Reproductive seasonality in African ungulates in relation to rainfall
Joseph O. Ogutu A B D , Hans-Peter Piepho A and Holly T. Dublin CA University of Hohenheim, Institute for Crop Science-340, Fruwirthstrasse 23, 70599 Stuttgart, Germany.
B International Livestock Research Institute, PO Box 30709, Nairobi, 00100, Kenya.
C The World Conservation Union (IUCN), c/o IUCN ESARO, Wasa Conservation Centre, PO Box 68200, Nairobi 00200, Kenya.
D Corresponding author. Email: jogutu2007@gmail.com
Wildlife Research 41(4) 323-342 https://doi.org/10.1071/WR13211
Submitted: 8 December 2013 Accepted: 12 September 2014 Published: 18 December 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Context: Reproductive seasonality in ungulates has important fitness consequences but its relationship to resource seasonality is not yet fully understood, especially for ungulates inhabiting equatorial environments.
Aims: We test hypotheses concerning synchronisation of conception or parturition peaks among African ungulates with seasonal peaks in forage quality and quantity, indexed by rainfall.
Methods: We relate monthly apparent fecundity and juvenile recruitment rates to monthly rainfall for six ungulate species inhabiting the Masai Mara National Reserve (Mara) of Kenya, using cross-correlation analysis and distributed lag non-linear models. We compare the phenology and synchrony of breeding among the Mara ungulates with those for other parts of equatorial East Africa, with bimodal rainfall and less seasonal forage variation, and for subtropical southern Africa, with unimodal rainfall distribution and greater seasonal forage variation.
Key results: Births were more synchronised for topi, warthog and zebra than for hartebeest, impala and giraffe in the Mara, and for impala and hartebeest in southern than in eastern Africa. This pattern is likely to reflect regional differences in climate and plant phenology, hider–follower dichotomy and grazing versus browsing. All six species except the browsing giraffe apparently time the conception to occur in one wet season and births to occur just before the onset or during the next wet season, so as to maximise high-quality forage intake during conception and parturition. Fecundity and recruitment rates among the African ungulates peak at intermediate levels of rainfall and are reduced at low or excessive levels of rainfall. Fecundity rate is most strongly positively correlated with rainfall pre-conception, during conception and during early gestation, followed by rainfall at about the time of parturition for all the grazers. For giraffe, fecundity rate is most strongly correlated with rainfall during the gestation period.
Conclusions: Rainfall seasonality strongly influences reproductive seasonality and juvenile recruitment among African ungulates. The interaction of the rainfall influence with life-history traits and other factors leads to wide interspecific and regional variation.
Implications: Global climate change, especially widening annual rainfall variation expected to result from global warming, could reduce the predictability of the timing of peak forage availability and quality based on meteorological cues, the length of time with adequate nutrition or both, and hence reduce reproductive success among tropical ungulates.
Additional keywords: cross-correlations, distributed lag non-linear models, juvenile recruitment, Mara–Serengeti ecosystem.
Introduction
Offspring of ungulates are born at a nutritionally optimal time for their survival, which is early in the growing season (spring), when food quality is highest to support peak maternal demands extending through late pregnancy and early (pre-weaning) lactation (Gaillard et al. 1993; Langvatn et al. 2004; Zerbe et al. 2012). In high latitudes, the timing is tightly controlled by photoperiod, and the birth season narrowly spans 2–3 weeks (Spinage 1973; Bradshaw and Holzapfel 2007). Calves born later show reduced survival (Côté and Festa-Bianchet 2001). The corresponding conception peak falls during autumn, after both males and females have accumulated substantial fat reserves through summer (Parker et al. 2009).
In rainfall-driven tropical savannas, the optimal period is less reliably predictable. Nevertheless, the birth peak should still correspond with the early growing season, i.e. shortly after the onset of the rainy season. For species with 8-month gestation, births should peak in early December following conceptions in early April. Longer gestation should be associated with early mating, whereas species dependent more on grass quantity than quality may give birth later.
Annual rainfall variation spreads the optimal period so that the reproductive peak should tend to be broader in rainfall-driven than in temperature-driven systems. Counteracting this is the benefit of predator-swamping for species with offspring that follow the mother after birth, penalising early or late-born young. Species that hide their young should thus show a broader spread of births than those with follower young (Estes 1976; Rutberg 1987).
For megaherbivores with gestation periods exceeding 1 year, the critical periods of late gestation, early lactation and weaning become more diffusely spread seasonally. In these circumstances, nutrition enabling oestrous cycling becomes the overriding influence, so that conceptions peak early in the rainy season, leading for certain species to a birth peak early in the dry season (Owen-Smith 1988).
The influence of annual rainfall variation on reproductive success (calves recruited per mother) potentially operates through the following three mechanisms: (1) maternal nutrition after parturition, affecting the maternal milk supply supporting neonatal growth; (2) maternal nutrition during late pregnancy, supporting the growth of the foetus and hence birth mass; and (3) maternal nutrition before conception, affecting fat stores and hence fertility (Jönsson 1997; Parker et al. 2009). Furthermore, rainfall conditions during growth from birth to maturity affect the age at first reproduction, with primiparous females potentially first conceiving later in the seasonal cycle than older females (adaptive benefits of early reproduction outweigh the costs of seasonally late birth).
Potentially confounding the patterns of rainfall influence on reproductive success is offspring mortality, alleviating nutritional demands on the mother before the next conception. However, mothers that have supported calves through an adverse rainy season may inadequately recover fat reserves by the usual conception time, delaying births.
In equatorial latitudes with reduced seasonal variation in rainfall, births should be more widely spread through the year than in subtropical latitudes with intense seasonality in rainfall. In tropical lowland savannas, primary production is limited mainly by rainfall because temperatures are high throughout the year (Rutherford 1980; Deshmukh 1984). Seasonal fluctuation in rainfall primarily determines seasonal changes in the quantity and quality of the vegetation. In particular, the alternation of wet and dry seasons determines seasonal changes of nutrient concentrations in the herb-layer vegetation (Boutton et al. 1988a). Because of this seasonality, dietary quantity and quality are highly variable, so that intakes of digestible nutrients by herbivores are highest during the wet season (Tolsma et al. 1987; Prins 1988; Georgiadis and McNaughton 1990). Consequently, the peak intakes of digestible nutrients by herbivores occur in the early wet season when forage quality is maximal.
Because rainfall governs vegetation productivity, biomass and quality in savannas, it is likely to affect reproductive seasonality of ungulates through its controlling influence on nutrition (Oftedal 1985; Pekins et al. 1998; Parker et al. 2009). A fundamental question in ecology thus concerns establishing the relationship between rainfall and reproductive seasonality as a crucial step in understanding how the rainfall influence operates.
Here we test predictions of several hypotheses relating seasonality of births among six ungulate species to seasonality in rainfall in the Mara–Serengeti ecosystem, with bimodal rainfall (Table 1).

|
The current analysis extends the earlier ones by Ogutu et al. (2008b, 2009, 2010, 2011) in three important respects. (i) It uses cross-correlation analysis and distributed lag non-linear models to explore potentially non-linear and delayed effects of monthly rainfall on monthly fecundity and juvenile recruitment rates. (ii) It tests predictions of new hypotheses on rainfall influences on the timing and spread of births in six species of ungulates beyond those tested in the previous analyses. (iii) The spread of births and rainfall influences on fecundity and juvenile recruitment rates are compared with patterns for the same and other ungulate species elsewhere, with a focus on eastern and southern Africa.
Materials and methods
Study area
The study was carried out in the Masai Mara National Reserve (1°13ʹ–1°45ʹS, 34°45ʹ–35°25ʹE), covering 1530 km2 in south-western Kenya. The area forms the northern-most section of the Mara–Serengeti ecosystem, covering some 25 000 km2 defined by the annual migratory movements of wildebeest (Connochaetes taurinus mearnsi), zebra and Thomson’s gazelles (Eudorcas thomsoni). More detailed accounts of the study area are provided elsewhere (Ogutu et al. 2008b, 2009).
Rainfall
In the Mara region of Kenya, rain falls in the wet (November–June) and dry (July–October) seasons. The wet season can be subdivided between the early wet-season (November–February, ‘short rains’) and the late wet-season (March–June, ‘long rains’) components. Rainfall is bimodal, with a secondary peak in December–January being separated from a primary peak in April–May by a short dry season in February (Ogutu et al. 2008a; Fig 1). The 8-month wet (mean ± 1 s.d., 785.0 ± 151.7 mm) and 4-month dry (213.7 ± 76.4 mm) seasons cover November–June and July–October, respectively. The annual rainfall total over 1965–2003 averaged 1010.1 ± 187.3 mm, with all months receiving no less than 46 mm of rainfall on average, including the dry-season months. Hence, the dry-season nutritional shortfalls for ungulates should be relatively low. Monthly rainfall was averaged over a network of 15 gauges spread over the study area to account for spatial variation and lagged to encompass the months of conception estimated by backdating recorded birth months by the gestation lengths of the six species. The gestation lengths are 5.5 months for warthog, 6.5–7 months for impala, 8 months for Coke’s hartebeest and topi, 12 months for zebra and 15 months for giraffe. Severe El Niño–Southern Oscillation (ENSO) droughts in 1993, 1997 and 1999–2000, plus mild droughts in 1991 and 1994 characterised rainfall during 1989–2003, whereas extreme floods, coincident with the strongest ENSO episode on instrumental record, occurred in 1997–98 (Ogutu et al. 2008a). Maximum daily temperatures tend to be lowest from May to August (Fig. 1).
Plant biomass peaks in June following the long rains, whereas the minimum plant biomass occurs in February in the Mara region (Boutton et al. 1988a). The maximum nutrient standing stocks occur during late-May–early June, corresponding to the peak in plant biomass (Boutton et al. 1988b). The standing crop of green grass peaks over April–June on average. Grazing pressure keeps the grass standing crop low through October–February. The production and growth rates of the grasses and concentration of nutrients in green leaves are typically highest during the short rains, lower during the long rains and negligible during the long dry season (Prins 1988). Live grasses, forbs and sedges have peak nutrient concentration and digestibility just after the short rains in December and in mid-March at the beginning of the long rains (Boutton et al. 1988b). Grass quality (crude protein) starts rising in September, before the rains but following a temperature rise, in a good year, to a peak in December (Boutton et al. 1988b). In general, the concentrations of nutrients are higher in young leaves of trees and grasses than in mature ones in African savannas (Tolsma et al. 1987). During April–June, most of the new vegetation has matured and increased the structural carbohydrate content of secondary cell walls, thus diluting nutrient concentrations (Boutton et al. 1988b; Georgiadis and McNaughton 1990). Moreover, nutrient concentrations in herbaceous forage decrease at high rainfall so that too much rainfall negatively affects grass quality (Boutton et al. 1988b; Georgiadis and McNaughton 1990) and hence reproductive performance of ungulates. Grass quality starts declining in April and remains lowest through May–July. As a result, the crude protein component of live grass falls below the 5% maintenance level required for ruminants in June and July (Boutton et al. 1988b).
There are marked interspecific differences in the phenology of grasses and trees, with some species having only one growth period at the beginning of the wet season, whereas others produce new leaves after showers even at the end of the wet season (Tolsma et al. 1987; Prins 1988). Consequently, different plant species possess the highest proportion of leaves and are selected by grazers at different times (Prins 1988). The variety of native grasses, forbs and shrubs that mature asynchronously extend the period in which green, nutritious forage is available to ungulates.
Population surveys and demography of the six ungulate species
The Masai Mara Ecological Monitoring Program conducted monthly vehicle counts of large herbivores from July 1989 to December 2003. The study area was subdivided into three census blocks using major rivers and roads, each with a fixed transect (Ogutu et al. 2008b, 2009). The three transects sampled 29.4% (450.4 km2) of the 1530 km2 Mara reserve. Counts were not conducted on all the three transects in 17 of the 174 months of monitoring and on one of the three transects during a further 6 months because of flooding, or logistical or financial constraints. The 17 months were distributed irregularly over 9 years and eight calendar months, as detailed in the captions to Figs S1–S5, available as Supplementary Material for this paper. Additional details on the transects and the counting procedure can be found elsewhere (Ogutu et al. 2008b, 2009).
Warthog, topi and hartebeest are resident grazers, zebra is a migratory, non-ruminant grazer, impala is a resident mixed grazer-browser, and giraffe is a resident browser in the Mara–Serengeti ecosystem. Topi and zebra young follow their mothers from birth. Impala is a hider species but lambs follow their mothers a few days after birth. Hartebeest calves hide for several days or longer. Warthog piglets mostly remain hidden in holes for a few weeks after birth, before following their mother all day, whereas giraffe calves lie out away from their mothers for 1–3 weeks after birth (Cumming 1975; Skinner and Chimimba 2005). The young are weaned after 4 (topi, warthog, impala and hartebeest), 11 (zebra) or 15–17 (giraffe) months, so that nursing ceases before conceptions. Females attain sexual maturity after 1 (impala), 1.5–2.5 (topi, hartebeest, warthog), 2.5 (zebra) or 5 (giraffe) years.
A combination of body size, coat colour, horn length and shape was used to classify juveniles into size classes (newborn, quarter, half, yearling and three-quarter size classes) during each count. Approximate ages in months of juveniles of each species in each size class are presented in Ogutu et al. (2009, 2011). Except for newborns and some quarter-grown males, individuals were sexed using the presence, size and shape of horns and other secondary sexual characters. Warthog and zebra were not sexed because it was too time-consuming to do so reliably under field conditions. Animals were highly visible because of open grasslands, minimising the likelihood of misclassification into age–sex classes. The apparent monthly fecundity rate (fecundity rate) for each species was estimated as the total number of newborns counted in each month, divided by the corresponding number of adult females (impala, topi, hartebeest and giraffe) or all adults (zebra and warthog). Juvenile recruitment rate was estimated likewise by replacing the number of newborn young with that of juveniles in the quarter size class for each species. To reduce chances of misclassification of fully grown adults, the adult class included three-quarter size animals (hartebeest, topi, giraffe), or adult plus yearling and three-quarter classes (impala, zebra and warthog). Consequently, the fecundity and recruitment rates may be viewed as apparent fecundity and apparent recruitment rates because of the biases. Even so, these data are valuable in documenting reproductive patterns of the six ungulate species monthly over 14.5 years, and can enrich our understanding of reproductive seasonality in equatorial ungulates and its relationship with rainfall variation.
Statistical models and analyses
Seasonal distribution of births
Prior to analysis, the missing counts were imputed using the transect-specific seasonal state–space model of Piepho and Ogutu (2007), separately for the newborn, juvenile (quarter size class) and breeding-age classes (Figs S1–S5). To establish how the distribution of births varied among months, we computed least-square means by regressing the count total of newborn calves against birth month, assuming a negative binomial error distribution with a log-link function, and used the logarithm of the count total of adult females or all adults (warthog, zebra) as an offset to derive fecundity rates. The penalised cubic B-spline smoother covariance structure with a third-order difference penalty on the spline coefficients was used to model temporal trends in fecundity rates (Ogutu et al. 2009, 2011, 2012). We then conducted simultaneous contrast tests of the least-square means between all pairs of the 12 months in a year or selected groups of the months and adjusted the tests for multiplicity using simulation adjustment and for unequal denominator degrees of freedom across estimates in the SAS Procedure GLIMMIX (SAS, version 9.3; SAS Institute, Cary, NC). These tests revealed unimodal distributions of births for all species but giraffe, for which a bimodal distribution was supported. Thus, we fitted a normal distribution to the distribution of fecundity rates, as follows:

where r is the fecundity rate, C is a scaling constant φ, σ2 are the parameters of the normal distribution, N(.,.) is the normal probability density function and ε are independent and identically distributed normal errors.
We estimated the timing of birth peaks (φ), birth synchrony (σ), and peak fecundity rate (θ) for the unimodal distribution, by using the cumulative normal-distribution function

where F(r) is the empirical cumulative distribution function of monthly fecundity rates.
We then estimated the time encompassing at least 80% of births from the month of the first-recorded birth by

where Z0.8 is the 80% quantile of the standard normal distribution, and the percentage of births occurring between months m1 and m2 by

where φ and σ are defined as in Eqn 2.
For giraffe, we tested for a bimodal distribution of births by using a two-component mixture of normal distributions, as follows:

where r is the fecundity rate, C is a scaling constant, φ1, φ2, σ12 and σ22 are the parameters of the two-component normal distributions, ρ is the mixing proportion, N(.,.) is the normal-probability density function and ε is an independent and identically distributed normal error. When ρ = 0, then Eqn 5 reduces to the unimodal distribution in Eqn 1. The timing of birth peaks and birth synchrony were estimated for the two-component normal distributions for giraffe by (φ1, φ2) and (σ12, σ22), respectively; and the peak fecundity rates by
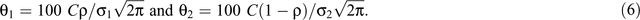
Models 1–6 were fit in the SAS procedure NLMIXED (SAS, version 9.3).
Relating monthly apparent fecundity and juvenile recruitment rates to rainfall
We investigated the relationship between monthly fecundity and juvenile recruitment rates and monthly rainfall using two statistical approaches. We then used the resultant relationships to establish the relative influence of rainfall, and hence forage availability, on reproductive success during the pre-conception, conception and gestation stages. First, we calculated the normalised cross-correlations between monthly fecundity rate and monthly rainfall over a range of leads and lags in rainfall encompassing the months pre-conception, during conception and post-conception for all the six species, to identify the time window over which resource cues, indexed by rainfall, exert the strongest positive influence on fecundity rate. Resource conditions backdated before conceptions potentially influence fertility, whereas the realised apparent fecundity may be modified additionally by conditions during gestation affecting fetal viability and neonatal survival dependent on birth mass. A further influence may come from the effective apparent fecundity in the preceding year, with poor calf production or survival relieving females of nurturing costs so that they are on a higher nutritional plane relative to rainfall in the next year. Second, because the relationship between fecundity rate and rainfall at each lag may be non-linear, we characterise the simultaneous variation in fecundity rate along both the lag and rainfall dimensions. This accounts for any potentially non-linear and delayed (lagged) effects of rainfall on fecundity rate. The maximum lag length was set to encompass the birth month, gestation, conception and pre-conception periods for each species.
The distributed lag non-linear model (DLNM)
A non-linear relationship between fecundity rate and rainfall can be specified using several different approaches. We adopt the distributed lag non-linear model (DLNM; Gasparrini et al. 2010; Gasparrini 2011; Gasparrini and Armstrong 2013) that uses smooth curves to describe the relationship between fecundity rate and rainfall because it generalises the other existing related approaches. The DLNM involves choosing a space of functions, called a basis, to characterise the relationship between fecundity rate and rainfall. The chosen basis determines the transformation of rainfall to produce a completely known transformation of rainfall called basis functions. The transformation results into a new set of transformed variables called basis variables. The matrix of the transformed variables can be included in the design matrix of a regression model to estimate the associated parameters. The delayed (lagged) effect of rainfall on fecundity rate is accounted for by specifying an additional virtual time dimension for the lags. The DLNM provides a detailed description of the time course of the relationship between fecundity rate and rainfall. The DLM incorporates the time dimension by including a parameter for rainfall at each lag up to the pre-specified maximum lag. We also consider the unconstrained DLNM that makes no assumptions about the shape of the association between fecundity rate and rainfall along lags and the relationship among the parameters. We provide the unconstrained DLNM merely for comparative purposes because it is well known to produce highly variable estimates because it does not account for multi-collinearity among lagged variables. We compare the unconstrained model with more parsimonious DLNMs that impose specific constraints on the shape of the distributed rainfall effects. The DLNM incorporates both non-linear and delayed effects of rainfall on fecundity rate. We similarly analysed juvenile recruitment using the DLNMs.
The DLNM we use for the time series of fecundity and recruitment rates can be written as a semi-parametric generalised additive model, as follows:
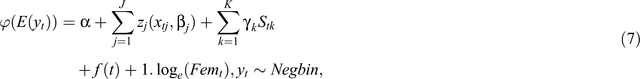
where E(yt) is the expectation of the fecundity or recruitment rate series (yt, t = 1,2, …, 174 months), ϕ is a monotonic link function. The functions zj characterise the relationships between the lagged variables xj and the linear predictor specified by the parameter vector βj. Sk denotes other predictors with linear effects and regression coefficient γk. We consider only season, a categorical variable with two levels in the Sk class of predictors. f(t) is a smooth function of time, modelling time trend in fecundity or recruitment rates. The logarithm of the number of females or all adults (warthog and zebra) used as an offset 1.loge(Femt) has a unit slope by construction.
The function z(xt, β) can be specified in various ways. The DLNM specification of this function is described in detail elsewhere (Gasparrini et al. 2010; Gasparrini 2011; Gasparrini and Armstrong 2013). We therefore first briefly summarise the general specification of the function z(xt, β) by Gasparrini et al. (2010) and Gasparrini and Armstrong (2013) and then describe how we specialised it to fit our data.
The DLNM can be formulated in terms of a lag-basis and cross-basis functions s(xt) of the N-dimensional vector of the time series of the response variable x = [x1, … xt, …, xN]T. s(xt) is defined in terms of the N × (L+1) matrix Q of the lagged time series, yielding qt = [x1, … xt-ℓ, …, xt-L]T. The matrix Q indirectly characterises the new lag dimension defined by the vector ℓ = [0, …, ℓ,…, L]T, where L is the maximum lag. On choosing the first basis with dimension õℓ to represent the association along the new lag space, we can use a (L+1) × õℓ basis matrix C by applying the specific basis functions to the lag vector ℓ. A compact and general representation of the lag-basis function S(xt) for DLNMs in terms of these components is

in which different choices of the basis used to generate C yield different models. The transformed variables in W = QC can be incorporated into the design matrix to estimate the õℓ-length parameter vector ç, in which Cç represents the lag-specific contributions. The non-linear extension of Eqn 8 to DLNMs is accomplished by choosing a second basis with dimension õx to model the relationship along the space of the predictor x, yielding the N × õx basis matrix Z by applying the related functions to x. Applied together with the transformation that defines the matrix of the lagged responses Q above, this results in a three-dimensional N × õx × (L+1) array  . The resulting parameterisation of the cross-basis function S(xt) for DLNMs becomes
. The resulting parameterisation of the cross-basis function S(xt) for DLNMs becomes

where rtjT is the vector of lagged response (monthly fecundity rates in our case) for time t, transformed using the basis function j, and c.k is the matrix of the basis variables for ℓ.
The more complex cross-basis for DLNMs in Eqn 9 generalises the lag-basis for DLNMs in Eqn 8. Model Eqns 8 and 9 may be fitted using the usual regression methods by including the cross-basis matrix W in the design matrix. The vector  of estimated parameters of the cross-basis function in Eqn 9 defines a simultaneously non-linear and lagged dependency, and its length õx × õℓ is the product of the dimensions of the bases for the two spaces. DLNMs are completely specified by the two bases chosen to generate the matrices Z and C.
of estimated parameters of the cross-basis function in Eqn 9 defines a simultaneously non-linear and lagged dependency, and its length õx × õℓ is the product of the dimensions of the bases for the two spaces. DLNMs are completely specified by the two bases chosen to generate the matrices Z and C.
Fitting the distributed lag non-linear model
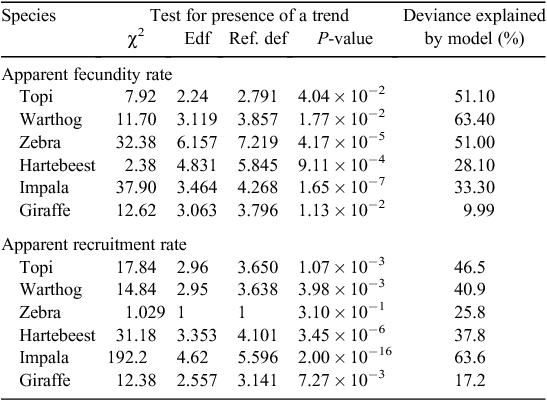
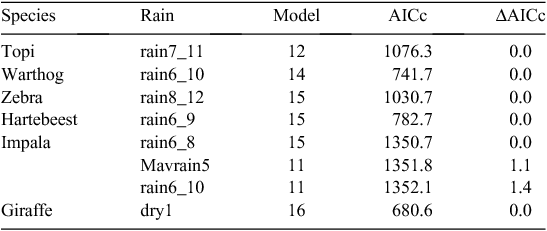
To fit the DLNMs, we used either a linear (hartebeest, impala, zebra and giraffe) or a second-degree polynomial (topi and warthog) basis function to describe the shape of the relationship between fecundity rate and rainfall along the rainfall dimension and a natural cubic-spline basis function for the lag dimension for all the species. The maximum lag was set to 16 months for topi, warthog, hartebeest and impala, 19 months for zebra and 23 months for giraffe, to encompass the birth month, gestation, conception and pre-conception periods for each species. The two basis functions were multiplied to produce a cross-basis (tensor product) function with ~7–10 degrees of freedom for transformed variables, depending on species. The transformed variables in the cross-product function constitute the covariates in z(xt, β) for which the parameter vector β is to be estimated. The number of newborn (response variable) was then regressed on all the 7–10 transformed variables plus season of births (wet or dry) and a cubic basis spline smoother of the running month of survey sampling, accounting for the temporal trend in fecundity rate, as explanatory variables using a generalised additive model. The logarithm of the number of adult females, or all adults (warthog and zebra), is specified as the offset in the model to derive the numerical fecundity rate. A negative binomial error distribution and a log-link function are specified for the number of newborns. The scale parameter of the negative binomial model, the regression coefficients for the intercept and each of the 7–10 transformed variables and the percentage of total deviance explained by the model are estimated by the restricted maximum likelihood method (Table 2).
|
The complete distributed lag non-linear model (Gasparrini et al. 2010) was fit in the R package dlnm (Gasparrini 2011). The overall cumulative association between fecundity rate and rainfall across all lag periods at which the rainfall effect was positive and greater than that for the reference value of 0 mm of rainfall was then re-expressed in terms of the one-dimensional basis functions initially chosen for the rainfall dimension (Gasparrini and Armstrong 2013). Using the results, we assessed the overall influence of rainfall on fecundity rate on the basis of the pattern of the lag-specific regression coefficients, overall cumulative association with rainfall and the patterns in the cross-correlation function. Juvenile recruitment was similarly analysed. Annotated SAS and R codes for fitting the models are provided in Supplementary material SM1 for this paper.
Relating apparent fecundity and juvenile recruitment rates to rainfall
Furthermore, we regressed the monthly fecundity rate against the moving average of monthly rainfall spanning the time window identified for each species by the cross-correlogram and the DLNM assuming a negative binomial error distribution for the number of newborn, a log-link function and the logarithm of the number of adult females or all adults (warthog and zebra) as the offset. We assessed the relative influences of (1) monthly rainfall at the time of conception versus around parturition on seasonal distribution of births and (2) of the late wet-season rainfall, primarily influencing the forage carried into the dry season versus that of the early wet season rainfall, contributing to fertility using model selection based on the corrected Akaike information criterion (AICc). We indexed forage availability and quality around the time of conception using blocks of monthly rainfall identified for each species above, as well as using rainfall components spanning the preceding dry (July–October), early dry (July–August), late dry (September–October), early wet (November–February) and late wet (March–June) seasons. For each rainfall component, we fitted a model assuming that fecundity rate increases exponentially, follows a humped relationship, or increases up to an asymptote and then stabilises with increasing rainfall. The models were (Ogutu et al. 2008b) as follows:






where μ is the logarithm of the expected abundance of the newborn or juveniles and x is the rainfall component.
These models were also fitted within a generalised linear model framework, assuming a negative binomial error distribution for the number of births, a log-link function and using the logarithm of the number of adult females or all adults (warthog and zebra) as an offset in the SAS procedure GLIMMIX. To further assess whether forage quality around the time of birth might have the greater influence on fecundity rate, we similarly related monthly fecundity rate to rainfall in the current season/quarter and to running means of monthly rainfall calculated over 2–6 months before and during the birth month. Support for all the rainfall components were assessed using AICc. The datasets on monthly fecundity and juvenile recruitment rates and rainfall are provided in Tables S2 and S3, respectively, available as Supplementary Material for this paper.
Results
Seasonal distribution of births
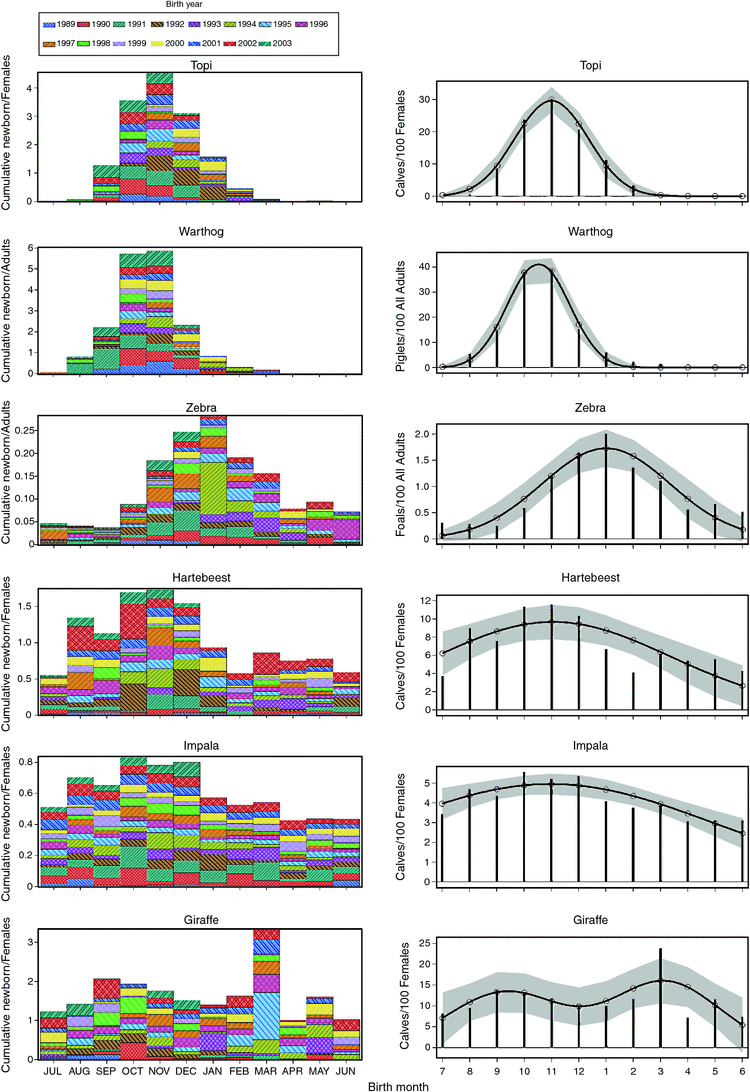
Fecundity rate varied seasonally for all the species (Figs 1, 2); a variation confirmed by contrasts of the least-square means (Tables 3, 4). Even so, the birth peak did not occur consistently 1–2 months after the onset of the early rains, nor did its timing clearly differ between the short- and long-grass grazers or between the tree and herbaceous browsers, as anticipated by H1. Instead, the birth peak was similarly timed for topi, hartebeest, impala and warthog, despite differences in grass height favoured, and later for zebra. Moreover, the expectation that births in the grazing topi, warthog, hartebeest and impala should peak in November–December (H2.1) was only partly supported. Topi births occurred primarily between September and February (99%), with a peak about the time of the commencement of the short rains in October–November, representing 55% of births (Tables 3, 4, Fig. 2). The corresponding peak in conception occurred before the late rains, through February–March. Warthog showed a very similar pattern, with 49% of piglets born during October–November, indicating a conception peak at the end of the late rains during April–May. Zebra births were also quite widely spread, but with a peak over December–January accounting for 31%. Because of the 12-month gestation and mating shortly after births, zebra conceptions occurred in the same months as births, i.e. during the early rains in the preceding year. For hartebeest, the peak birth months are likewise October–November, implying that conception peaks in February–March, but births were more widely spread so that only 24.5% occurred during October–November. Impala births were spread throughout the year, with the peak in the 3 months of October–December accounting for only 31.5% compared with the null value of 25%, corresponding with a conception peak through March–May. The expectation that births should have a broader spread in hartebeest with hiding young than in topi with follower young (H2.2) was supported. The birth peak in zebra that is less dependent on grass quality owing to a non-ruminant digestive physiology and has follower young was surprisingly narrower than expected by H2.3. The observed spread of births therefore deviates from expectations for impala and hartebeest, with a broad spread of births, and zebra, with a quite narrow spread. For giraffe, births showed double peaks, with a sharper peak in March and a more diffuse peak through September–November, implying that conception peaks in December and June–August. The diffuse birth peak for giraffe agrees with the prediction of H2.4.
Monthly rainfall influence on fecundity and juvenile recruitment rates
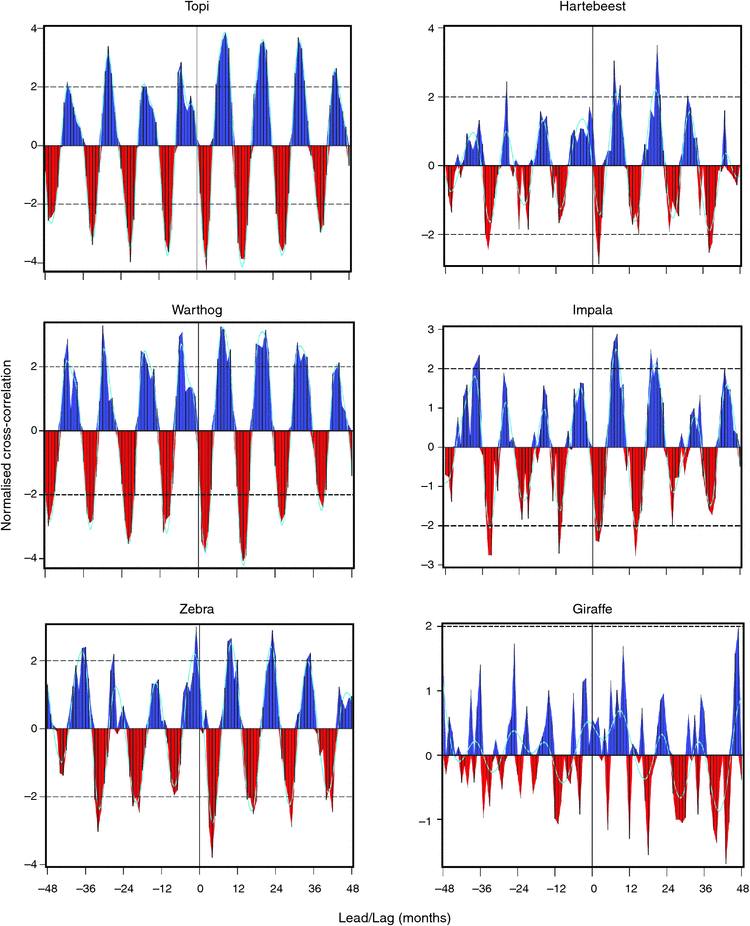
The cross-correlations between fecundity rate and monthly rainfall showed a quasi-cyclic pattern for all six species. The cross-correlations were positive at lags 6–11 for topi, 6–10 for warthog and impala, 8–12 for zebra, 7–10 for hartebeest and 0–11 for giraffe (Fig. 3). Additionally, the cross-correlations were positive for the birth month for zebra, hartebeest and giraffe, but not for the other three species (Fig. 3).
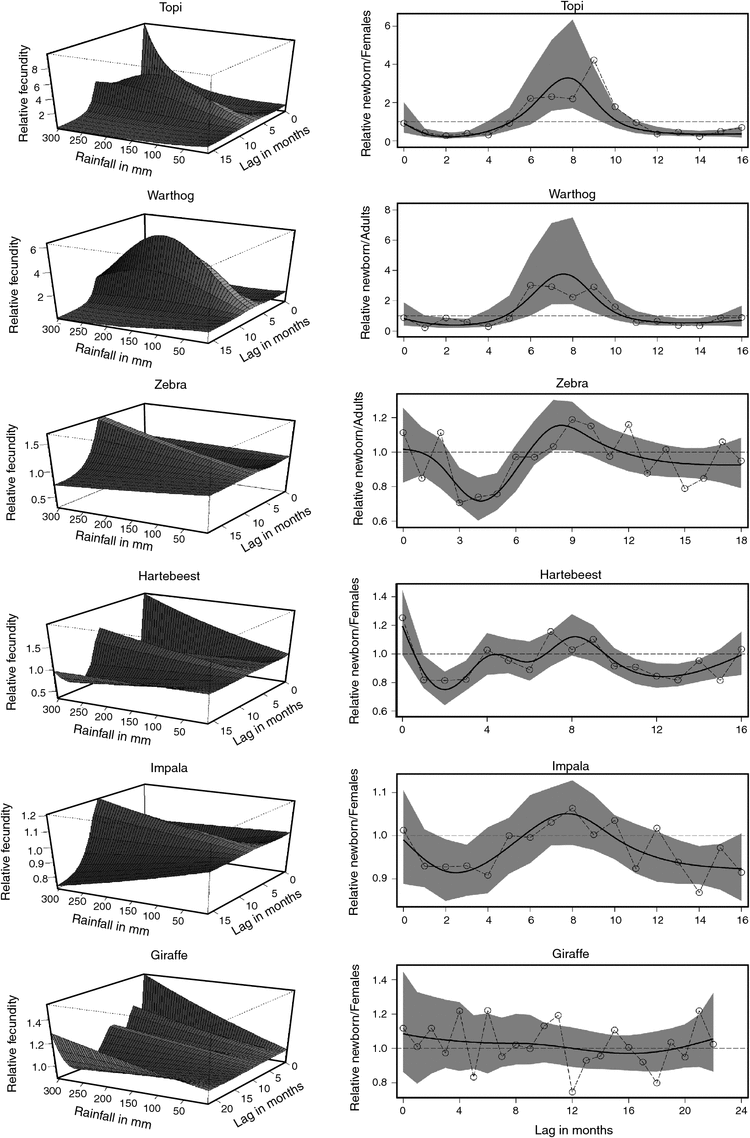
The DLNM showed that rainfall influence on fecundity rate for all six species is non-linear and distributed over several months before the birth month, similar to the pattern found by the cross-correlograms (Fig. 4, left panels). The distribution of rainfall effect on fecundity rate across lags, on the basis of both the unconstrained and constrained models, showed that rainfall effect is positive at lags 6–11 for topi, 6–10 for warthog and impala, 8–12 for zebra, 7–9 for hartebeest and 0–11 for giraffe (Fig. 4, right panels). Specifically, fecundity rate was most strongly positively influenced by rainfall (1) 2–3 months pre-conception, during conception and 1–2 months post-conception for topi, (2) 3–4 months pre-conception, during conception and 1 month post-conception for warthog, (3) during conception and 4–5 months post-conception for zebra, (4) 1 month pre-conception, during conception and 1 month post-conception for hartebeest, (5) 2–3 months pre-conception, during conception and 1 month post-conception for impala, and (6) from 5 months post-conception up to the birth month for giraffe. Also, for giraffe, rainfall had small but positive effects distributed over 12 lags from early gestation to birth (Fig. 4). Rainfall effect on fecundity was also positive immediately before and during the birth month for zebra, hartebeest and impala and increased toward positive values in the birth month for topi and warthog. Furthermore, the rainfall effect peaked at lags 8–9 for topi and zebra, 7–8 for warthog and impala and lag 8 for hartebeest but showed no evident peak for giraffe (Fig. 4, right panels). The peaks suggested a peak in rainfall influence around the time of conception for topi, warthog, impala and hartebeest, but not for zebra and giraffe. The fecundity rates decreased with increasing rainfall at all the other lags for all the species. Fecundity rates for zebra and hartebeest similarly decreased as rainfall increased, except in the birth month when they increased with rainfall. The correlation between fecundity rate and rainfall was also zero or negative at all the other lags but 0–11 for giraffe (Fig. 4).
The established patterns of rainfall influence support the hypothesis that adequate maternal fat store is crucial for conception and hence timing of births, or equally, that insufficient nutrition suppresses or delays oestrous cycling, as postulated by H3.1. The patterns accord with the prediction of H3.2 that adequate nutrition at the time of conception is a stronger predictor of timing of births in grazing African ungulates than is nutrition at the time of parturition.
When the bidimensional predictions of the DLNM were reduced to the rainfall dimension only, the effect of rainfall on fecundity rate over the range of lags at which rainfall effect is positive showed a humped relationship with a peak at ~150 mm for topi and warthog, but exponentially increasing relationships for the other species (Fig. 5). Warthog fecundity responded more strongly to a unit increase in rainfall than did topi fecundity, but both warthog and topi responded more strongly to rainfall than did the other species (Fig. 5).
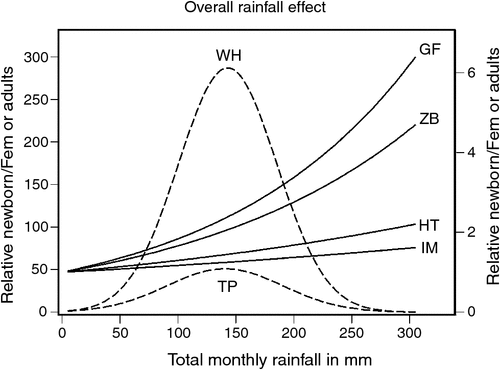
|
Regression of fecundity rate on moving averages calculated over blocks of monthly rainfall identified by the cross-correlograms and the DLNMs had stronger support, based on the Akaike information criterion, than all the seasonal and quarterly rainfall components for all the species (Table 5). These results further support the expectations that rainfall and adequate nutrition at the time of conception have stronger influences on the timing of births than those at the time of parturition (H3.1 and H3.2). The correlation between fecundity rate and the dry-season rainfall preceding births was somewhat stronger for the browsing giraffe than for the five grazers (Table 5). Monthly rainfall averaged over 5 months (warthog and impala) or 6 months (topi, zebra, and hartebeest) before, and including, the birth month had humped relationships with fecundity rate and the second-strongest support (Table 5). As well, fecundity rate was only weakly correlated with rainfall in the wet or dry season preceding births, implying that forage quality affecting fetal growth does not have an overriding influence on timing of births as contemplated by H3.3.
|
The cross-correlations for recruitment rates showed patterns broadly similar to those for fecundity rates and peaked at lags 10–13 months for topi, 10–14 for warthog, 12–15 for zebra, 10–12 for hartebeest, and 7–10 for giraffe, but showed no clear peak for impala (Fig. S6, available as Supplementary Material for this paper). The distribution of rainfall effect on juvenile recruitment along lags in months showed two peaks, with a major peak occurring about the time of conception and a minor peak about the time of birth. The peaks were more marked for the five grazing species than for the browsing giraffe. The major peak for giraffe occurred during the gestation, as opposed to the conception period for the grazers (Fig. S7, available as Supplementary Material for this paper). Rainfall in the current and in the preceding 5 months had a positive effect on juvenile recruitment in topi, warthog, zebra and hartebeest. But for impala, only rainfall in the current and the preceding 1 month had a positive effect on juvenile recruitment. Giraffe showed a positive rainfall influence in the preceding 2 months (Fig. S7), a pattern inconsistent with the prediction of H3.4. When the bidimensional relationship with rainfall was reduced to the rainfall dimension only, juvenile recruitment had a humped overall relationship with rainfall for five of the six species. Juvenile recruitment peaked at ~100 mm of rainfall for topi, hartebeest and giraffe, and at ~150 mm for warthog and zebra. Impala recruitment was apparently an exception and declined with increasing rainfall (Fig. S8, available as Supplementary Material for this paper).
Discussion
Seasonal distribution of births
There is considerable interspecific variation in birth distribution, such that births are seasonally restricted for topi and warthog, intermediate for zebra, span the whole year with only minor peaks for hartebeest and impala, and are effectively aseasonal for giraffe. Births by many topi and warthog begin a month before the commencement of the early rains, associated with the time when grass quality starts to rise (Boutton et al. 1988b), so that early lactation is associated with the time when nutritious young grass foliage becomes available at the start of the rains. Even so, many topi and warthog births occur in July–September, well before the early rains, or in January–February, toward the end of the early rains. The minor birth peaks shown by hartebeest and impala also coincided with the period just before the early rains, whereas the birth peak for zebra occurred 2 months later, well into the early rains. Timing of peak births for zebra hence resembled that for the Mara–Serengeti wildebeest, which also occurs toward the end of the early rains in January–March, although with a much stronger peak in February (Sinclair et al. 2000). The peak maternal demand for both species also comes during the long rains. The interspecific distinctions in the distribution of births portray complex interactions between manifold life-history traits such as gestation length, hider–follower and grazer–browser dichotomies and ruminant–non-ruminant digestive physiologies, with ecological constraints, notably rainfall, in shaping seasonality of births in African ungulates.
Monthly rainfall influence on fecundity and recruitment rates
Rainfall influence on fecundity rate for all the five grazers was positive and strongest during the pre-conception, conception and early gestation periods. For giraffe, rainfall had a relatively small but positive and increasing influence on fecundity rate, from 5 months post-conception to the birth month. The strong and positive influence of rainfall during the pre-conception period for topi, impala and warthog is likely to reflect its influence on accumulation of fat reserves by females. The quasi-cyclic pattern of the correlation between rainfall and fecundity rate and the increase in this correlation during late pregnancy for all six species implies a positive rainfall influence about the time of birth and early lactation when maternal energy demands are high. The cross-correlation patterns for recruitment support the view that rainfall influence on the timing of reproduction is also strong and positive during the early juvenile stages. Juvenile recruitment in impala that was weakly negatively correlated with rainfall was the only apparent exception to this pattern.
The blocks of months with positive rainfall influence identified by the DLNM and the timing of peak conception and parturition months for the Mara ungulates are consistent with the notion that conception and early gestation are timed to coincide with high-quality forage months in the preceding wet season, and parturition and lactation are timed to coincide with, or to occur just before, months with favourable forage-quality conditions in the current wet season. So, for example, the conception peak in November–January and the birth peak in November–January a year later enable zebra with a 12-month gestation length to enjoy conditions of high-quality forage at conception, early gestation, parturition and lactation.
The humped overall relationships between fecundity rate and rainfall imply that conceptions will be low or suppressed (1) by low rainfall during dry seasons and droughts when grasses are dry or fibrous, or (2) by excessive rainfall during the peak wet-season months and floods when grass quality declines. The humped relationship implies, furthermore, that substantial reductions or increases in rainfall, for example, as a result of climate change, will shift fecundity rate in topi and warthog away from their optima. The higher synchronisation of reproduction in topi and warthog than in the other four species suggests greater sensitivity to seasonal variation in forage quality, possibly through suppression of oestrous cycling at low forage quality. It is likely to ensure a tighter match between the timing of conception and lactation and the periods with optimal forage conditions. The increase in fecundity rate with rainfall in zebra, impala, hartebeest and giraffe also implies fewer conceptions during dry seasons or droughts. Low fecundity rate for topi and warthog at both low and high rainfall values implies fewer births during both drought and excessive rainfall conditions.
The relationships with rainfall further support the suggestions that high but not excessive rainfall enhances conception and early fetal growth by promoting sufficient supply of high-quality forage, accumulation of fat reserves and attainment of peak physical condition necessary for conception (Talbot and Talbot 1963; du Plessis 1972, Estes 1976; Prins 1996; Bro-Jørgensen 2001). The positive rainfall influence at the time of parturition portrays increased demand for high-quality forage during late pregnancy and early lactation when energetic demands peak. The relationships with rainfall, moreover, support the following three suggestions: (1) years with more but not excessive rainfall will have more conceptions, successful fetal development and births than years with less rainfall; (2) both the amount and spread of rainfall over several consecutive months are crucial in shaping reproductive seasonality of births among the ungulates; and (iii) a decrease in rainfall owing to climate change would reduce fecundity of births for all six species, whereas a large increase in rainfall would likely reduce fecundity of births in topi and warthog more than for the other four species.
The patterns of rainfall influence reinforce the general view that rainfall exerts strong controls on the timing and synchrony of births in African mammals (Moss 2001; Moe et al. 2007; Ryan et al. 2007; Wittemyer et al. 2007a; Ogutu et al. 2010) through its influence on the phenology, productivity and quality of vegetation (Rutherford 1980; Deshmukh 1984; Boutton et al. 1988b). The results suggest that rainfall controls the timing and success of conception not only through its influence on adequate nutrition before and during conception (Talbot and Talbot 1963; Estes 1976) but also during gestation and at the time of birth, depending on species. Direct support for this suggestion comes from Kenyan Samburu elephants followed for 7 years, 86% of which conceived after the peak in vegetation productivity, with the highest conception rate occurring during the unusually high El Niño rains in 1997–98 and no conception occurring during the subsequent La Niña drought phase in 2000 (Wittemyer et al. 2007a). Moreover, the timing of birth peaks in the resident wildebeest population of the western Serengeti occurs in January, a month earlier than in the main migratory population (Ndibalema 2009), being adjusted to the earlier onset of the rainy season. In the nearby Ngorongoro Crater, there is a difference of up to 3 weeks in the timing of calving peaks in the resident subpopulations of wildebeest on opposite sides of the crater, reflecting microclimatic differences (Estes 1976).
The results are also consistent with the notion that rainfall governs the nutritional quality of forage for ungulates at the time of birth and during lactation when nutritional demands on mothers are highest. Consequently, African ungulates consume high-quality forage (in the wet season) before ovulation, and some fail to conceive during seasons with inadequate nutrition. This reflects failure to transcend the threshold level in body condition, below which conceptions fail to materialise (Lewis and Kappeler 2005), and a capital breeding reproductive strategy, in which energy buffers are stored before initiation of reproductive events (Stearns 1992; Jönsson 1997; Stephens et al. 2009).
Why are births synchronised for some species but not others? On theoretical grounds, natural selection should favour mating at the most favourable time of year, when a population is in peak condition, to maximise offspring recruitment. The relationships we found between fecundity and juvenile recruitment rates and rainfall suggest that birth peaks are timed not only to match peaks in high-quality nutrition after parturition (du Plessis 1972; Grimsdell 1973; Sinclair 1977; Carmichael et al. 1977; Prins 1996; Moe et al. 2007), but also to enable ungulates to anticipate habitat conditions at the time of birth, on the basis of experienced ecological conditions at the time of conception (Wittemyer et al. 2007a; Ogutu et al. 2010). However, high interannual variability and unpredictability of rainfall in African savannas probably prevents some individuals from reliably predicting forage quality at the time of parturition on the basis of conditions at the time of conception. Nevertheless, tropical ungulates attempt to surmount this constraint by adjusting timing of conception to track interannual variation in the distribution and amount of rainfall. As a result, conceptions are likely to occur, on average, when conditions are auspicious. Also, the expected time of peak parturition coincides with the period with the highest probability of ample supplies of high-quality resources. Consequently, females receive high-quality nutrition to meet the high energetic and nutritional demands of post-partum maternal care during early lactation. Breeding males and females then enter the period of the rut in peak nutritional condition (Talbot and Talbot 1963).
Our results further suggest that grazing ungulates living in variable environments, such as the Mara–Serengeti, are likely to maximise their use of experienced ecological conditions pre-conception and at the time of conception, to adaptively fine-tune their prediction of probabilistic (expected) forage availability during parturition when forage quality is hard to predetermine because of high interannual variability in seasonal forage distribution (Wittemyer et al. 2007a; Ogutu et al. 2010). Even though there is no way of reliably predicting what conditions will be like many months beforehand in such stochastic environments, by having conceptions during nutritionally favourable periods and/or integrating resource cues over blocks of several successive months, rather than relying on rigidly fixed blocks of months, African ungulates bank on maximising the expected reproductive success if conditions are favourable at the end of gestation.
The seasonal phenology of births, resulting in parturition at the average onset of elevated forage quality and helping synchronise the highest energy costs of early lactation with maximum forage quality, is typical of the income breeding strategy (Jönsson 1997; Festa-Bianchet et al. 1998). The high variability in forage availability and quality in African savannas both within and among years has probably predisposed African ungulates to use a mixed capital-income breeding strategy to integrate energy allocation from stores (capital strategy) to create energy buffers for periods of nutritional stress, and a facultative feeding (income strategy) to replenish energy stores depleted in the course of gestation and lactation. The low maximum seasonal forage production in savannas compared with the seasonal superabundance in north temperate regions also makes capital inputs essential to savanna ungulates, despite their smaller fat stores.
Using such a mixed strategy, African ungulates are likely to rely on both energy stores and available forage during post-parturition lactation to enhance calf recruitment. Such switching between stored and procured resources or a mixed energy allocation strategy to surmount energy constraints and ensure successful reproduction has been suggested for other large mammals (Lewis and Kappeler 2005; Wittemyer et al. 2007a; Servanty et al. 2009).
Whereas our analysis showed that rainfall before, during and after conception influences the seasonal timing of births, reproductive success, in particular survival of juveniles, is also dependent on rainfall during the wet-season months at the time of birth, influencing the amount of grass carried forward through the dry season for all the five grazers. But support for the cumulative wet-season rainfall component was far weaker than that for the blocks of monthly rainfall identified by the DLNMs for all the six species. Owen-Smith (cited by Novellie 1986) argued that rainfall pre-conception has no influence on calf production by kudus (Tragelaphus strepsiceros) in Kruger Park, because many calves are produced when high rainfall follows drought conditions pre-conception. Also, for Kudu in Kruger Park rainfall in the dry season spanning the gestation period before birth has the greatest influence on population change, rather than the prior wet-season rainfall preceding conception. However, among all the Mara ungulates except the browsing giraffe, the dry-season rainfall component received consistently less support than did the wet-season rainfall component.
Our findings contrast with those of research conducted in northern temperate regions on Dall sheep (Ovisdalli, Rachlow and Bowyer 1991), moose (Alces alces, Bowyer et al. 1998; Garel et al. 2009), roe deer (Capreolus capreolus, Linnell and Andersen 1998) and reindeer (Rangifer tarandus, Post et al. 2003), suggesting that synchronisation of the mother and newborn requirements with high nutritional conditions after parturition (Dauphiné and McClure 1974; Bunnell 1982; Thompson and Turner 1982; Rutberg 1987; Risenhoover and Bailey 1988; Bowyer 1991; Keech et al. 2000; Côté and Festa-Bianchet 2001) is the overriding factor in the timing of births.
Climatic changes to precipitation regimes may alter the onset of seasonal vegetation green-up (Pettorelli et al. 2005), with adverse consequences for maternal body condition and juvenile recruitment. Given the pervasive rainfall influences, interannual variation in plant phenology caused by climatic variability may induce variation in the timing of parturition (Gunn and Doney 1975; Post et al. 2003), number of calves born (Post and Forchammer 2008 ; Burthe et al. 2011; Moyes et al. 2011), growth and survival of juveniles (Clutton-Brock et al. 1982; Weladji and Holand 2003; Pettorelli et al. 2007; Burthe et al. 2011), as has been documented for temperate ungulates. Nevertheless, the high interannual variation in seasonal reproductive patterns implies high flexibility in tracking interannual variation in the distribution of forage and that African equatorial ungulates could be more resilient to global warming than temperate ungulates dependent on more predictable resource cues. Even so, increasing rainfall variation, associated with more frequent and intense droughts, could lower conceptions, calf survival and hence overall reproductive success in African ungulates.
Regional variation in reproductive seasonality with rainfall among African ungulates
The seasonal patterns of birth shown by all the grazing Mara ungulates are only partially consistent with the synchronisation of the energetically demanding period of early lactation and calf growth with the early rains when the expectation of high-quality forage is highest. But highly variable seasonal distribution of rainfall among years results in variable seasonal distribution of conception and births among years. Moreover, given the positive association of births with temporally variable rains and the considerable regional variation in rainfall seasonality, it is not surprising that African ungulates exhibit striking regional variation in reproductive seasonality. The seasonal patterns we documented in the Mara are broadly similar to those shown by these same species elsewhere in East Africa (Leuthold and Leuthold 1975; Rodgers 1984; Sinclair et al. 2000), in particular the wide seasonal spread of births and the high interannual variability in birth distributions.
However, there are also important interspecific and regional distinctions in the timing and synchrony of births. Thus, topi and warthog births peak a month earlier in the Serengeti (Sinclair et al. 2000) than in the Mara, reflecting regional differences in the timing of rainfall. Nevertheless, tsessebe (D. l. lunatus) shows a narrow birth peak extending through September–December from the late dry season through the early rains in South Africa (Fairall 1968), very similar to the pattern shown by the conspecific topi in the Mara. Novellie (1986) suggested that rain falling up to 6–12 months before the peak conception period, rather than during the period of pregnancy and lactation, is most strongly correlated with, and thus appears to influence, the lambing percentage in bontebok (D. dorcas dorcas), in the winter-rainfall region of South Africa. This pattern differs from the pattern we found for the congeneric Mara–Serengeti topi. Novellie (1986) suggested further that nutrient availability before the mating season determines the physiological condition and hence the probability of conception by female bontebok. Warthog also breeds seasonally in southern Africa and has a birth peak over October–November (Fairall 1968; Mason 1986). However, where there is rainfall year-round (e.g. in western Uganda, Zaire and Congo Brazzaville), warthog give birth in all months (Brown 1936; Clough 1969).
Zebra births also peak in December–March in Serengeti Park (Sinclair et al. 2000) and in the Amboseli ecosystem of Kenya, before the onset of the long rains (Simon 1962, p. 22). Burchell’s zebra foals peak earlier in December–January during the middle of the southern African wet season (Smuts 1974, cited by Skinner and Chimimba 2005). But the Cape mountain zebra (Equus zebra zebra), which occupies the winter rainfall region of South Africa, foals year-round, although with a peak in summer (Penzhorn 1985), because grass growth is more responsive to the summer-rainfall component. As a result, low precipitation during spring and early summer is significantly associated with the majority of conceptions being late, whereas high precipitation is associated with a majority of early conceptions among mountain zebra (Penzhorn 1985).
The red hartebeest (A. b. caama) and impala both show narrow birth seasons in South Africa, but reproduce year-round in the Mara–Serengeti, unlike the patterns for warthog, zebra and giraffe that differ little from those shown in South Africa. Hartebeest shows a birth peak during October–November in southern Africa, coinciding with the start of the rains, during which 82% of calves are born (Skinner et al. 1973; Skinner et al. 1974; Anderson 1979). Conception in hartebeest is related to rainfall such that high conception and juvenile recruitment follow exceptionally wet years with abundant forage. The same applies to giraffe and Grant’s (Nanger granti) and Thomson’s gazelles in Kenya (Field and Blankenship 1973).
For impala, the birth peak in southern Africa is very narrow, with 80% of lambs typically born within a 2-week period extending from late November into early December (Skinner et al. 1974, 2002; Moe et al. 2007). This is linked to the greater rainfall seasonality in southern Africa. The impala rut generally follows the months of heavy rainfall and increased food quality (Fairall 1972; Murray 1982). Thus, at Sengwa in Zimbabwe, impala conceptions peak at the end of the rains in April–May and births peak at the beginning of the rains in November–December (Murray 1982). Food quality is highest 1–2 months after the birth peak, implying that breeding is timed to favour adult and juvenile survival (Murray 1982). This pattern supports the argument that it is the wider spread of rainfall and hence resources in equatorial Africa that largely underpin the wider spread of births there.
Similarly to hartebeest and impala, sable antelope (Hippotragus niger) is a strictly seasonal breeder, with 2–3 months calving season in southern Africa, but with peaks that vary geographically from South Africa (February–March; Fairall 1968), Zambia (June–September; Ansell 1960, 1963), Zimbabwe (March; Child and Wilson 1964; Wilson, 1969), Botswana (January–early February; Child 1968) to Angola (Estes and Estes 1974); however, it breeds throughout the year near the equator in Kenya (Sekulic 1978). Consequently, the calving seasons of sable also seem timed to coincide with the peak of the growing season and peak availability of nutritious forage for lactating females (Wilson and Hirst 1977).
The year-round distribution of rainfall, with rainfall even in the dry-season months, in equatorial Africa, plus hiding young thus is likely to underlie the year-round breeding by hartebeest, impala and sable in East Africa compared to southern Africa. However, that topi has only one mating season in East Africa, whereas hartebeest there breeds year-round is hard to explain, but could be due to hiding young and lower sensitivity of hartebeest to forage quality and hence variation in rainfall. Moreover, topi offspring develop so quickly that they are independent at 1 year, whereas hartebeest young accompany their mothers for up to 2 years. Hartebeest dams become pregnant again within a month, and may be followed by up to three offspring. Also, topi does not strictly represent follower young to the same extent as does wildebeest or even the congeneric blesbok (Damaliscus pyrgargus). Rather, the topi system is transitional between the hartebeest hider system and the fully developed follower system of the wildebeest, so that topi young go through an abbreviated hiding stage in the Mara–Serengeti woodlands. By contrast, roan antelope (Hippotragus equines, Wilson and Hirst 1977) and waterbuck (Kobus ellipsyprimnus, Spinage 1969; Sinclair et al. 2000; Ogutu et al. 2011), both grazers that are far larger in body size and have longer gestation periods (waterbuck: 9.3 months (280 days, Smithers 1983) and roan antelope: 9–9.5 months (268–286 day, mean 277 days, Chardonnet and Crosmary 2013)) than topi, hartebeest or C.t mearnsi, but which prefer moist savannas where dry-season conditions are less severe, are aseasonal breeders in both East and South Africa.
Calving by giraffe is bimodal and peaks during December–March and August–October in southern Africa as in Mara (Fairall 1968; Hall-Martin et al. 1975), despite the expectation that births should be more synchronised in more seasonal environments that receive rainfall in fewer months (Anderson 1979). Our results agree with the finding of Bercovitch and Berry (2010) that female giraffe produces infants throughout the year, and contrast with the finding of Hall-Martin et al. (1975) that conception in giraffe is most strongly correlated with rainfall a month before and during conception.
Studies of several other populations of African ungulates have provided further evidence that rainfall seasonality strongly influences their reproductive seasonality through its influence on food availability and quality around the time of conception, birth or both. Thus, the wildebeest mating peaks also coincide with times of surplus food, e.g. April–June for the Mara (Talbot and Talbot 1963). Wildebeest calving peaks in the early rains from mid-November to December in Kruger Park (Fairall 1968), September–October in Zambia (Ansell 1960), January–March in Mara (Talbot and Talbot 1963) and February–March in Serengeti (Sinclair et al. 2000).
Buffalo (Syncerus caffer) births are also seasonal (Grimsdell 1973; Sinclair 1977; Prins 1996; Sinclair et al. 2000) and peak at the height of the wet-season rains in March–May in Serengeti (Sinclair et al. 2000) and earlier in January–March in South Africa, corresponding to mating peaks in March–May (Fairall 1968; Ryan et al. 2007). Buffalo births in South Africa are most strongly correlated with monthly rainfall 13 months before the birth month, which is about the time of conception (Ryan et al. 2007). The correlations of births with rainfall are positive both at lags 0–3 and 11–15 months from the birth month, suggesting that conception is influenced by rainfall and that the gestation period for buffalo ensures that the same high-quality conditions are realised at parturition 340 days later (Ryan et al. 2007). The spread of monthly rainfall influence over the current and the preceding wet-season months for buffalo is similar to that for the Mara–Serengeti ungulates.
Conceptions in the red lechwe (Kobus leche leche) peak during the rains in (December–March), leading to peak births between August and October, just before the onset of the rains, suggesting that nutrition is the most likely determinant of reproductive seasonality in female lechwe in the Linyati Swamp in northern Botswana (Williamson 1991). Lechwe deviates from the majority of African ungulates because forage quality and quantity peak in its wetland habitats in the dry season, after the floods have receded (Nefdt 1996). Consequently, the majority of lechwe births occur in the dry season (Nefdt 1996). The Kafue lechwe also mates during the dry part of the year when grass quality and quantity are lowest, but calves are born when grass is most abundant (Nefdt 1996). Rising water levels, and not rainfall, thus provide the proximate cue for mating in Kafue lechwe (Nefdt 1996). Among Puku (Kobus vardoni) occupying Luanga Valley, Zambia, births are seasonal, and 67% of births occur from January to April during the wet season, when food quality and availability are greatest, thus ensuring maximum calf survival (Rosser 1989). Puku also hides its young calves from predators in the thick herb layer growing in the wet season (Rosser 1989). Calf production in white-eared kob (Kobus kob leucotis) is also synchronous but occurs in the late wet season, well after the period of peak food abundance, when the kobs are migrating to their northern dry-season range (Fryxell 1987). Predation is likely the proximate factor affecting survival of the young and the migration to the swamps, where predators are fewer, may serve to lessen calf mortality (Fryxell 1987).
However, lambing in Kudu, a browser like giraffe, is positively correlated with rainfall during the period of pregnancy and lactation and not with rainfall before the conception period (Owen-Smith, cited by Novellie 1986), being similar to the pattern shown by the Mara–Serengeti giraffe. In wild female African elephants, mixed grazer-browsers, the onset of the rainy season, when primary productivity and the quality of food sharply increase, appears to serve as the energetic stimulus driving increased progestin levels and the onset of reproductive activity (Wittemyer et al. 2007b).
Where seasonal occurrence of rainfall varies widely among years as in arid south-western African savannas, ungulates such as the oryx (Oryx spp.; Morrow et al. 1999; Wronski et al. 2011) and springbok (Antidorcas marsupialis; Skinner et al. 2001) reproduce year-round.
Conclusions
Birth peaks did not consistently occur 1–2 months after the onset of the early rains when forage quality peaks, as expected. Even though the five grazing ungulate species produce many young at the start of the early rains, particularly topi, warthog and zebra, many young are also born outside the anticipated 1–2 months at the onset of the early rains. Topi, hartebeest and impala births peak in November–December, whereas warthog births peak somewhat earlier, in October–November. The hider–follower dichotomy did not explain much of the variation in timing of birth peaks because, even though hartebeest with hiding young had a broader birth peak than did topi with following young, hartebeest had a narrower peak than impala, which also has hiding young. Moreover, zebra showed a broad birth season despite having follower young, likely because it is a non-ruminant less dependent on food quality and has a 12-month gestation period. Our analysis demonstrated that the birth peak is broader in East Africa with bimodal rainfall than in South Africa with unimodal rainfall only for the three species that are seasonal breeders in South Africa, comprising hartebeest, impala and sable antelope. Gestation length also has limited power in explaining reproductive seasonality among African ungulates because giraffe with the longest gestation period of the six species, and impala and hartebeest with far shorter gestation periods, all showed little reproductive seasonality.
For the grazing ungulates, poor nutrition pre-conception is likely to suppress or delay conception because females take longer to build up adequate fat reserves, whereas good nutrition pre-conception enhances or advances oestrus. The rainfall influence on fecundity rate pre-conception, at conception and during early gestation was stronger than that around parturition for all five grazers. The lagged blocks of monthly rainfall had the strongest influence of all the rainfall components considered, implying that rainfall had delayed effects on fecundity rate spanning several months. The rainfall influence on juvenile recruitment rate established for all of the species supported the prediction that the effect of rainfall should be strong soon after birth when post-natal growth requires high-quality forage. Moreover, conception peaks appeared timed to match cumulative past vegetation conditions, as indexed by past rainfall, whereas birth peaks appeared to match vegetation quality peaks and, hence, current rates of nutritional gain.
Our findings suggest that African ungulates adaptively fine-tune their breeding to match local phenology of food quality and quantity, by relying on rainfall during crucial stages in their reproductive cycles. The ungulate species, especially the grazers, exploit the bimodal rainfall distribution by timing conception to occur in one wet season and births to occur just before the onset of, or during the next wet season, to maximise high quality forage intake around both conception and parturition. For the browsing giraffe, rainfall had little influence pre-conception or during conception. The seasonal reproductive patterns showed high levels of flexibility across years. The timing of reproductive activity among the ungulates is variable and less predictable, reflecting a strong interaction between ecological variability and life-history constraints in shaping the timing of reproductive events. Our results also suggest that the hypothesis that parturition peaks are timed so that calves can be weaned on the palatable and high-quality new grass that appears at the beginning of the rains does not fully explain why seasonal rainfall variation is so weakly influential for aseasonal breeders with similar gestation lengths and feeding styles as their sympatric seasonal breeders of similar size.
Acknowledgements
The Masai Mara Ecological Monitoring Program was designed and supervised by Dr Holly T. Dublin and executed by Messes Paul Chara, John Naiyoma, Charles Matankory and Alex Obara. It was funded by the World Wide Fund for Nature–East Africa Program (WWF-EARPO) and Friends of Conservation (FOC). The program also received financial, material or logistical support from WWF–US, WWF–Sweden, the Darwin Initiative (DICE), the University of British Columbia, United States Fish and Wildlife Service, Kenya Wildlife Service, Cottar’s Camp, Kichwa Tembo, Keekorok Lodge/Balloon Safaris and Kerr and Downey Safaris. Data analysis and writing were supported by the National Science Foundation (NSF) through Grant Nos: BCS 0709671 and DEB-0342820 and a grant from the Belgian government (DGIC BEL011) to the International Livestock Research Institute. JO was supported by an Alexander von Humboldt Research Fellowship at the Bioinformatics Unit, Institute of Crop Science, University of Hohenheim, Stuttgart, Germany, and by the International Livestock Research Institute (ILRI). WWF–EARPO, the Kenya Meteorological Department and Prof. K. Holekamp provided the rainfall data. We thank Drs A. R. E Sinclair, R. Hilborn and S. A. R. Mduma for advice on ageing and sexing animals, Drs N. Owen-Smith and R. D. Estes and two anonymous reviewers for suggestions that helped improve an earlier version of this paper. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 641918.
References
Anderson, J. L. (1979). Reproductive seasonality of the nyala Tragelaphus angasi: the interaction of light, vegetation phenology, feeding style and reproductive physiology. Mammal Review 9, 33–46.| Reproductive seasonality of the nyala Tragelaphus angasi: the interaction of light, vegetation phenology, feeding style and reproductive physiology.Crossref | GoogleScholarGoogle Scholar |
Ansell, W. F. H. (1960). The breeding of some larger mammals in northern Rhodesia. Proceedings of the Zoological Society of London 134, 251–274.
| The breeding of some larger mammals in northern Rhodesia.Crossref | GoogleScholarGoogle Scholar |
Ansell, W. F. H. (1963). Additional breeding data on northern Rhodesian mammals. Puku 1, 9–19.
Bercovitch, F. B., and Berry, P. S. M. (2010). Reproductive life history of Thonicroft’s giraffe in Zambia. African Journal of Ecology 48, 535–538.
| Reproductive life history of Thonicroft’s giraffe in Zambia.Crossref | GoogleScholarGoogle Scholar |
Boutton, T. W., Tieszen, L. L., and Imbamba, S. K. (1988a). Biomass dynamics of grassland vegetation in Kenya. African Journal of Ecology 26, 89–101.
| Biomass dynamics of grassland vegetation in Kenya.Crossref | GoogleScholarGoogle Scholar |
Boutton, T. W., Tieszen, L. L., and Imbamba, S. K. (1988b). Seasonal changes in the nutrient of East African grassland vegetation. African Journal of Ecology 26, 103–115.
| Seasonal changes in the nutrient of East African grassland vegetation.Crossref | GoogleScholarGoogle Scholar |
Bowyer, R. T. (1991). Timing of parturition and lactation in southern mule deer. Journal of Mammalogy 72, 138–145.
| Timing of parturition and lactation in southern mule deer.Crossref | GoogleScholarGoogle Scholar |
Bowyer, R. T., Kie, J. G., and Van Ballenberghe, V. (1998). Habitat selection by neonatal black-tailed deer: climate, forage, or risk of predation? Journal of Mammalogy 79, 415–425.
| Habitat selection by neonatal black-tailed deer: climate, forage, or risk of predation?Crossref | GoogleScholarGoogle Scholar |
Bradshaw, W. E., and Holzapfel, C. M. (2007). Evolution of animal photoperiodism. Annual Review of Ecology Evolution and Systematics 38, 1–25.
| Evolution of animal photoperiodism.Crossref | GoogleScholarGoogle Scholar |
Bro-Jørgensen, J. (2001). Lek-breeding in topi antelopes (Damaliscus lunatus). PhD Thesis. University College, London.
Brown, C. E. (1936). Rearing wild animals in captivity and gestation periods. Journal of Mammalogy 17, 10–13.
| Rearing wild animals in captivity and gestation periods.Crossref | GoogleScholarGoogle Scholar |
Bunnell, F. L. (1982). The lambing period of mountain sheep: synthesis, hypotheses, and tests. Canadian Journal of Zoology 60, 1–14.
| The lambing period of mountain sheep: synthesis, hypotheses, and tests.Crossref | GoogleScholarGoogle Scholar |
Burthe, S. A., Butler, A., Searle, K. R., Hall, S. J. G., Thackeray, S. J., and Wanless, S. (2011). Demographic consequences of increased winter births in a large aseasonally breeding mammal (Bos taurus) in response to climate change. Journal of Animal Ecology 80, 1134–1144.
| Demographic consequences of increased winter births in a large aseasonally breeding mammal (Bos taurus) in response to climate change.Crossref | GoogleScholarGoogle Scholar |
Carmichael, I. H., Patterson, L., Dräger, N., and Breton, D. A. (1977). Studies on reproduction in the African buffalo (Syncerus caffer) in Botswana. South African Journal of Wildlife Research 7, 45–52.
Chardonnet, P., and Crosmary, W. (2013). Pigs, hippopotamuses, chevrotain, giraffes, deer and bovids. In ‘Mammals of Africa’. (Eds J. Kingdon and M. Hoffmann) pp. 548–556. (Bloomsbury: Londres.).
Child, G. (1968). An ecological survey of north-eastern Botswana. UNDP/FAO Project no. TA 2563. Report to the Government of Botswana. FAO Rome..
Child, G., and Wilson, V. J. (1964). Observations on ecology and behaviour of roan and sable in three tsetse control areas. Arnoldia Rhodesia 16, 1–8.
Clough, G. (1969). Some preliminary observations on reproduction in the warthog, Pharcochoerus aethiopicus Pallas. Journal of Reproduction and Fertility 6, 323–337.
Clutton-Brock, T. H., Guinness, F. E., and Albon, S. D. (1982). Red deer, behaviour and ecology of two sexes. (University of Chicago Press: Chicago, IL.)
Côté, S. D., and Festa-Bianchet, M. (2001). Birthdate, mass and survival in mountain kids, effects of maternal characteristic and forage quality. Oecologia 127, 230–238.
| Birthdate, mass and survival in mountain kids, effects of maternal characteristic and forage quality.Crossref | GoogleScholarGoogle Scholar | 24577654PubMed |
Cumming, D. H. (1975). ‘A field study of the ecology and behaviour of warthog. Vol. 7.’ (Trustees of the National Museums and Monuments of Rhodesia: Salisbury, Rhodesia.)
Dauphiné, T. C. J., and McClure, R. L. (1974). Synchronous mating in Canadian barren-ground caribou. The Journal of Wildlife Management 38, 54–66.
| Synchronous mating in Canadian barren-ground caribou.Crossref | GoogleScholarGoogle Scholar |
Deshmukh, I. K. (1984). A common relationship between precipitation and grassland peak biomass for East and southern Africa. African Journal of Ecology 22, 181–186.
| A common relationship between precipitation and grassland peak biomass for East and southern Africa.Crossref | GoogleScholarGoogle Scholar |
Du Plessis, S. S. (1972). Ecology of blesbok with special reference to productivity. Wildlife Monographs. No. 30. pp. 69. (The Wildlife Society: Washington, DC.)
Estes, R. D. (1976). The significance of breeding synchrony in the wildebeest. East African Wildlife Journal 14, 135–152.
| The significance of breeding synchrony in the wildebeest.Crossref | GoogleScholarGoogle Scholar |
Estes, R. D., and Estes, R. K. (1974). The biology and conservation of the giant sable antelope (Hippotragus niger variani Thomas, 1916). Proceedings. Academy of Natural Sciences of Philadelphia 126, 73–104.
Fairall, N. (1968). The reproductive seasons of some mammals in the Kruger National Park. Zoologica Africana 3, 180–210.
Fairall, N. (1972). Behavioural aspects of the reproductive physiology of the impala, Aepyceros melampus (Litch.). Zoologica Africana 7, 167–174.
Festa-Bianchet, M., Gaillard, J.-M., and Jorgenson, J. T. (1998). Mass and density-dependent reproductive success and reproductive costs in a capital breeder. American Naturalist 152, 367–379.
| Mass and density-dependent reproductive success and reproductive costs in a capital breeder.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M%2FpvFemsg%3D%3D&md5=e3a6028f89ece23e2286085d3443f797CAS | 18811445PubMed |
Field, C. R., and Blankenship, L. H. (1973). Nutrition and reproduction of Grant’s and Thomson’s gazelles, Coke’s hartebeest and giraffe in Kenya. Journal of Reproduction and Fertility 19, 287–301.
| 1:STN:280:DyaE2c7ivVWnsQ%3D%3D&md5=002ff2ca2142a91db1c33cf6085730ecCAS | 4522381PubMed |
Fryxell, J. M. (1987). Seasonal reproduction of white-eared kob in Boma National Park, Sudan. African Journal of Ecology 25, 117–124.
| Seasonal reproduction of white-eared kob in Boma National Park, Sudan.Crossref | GoogleScholarGoogle Scholar |
Gaillard, J.-M., Delorme, D., Julien, J.-M., and Tatin, D. (1993). Timing and synchrony of births in roe deer. Journal of Mammalogy 74, 738–744.
| Timing and synchrony of births in roe deer.Crossref | GoogleScholarGoogle Scholar |
Garel, M., Solberg, E. J., Sæther, B.-E., Grøtan, V., Tufto, J., and Heim, M. (2009). Age, size, and spatiotemporal variation in ovulation patterns of a seasonal breeder, the Norwegian moose (Alces alces). American Naturalist 173, 89–104.
| Age, size, and spatiotemporal variation in ovulation patterns of a seasonal breeder, the Norwegian moose (Alces alces).Crossref | GoogleScholarGoogle Scholar | 19072136PubMed |
Gasparrini, A. (2011). Distributed lag linear and non-linear models in R: the package dlnm. Journal of Statistical Software 43, 1–20.
| 22003319PubMed |
Gasparrini, A., and Armstrong, B. (2013). Reducing and meta-analyzing estimates from distributed lag non-linear models. BMC Medical Research Methodology 13, 1.
| Reducing and meta-analyzing estimates from distributed lag non-linear models.Crossref | GoogleScholarGoogle Scholar | 23297754PubMed |
Gasparrini, A., Armstrong, B., and Kenward, M. G. (2010). Distributed lag non-linear models. Statistics in Medicine 29, 2224–2234.
| Distributed lag non-linear models.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjpt1ChtQ%3D%3D&md5=1a70eada71948c29a61d8f1661026c56CAS | 20812303PubMed |
Georgiadis, N., and McNaughton, S. J. (1990). Elemental and fibre contents of savanna grasses: variation with grazing, soil type, season and species. Journal of Applied Ecology 27, 623–634.
| Elemental and fibre contents of savanna grasses: variation with grazing, soil type, season and species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhs1akt7s%3D&md5=b537e255e154a14f53b71a5bf4c9f638CAS |
Grimsdell, J. J. R. (1973). Reproduction in the African buffalo (Syncerus caffer) in western Uganda. Journal of Reproduction and Fertility 19, 303–318.
| 1:STN:280:DyaE2c7ivVWntg%3D%3D&md5=f0365b1544cc9840b7bcd13be48c0719CAS |
Gunn, R. G., and Doney, J. M. (1975). The interaction of nutrition and physical condition at mating on ovulation rate and early embryo mortality in Scottish blackface ewes. Journal of Agricultural Science (Cambridge) 85, 465–470.
| The interaction of nutrition and physical condition at mating on ovulation rate and early embryo mortality in Scottish blackface ewes.Crossref | GoogleScholarGoogle Scholar |
Hall-Martin, A. J., Skinner, J. D., and Van Dyk, J. M. (1975). Reproduction in the giraffe in relation to some environmental factors. East African Wildlife Journal 13, 237–248.
| Reproduction in the giraffe in relation to some environmental factors.Crossref | GoogleScholarGoogle Scholar |
Ims, R. A. (1990). On the adaptive value of reproductive synchrony as a predator-swamping strategy. American Naturalist 136, 485–498.
| On the adaptive value of reproductive synchrony as a predator-swamping strategy.Crossref | GoogleScholarGoogle Scholar |
Jönsson, K. I. (1997). Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66.
| Capital and income breeding as alternative tactics of resource use in reproduction.Crossref | GoogleScholarGoogle Scholar |
Keech, M. A., Bowyer, R. T., Ver Hoef, J. M., Boertje, R. D., Dale, B. W., and Stephenson, R. (2000). Life-history consequences of maternal condition in Alaskan moose. The Journal of Wildlife Management 64, 450–462.
| Life-history consequences of maternal condition in Alaskan moose.Crossref | GoogleScholarGoogle Scholar |
Langvatn, R., Mysterud, A., Stenseth, N. C., and Yoccoz, N. G. (2004). Timing and synchrony of ovulation in red deer constrained by short northern summers. American Naturalist 163, 763–772.
| Timing and synchrony of ovulation in red deer constrained by short northern summers.Crossref | GoogleScholarGoogle Scholar | 15122493PubMed |
Leuthold, W., and Leuthold, B. M. (1975). Temporal patterns of reproduction in ungulates of Tsavo East National Park, Kenya. East African Wildlife Journal 13, 159–169.
| Temporal patterns of reproduction in ungulates of Tsavo East National Park, Kenya.Crossref | GoogleScholarGoogle Scholar |
Lewis, R. J., and Kappeler, P. M. (2005). Are Kirindy Sifaka capital or income breeders? It depends. American Journal of Primatology 67, 365–369.
| Are Kirindy Sifaka capital or income breeders? It depends.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2Mnhs1Srtg%3D%3D&md5=ffe13b9640109b33c7e43bf1a0e2dd9fCAS | 16287106PubMed |
Linnell, J. D. C., and Andersen, R. (1998). Timing and synchrony of birth in a hider species, the roe deer Capreolus capreolus. Journal of Zoology 244, 497–504.
| Timing and synchrony of birth in a hider species, the roe deer Capreolus capreolus.Crossref | GoogleScholarGoogle Scholar |
Mason, D. R. (1986). Reproduction in the male warthog Pharcocoerus aethiopicus from Zululand, South Africa. South African Journal of Zoology 21, 39–47.
Moe, S. R., Rutina, L. P., and du Toit, J. T. (2007). Trade-off between resource seasonality and predation risk explains reproductive chronology in impala. Journal of Zoology 273, 237–243.
| Trade-off between resource seasonality and predation risk explains reproductive chronology in impala.Crossref | GoogleScholarGoogle Scholar |
Morrow, C. J., Wildt, D. E., and Monfort, S. L. (1999). Reproductive seasonality in the female scimitar-horned oryx (Oryx dammah). Animal Conservation 2, 261–268.
Moss, C. J. (2001). The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. Journal of Zoology 255, 145–156.
| The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya.Crossref | GoogleScholarGoogle Scholar |
Moyes, K., Nussey, D. H., Clements, M. N., Guinness, F. E., Morris, A., Morris, S., Pemberton, J. M., Kruuk, L. E. B., and Clutton-Brock, T. H. (2011). Advancing breeding phenology in response to environmental change in a wild red deer population. Global Change Biology 17, 2455–2469.
| Advancing breeding phenology in response to environmental change in a wild red deer population.Crossref | GoogleScholarGoogle Scholar |
Murray, M. G. (1982). The rut of the impala: aspects of seasonal mating under tropical conditions. Zeitschrift für Tierpsychologie 59, 319–337.
| The rut of the impala: aspects of seasonal mating under tropical conditions.Crossref | GoogleScholarGoogle Scholar |
Ndibalema, V. G. (2009). A comparison of sex ratio, birth periods and calf survival among Serengeti wildebeest sub-populations, Tanzania. African Journal of Ecology 47, 574–582.
| A comparison of sex ratio, birth periods and calf survival among Serengeti wildebeest sub-populations, Tanzania.Crossref | GoogleScholarGoogle Scholar |
Nefdt, R. J. C. (1996). Reproductive seasonality in Kafue lechwe antelope. Journal of Zoology 239, 155–166.
| Reproductive seasonality in Kafue lechwe antelope.Crossref | GoogleScholarGoogle Scholar |
Novellie, P. (1986). Relationships between rainfall, population density and the size of the bontebok lamb crop in the Bontebok National Park. South African Journal of Wildlife Research 16, 39–46.
Oftedal, O. T. (1985). Pregnancy and lactation. In ‘Bioenergetics of wild herbivores’. (Eds R. J. Hudson and R. G. White.) pp. 215–238. (CRC Press: Boca Raton, FL.)
Ogutu, J. O., Piepho, H.-P., Dublin, H. T., and Bhola, Ogutu, J. O., Piepho, H.-P., Dublin, H. T., and Bhola, (2008a). El Niño–Southern Oscillation, rainfall, temperature and normalized difference vegetation index fluctuations in the Mara–Serengeti ecosystem. African Journal of Ecology 46, 132–143.
| El Niño–Southern Oscillation, rainfall, temperature and normalized difference vegetation index fluctuations in the Mara–Serengeti ecosystem.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Piepho, H.-P., Dublin, H. T., Bhola, N., and Reid, R. S. (2008b). Rainfall influences on ungulate population abundance in the Mara–Serengeti ecosystem. Journal of Animal Ecology 77, 814–829.
| Rainfall influences on ungulate population abundance in the Mara–Serengeti ecosystem.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cvgsFynug%3D%3D&md5=c46969a9525582191775d3353a658e4dCAS | 18422558PubMed |
Ogutu, J. O., Piepho, H.-P., Dublin, H. T., Bhola, N., and Reid, R. S. (2009). Dynamics of Mara–Serengeti ungulates in relation to land use changes. Journal of Zoology 278, 1–14.
| Dynamics of Mara–Serengeti ungulates in relation to land use changes.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Piepho, H.-P., Dublin, H. T., Bhola, N., and Reid, R. S. (2010). Rainfall extremes explain interannual shifts in timing and synchrony of calving in topi and warthog. Population Ecology 52, 89–102.
| Rainfall extremes explain interannual shifts in timing and synchrony of calving in topi and warthog.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Piepho, H.-P., Dublin, H. T., Bhola, N., and Reid, R. S. (2011). Dynamics of births and juvenile recruitment in Mara–Serengeti ungulates in relation to climatic and land use changes. Population Ecology 53, 195–213.
| Dynamics of births and juvenile recruitment in Mara–Serengeti ungulates in relation to climatic and land use changes.Crossref | GoogleScholarGoogle Scholar |
Ogutu, J. O., Owen-Smith, N., Piepho, H.-P., Kuloba, B., and Endebe, J. (2012). Dynamics of ungulates in relation to climatic and land use changes in an insularized African savanna ecosystem. Biodiversity and Conservation 21, 1033–1053.
| Dynamics of ungulates in relation to climatic and land use changes in an insularized African savanna ecosystem.Crossref | GoogleScholarGoogle Scholar |
Owen-Smith, R. N. (1988). ‘Mega Herbivores: the Influence of Very Large Body Size on Ecology.’ (Cambridge University Press: Cambridge, UK.)
Owen-Smith, N. (2002). ‘Adaptive Herbivore Ecology. From Resources to Populations in Variable Environments.’ (Cambridge University Press: Cambridge, UK.)
Parker, K. L., Barboza, P. S., and Gillingham, M. P. (2009). Nutrition integrates environmental responses of ungulates. Functional Ecology 23, 57–69.
| Nutrition integrates environmental responses of ungulates.Crossref | GoogleScholarGoogle Scholar |
Pekins, P. J., Smith, K. S., and Mautz, W. W. (1998). The energy costs of gestation in white-tailed deer. Canadian Journal of Zoology 76, 1091–1097.
| The energy costs of gestation in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Penzhorn, B. I. (1985). Reproductive characteristics of a free-ranging population of Cape Mountain zebra (Equus zebra zebra). Journal of Reproduction and Fertility 73, 51–57.
| Reproductive characteristics of a free-ranging population of Cape Mountain zebra (Equus zebra zebra).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M7gslyhtw%3D%3D&md5=f8ad53ef926f680b2d8a75c5da4ecff8CAS |
Pettorelli, N., Weladji, R. B., Holand, Ø., Mysterud, A., Breie, H., and Stenseth, N. C. (2005). The relative role of winter conditions: linking climate and landscape-scale plant phenology to alpine reindeer body mass. Biology Letters 1, 24–26.
| The relative role of winter conditions: linking climate and landscape-scale plant phenology to alpine reindeer body mass.Crossref | GoogleScholarGoogle Scholar | 17148119PubMed |
Pettorelli, N., Pelletier, F., von Hardenberg, A., Festa-Bainchet, M., and Côté, S. D. (2007). Early onset of vegetation growth vs rapid green-up: impacts on juvenile mountain ungulates. Ecology 88, 381–390.
| Early onset of vegetation growth vs rapid green-up: impacts on juvenile mountain ungulates.Crossref | GoogleScholarGoogle Scholar | 17479756PubMed |
Piepho, H.-P., and Ogutu, J. O. (2007). Simple state space models in a mixed model framework. The American Statistician 61, 224–232.
| Simple state space models in a mixed model framework.Crossref | GoogleScholarGoogle Scholar |
Post, E., and Forchammer, M. C. (2008). Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Transactions of the Royal Society of London B 363, 2369–2375.
Post, E., Bøving, P. S., Perdersen, C., and MacArthur, M. A. (2003). Synchrony between caribou calving and plant phenology in depredated and non-depredated populations. Canadian Journal of Zoology 81, 1709–1714.
| Synchrony between caribou calving and plant phenology in depredated and non-depredated populations.Crossref | GoogleScholarGoogle Scholar |
Prins, H. H. T. (1988). Plant phenology patterns in Lake Manyara National Park, Tanzania. Journal of Biogeography 15, 465–480.
| Plant phenology patterns in Lake Manyara National Park, Tanzania.Crossref | GoogleScholarGoogle Scholar |
Prins, H. H. T. (1996). ‘Ecology and Behaviour of the African Buffalo: Social Inequality and Decision Making.’ (Chapman and Hall: New York.)
Rachlow, J. L., and Bowyer, R. T. (1991). Interannual variation in timing and synchrony of parturition in Dall’s sheep. Journal of Mammalogy 72, 487–492.
| Interannual variation in timing and synchrony of parturition in Dall’s sheep.Crossref | GoogleScholarGoogle Scholar |
Risenhoover, K., and Bailey, J. (1988). Growth rates and birthing period of bighorn sheep in low-elevation environments in Colorado. Journal of Mammalogy 69, 592–597.
| Growth rates and birthing period of bighorn sheep in low-elevation environments in Colorado.Crossref | GoogleScholarGoogle Scholar |
Rodgers, W. A. (1984). Warthog ecology in east Tanzania. Mammalia 48, 327–350.
| Warthog ecology in east Tanzania.Crossref | GoogleScholarGoogle Scholar |
Rosser, A. M. (1989). Environmental and reproductive seasonality of puku, Kobus vardoni, in Luanga Valley, Zambia. African Journal of Ecology 27, 77–88.
| Environmental and reproductive seasonality of puku, Kobus vardoni, in Luanga Valley, Zambia.Crossref | GoogleScholarGoogle Scholar |
Rutberg, A. T. (1987). Adaptive hypotheses of birth synchrony in ruminants: an interspecific test. American Naturalist 130, 692–710.
| Adaptive hypotheses of birth synchrony in ruminants: an interspecific test.Crossref | GoogleScholarGoogle Scholar |
Rutherford, M. C. (1980). Annual plant production-precipitation relations in arid and semi-arid regions. South African Journal of Science 76, 53–56.
Ryan, S. J., Knechtel, C. V., and Getz, W. M. (2007). Ecological cues, gestation length, and birth timing in African buffalo (Syncerus caffer). Behavioral Ecology 18, 635–644.
| Ecological cues, gestation length, and birth timing in African buffalo (Syncerus caffer).Crossref | GoogleScholarGoogle Scholar |
Sekulic, R. (1978). Seasonality of reproduction in the sable antelope. East African Wildlife Journal 16, 177–182.
| Seasonality of reproduction in the sable antelope.Crossref | GoogleScholarGoogle Scholar |
Servanty, S., Gaillard, J.-M., Toïgo, C., Brandt, S., and Baubet, E. (2009). Pulsed resources and climate-induced variation in the reproductive traits of wild boar under high hunting pressure. Journal of Animal Ecology 78, 1278–1290.
| Pulsed resources and climate-induced variation in the reproductive traits of wild boar under high hunting pressure.Crossref | GoogleScholarGoogle Scholar |
Simon, N. (1962). ‘Between the Sunlight and the Thunder: the Wild Life of Kenya.’ (Collins: London.)
Sinclair, A. R. E. (1977). ‘The African Buffalo.’ (University of Chicago Press: Chicago, IL.)
Sinclair, A. R. E., Mduma, S. A. R., and Arcese, P. (2000). What determines phenology and synchrony of ungulate breeding in Serengeti? Ecology 81, 2100–2111.
| What determines phenology and synchrony of ungulate breeding in Serengeti?Crossref | GoogleScholarGoogle Scholar |
Skinner, J. D., and Chimimba, C. T. (2005). ‘The Mammals of the Southern African Subregion.’ (Cambridge University Press: Cambridge, UK).
Skinner, J. D., Van Zyl, J. H., and Van Heerden, J. A. H. (1973). The effect of season on reproduction in the black wildebeest and red hartebeest in South Africa. Journal of Reproduction and Fertility 19, 101–110.
| 1:STN:280:DyaE2c7ivVWhtg%3D%3D&md5=1812bf3369eb993f99ec2b52c9292661CAS | 4522365PubMed |
Skinner, J. D., Van Zyl, J. H., and Oates, L. C. (1974). The effect of season on the breeding cycle of plains antelope of the western Transvaal highveld. Journal of South African Wildlife Management Association 4, 15–23.
Skinner, D. C., Richter, T. A., Malpaux, B., and Skinner, J. D. (2001). Annual ovarian cycles in an aseasonal breeder, the Springbok (Antidorcas marsupialis). Biology of Reproduction 64, 1176–1182.
| Annual ovarian cycles in an aseasonal breeder, the Springbok (Antidorcas marsupialis).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1aru7s%3D&md5=207c5d27ff010ce7a476fc6dc1d7c063CAS | 11259265PubMed |
Skinner, J. D., Moss, D. G., and Skinner, D. C. (2002). Inherent seasonality in the breeding seasons of African mammals: evidence from captive breeding. Transactions of the Royal Society of South Africa 57, 25–34.
| Inherent seasonality in the breeding seasons of African mammals: evidence from captive breeding.Crossref | GoogleScholarGoogle Scholar |
Smithers, R. H. N. (1983) ‘The Mammals of the Southern African Subregion.’ (University of Pretoria: Pretoria.)
Spinage, C. A. (1969). Reproduction in the Uganda defassa waterbuck, Kobus defassa ugandae Neumann. Journal of Reproduction and Fertility 18, 445–457.
| Reproduction in the Uganda defassa waterbuck, Kobus defassa ugandae Neumann.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1M3hsVGmtg%3D%3D&md5=a05767bcbc68e4c6f801e8fd2785b71aCAS | 5788217PubMed | 5788217PubMed |
Spinage, C. A. (1973). The role of photoperiodism in the seasonal breeding of tropical African ungulates. Mammal Review 3, 71–83.
| The role of photoperiodism in the seasonal breeding of tropical African ungulates.Crossref | GoogleScholarGoogle Scholar |
Stearns, S. C. (1992). ‘The Evolution of Life Histories.’ (Oxford University Press: Oxford, UK.)
Stephens, P. A., Boyd, I. L., McNamara, J. M., and Houston, A. I. (2009). Capital breeding and income breeding: their meaning, measurement and worth. Ecology 90, 2057–2067.
| Capital breeding and income breeding: their meaning, measurement and worth.Crossref | GoogleScholarGoogle Scholar | 19739368PubMed |
Talbot, L. M., and Talbot, M. H. (1963). ‘The Wildebeest in Western Maasailand, East Africa.’ Wildlife Monographs. No. 12. (The Wildlife Society: Washington, DC.)
Thompson, R. W., and Turner, J. C. (1982). Temporal geographic variation in the lambing season of bighorn sheep. Canadian Journal of Zoology 60, 1781–1793.
| Temporal geographic variation in the lambing season of bighorn sheep.Crossref | GoogleScholarGoogle Scholar |
Tolsma, D. J., Ernst, W. H. O., Verweij, R. A., and Vooijs, R. (1987). Seasonal variation of nutrient concentrations in a semi-arid savanna ecosystem in Botswana. Journal of Ecology 75, 755–770.
| Seasonal variation of nutrient concentrations in a semi-arid savanna ecosystem in Botswana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXjs1OmtQ%3D%3D&md5=dd4b47e7b2ba2fc18931646ea027029dCAS |
Weladji, R. B., and Holand, Ø. (2003). Global climate change and reindeer: effects of winter weather on the autumn weight and growth of calves. Oecolgia 136, 317–323.
| Global climate change and reindeer: effects of winter weather on the autumn weight and growth of calves.Crossref | GoogleScholarGoogle Scholar |
Williamson, D. T. (1991). Condition, growth and reproduction in female red lechwe (Kobus leche leche Gray 1850). African Journal of Ecology 29, 105–117.
| Condition, growth and reproduction in female red lechwe (Kobus leche leche Gray 1850).Crossref | GoogleScholarGoogle Scholar |
Wilson, V. J. (1969). The large mammals of the Matapos National Park. Arnoldia Rhodeia 4, 1–32.
Wilson, D. E., and Hirst, S. M. (1977).’ Ecology and Factors Limiting Roan and Sable Antelope Populations in South Africa.’ Wildlife Monographs No 54. (The Wildlife Society: Washington, DC.)
Wittemyer, G., Rasmussen, H. B., and Douglas-Hamilton, I. (2007a). Breeding phenology in relation to NDVI variability in free-ranging African elephant. Ecography 30, 42–50.
| Breeding phenology in relation to NDVI variability in free-ranging African elephant.Crossref | GoogleScholarGoogle Scholar |
Wittemyer, G., Ganswindt, A., and Hodges, K. (2007b). The impact of ecological variability on the reproductive endocrinology of wild female African elephants. Hormones and Behavior 51, 346–354.
| The impact of ecological variability on the reproductive endocrinology of wild female African elephants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXis1Oktr4%3D&md5=0d938d7e3124632bbdd90e051a09bb19CAS | 1:CAS:528:DC%2BD2sXis1Oktr4%3D&md5=0d938d7e3124632bbdd90e051a09bb19CAS | 17320085PubMed | 17320085PubMed |
Wronski, T., Lerp, H., and Ismail, K. (2011). Reproductive biology and life history traits of Arabian oryx (Oryx leucoryx) founder females reintroduced to Mahazat as-Sayd, Saudi Arabia. Mammalian Biology-Zeitschrift für Säugetierkunde 76, 506–511.
Zerbe, P., Marcus, C., Codron, D., Lackey, L. B., Rensch, E., Streich, J. W., Hatt, J.-M., and Müller, D. W. H. (2012). Reproductive seasonality in captive wild ruminants: implications for biogeographical, photoperiodic control, and life history. Biological Reviews of the Cambridge Philosophical Society 87, 965–990.
| Reproductive seasonality in captive wild ruminants: implications for biogeographical, photoperiodic control, and life history.Crossref | GoogleScholarGoogle Scholar | 22780447PubMed | 22780447PubMed |



 = 1.4473 (95% limits: 1.1146–1.780),
= 1.4473 (95% limits: 1.1146–1.780),  = 9.2 (8.1–10.3),
= 9.2 (8.1–10.3), 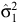 = 1.9181 (0.5510–3.2853),
= 1.9181 (0.5510–3.2853), 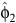 = 3.4 (2.0–4.7),
= 3.4 (2.0–4.7), 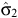 = 2.0736 (0.3822–3.7649) and ρ22 = 0.5198 (0.1913–0.8483). The percentage peak fecundity rate was 15.7% for the first component and 13.4% for the second component.
= 2.0736 (0.3822–3.7649) and ρ22 = 0.5198 (0.1913–0.8483). The percentage peak fecundity rate was 15.7% for the first component and 13.4% for the second component.