Effects of a GnRH vaccine on the movement and activity of free-living wild boar (Sus scrofa)
Roger J. Quy A , Giovanna Massei A , Mark S. Lambert A D , Julia Coats A , Lowell A. Miller B C and David P. Cowan AA National Wildlife Management Centre, Animal Health and Veterinary Laboratories Agency, Sand Hutton, York, YO41 1 LZ, UK.
B USDA APHIS National Wildlife Research Center, 4101 Laporte Avenue, Fort Collins, CO 80521, USA.
C Present address: Circle M Products, 12242 County Road 66, Greeley, CO 80631, USA.
D Corresponding author. Email: mark.lambert@ahvla.gsi.gov.uk
Wildlife Research 41(3) 185-193 https://doi.org/10.1071/WR14035
Submitted: 21 February 2014 Accepted: 30 June 2014 Published: 7 August 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Context: Fertility control is being promoted as a non-lethal means of managing wildlife populations. We recently evaluated a single-dose injectable immunocontraceptive vaccine (GonaCon™) on captive female wild boar for effectiveness and potential side effects; reproductive output was inhibited for 4–6 years, with no obvious detrimental effects on physiology and behaviour.
Aims: We injected individual free-living wild boar individuals with the fertility-control vaccine GonaCon™ to examine its effectiveness (measured as raised levels of GnRH antibodies) and looked for potential changes in movement and activity patterns.
Methods: We trapped, fitted telemetry devices to, and released wild boar individuals living in woodland in the West Midlands region of England between 2006 and 2010. We compared data on movements and activity among 10 adult females treated with the vaccine and 11 controls treated with saline only. We measured anti-GnRH antibody titres in six recaptured boar individuals as an indicator of the effectiveness of the vaccine.
Key results: Post-treatment GnRH antibody titres varied among the boar individuals; four of five treated sows resampled between 9 and 30 weeks post-injection had antibody titres high enough to block reproduction (detectable at 1 : 32 000–1 : 64 000 dilution). At least three treated females were pregnant at the time of vaccination; there was no subsequent evidence that the vaccine interfered with pregnancy. According to the distances moved per hour over a 24-h cycle and the daily activity cycle in relation to season, there were no differences in the behaviour of treated and control females that were likely to be biologically significant. The behaviour of two treated females monitored soon after vaccination and again 12 months later also showed no major differences.
Conclusions: Free-living wild boar responded to treatment with a 1.0-mL (1000 µg) dose of an anti-GnRH vaccine and no major adverse effects on activity and movement were subsequently detected.
Implications: Our results indicated that the vaccine could be more widely evaluated in the field against overabundant or nuisance populations. Such populations are increasingly found in urban areas and parks, where culling may not be an option. We suggest that further refinement of this approach for managing wild boar populations, including development of an oral vaccine, are warranted.
Introduction
In the UK, feral wild boar (Sus scrofa) populations have become established following escapes from farms (Wilson 2003). The species originally became extinct in the UK in the 13th century (Yalden 2010) and the recent (unplanned) reintroductions have been associated with agricultural damage, potential for maintenance and transmission of livestock diseases, negative conservation impacts, and risks to public safety, including collisions with vehicles (Wilson 2005). Similar problems have been reported from feral-pig populations globally (Massei et al. 2011).
Although the different feral populations of wild boar in the UK are widely separated and relatively small at present, model simulations predict an expansion in range and abundance (Holland et al. 2007, 2009). Consequently, management options have been considered (Wilson 2005), including fertility control, which potentially offers a non-lethal means of managing overabundant, free-ranging animals. Over the past 15–20 years, immunocontraceptive vaccines have been developed and one such vaccine (GonaCon™) inhibits reproductive activity by reducing the circulating level of the gonadotrophin-releasing hormone (GnRH), thereby reducing other reproductive hormones (Miller et al. 2004, 2008; Fagerstone et al. 2006).
As well as the effectiveness of fertility-control agents, it is also important that these agents have no significant negative side effects on physiology or behaviour. Some minor behavioural changes might not be associated with negative consequences; for example, it might be expected that temporarily or permanently non-reproducing females might have lower energy requirements, and hence may be less active, than breeding females. However, other changes in behaviour, such as increased aggression would clearly be undesirable. Some studies have reported behavioural differences following the use of contraceptives in wildlife populations; hormonally sterilised ricefield rats (Rattus argentiventer), for example, tended to lose their territories, as indicated by the percentage of rats changing burrows, more than did surgically sterilised (by tubal ligation) and control rats (Jacob et al. 2004). However, when Gray and Cameron (2010) reviewed studies on the side effects of contraceptive treatments in wildlife, they found little evidence of effects on movements and activities. No differences were observed in the crepuscular activities of untreated female eastern grey kangaroos (Macropus giganteus) compared with those of animals treated with the GnRH agonist deslorelin (Woodward et al. 2006). No differences in space-use patterns and fidelity to seasonal breeding ranges were observed in ovariectomised brushtail possums (Trichosurus vulpecula) (Ramsey 2007) or surgically sterilised vixens (Vulpes vulpes) (Saunders et al. 2002). The long-term effectiveness and potential side effects of GonaCon™ were recently evaluated in captive female wild boar (Massei et al. 2012); 11 of 12 females did not give birth for at least 4 years (and some for 6 years) post-vaccination, but no effects were seen on bodyweight, haematology, biochemistry and on the social ranking of females that were housed together (there were no apparent ill effects of the vaccine on the single treated sow that did give birth or her piglets). However, there have been very few studies of the effect of contraceptive vaccines on the physiology and behaviour of free-living animals. The present study was initiated to extend the evaluation of an immunocontraceptive vaccine to its effects on free-living wild boar. Specifically, we aimed to detect changes, if any, in daily activity cycles and movements, owing to female boar being rendered anoestrous and infertile.
Materials and methods
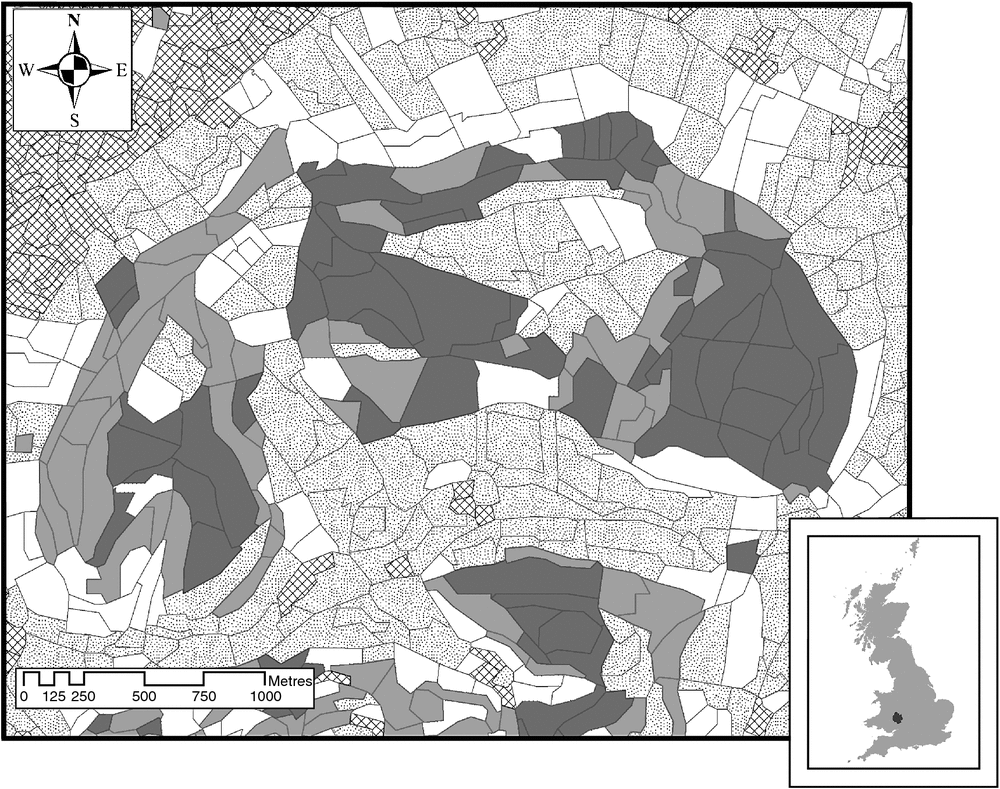
The study site, located to the south-east of the town of Ross-on-Wye (Herefordshire, UK), comprised two main blocks of woodland (218 ha and 109 ha) joined along part of their northern boundary and surrounded predominantly by farmland (Fig. 1). The woodland contained a mixture of coniferous and deciduous trees and was managed for timber production. The Centre for Ecology and Hydrology (CEH) Land Cover Map 2007 (CEH, Wallingford, UK) broad habitat categories represented within the study area were coniferous woodland (430.5 ha, 16.4%) broad-leaved, mixed and yew woodland (378.6 ha, 14.4%), arable and horticulture (1038.5 ha, 39.7%), built up areas and gardens (266.1 ha, 10.2%), acid grassland (30.7 ha, 1.2%), dwarf shrub heath (5.3 ha, 0.2%), improved grassland (257.4 ha, 9.8%) and rough low-productivity grassland (210.9 ha, 8.1%).The boar population in the woods was thought to have originated from escapes from a farm in the late 1990s (Wilson 2005). The size of the population was not known but was controlled intermittently by shooting organised by local landowners.
Wild boar individuals were trapped between July 2006 and August 2010 and trapping sessions were conducted approximately every 2 weeks between January and August each year. Attempts at trapping in the autumn and early winter were not successful perhaps because of the availability of abundant natural food (e.g. acorns, sweet chestnuts and hazel nuts), as was the case in a previous study at this site (CSL 2005).
Up to 14 single-capture traps (either 221 × 86 × 129 (height) cm or 184 × 67 × 92 (height) cm) and one multi-capture (corral) trap were distributed across the two woods at sites where fresh signs of boar activity (wallows, trails, rooting) were found. Traps were prebaited with maize for up to 1 week and then set for 2 days, with each trap checked twice a day.
Only females weighing more than 40 kg or older than 7–9 months (as established from patterns of tooth eruption and replacement; Bouldoire and Vassant 1989) were used for the study and juveniles and adult males were released. Each adult female was anaesthetised using a mixture of 2.0–3.0 mL Zoletil® (tiletamine–zolazepam; Virbac S.A., Carros, France), 0.2–0.3 mL Zalopine® (medetomidine hydrochloride; Orion Corporation, Espoo, Finland) and 0.5–1.0 mL of Torbugesic® (butorphanol tartrate; Fort Dodge Animal Health, Southampton, UK) administered via a dart gun; anaesthesia was reversed by injecting 4.0 mL of Antisedan® (atipamezole hydrochloride; Pfizer Animal Health, New York, NY, USA). A blood sample was collected from each unconscious female and the neck and belly circumferences were measured; to minimise the handling of large wild boar, bodyweights were not routinely recorded. To facilitate identification during subsequent recaptures, each individual was fitted with a colour-coded and numbered ear tag (Allflex, Dallas, TX, USA), supplemented in the last year of the study with a uniquely numbered microchip implanted under the skin (‘Identichip™’ Animalcare, York, UK). Half of the females were assigned to the treatment group and injected intramuscularly with 1.0 mL GonaCon™ containing 1000 µg of a GnRH vaccine (NWRC, Fort Collins, CO, USA) and the other half were assigned to the control group and injected with 1.0 mL of saline solution. The site of injection was recorded for all animals.
The effectiveness of the vaccine was determined by quantifying anti-GnRH antibody titres in blood samples of females retrapped after vaccination and by examining the reproductive status of the females (lactating teats or dry teats, presence of piglets in the same trap). We set traps with the aim of sampling the population every 2 weeks, and prebaited traps during the intervening weeks. The enzyme-linked immunosorbent assay (ELISA; NWRC, Fort Collins, CO, USA) was used to measure anti-GnRH antibody titres, following the method of Massei et al. (2012).
Each GPS unit (Quantum 5000, Telemetry Solutions, Concord, CA, USA, or Tellus Remote UHF, Followit, Lindesberg, Sweden) was attached to a collar of polyester webbing coated with urethane, and weighed 600–700 g. Collars also contained a VHF transmitter operating at a frequency between 173.000–173.999 MHz, activity sensors and a mortality indicator. The activity sensors detected motion in two different planes and operated during a 1-min period immediately before, during or after a scheduled GPS fix; the level of activity was determined from the total number of sensor activations. A drop-off mechanism incorporated into each collar was preprogrammed to activate automatically after a set period of time if a tagged animal could not be recaptured to replace its collar. This avoided the collar becoming too tight as the animal grew and also allowed the GPS unit to be retrieved by tracking its VHF signal. Some animals were also fitted with an ear-tag transmitter (Biotrack Ltd, Dorset, UK) that operated on a different frequency; this enabled animals to be located after an automatic collar drop-off. Two animals were retrapped and fitted with new collars 3 and 7 months, respectively, after the original collar had dropped off.
The schedule of fixes uploaded to each collar varied from 9 to 36 fixes per day, with 0.5–2-h intervals at night and 1–4-h intervals during the day when wild boars were less active. GPS fixes were downloaded periodically from each collar via a UHF (433 MHz) wireless link to a laptop computer. Activity data were downloaded wirelessly at the same time as the GPS fixes or separately via a wired connection when an animal was recaptured or the discarded collar recovered.
As the data were accumulated over a 4-year period, differences between the activity and movement (hourly distance travelled) of treated animals and controls were examined by combining the data from all years and then grouping into 3-month periods corresponding approximately to seasons, namely December–February (winter), March–May (spring), June–August (summer) and September–November (autumn). Analysing by time period avoided the possible confounding effect of seasonal variations in activity and movement that have been found in this population (CSL 2005) and in hunted populations elsewhere (Keuling et al. 2008a, 2008b). If an animal was tracked across seasons, then the data were split into the appropriate time periods. The first 3–4 days of data collected immediately after release were omitted from all analyses in case the behaviour of individual animals was affected by recovery from the anaesthesia.
For comparing activity patterns within each season, the daily activity cycle for each boar was determined from the number of sensor activations each hour for each day that data were collected (an activity count was stored in the GPS unit even when a positional fix was not obtained). The method of Murtaugh (2007) was followed to fit a no-intercept regression to the activity counts. As the two types of GPS collars used in the study recorded activity on different scales, the counts were first transformed to z-scores (5 was added to remove negative numbers). The resulting coefficient estimates were sample mean scores and standard errors for each hour for each boar during a season. For each season, the results were summarised for treated and control groups separately by calculating the weighted average of the regression coefficients with weights being proportional to the reciprocals of the squared standard errors for individual fits (Murtaugh 2007).
To compare daily movement patterns within each season, the straight-line distance between two fixes that were 1 h apart was calculated. No attempt was made to filter fixes for accuracy based on the positional dilution of precision (PDOP) value, because this has previously been found to be unnecessary (Cargnelutti et al. 2007); however, fixes obviously inaccurate were omitted. Using the regression approach above, coefficient estimates were sample mean distances moved each hour throughout the day during a season for each animal. Similarly, results were summarised for each group of boar using the weighted average.
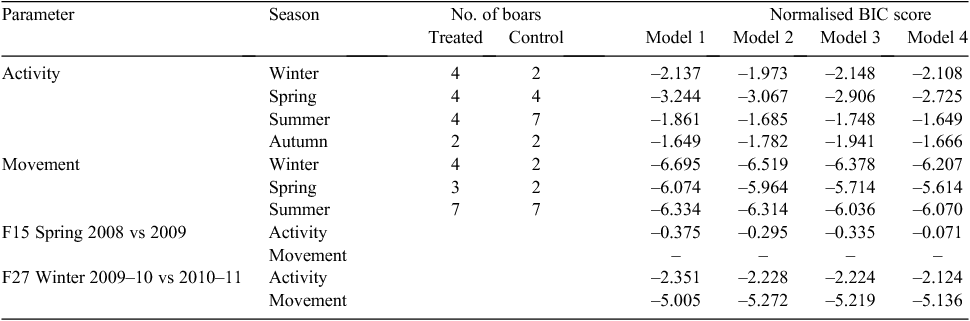
To test for differences between the daily patterns of activity and movement for treated and control groups of wild boar each season, the approach used by Schwartz et al. (2010) was followed in which four regression models were fitted for each comparison. The response variable was the difference in the mean activity count (z-score) or distance moved between the two groups (treated, control). The first model (intercept-only) assumed uncorrelated errors, where the dependent variable was the difference at Time t (t = 0,…., 23 h), the second was the same, but accounted for correlated errors with a first-order autoregressive AR(1) process. In the third and fourth models, two independent (temporal) variables were included by treating hour as a circular variable, transforming to radians and taking the sine and cosine. Errors were treated as uncorrelated in Model 3, but Model 4 accounted for correlated errors as in Model 2. Models were fitted using SPSSv19 and compared using the Bayesian information criterion (BIC) (Schwarz 1978). Similar to Schwartz et al. (2010), in group comparisons, if the BIC scores were lowest for Models 1 and 2, (i.e. adding the two temporal variables did not improve the fit of the model), it was concluded that the difference between the two groups (treated, control) did not significantly vary during the 24-h cycle, and hence there were no differences between the two groups in the pattern of daily activity or movement described by the shape of the plotted curves. If lower BIC scores were given by Models 3 and 4, this would indicate differences in the pattern of daily activity or movement cycles between the treated and control groups, i.e. a shift in movement or activity toward a particular part of the day for one of the groups. Additionally, it was concluded that the overall activity or movement levels (described by the amplitude of the plotted curves) did not differ between treated and control groups if the 95% confidence interval for the mean difference overlapped zero. The four models were also used to compare, separately, the activity and movements of two treated females that were monitored in the same season in consecutive years. To allow comparisons with other studies, seasonal home ranges were calculated as 100% minimum convex polygons (MCPs) to describe the maximum space used by each animal with >54 location fixes (Keuling et al. 2008a) using Ranges8 (Kenward et al. 2008). We tested for differences in home-range size between treated and control groups within seasons, using Mann–Whitney U-tests in Genstat version 16.1 (Payne 2009).
Results
Between 2006 and 2010, in total, 17 boar individuals were treated with GonaCon™ and 17 control animals were injected with saline. Movement and/or activity data were collected from 10 treated and 11 control animals – data were lost when some of the GPS collars malfunctioned. The mean length of time that the movements and activity of tagged boar were monitored continuously, post-treatment was 8.7 weeks (range 1–53 weeks); one female was monitored intermittently over a period of 72 weeks. Two animals were retrapped and fitted with new collars at 3 and 7 months, respectively, after the original collar had dropped off.
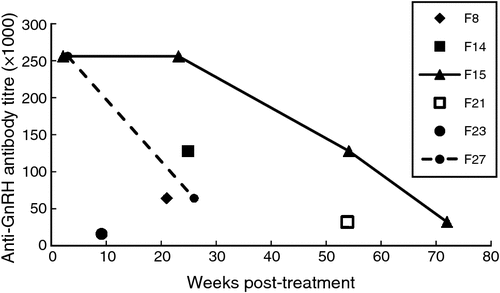
Blood samples were obtained at various time points between 2 and 72 weeks post-vaccination from six treated females that were recaptured at least once; one female (F27) was recaptured twice and another (F15) four times. At least three females (F14, F23 and F27) were pregnant when treated, as indicated by the presence of dependent young trapped with the lactating mother a few months after vaccination (F14 and F23), or by the temporary decrease in activity while farrowing, confirmed when the sow was later trapped with dependent young (F27). Compared with the anti-GnRH antibody titres that induced and maintained infertility in other species (antibodies detectable at ≥1 : 64 000 dilution, e.g. Miller et al. 2000), the titres of four of five vaccinated wild boar individuals resampled at 20–30 weeks were high enough to suggest that GonaCon™ had rendered these sows infertile (Fig. 2). Two treated sows resampled at 54 weeks had GnRH antibodies detectable at 1 : 32 000–1 : 12 8000 dilution; hence, one or both of these were likely to be infertile; in a previous study on captive wild boar, one of two females with a titre of 1 : 32 000 dilution did not reproduce (Massei et al. 2012). One of the two treated sows resampled at 54 weeks was resampled again at 72 weeks; GnRH antibody titres were detected at 1 : 32 000 dilution, suggesting that this animal may still have been infertile at that time.
|
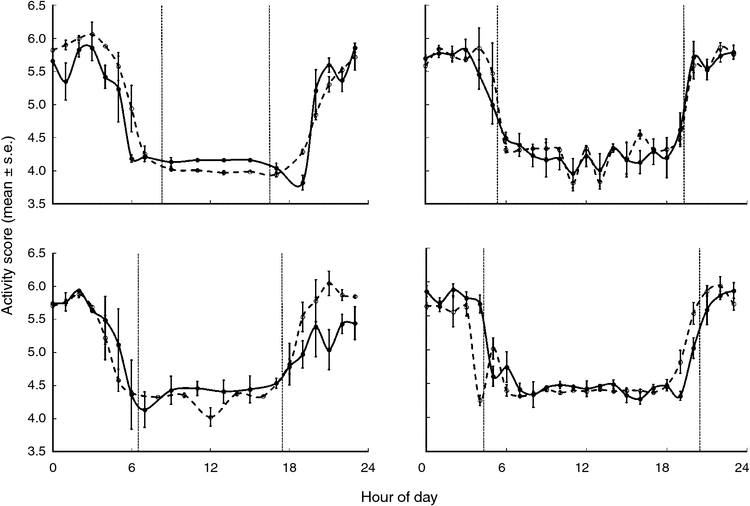
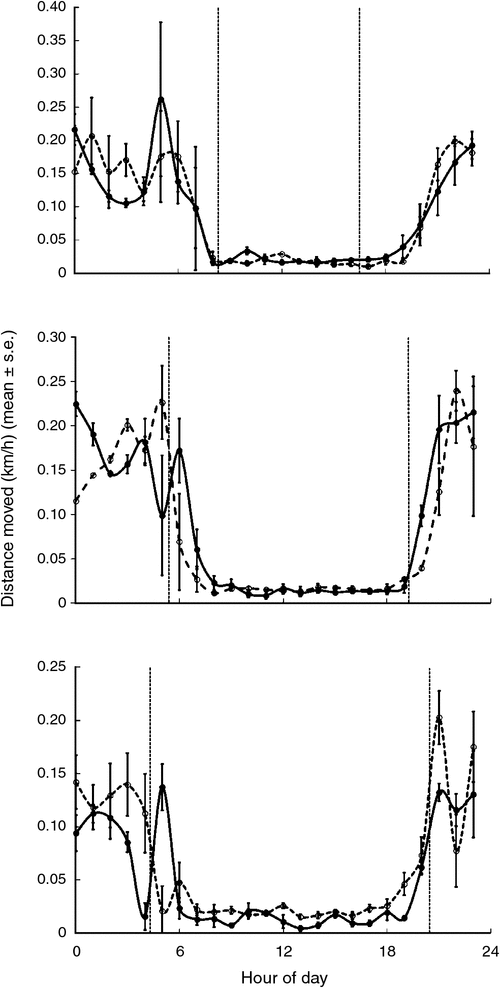
The activity and movement plots showed that the wild boars were predominantly nocturnal (Figs 3, 4). In all group comparisons of activity and movement, models that assumed uncorrelated errors (Models 1 or 3) returned lower BIC scores than did those that accounted for correlated errors (Table 1). Model 1 returned the lowest BIC scores for movement (hourly distance travelled) in winter, spring and summer (we did not have sufficient data for autumn). Similarly, Model 1 returned the lowest BIC score for boar activity levels in spring and summer. Hence, the difference in the mean activity count (z-score) or distance moved between treated and control groups did not significantly vary during the 24-h cycle between treated and control animals during these seasons. Model 3 returned the lowest BIC scores for activity in winter and autumn, suggesting differences between the patterns of activity cycles for treated and control animals during these seasons. Temporal activity and movement patterns of two treated animals in the same season soon after vaccination and 1 year apart (F15, F27) were not significantly different, with the lowest BIC scores returned for Models 1 or 2 (Table 1).
|
|
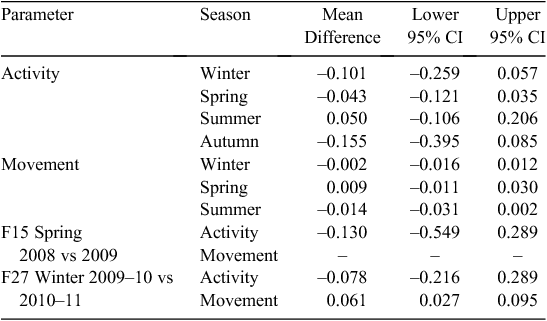
The level (i.e. amplitude) of activity and movement during all seasons for which we had sufficient data for comparisons did not differ between treated and control groups (i.e. 95% confidence limits for the mean difference included zero; Table 2). Comparisons of activity and movement levels for F15 and F27 in the same season 1 year apart are also shown in Table 2; there was no significant difference in the level of activity of F15 between spring 2008 and spring 2009, or in the level of activity of F27 between winter 2009–10 and winter 2010–11, although a significant difference (a reduction) in movement (hourly distance travelled) by F27 was found between winter 2009–10 and winter 2010–11.
|
We captured a total of 38 347 location fixes for 21 wild boar individuals, including 10 treated and 11 controls. Some animals were tracked in multiple seasons within years. Two animals (F15 and F27) were tracked in the same season in different years; for these two animals, data from the year with the greatest number of location fixes was used in the analyses. The mean number of location fixes captured for treated animals was 850 (n = 4, range 147–1838) in winter, 2439 (n = 4, range 791–5199) in spring, 427 (n = 8, range 80–1673) in summer and 1622 (n = 2, range 95–3149) in autumn. The mean number of location fixes captured for control animals was 1040 (n = 3, range 132–2152) in winter, 1309 (n = 3, range 444–2102) in spring, 1115 (n = 9, range 94–3004) in summer and 1424 for one animal in autumn. One animal with <54 location fixes was excluded from the analyses. Mean (±s.d.) 100% MCP home ranges for treated animals were 160.7 (±48.3) ha, 303.8 (±151.3) ha, 124.6 (±57.6) ha and 168.6 (±3.9) ha for winter, spring, summer and autumn respectively. Mean (±s.d.) 100% MCP home ranges for control animals were 168.2 (±156.7) ha, 220.4 (±57.0) ha and 263.9 (±158.1) ha for winter, spring and summer respectively (the single control animal in autumn had a home range of 253.9 ha). There was no difference between home range of treated and control animals in winter (Mann–Whitney U = 5.0, P = 0.857) or spring (Mann–Whitney U = 5.0, P = 0.857), but controls had larger home ranges than did treated animals in summer (Mann–Whitney U = 13.0, P = 0.050). We had insufficient data to test for differences between treated and control animals in autumn.
Discussion
We trapped free-living wild boars and injected them with a contraceptive vaccine at a dose level previously shown to induce long-term infertility in captive wild boar; study animals were then periodically retrapped. We confirmed that free-living wild boar responded to GonaCon™ by producing GnRH antibodies at levels that are known to be associated with infertility in captive wild boar and feral pigs (Miller et al. 2003; Killian et al. 2006; Massei et al. 2008, 2012). It also appeared that, as previously found in bison (Miller et al. 2004), GonaCon™ vaccination did not affect existing pregnancy. Farrowing in wild boar mainly occurs between February and June (Gethöffer et al. 2007) and females become pregnant between December and March. Five females treated during pregnancy produced litters and at least four of these animals (one was not recaptured) also developed antibody titres that putatively should have prevented further pregnancies. Although these results are encouraging, the relatively small sample size of treated animals that were retrapped precludes the possibility of drawing robust conclusions on the long-term effectiveness of the vaccine in free-living boar, as well as on the proportion of females that respond to this contraceptive. Both these parameters may vary among species, and the context of the treatment. For example, GonaCon™ appears to be particularly effective in white-tailed deer (Odocoileus virginianus); a single vaccination blocked reproduction in 80–100% of treated does for 5 years (Killian et al. 2008a). Efficacy of a similar treatment in captive mares (Equus caballus) declined from 94% to 40% over 4 years, (Killian et al. 2008b), and efficacy was lower for feral mares taken from the same population (Gray et al. 2010), although fertility rates of treated animals were still significantly lower than those of controls.
Compared with the results obtained with captive wild boar animals, where some sows were rendered infertile for at least 6 years, infertility in the treated free-living animals might not have lasted as long; titres in female F15 had dropped to 1 : 32 000 after 18 months, a level that in captive wild boar was associated with returning fertility (Massei et al. 2012). The declining pattern of antibody titres observed in that animal suggested that infertility much beyond 72 weeks would have been unlikely, although in captive wild boar, antibody titres declined over the first 12–24 months post-injection, then remained at a sufficient (low) level to block reproduction for several years (Massei et al. 2012).
We monitored behaviour of treated and control animals using GPS tags. We looked for differences between treated and control groups in patterns of activity and distances travelled during the 24-h cycle; if there was no temporal effect (i.e. if the difference between groups was either zero or consistent over the 24-h cycle), we concluded that there was no treatment effect. We found that treated sows tended to be less active than controls during the evening and night time during autumn and winter. Wild boars in our study area were in general more active during night time, and hence the difference between groups was most likely to be detected at night during this peak activity period (boar tended to lay up in thick cover during the day); the timing of the difference probably reflects an increase in (reproductive) activity of control females in autumn associated with the start of the breeding season (Henry 1968; Mauget 1982) However, there was no difference in the overall level of activity between treated and control sows, and there was no significant difference in temporal movement patterns (hourly distance moved) between the two groups for all three seasons for which we had sufficient data to make valid comparisons. For the two individual (treated) animals for which we had sufficient long-term data to make comparisons, there were no significant differences in levels of activity between two consecutive seasons 1 year apart. One of these animals (F27) appeared to move shorter distances in Year 2, although we noted that this animal sustained an injury to a rear limb between seasons, or this could have been associated with age (Keuling et al. 2008a, 2009). Pregnancy-associated changes in the home-range size and activity are well known in wild boar (e.g. Mauget 1984) and were a confounding factor that might have affected the comparison between both treated and control animals. The relative amounts of data (fixes and activity counts) obtained from pregnant and non-pregnant females and the use of weighting in the method of analysis meant that the effects of pregnancy could not be clearly determined. Also, with very small sample sizes, the power of the statistical tests was low. Nevertheless, gross effects over the short term (2–6 months) should still have been detectable. The mean proportion of time spent active each day was similar to that observed in other populations of wild boar (Massei et al. 1997; Russo et al. 1997; Keuling et al. 2008b). Furthermore, (MCP) home-range sizes of treated wild boar were similar to those found for wild boar from previous studies (Massei et al. 1997; Keuling et al. 2008a), and were not significantly different from control animals for two of three seasons. Treated animals had smaller home ranges than did controls in summer; there was large variation between individuals and the significance level was borderline, although the difference could reflect reduced foraging by treated (non-breeding) animals approaching the breeding season. We suggest, therefore, that there were no major behavioural impacts associated with anti-GnRH vaccination of individual animals; rather the differences were subtle, and therefore unlikely to have negative welfare implications or other undesirable consequences. Our results are, therefore, broadly consistent with other studies that found no effect on behaviour following contraceptive treatment (e.g. Gray and Cameron 2010; Massei et al. 2012). However, the effect of anti-GnRH vaccination on the behaviour and structure of social groups of wild boar was not tested. Similarly, any treatment effect of the vaccine on individuals could have been masked by social effects (i.e. the behaviour of a treated individual could have been influenced by the behaviour of the untreated majority within a social group). Development of an oral vaccine would enhance the utility of the technique, and would facilitate wider testing, particularly on entire social groups. The risks to non-target animals from wider use of the GnRH vaccine are low; a species-specific system for delivery of baits to wild boar has been developed that could prevent uptake of baits by non-target species (Massei et al. 2010; Campbell et al. 2011), and consumption of meat from GnRH-vaccinated animals poses no risk to non-target wildlife or humans (APHIS 2006).
We conclude that injection of GonaCon™ raised GnRH antibodies in individual free-living wild boar animals, with no obvious negative consequences. However, a larger number of animals monitored over a longer period would be required to draw robust conclusions on the long-term effectiveness of GonaCon™ in feral populations of this species.
Acknowledgements
This study was funded by the Department for Environment, Food and Rural Affairs under project WM0408. The study was carried out under a UK Home Office licence in accordance with the Animals (Scientific Procedures) Act 1986 and was approved by The Food and Environment Research Agency’s Ethical Review Process.
References
APHIS (2006). ‘GonaCon™—Birth Control for Deer: Questions and Answers. USDA–APHIS Fact Sheets on Wildlife Damage Management. Paper 7.’ Available at http://digitalcommons.unl.edu/usdaaphisfactsheets/7 [verified 8 July 2014].Bouldoire, J.-L., and Vassant, J. (1989). ‘Le Sanglier.’ (Hatier: Paris.)
Campbell, T. A., Long, D. B., and Massei, G. (2011). Efficacy of the boar-operated-system to deliver baits to feral swine. Preventive Veterinary Medicine 98, 243–249.
| Efficacy of the boar-operated-system to deliver baits to feral swine.Crossref | GoogleScholarGoogle Scholar | 21176854PubMed |
Cargnelutti, B., Coulon, A., Hewison, A. J. M., Goulard, M., Angibault, J.-M., and Morellet, N. (2007). Testing global positioning system performance for wildlife monitoring using mobile collars and known reference points. The Journal of Wildlife Management 71, 1380–1387.
| Testing global positioning system performance for wildlife monitoring using mobile collars and known reference points.Crossref | GoogleScholarGoogle Scholar |
CSL (2005). Investigating capture and removal strategies for wild boar at low density. Central Science Laboratory (now National Wildlife Management Centre, AHVLA) project report to Defra. Available at http://randd.defra.gov.uk/Document.aspx?Document=WM0308_2753_FRP.doc [verified 8 July 2014].
Fagerstone, K. A., Miller, L., Bynum, K., Eisemann, J., and Yoder, C. (2006). When, where and for what wildlife species will contraception be a useful management approach. In ‘Proceedings of the 22nd Vertebrate Pest Conference’ 2006. (Eds R. Timm and J. O’Brien.) pp. 45–54. (University of California: Davis, CA.)
Gethöffer, F., Sodeikat, G., and Pohlmeyer, K. (2007). Reproductive parameters of wild boar (Sus scrofa) in three different parts of Germany. European Journal of Wildlife Research 53, 287–297.
| Reproductive parameters of wild boar (Sus scrofa) in three different parts of Germany.Crossref | GoogleScholarGoogle Scholar |
Gray, M. E., and Cameron, E. Z. (2010). Does contraceptive treatment in wildlife result in side effects? A review of quantitative and anecdotal evidence. Reproduction 139, 45–55.
| Does contraceptive treatment in wildlife result in side effects? A review of quantitative and anecdotal evidence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovVWntQ%3D%3D&md5=e46bcf046f2a32f7203c7215ace1595fCAS | 19656957PubMed |
Gray, M.E., Thain, D.S., Cameron, E.Z., and Miller, L.A. (2010). Multi-year fertility reduction in free-roaming feral horses with single-injection immunocontraceptive formulations. Wildlife Research 37, 475–481.
| Multi-year fertility reduction in free-roaming feral horses with single-injection immunocontraceptive formulations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVCht7%2FI&md5=c3e9661701e7683e8c26875df48ef2d1CAS |
Henry, V. (1968). Length of estrous cycle and gestation in European wild hogs. The Journal of Wildlife Management 32, 406–408.
| Length of estrous cycle and gestation in European wild hogs.Crossref | GoogleScholarGoogle Scholar |
Holland, E.P., Aegerter, J.N., and Smith, G.C. (2007). Spatial sensitivity of a generic population model, using wild boar (Sus scrofa) as a test case. Ecological Modelling 205, 146–158.
| Spatial sensitivity of a generic population model, using wild boar (Sus scrofa) as a test case.Crossref | GoogleScholarGoogle Scholar |
Holland, E.P., Burrow, J.F., Dytham, C., and Aegerter, J.N. (2009). Modelling with uncertainty: introducing a probabilistic framework to predict animal population dynamics. Ecological Modelling 220, 1203–1217.
| Modelling with uncertainty: introducing a probabilistic framework to predict animal population dynamics.Crossref | GoogleScholarGoogle Scholar |
Jacob, J., Matulessy, J., and Sudarmaji, (2004). Effects of imposed sterility on movement patterns of female ricefield rats. The Journal of Wildlife Management 68, 1138–1144.
| Effects of imposed sterility on movement patterns of female ricefield rats.Crossref | GoogleScholarGoogle Scholar |
Kenward, R., Walls, S., South, A., and Casey, N. (2008). ‘Ranges8: For the Analysis of Tracking and Location Data. Online Manual.’ (Anatrack Limited: Wareham, UK.)
Keuling, O., Stier, N., and Roth, M. (2008a). Annual and seasonal space use of different age classes of female wild boar Sus scrofa L. European Journal of Wildlife Research 54, 403–412.
| Annual and seasonal space use of different age classes of female wild boar Sus scrofa L.Crossref | GoogleScholarGoogle Scholar |
Keuling, O., Stier, N., and Roth, M. (2008b). How does hunting influence activity and spatial usage in wild boar Sus scrofa L.? European Journal of Wildlife Research 54, 729–737.
| How does hunting influence activity and spatial usage in wild boar Sus scrofa L.?Crossref | GoogleScholarGoogle Scholar |
Keuling, O., Stier, N., and Roth, M. (2009). Commuting, shifting or remaining? Different spatial utilisation patterns of wild boar Sus scrofa L. in forest and field crops during summer. Mammalian Biology – Zeitschrift für Säugetierkunde 74, 145–152.
| Commuting, shifting or remaining? Different spatial utilisation patterns of wild boar Sus scrofa L. in forest and field crops during summer.Crossref | GoogleScholarGoogle Scholar |
Killian, G., Miller, L., Rhyan, J., and Doten, H. (2006). Immunocontraception of Florida feral swine with a single‐dose GnRH vaccine. American Journal of Reproductive Immunology 55, 378–384.
| Immunocontraception of Florida feral swine with a single‐dose GnRH vaccine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xltlehtr4%3D&md5=96dc1957bf413139c6c203978f57dea2CAS | 16635212PubMed |
Killian, G., Wagner, D., Fagerstone, K., and Miller, L. (2008a). Long-term efficacy and reproductive behavior associated with GonaCon™ use in white-tailed deer (Odocoileus virginianus). In ‘Proceedings of the 23rd Vertebrate Pest Conference’. (Eds R. Timm and M. Maldon.) pp. 240–243. (University of California: Davis, CA.)
Killian, G., Thain, D., Diehl, N.K., Rhyan, J., and Miller, L. (2008b). Four-year contraception rates of mares treated with single-injection porcine zona pellucida and GnRH vaccines and intrauterine devices. Wildlife Research 35, 531–539.
| Four-year contraception rates of mares treated with single-injection porcine zona pellucida and GnRH vaccines and intrauterine devices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1KrtLjJ&md5=3d6115876f5da47fd33626bde8b97532CAS |
Massei, G., Coats, J., Quy, R., Storer, K., and Cowan, D. P. (2010). The boar‐operated‐system: a novel method to deliver baits to wild pigs. The Journal of Wildlife Management 74, 333–336.
| The boar‐operated‐system: a novel method to deliver baits to wild pigs.Crossref | GoogleScholarGoogle Scholar |
Massei, G., Cowan, D. P., Coats, J., Bellamy, F., Quy, R., Pietravalle, S., Brash, M., and Miller, L. A. (2012). Long-term effects of immunocontraception on wild boar fertility, physiology and behaviour. Wildlife Research 39, 378–385.
| Long-term effects of immunocontraception on wild boar fertility, physiology and behaviour.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvFKluro%3D&md5=7a6e0b9ff0fbc646688d3b395ee86e63CAS |
Massei, G., Cowan, D.P., Coats, J., Gladwell, F., Lane, J.E., and Miller, L.A. (2008). Effect of the GnRH vaccine GonaCon on the fertility, physiology and behaviour of wild boar. Wildlife Research 35, 540–547.
| Effect of the GnRH vaccine GonaCon on the fertility, physiology and behaviour of wild boar.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1KrtLjP&md5=385f446f988e658008ec2bb3bc9bdf9cCAS |
Massei, G., Genov, P. V., Staines, B. W., and Gorman, M. L. (1997). Factors influencing home range and activity of wild boar (Sus scrofa) in a Mediterranean coastal area. Journal of Zoology 242, 411–423.
| Factors influencing home range and activity of wild boar (Sus scrofa) in a Mediterranean coastal area.Crossref | GoogleScholarGoogle Scholar |
Massei, G., Roy, S., and Bunting, R. (2011). Too many hogs? A review of methods to mitigate impact by wild boar and feral pigs. Human-Wildlife Interactions 5, 79–99.
Mauget, R. (1982). Seasonality of reproduction in the wild boar. In ‘Control of pig reproduction’. (Eds D. Cole and G. Foxcroft.) pp. 509–526. (Butterworth: London.)
Mauget, R. (1984). Rythme d’activité et budget-temps chez le sanglier européen (Sus scrofa L.). In ‘Symposium International sur le Sanglier’, 1984, Toulouse (France). (Eds F. Spitz and D. Pépin.) pp. 79–92. (Les Colloques de l’INRA 22, Paris, France)
Miller, L. A., Johns, B. E., and Killian, G. J. (2000). Immunocontraception of white-tailed deer with GnRH vaccine. American Journal of Reproductive Immunology 44, 266–274.
| 1:STN:280:DC%2BD3M7lt1Gnug%3D%3D&md5=5aa1f69018434954dc564589cd030e65CAS | 11125787PubMed |
Miller, L., Rhyan, J., and Killian, G. (2003). Evaluation of GnRH contraceptive vaccine using domestic swine as a model for feral hogs. In ‘Proceedings of the 10th Wildlife Damage Management Conference’. (Eds K. A. Fagerstone and G. A. Witmer.) pp. 120–127. (University of Nebraska: Lincoln, NE)
Miller, L. A., Gionfriddo, J. P., Fagerstone, K. A., Rhyan, J. C., and Killian, G. J. (2008). The single-shot GnRH immunocontraceptive vaccine (GonaCon™) in white-tailed deer: comparison of several GnRH preparations. American Journal of Reproductive Immunology 60, 214–223.
| The single-shot GnRH immunocontraceptive vaccine (GonaCon™) in white-tailed deer: comparison of several GnRH preparations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFKrsbzP&md5=19e6d7112adcfe8e7a7812e8184190a8CAS | 18782282PubMed |
Miller, L. A., Rhyan, J. C., and Drew, M. (2004). Contraception of bison by GnRH vaccine: a possible means of decreasing transmission of brucellosis in bison. Journal of Wildlife Diseases 40, 725–730.
| Contraception of bison by GnRH vaccine: a possible means of decreasing transmission of brucellosis in bison.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsV2ht74%3D&md5=5cac4a34acdac104a3c6b5212af1556cCAS | 15650090PubMed |
Murtaugh, P. A. (2007). Simplicity and complexity in ecological data analysis. Ecology 88, 56–62.
| Simplicity and complexity in ecological data analysis.Crossref | GoogleScholarGoogle Scholar | 17489454PubMed |
Payne, R. W. (2009). Genstat. Wiley Interdisciplinary Reviews: Computational Statistics 1, 255–258.
| Genstat.Crossref | GoogleScholarGoogle Scholar |
Ramsey, D. (2007). Effects of fertility control on behavior and disease transmission in brushtail possums. The Journal of Wildlife Management 71, 109–116.
| Effects of fertility control on behavior and disease transmission in brushtail possums.Crossref | GoogleScholarGoogle Scholar |
Russo, L., Massei, G., and Genov, P. V. (1997). Daily home range and activity of wild boar in a Mediterranean area free from hunting. Ethology Ecology and Evolution 9, 287–294.
| Daily home range and activity of wild boar in a Mediterranean area free from hunting.Crossref | GoogleScholarGoogle Scholar |
Saunders, G., McIlroy, J., Berghout, M., Kay, B., Gifford, E., Perry, R., and Van de Ven, R. (2002). The effects of induced sterility on the territorial behaviour and survival of foxes. Journal of Applied Ecology 39, 56–66.
| The effects of induced sterility on the territorial behaviour and survival of foxes.Crossref | GoogleScholarGoogle Scholar |
Schwartz, C. C., Cain, S. L., Podruzny, S., Cherry, S., and Frattaroli, L. (2010). Contrasting activity patterns of sympatric and allopatric black and grizzly bears. The Journal of Wildlife Management 74, 1628–1638.
| Contrasting activity patterns of sympatric and allopatric black and grizzly bears.Crossref | GoogleScholarGoogle Scholar |
Schwarz, G. (1978). Estimating the dimension of a model. Annals of Statistics 6, 461–464.
| Estimating the dimension of a model.Crossref | GoogleScholarGoogle Scholar |
Wilson, C. J. (2003). Distribution and status of feral wild boar Sus scrofa in Dorset, southern England. Mammal Review 33, 302–307.
| Distribution and status of feral wild boar Sus scrofa in Dorset, southern England.Crossref | GoogleScholarGoogle Scholar |
Wilson, C. J. (2005). Feral wild boar in England; status, impact and management: a report on behalf of Defra European Wildlife Division. Defra, London, UK.
Woodward, R., Herberstein, M.E., and Herbert, C.A. (2006). Fertility control in female eastern grey kangaroos using the GnRH agonist deslorelin. 2. Effects on behaviour. Wildlife Research 33, 47–55.
| Fertility control in female eastern grey kangaroos using the GnRH agonist deslorelin. 2. Effects on behaviour.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitVOhtrs%3D&md5=fbf3687c78c545f9fefb3b254696505fCAS |
Yalden, D. (2010). ‘The History of British Mammals.’ (Poyser Natural History: London, UK)