Fauna and vegetation responses to fire and invasion by toxic cane toads (Rhinella marina) in an obligate seeder-dominated tropical savanna in the Kimberley, northern Australia
Ian J. Radford A B and Richard Fairman AA Department of Parks and Wildlife, Western Australia, PO Box 942 (Lot 248 Ivanhoe Road), Kununurra, WA 6743, Australia.
B Corresponding author. Email: ian.radford@dpaw.wa.gov.au
Wildlife Research 42(4) 302-314 https://doi.org/10.1071/WR14259
Submitted: 17 December 2014 Accepted: 10 June 2015 Published: 3 August 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Context: Changed fire regimes are an important threatening process to savanna biodiversity. Fire-sensitive vegetation such as pindan and its fauna may be particularly susceptible to fire impacts. Invasion by alien species is an additional threatening process. The toxic anuran Rhinella marina is a well publicised invader of savannas. Little is known of impacts in many habitats.
Aims: To test the hypotheses (1) that fire responses among pindan fauna are greater than general savanna responses, and (2) that cane toad-invasion impacts will be reduced relative to riparian habitats.
Methods: Reptiles, frogs, invertebrates and mammals were surveyed seven times from 2008 to 2012, four times before and three times following R. marina invasion. Time since last fire was recorded during each survey. Vegetation change was measured.
Key results: Pindan vegetation structural recovery took 4–5 years, whereas fauna recovery took only 1 year. Ground active agamids, combined Scincidae, fossorial skinks and ground-layer invertebrates responded positively to recent fire. Skinks of Ctenotus spp. declined in size after fire. Short-term fauna responses reflect rapid re-establishment of herbaceous cover. Fauna responses were detected following R. marina invasion, including increases in frogs of Uperoleia spp. and skinks of Carlia spp., and decreases in Lerista griffini and ground-layer invertebrates. Insufficient data were available to test for responses among large predators; however, >50% lower Varanus spp. trap success occurred post-invasion. No invasion response was detected among small mammals.
Conclusions: Pindan fauna fire responses were similar to those of savannas. Fauna responses to Rhinella marina invasion were relatively minor compared with those previously reported in riparian habitats and this may be related to the lower abundance of the invader here than in previous studies in riparian or more fertile habitats.
Implications: The dominant obligate seeding tree in pindan woodland, A. tumida, requires >4 years with no high-intensity fires for re-establishment of the dominant tree. Fire management should aim to minimise extensive fires to reduce impacts on fire-sensitive fauna. Persistence of large predators after cane-toad invasion suggests possible refuge value of low-productivity pindan savannas.
Introduction
Fire responses among plant communities and their faunas are highly variable. Fire responses range from radical change in vegetation and fauna community structure followed by extended post-fire recovery (e.g. Fox 1982; Masters 1996; Letnic et al. 2004), through to plant and animal communities that have little apparent fire response, or have extremely rapid recovery (Williams et al. 1999; Andersen and Muller 2000; Corbett et al. 2003; Russell-Smith et al. 2003; Andersen et al. 2005; Andersen et al. 2010; Radford 2012).
Generally, it would be expected that post-fire succession would be more pronounced and diverse in climatic zones and vegetation types where recovery time is slower. Thus, flammable-desert or -heath vegetation that requires many years to recover ‘climax’ vegetation or particular vegetation structures may be expected to have a greater range of plant- and animal-assemblage successional stages (Fox 1982; Masters 1996; Haslem et al. 2012), than do vegetation types that rapidly recover after fire (Andersen et al. 2005; Radford and Andersen 2012; Andersen et al. 2014a). However, there can be a disconnect between floral and faunal community fire responses, or among taxa within communities, such that different successional trajectories are observed among different taxonomic groups independently of vegetation recovery (Andersen et al. 2005, 2012; Clarke 2008; Taylor et al. 2013).
Fire-response differences among taxa probably relate directly to the habitat attributes that particular taxa respond to. So ants and other invertebrate groups that respond positively to open savanna habitat may be unaffected by fire because they perceive savanna as open irrespective of whether the site is recently burnt or not (Andersen and Muller 2000; Andersen et al. 2010, 2014a). In contrast, larger fauna such as mammals may be severely affected by the same fire regime (Andersen et al. 2005, 2012; Legge et al. 2008). Differences in recovery rates and post-fire seral stages may be important for conservation management because particular species or groups may respond only to a subset of post-fire serial stages or habitat attributes (Andersen et al. 2012, 2014b; Radford et al. 2015).
Savanna vegetation, as one of the more regularly flammable vegetation types with development of an annual fuel load, might be expected to have rapid recovery of flora and fauna after fire events. And, in fact, many elements of the flora and fauna of tropical savannas are highly resilient to regular fires (Andersen and Muller 2000; Corbett et al. 2003; Russell-Smith et al. 2003; Andersen et al. 2005; Radford 2012), namely, plants through high percentages of resprouting species in frequently burnt savannas that rapidly recover pre-fire above-ground biomass (Clarke et al. 2015) and animals, presumably, through the rapid recovery of their habitat structures and resource bases (Radford 2012; Radford and Andersen 2012). However, not all savanna vegetation types are so rapid in their recovery of pre-fire condition. There are several vegetation components making up a small proportion within the savanna matrix that take much longer to recover after fire. These include vegetation components dominated by obligate-seeder species the adult stands of which are killed by canopy scorch. Such elements of savanna vegetation in northern Australia include Callitris intratropica (Bowman et al. 2001; Prior et al. 2011), tropical heath communities (Russell-Smith et al. 1998) and savanna woodlands dominated by Acacia spp. (Woinarski and Fisher 1995a, 1995b). Aside from a small number of one-off descriptive studies of floral and faunal communities associated with these savanna subelements (Woinarski and Fisher 1995a, 1995b; Trauernicht et al. 2013; Radford et al. 2013), little is known of the dynamics of fire responses among most of these vegetation types and their associated biota (Russell-Smith et al. 2010).
The present study primarily aims to describe fire responses of terrestrial animal assemblages and vegetation structure associated within pindan (Acacia tumida) savanna woodlands of the wet–dry tropical Kimberley region of northern Western Australia, so as to inform fire management. Acacia tumida is an obligate seeder with distributions across the tropical Kimberley. Much of the pindan woodland area is thought to be degraded in terms of biodiversity values through exacerbation of fire regimes, particularly through large areas of the western Kimberley (McKenzie 1981; Kenneally et al. 1996). Truncation of fire regimes are thought to threaten the vegetation type itself because, being an obligate seeder, this species requires a minimum time interval to reproduce before the next fire. Despite apparent threats to this vegetation type, there has been little investigation of fire responses among component biota. Description of fauna composition of a similar lancewood (Acacia shirleyi) woodland in the Northern Territory suggested that the biota was made up predominantly of fire-resilient savanna taxa (Woinarski and Fisher 1995a, 1995b). This is similar to the finding by Radford et al. (2013) that, despite being a fire-sensitive vegetation type (dominated by a fire killed species), Callitris intratropica stands were found to harbour predominantly fire-resilient fauna.
It is unclear whether similar fauna would be present in pindan woodlands of the Kimberley region, or what the dynamics of their fire responses might be. We postulate that delayed recovery of pre-fire vegetation structures in this woodland (obligate seeding) will lead to more gradual recovery of fauna assemblages than in a more typical resprouter-dominated savanna. The present study aims to fill this gap in knowledge to inform fire managers of any specific fire-regime requirements, and also to add to the sum total of fire-response data for little known subelements within savanna vegetation (Radford et al. 2013).
Another significant threat to savanna biodiversity in northern Australia is invasion by the toxic anuran Rhinella marina (the cane toad; Shine 2010; Woinarski et al. 2011; Ziembicki et al. 2015). This species has several documented impacts among savanna ecosystems (Shine 2010). These include reductions among top predators as a result of poisoning (Shine 2010; Pearson et al. 2014), evolution of changed morphology in response to selective pressures through toad-related mortality (Phillips and Shine 2005), reduced invertebrate abundance (Greenlees et al. 2006) and cascading ecosystem responses, such as increases in meso-predator numbers, to functional loss of top predators (Doody et al. 2006, 2013). However, impacts by cane toads are variable, with inconsistent responses being recorded among different habitats and even among sites for particular species (Shine 2010; Ujvari et al. 2011). Many savannas with documented impacts have been associated with higher productivity or riparian habitats (e.g. Greenlees et al. 2006; Doody et al. 2009). It is unclear whether impacts would be generally less pronounced in areas with lower incidence of and carrying capacity for cane toads. Timing of invasion by cane toads in 2011 during this fire-response study has allowed the opportuntiy to address the question of whether pindan fauna are adversely affected by cane-toad invasion in a drier and less productive savanna type compared with other habitats.
Materials and methods
Study area and sites
The study was conducted inside and adjoining Mirima National Park near Kununurra, in the East Kimberley region of Western Australia. The region experiences a tropical monsoonal climate, with high temperatures year round (daily mean maximum 29.6–36.0°C), and medium rainfall (913 mm annual rainfall) occurring predominantly from November to April. Pindan woodlands are characterised by varying dominance of A. tumida and A. platycarpa in the mid-storey, with a sparse emergent top-story of eucalypt (e.g. Eucalyptus tetrodonta, E. miniata, Corymbia polycarpa) and non-eucalypt tree species (e.g. Erythrophleum chlorostachys, Gevillea agrifolia, Adansonia gregori) on sand or loamy sand substrates. The grass layer is co-dominated by annual Sorghum stipoidium and perennial hummock and/or tussock grasses, including Triodia spp. and Aristida spp. Pindan woodland forms the dominant vegetation type in non-rocky habitats within Mirima National Park (Kununurra).
Surveys
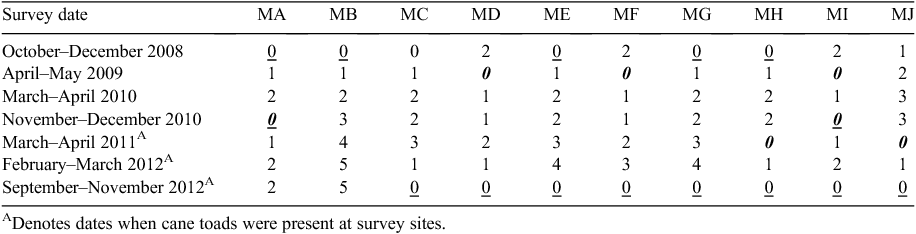
Ground-layer fauna was surveyed repeatedly at 10 pindan sites from October 2008 through to November 2012. Seven fauna surveys were conducted during the study, taking place in October–December 2008, March–May 2009, April–May 2010, November–December 2010, March–April 2011, February–April 2012 and October–December 2012. All surveys were undertaken during the hot, humid periods, either approaching the start of the wet season, or during or just after wet season, so that reptile, frog and invertebrate activity would be greatest. Sites were established between 100 m and 1700 m apart, which, for most small ground-dwelling vertebrates and invertebrates found in surveys (<100 mm in length or <100 g in weight), makes site survey data independent because few individuals were likely to travel among sites. Survey sites were initially chosen in 2008 to represent a range of post-fire intervals (0 years, 1 year and ≥2 years post-fire; Table 1). With the occurrence of annual fires throughout the study period, site-specific post-fire interval changed frequently during the study, with 21 survey observations of 0 years post-fire, 18 surveys for 1 year, 19 surveys for 2 years, 7 surveys for 3 years, 3 surveys for 4 years and 2 surveys 5 years post-fire (Table 1). Fire regime was characterised not only by years since the most recent fire (post-fire interval), but also fire intensity (high intensity for tree-crown scorch or low intensity where tree crowns were not scorched). Fire events before and during survey periods ranged from low-intensity prescribed fires (scorch height <3 m and <100% grass-layer vegetation consumed, Williams et al. 1999) during wet–dry transition periods (May 2008, April 2011), to high intensity late dry-season wildfires (August, October 2008, October 2012) (scorch height >12 m, 100% consumption of grass layer; Williams et al. 1999; Table 1). Prescribed management fires, either to protect or to burn particular sites before surveys, or as part of the greater fire management of the area, were applied in April–May 2009, November–December 2010 and March–April 2011 (Table 1). All other fires were savanna wildfires either caused by arson or accident.
|
Vegetation structure
Vegetation was assessed during five of the seven trapping sessions. During all vegetation measurements, total projected tree (>4 m) and shrub (1–4 m) canopy cover was recorded as a percentage using the bitterlich method (Lindsey et al. 1958). In addition, herbaceous ground-layer vegetation cover was assessed using a point-intersect method on a pre-marked staff. Vegetation cover was classified at point intercepts according to the ground-cover categories perennial grass (Triodia spp., Aristida spp.), annual grass (annual Sorghum), dicot (forbs and subshrubs), leaf and woody litter and bare ground. Estimation was based on placement of a 1.5-m graduated stick with 10-cm increments at 16 within-site locations (240 points). Locations for placement of the stick were chosen on the diagonal from each of the four corners of the trapping grid, and were placed five paces in a line from the corner of the plot towards the next diagonal trap placement (4 in a line from each corner). Ground-cover categories were then calculated on the basis of percentages derived from intercept points.
Reptile, frog and ground-layer invertebrates
Reptiles, frogs and large ground-dwelling invertebrates (>5 mm long) were sampled using funnel traps during all seven surveys. In total, 18 funnel traps (15 × 15 × 70 cm, with 5-cm entrance diameter) were placed in a 6 × 3 trapping grids, each trap 20 m apart (grid area 0.6 ha). Each funnel was placed in a shallow (10–20 cm deep) trench 6 m long that attracted and guided fauna into the funnel trap from either end as per Radford (2012). This worked in a way similar to drift fences. Traps were opened for seven nights for the first two surveys and for four nights thereafter. Traps were checked daily as soon as possible after sunrise during surveys, to avoid heat stress- or desiccation-related deaths among captured animals. Shade from hessian sacks, branches or grass tufts were also used on top of each trap to reduce fauna mortality resulting from overheating. In very hot dry weather, funnel traps were watered (3–5 L every day) to prevent heat-related deaths in traps through evaporative cooling. Percentage trap success (number of captures/number of trap-nights × 100) was used for all fauna-abundance comparisons, with individual animals being marked using permanent marker pens so that recaptures were not included in totals. Reptiles and frogs were identified to species using standard fauna keys (Storr et al. 1983, 1999, 2002; Wilson and Swan 2003). Invertebrates were identified to class (Chilopoda (centipedes)), order (e.g. Coleoptera (beetles), Blattodea (cockroaches), Scorpiones (scorpions), Araneae (spiders)), suborder (e.g. Caelifera (grasshoppers), Ensifera (crickets)) or to family (e.g. Carabidae (carabid beetles) where readily recognisable in the field). All fauna was measured for combined head and body length and reptiles were measured for snout–vent length and nose–tail length as a measure of animal size. All animals were released on-site after processing.
Mammals were sampled during the latter five fauna surveys using Elliott and pit-fall traps. Eighteen Elliott traps (9 medium (9 × 10 × 33 cm) and 9 large (15 × 15.5 × 46 cm)) were placed near each funnel trap, making up a duplicate mammal-trapping grid. Elliott traps were baited with a mixture of peanut butter and oats and left open for four nights as per funnel traps. Four pit-fall traps were set up in the four corners of each survey site. Each pit-fall trap was made up of a 20-L bucket buried flush with the ground. Four shallow trenches (10–20 cm deep and 4 m long) radiating into pit-falls were used to direct animal movements into the traps. Like funnel traps, Elliott traps were shaded from the sun by using hessian sacks or leaf material to prevent overheating. Artificial shade was provided in pit traps in the form of loose, moist soil and polystyrene trays to reduce direct sun exposure. All traps were checked as soon as possible after sunrise to reduce risk of heat-related injury/death among trapped animals. Mammal weight, head length, pes length and, in the case of rodent species, combined head–body and tail length were measured to facilitate species identification. Mammal identification was based on Menkhorst and Knight (2004). Mammals were earmarked using permanent marker pens to allow accurate counts of individuals of each species per survey to be made. Although reptiles, frogs and invertebrates were also captured in pit-fall traps, only mammal records from pits are included in this analysis.
Statistical analysis
Nested repeated-measures ANOVA (general linear model; MINITAB 14, Minitab Inc. U.S.A.) was used to test for differences in univariate measures of vegetation, habitat attributes and fauna groups among sites, post-fire intervals, pre- and post-invasion (by toxic cane toads) and among survey dates. Because we were using repeated survey of permanent sites that were burnt periodically throughout the study, post-fire interval was nested within sites for these analyses. Fauna data were divided into surveys before and after cane-toad invasion, and survey date was nested within these periods for analysis. Additional factors, including the intensity of site fires before survey (high intensity with A. tumida tree leaf scorch; low intensity with ground-layer vegetation scorched) and rainfall in the 3 months before survey (fauna only) were included within the ANOVA as covariates. Because of the non-independence of repeated site measurements and post-fire interval in a nested design, the ANOVA error term could not be used to calculate sums of squares. All variables except for mammal data were arc-sin (square-root) transformed to homogenise variances for percentage data.
Results
Vegetation
Pindan savanna woodlands at survey sites had a mean tree cover of 18.8% and perennial grass cover of 18.7%, shrub cover of 4.7%, annual grass cover of 8.7%, forb cover of 9.1% and leaf and woody litter cover of 37.3%. Time since most recent fire ranged from 0 to 5 years, with a mean time since last fire (TSLF) of 1.7 years. The trees and shrubs making up the greatest components of canopy cover were Acacia tumida (13.8%), followed by Eucalyptus tetrodonta (2.7%), E. miniata (1.6%), Erythrophleum chlorostachys (1.4%), Grevillea agrifolia (1.3%) and Scaevola browniana (1.1%).
Exothermic fauna
During seven surveys at 10 sites over a total of 6120 trap-nights, 1085 reptiles, 194 frogs and 1375 invertebrates were trapped. Exothermic vertebrate trap success (TS) was 20.8%. The most common component of vertebrate assemblages were species of Scincidae, with a combined trap success of 12.8% (and 61.3% of captures). Lerista griffini (sand swimmer skink), Carlia spp., Ctenotus spp., Eremiascincus isolepis and Morethia ruficauda were the most common taxa. Frogs were the next most common vertebrate group, with 3.4% TS, and made up 16% of animals captured. The most common frogs were Platyplectrum ornatum and Uperoleia spp. Gekkonidae (2.2% TS) and Agamidae (1.6% TS) were also common groups, making up 10.8% and 7.9% of vertebrates, respectively. Heteronotia binoei was the most common gecko and Diporiphora magna and D. pindan were the most common agamids. Larger reptiles including Varanus spp. (n = 24), Elapidae (n = 25), Pygopodidae (n = 1) and Ramphotyphlops (n = 2) were trapped rarely (<0.8% TS) and made up 3.8% of vertebrate captures. Small numbers preclude this latter group from formal analysis of trends. Total exothermic vertebrate species richness was 6.2 sites survey–1. Invertebrates had combined TS of 22.5%, with the most common groups being carabid Coleoptera (27.4% of invertebrates captured), Blattodea (21.4%), Chilopoda (20.0%), Araneae (5.9%), Gryllidae (5.6%), other non-carabid Coleoptera (5.0%) and Scorpiones (4.4%).
Mammal fauna
During five surveys and 4240 trap-nights (Elliotts plus pit-fall traps), 80 small mammals were captured (1.8% TS) in total. The most common species was the rodent Pseudomys delicatulus (n = 52). Other rodents captured included Rattus tunneyi (n = 12), P. nanus (n = 10) and Zyzomys argurus (n = 3). Three small dasyurid species Planigale ingrami were also recorded during the study (n = 3).
Fire responses of vegetation structure
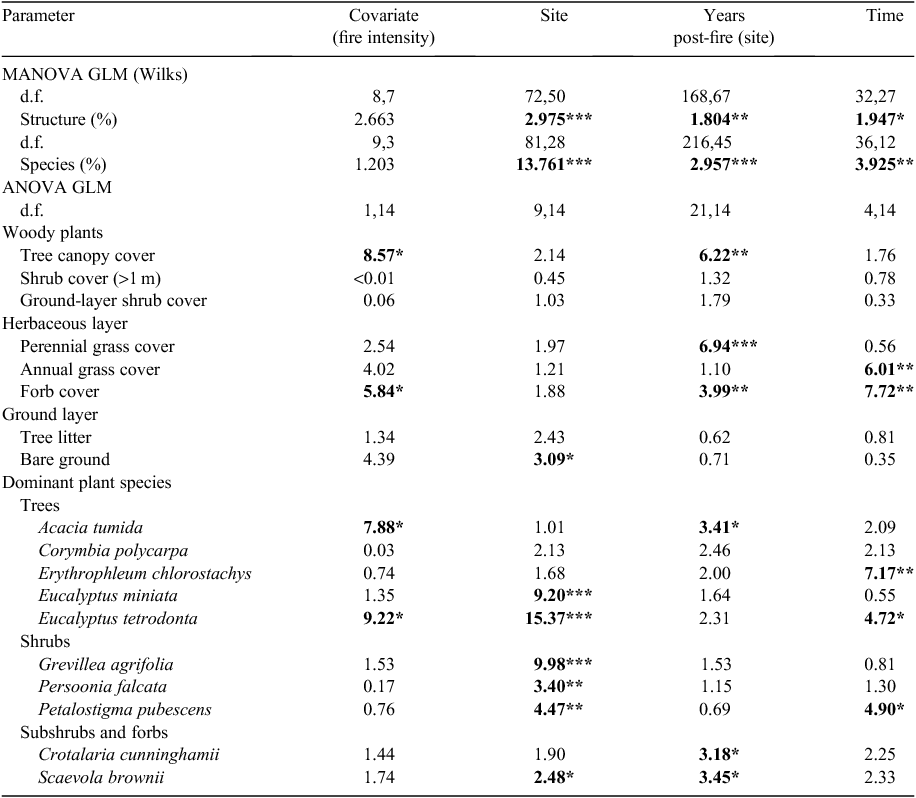
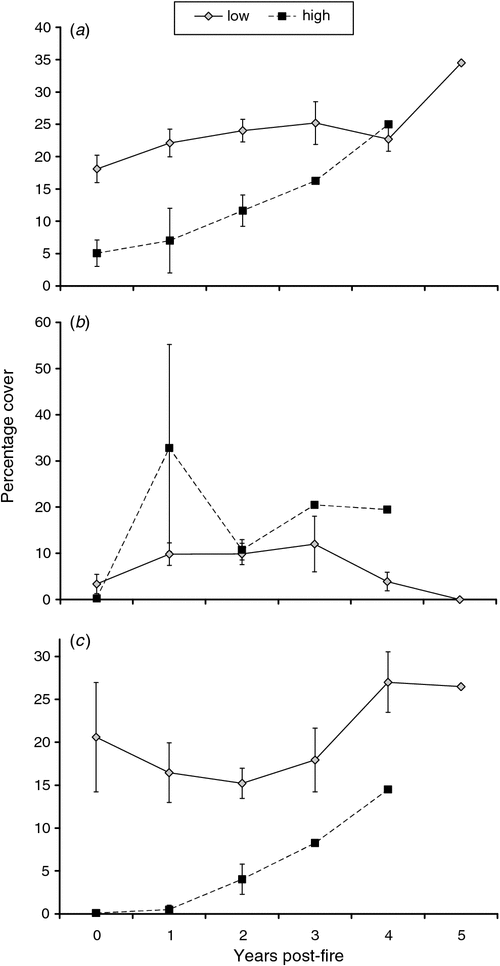
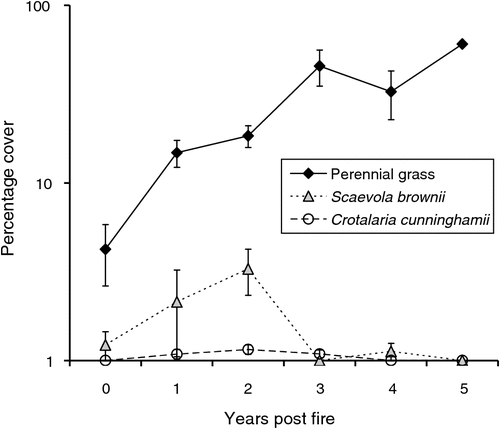
Vegetation structure and assemblage were significantly influenced by site, TSLF and survey date, but there was no effect of fire intensity on structural composition (Table 2). Tree cover, perennial grass cover and forb cover were influenced by TSLF (Table 2). Tree and forb cover were influenced by fire intensity (Table 2). Tree and perennial grass cover increased with post-fire interval from zero to 5 years (Figs 1a, 2, Table 2). Forb cover increased from zero in the year of the fire to 1 year post-fire and then declined 4 and 5 years post-fire (Fig. 1b, Table 2). Tree cover was greater at sites experiencing low-intensity fires, whereas forb cover was higher at these sites (Fig. 1a, b, Table 2). Tree cover after high-intensity fire attained values of sites affected only by low-intensity fire after 4 or 5 years post-fire (Fig. 1a). Additional factors significantly affecting pindan vegetation and habitat structure included site-specific (edaphic) effects (e.g. on percentage of bare ground) and survey date (annual grass, forb cover) relating to seasonal change (wet–dry transition or buildup season and rainfall; Table 2). In all, 4 of 10 common plant species in pindan woodland were significantly affected by fire (Table 2). Cover of the dominant tree species, A. tumida, was significantly influenced by post-fire interval and fire intensity (Table 2). Acacia tumida cover was reduced almost to zero after intense fire (because of tree mortality) and increased with post-fire interval after both high- and low-intensity fires (Fig. 1c). Acacia tumida cover had fully recovered after a high-intensity fire 4–5 years post-fire (Fig. 1c) and, by this stage, trees were mature and able to produce seeds. Covers of the forbs Crotalaria cunninghamii and Scaevola brownii were influenced by post-fire interval (Table 2), with both attaining maximum cover at intermediate post-fire intervals (2 years post-fire; Fig. 2). In addition to fire-related effects, the covers of E. miniata, E. tetrodonta, G. agrifolia, Persoonia falcata, Petalostigma pubescens and S. cunninghamii were significantly related to site-specific factors (edaphic). Cover of 3 of 10 common plant species was influenced by survey date, indicating seasonal effects (rainfall and season; Table 2).
|
|
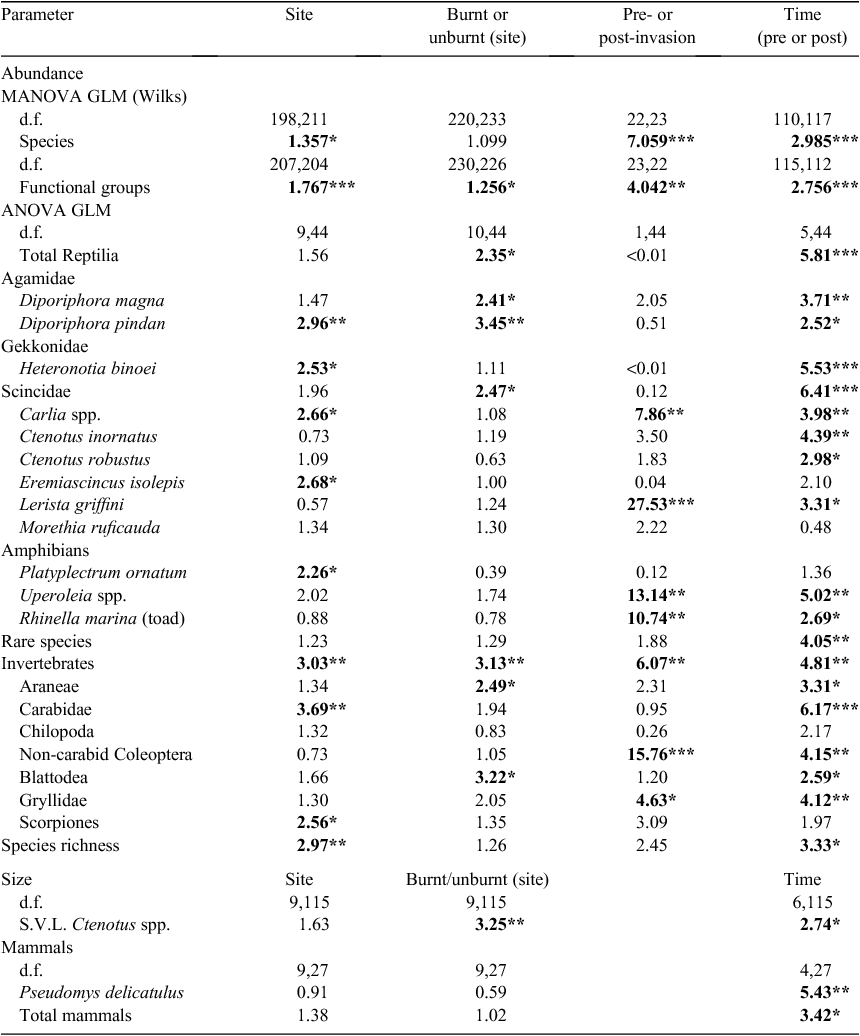
Fauna fire response
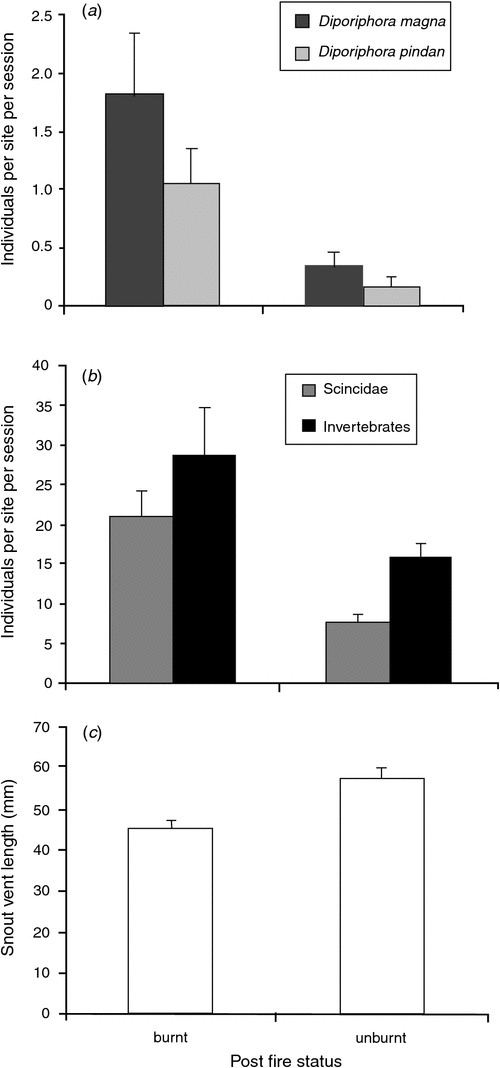
Greatest responses among fauna groups were to temporal or seasonal variability (survey date) and spatial variability (site-related factors) rather than to either fire or invasion (Table 3). Nonetheless, fire responses were detected. Unlike vegetation, fauna groups did not show a gradual or successional response to post-fire intervals from zero to 5 years post-fire (P > 0.05). Instead, fauna groups for which fire responses were evident partitioned differences between the year of the burn and all other TSLF (Table 3). Fire-response groups included two agamid species (D. magna and D. pindan), combined Scincidae, combined reptiles, Araneae, Blattodea and combined invertebrates (Table 3, Fig. 3a, b). In all cases, abundance (trap success) was higher at sites in the year of the fire than at all other TSLF (Fig. 3a, b). The exception to this were skinks of Ctenotus spp. (C. inornatus and C. robustus), which, although present at similar abundance in the year of a fire and at other times, were on average smaller (shorter snout–vent length) in the year of the fire (Table 3, Fig. 3c).
|
Invasion response
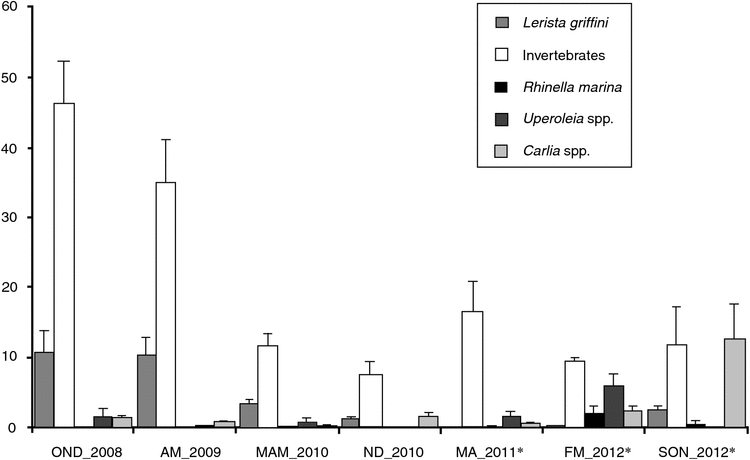
Combined fauna assemblages differed between surveys before and after toad invasion (Table 3). Mean trap success of the burrowing skink Lerista griffini, Coleoptera, Gryllidae, and total invertebrates was lower post-invasion, whereas abundance of skinks of Carlia spp., frogs of Uperoleia spp. and Rhinella marina (cane toad) was higher (Table 3, Fig. 4). Inter-survey variability was high within pre- and post-invasion periods (Fig. 4). Lerista griffini and invertebrate trap success was greatest during the first two pre-invasion surveys (2008/2009) and was generally lower thereafter, irrespective of pre- and post-invasion intervals (Fig. 4). Similarly, high mean TS of Carlia spp. and Uperoleia spp. occurred only during a single post-invasion survey, rather than generally across all post-toad surveys (Fig. 4).
Statistical analysis of trends among large predators including Varanus spp. (i.e. V. gouldii (10 pre-/4 post-invasion), 1 V. acanthurus (5/1) and V. mitchelli (0/1), V. scalaris (1/0), V. tristis (2/0)) and snakes (Brachyurophis roperi (9/1), Psuedonaja nuchalis (2/3), Pseudechis australis (1/5), P. weigeli (0/1), Acanthophis praelongus (1/0), Suta punctata (2/0)) was precluded by low number of captures throughout the study. However, trap success for total Varanus spp. was reduced by >50% (from 0.56% to 0.27%) after R. marina had established at survey sites. In contrast to Varanus, total snake trap success was identical in pre- and post-invasion surveys (0.46%), although the burrowing snake Brachyurophis roperi had a >80% reduction in trap success (from 0.28% to 0.05%) and mulga snakes Pseudechis spp. actually increased by 86% post-invasion (from 0.03% to 0.28%).
Discussion
Vegetation fire response
Australian savannas, generally, are dominated by resprouting species (Clarke et al. 2015). Dominance by resprouting trees and shrubs has resulted in relative structural and compositional resilience in Australian savannas (Williams et al. 1999; Russell-Smith et al. 2003; Radford et al. 2008), although long-term absence of fire can result in major changes (Woinarski et al. 2004). In contrast to resprouter-dominated savannas, more fire-responsive elements within savanna vegetation, including obligate seeders, have more pronounced responses. Callitris intratropica is often cited as a species declining within the savanna matrix because of its inability to resprout after canopy scorch with high-intensity fire (Bowman et al. 2001; Trauernicht et al. 2013). Obligate-seeder heath species on the Arnhem Land Plateau are also considered under threat from high frequency fire regimes where maturation periods exceed interfire intervals (Russell-Smith et al. 2003).
In the present study, the dominant tree, A. tumida, was killed by high-intensity fires (scorch >8 m) and seedling emergence occurred in the following wet season, making this an obligate-seeding species (Clarke et al. 2015). This species, and also A. platycarpa, another characteristic pindan species with basal regrowth, are largely absent from the tree canopy for 3–5 years after fire. Other dominant pindan species were predominantly epicormic resprouters, and either re-established canopies within a single year (e.g. Eucalyptus tetrodonta, E. miniata, Erythrophleum chlorostachys, Petalostigma pubescens and Adansonia gregori) or in the case of some shrubs (e.g. Grevillea agrifolia, Persoonia falcata) were able to re-establish from basal regrowth within 1 or 2 years. Re-establishment of pre-fire perennial hummock and tussock grass canopies, like that for A. tumida trees, took ~3 years. In the interim period (1–2 years post-fire), forbs (e.g. Spermacoce spp., Gomphrena canescens) and subshrubs (e.g. Crotalaria cunninnghamii, Scaevola brownii) increased their cover values, presumably taking advantage of increased light and reduced competition for soil resources. Annual Sorghum grass, although it did not increase in cover, increased in height and biomass (no data collected) in the period from 1 to 2 years post-fire and before tree canopy and perennial grass re-establishment. Pindan woodlands, therefore, followed distinct successional stages post-fire, with initial increases in biomass and cover of annual grasses and forbs, and mass emergence of A. tumida seedlings, 1–2 years post-fire, followed by re-establishment of the perennial grass layer, shrub and tree canopies between 3 and 5 years, and an associated decline in forb and annual grass cover. This successional pattern is similar, although more rapid to that seen in central Australian arid grasslands (Masters 1996; Letnic et al. 2004). This 3–5-year post-fire succession differs from typical resprouter-dominated savannas in northern Australia where pre-fire tree and perennial grass cover is largely re-established 1 or 2 years after fire (Williams et al. 1999; Cook 2003).
It should be noted here that the present study cannot claim to elucidate patterns of vegetation or structural development of pindan woodlands beyond 5 years post-fire. This is due to the absence of any longer-term unburnt sites in the study. Observation of long-unburnt pindan woodland at Derby in the western Kimberley with >40 years without fire suggested that there is a gradual loss of perennial grass cover and annual grass biomass as A. tumida and shrub canopies develop over time (I. J. Radford, unpubl. data). There is also an increase in some forb species (e.g. Pterocaulon spp.). One of the more prominent features of long-unburnt pindan sites is the development of a dense Calytrix exstipulata shrub layer (I. J. Radford, unpubl. data). This is a fire-sensitive species killed by canopy scorch and, therefore, usually restricted to fire-sheltered habitats (Radford et al. 2013). It is not known whether long-unburnt pindan woodland contains unique botanical biodiversity. However, similar long-term unburnt Callitris intratropica stands (another fire-sensitive species) were found to contain no higher species richness of fire-sensitive plants (aside from C. exstipulata) than were surrounding savannas (Radford et al. 2013). Although C. intratropica stands elsewhere reportedly contain high plant diversity compared with surrounding savannas (Trauernicht et al. 2013), long-unburnt fire-sensitive savannas appeared to contain no particular concentration of fire-sensitive, threatened or non-savanna (e.g. rainforest) taxa (Radford et al. 2013). This latter study was conducted in the same East Kimberley region as the present study.
Fauna fire responses
Savanna faunas both in Australia (Andersen and Muller 2000; Corbett et al. 2003; Andersen et al. 2005, 2010, 2014a, 2014b; Radford 2012; Radford and Andersen 2012) and elsewhere worldwide (Gillon 1983; Parr and Chown 2003; Parr et al. 2004) are often thought to be relatively fire resilient, consistent with fire being intrinsic to this biome (Bond et al. 2005). Despite this, some savanna groups are more fire-sensitive than others, for instance, small mammals (Andersen et al. 2005; Woinarski et al. 2010, 2011) and granivorous finches in northern Australia (Woinarski and Legge 2013). The few known fauna records from fire-sensitive savanna vegetation in northern Australia suggest that they contain typical savanna fauna, rather than specialised assemblages characterised by rainforest or fire-sensitive taxa (Woinarski and Fisher 1995a, 1995b; Radford et al. 2013). Pindan fauna documented in the present study were also found to be predominantly typical savanna species (e.g. Trainor and Woinarski 1994; Corbett et al. 2003; Radford 2012).
The predominance of typical savanna species in the present study may explain the apparently minor and short-lived fire responses documented here. Establishment of some vegetation ground cover by forbs and annual grasses after a single wet season apparently provided sufficient habitat for recovery of the majority of these species of fauna. In the year of the fire, ground-active running predators, such as Diporiphora spp., may benefit from increased hunting efficiency through the loss of herbaceous-layer vegetation, leading to their increased trap success. Increased predator activity after fire removal of vegetation cover has often been observed in savannas. Species including raptors (Braithwaite and Estbergs 1987; Corbett et al. 2003), the frilled lizard (Corbett et al. 2003), native mammals including Dasyurus hallucatus and Isoodon auratus (Radford 2012) and introduced mammalian predators Felis catus and Canis dingo (McGregor et al. 2014; Leahy et al. unpubl. data) are known to respond positively to removal of ground-layer vegetation by fire. Similar post-fire increases in ground-active reptilian predators have been observed in central Australian spinifex grasslands, although this was for more extended periods due to the more gradual re-establishment of ground-layer vegetation in arid environments (Masters 1996; Letnic et al. 2004; Haslem et al. 2012).
Increased post-fire activity among other ground-dwelling reptiles, skinks and invertebrates (e.g. spiders, beetles, cockroaches, crickets) soon after fire may reflect changes in trap encounters owing to changes to habitat structure, rather than population trends. There was little time for population responses to fire during surveys, which took place as few as 2 days after fire. It is likely that reduced vegetation complexity and cover at ground level soon after fire increased the ease of movement of many of these small ground-dwelling fauna, leading to more frequent trap encounters. Similar increases among skinks and some invertebrates have been observed previously (Gillon 1983; Andersen and Muller 2000; Radford 2012). However, like many other fire-ecology studies from Australian savannas, no major compositional changes were detected among fauna either in the year of the fire (Andersen and Muller 2000; Corbett et al. 2003; Andersen et al. 2005; Radford and Andersen 2012), or over longer periods necessary for full recovery of woody vegetation structures.
Higher predator activity post-fire, and also exposure to greater heat stress with low ground cover, may explain a reduction in the mean size (length) of large species of Ctenotus skinks (C. robustus and C. inornatus) after fire. Skinks of Ctenotus spp. are large, often attaining total lengths of >300 mm, with individuals as long as 380 mm being observed. Such animals would be more visible to predators in post-fire environments than are smaller skinks and invertebrates, and may be subject to heightened predation levels (Radford 2012). In addition, some Ctenotus spp. have strong habitat preferences for high-cover habitats (Masters 1996), possibly to balance their thermodynamic requirements as well as to avoid predators (Bennett and John-Alder 1986). Such requirements may make immediate post-fire environments hostile to large skinks and result in a temporary loss of larger individuals from the population. However, as with other fire responses among pindan fauna, mean size increased back to pre-fire levels after a single wet season.
The present study may have under-estimated fire impacts for some elements of the pindan fauna. One of the key groups identified in northern Australia as having predominantly negative responses to fire is the group of small and medium-sized mammals including rodents and marsupial species up to 5 kg in size (Andersen et al. 2005; Woinarski et al. 2010, 2011; Ziembicki et al. 2015). However, small mammals made up only a small component of pindan survey captures, reflecting generally low abundance and diversity of mammals in this rainfall zone (<1000 mm) across the Western Australian Kimberley region (Radford et al. 2014). As is typical for most habitats in this rainfall zone (Radford et al. 2014), only rodent species and small dasyurids (<35 g) were recorded at pindan sites. Historical loss of many of these mammals as a functional component of savannas, plus low numbers captured in the study, means that we may have under-estimated the true impact of fire on the original mammal assemblages in pindan woodlands. We may have under-estimated responses of pindan fauna characteristic of arboreal habitats. Arboreal specialists are likely to respond to loss of habitat after intense fire because of the loss of A. tumida and A. platycarpa trees, which make up a major component of the tree canopy. Frequent fires may have negative effects on the availability of larger trees with nest hollows (Williams et al. 1999; Russell-Smith et al. 2003; Radford et al. 2008) and associated hollow-dependent fauna (Firth et al. 2006; Brazill-Boast et al. 2010, 2011, 2013), although this was not assessed at survey sites in the present study. Pindan specialist fauna, for instance, invertebrates that feed exclusively on A. tumida and A. playcarpa, are likely to be negatively affected by high-intensity fires causing widespread tree mortality but were not recorded in the present study. Finally, we were unable to document long-term responses (>5 year) to an absence of fire because we surveyed no older sites. A long-term absence of fire in savannas is known to result in radical assemblage responses among some fauna (e.g. forest-specialist ants; Woinarski et al. 2004; Andersen et al. 2012, 2014b). However, the only known data that detail fauna assemblages from long-unburnt (>40 years) pindan woodland (C. Palmer, unpubl. data, 2001) indicate a generalist savanna fauna similar to that documented in the present study at more frequently burnt sites. Specialist survey of these additional fauna groups (e.g. arboreal species, specialist Acacia spp. feeders) would be required to more fully assess the longer-term effects of the absence of fire in pindan woodlands.
Response of pindan fauna to cane-toad invasion
Cane-toad invasion has led to a range of impacts among different savanna fauna across northern Australia and internationally; however, impacts have often been inconsistent (Shine 2010; Ujvari et al. 2011). Post-invasion impacts have been recorded for invertebrates (Greenlees et al. 2006), adult frogs (Catling et al. 1999; Greenlees et al. 2007, 2010) and tadpoles (Crossland et al. 2008) from several separate studies. Toad invasion is generally thought to cause major impacts on large predatory varanid lizards and elapid snakes (Phillips et al. 2003; Smith and Phillips 2006; Shine 2010; Pearson et al. 2014). Cascading effects on medium-sized predators such as agamid lizards have also been recorded after poisoning of top predators (e.g. V. panoptes) in some instances (Doody et al. 2013).
Some post-invasion changes to assemblages of pindan fauna were observed in the present study, but these differed from previous studies in the taxa apparently affected. Ground-layer invertebrates and Lerista griffini, a fossorial skink that dominated pre-invasion vertebrate faunas, both decreased in abundance after cane-toad invasion, whereas skinks of Carlia spp. and frogs of Uperoleia spp. increased in abundance. Given design restrictions of the present study (an absence of non-invaded experimental control sites), it is impossible to fully separate invasion from temporal effects. Several of the changes to fauna documented here may be attributable as much to particular seasonal conditions (for instance, the highest Uperoleia spp. trap success was recorded during a period of very high rainfall) as to impacts of invasion. In addition, high inter-survey variation within both pre- and post-invasion periods makes it difficult to determine whether variation is stochastic or directional. Nonetheless, the possibility remains that changes observed here are actually related to perturbations to trophic interactions caused by the arrival of cane toads. Although it is hard to identify the precise trophic pathways leading to declines or increases among particular fauna in the present study, the results are consistent with those of previous studies (e.g. Doody et al. 2009, 2013) and our own anecdotal observations (see below), namely, that apparent impacts on large predators such as varanids could lead to cascading effects on pindan faunas. In this case, reductions among varanids could lead to a reduced predation pressure on prey species (e.g. skinks of Carlia spp. and Uperoleia spp.). In turn, increases among prey groups could lead to cascading effects on availability of resources for invertebrate species and fossorial species (the skink L. griffini and snake B. roperi) relying on inputs from ground-layer trophic interactions.
Obviously these hypotheses are highly speculative and would require manipulative tests to demonstrate. Nonetheless, we would predict that if these trophic cascades are operating in pindan savannas as a response to cane-toad invasion, future surveys should continue to record similarly altered assemblages as long as cane toads remain at a similar abundance. Alternatively, continued stochastic changes among components of fauna over time would indicate a much more chaotic ecosystem governed by seasonal conditions. Unlike other studies, which have documented short-term changes associated with the timing of invasion from single surveys (Catling et al. 1999), the present study with multiple pre- and post-invasion surveys should have greater ability to discriminate between seasonal variation and directional trends. However, as noted by Ujvari et al. (2011), replication of impact assessment both spatially and temporally is necessary if we are to gain a full appreciation of impacts of cane toads across highly variable tropical savannas across northern Australia. Further surveys into medium and long-term time scales (5–10 years) may be necessary for us to demonstrably elucidate post-invasion impacts of cane toads in savanna ecosystems.
Despite repeated documentation of cane-toad impacts on large predatory varanids and elapids from several studies (e.g. Phillips et al. 2003; Smith and Phillips 2006; Shine 2010; Pearson et al. 2014), we were unable to detect any differences in abundance of these groups post-invasion. This is, to a large extent, due to very small numbers of these larger animals captured during our surveys (total n = 49). Small numbers are partially due to the relatively small size of traps (funnels 15 cm wide and 70 cm long compared with Varanus spp. and Elapidae being up to 140 cm long), but also to large home ranges of top predators probably reduce trap encounters in these low-productivity environments. Notwithstanding this important caveat, we can make several observations relevant to impacts by cane toads on these larger predators. Appearance of toads at survey sites and within trap records coincides with several apparently uninjured varanids (Varanus gouldii) and elapids (Pseudechis australis) being observed dead at survey sites. This supports repeated observations that initial appearance of toads is often associated with mortality events through lethal poisoning on attempted ingestion (Pearson et al. 2014). Large varanids and elapids as a group continued to be recorded during post-invasion surveys; however, fewer than half as many varanids were captured post-invasion as pre-invasion (accounting for differences in trap effort). This suggests that there was a negative, if a muted, impact, of toad invasion at least on varanid species.
Low numbers of cane toads trapped in pindan surveys (only 27 toads captured during 2160 trap-nights) may have mitigated invasion impacts relative to other studies. Studies documenting invasion impacts, such as on the Daly River (Doody et al. 2006, 2013) or the Kakadu floodplains (Greenlees et al. 2006), may have had greater impacts simply because of the much greater number of cane toads present in these wetter and more productive riparian habitats. The small number of toads observed and captured during pindan surveys contrasts with observations of extremely high densities attained during invasion in many tropical areas (Freeland 1986; Lever 2001; Shine 2010) and very large numbers of toxic prey in these habitats could easily account for much greater impacts among predators. In contrast, pindan savannas that provide little access to permanent or ephemeral water and very low habitat productivity owing to sandy soils may simply not be able to support high cane-toad densities. Results presented here confirmed findings by Shine (2010) and Ujvari et al. (2011) that impacts are not consistent across habitat types. Low apparent invasion impacts in low-productivity non-riparian savanna habitats, including pindan woodlands, may play an important role in the persistence of toad-susceptible species, including varanids, by providing refuge habitat with low toad densities and, therefore, low toad-encounter rates.
Management implications
Being an obligate seeder-dominated vegetation type that takes 4–5 years for re-establishment of mature reproductive trees, pindan woodland needs to have a minimum interval of 5–6 years between intense canopy-killing fires. Although seed banks are not necessarily depleted after a single post-fire re-establishment event (I. J. Radford, unpubl. data), continued inter-fire intervals of <4 years will inevitably deplete the regenerative capacity of pindan woodland. Because of the frequency of fires in surrounding vegetation, the most prudent way to achieve longer fire intervals in this vegetation is through annual low-intensity patchy fires in pindan habitat. Such fires can be ignited during wet-season and very early dry-season periods (when fires self-extinguish overnight) because of the presence of highly flammable grasses of Triodia spp. and senescent perennial grasses from the previous dry season if fires did not occur. Low-intensity fire did not kill mature A. tumida trees, allowed continued seed production and mitigated the effects of late-season fires by reducing fire extent and intensity.
Despite requirements for longer inter-fire intervals for pindan woodland itself, specialised fire management of pindan fauna is not required on the basis of fire responses. Fauna responses here were more typical of responses of savanna fauna generally, than of responses of fauna in slower-regenerating vegetation such as arid grasslands (Masters 1996; Letnic et al. 2004). Fauna responses here appeared to be muted and somewhat similar to generalised responses of savanna faunas (Andersen and Muller 2000; Corbett et al. 2003). This reflects findings among similar fire-sensitive savanna types including Callitris intratropica (Radford et al. 2013), A. shirleyi and A. tumida woodlands (Woinarski and Fisher1995a, 1995b) where faunas were predominantly savanna generalists, rather than obligate woodland specialists. However, savanna fire management, generally, should aim to minimise the burning of extensive areas during any single fire. Recent evidence suggests that reducing fire size and/or extent, and retaining key vegetation-cover attributes is probably the most important feature of fire mosaics to maintain threatened biodiversity including mammals (Lawes et al. 2015; Radford et al. 2015). Although many of the species (e.g. critical weight-range mammals) identified as benefiting from retention of ground-vegetation cover and low-intensity patchy fires no longer persist as major functional elements in savannas in the medium- and low-rainfall Kimberley region (Radford et al. 2014), refuge populations occur in some fire-protected habitats (e.g. Isoodon macrourus occurs in the outskirts of Kununurra adjacent to pindan woodlands and Dasyurus hallucatus exists in some gorges). Such populations provide regional foci for re-establishment of populations under more benign fire regimes or abatement of other threatening processes.
Acknowledgements
Financial support, field sites and support facilities were provided by the Department of Parks and Wildlife (and its predecessors). Technical assistance was provided by Daphne Bocciarelli, Jai Latham, Len Terry, T. Robins and T. Howard. Comments on drafts of the manuscript were made by Lachie McCaw, Leonie Valentine, Ben Corey, Sean Doody, John Woinarski and anonymous reviewers. Statistical advice was given by Matthew Williams. Research was conducted under DEC Animal Ethics Approval no. 23/2007.
References
Andersen, A. N., and Muller, W. J. (2000). Arthropod responses to experimental fire regimes in an Australian tropical savannah: ordinal level analysis. Austral Ecology 25, 199–209.| Arthropod responses to experimental fire regimes in an Australian tropical savannah: ordinal level analysis.Crossref | GoogleScholarGoogle Scholar |
Andersen, A. N., Cook, G. D., Corbett, L. K., Douglas, M. M., Eager, R. W., Russell-Smith, J., Setterfield, S. A., Williams, R. J., and Woinarski, J. C. Z. (2005). Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment. Austral Ecology 30, 155–167.
| Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment.Crossref | GoogleScholarGoogle Scholar |
Andersen, A. N., Lanoue, J., and Radford, I. (2010). The ant fauna of the remote Mitchell Falls area of tropical north-western Australia: biogeography, environmental relationships and conservation significance. Journal of Insect Conservation 14, 647–661.
| The ant fauna of the remote Mitchell Falls area of tropical north-western Australia: biogeography, environmental relationships and conservation significance.Crossref | GoogleScholarGoogle Scholar |
Andersen, A. N., Woinarski, J. C. Z., and Parr, C. L. (2012). Savanna burning for biodiversity: fire management for faunal conservation in Australian tropical savannas. Austral Ecology 37, 658–667.
| Savanna burning for biodiversity: fire management for faunal conservation in Australian tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Andersen, A. N., Bocciarelli, D., Fairman, R., and Radford, I. J. (2014a). Conservation status of ants in an iconic region of monsoonal Australia: levels of endemism and responses to fire in the eastern Kimberley. Journal of Insect Conservation 18, 137–146.
| Conservation status of ants in an iconic region of monsoonal Australia: levels of endemism and responses to fire in the eastern Kimberley.Crossref | GoogleScholarGoogle Scholar |
Andersen, A. N., Ribbons, R. R., Pettit, M., and Parr, C. L. (2014b). Burning for biodiversity: highly resilient ant communities respond only to strongly contrasting fire regimes in Australia’s seasonal tropics. Journal of Applied Ecology 51, 1406–1413.
| Burning for biodiversity: highly resilient ant communities respond only to strongly contrasting fire regimes in Australia’s seasonal tropics.Crossref | GoogleScholarGoogle Scholar |
Bennett, A. F., and John-Alder, H. (1986). Thermal regulation of some Australian skinks. Copeia 1986, 57–64.
| Thermal regulation of some Australian skinks.Crossref | GoogleScholarGoogle Scholar |
Bond, W. J., Woodward, F. I., and Midgley, G. F. (2005). The global distribution of ecosystems in a world without fire. New Phytologist 165, 525–538.
| The global distribution of ecosystems in a world without fire.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M%2Fpt1OktQ%3D%3D&md5=4f49c878e1dd5f48a64f02c989c41bdfCAS | 15720663PubMed |
Bowman, D. M. J. S., Price, O., Whitehead, P. J., and Walsh, A. (2001). The ‘wilderness effect’ and the decline of Callitris intratropica on the Arnhem Land Plateau, northern Australia. Australian Journal of Botany 49, 665–674.
| The ‘wilderness effect’ and the decline of Callitris intratropica on the Arnhem Land Plateau, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Braithwaite, R., and Estbergs, J. (1987). Fire-birds of the Top-End. Australian Natural History 22, 299–302.
Brazill-Boast, J., Pryke, S. R., and Griffith, S. C. (2010). Nest-site utilisation and niche overlap in two sympatric, cavity-nesting finches. Emu 110, 170–177.
| Nest-site utilisation and niche overlap in two sympatric, cavity-nesting finches.Crossref | GoogleScholarGoogle Scholar |
Brazill-Boast, J., Pryke, S. R., van Rooij, E., and Griffith, S. C. (2011). Interference from long-tailed finches constrains reproduction in the endangered Gouldian finch. Journal of Animal Ecology 80, 39–48.
| Interference from long-tailed finches constrains reproduction in the endangered Gouldian finch.Crossref | GoogleScholarGoogle Scholar | 20880021PubMed |
Brazill-Boast, J., Pryke, S. R., and Griffith, S. C. (2013). Provisioning habitat with custom-designed nest-boxes increases reproductive success in an endangered finch. Austral Ecology 38, 405–412.
| Provisioning habitat with custom-designed nest-boxes increases reproductive success in an endangered finch.Crossref | GoogleScholarGoogle Scholar |
Catling, P. C., Hertog, A., Burt, R. J., Forrester, R. I., and Wombey, J. C. (1999). The short-term effect of cane toads (Bufo marinus) on native fauna in the Gulf Country of the Northern Territory. Wildlife Research 26, 161–185.
| The short-term effect of cane toads (Bufo marinus) on native fauna in the Gulf Country of the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Clarke, M. F. (2008). Catering for the needs of fauna in fire management: science or just wishful thinking? Wildlife Research 35, 385–394.
| Catering for the needs of fauna in fire management: science or just wishful thinking?Crossref | GoogleScholarGoogle Scholar |
Clarke, P. J., Lawes, M. J., Murphy, B. F., Russell-Smith, J., Nano, C. E. M., Bradstock, R., Enright, N. J., Fontaine, J. B., Gosper, C. R., Radford, I., Midgley, J. J., and Gunton, R. (2015). A synthesis of postfire recovery traits of woody plants in Australian ecosystems. The Science of the Total Environment , .
| A synthesis of postfire recovery traits of woody plants in Australian ecosystems.Crossref | GoogleScholarGoogle Scholar | 25887372PubMed |
Cook, G. D. (2003). Fuel dynamics, nutrients, and atmospheric chemistry. In ‘Fire in Tropical Savannas: the Kapalga Experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams.) pp. 47–58. (Springer-Verlag: New York.)
Corbett, L. C., Andersen, A. N., and Muller, W. J. (2003). Terrestrial vertebrates. In ‘Fire in Tropical Savannas: the Kapalga Experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams.) pp. 126–152. (Springer-Verlag: New York.)
Crossland, M. R., Brown, G. P., Anstis, N., Shilton, C. M., and Shine, R. (2008). Mass mortality of native anuran tadpoles in tropical Australia due to the invasive cane toad (Bufo marinus). Biological Conservation 141, 2387–2394.
| Mass mortality of native anuran tadpoles in tropical Australia due to the invasive cane toad (Bufo marinus).Crossref | GoogleScholarGoogle Scholar |
Doody, J. S., Green, B., Sims, R., Rhind, D., West, P., and Steer, D. (2006). Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pig-nosed turtles (Carettochelys insculpta). Wildlife Research 33, 349–354.
| Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pig-nosed turtles (Carettochelys insculpta).Crossref | GoogleScholarGoogle Scholar |
Doody, J. S., Green, B., Rhind, D., Castellano, C. M., Sims, R., and Robinson, T. (2009). Population-level declines in Australian predators caused by an invasive species. Animal Conservation 12, 46–53.
| Population-level declines in Australian predators caused by an invasive species.Crossref | GoogleScholarGoogle Scholar |
Doody, J. S., Castellano, C. M., Rhind, D., and Green, B. (2013). Indirect facilitation of a native mesopredator by an invasive species: are cane toads re-shaping tropical riparian communities? Biological Invasions 15, 559–568.
| Indirect facilitation of a native mesopredator by an invasive species: are cane toads re-shaping tropical riparian communities?Crossref | GoogleScholarGoogle Scholar |
Firth, R. S. C., Woinarski, J. C. Z., and Noske, R. A. (2006). Home range and den characteristics of the brush-tailed rabbit-rat (Conilurus penicillatus) in the monsoonal tropics of the Northern Territory, Australia. Wildlife Research 33, 397–407.
| Home range and den characteristics of the brush-tailed rabbit-rat (Conilurus penicillatus) in the monsoonal tropics of the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Fox, B. J. (1982). Fire and mammalian secondary succession in an Australian coastal heath. Ecology 63, 1332–1341.
| Fire and mammalian secondary succession in an Australian coastal heath.Crossref | GoogleScholarGoogle Scholar |
Freeland, W. J. (1986). Populations of cane toad, Bufo marinus, in relation to time since colonisation. Australian Wildlife Research 13, 321–329.
| Populations of cane toad, Bufo marinus, in relation to time since colonisation.Crossref | GoogleScholarGoogle Scholar |
Gillon, D. (1983). The fire problem in tropical savannas. In ‘Ecosystems of the World. Vol. 13: Tropical Savannas’. (Ed. F. Bourliere.) pp. 617–641. (Elsevier: Amsterdam.)
Greenlees, M. J., Brown, G. P., Webb, J. K., Phillips, B. L., and Shine, R. (2006). Effects of an invasive anuran [the cane toad (Bufo marinus)] on the invertebrate fauna of a tropical Australian floodplain. Animal Conservation 9, 431–438.
| Effects of an invasive anuran [the cane toad (Bufo marinus)] on the invertebrate fauna of a tropical Australian floodplain.Crossref | GoogleScholarGoogle Scholar |
Greenlees, M. J., Brown, G. P., Webb, J. K., Phillips, B. L., and Shine, R. (2007). Do invasive cane toads (Chaunus marinus) compete with Australian frogs (Cyclorana australis)? Austral Ecology 32, 900–907.
| Do invasive cane toads (Chaunus marinus) compete with Australian frogs (Cyclorana australis)?Crossref | GoogleScholarGoogle Scholar |
Greenlees, M. J., Phillips, B. L., and Shine, R. (2010). Adjusting to a toxic invader: native Australian frogs learn not to prey on cane toads. Behavioural Ecology 21, 966–971.
Haslem, A., Avitabile, S. C., Taylor, R. S., Kelly, L. T., Watson, S. J., Nimmo, D. G., Kenny, S. A., Callister, K. E., Spence-Bailey, L. M., Bennett, A. F., and Clarke, M. F. (2012). Time-since-fire and inter-fire interval influence hollow availability for fauna in a fire-prone system. Biological Conservation 152, 212–221.
| Time-since-fire and inter-fire interval influence hollow availability for fauna in a fire-prone system.Crossref | GoogleScholarGoogle Scholar |
Kenneally, K. F., Edinger, D. C., and Willing, T. (1996). ‘Broome and Beyond: Plants and People of the Dampier Peninsula, Kimberley, Western Australia.’ (Western Australian Department of Conservation and Land Management: Perth.)
Lawes, M., Murphy, B., Fisher, A., Woinarski, J., Edwards, A., and Russell-Smith, J. (2015). Small mammals decline with increasing fire extent in northern Australia: evidence from long-term monitoring in Kakadu National Park. International Journal of Wildland Fire , .
| Small mammals decline with increasing fire extent in northern Australia: evidence from long-term monitoring in Kakadu National Park.Crossref | GoogleScholarGoogle Scholar |
Legge, S., Murphy, S., Heathcote, J., Flaxman, E., Augusteyn, J., and Crossman, M. (2008). The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia. Wildlife Research 35, 33–43.
| The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., Dickman, C. R., Tischler, M. K., Tamayo, B., and Beh, C. L. (2004). The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. Journal of Arid Environments 59, 85–114.
| The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Lever, C. (2001). ‘The Cane Toad: the History and Ecology of a Successful Colonist.’ (Westbury Academic and Scientific Publishing: Otley, UK.)
Lindsey, A. A., Barton, J. D., and Miles, S. R. (1958). Field efficiencies of forest sampling methods. Ecology 39, 428–444.
| Field efficiencies of forest sampling methods.Crossref | GoogleScholarGoogle Scholar |
Masters, P. (1996). The effects of fire-driven succession on reptiles in spinifex grasslands at Uluru National Park, Northern Territory. Wildlife Research 23, 39–48.
| The effects of fire-driven succession on reptiles in spinifex grasslands at Uluru National Park, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
McGregor, H. W., Legge, S., Jones, M. E., and Johnson, C. N. (2014). Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS One 9, e109097.
| Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats.Crossref | GoogleScholarGoogle Scholar | 25329902PubMed |
McKenzie, N. L. (1981). Mammals of the Phanerozoic Southwest Kimberley, Western Australia: biogeography and recent changes. Journal of Biogeography 8, 263–280.
| Mammals of the Phanerozoic Southwest Kimberley, Western Australia: biogeography and recent changes.Crossref | GoogleScholarGoogle Scholar |
Menkhorst, P., and Knight, F. (2004). ‘A Field Guide to the Mammals of Australia.’ (Oxford University Press: Melbourne.)
Parr, C. L., and Chown, S. L. (2003). Burning issues for conservation: a critique of faunal fire research in southern Africa. Austral Ecology 28, 384–395.
| Burning issues for conservation: a critique of faunal fire research in southern Africa.Crossref | GoogleScholarGoogle Scholar |
Parr, C. L., Robertson, H. G., Biggs, H. C., and Chown, S. L. (2004). Response of African savanna ants to long-term fire regimes. Journal of Applied Ecology 41, 630–642.
| Response of African savanna ants to long-term fire regimes.Crossref | GoogleScholarGoogle Scholar |
Pearson, D. J., Webb, J. K., Greenlees, M. J., Phillips, B. L., Bedford, G. S., Brown, G. P., Thomas, J., and Shine, R. (2014). Behavioural responses of reptile predators to invasive cane toads in tropical Australia. Austral Ecology 39, 448–454.
| Behavioural responses of reptile predators to invasive cane toads in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Phillips, B. L., and Shine, R. (2005). The morphology, and hence impact, of an invasive species (the cane toad, Bufo marinus): changes with time since colonisation. Animal Conservation 8, 407–413.
| The morphology, and hence impact, of an invasive species (the cane toad, Bufo marinus): changes with time since colonisation.Crossref | GoogleScholarGoogle Scholar |
Phillips, B. L., Brown, G. P., and Shine, R. (2003). Assessing the potential impact of cane toads on Australian snakes. Conservation Biology 17, 1738–1747.
| Assessing the potential impact of cane toads on Australian snakes.Crossref | GoogleScholarGoogle Scholar |
Prior, L. D., McCaw, W. L., Grierson, P. F., Murphy, B. P., and Bowman, D. M. J. S. (2011). Population structures of the widespread Australian conifer Callitris columellaris are a bio-indicator of continental environmental change. Forest Ecology and Management 262, 252–262.
| Population structures of the widespread Australian conifer Callitris columellaris are a bio-indicator of continental environmental change.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J. (2012). Threatened mammals become more predatory after fire in a rocky tropical savanna. Austral Ecology 37, 926–935.
| Threatened mammals become more predatory after fire in a rocky tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J., and Andersen, A. N. (2012). Effects of fire on grass-layer savanna macro-invertebrates as key food resources for insectivorous vertebrates in northern Australia. Austral Ecology 37, 733–742.
| Effects of fire on grass-layer savanna macro-invertebrates as key food resources for insectivorous vertebrates in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J., Grice, A. C., Abbott, B. N., Nicholas, D. M., and Whiteman, L. (2008). Impacts of wet and dry season prescribed burning on invasive Cryptostegia grandiflora populations and on associated tropical woodland, and riparian forest in north Queensland, Australia. Austral Ecology 33, 151–167.
| Impacts of wet and dry season prescribed burning on invasive Cryptostegia grandiflora populations and on associated tropical woodland, and riparian forest in north Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J., Andersen, A. N., Graham, G., and Trauernicht, C. (2013). The fire-refuge value of patches of a fire-sensitive tree in fire-prone savannas: Callitris intratropica in northern Australia. Biotropica 45, 594–601.
| The fire-refuge value of patches of a fire-sensitive tree in fire-prone savannas: Callitris intratropica in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J., Dickman, C. R., Start, A. N., Palmer, C., Carnes, K., Everitt, C., Fairman, R., Graham, G., Partridge, P., and Thomson, A. (2014). Mammals of Australia’s tropical savannas: a conceptual model of assemblage structure and regulatory factors in the Kimberley region. PLoS One 9, e92341.
| Mammals of Australia’s tropical savannas: a conceptual model of assemblage structure and regulatory factors in the Kimberley region.Crossref | GoogleScholarGoogle Scholar | 24670997PubMed |
Radford, I. J., Gibson, L. A., Corey, B., Carnes, K., and Fairman, R. (2015). Influence of fire mosaics, habitat characteristics and cattle disturbance on mammals in fire-prone savanna landscapes of the northern Kimberley. PLoS One 10, e0130721.
| Influence of fire mosaics, habitat characteristics and cattle disturbance on mammals in fire-prone savanna landscapes of the northern Kimberley.Crossref | GoogleScholarGoogle Scholar | 26121581PubMed |
Russell-Smith, J., Ryan, P. G., Klessa, D., Waight, G., and Harwood, R. (1998). Fire regimes, fire-sensitive vegetation and fire management of the sandstone Arnhem Plateau, monsoonal northern Australia. Journal of Applied Ecology 35, 829–846.
| Fire regimes, fire-sensitive vegetation and fire management of the sandstone Arnhem Plateau, monsoonal northern Australia.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Whitehead, P. J., Cook, G. D., and Hoare, J. L. (2003). Response of Eucalyptus-dominated savanna to frequent fires: lessons from Munmarlary, 1973–1996. Ecological Monographs 73, 349–375.
| Response of Eucalyptus-dominated savanna to frequent fires: lessons from Munmarlary, 1973–1996.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Yates, C. P., Brock, C., and Westcott, V. C. (2010). Fire regimes and interval-sensitive vegetation in semi-arid Gregory National Park, northern Australia. Australian Journal of Botany 58, 300–317.
Shine, R. (2010). The ecological impact of invasive cane toads (Bufo Marinus) in Australia. The Quarterly Review of Biology 85, 253–291.
| The ecological impact of invasive cane toads (Bufo Marinus) in Australia.Crossref | GoogleScholarGoogle Scholar | 20919631PubMed |
Smith, J. G., and Phillips, B. L. (2006). Toxic tucker: the potential impact of cane toads on Australian reptiles. Pacific Conservation Biology 12, 40–49.
Storr, G. M., Smith, L. A., and Johnstone, R. E. (1983). ‘Lizards of Western Australia. II. Dragons and Monitors.’ (Western Australian Museum: Perth.)
Storr, G. M., Smith, L. A., and Johnstone, R. E. (1999). ‘Lizards of Western Australia. I. Skinks.’ (Western Australian Museum: Perth.)
Storr, G. M., Smith, L. A., and Johnstone, R. E. (2002). ‘Snakes of Western Australia.’ (Western Australian Museum: Perth.)
Taylor, R. S., Watson, S. J., Bennett, A. F., and Clarke, M. F. (2013). Which fire management strategies benefit biodiversity? A landscape-perspective case study using birds in mallee ecosystems of south-eastern Australia. Biological Conservation 159, 248–256.
| Which fire management strategies benefit biodiversity? A landscape-perspective case study using birds in mallee ecosystems of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Trainor, C. R., and Woinarski, J. C. Z. (1994). Responses of lizards to three experiments fires in the savanna forests of Kakadu National Park. Wildlife Research 21, 131–147.
| Responses of lizards to three experiments fires in the savanna forests of Kakadu National Park.Crossref | GoogleScholarGoogle Scholar |
Trauernicht, C., Murphy, B. P., Tangalin, N., and Bowman, D. M. J. S. (2013). Cultural legacies, fire ecology, and environmental change in the Stone Country of Arnhem Land and Kakadu National Park, Australia. Ecology and Evolution 3, 286–297.
| Cultural legacies, fire ecology, and environmental change in the Stone Country of Arnhem Land and Kakadu National Park, Australia.Crossref | GoogleScholarGoogle Scholar | 23467505PubMed |
Ujvari, B., Shine, R., and Madsen, T. (2011). Detecting the impact of invasive species on native fauna: cane toads (Bufo marinus), frillneck lizards (Chlamydosaurus kingii) and the importance of spatial replication. Austral Ecology 36, 126–130.
| Detecting the impact of invasive species on native fauna: cane toads (Bufo marinus), frillneck lizards (Chlamydosaurus kingii) and the importance of spatial replication.Crossref | GoogleScholarGoogle Scholar |
Williams, R. J., Cook, G. D., Gill, A. M., and Moore, P. H. R. (1999). Fire regime, fire intensity and tree survival in a tropical savanna in northern Australia. Austral Ecology 24, 50–59.
| Fire regime, fire intensity and tree survival in a tropical savanna in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Wilson, S., and Swan, G. (2003). ‘A Complete Guide to Reptiles of Australia.’ (Reed New Holland: Sydney.)
Woinarski, J. C. Z., and Fisher, A. (1995a). Wildlife of lancewood (Acacia shirleyi) thickets and woodlands in northern Australia. 1. Variation in vertebrate species composition across the environmental range occupied by lancewood vegetation in the Northern Territory. Wildlife Research 22, 379–411.
| Wildlife of lancewood (Acacia shirleyi) thickets and woodlands in northern Australia. 1. Variation in vertebrate species composition across the environmental range occupied by lancewood vegetation in the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., and Fisher, A. (1995b). Wildlife of lancewood (Acacia shirleyi) thickets and woodlands in northern Australia. 2. Comparisons with other environments of the region (acacia woodlands, Eucalyptus savanna woodlands and monsoon rainforests). Wildlife Research 22, 413–443.
| Wildlife of lancewood (Acacia shirleyi) thickets and woodlands in northern Australia. 2. Comparisons with other environments of the region (acacia woodlands, Eucalyptus savanna woodlands and monsoon rainforests).Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., and Legge, S. (2013). The impacts of fire on birds in Australia’s tropical savannas. Emu 113, 319–352.
| The impacts of fire on birds in Australia’s tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Risler, J., and Kean, L. (2004). Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecology 29, 156–176.
| Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Armstrong, M., Brennan, K., Fisher, A., Griffiths, A. D., Hill, B., Milne, D., Ward, S., Watson, M., Winderlich, S., and Young, S. (2010). Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildlife Research 37, 116–126.
| Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Legge, S., Fitzsimons, J. A., Traill, B. J., Burbidge, A. A., Fisher, A., Firth, R. S. C., Gordon, I. J., Griffiths, A. D., Johnson, C., McKenzie, N. L., Palmer, C., Radford, I., Rankmore, B., Ritchie, E., Ward, S., and Ziembicki, M. (2011). The disappearing mammal fauna of northern Australia: context, cause, and response. Conservation Letters 4, 192–201.
| The disappearing mammal fauna of northern Australia: context, cause, and response.Crossref | GoogleScholarGoogle Scholar |
Ziembicki, M., Woinarski, J. C. Z., Fisher, A., Burbidge, A. A., Legge, S., Murphy, B., Radford, I., Lawes, M., Webb, J., Perry, J., Johnson, C. N., Reardon, T., Gillespie, G., Griffiths, T., Ritchie, E., Smith, J., Tuft, K., McGregor, H., and Kanowski, J. (2015). Stemming the tide: progress towards resolving the causes of decline and implementing management responses for the disappearing mammal fauna of northern Australia. Therya 6, 169–225.