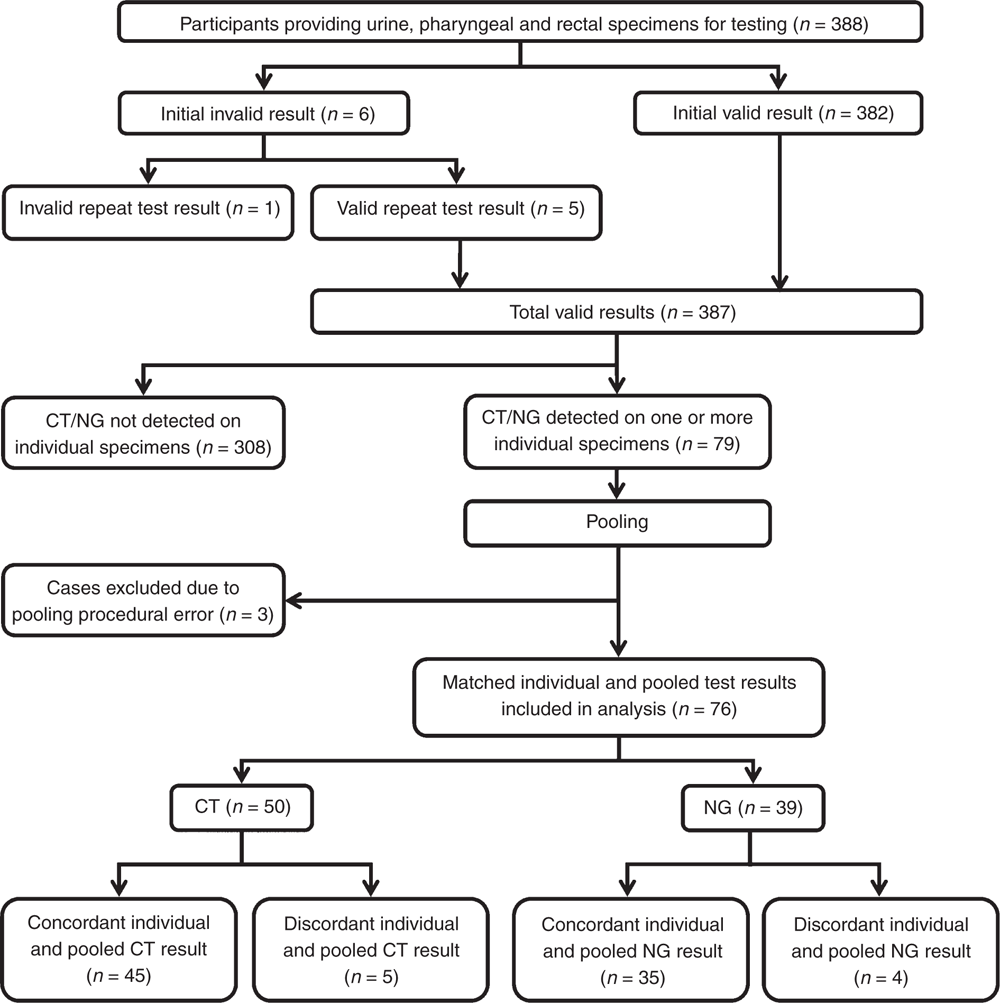
In all, 388 participants provided three individual anatomical specimens. On initial individual testing, 1.5% (6/388) of specimens returned an invalid result (one urine, one rectal, four pharyngeal). Of these, 83.3% (5/6) produced a valid result on retesting. Only one pharyngeal specimen returned a second invalid result, and this participant’s result was excluded from the analyses. Fig. 1 provides a summary of the study flow, with results of individual and pooled tests included in the concordance analysis.
CT and/or NG was detected in one or more individual anatomical specimens for 79 participants (20.4%). In all, 76 of the 79 participants with infection detected (96.2%) were eligible for inclusion in the pooling evaluation study; three (3.8%) participants were excluded due to specimen processing errors. Of the 76 eligible participants, 37 (48.7%), 26 (34.2%) and 13 (17.1%) had CT, NG or both CT and NG respectively. The median age of the 76 participants was 29 years (interquartile range (IQR) 24.0–37.75 years). Seventy-four participants (97.4%) identified as a male, with 67 of 74 (90.5%) reporting sex exclusively with men, and a further five (6.8%) reporting both male and female sexual partners. Of the remaining 2 of 76 participants (2.6%), one identified as female and one identified as transgender. Participants from sex-on-premises venues accounted for 38 of the 76 eligible for the pooling study (50.0%).
Performance of pooled testing
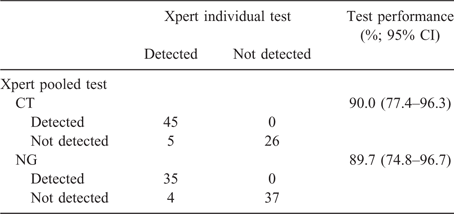
The agreement (κ) between individual and pooled results was 0.860 (95% CI 0.742–0.979) for CT and 0.895 (95% CI 0.795–0.995) for NG. Sensitivity and negative predictive value data are provided in Tables 1 and 2. Of note are the sensitivities of the pooling method for CT and NG (90.0% and 89.7% respectively). Eight of 76 specimens with CT and/or NG present were not detected using the pooling method. These comprised five rectal swabs by individual CT testing processed on Day 0 (n = 1) and Day 1 (n = 4) after individual specimen processing, and four pharyngeal swabs that detected NG tested on Day 0 (n = 1), Day 1 (n = 2) and Day 2 (n = 1) after individual specimen processing.
|

|
The Ct values for these specimens are provided in Tables S1 and S2, available as Supplementary Material to this paper. The Ct values for individual specimens (median 37.5 cycles for CT; 32.9 and 34.1 cycles for NG, given Xpert has two targets for gonorrhoea, NG2 and NG4) were significantly higher for rectal CT (P = 0.001) and pharyngeal NG (NG2, P = 0.003; NG4, P = 0.002) than those observed for specimens providing ‘detected’ results by pooling (Table S3). This suggests low bacterial loads are more likely to lead to false-negative results when specimens are pooled.
Eight swab specimens with relatively late Ct values (i.e. low bacterial loads) provided nine false-negative results via the pooling strategy (one individual specimen was positive for both CT and NG and negative for both on pooling).
The results of the additional laboratory experiment are summarised in Table S4. Nine, four and one of 15 replicates from Approaches A, B and C respectively provided ‘detected’ results for CT. The detection of replicates using Approach A was not significantly greater than that using Approach B (P = 0.14). Approach C detected significantly fewer replicates than Approach A (P = 0.0067), and although Approach B detected more replicates than Approach C, the difference was not statistically significant (P = 0.330).
Discussion
Overall, the results of the present study indicate that the pooling of specimens to increase the throughput of CT and NG testing at or near to the POC is feasible, but comes at a cost to assay sensitivity. Notably, the use of pooling resulted in Xpert sensitivity decreasing to approximately 90% for both CT and NG. These sensitivity data are similar to sensitivities reported by previous laboratory-based studies examining the use of pooling on both the Aptima Combo 2 and Abbott Real-time CT/NG assays (90–92%),14,15 suggesting there may be inherent limitations to the pooling approach that may affect all CT and NG molecular assays. Results from the present study appear to suggest overdilution of swab specimens containing low CT and NG DNA loads is a key problem. This is particularly relevant when people only have an extragenital infection, especially asymptomatic pharyngeal or rectal infections, both of which may harbour lower organism loads than urogenital infections,24 and when relatively large volumes of CT- and NG-negative urine are pooled with the rectal and pharyngeal swab specimens. The role of urea and organic acid inhibitors contained in neat urine, and their potential effects on test sensitivity, cannot be excluded when 7 mL is combined with the two swabs from different anatomical sites.
The issue of lower organism loads impeding detection by the pooling approach was further demonstrated by our additional pooling experiment. Of note, significantly more replicates were detected by Approach A (which was representative of testing individual swab specimens) than with Approach C (which used the same pooling volumes as per our clinical specimen testing). Although decreasing the volume of urine in Approach B (1 mL instead of 7 mL) did show some improvement compared with Approach C, the results were not statistically significant. Nevertheless, the results obtained using Approach B are encouraging and suggest further research around pooling volumes is warranted. We will be using Approach B in parallel with individual testing in our ongoing POC studies. Future studies may also want to consider the value of pooling only two specimens, such as urine and a rectal swab, as a means of improving CT and NG detection, especially if resources are limited and the need for separate treatment modalities for urogenital and anorectal CT and NG infections are to be maintained.
Notwithstanding the results described above, POC testing (as opposed to laboratory-based methods) can still achieve unquestionable benefits in terms of screening and the of number of people subsequently treated, even if test sensitivity is lower than clinically preferred. In fact, previous mathematical modelling has indicated POC tests of moderate sensitivity (as low as 50%) can still be of value, particularly in populations where people are unlikely to return for treatment or where the delay in treatment would result in significant STI transmission.25 Thus, the 90% sensitivity achieved here could still be quite advantageous, especially for individuals who have an asymptomatic STI, and ongoing transmission can be interrupted. The potential cost savings for low-resource settings using a pooling methodology would also be significant. Results from the additional laboratory-based experiment suggest the sensitivity for CT and NG detection could be improved using a reduction in urine volume from 7 to 1 mL in the preparation of the pooled specimen. A refined pooling method will be implemented in Phase 2 of this study to determine whether test sensitivity increases as a result of this volume reduction.
Of further interest in this study is the overall κ agreement between the individual and pooled sampling results which was high when testing was conducted by trained peer lay providers near the POC. Initially we were concerned that asking lay providers to implement a pooling approach could unnecessarily complicate POC testing methods and lead to sample handling errors. Overall, study results and testing in the hands of lay providers are promising and further highlight the feasibility of using pooled specimens near to the POC. The study results also highlight the importance of offering testing services that cover both urogenital and extragenital infections. If only urine specimens from these 76 participants had been tested, then 82.0% (41/50) of CT infections and 84.6% (33/39) of NG infections would have been missed.
We were also intrigued by the fact that two individual pharyngeal specimens that provided NG ‘detected’ results and were negative by pooling actually delivered Ct values for one NG target each in the pooled specimen (see Table S2; note, the Xpert NG test has two NG detection targets and both need to react to report a ‘detected’ NG result).
These results suggest the false-negative pooled specimens were on the edge of the detection limit of the Xpert assay and DNA could not be reliably detected for both NG targets.
This study has several limitations. First, due to a lack of funding, the specimens were not forwarded to a laboratory for comparative CT and NG testing. Therefore, we were unable to assess the performance of the trained lay providers or Xpert results against laboratory-based nucleic acid amplification (NAAT) testing. Similarly, only participants’ specimens with infection detected in any one of their individual specimens progressed to the pooling strategy. For this reason we were unable to perform any assessment of the specificity or negative predictive value of pooled samples (i.e. by testing of negative specimens) and our future studies need to take this into account. Similarly, the risk of sample contamination, environmental or otherwise, during the pooling process could not be investigated, but this risk was mitigated by participation in and satisfactory results from quality assurance testing.
The sensitivity of pooled CT and NG testing compared with the testing of individual anatomical specimens when using Xpert in the hands of trained lay providers was 90.0% (95% CI 77.4–96.3) for CT and 89.7% (95% CI 74.8–96.7) for NG. Overall, these pilot data suggest specimen pooling is feasible near to the POC when clinical testing guidelines are followed, and that significant time and costs could potentially be saved when screening at-risk populations for CT and NG, plus asymptomatic individuals and those living in low-resource settings. A second study will be conducted to determine if a reduction in the pooled urine volume improves test sensitivity for CT and NG detection near the POC.
Conflicts of interest
None declared.
Acknowledgements
The authors thank the participants and staff members who participated in this research, and acknowledge the collaborative involvement of sexual health and general practice services to which participants were referred. The authors acknowledge members of the Urban GeneXpert Trial Advisory Committee, namely Diane Rowling and Julian Langton-Lockton (Metro North Sexual Health and HIV Service), Rayden Rivett (Cepheid, Australia), Cassio Oliveira (Queensland Positive People), Melissa Warner (HIV Foundation Queensland and Communicable Diseases Branch, Department of Health, Queensland) and Rebecca Guy (Kirby Institute, UNSW, Sydney). The authors also thank The University of Queensland Centre for Clinical Research (UQCCR) for laboratory access to conduct the pooling experiment using its GeneXpert-XVI instrument. Funding for the study was provided by Metro North Hospital and Health Service and the HIV Foundation Queensland. Cepheid provided the 16-module GeneXpert-XVI instrument and some Xpert CT/NG assay cartridges free of charge.
References
[1] Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10 e0143304| Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting.Crossref | GoogleScholarGoogle Scholar | 26646541PubMed |
[2] Tongtoyai J, Todd CS, Chonwattana W, Pattanasin S, Chaikummao S, Varangrat A, et al. Prevalence and correlates of Chlamydia trachomatis and Neisseria gonorrhoeae by anatomic site among urban Thai men who have sex with men. Sex Transm Dis 2015; 42 440–9.
| Prevalence and correlates of Chlamydia trachomatis and Neisseria gonorrhoeae by anatomic site among urban Thai men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 26165436PubMed |
[3] van Liere GAFS, Dukers-Muijrers NHTM, Levels L, Hoebe CJPA. High proportion of anorectal Chlamydia trachomatis and Neisseria gonorrhoeae after routine universal urogenital and anorectal screening in women visiting the sexually transmitted infection clinic. Clin Infect Dis 2017; 64 1705–10.
| 28369227PubMed |
[4] van Liere GA, van Rooijen MS, Hoebe CJ, Heijman T, de Vries HJ, Dukers-Muijrers NH. Prevalence of and factors associated with rectal-only chlamydia and gonorrhoea in women and in men who have sex with men. PLoS One 2015; 10 e0140297
| Prevalence of and factors associated with rectal-only chlamydia and gonorrhoea in women and in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 26713628PubMed |
[5] Patton ME, Kidd S, Llata E, Stenger M, Braxton J, Asbel L, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men – STD Surveillance Network, United States, 2010–2012. Clin Infect Dis 2014; 58 1564–70.
| 24647015PubMed |
[6] Rank RG, Yeruva L. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 2014; 82 1362–71.
| Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection.Crossref | GoogleScholarGoogle Scholar | 24421044PubMed |
[7] Llata E, Braxton J, Asbel L, Chow J, Jenkins L, Murphy R, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol 2018; 132 692–7.
| Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse.Crossref | GoogleScholarGoogle Scholar | 30095784PubMed |
[8] Australasian Sexual Health Alliance. Australian STI management guidelines for use in primary care: MSM – men who have sex with men. 2016. Available online at: http://www.sti.guidelines.org.au/populations-and-situations/msm#testing-advice [verified 22 March 2018].
[9] van Liere GA, Hoebe CJ, Dukers-Muijrers NH. Evaluation of the anatomical site distribution of chlamydia and gonorrhoea in men who have sex with men and in high-risk women by routine testing: cross-sectional study revealing missed opportunities for treatment strategies. Sex Transm Infect 2014; 90 58–60.
| Evaluation of the anatomical site distribution of chlamydia and gonorrhoea in men who have sex with men and in high-risk women by routine testing: cross-sectional study revealing missed opportunities for treatment strategies.Crossref | GoogleScholarGoogle Scholar | 24106338PubMed |
[10] Chandra NL, Broad C, Folkard K, Town K, Harding-Esch EM, Woodhall SC, et al. Detection of Chlamydia trachomatis in rectal specimens in women and its association with anal intercourse: a systematic review and meta-analysis. Sex Transm Infect 2018; 94 320–6.
| Detection of Chlamydia trachomatis in rectal specimens in women and its association with anal intercourse: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 29431148PubMed |
[11] Bazan JA, Carr Reese P, Esber A, Lahey S, Ervin M, Davis JA, et al. High prevalence of rectal gonorrhea and chlamydia infection in women attending a sexually transmitted disease clinic. J Womens Health (Larchmt) 2015; 24 182–9.
| High prevalence of rectal gonorrhea and chlamydia infection in women attending a sexually transmitted disease clinic.Crossref | GoogleScholarGoogle Scholar |
[12] Javanbakht M, Gorbach P, Stirland A, Chien M, Kerndt P, Guerry S. Prevalence and correlates of rectal chlamydia and gonorrhea among female clients at sexually transmitted disease clinics. Sex Transm Dis 2012; 39 917–22.
| Prevalence and correlates of rectal chlamydia and gonorrhea among female clients at sexually transmitted disease clinics.Crossref | GoogleScholarGoogle Scholar | 23191945PubMed |
[13] Ding A, Challenor R. Rectal chlamydia in heterosexual women: more questions than answers. Int J STD AIDS 2014; 25 587–92.
| Rectal chlamydia in heterosexual women: more questions than answers.Crossref | GoogleScholarGoogle Scholar | 24352134PubMed |
[14] Sultan B, White JA, Fish R, Carrick G, Brima N, Copas A, et al. The ‘3 in 1’ study: pooling self-taken pharyngeal, urethral, and rectal samples into a single sample for analysis for detection of Neisseria gonorrhoeae and Chlamydia trachomatis in men who have sex with men. J Clin Microbiol 2016; 54 650–6.
| The ‘3 in 1’ study: pooling self-taken pharyngeal, urethral, and rectal samples into a single sample for analysis for detection of Neisseria gonorrhoeae and Chlamydia trachomatis in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 26719439PubMed |
[15] Thielemans E, Wyndham-Thomas C, Henrard S, De Vleeschouwer A, Steensels D, Montesinos I, et al. Screening for Chlamydia trachomatis and Neisseria gonorrhoeae infections in men who have sex with men: diagnostic accuracy of nucleic acid amplification test on pooled urine, anorectal, and pharyngeal specimens. Sex Transm Dis 2018; 45 195–8.
| Screening for Chlamydia trachomatis and Neisseria gonorrhoeae infections in men who have sex with men: diagnostic accuracy of nucleic acid amplification test on pooled urine, anorectal, and pharyngeal specimens.Crossref | GoogleScholarGoogle Scholar | 29419710PubMed |
[16] Speers DJ, Chua I-LJ, Manuel J, Marshall L. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis from pooled rectal, pharyngeal and urine specimens in men who have sex with men. Sex Transm Infect 2018; 94 293–7.
| Detection of Neisseria gonorrhoeae and Chlamydia trachomatis from pooled rectal, pharyngeal and urine specimens in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 29066627PubMed |
[17] Guy RJ, Natoli L, Ward J, Causer L, Hengel B, Whiley D, et al. A randomised trial of point-of-care tests for chlamydia and gonorrhoea infections in remote Aboriginal communities: Test, Treat ANd GO – the ‘TTANGO’ trial protocol. BMC Infect Dis 2013; 13 485
| A randomised trial of point-of-care tests for chlamydia and gonorrhoea infections in remote Aboriginal communities: Test, Treat ANd GO – the ‘TTANGO’ trial protocol.Crossref | GoogleScholarGoogle Scholar | 24138699PubMed |
[18] Natoli L, Guy RJ, Shephard M, Causer L, Badman SB, Hengel B, et al. ‘I do feel like a scientist at times’: a qualitative study of the acceptability of molecular point-of-care testing for chlamydia and gonorrhoea to primary care professionals in a remote high STI burden setting. PLoS One 2015; 10 e0145993
| ‘I do feel like a scientist at times’: a qualitative study of the acceptability of molecular point-of-care testing for chlamydia and gonorrhoea to primary care professionals in a remote high STI burden setting.Crossref | GoogleScholarGoogle Scholar | 26713441PubMed |
[19] Badman SG, Willie B, Narokobi R, Gabuzzi J, Pekon S, Amos-Kuma A, et al. A diagnostic evaluation of a molecular assay used for testing and treating anorectal chlamydia and gonorrhoea infections at the point-of-care in Papua New Guinea. Clin Microbiol Infect 2019; 25 623–7.
| A diagnostic evaluation of a molecular assay used for testing and treating anorectal chlamydia and gonorrhoea infections at the point-of-care in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 30107282PubMed |
[20] Cepheid. Xpert CT/NG urine specimen collection (first catch). 2012. Available online at: http://www.cepheid.com/administrator/components/com_productcatalog/library-files/21a33f55a6b1006f25fb9f479f1605ea-cabdac1a14bebd60d86aee0a84572cad-301-1792--Rev.%20A%20CT-NG%20Urine%20Sample%20Collection.pdf [verified 6 March 2018].
[21] Cepheid. Xpert CT/NG patient collected vaginal swab specimen collection. 2012. Available online at: http://www.cepheid.com/administrator/components/com_productcatalog/library-files/ada65ce36564c440d72bf9050200f33d-2f31dbd93ccc1a6c005c675af413b3e5-301-1790--Rev.%20A%20CT-NG%20Endocervical%20Sample%20Collection.pdf [verified 6 March 2018].
[22] Cepheid. XPERT CT/NG datasheet. 2018. Available online at: http://www.cepheid.com/administrator/components/com_productcatalog/library-files/c79b33a30737d79ec2275420913b888f-e6faea01628d163d8116a0af9ee201b4-CTNG-DATASHEET-400-02.pdf [verified 6 March 2018].
[23] Kirkwood BR, Sterne JAC. Essential medical statistics. 2nd edn. Malden: Blackwell Science; 2003.
[24] Priest D, Ong JJ, Chow EPF, Tabrizi S, Phillips S, Bissessor M, et al. Neisseria gonorrhoeae DNA bacterial load in men with symptomatic and asymptomatic gonococcal urethritis. Sex Transm Infect 2017; 93 478–81.
| Neisseria gonorrhoeae DNA bacterial load in men with symptomatic and asymptomatic gonococcal urethritis.Crossref | GoogleScholarGoogle Scholar | 28148678PubMed |
[25] Vickerman P, Watts C, Alary M, Mabey D, Peeling RW. Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex Transm Infect 2003; 79 363–7.
| Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women.Crossref | GoogleScholarGoogle Scholar | 14573829PubMed |
* These authors contributed equally to this paper as joint first authors.
† These authors are joint last authors.