Uptake, yield and resource requirements of screening for asymptomatic sexually transmissible infections among HIV-positive people attending a hospital outpatient clinic
Stephen Ritchie A B D , Rebecca Henley A , Jackie Hilton C , Rupert Handy A , Joan Ingram A , Susan Mundt A , Mitzi Nisbet A , Mark Thomas A B and Simon Briggs AA Adult Infectious Disease Service, Auckland District Health Board, Auckland 1023, New Zealand.
B Department of Molecular Medicine and Pathology, University of Auckland, Auckland 1023, New Zealand.
C Auckland Sexual Health Service, Auckland District Health Board, Auckland 1023, New Zealand.
D Corresponding author. Email: s.ritchie@auckland.ac.nz
Sexual Health 11(1) 67-72 https://doi.org/10.1071/SH13167
Submitted: 15 October 2013 Accepted: 5 February 2014 Published: 12 March 2014
Abstract
Background: We performed a prospective audit of screening for asymptomatic sexually transmissible infections (STIs), during an intensive effort to screen all patients at our hospital-based HIV clinic. We aimed to measure the effectiveness and resource implications of our screening program. Methods: All outpatients who attended during an 8-month period were invited to take part in opt-out screening for chlamydia (Chlamydia trachomatis), gonorrhoea (Neisseria gonorrhoeae) and syphilis. Participants completed a brief questionnaire, were asked about current symptoms of STIs and self-collected specimens for laboratory testing. Results: The majority (535 out of 673, 80%) of the patients who were asked to participate provided specimens for screening. No chlamydia, gonorrhoea or syphilis infections were identified in women (n = 91) or in heterosexual men (n = 76). In contrast, 34 out of 368 (10%) of men who have sex with men tested positive (chlamydia, 25; gonorrhoea, 2; chlamydia and gonorrhoea, 2; syphilis, 5). The laboratory cost of diagnosing each case of rectal chlamydia or gonorrhoea (NZ$664) was substantially lower than the cost of diagnosing each case of urethral infection (NZ$5309). Conclusions: There was high uptake of screening among our clinic population, who preferred screening to be performed at the hospital clinic. The yield of screening men who have sex with men warrants continued annual screening for rectal gonorrhoea and chlamydia and for syphilis.
Additional keywords: chlamydia, costs, gonorrhoea, men who have sex with men, New Zealand, syphilis.
Introduction
Authorities such as the British HIV Association and the Centers for Disease Control advocate testing people living with HIV and AIDS (PLWHA) at regular intervals for asymptomatic sexually transmissible infections (STIs).1,2
One rationale for this recommendation is to reduce the transmission of HIV infection. There is no direct evidence that demonstrates a reduction in HIV transmission following screening and treatment of asymptomatic STIs; however, HIV transmission risk is increased in the presence of other STIs3 and transmission of other STIs is increased when the source person is HIV-positive.4 Furthermore, identification and treatment of asymptomatic STIs can reduce the sequelae (e.g. tertiary syphilis or pelvic inflammatory disease) and provide an opportunity for counselling to reduce the risk of future STI acquisition.5
Several studies have shown high rates of asymptomatic STI among men who have sex with men (MSM). For example, the prevalence of chlamydia (Chlamydia trachomatis) infection was 5% among MSM who attended a genitourinary medicine clinic in London,6 7% among HIV-positive MSM who attended the Melbourne Sexual Health Centre7 and 9% among HIV-positive MSM who attended clinics in Amsterdam or Rotterdam.8
The annual screening rate for STIs among PLWHA, in some settings, is low. In Melbourne, Australia, only 18% of HIV-positive MSM attending a hospital infectious disease clinic were screened for chlamydia and gonorrhoea (Neisseria gonorrhoeae) during a twice-yearly screening period;7 in eight large HIV clinics in six cities in the United States, the annual screening rate for rectal chlamydia and gonorrhoea among HIV-positive MSM was less than 10%.9
The Adult Infectious Disease Service at Auckland City Hospital cares for a large number of people living with HIV infection in the Greater Auckland and Northland regions of New Zealand (an adult population of 1.35 million). All new clinic patients are screened for hepatitis A, B and C virus coinfection; those susceptible to hepatitis A or B are immunised. Women are advised to have annual cervical screening, which has been the subject of a previous audit.10 At the time the current study was performed, no routine screening for human papillomavirus infection among MSM was performed. Syphilis serology is performed annually.
For several years, our service recommended that all PLWHA who attend the outpatient clinic have annual STI screening performed either by their general practitioner or at an Auckland Sexual Health Service (ASHS) clinic. We perceived the uptake of this recommendation to be low. We were also aware of instances when syphilis serology had not been performed adequately. Thus, we initiated a systematic STI screening program offered to all clinic attendees with HIV infection. We performed a prospective audit of this program to measure the screening uptake, prevalence of STIs and resource implications of screening for asymptomatic infection caused by gonorrhoea, chlamydia and Treponema pallidum.
Materials and methods
The Auckland City Hospital adult HIV clinic cared for 740 people (as of 1 January 2012); patients are usually reviewed every 3 or 6 months.
We endeavoured to include all clinic patients who attended clinic between 1 January 2012 and 31 August 2012. We expected that most patients would be seen at least once during this period. Patients were only included in the study once. On arrival at the clinic, patients were given an information sheet explaining the rationale of the screening program to read while they were in the waiting room. As STI screening was considered part of routine clinic care, we did not request written consent to participate.
During the clinic visit, the screening program was explained further by the clinic doctor or nurse, and patients were invited to take part in screening on an opt-out basis. Attempts were made to ask all clinic patients (including those who did not want to have screening performed) four questions: if they believed they were at risk of an STI, whether they had been screened for STIs in the previous year, whether they had ever been diagnosed with an STI (other than HIV infection) and their preferred STI screening site (hospital clinic, sexual health clinic, primary care or other). Patients who consented to be screened were then asked a list of specific questions about whether they had recently experienced symptoms consistent with an STI. Finally, specimens were collected according to gender and sexual practices.
Collection of specimens
Asymptomatic women were asked to self-collect a vaginal swab to test for infection with chlamydia and gonorrhoea. Asymptomatic heterosexual men were asked to provide a first-catch urine specimen to test for infection with chlamydia and gonorrhoea. Asymptomatic MSM were asked to provide a first-catch urine specimen and to self-collect a rectal swab to test for infection with chlamydia and gonorrhoea; in addition, a clinician collected a throat swab to test for gonorrhoea.
To avoid the need for an extra venepuncture, serological testing for syphilis was performed on blood samples collected from STI screening participants before the next clinic visit, along with other routine blood tests. Patients with syphilis screening performed in the preceding 6 months did not undergo repeat syphilis screening.
Testing of specimens
Vaginal swabs, urine samples and rectal swabs were tested for chlamydia and gonorrhoea by nucleic acid amplification testing (NAAT: BD ProbeTec, Becton Dickinson and Co., Sparks, MD, USA) using strand displacement amplification. Pharyngeal swabs were tested for gonorrhoea by culture on New York City agar (Fort Richard, Auckland, New Zealand) using routine laboratory procedures.
Serum samples were tested for evidence of syphilis by screening immunoassay. Positive results were followed by a rapid plasma reagin (RPR) test and a T. pallidum haemagglutination assay-specific serological test. A positive STI screen for syphilis was defined as any new diagnosis of syphilis and required a positive T. pallidum haemagglutination assay and elevated RPR tests.
Clinical follow up
Patients who reported symptoms consistent with an STI or who had positive results from screening tests were referred to an ASHS clinic, where treatment and contact tracing were provided. An HIV nurse specialist recorded all patients’ results in a database and ensured that all participants with symptoms or positive STI screening tests attended an ASHS clinic within 2 weeks.
Resource requirements
The cost per test at the Auckland City Hospital laboratory was: chlamydia NAAT, NZ$32.46; gonorrhoea NAAT, NZ$12.92; gonorrhoea culture, NZ$17.83; treponemal screen, NZ$14.79. Chlamydia and gonorrhoea NAATs were performed in tandem on all specimens. The laboratory cost of each positive test result was calculated from the total cost of all of the tests performed divided by the number of positive tests. Each clinician was asked to indicate how long it took to explain the screening procedures to each patient.
Statistical analysis
Differences between categorical variables were tested using the χ2-test. The distribution of patients’ ages was nonparametric and the Mann–Whitney U-test was used to test for statistical significance. A P-value of <0.05 was considered to represent statistical significance.
Ethical approval for our clinical audit was obtained from the Northern X Health and Disability Ethics Committee of the New Zealand Ministry of Health.
Results
Participants
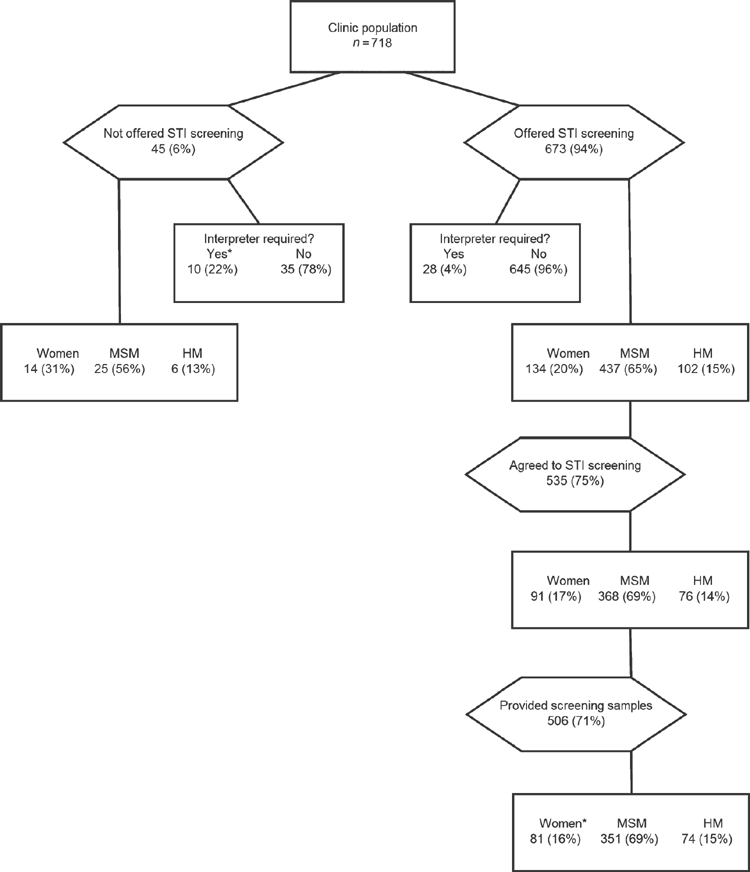
During the study period, 718 PLWHA attended the clinic, 673 (94%) patients were offered STI screening, 562 (78%) patients completed the questionnaire, 535 (75%) patients consented to screening and 506 (70%) patients provided all of the samples required for screening (Fig. 1). The median age of the patients was 46 (interquartile range (IQR): 39–52) and did not differ among those offered STI screening, those who agreed to STI screening and those who provided samples for screening. Only a small proportion (117 out of 673, 17%) of those offered STI screening had been screened for STIs in the preceding 12-month period.
STI screening was less likely to be offered to patients who required an interpreter (Table 1, P < 0.01); however, patients who required an interpreter were as likely to agree to screening as patients who did not require an interpreter. There were no differences in the ethnicities of patients offered STI screening or not offered screening (Table S1, available as Supplementary Material to this paper).
|
Women were as likely as heterosexual men and MSM to be offered screening, but there was a trend for women to decline testing more often than men (P = 0.07). Women were less likely than men to provide samples for laboratory testing (P = 0.03). There were no significant differences in age or ethnicities between women who submitted samples for testing and women who declined STI screening or did not submit samples for testing.
Twenty-three of the 60 people (36%) who answered the questionnaire but declined STI screening declined because they had STI screening performed during the previous year.
Questionnaire
Of the 372 MSM who completed the questionnaire, 219 (59%) reported a previous STI (other than HIV infection) and 128 (34%) reported that they considered themselves to be at risk of an STI. In contrast, of 190 heterosexual men and women who completed the questionnaire, only 56 (30%) reported a previous STI (other than HIV infection, P < 0.01) and only 24 (13%) reported that they considered themselves to be at risk of an STI (P < 0.01).
Of the 309 European people who completed the questionnaire, 104 (34%) reported that they considered themselves at risk of STI. However, a smaller proportion of African people (12 out of 101, 12%) reported that they considered themselves to be at risk of STI (P < 0.01). Likewise, a history of STIs was reported by 173 out of 309 (73%) of European patients but by only 31 out of 101 (31%) of African patients (P < 0.01).
Symptoms suggestive of a current STI were reported by 7 out of 372 (2%) MSM, and by 4 out of 190 (2%) heterosexual men and women. Overall, 414 out of 562 (73%) patients stated that the infectious disease clinic was their preferred site for STI screening, 101 out of 562 (18%) preferred to visit their primary care provider for STI screening and 51 out of 562 (9%) preferred to visit an ASHS clinic for STI screening.
Testing for STIs
Almost all (362 out of 365, 99%) MSM who agreed to participate in STI screening had a throat swab collected by the clinic doctor or nurse. Almost all (503 out of 535, 94%) of those who agreed to participate in STI screening provided self-collected swabs, urine samples or both. Ten women did not submit self-collected vaginal swabs, two heterosexual men did not submit a urine sample and 17 MSM did not submit either a urine test or rectal swab for testing. A further three patients who agreed to take part in STI screening went to an ASHS clinic and had testing performed there. During the 12-month period of the audit, syphilis serology was performed in 397 out of 535 (74%) of those who agreed to participate in STI screening.
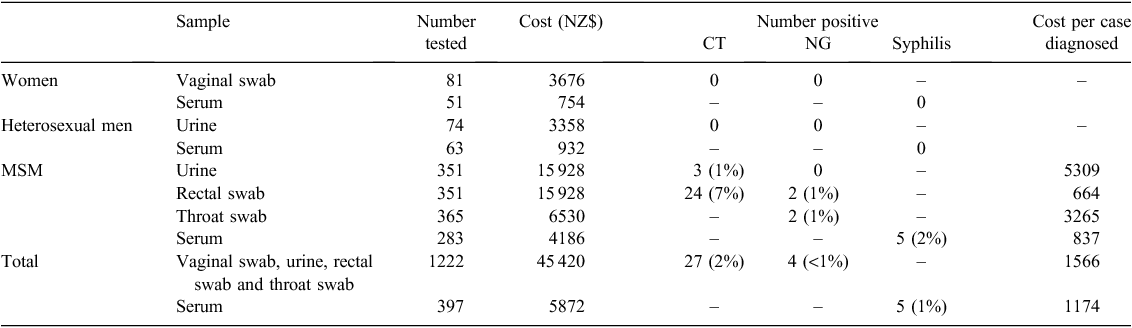
No STI was diagnosed as the result of testing 269 samples provided by 155 heterosexual men and women (Table 1). In contrast, 34 out of 365 (10%) MSM had one or more STI diagnosed as the result of STI screening: 22 out of 351 (6%) rectal swabs and 3 out of 351 (1%) urine samples provided by MSM were positive for chlamydia alone, 2 out of 352 (1%) rectal swabs provided by MSM were positive for both chlamydia and gonorrhoea, 2 out of 362 (1%) throat swabs collected from MSM were positive for gonorrhoea and 5 out of 283 (1%) serum samples collected from MSM diagnosed new syphilis infections. Four patients with a syphilis infection had previously had negative tests and one had not had syphilis screening previously.
All 29 MSM with chlamydia or gonorrhoea infection were referred to an ASHS clinic for further assessment and treatment. Another six men and four women who reported symptoms consistent with a current STI but did not have any STI diagnosed on further testing were referred to an ASHS clinic. These symptomatic people were of similar ages (median age: 41 years, IQR: 32–51) to the men and women who did not have symptoms or a positive STI screening test (median age: 47 years, IQR: 39–54; P = 0.09). One of the women and five of the men considered themselves to be at risk of STI (the symptoms and diagnoses of these people are summarised in Table S2). Three MSM had an STI diagnosed: two cases associated with human papillomavirus infection and one with herpes simplex virus infection.
In total, 34 out of 368 (10%) MSM who were screened for an STI tested positive for recent syphilis, gonorrhoea, chlamydia or any combination of these STIs. The MSM who tested positive were younger (median age: 43 years, IQR: 32–49) than the MSM who tested negative (median age: 48 years, IQR: 41–56; P = 0.01). There was a trend that MSM who tested positive for an STI were more likely to consider themselves at risk of STI (18 out of 34, 53%) than MSM who tested negative for an STI (103 out of 334, 31%) (P = 0.06). The proportions of MSM who reported a past STI or who had an STI screen in the previous 12 months did not differ between those who tested positive and those who tested negative for an STI.
Resource requirements
The clinicians estimated that the amount of clinic time used in discussions related to screening was less than 5 min in 420 out of 535 (78%) consultations, 5–10 min in 108 out of 535 (21%) consultations and more than 10 min in 7 out of 535 (1%) consultations. Prolonged discussions related to screening more often occurred with patients who required the use of an interpreter (P < 0.01).
The cost of laboratory testing for a female or a heterosexual male was NZD$60 and testing for a MSM was $123. The cost of each laboratory test per positive result is shown in Table 1.
The HIV specialist nurse who coordinated the screening program devoted 5 h each week to packaging swabs and specimen jars with test request forms in readiness for each clinic, maintaining a study database and ensuring that patients with positive test results were reviewed at an ASHS clinic within 2 weeks.
Discussion
The implementation of an STI screening program in our clinic resulted in 70% of our patients being tested. This uptake compares favourably with rates in other ‘real-world’ clinics: 42% in two clinics in Amsterdam and Rotterdam,8 and 52% in a dedicated nurse-led STI clinic for HIV-positive people in London.11 We found that an improvement in the number of people who have annual syphilis serology testing performed was required: only 74% of our clinic population had syphilis serology during the 12-month period of this audit.
The screening program resulted in a treatable STI being diagnosed in 10% of MSM. Only one of these 34 patients reported symptoms suggestive of an STI, emphasising the importance of routine screening for these infections rather than testing based on patient-reported symptoms.
We did not detect any STIs in any of the heterosexual men or women attending our clinic. Most of the heterosexual men and women with HIV infection who attend our clinic have emigrated from Africa or South-east Asia, and although we have not conducted surveys of sexual behaviour in this population, it is our impression that most have had only a few sexual partners since their arrival in New Zealand. The low prevalence of STIs among heterosexual HIV-positive people in a study conducted in the Netherlands has been used as an argument against routine screening of this group.12
The resources required to initiate this screening program were not inconsequential. The extra time spent during each clinic visit was minimal; however, 5 h per week were required to maintain the screening kits and to check through all the laboratory results carefully and arrange further follow up when required. The most significant laboratory cost was for NAAT. Approximately NZD$35 000 was spent on NAAT for MSM. Among MSM, each of the 24 episodes of rectal chlamydia or gonorrhoea cost $664 to diagnose. However, the diagnostic yield of NAAT was higher for rectal swabs than urine: each case of urethral chlamydia cost $5309 to diagnose. We acknowledge that the simplistic costing that we have performed cannot take into account other important savings such as those associated with preventing STI transmission following treatment and counselling.
We found that a minority of patients (45 out of 718, 6%) were not offered STI screening during the study period. We do not know whether STI screening was offered and declined, but not documented, or whether this was due to omission. However, a high proportion of these patients required interpreters, which raises the possibility that screening was not discussed due to time constraints. A database linked to the electronic records is under development at our clinic and we expect that electronic patient recall will enable almost all clinic patients to be screened in the future.13
The main limitation of our audit was our inability to collect detailed information about participants’ sexual behaviours and networks that would enable a search for risk factors for STI. To do so would have required written informed consent, which we worried might reduce the uptake of our screening program. However, almost half of MSM who tested positive for STI did not consider themselves to be at risk for STI. This finding indicates that it is important to screen all MSM. It is unlikely that a detailed search for risk factors would enable us to develop a questionnaire that would allow targeted screening of a subset of MSM.
Our audit has provided useful information that will enable us to focus future annual STI screening. We plan to offer screening only to MSM, with annual rectal chlamydia and gonorrhoea NAAT and syphilis serology. In order to maintain the adherence to STI screening and to ensure that syphilis serology is performed, we plan to use a single stamp listing these tests together with CD4 count and HIV viral load that can be applied to the laboratory request form. Patients will be asked to have these tests performed at the community laboratory before their next clinic visit, to allow the results of these tests to be available by the time the patient attends the clinic. These changes to the screening process, together with the familiarity that patients develop with repeated screening, will greatly reduce the resources required.
Conflicts of interest
None declared.
Acknowledgements
The authors thank the staff at the Auckland Sexual Health Service for their contribution to the management of patients with positive results. This study was supported by Janssen as an investigator-initiated study.
References
[1] Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institutes of Health, Infectious Diseases Society of America. Incorporating HIV prevention into the medical care of persons living with HIV. Morb Mortal Wkly Rep 2003; 52 1–24.[2] Fakoya A, Lamba H, Mackie N, Nandwani R, Brown A, Bernard EJ, et al. UK guidelines for the management of sexual and reproductive health (SRH) of people living with HIV infection. London: British HIV Association; 2008. Available online at: http://www.bhiva.org/UKGuidelines2008.aspx [verified February 2014].
[3] Martin HL, Nyange PM, Richardson BA, Lavreys L, Mandaliya K, Jackson DJ, et al Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis 1998; 178 1053–9.
| Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1.Crossref | GoogleScholarGoogle Scholar | 9806034PubMed |
[4] McClelland RS, Lavreys L, Katingima C, Overbaugh J, Chohan V, Mandaliya K, et al Contribution of HIV-1 infection to acquisition of sexually transmitted disease: a 10-year prospective study. J Infect Dis 2005; 191 333–8.
| Contribution of HIV-1 infection to acquisition of sexually transmitted disease: a 10-year prospective study.Crossref | GoogleScholarGoogle Scholar | 15633091PubMed |
[5] Johnson WD, Hedges LV, Diaz RM. Interventions to modify sexual risk behaviors for preventing HIV infection in men who have sex with men. Cochrane Database Syst Rev 2002;
| Interventions to modify sexual risk behaviors for preventing HIV infection in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[6] Ivens D, MacDonald K, Bansi L, Nori A. Screening for rectal chlamydia infection in a genitourinary medicine clinic. Int J STD AIDS 2007; 18 404–6.
| Screening for rectal chlamydia infection in a genitourinary medicine clinic.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2szpt1ejsw%3D%3D&md5=696cdeb67ab13a802b4c1c27f89fbc3bCAS | 17609031PubMed |
[7] Teague R, Mijch A, Fairley CK, Sidat M, Watson K, Boyd K, et al Testing rates for sexually transmitted infections among HIV-infected men who have sex with men attending two different HIV services. Int J STD AIDS 2008; 19 200–2.
| Testing rates for sexually transmitted infections among HIV-infected men who have sex with men attending two different HIV services.Crossref | GoogleScholarGoogle Scholar | 18397563PubMed |
[8] Heiligenberg M, Rijnders B, Schim van der Loeff MF, de Vries HJC, van der Meijden WI, Geerlings SE, et al High prevalence of sexually transmitted infections in HIV-infected men during routine outpatient visits in the Netherlands. Sex Transm Dis 2012; 39 8–15.
| High prevalence of sexually transmitted infections in HIV-infected men during routine outpatient visits in the Netherlands.Crossref | GoogleScholarGoogle Scholar | 22183837PubMed |
[9] Hoover KW, Butler M, Workowski K, Carpio F, Follansbee S, Gratzer B, et al STD screening of HIV-infected MSM in HIV clinics. Sex Trans Dis 2010; 37 771–776.
| STD screening of HIV-infected MSM in HIV clinics.Crossref | GoogleScholarGoogle Scholar |
[10] Grewal J, Lowe M, Gerrard H, Henley R, Perkins N, Briggs S. Audit of cervical screening in women with HIV infection in the Auckland and Northland regions of New Zealand. N Z Med J 2010; 123 71–8.
| 20717179PubMed |
[11] Hamlyn E, Barrett S, Kelsey J, Lockyer S, Welz T, Poulton M. Improvement in screening for sexually transmitted infections in HIV-positive patients following implementation of a nurse-led clinic. Int J STD AIDS 2007; 18 424–6.
| Improvement in screening for sexually transmitted infections in HIV-positive patients following implementation of a nurse-led clinic.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2szpt1ejug%3D%3D&md5=44112ed4d385ffe4fc4f45b1ffbdd7f2CAS | 17609038PubMed |
[12] Heiligenberg M, van der Loeff MFS, de Vries HJC, Speksnijder AGCL, Geerlings SE, Coutinho R, et al Low prevalence of asymptomatic sexually transmitted infections in HIV-infected heterosexuals visiting an HIV clinic in the Netherlands. AIDS 2012; 26 646–649.
| Low prevalence of asymptomatic sexually transmitted infections in HIV-infected heterosexuals visiting an HIV clinic in the Netherlands.Crossref | GoogleScholarGoogle Scholar | 22210633PubMed |
[13] Brook G, McSorley J, Shaw A. Retrospective study of the effect of enhanced systematic sexually transmitted infection screening, facilitated by the use of electronic patient records, in an HIV-infected cohort. HIV Med 2013; 14 347–53.
| Retrospective study of the effect of enhanced systematic sexually transmitted infection screening, facilitated by the use of electronic patient records, in an HIV-infected cohort.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3svgsFClsA%3D%3D&md5=293d998827d0b314812c83905c5a69f4CAS | 23432731PubMed |