Mpox knowledge, vaccination and intention to reduce sexual risk practices among men who have sex with men and transgender people in response to the 2022 mpox outbreak: a cross-sectional study in Victoria, Australia
Eric P. F. Chow A B C * , Ranjit S. Samra A , Catriona S. Bradshaw
A B C * , Ranjit S. Samra A , Catriona S. Bradshaw  A B C , Marcus Y. Chen
A B C , Marcus Y. Chen  A B , Deborah A. Williamson
A B , Deborah A. Williamson  D E F , Janet M. Towns
D E F , Janet M. Towns  A B , Kate Maddaford
A B , Kate Maddaford  A B , Finn Mercury A and Christopher K. Fairley
A B , Finn Mercury A and Christopher K. Fairley  A B
A B
A Melbourne Sexual Health Centre, Alfred Health, Melbourne, Vic., Australia.
B Central Clinical School, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, Vic., Australia.
C Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, Vic., Australia.
D Victorian Infectious Diseases Reference Laboratory, The Royal Melbourne Hospital at The Peter Doherty Institute for Infection and Immunity, Melbourne, Vic., Australia.
E Department of Infectious Diseases, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Melbourne, Vic., Australia.
F Walter and Eliza Hall Institute, Melbourne, Vic., Australia.
Abstract
The first mpox case was reported in May 2022 in Australia. Most cases have been diagnosed in men who have sex with men (MSM). This study aimed to examine community understanding of mpox, attitudes towards vaccination, and potential changes in sexual practices surrounding the mpox outbreak among MSM and transgender people in Victoria, Australia.
Participants were recruited from sexual health clinics and communities in Victoria, Australia, in August–October 2022. Participants were asked about their understanding and knowledge of mpox, vaccination uptake and intentions to change sexual practices. Univariable and multivariable logistic regression was performed to examine the factors associated with mpox vaccine uptake.
Most participants (97.8%, 525/537) had heard about mpox and 10.5% (55/525) knew someone who had had mpox. Of the 12 mpox knowledge questions, the median score of correct answers was 10 (IQR = 8–11) out of a maximum of 12. More than a third (36.6%, 191/522) had been vaccinated against mpox. MSM who had a good knowledge of mpox had the highest odds of receiving mpox vaccine compared with those who had poor knowledge (aOR = 4.05; 95% CI: 1.54–10.61). To prevent mpox, half reported they would reduce having sex with casual partners, stop having chemsex (used drugs for the purpose of sex), stop attending sex-on-premises-venues, and stop having group sex. A quarter reported they would increase condom use for anal sex.
One-third of high-risk participants and a substantial proportion of participants intended to reduce or stop certain practices, which may explain the large reduction in mpox cases.
Keywords: control, epidemiology, gay men, harm reduction, monkeypox, mpox, outbreak, prevention, sexual behaviour, sexual practice, sexually transmitted disease, sexually transmitted infection, STIs, vaccination, vaccine.
Introduction
Mpox (also previously known as monkeypox or MPX) is a zoonotic orthopoxvirus, previously endemic in central or western Africa.1–3 Mpox is endemic countries is primarily spread through animal-to-human contact or human-to-human via close skin-to-skin or household contacts, and it is not a typical sexually transmitted infection (STI).4,5 However, outbreaks of human-to-human mpox cases were reported in several non-endemic European countries from May 2022 with a rapid increase in the number of these cases, that occurred predominantly among gay, bisexual, and other men who have sex with men (MSM).6 Given the multi-country outbreak of mpox,7 the World Health Organization (WHO) declared ongoing mpox outbreaks in multiple countries a Public Health Emergency on 23 July 2022.8 As of 23 November 2022, there were 80 850 confirmed cases and 55 deaths among 110 countries.9
The first mpox case in Australia was reported in May 2022. On 28 July 2022, Australia declared the mpox outbreak as a Communicable Disease Incident of National Significance in Australia. As of 10 November 2022, there were 141 notified mpox cases in Australia, almost half (n = 69) were reported in Victoria;10 and most cases had been diagnosed among MSM.
Data have shown that the first-generation smallpox vaccines are effective (~85%) at cross-protecting against mpox;11 several countries (including Australia) have used the third-generation smallpox vaccine (modified vaccinia Ankara – Bavarian Nordic, MVA-BN) in their mpox vaccination programs in response to this mpox outbreak; however, the effectiveness of the third-generation vaccine against mpox in human is still not completely clear. Although some studies have demonstrated a relatively low level of virus-neutralising antibodies after MVA-BN vaccination,12 it is anticipated that these vaccination programs would be effective without having the definite evidence.
In Victoria, free mpox vaccines (JYNNEOS vaccine) have been available to eligible individuals since 12 August 2022. The primary aim of the mpox vaccines was used for pre-exposure prophylaxis in this campaign; however, vaccines can also be given as post-exposure prophylaxis if individuals have close contacts of mpox cases within 4 days.13 Because the initial stocks of the vaccine were limited, the first phase of the Victorian mpox vaccination program targeted higher risk sexually active MSM and transgender people who had had at least one STI in the past 12 months, or attending sex-on-premises venues (SOPV) or who were intending to travel to Europe or North America before 31 October 2022.14 This first phase of vaccination was only able to provide the first dose to 3500 MSM.15,16 The second phase of vaccination commenced in November 2022 targeting a wider population who were at-risk of mpox. Furthermore, several Australian community-based organisations in collaborations with the Department of Health have also launched public health education messaging on mpox via social media to increase the awareness of mpox and uptake of mpox vaccination.
At the beginning of this mpox outbreak, the primary mode of transmission was unclear although there has been cumulative evidence suggesting that mpox is primarily spread through sexual contact in this mpox outbreak.5,17–20 Furthermore, people who are diagnosed with mpox in this outbreak usually present with genital or anal lesions,19,20 which is not usually seen in previous mpox outbreaks in central or western Africa. It is also estimated about 14% of the severe mpox cases in this outbreak have required hospitalisation globally.21 With the new natural history and clinical presentation of mpox in this outbreak, it is reasonably hypothesised that people may change their sexual practices in order to reduce their risk of contracting mpox as a prevention strategy in the absence of widely available vaccines, and this approach was also seen in other pandemics such as HIV and coronavirus disease 2019 (COVID-19).22,23 Most international studies on mpox vaccination examining the willingness and determinants of receiving mpox vaccination but there have been very limited studies examining the changes in sexual practices during the mpox outbreak.24–29 A better understanding of the sexual practices of at-risk population may help to adjust prevention policies or strategies. This study aimed to examine community understanding of mpox, attitudes towards vaccination, and potential changes in sexual practices due to the mpox outbreak among MSM and transgender people in Victoria, Australia.
Materials and methods
Study design and study sample
An online anonymous survey using Qualtrics software (Provo, USA) was conducted between 24 August 2022 and 23 October 2022. Individuals were eligible if they were: (1) a man or trans woman who had sex with men; (2) at least 18 years old; and (3) currently living in Victoria, Australia. Females were not eligible to participate because they were not eligible for the initial mpox vaccination. The front page of the survey provided a description of the study including the aim of the study and involvement. A participant information sheet was also provided on the front page of the survey. Ethics approval was obtained from the Alfred Hospital Ethics Committee, Melbourne, Australia (494/22).
Participants were recruited from a sexual health clinic and the community in Victoria. For clinic recruitment, individuals who attended the Melbourne Sexual Health Centre (MSHC) during the study period and met the eligibility criteria received a single short message service (SMS) invitation to participate in the survey. This SMS included a brief statement of the study and the survey link. For community recruitment, recruitment flyers were posted on social media (i.e. Twitter and Facebook) via existing networks and LGBTQIA+ community, particularly to those in regional areas.
Eligible individuals were required to confirm they met the eligibility criteria, had read the participant information sheet, and consented to participate in the study. Individuals could select the ‘Agree’ button on the front page if they consented to participate; otherwise, they had the option to select ‘Disagree’ if they did not want to participate. Participants had the option to enter into a lucky draw for an AUD50 electronic voucher; and a total of 10 prizes were given.
Data collection and measures
The survey comprised three main sections. The first section collected demographic characteristics (e.g. age, country of birth, education level), and sexual health and practices (e.g. HIV status, PrEP use, STI diagnoses in the past 12 months, intention to travel overseas and planning to have casual sex while travelling). The second section asked about mpox knowledge. Participants were first asked whether they had heard of mpox, but they were not asked about mpox knowledge if they had never heard of mpox. This section comprised 12 true or false statements about the mpox outbreak, transmission, symptoms, and prevention. The knowledge questions were developed from a previous survey examining mpox knowledge among general practitioners in Indonesia,30 and we modified the questions so that they referred to Australia and could be read by laypersons. Participants were also asked to score on a scale from 0 (not at all concerned) to 10 (very concerned), about how concerned they were about catching mpox; and also from 0 (not sick at all) to 10 (extremely sick), about how sick they thought individuals would get if they caught mpox. The third section included questions about whether they had received or intention to receive the mpox vaccine, and also whether they would be willing to change their current sexual practices because of the mpox outbreak.
During the mpox outbreak, one-third of the Australian mpox cases were diagnosed at MSHC. The first mpox case at MSHC was diagnosed in June 2022. We also extracted the weekly number of mpox cases diagnosed at MSHC between June 2022 and October 2022 (i.e. end of survey recruitment).
Statistical analyses
In this analysis, we included participants who had completed the questions to key variables for analyses (e.g. mpox knowledge, vaccination uptake and vaccination intention). Descriptive statistics were used to report the frequency and proportion of study variables. Participants were asked whether they had received the mpox vaccine during the mpox vaccination campaign, or whether they had intention to receive the vaccine, and they could select ‘yes’, ‘no’, ‘I do not know’ or ‘prefer not to say’, and these options are adopted from previous vaccination intention studies.31,32 Of the 12 mpox knowledge questions, participants scored one point for a correct statement and zero points for an incorrect statement, so total scores for all 12 statements ranged from 0 to 12. The median and interquartile range (IQR) of the total knowledge score was calculated.
Two separate logistic regression analyses were performed to identify the characteristics (e.g. age, HIV status, PrEP use, history of other vaccinations, intention to travel overseas and planning to have casual sex while travelling) that were associated with: (1) mpox vaccine uptake (i.e. dependent variable); and (2) mpox vaccination intention (i.e. dependent variable). Participants who were unsure or preferred not to say whether they had received the mpox vaccine or intended to receive the mpox vaccine were excluded from both logistic regression analyses, as per previous vaccination uptake studies.33,34 Vaccinated participants were excluded from the mpox vaccination intention analysis. We performed univariable logistic regression separately with all the explanatory variables, these variables were selected because they were identified in the literature as known risk factors associated with mpox or a priori knowledge. The Box–Tidwell test was used to check for the linearity assumption between continuous independent variables and logit transformation of the dependent variable. Due to the non-linearity relationships between the continuous independent and logit transformation of the dependent variable, we categorised age into four categories (18–24 years; 25–34 years; 35–44 years; ≥45 years) as per the Australian Bureau of Statistics’ standard 10 year groupings.35 The number of partners was categorised into five categories (0–1; 2–5; 6–10; 11–20; ≥21) as per the Gay Community Periodic Survey.36 The mpox knowledge into three categories (poor; moderate; high), where poor was defined as individuals who scored 0–50% (i.e. 0–6 score), moderate as individuals who scored 51–75% (i.e. 7–9 score), and good as individuals who scored 76–100% (10–12 score), as per previous studies.37 Independent variables with P-value less than 0.20 in the univariable logistic regression were included in the multivariable logistic regression using backward elimination. The overall significance of categorical variables was used for model selection. Any independent variables with a P-value less than 0.05 were retained in the final model. Crude and adjusted odds ratios (OR) and their corresponding 95% confidence intervals (CI) were reported. We used Hosmer–Lemeshow test to assess the goodness of fit and C-statistics was used to assess the strength of fit of the multivariable logistic regression models. All statistical analyses were conducted in Stata (ver. 17, College Station, TX, USA). All figures were generated in R (ver. 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 563 eligible participants consented to participate in the study and 537 (95.4%) completed the questions to key variables for analyses and were included in the final analysis; of these, 531 (98.9%) had complete data. Table 1 shows the median age of the participants was 33 years (IQR 28–42) and almost all were cisgender men (99.1%, 532/537). Most were highly educated with 69.8% (375/537) completed a university degree. Two-thirds of the participants (68.9%, 370/537) were recruited from a sexual health clinic, and one-third (31.1%, 167/537) were recruited from the community. The median number of male sexual partners in the past 12 months was 10 (IQR 4–20), and 42.1% (226/537) reported having an STI diagnosis other than HIV in the past 12 months.
| Variable | n | % | |
|---|---|---|---|
| Age (years), median (IQR)A | 33 | 28–42 | |
| Gender, n (%) | |||
| Cisgender men | 532 | 99.1 | |
| Transgender men | 4 | 0.7 | |
| Transgender women | 1 | 0.2 | |
| Country of birth, n (%) | |||
| Australia | 287 | 53.4 | |
| Overseas | 244 | 45.4 | |
| Prefer not to say/missing | 6 | 1.1 | |
| Highest level of education, n (%) | |||
| Primary/secondary school | 72 | 13.4 | |
| Certificates/diplomas/apprenticeships | 90 | 16.8 | |
| University | 375 | 69.8 | |
| Source of recruitment, n (%) | |||
| Community/social media | 167 | 31.1 | |
| Sexual health clinics | 370 | 68.9 | |
| HIV status and PrEP use, n (%) | |||
| Living with HIV | 47 | 8.8 | |
| Currently taking HIV PrEP | 267 | 49.7 | |
| Not living with HIV and not taking PrEP | 222 | 41.3 | |
| Prefer not to say | 1 | 0.2 | |
| Currently working as a sex worker, n (%) | |||
| No | 528 | 98.3 | |
| Yes | 6 | 1.1 | |
| Prefer not to say | 3 | 0.6 | |
| Number of male sexual partners in the past 12 months, median (IQR)A | 10 | 4–20 | |
| Had been diagnosed with an STI other than HIV in the past 12 months, n (%) | |||
| No | 309 | 57.5 | |
| Yes | 226 | 42.1 | |
| Prefer not to say | 2 | 0.4 | |
| Had used drugs in the past 12 months, n (%) | |||
| No | 357 | 66.5 | |
| Yes | 175 | 32.6 | |
| Prefer not to say | 5 | 0.9 | |
| Group sex in the past 12 months, n (%) | |||
| No | 336 | 62.6 | |
| Yes | 201 | 37.4 | |
ATwo participants did not report age or the number of male sexual partners.
Most participants (97.8%, 525/537) had heard about mpox, 10.5% (55/525) knew someone who had had mpox, and a small proportion (1.3%, 7/525) had had close contact with someone who was diagnosed with mpox. Of the 12 mpox knowledge questions, the median score of correct answers was 10 (IQR 8–11). Table 2 shows that more than one-third of the participants (38.3%, 201/525) stated mpox was a newly discovered virus. Most participants correctly identified mpox could be transmitted through sexual contact with an infected person (94.7%, 497/525), and infected individuals would have flu-like symptoms (88.1%, 461/523) and ulcers, blisters or sores (97.3%, 509/523). More than two-thirds of participants (68.7%, 360/524) correctly identified the smallpox vaccine was thought to be effective against mpox. However, 29.8% (156/524) participants reported there was no effective vaccine, nor did they not know any vaccine that was effective against mpox. Only 5.0% (26/525) of participants did not know how mpox could be transmitted. The median score in relation to the concern about catching mpox was 6 (IQR 4–8), with 17.7% (91/515) scoring 10 (very concerned). The median score of the perceived severity of sickness when the individuals had mpox was 7 (IQR 5–8), with 13.3% (69/517) scored 10 (extremely sick).
| Statements | n | % | |
|---|---|---|---|
| Mpox is a newly discovered virus | |||
| True | 201 | 38.3 | |
| FalseA | 324 | 61.7 | |
| There are cases of human mpox cases occurring in Australia now | |||
| TrueA | 519 | 98.9 | |
| False | 6 | 1.1 | |
| Mpox can be transmitted through breathing in respiratory droplets from an infected person | |||
| TrueA | 320 | 61.0 | |
| False | 205 | 39.0 | |
| Mpox can be transmitted through tongue kissing | |||
| TrueA | 412 | 78.5 | |
| False | 113 | 21.5 | |
| Mpox can be transmitted through sexual contact with an infected person | |||
| TrueA | 497 | 94.7 | |
| False | 28 | 5.3 | |
| Mpox can be transmitted through contact with contaminated clothing, bedding or towels | |||
| TrueA | 390 | 74.3 | |
| False | 135 | 25.7 | |
| Ulcers, blisters or sores on the skin and/or genitals are one of the signs or symptoms of human mpox | |||
| TrueA | 509 | 97.3 | |
| False | 14 | 2.7 | |
| Flu-like illness (fever, chills, headache) is one of the signs or symptoms of human mpox | |||
| TrueA | 461 | 88.1 | |
| False | 62 | 11.9 | |
| A vaccine exists to prevent human mpox | |||
| TrueA | 474 | 90.6 | |
| False | 49 | 9.4 | |
| Mpox is a self-limiting infection (i.e. can be resolved without taking any form of medicine), N = 521 | |||
| TrueA | 351 | 67.4 | |
| False | 170 | 32.6 | |
| If an individual has mpox, the individual needs to abstain from sex, N = 522 | |||
| YesA | 489 | 93.7 | |
| No | 9 | 1.7 | |
| I do not know | 24 | 4.6 | |
| Vaccine is thought to be effective against human mpox (N = 524) | |||
| COVID-19 vaccine | 9 | 1.7 | |
| Flu vaccine | 10 | 1.9 | |
| Hepatitis A/B vaccine | 13 | 2.5 | |
| Human papillomavirus vaccine | 24 | 4.6 | |
| Smallpox vaccineA | 360 | 68.7 | |
| No vaccine is effective against mpox | 59 | 11.3 | |
| I don’t know if any vaccine is effective against mpox | 97 | 18.5 | |
ACorrect response for the statement.
One-third of the participants (36.6%, 191/522) reported they had had the mpox vaccine, 59.8% (312/522) had not had the mpox vaccine and 3.6% (19/522) were unsure. Compared with participants who were not PrEP users, PrEP users (aOR 3.08, 95% CI: 1.82–5.20) had higher odds of being vaccinated against mpox after adjusting for other potential confounders (Table 3). Furthermore, individuals with the following characteristics also had higher odds of being vaccinated against mpox: aged 35–44 years compared to those aged 18–24 years (aOR 2.61; 95% CI: 1.09–6.22), those who had completed certificates/diplomas/apprenticeships compared to those completed primary/secondary school (aOR 2.54; 95% CI: 1.11–5.81), those being recruited from sexual health clinic compared to community (aOR 1.79; 95% CI: 1.10–2.92), those who had an STI diagnosis in the past 12 months (aOR 1.80; 95% CI: 1.12–2.90) and those who had a good mpox knowledge compared to poor knowledge (aOR 4.05; 95% CI: 1.54–10.61). The Hosmer–Lemeshow test showed that there was no evidence of lack of fit in the multivariable logistic regression model (χ2 = 266.94, P = 0.625) and the C-statistic of 0.796 suggested a high degree of model strength.
| Characteristics | n/N (%) | OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Age (years) | 0.001A | 0.147A | ||||
| 18–24 | 12/54 (22.2%) | 1 | Ref | 1 | Ref | |
| 25–34 | 68/213 (31.9%) | 1.64 (0.81–3.32) | 0.167 | 0.99 (0.43–2.27) | 0.973 | |
| 35–44 | 63/128 (49.2%) | 3.39 (1.64–7.03) | 0.001 | 2.61 (1.09–6.22) | 0.031 | |
| ≥45 | 45/101 (44.6%) | 2.81 (1.33–5.97) | 0.007 | 1.41 (0.58–3.43) | 0.444 | |
| Gender | ||||||
| Cisgender men | 187/491 (39.1%) | 1 | Ref | |||
| Transgender peopleB | 1/5 (20.0%) | 0.41 (0.05–3.66) | 0.422 | |||
| Country of birth | ||||||
| Australia | 100/261 (38.3%) | 1 | Ref | |||
| Overseas/prefer not to say/missing | 88/235 (37.4%) | 0.96 (0.67–1.39) | 0.842 | |||
| Highest level of education | ||||||
| Primary/secondary school | 21/68 (30.9%) | 1 | Ref | 1 | Ref | |
| Certificates/diplomas/apprenticeships | 37/74 (50.0%) | 2.24 (1.13–4.45) | 0.022 | 2.54 (1.11–5.81) | 0.027 | |
| University | 130/354 (36.7%) | 1.30 (0.74–2.27) | 0.358 | 1.30 (0.65–2.59) | 0.457 | |
| Source of recruitment | ||||||
| Community/social media | 40/153 (26.1%) | 1 | Ref | 1 | Ref | |
| Sexual health clinics | 148/343 (43.1%) | 2.14 (1.41–3.26) | <0.001 | 1.79 (1.11–2.92) | 0.019 | |
| HIV status and PrEP use | ||||||
| Non-PrEP users | 37/206 (18.0%) | 1 | Ref | 1 | Ref | |
| PrEP users | 129/246 (52.4%) | 4.57 (2.29–9.10) | <0.001 | 3.08 (1.82–5.20) | <0.001 | |
| Living with HIV | 22/44 (50.0%) | 5.04 (3.26–7.78) | <0.001 | 2.18 (0.99–4.81) | 0.053 | |
| Currently working as a sex worker | ||||||
| No | 184/488 (37.7%) | 1 | Ref | |||
| Yes | 1/3 (33.3%) | 2.48 (0.41–14.97) | 0.323 | |||
| Prefer not to say | 3/5 (60.0%) | 0.83 (0.07–9.17) | 0.876 | |||
| Number of male sexual partners in the past 12 months | <0.001A | 0.060A | ||||
| 0–1 | 7/41 (17.1%) | 1 | Ref | 1 | Ref | |
| 2–5 | 29/127 (22.8%) | 1.44 (0.58–3.58) | 0.436 | 0.78 (0.29–2.15) | 0.636 | |
| 6–10 | 34/111 (30.6%) | 2.14 (0.86–5.32) | 0.100 | 0.81 (0.29–2.26) | 0.690 | |
| 11–20 | 47/107 (43.9%) | 3.80 (1.55–9.35) | 0.004 | 0.96 (0.34–2.73) | 0.944 | |
| ≥21 | 71/110 (64.5%) | 8.84 (3.59–21.80) | <0.001 | 2.64 (0.93–7.48) | 0.068 | |
| Mpox knowledge score | 0.001A | 0.005A | ||||
| Poor (0–50%, 0–6) | 7/42 (16.7%) | 1 | Ref | 1 | Ref | |
| Moderate (51–75%, 7–9) | 45/153 (29.4%) | 2.08 (0.86–5.04) | 0.103 | 2.09 (0.77–5.69) | 0.150 | |
| Good (76–100%, 10–12) | 136/301 (45.2%) | 4.12 (1.77–9.57) | 0.001 | 4.05 (1.54–10.61) | 0.004 | |
| STI diagnoses in the past 12 months | ||||||
| No | 74/286 (25.9%) | 1 | Ref | 1 | Ref | |
| Yes | 114/210 (54.3%) | 3.40 (2.33–4.97) | <0.001 | 1.80 (1.12–2.90) | 0.016 | |
| Drug use in the past 12 months | ||||||
| No | 112/336 (33.3%) | 1 | Ref | |||
| Yes | 74/155 (47.7%) | 1.83 (1.24–2.69) | 0.002 | |||
| Prefer not to say | 2/5 (40.0%) | 1.33 (0.22–8.09) | 0.755 | |||
| Casual partners in the past 12 months | ||||||
| No | 11/60 (18.3%) | 1 | Ref | |||
| Yes | 177/436 (40.6%) | 3.04 (1.54–6.02) | 0.001 | |||
| Group sex in the past 12 months | ||||||
| No | 94/310 (30.3%) | 1 | Ref | |||
| Yes | 94/186 (50.5%) | 2.35 (1.61–3.42) | <0.001 | |||
| Used drugs for the purpose of sex in the past 12 months | ||||||
| No | 164/442 (37.1%) | 1 | Ref | |||
| Yes | 24/54 (44.4%) | 1.36 (0.77–2.40) | 0.295 | |||
| Attended SOPV in the past 12 months | ||||||
| No | 86/295 (29.2%) | 1 | Ref | |||
| Yes | 102/201 (50.7%) | 2.50 (1.72–3.64) | <0.001 | |||
| Condomless anal sex in the past 12 months | ||||||
| No | 25/127 (19.7%) | 1 | Ref | |||
| Yes | 163/369 (44.2%) | 3.23 (1.99–5.23) | <0.001 | |||
| Planning travelling overseas to the UK, Europe or North America before 31 October 2022 | ||||||
| No | 162/431 (37.6%) | 1 | Ref | |||
| Yes, and planned to have casual sex | 23/43 (53.5%) | 1.91 (1.02–3.59) | 0.044 | |||
| Yes, and did not plan to have casual sex | 1/8 (12.5%) | 0.24 (0.03–1.95) | 0.180 | |||
| Yes, and did not know whether they would have casual sex | 2/14 (14.3%) | 0.28 (0.06–1.25) | 0.095 | |||
Note: there were 522 participants reported mpox vaccination status, and 26 participants were excluded from this analysis because 19 were unsure whether they had had the mpox vaccine and seven did not have complete data (e.g. age or number of male partners) for the multivariable analyses.
CI, confidence intervals; OR, odds ratio; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infections; SOPV, sex-on-premises venues; n, number of participants who had received mpox vaccine; N, number of participants in the category.
AP for trend.
BDue to the small number of transgender people, this category includes four transgender men and one transgender woman.
Of the 312 participants who did not have the mpox vaccine at the time of the survey, 68.3% (213/312) reported they intended to get vaccinated, 8.0% (25/312) reported they would not get vaccinated, 23.4% (73/312) were unsure whether they would get vaccinated, and 0.3% (1/312) preferred not to answer. Eighteen participants planned to have casual sex while travelling overseas (i.e. UK, Europe or North America) before 31 October 2022, and all (100%) intended to receive the mpox vaccine. The multivariable analysis showed that PrEP users (aOR = 3.40; 95% CI: 1.21–9.53) and those who had attended SOPV in the past 12 months (aOR = 3.84; 95% CI: 1.10–13.44) had higher odds of having the intention to receive the mpox vaccine (Table 4).
| Characteristics | n/N (%) | OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Age (years) | 0.855A | |||||
| 18–24 | 24/28 (85.7%) | 1 | Ref | |||
| 25–34 | 103/113 (91.2%) | 1.72 (0.50–5.94) | 0.394 | |||
| 35–44 | 42/49 (85.7%) | 1.00 (0.27–3.77) | 1.000 | |||
| ≥45 | 41/45 (91.1%) | 1.71 (0.39–7.46) | 0.477 | |||
| Gender | ||||||
| Cisgender men | 209/233 (89.7%) | 1 | Ref | |||
| Transgender peopleB | 1/2 (50.0%) | 0.11 (0.01–1.90) | 0.130 | |||
| Country of birth | ||||||
| Australia | 107/120 (89.2%) | 1 | Ref | |||
| Overseas/prefer not to say/missing | 103/115 (89.6%) | 0.96 (0.42–2.20) | 0.921 | |||
| Highest level of education | ||||||
| Primary/secondary school | 30/34 (88.2%) | 1 | Ref | |||
| Certificates/diplomas/apprenticeships | 23/28 (82.1%) | 0.61 (0.15–2.54) | 0.501 | |||
| University | 157/173 (90.8%) | 0.31 (0.41–4.19) | 0.651 | |||
| Source of recruitment | ||||||
| Community/social media | 67/80 (83.8%) | 1 | Ref | |||
| Sexual health clinics | 143/155 (92.3%) | 2.31 (1.00–5.34) | 0.050 | |||
| HIV status and PrEP use | ||||||
| Non-PrEP users | 98/118 (83.1%) | 1 | Ref | 1 | Ref | |
| PrEP users | 95/100 (95.0%) | 3.88 (1.40–10.75) | 0.009 | 3.40 (1.21–9.53) | 0.020 | |
| Living with HIV | 17/17 (100%) | NA | NA | NA | NA | |
| Currently working as a sex worker | ||||||
| No | 208/233 (89.3%) | 1 | Ref | |||
| Yes | 2/2 (100%) | NA | NA | |||
| Prefer not to answer | ||||||
| Number of male sexual partners in the past 12 months | 0.011A | |||||
| 0–1 | 13/19 (68.4%) | 1 | Ref | |||
| 2–5 | 63/72 (87.5%) | 3.23 (0.98–10.65) | 0.054 | |||
| 6–10 | 55/60 (91.7%) | 5.08 (1.34–19.23) | 0.017 | |||
| 11–20 | 48/51 (94.1%) | 7.38 (1.62–33.61) | 0.010 | |||
| ≥21 | 31/33 (93.9%) | 7.15 (1.27–40.21) | 0.025 | |||
| Mpox knowledge score | ||||||
| Poor (0–50%, 0–6) | 17/20 (85.0%) | 1 | Ref | |||
| Moderate (51–75%, 7–9) | 66/79 (83.5%) | 0.90 (0.23–3.50) | 0.875 | |||
| Good (76–100%, 10–12) | 127/136 (93.4%) | 2.49 (0.61–10.11) | 0.202 | |||
| STI diagnoses in the past 12 months | ||||||
| No | 132/152 (86.8%) | 1 | Ref | |||
| Yes | 78/83 (94.0%) | 2.36 (0.85–6.55) | 0.098 | |||
| Drug use in the past 12 months | ||||||
| No | 151/169 (89.4%) | 1 | Ref | |||
| Yes | 56/63 (88.9%) | 0.95 (0.38–2.41) | 0.920 | |||
| Prefer not to answer | 3/3 (100%) | – | ||||
| Casual partners in the past 12 months | ||||||
| No | 24/30 (80.0%) | 1 | Ref | |||
| Yes | 186/205 (90.7%) | 2.45 (0.89–6.73) | 0.083 | |||
| Group sex in the past 12 months | ||||||
| No | 135/157 (86.0%) | 1 | Ref | |||
| Yes | 75/78 (96.2%) | 4.07 (1.18–14.06) | 0.026 | |||
| Used drugs for the purpose of sex in the past 12 months | ||||||
| No | 187/208 (89.9%) | 1 | Ref | |||
| Yes | 23/27 (85.2%) | 0.65 (0.20–2.05) | 0.457 | |||
| Attended SOPV in the past 12 months | ||||||
| No | 131/153 (85.6%) | 1 | Ref | 1 | Ref | |
| Yes | 79/82 (96.3%) | 4.42 (1.28–15.25) | 0.019 | 3.84 (1.10–13.44) | 0.036 | |
| Condomless anal sex in the past 12 months | ||||||
| No | 66/76 (86.8%) | 1 | Ref | |||
| Yes | 144/159 (90.6%) | 1.45 (0.62–3.41) | 0.388 | |||
| Planning travelling overseas to the UK, Europe or North America before 31 October 2022 | ||||||
| No | 178/201 (88.6%) | 1 | Ref | |||
| Yes, and planned to have casual sex | 18/18 (100%) | NA | NA | |||
| Yes, and did not plan to have casual sex | 4/5 (80.0%) | 0.52 (0.06–4.83) | 0.563 | |||
| Yes, and did not know whether they would have casual sex | 10/11 (90.9%) | 1.29 (0.16–10.56) | 0.811 | |||
Note: there were 312 unvaccinated participants, and 77 participants were excluded from this analysis because 73 were unsure whether they would get vaccinated and four did not have complete data (e.g. age or number of male partners) for the multivariable analyses.
CI, confidence intervals; OR, odds ratio; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infections; SOPV, sex-on-premises venues; n, number of unvaccinated participants intended to receive mpox vaccine; N, number of unvaccinated participants in the category.
AP for trend.
BDue to the small number of transgender people, this category includes four transgender men and one transgender woman.
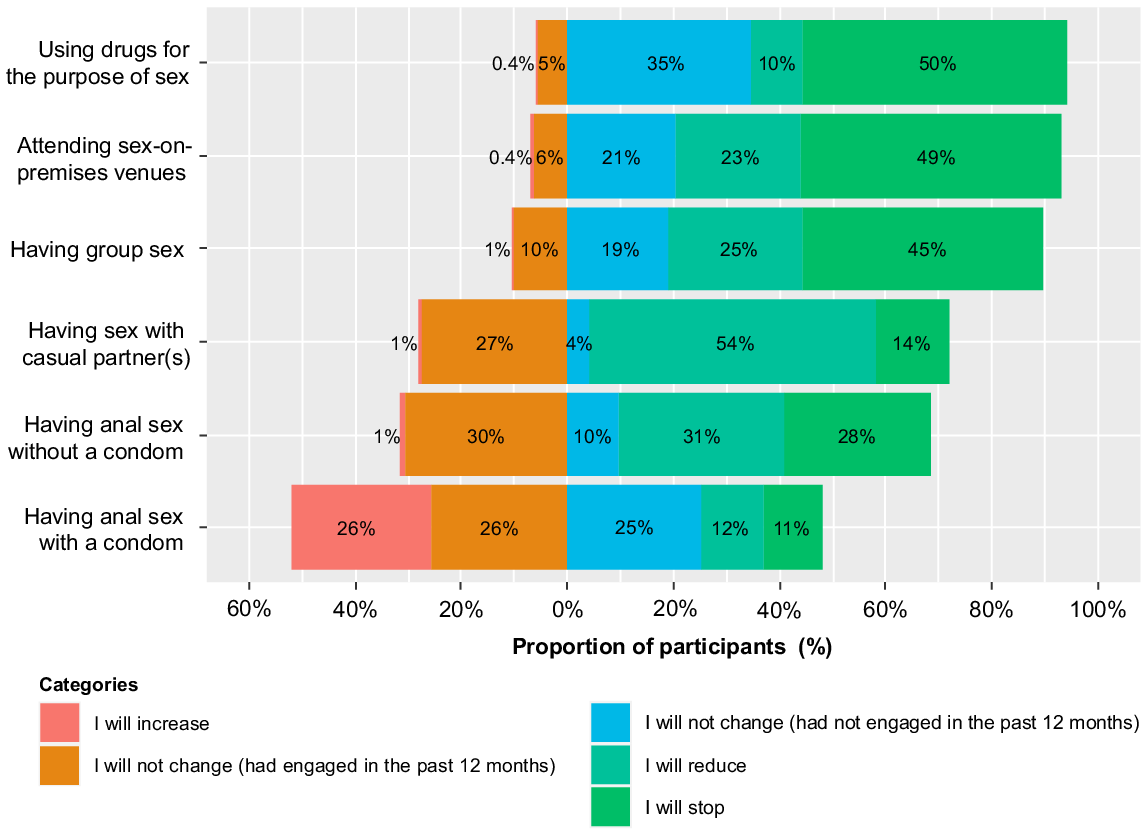
Fig. 1 shows that a substantial proportion of participants would either reduce or stop some sexual activities to prevent mpox. Most reported they would reduce having sex with casual partners (53.9%, 280/519), stop having chemsex (49.8%, 254/510), stop attending SOPV (49.3%, 253/513), and stop having group sex (45.3%, 233/514). One-quarter (26.2%, 134/512) reported they would increase condom use for anal sex but half (51.2%, 262/512) would not change.
Proportion of study participants who would change their sexual practices because of the mpox outbreak.
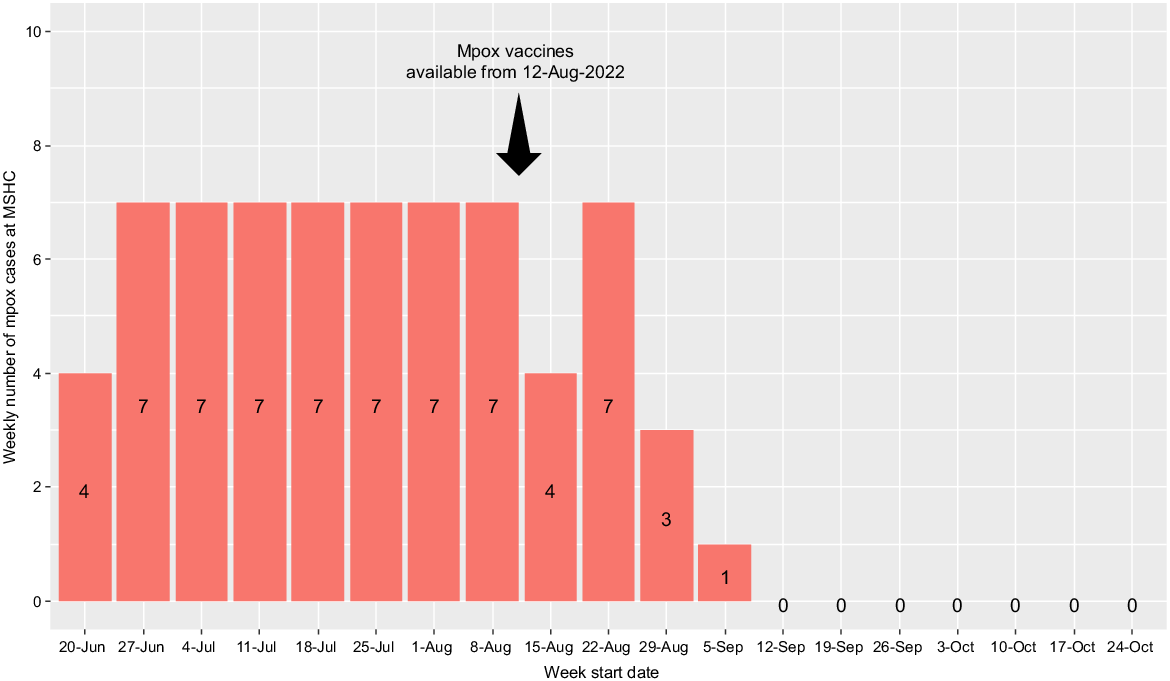
During the study period, 60.9% (42/69) of the mpox cases in Victoria were diagnosed at MSHC. The weekly mpox cases diagnosed at MSHC peaked in late July and August, and it dropped significantly in early September after the implementation of the first phase of mpox vaccination program in mid-August (Fig. 2).
Discussion
This cross-sectional study showed more than two-thirds of unvaccinated MSM intended to receive the mpox vaccine. Our data has shown that the majority of MSM had good knowledge of mpox (i.e. averaging 10 questions correct out of 12). The good knowledge and high awareness of mpox reflect the success of timely online public health education, messaging and lay media coverage, and we found that having a good knowledge of mpox is the leading factor that is associated with mpox vaccination uptake. However, we found that the perceived severity of mpox was relatively high in our study compared to what clinical reports indicate about disease severity and this may be associated with the dissemination of misinformation through social media platforms.38,39 The high proportion of MSM who were willing to reduce their sexual risk anecdotally may have contributed to fewer mpox cases given reductions occurred before substantial vaccination had occurred.
Cases of human-to-human transmission of mpox increased rapidly among the MSM community in several non-endemic European countries from May 2022 but declines in the number of cases in many countries began before substantial vaccine doses were given. For example, in the US, there was already a large decline in mpox cases at the beginning of August but their vaccination program was only rolled out from early July with a peak of uptake in mid-August.40–43 Similarly in the UK, mpox cases began to decline around mid-July but vaccinations were only rolled out from late June with a peak of uptake in late July.44,45 Lastly in Australia, cases declined in late August but the vaccination program only started in mid/late August.40
The rapid increase and decline of mpox cases could be explained by several factors that may have preceded substantial vaccination coverage in the general MSM population. First, mpox vaccine uptake may have been highly concentrated in high-risk individuals and therefore vaccination may have had a more significant effect than would be expected from the relatively low initial coverage in general MSM population. It is estimated that the basic reproduction number (R0) for the 2022 mpox outbreak is approximately 1.39 (95% CI: 1.37–1.42),46 meaning one infected person can infect, on average, 1.4 new people. The herd immunity threshold can also be estimated by the –equation 1 – 1/R0.47 Based on the estimated R0 from previous studies, it is estimated that at least 28% (i.e. 1 – 1/1.39 = 0.28) of the population needs to be vaccinated to end the mpox outbreak. In Victoria, the first phase of mpox vaccination program started in mid-August 2022 with a limited stock of 3500 doses of mpox vaccines, suggesting <10% of gay men living in Victoria would be able to access the mpox vaccines (i.e. estimated 36 000 gay men living in Victoria).15,48 Our study has shown that 37% of the study participants who were at substantial STI risk, had been vaccinated against mpox, and vaccinated participants are over-represented among those who had an STI and attended SOPV in the past 12 months, which were the eligibility criteria for the first phase of mpox vaccination although these individual’s risk factors are not associated with vaccination uptake in the multivariable analysis. A UK-based mathematical model has also predicted substantial second waves of mpox in the absence of mpox vaccination and reversion in sexual practices;49 therefore, continuing to vaccinate at-risk populations is important to prevent a rebound of mpox cases.
Second, the willingness to change sexual practices may also explain the rapid reductions in mpox cases. Our findings showed that the majority of MSM would consider harm reduction strategies (i.e. reducing or stopping at-risk sexual practices such as group sex, casual sex, chemsex and attending SOPV) to prevent mpox. This is also consistent with the findings from a US study carried out in August 2022 among 797 MSM showing almost half reported reducing the number of sexual partners, one-time sexual partners, group sex, and meeting a partner for sex via a dating app or at a SOPV since the mpox outbreak;50 while another half also reported they did not change their sexual practices but it is unclear whether these individuals have engaged in these activities before the mpox outbreak. Reducing the number of sexual partners (i.e. contact with susceptible or infected individuals) will reduce the R0; and the magnitude of the intended reduction is likely to substantially reduce transmission. A US-based mathematical model has estimated that one-time sexual partners account for approximately 50% of mpox transmission among MSM; and a 40% reduction in one-time sexual partners could potentially delay the spread of the mpox outbreak and reduce the proportion of infected MSM by 20–31%.51 The willingness to reduce sexual risk practices may be due to the fear and anxiety of acquiring mpox.52 Numerous clinical pictures of people with an extreme presentation of mpox (particularly those with obvious rash lesions on the face) were disseminated through the media at the beginning of the outbreak. This might have created fear of catching mpox and thus changed practices to reduce the risk of contracting mpox. Similar observations in reducing sexual risk practices at the beginning of the AIDS epidemic in the 1980s were also noted and resulted in the reductions in other STIs.22,53 Additionally, the stigma of the mpox outbreak reinforces the homophobic and racist stereotypes in the MSM community, particularly among those who are vaccinated or have been diagnosed with mpox.54 Stigmatisation can be a major barrier for individuals to seek health care for mpox vaccination or treatment. Lessons learnt from past epidemic such as HIV can be applied to reduce stigma in mpox and protect the vulnerable community.55
There is other evidence suggesting that the reproductive rate of STIs has been reduced by reductions in sexual practices. The UK noted that the reduction in mpox cases was temporally associated with declines in lymphogranuloma venereum and Shigella diagnoses suggesting that reductions in STI risk were driven by mpox cases.44 Observations that the R0 for mpox reduced as the epidemic progressed also support this observation.44 It would be reasonable to assume however that the reductions in sexual risk were temporary and that the mpox vaccine roll-out will now be the principal reason that no relapse in the outbreak has occurred.
Third, it is possible only a relatively small core group was driving mpox transmission in Australia and that this small core group became immune from natural infection early in the epidemic. This possibility is supported by a Dutch-based mathematical modelling study has shown that the high infection-induced immunity due to core group is sufficient to fade out the mpox outbreak in the absence of mpox vaccination program.56
There are several limitations to this study. First, participants were recruited from a convenience sample of MSM attending a sexual health service, connecting to social media or the LGBTQIA+ community. Individuals who have been vaccinated against mpox, are more concerned about mpox, more sexually active or have better sexual health knowledge and awareness might be more likely to participate and complete the survey. The high level of mpox vaccination in this group suggests that the early vaccination program appropriately targeted higher risk individuals. Second, recall bias and social desirability bias might have occurred of self-reported sexual practices and vaccination status.57 Third, due to the limited stock of the vaccines, some individuals might not be eligible or have access to the mpox vaccines; however, we did not ask unvaccinated study participants whether they had issues or difficulties in accessing the mpox vaccines. Fourth, we asked participants whether they would change their sexual practices to prevent mpox infection, which reflects their intention or willingness to change rather than the actual behavioural changes.
Australian health departments and local community-based organisations released timely public health education and messaging about mpox to the community. The education and messaging included the mode of mpox transmission, mpox-related symptoms, prevention, treatment and vaccination. Our study suggests that the education and messaging together with the widespread media attention the outbreak received resulted in a small and brief mpox outbreak. Whether this was related to sufficient vaccine coverage of high-risk individuals, changes in sexual practices and risk or a combination of these factors is hard to determine. Digital media provide a convenient and easy access platform for people accessing related information and individuals may also rely on these platforms for health-related information.58–60 However, fake news and misleading content can also be disseminated through these channels easily;38,39,61–63 and this misleading content not only affects individuals’ health related to the disease (e.g. vaccine hesitancy) but also their mental health (e.g. anxiety).64–66 Leading health organisations or local health authorities should monitor these social media and remove harmful and misleading health information for future disease outbreaks or pandemics.39 Lastly, culturally appropriate public health communication and messaging are important to reduce stigmatisation and discrimination about the disease or affected communities.67,68
Conflicts of interest
EPFC is an Associate Editor of Sexual Health, but was blinded from the peer review process for this manuscript. The authors declare no other conflicts of interest.
Declaration of funding
EPFC and DAW are each supported by an Australian National Health and Medical Research Council (NHMRC) Emerging Leadership Investigator Grant (GNT1172873 and GNT1174555, respectively). CKF and CSB are each supported by an Australian NHMRC Leadership Investigator Grant (GNT1172900 and GNT1173361, respectively). JMT and MYC are supported by Australian National Health and Medical Research Council (NHMRC) Partner Project Grant (GNT2003399).
Author contributions
EPFC, RSS, CSB, MYC, DAW and CKF conceived and designed the study. EPFC performed data analyses and wrote the first draft of the manuscript. KM and FM were involved in study recruitment. FM designed the study materials. JMT extracted and reviewed the number of mpox cases at the Melbourne Sexual Health Centre. All authors provided data interpretation, revised the manuscript for intellectual content, and approved the final version of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgements
We thank the Victorian LGBTQAI+ community groups (e.g. Geelong Rainbow Inc.) for disseminating the advertising flyers through their networks and also assisting with study recruitment.
References
1 Durski KN, McCollum AM, Nakazawa Y, Petersen BW, Reynolds MG, Briand S, et al. Emergence of Monkeypox – West and Central Africa, 1970–2017. MMWR Morb Mortal Wkly Rep 2018; 67(10): 306-10.
| Crossref | Google Scholar |
2 Foster SO, Brink EW, Hutchins DL, Pifer JM, Lourie B, Moser CR, et al. Human monkeypox. Bull World Health Organ 1972; 46(5): 569-76.
| Google Scholar |
3 Breman JG, Kalisa-Ruti , Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970–79. Bull World Health Organ 1980; 58(2): 165-82.
| Google Scholar |
4 Titanji BK, Tegomoh B, Nematollahi S, Konomos M, Kulkarni PA. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis 2022; 9(7): ofac310.
| Crossref | Google Scholar |
5 Allan-Blitz L-T, Klausner JD. Current evidence demonstrates that monkeypox is a sexually transmitted infection. Sex Transm Dis 2023; 50(2): 63-5.
| Crossref | Google Scholar |
6 Vaughan AM, Cenciarelli O, Colombe S, Alves de Sousa L, Fischer N, Gossner CM, et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European Region, 7 March to 23 August 2022. Euro Surveill 2022; 27(36): 2200620.
| Crossref | Google Scholar |
7 World Health Organization. Multi-country monkeypox outbreak in non-endemic countries. Geneva: World Health Organization; 2022. Available at https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 [cited 24 November 2022]
8 World Health Organization. WHO Director-General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern. Geneva: World Health Organization; 2022. Available at https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern [cited 24 November 2022]
9 Centers for Disease Control and Prevention. 2022 Monkeypox Outbreak Global Map – Data as of 23 Nov 2022 5:00 PM EDT. Atlanta: Centers for Disease Control and Prevention; 2022. Available at https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html [cited 28 November 2022]
10 Victoria Department of Health. More monkeypox vaccines arrive in Victoria. Melbourne: Victoria State Government Department of Health; 2022. Available at https://www.health.vic.gov.au/media-releases/more-monkeypox-vaccines-arrive-in-victoria [cited 23 June 2022]
11 Poland GA, Kennedy RB, Tosh PK. Prevention of monkeypox with vaccines: a rapid review. Lancet Infect Dis 2022; 22: E349-58.
| Crossref | Google Scholar |
12 Zaeck LM, Lamers MM, Verstrepen BE, Bestebroer TM, van Royen ME, Gotz H, et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med 2023; 29(1): 270-8.
| Crossref | Google Scholar |
13 Victoria Department of Health. Mpox (monkeypox). Melbourne: The Victorian Government; 2023. Available at https://www.health.vic.gov.au/infectious-diseases/mpox-monkeypox [cited 12 April 2023]
14 Victoria State Government Department of Health. Victorian monkeypox vaccination program interim guidelines – version 4.3. Melbourne: Victoria State Government Department of Health; 2022. Available at https://www.health.vic.gov.au/sites/default/files/2022-09/victorian-monkeypox-vaccination-program-interim-guidelines-v4.3%20.docx [cited 24 November 2022]
15 Premier of Victoria. Monkeypox vaccines arrive in Victoria. Melbourne: The Victorian Government; 2022. Available at https://www.premier.vic.gov.au/monkeypox-vaccines-arrive-victoria [cited 12 December 2022]
16 Chow EPF, Chen MY, Bradshaw CS, Towns JM, Fairley CK. Accessing first doses of mpox vaccine made available in Victoria, Australia. Lancet Reg Health West Pac 2023; 31: 100712.
| Crossref | Google Scholar |
17 Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill 2022; 27(22): 2200421.
| Crossref | Google Scholar |
18 Philpott D, Hughes CM, Alroy KA, Kerins JL, Pavlick J, Asbel L, et al. Epidemiologic and clinical characteristics of monkeypox cases - United States, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep 2022; 71(32): 1018-22.
| Crossref | Google Scholar |
19 Tarin-Vicente EJ, Alemany A, Agud-Dios M, Ubals M, Suner C, Anton A, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet 2022; 400(10353): 661-9.
| Crossref | Google Scholar |
20 Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries – April-June 2022. N Engl J Med 2022; 387(8): 679-91.
| Crossref | Google Scholar |
21 DeWitt ME, Polk C, Williamson J, Shetty AK, Passaretti CL, McNeil CJ, et al. Global monkeypox case hospitalisation rates: a rapid systematic review and meta-analysis. EClinicalMedicine 2022; 54: 101710.
| Crossref | Google Scholar |
22 Judson FN. Fear of AIDS and gonorrhoea rates in homosexual men. Lancet 1983; 322(8342): 159-60.
| Crossref | Google Scholar |
23 Chow EPF, Hocking JS, Ong JJ, Phillips TR, Schmidt T, Buchanan A, et al. Brief report: changes in PrEP use, sexual practice, and use of face mask during sex among MSM during the second wave of COVID-19 in Melbourne, Australia. J Acquir Immune Defic Syndr 2021; 86(2): 153-6.
| Crossref | Google Scholar |
24 Dukers-Muijrers NHTM, Evers Y, Widdershoven V, Davidovich U, Adam PCG, Op de Coul ELM, et al. Mpox vaccination willingness, determinants, and communication needs in gay, bisexual, and other men who have sex with men, in the context of limited vaccine availability in the Netherlands (Dutch Mpox-survey). Front Public Health 2023; 10: 1058807.
| Crossref | Google Scholar |
25 Fu L, Sun Y, Li Y, Wang B, Yang L, Tian T, et al. Perception of and vaccine readiness towards Mpox among men who have sex with men living with HIV in China: a cross-sectional study. Vaccines 2023; 11(3): 528.
| Crossref | Google Scholar |
26 Reyes-Uruena J, D’Ambrosio A, Croci R, Bluemel B, Cenciarelli O, Pharris A, et al. High monkeypox vaccine acceptance among male users of smartphone-based online gay-dating apps in Europe, 30 July to 12 August 2022. Euro Surveill 2022; 27(42): 2200757.
| Crossref | Google Scholar |
27 Ulloque-Badaracco JR, Alarcon-Braga EA, Hernandez-Bustamante EA, Al-Kassab-Cordova A, Benites-Zapata VA, Bonilla-Aldana DK, et al. Acceptance towards Monkeypox vaccination: a systematic review and meta-analysis. Pathogens 2022; 11(11): 1248.
| Crossref | Google Scholar |
28 Wang H, d’Abreu de Paulo KJI, Gultzow T, Zimmermann HML, Jonas KJ. Monkeypox self-diagnosis abilities, determinants of vaccination and self-isolation intention after diagnosis among MSM, the Netherlands, July 2022. Euro Surveill 2022; 27(33): 2200603.
| Crossref | Google Scholar |
29 Wang H, d’Abreu de Paulo KJI, Gultzow T, Zimmermann HML, Jonas KJ. Perceived monkeypox concern and risk among men who have sex with men: evidence and perspectives from The Netherlands. Trop Med Infect Dis 2022; 7(10): 293.
| Crossref | Google Scholar |
30 Harapan H, Setiawan AM, Yufika A, Anwar S, Wahyuni S, Asrizal FW, et al. Knowledge of human monkeypox viral infection among general practitioners: a cross-sectional study in Indonesia. Pathog Glob Health 2020; 114(2): 68-75.
| Crossref | Google Scholar |
31 Stead M, Jessop C, Angus K, Bedford H, Ussher M, Ford A, et al. National survey of attitudes towards and intentions to vaccinate against COVID-19: implications for communications. BMJ Open 2021; 11(10): e055085.
| Crossref | Google Scholar |
32 Ogilvie GS, Gordon S, Smith LW, Albert A, Racey CS, Booth A, et al. Intention to receive a COVID-19 vaccine: results from a population-based survey in Canada. BMC Public Health 2021; 21(1): 1017.
| Crossref | Google Scholar |
33 Sims A, Archie-Booker E, Waldrop RT, Claridy M, Gerbi G. Factors associated with human papillomavirus vaccination among women in the United States. ARC J Public Health Community Med 2018; 3(1): 6-12.
| Crossref | Google Scholar |
34 McLendon L, Puckett J, Green C, James J, Head KJ, Yun Lee H, et al. Factors associated with HPV vaccination initiation among United States college students. Hum Vaccin Immunother 2021; 17(4): 1033-43.
| Crossref | Google Scholar |
35 Australian Bureau of Statistics. Age standard. Canberra, Australia: Australian Bureau of Statistics; 2014. Available at https://www.abs.gov.au/statistics/standards/age-standard/latest-release [cited 2 June 2023]
36 Broady T, Chan C, MacGibbon J, Bavinton B, Mao L, McKenzie T, et al. Gay community periodic survey: Melbourne 2022. Sydney: Centre for Social Research in Health, UNSW Sydney; 2022. doi:10.26190/p2gh-n362
37 Baig M, Jameel T, Alzahrani SH, Mirza AA, Gazzaz ZJ, Ahmad T, et al. Predictors of misconceptions, knowledge, attitudes, and practices of COVID-19 pandemic among a sample of Saudi population. PLoS ONE 2020; 15(12): e0243526.
| Crossref | Google Scholar |
38 Ortiz-Martínez Y, Sarmiento J, Bonilla-Aldana DK, Rodríguez-Morales AJ. Monkeypox goes viral: measuring the misinformation outbreak on Twitter. J Infect Dev Ctries 2022; 16(7): 1218-20.
| Crossref | Google Scholar |
39 Ortiz-Martínez Y, Galvis-Cataño LM, Arias-Rodríguez D, Romero-Dager C, Bonilla-Aldana DK, Rodriguez-Morales AJ. YouTube and 2022 Monkeypox outbreak: opportunities for awareness and infection control. J Hosp Infect 2022;
| Crossref | Google Scholar |
40 Centers for Disease Control and Prevention. U.S. Monkeypox case trends reported to CDC – Data as reported to CDC as of 16 Nov 2022 2:00 PM EDT. Atlanta: Centers for Disease Control and Prevention; 2022. Available at https://www.cdc.gov/poxvirus/monkeypox/response/2022/mpx-trends.html [cited 25 November 2022]
42 Abbott L. ‘Well done!’ Monkeypox cases drop as Sutton lauds community response. The Age 2022;
| Google Scholar |
43 Centers for Disease Control and Prevention. Monkeypox vaccine administration in the U.S. – Data as of November 15 2022 4:00 AM EDT. Atlanta: Centers for Disease Control and Prevention; 2022. Available at https://www.cdc.gov/poxvirus/monkeypox/response/2022/vaccines_data.html [cited 28 November 2022]
44 UK Health Security Agency. Investigation into monkeypox outbreak in England: technical briefing 8. London: UK Health Security Agency; 2022. Available at https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-8 [cited 24 November 2022]
45 UK Health Security Agency. Monkeypox outbreak: vaccination strategy. London: UK Health Security Agency; 2022. Available at https://www.gov.uk/guidance/monkeypox-outbreak-vaccination-strategy [cited 28 November 2022]
46 Du Z, Shao Z, Bai Y, Wang L, Herrera-Diestra JL, Fox SJ, et al. Reproduction number of monkeypox in the early stage of the 2022 multi-country outbreak. J Travel Med 2022; 29: taac099.
| Crossref | Google Scholar |
47 Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature 1985; 318(6044): 323-9.
| Crossref | Google Scholar |
48 Callander D, Mooney-Somers J, Keen P, Guy R, Duck T, Bavinton BR, et al. Australian ‘gayborhoods’ and ‘lesborhoods’: a new method for estimating the number and prevalence of adult gay men and lesbian women living in each Australian postcode. Int J Geogr Inf Sci 2020; 34(11): 2160-76.
| Crossref | Google Scholar |
49 Brand S, Cavallaro M, Cumming F, Turner C, Florence I, Blomquist P, et al. The role of vaccination and public awareness in forecasts of monkeypox incidence in the United Kingdom. Res Sq 2022;
| Crossref | Google Scholar |
50 Delaney KP, Sanchez T, Hannah M, Edwards OW, Carpino T, Agnew-Brune C, et al. Strategies adopted by gay, bisexual, and other men who have sex with men to prevent monkeypox virus transmission – United States, August 2022. MMWR Morb Mortal Wkly Rep 2022; 71(35): 1126-30.
| Crossref | Google Scholar |
51 Spicknall IH, Pollock ED, Clay PA, Oster AM, Charniga K, Masters N, et al. Modeling the impact of sexual networks in the transmission of monkeypox virus among gay, bisexual, and other men who have sex with men – United States, 2022. MMWR Morb Mortal Wkly Rep 2022; 71(35): 1131-5.
| Crossref | Google Scholar |
52 Farahat RA, Yassin MA, Al-Tawfiq JA, Bejan CA, Abdelazeem B. Public perspectives of monkeypox in Twitter: a social media analysis using machine learning. New Microbes New Infect 2022; 49-50: 101053.
| Crossref | Google Scholar |
53 Chow EPF, Grulich AE, Fairley CK. Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV. Lancet HIV 2019; 6(6): e396-e405.
| Crossref | Google Scholar |
54 UNAIDS. UNAIDS warns that stigmatizing language on Monkeypox jeopardises public health. Geneva: The Joint United Nations Programme on HIV and AIDS; 2022. Available at https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2022/may/20220522_PR_Monkeypox [cited 12 December 2022]
55 Yang Z, Liu X, Zhu Z, Zhang L, Han S, Fu Y, et al. Combating stigma and health inequality of monkeypox: experience from HIV. Infect Drug Resist 2022; 15: 5941-3.
| Crossref | Google Scholar |
56 Xiridou M, Miura F, Adam P, Op de Coul E, de Wit J, Wallinga J. The fading of the mpox outbreak among men who have sex with men: a mathematical modelling study. medRxiv 2023; 2023.01.31.23285294 2023.01.31.23285294.
| Crossref | Google Scholar |
57 Chow EPF, Fairley CK, Wigan R, Hocking JS, Garland SM, Cornall AM, et al. Accuracy of self-reported human papillomavirus vaccination status among gay and bisexual adolescent males: cross-sectional study. JMIR Public Health Surveill 2021; 7(12): e32407.
| Crossref | Google Scholar |
58 Smailhodzic E, Hooijsma W, Boonstra A, Langley DJ. Social media use in healthcare: a systematic review of effects on patients and on their relationship with healthcare professionals. BMC Health Serv Res 2016; 16(1): 442.
| Crossref | Google Scholar |
59 D’Souza RS, D’Souza S, Strand N, Anderson A, Vogt MNP, Olatoye O. YouTube as a source of medical information on the novel coronavirus 2019 disease (COVID-19) pandemic. Glob Public Health 2020; 15(7): 935-42.
| Crossref | Google Scholar |
60 Ortiz-Martínez Y, Ortiz-Martínez HM. TikTok and its importance in monkeypox public health engagement. J Adolesc Health 2023; 72(2): 312.
| Crossref | Google Scholar |
61 Li HO-Y, Pastukhova E, Brandts-Longtin O, Tan MG, Kirchhof MG. YouTube as a source of misinformation on COVID-19 vaccination: a systematic analysis. BMJ Glob Health 2022; 7(3): e008334.
| Crossref | Google Scholar |
62 Li HO-Y, Bailey A, Huynh D, Chan J. YouTube as a source of information on COVID-19: a pandemic of misinformation? BMJ Glob Health 2020; 5(5): e002604.
| Crossref | Google Scholar |
63 Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav 2021; 5(3): 337-48.
| Crossref | Google Scholar |
64 Ahmed SK, Abdulqadir SO, Omar RM, Abdullah AJ, Rahman HA, Hussein SH, et al. Knowledge, attitude and worry in the Kurdistan Region of Iraq during the Mpox (Monkeypox) outbreak in 2022: an online cross-sectional study. Vaccines 2023; 11(3): 610.
| Crossref | Google Scholar |
65 Farahat RA, Head MG, Tharwat S, Alabdallat Y, Essar MY, Abdelazeem B, et al. Infodemic and the fear of monkeypox: call for action. Trop Med Health 2022; 50(1): 63.
| Crossref | Google Scholar |
66 Zenone M, Caulfield T. Using data from a short video social media platform to identify emergent monkeypox conspiracy theories. JAMA Netw Open 2022; 5(10): e2236993.
| Crossref | Google Scholar |
67 Taylor L. Monkeypox: WHO to rename disease to prevent stigma. BMJ 2022; 377: o1489.
| Crossref | Google Scholar |
68 Manirambona E, Shomuyiwa DO, Musa SS, Lucero-Prisno DE, III. Monkeypox among men who have sex with men in Africa: the need for testing and vaccination beyond stigma. J Med Virol 2023; 95: e28121.
| Crossref | Google Scholar |