Drought-driven change in wildlife distribution and numbers: a case study of koalas in south west Queensland
Leonie Seabrook A E , Clive McAlpine A B , Greg Baxter A B , Jonathan Rhodes A B , Adrian Bradley C and Daniel Lunney DA The University of Queensland, Landscape Ecology and Conservation Group, School of Geography, Planning & Environmental Management, Brisbane, Queensland 4072, Australia.
B The University of Queensland, The Ecology Centre, Brisbane, Queensland 4072, Australia.
C The University of Queensland, School of Biomedical Sciences, Brisbane, Queensland 4072, Australia.
D Office of Environment and Heritage NSW, PO Box 1967, Hurstville, New South Wales 2220, Australia and School of Biological Sciences and Biotechnology, Murdoch University, Murdoch, Western Australia 6150, Australia.
E Corresponding author. Email: l.seabrook@uq.edu.au
Wildlife Research 38(6) 509-524 https://doi.org/10.1071/WR11064
Submitted: 1 April 2011 Accepted: 16 August 2011 Published: 11 November 2011
Abstract
Context: Global climate change will lead to increased climate variability, including more frequent drought and heatwaves, in many areas of the world. This will affect the distribution and numbers of wildlife populations. In south-west Queensland, anecdotal reports indicated that a low density but significant koala population had been impacted by drought from 2001–2009, in accord with the predicted effects of climate change.
Aims: The study aimed to compare koala distribution and numbers in south-west Queensland in 2009 with pre-drought estimates from 1995–1997.
Methods: Community surveys and faecal pellet surveys were used to assess koala distribution. Population densities were estimated using the Faecal Standing Crop Method. From these densities, koala abundance in 10 habitat units was interpolated across the study region. Bootstrapping was used to estimate standard error. Climate data and land clearing were examined as possible explanations for changes in koala distribution and numbers between the two time periods.
Key results: Although there was only a minor change in distribution, there was an 80% decline in koala numbers across the study region, from a mean population of 59 000 in 1995 to 11 600 in 2009. Most summers between 2002 and 2007 were hotter and drier than average. Vegetation clearance was greatest in the eastern third of the study region, with the majority of clearing being in mixed eucalypt/acacia ecosystems and vegetation on elevated residuals.
Conclusions: Changes in the area of occupancy and numbers of koalas allowed us to conclude that drought significantly reduced koala populations and that they contracted to critical riparian habitats. Land clearing in the eastern part of the region may reduce the ability of koalas to move between habitats.
Implications: The increase in hotter and drier conditions expected with climate change will adversely affect koala populations in south-west Queensland and may be similar in other wildlife species in arid and semiarid regions. The effect of climate change on trailing edge populations may interact with habitat loss and fragmentation to increase extinction risks. Monitoring wildlife population dynamics at the margins of their geographic ranges will help to manage the impacts of climate change.
Additional keywords: climate variability, distribution, faecal standing crop method, habitat loss, Phascolarctos cinereus.
Introduction
The geographic range of a species is broadly governed by the presence of environmental conditions suitable for that species and its ability to disperse to new areas (Gaston 2009). However, within a geographic range, there is a complex internal structure of distribution, often termed the area of occupancy, which reflects finer scale variations in habitat suitability, local and meta-population dynamics and legacies of past events (Brown et al. 1996; Gaston and Fuller 2009). This fine scale structure leads to considerable variations in the density and numbers of local populations within the area of occupancy (Gaston and Fuller 2009). Changes in environmental conditions, such as climate variability and loss of habitat, affect population dynamics, including dispersal ability, breeding success and mortality rates, and alter population density either temporarily or permanently (Fahrig 2003; Hughes 2003; Parmesan 2006). These changes can occur cyclically, with populations expanding or contracting as environmental conditions fluctuate, but if conditions alter too much, there may be more permanent effects on populations (Thomas et al. 2006; Piessens et al. 2009).
Climate is one of the major determinants of the distribution of species (Caughley et al. 1988). Climate change is affecting species distributions, leading to range shifts and changes in density and numbers within geographic ranges (Parmesan and Yohe 2003; Wilson et al. 2004; Thomas et al. 2006). Species will survive climate change either by adapting or moving to remain within suitable environmental conditions. The physiological capacity of a species to adapt is based on its phenotypic plasticity (the ability of an individual to change its physiological properties or behaviour in response to environmental conditions) or microevolutionary changes over short time spans (Fuller et al. 2010). There is evidence that evolutionary and physiological adaptations are occurring in several species in response to climate change, although there is little evidence that these adaptations will occur rapidly enough to allow occupation of previously unsuitable climatic regions (Parmesan 2006). During the process of climate-induced range shifts, trailing edge populations become increasingly fragmented, while leading edge populations tend to expand along a broad front (Thomas 2010). Some trailing edge populations will survive in refugia where local and regional variations in climate provide favourable conditions (Hampe and Petit 2005). However, many will be vulnerable to local declines arising from climate variability and weather extremes, particularly drought and heatwaves, which may affect a fauna species directly or change habitat quality and resource availability (Adams 2010; Albright et al. 2010). Monitoring changes in distribution and numbers at the limit of a species’ range during conditions that mimic those predicted under climate change scenarios will help identify and manage the effects of climate change (Adams 2010).
Habitat loss and fragmentation are also important drivers of changes in species distribution and numbers (Fahrig 2003; Wiegand et al. 2005). Habitat fragmentation has a range of effects on forest-dependent mammals, including: decreases in habitat amount and quality; lower breeding and dispersal success; and increased risk of mortality due to predation or collisions with motor vehicles (Andrén 1994; Cogger et al. 2003; Fahrig 2003; McAlpine et al. 2006a). Changes in distribution and numbers may lag behind habitat clearance, resulting in an extinction debt (Tilman et al. 1994; Mouquet et al. 2011). The 2006 State of the Environment report for Australia identified that loss of native vegetation continues to be one of the greatest threats to biodiversity (Beeton et al. 2006). The combination of climate change and habitat fragmentation may affect the long-term viability of many species (Travis 2003).
Koalas (Phascolarctos cinereus) are tree-dwelling marsupial folivores, endemic to Australia, which feed almost exclusively on a limited variety of Eucalyptus, Corymbia and Angophora species. They are found across a broad geographic range in eastern and south-east Australia, occurring in the states of Queensland, New South Wales, Victoria and South Australia (Martin and Handasyde 1999). The distribution of the koala is limited by climatic factors, the presence of suitable habitat and the ability to colonise new areas (Martin and Handasyde 1999; Adams-Hosking et al. 2011). Since the European settlement of Australia, changes in distribution have occurred mainly from broad-scale habitat loss, but historical events such as pelt hunting, bushfires and disease have also contributed to local and regional population extinctions over time (Melzer et al. 2000; Phillips 2000). In Queensland, a series of state-wide community surveys indicate that, between 1928 and 1987 when the first and last state-wide surveys were conducted, the range of the koala has progressively contracted to the south and east, with a reduction of ~27% in their extent of occurrence and 31% in the area of occupancy (Gordon et al. 2006). The survey results also show changes in the densities of local koala populations.
The first koala survey in Queensland was carried out in 1928 by the Nature Lovers League to assess the impact of an open hunting season in 1927 for koala pelts (Gordon et al. 2006). Nearly all replies from local councils and Dingo Boards contained the same message, namely that koalas had become scarce or absent in their districts since the open season. In 1967, the Wildlife Preservation Society of Queensland carried out a survey through primary schools across Queensland, asking about current and historic sightings of koalas (Kikkawa and Walter 1968). Results pointed to a range contraction from the north and west. Koala deaths were attributed mainly to bushfires and disease, with some mention of past hunting (Kikkawa and Walter 1968). Campbell et al. (1979) reported on a second survey carried out in 1977 by the Wildlife Preservation Society of Queensland. This survey found that although the distribution did not appear to have changed significantly since 1967, many districts reported lower numbers of koalas. Patterson (1996) compared koala distribution from a 1986–1988 survey with the 1967 and 1977 surveys. Koala sightings showed a marked range contraction from the west in the southern half of Queensland and some contraction to the south (Patterson 1996). The contraction was attributed primarily to habitat loss, fragmentation and the associated decline in habitat quality.
Within the koalas’ broad range, their area of occupation and densities are patchy and depend on the presence of favoured tree species and fertile soils with higher levels of soil nutrients and soil moisture (Bryan 1997; Sullivan et al. 2003a). Population densities vary from 9 per ha in parts of Victoria to less than 0.001 per ha in inland Queensland (Melzer et al. 2000). In Queensland, densities vary from 0.02–2 koalas per ha in Springsure (Gordon et al. 1990; Melzer and Lamb 1994), to 0.6–1.6 per ha in Oakey (Gordon et al. 1990), and 0.4 koala per ha in coastal Redlands Shire (White and Kunst 1990).
Local koala populations face several threats, including loss and fragmentation of habitat (Melzer et al. 2000; McAlpine et al. 2006a; McAlpine et al. 2006b), car strikes and dog attacks (Dique et al. 2003; Lunney et al. 2007), and disease, which can lead either to death or infertility (Gordon et al. 1990; Hanger and Loader 2009). Koalas are susceptible to climatic extremes, particularly heatwaves and droughts, which also affect the quality of nutrients and moisture available in their diet (Cork and Braithwaite 1996; Moore and Foley 2000). There is evidence that koalas can modify their behaviour and physiology to increase water intake if necessary (Krockenberger 2003; Ellis et al. 2010), but climatic stress on trees can lead to widespread leaf fall and subsequent crashes in koala populations (Gordon et al. 1988; Gordon et al. 1990). For example, Gordon et al. (1990) found, in northern and western Queensland, that the variability in rainfall and its effect on food sources was probably the most important factor affecting koala populations, although in the eastern region it was high infertility and prevalence of cystitis.
In the mid 1990s, Sullivan et al. (2003a, 2004) conducted the first broad-scale study of koala distribution and abundance in the Mulgalands bioregion of south-west Queensland. They estimated that the total population ranged from 44 593 to 77 567. Densities varied across the region from 0.0007 to 2.5 per ha, with the highest densities occurring in riverine and residual habitats in the eastern Mulgalands. Since that study was completed, south-west Queensland suffered a severe and prolonged drought (Hughes 2003; Beeton et al. 2006) and anecdotal evidence suggested that regional koala numbers had declined.
This study aimed to update our understanding of the distribution and numbers of koalas in south-west Queensland and compare the results with those of the study by Sullivan et al. (2003a, 2004) Several years of severe drought provided conditions similar to those predicted by climate change scenarios, and an opportunity to assess the likely impacts of future climate change on koala populations. We identified koala distribution using a community survey and faecal pellet survey. A Faecal Standing Crop Method was used to estimate population densities. From these densities, the numbers of koalas in 10 habitat units were interpolated across the study region. We examined climate data and the area of vegetation clearing as possible explanations for changes in koala distribution and numbers between the two studies.
Materials and methods
Study area
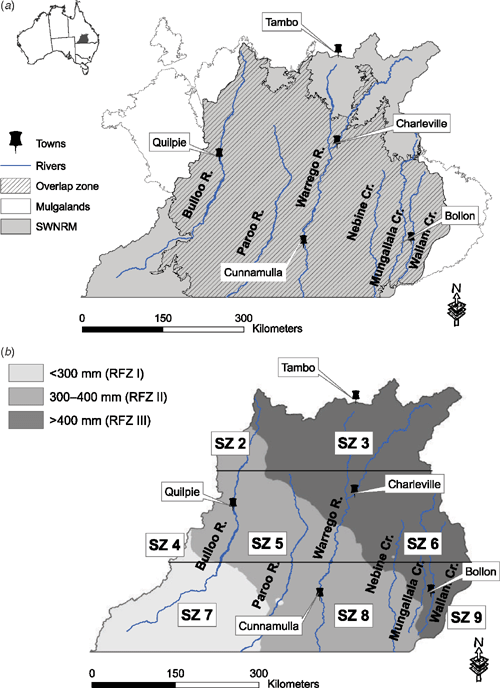
The study area was the South West Natural Resource Management (SWNRM) region, covering 187 000 km2 of southern inland Queensland, Australia (Fig. 1a). Average annual rainfall ranges between 250 mm in the south-west and 550 mm in the north-east (Fig. 1b). The mean daily maximum and minimum temperatures for Charleville, the region’s largest town, are 28°C and 13.5°C respectively. Elevation varies from 580 m above sea level in the north-east to 100 m above sea level in the south. Regional drainage patterns are from north to south, with the Warrego and Paroo Rivers along with the Nebine, Wallam and Mungallala Creeks forming part of the Murray–Darling Basin (Sattler and Williams 1999). The Bulloo River in the west of the region has an internal drainage basin. The dominant landforms are plains with generally sandy, infertile soils. The major plains vegetation communities comprise mulga (Acacia aneura) with poplar box (Eucalyptus populnea) and other eucalypt species co-dominant in the higher rainfall areas to the east and north (Sattler and Williams 1999). Separating the plains are low residual ranges and plateaus, with shallow red earth soils. Residual vegetation has some eucalypt communities of Thozet’s box (E. thozetiana) or grey box (E. microcarpa), but it is dominated by Acacia species (Table 1). There are significant floodplains, dominated by poplar box and coolabah (E. coolabah). Riparian vegetation, comprising river red gum (E. camaldulensis) and coolabah occurs as narrow strips along waterways. Land use in the region is predominantly cattle grazing. Although the SWNRM region does not correspond exactly with the Mulgalands bioregion used in Sullivan’s study (Sullivan et al. 2002), the area common to both (the overlap zone) covers 145 537 km2, or 78% of the Mulgalands bioregion (Fig. 1a).
|

|
Koala distribution
We used a combination of two independent survey designs – a community survey and faecal pellet survey – to assess the distribution and numbers of koalas in south-west Queensland.
Community surveys
Community surveys are an effective way to obtain presence or absence data relatively cheaply and to provide access to private land (Lunney et al. 2009). Community surveys have been used to obtain locations across wide areas for several easily recognisable species, including spotted-tailed quolls (Dasyurus maculatus) (Lunney and Matthews 2001), platypus (Ornithorhynchus anatinus) (Otley 2001), and bandicoots (Isoodon macrourus) (FitzGibbon and Jones 2006). National and state-wide assessments of koala distribution have all used surveys targeting sections of the community (Kikkawa and Walter 1968; Campbell et al. 1979; Phillips 1990; Reed et al. 1990; Patterson 1996; Lunney et al. 2009). Surveys are useful at identifying outlying records where animals are found on a transient basis (Lunney and Matthews 2001). Koalas are difficult to spot in the wild, but there is no confusion with other species, and koalas are popular with people in the local community, making community surveys a valuable and repeatable source of information about this iconic species.
A community survey was distributed as unaddressed mail to 680 rural landholders in the SWNRM region. Respondents were asked whether koalas occurred in their area, when koalas had last been sighted, and whether they thought koala numbers were changing. They were provided with a map of the region and asked to mark their sightings. Questions were also included on other species including common brushtail possums (Trichosurus vulpecula) which use similar habitat, dingos or wild dogs (Canis lupus) and foxes (Vulpes vulpes). Commonly sighted species have been used in previous surveys to assess where koalas were either genuinely not present or were not noticed (Lunney et al. 2009). We did not follow up the mail survey to increase the rate of return. Although the survey was not sent to people living in towns, there was media coverage of the research project including contact details of the research team.
Faecal pellet surveys
Identifying species presence can be done through direct observation, but in many cases indirect signs such as scats or tracks are used (Wilson and Delahay 2001; Telfer et al. 2006). Surveys that count koalas through direct observation take many person hours and, although they are used in the more densely populated east coast koala populations and in smaller study areas (Gordon et al. 1990; Melzer and Lamb 1996; Dique et al. 2004), they are not practical for large areas with low-density populations (Sullivan et al. 2002). Faecal pellet surveys are a well-established method of determining koala presence, and thus distribution and habitat selection (Phillips and Callaghan 2000; McAlpine et al. 2006b). Koala faecal pellets are relatively long-lasting and easily distinguishable from the pellets of other mammals in the region, such as goats (Capra hircus) or common brushtail possums, either by external and internal appearance, or smell. There are consequences in using indirect signs for detecting species occupancy of sites, including false absences if signs decay rapidly or false positives if pellets last many months (Rhodes et al. 2011). The issue of false absences was addressed through field-ageing trials of pellets during the survey period. The issue of false positives could not be determined during one-off surveys, but in many survey sites there had been a significant rain and flooding event at the start of 2009 (five months before the start of field surveys), which would have washed away older pellets. We acknowledge that the absence of pellets does not necessarily equate to the absence of koalas. However, both Sullivan (2000) and Munks et al. (1996) found that direct observation was not practical for the low-density and patchily distributed koalas of western Queensland because of the length of time needed to carry out reliable counts. In addition, Sullivan (2000) found that there was only a slight underestimate of koala density when using faecal pellet surveys rather than direct observation.
The presence of fresh faecal pellets allowed an estimate to be made of koala densities and numbers using a Faecal Standing Crop Method (FSCM) (Sullivan et al. 2002; Sullivan et al. 2004). FSCM has been used widely for estimating the density and numbers of species such as deer (Cervus elephus and Capreolus capreolus), elephant (Loxodonta africana) and kangaroos (Macropodid spp.) (Johnson and Jarman 1987; Latham et al. 1996; Mayle 1996; Barnes 2001; Bennett et al. 2005). It was adapted for use with koalas in the Mulgalands by Sullivan et al. (2002). Provided that pellets can be reliably aged, and the daily production rate is known, the FSCM is a useful tool for assessing the density of a cryptic and wide spread species, such as the koala (Sullivan et al. 2004).
We followed a similar sampling strategy to Sullivan et al. (2002, 2003a, 2003b). Vegetation communities were classified into 10 habitat units based on landform and dominant vegetation (Table 1). We used the Regional Ecosystem mapping Version 5 supplied by the Queensland Herbarium (2005). The region was also stratified into eight sampling zones based on differences in rainfall on an east–west gradient, and in latitude on the north–south gradient, following Sullivan et al. (2003b) (Fig. 1b). The difference in study regions between this work and that of Sullivan meant that none of Sullivan’s sample zone 1 overlapped with the SWNRM region. We did not survey sites in the extreme south-west of the region because both Sullivan et al. (2004) and the community survey indicated that no koalas had been present for many decades. In addition, surveys in the southern part of Sample Zone 8 (SZ 8) (Fig. 1b), close to the New South Wales border, were prevented by heatwaves in December 2009 followed by flooding in January–March 2010. Evidence from the community survey and personal communication with Queensland Parks and Wildlife rangers in Charleville and Culgoa Flood Plain National Park indicate that koalas are seldom present in this area, and the lack of faecal pellet surveys did not, in our view, make a significant difference to the population estimates.
Based on the findings of Gordon et al. (1988) and Munks et al. (1996), koalas in semiarid regions are more commonly found in riverine habitat (habitat unit 1), although they do utilise other habitat for both feeding and resting (Witt and Pahl 1994; Melzer and Lamb 1996; Ellis et al. 2002). In the Mulgalands, Sullivan et al. (2004) found the highest densities to be in riverine habitats, although residual landforms also formed important habitat in the north (Sullivan et al. 2003a; Sullivan et al. 2003b). Furthermore, Rhodes et al. (2006) recommended surveying in high-quality habitat if declines in population are suspected because the higher densities of individuals living there allow a greater chance of detecting declines. For these reasons, we sampled more sites in riverine than in other habitats. Within each habitat unit, between 30 and 50 field sites, at least 3 km apart, were randomly generated using Hawth’s Analysis Tools 3.26 (http://www.spatialecology.com/htools/) in ArcGIS 9.3 (www.esri.com). In habitat unit 1 (riverine habitat with river red gums present), 100 sites were generated. The distribution of sites was examined and where possible two to three sites were chosen in a locality or on a single property to maximise the efficiency of the time taken on field surveys. In total, we surveyed 200 sites in the SWNRM region in south-west Queensland for faecal pellets between May and November 2009.
The faecal-pellet survey method incorporated elements from the spot-assessment technique of Phillips and Callaghan (2000) and from Sullivan et al. (2002). A central tree closest to the GIS-generated point was flagged and it, plus the closest 29 Eucalyptus, Corymbia or Angophora trees with a DBH (diameter at breast height over bark) greater than 10 cm, formed a site. Site characteristics, including tree species, height and DBH were recorded. The area of the site was calculated by measuring the radius of the circle of trees forming the site. Following the experience of Sullivan et al. (2002), searches for faecal pellets were carried out using two methods. First, under all species except river red gums, a basal search in a radius of 1 m around the trunk of the tree was conducted. If koala pellets were detected in the basal search, then a second extended search using 1 m quadrats was carried out under the canopy in order that a density could be calculated. During quadrat searches, the area of one-quarter of the canopy was searched, using up to six quadrats. For example, if one-quarter of the canopy was estimated at 3 m2, then three quadrats were randomly placed under the canopy. For river red gums, all pellet searches were done using quadrats. The growth form of riverine trees, particularly river red gum, was often multi-stemmed with spreading branches and an asymmetrical canopy, and there was a greater chance of false absences under these trees if only a basal search was used (Sullivan et al. 2002). Since riverine habitats support the greatest densities of koalas, it was important to reduce the possibility of under-estimating densities in these habitats by falsely recording pellets as absent under a tree.
Koala density calculations
For the calculation of density, and hence koala abundance, we used the Faecal Standing Crop Method (Sullivan et al. 2002; Sullivan et al. 2004). The FSCM requires three types of data: the number of pellets present, how many pellets are produced daily, and the age of the pellets. The number of pellets was estimated by counting the fresh pellets found in the quadrat search, adjusting this by a visibility correction factor taken from Sullivan et al. (2004), which was based on the percentage of ground cover in the quadrats, and multiplying the adjusted number of pellets by the canopy area of the tree to give an estimated total pellet count. Daily pellet production was based on the findings of Sullivan et al. (2004), and ranged from 138.2–163.3 pellets. The maximum pellet age was estimated from field trials and was based on external appearance and internal odour. Fresh koala pellets have a characteristic sheen and a greenish colour, which disappears after 1–3 days depending on weather conditions. They also have a strong smell of eucalyptus oils when broken open. Over a period of days or weeks, depending on weather conditions, the characteristic eucalyptus smell fades. To assess the age at which pellets lost their internal odour of eucalyptus oils, fresh pellets were stored in a domestic refrigerator and three to four were placed outside at the base of a eucalypt trunk at 3-day intervals by an employee of SWNRM in Charleville (Sullivan et al. 2002). The age at which the smell faded was used to assign an age range to the fresh pellets found in field surveys. This included a margin for error of 4–5 days, so a pellet that lost its internal odour at 10 days might be allocated a minimum age of 7 days and a maximum age of 12 days.
Koala density was calculated for each tree at a site by applying the equation:
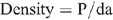
Where P = estimated total pellet count, d = daily pellet production and a = maximum pellet age (Sullivan et al. 2004). Site densities were calculated by summing the pellet count under all trees in the site.
Estimating koala numbers
The number of koalas was calculated for each habitat unit within each of the eight sampling zones where sites with fresh pellets were located, using an area weighted average interpolated from the site densities. To enable a more accurate comparison with the population estimates of Sullivan et al. (2004), the calculation of koala numbers was limited to the area of the overlap zone between the SWNRM region and the Mulgalands Bioregion (Fig. 1a). Following Sullivan et al. (2004), we used an inverse weighted distance (IWD) method in the ArcGIS Spatial Analyst tool to interpolate a density surface from the sites, using a power of 2, which gives greater weight to nearby sites. If only one site in a habitat unit and sample zone contained fresh pellets, then the IWD surface was generated for a distance of 50 km around that site. We generated a minimum, mean and maximum IDW surface for the habitat units in each sample zone. The density surfaces were then reclassified into 20 classes using Jenks Natural Breaks option to give an attribute table that could be multiplied by the area of the habitat units (Sullivan et al. 2004). Each habitat unit was converted from polygons into a raster grid, with a cell size of 50 m, based on the percentage cover of the different regional ecosystems making up the habitat unit. This step gave the most accurate measure of the area covered by each habitat unit because one regional ecosystem could contain a mixture of two to three different habitat units. We then calculated the area of each reclassified density surface in the habitat unit in which the sites occurred. Finally, the reclassified IDW surface was then converted back to density classes and multiplied by the tabulated area of the habitat unit to estimate the number of koalas for that habitat unit within a particular sample zone.
To obtain a measure of the accuracy of our parameter estimates, we used a bootstrap method to estimate a standard error of koala abundance (Efron and Tibshirani 1986; Sullivan et al. 2004). For each site with fresh pellets, 500 bootstrap estimates were generated for the total pellet count after adjusting for visibility. Fifty estimates were selected at random for each site and the equation to calculate site densities shown above was applied. Minimum, mean and maximum IWD surfaces were generated, values reclassified, and areas tabulated as described above and in Sullivan et al. (2004). In two habitat units, our estimate of koala numbers was less than 100 koalas, so we did not generate bootstrap estimates because the difference in total numbers would have been minimal. Once numbers had been calculated for the 50 bootstrap estimates, the standard error of the means were calculated together with the 95% confidence interval.
Weather conditions and land clearing
We examined historical climate data for the region and estimated the area of land clearing to assess whether either or both of these factors might have contributed to a change in koala populations between 1995 and 2009. Although we cannot unequivocally link climatic conditions or land clearing with changes in koala populations, there is evidence from previous work that allows us to assign their effects to our findings (Gordon et al. 1988; Cogger et al. 2003; McAlpine et al. 2006b; Ellis et al. 2010).
Anecdotal evidence from several landholders mentioned the local disappearance of koalas during the severe drought of 2002–2007, with 2002 and 2006 being mentioned in particular. Evidence from previous studies indicates that hot days and low rainfall can have severe impacts upon koala populations, either directly by causing physiological stress or indirectly by affecting the nutrient and water content in eucalypt leaves (Gordon et al. 1988; Clifton et al. 2007; Ellis et al. 2010). We examined climate data from the Bureau of Meteorology for the major towns across the region from 1990 to 2009 to see if there had been significant changes in climatic conditions. We totalled the amount of summer rainfall in the six hottest months (1 October to 31 March), and counted the number of days over 40°C over the same period. This temperature threshold was chosen for several reasons. In their study of koala metabolism and heat balance, Degabriele and Dawson (1979) stated that the body temperature, respiratory rate and evaporative water loss of koalas rose rapidly when air temperatures were above 30°C. Bioclimatic modelling conducted by Adams-Hosking et al. (2011) identified that koalas occur in areas with maximum summer temperatures of under 37.7°C. However, Ellis et al. (2010) found that, in central Queensland, the temperatures in trees used by koalas for shelter in the summer could be around 2°C cooler than ambient air temperature, thereby moderating the effect on thermoregulation. We accordingly picked 40°C as an air temperature that would result in significant physiological stress for koalas.
Another major cause of koala population decline is land clearing and habitat fragmentation (Cogger et al. 2003; Sullivan et al. 2003b; McAlpine et al. 2006b). The Statewide Landcover and Trees Study (SLATS) uses Landsat Thematic Mapper satellite images to assess changes in woody vegetation over a given time period (for methods see DNRW 2008). We used data from SLATS for the years from 1995–2008 to quantify woody vegetation clearance in the different habitat units. The data were in the form of a GIS point file, with each point showing the rate of clearing per annum for the 25 m2 Landsat pixel where woody vegetation had been cleared during the relevant time period. A spatial join was used to combine data from the SLATS point files with the habitat unit polygons derived from remnant regional ecosystem mapping (Queensland Herbarium 2005). Where SLATS points occurred in areas that were classified as cleared in the remnant regional ecosystem mapping, the pre-clearing regional ecosystem data were used to assign a habitat unit to that point. The area cleared in hectares was multiplied by the percentage of each habitat unit of that point, and the totals were summed to get the total area of clearing by habitat unit since 1995.
Results
Distribution of koalas from community surveys and field surveys
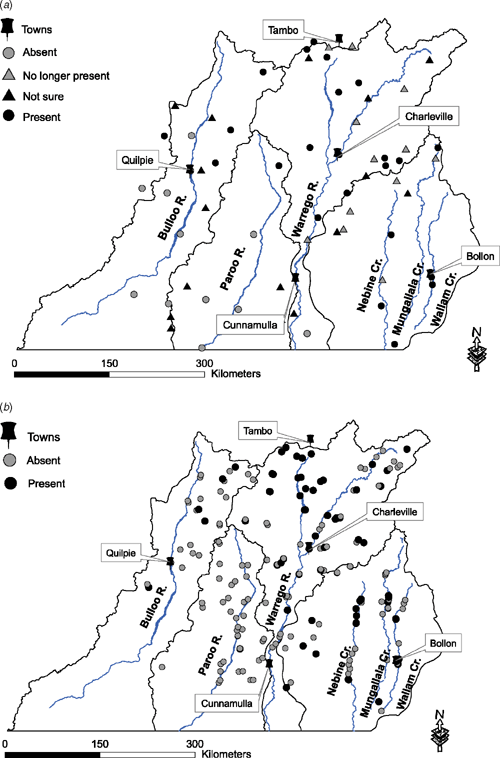
A total of 63 community surveys were returned (9.2%). The distribution of records of koala occurrence was evenly spread across the study area (Fig. 2a) and broadly agreed with the results of the faecal pellet surveys. Due to the low number of returns, the community survey was used only to map distribution according to the reported sightings, but not analysed further.
|
The distribution of koalas shown by the field survey was similar to the community survey (Fig. 2b). The most densely occupied area was between Charleville and Tambo, where all the sites had some signs of koala presence. There was a patchier occurrence elsewhere in the north and eastern sections of the region. Koala pellets were found at only one site in the south-west of the region (Fig. 2b).
Nearly half of the field sites in habitat units 1, 2 and 9 had faecal pellets present, with fresh pellets present at 70% of these sites (Table 2). No pellets were found in habitat units 3, 4, and 8, although the number of sites surveyed in these habitat units were limited due to difficulties accessing them in the field or because there were not enough trees of the genera Eucalyptus, Corymbia or Angophora present at the site. The difference between the number of sites with fresh pellets in the SWNRM region (48) and those in the overlap region (27) (Table 2) was due to the densely occupied sites between Charleville and Tambo on the Ward River falling outside the overlap zone with the Mulgalands Bioregion (Fig. 2b).
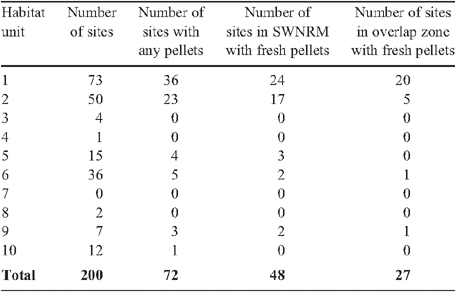
|
Estimated koala density and numbers
In 2009, koala density estimates varied from 0.002 to 1.85 koalas per ha. Average densities in 2009 in Rainfall Zone 3 in the north-eastern part of the region were 1.3 ± 0.03 and in Rainfall Zone 2 were 0.04 ± 0.02 (see Fig. 1b for Rainfall Zone locations). Sullivan et al. (2004) found that koala densities varied across the region from 0.0007–2.5 per ha with average densities in Rainfall Zone 3 being 1.8 ± 0.06 and in Rainfall Zone 2 being 0.02 ± 0.01
The bootstrap estimates of total koala numbers in the overlap zone ranged from 9843 to 13 430 (±95% confidence interval), with the mean of 11 634 (±904 standard error) (Table 3). The overall decline compared with the numbers estimated by Sullivan et al. (2004) in 1995 was 80% (Table 3).
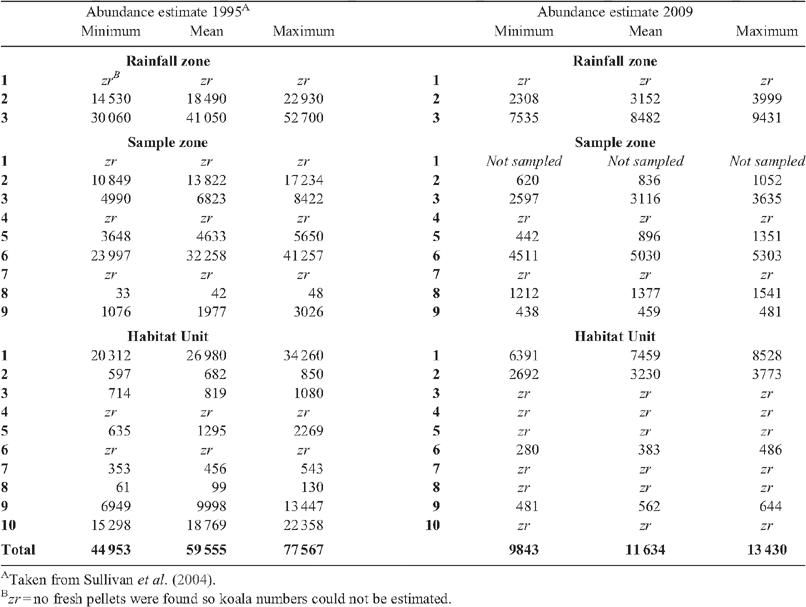
|
Weather conditions and area of land cleared
Weather records collected by the Bureau of Meteorology show that, between 1989 and 2009, the average number of days over 40°C over the summer months (October–March) was only slightly higher than the long-term average since 1950. However, between 2002 and 2007, Charleville, Bollon and Tambo had more than double the average number of hot days, with a peak across most of the region in 2006 (Fig. 3). There was also a much lower-than-average annual rainfall, particularly in 2002–2003 (Fig. 3). The combination of an above-average number of hot days and low summer rainfall was most evident in Charleville and Bollon between 2002 and 2006 (see Fig. 1a for locations). In Quilpie and Cunnamulla, the climate is both hotter and drier than further east (Fig. 3). These conditions are probably close to the climatic limits of the koala, and would contribute to determining the western boundary of its range. Koalas were uncommon near these towns, although there was one site with old faecal pellets south-west of Quilpie (Fig. 2b).
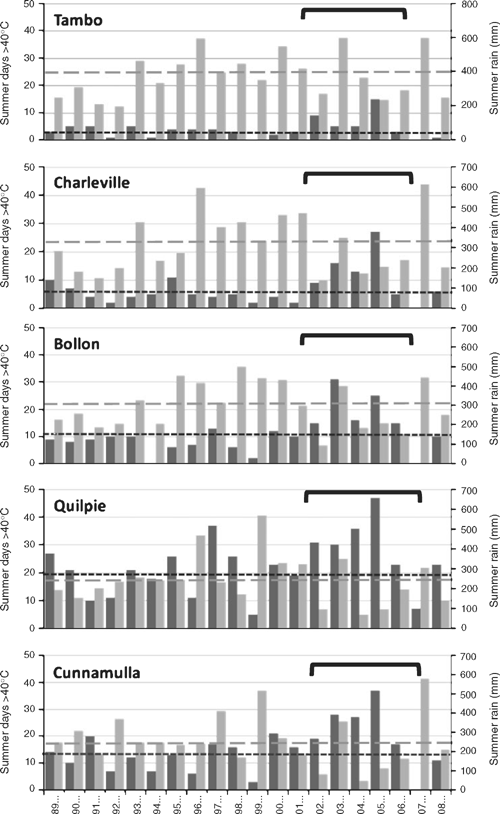
|
The greatest amount of clearing had occurred in the eastern third of the region, with a trend of increasing clearing towards the south. Across all habitat units, 11.72% of clearing was in SZ 3, 18.17% in SZ 6 and 22.6% in SZ 9 (Table 4 and see Fig. 1 for zone locations). The habitat units with the greatest percentage of clearing were HU 5 (20.2%) (poplar box and silver-leaved ironbark: E. melanophloia) and HU 9 (16%) (Thozet’s box and Dawson gum: E. cambageana). Sullivan et al. (2003a) found Thozet’s box and poplar box formed an important part of the diet of koalas in south-west Queensland, and these levels of clearing may have contributed to the decline in koala population size between 1995 and 2009 (Table 3) through loss of habitat and by limiting the ability of koalas to disperse safely away from creek lines.
Discussion
The effect of climate change on the trailing edge of species distribution is predicted to lead to increasingly fragmented populations, some of which may survive in small refugia due to local and regional variations in climate but many of which will face local extinction from extreme events (Parmesan 2006; Thomas et al. 2006; Gaston and Fuller 2009). Trailing-edge populations have been shown to be critical to the long-term survival of species because they may contain individuals that can adapt to changing climatic conditions (Wilson et al. 2004; Hampe and Petit 2005; Thomas et al. 2006). This project assessed the distribution and numbers of koalas in south-west Queensland following a severe drought from 2001 to 2007 and compared the results with a previous study carried out in the mid 1990s. Koalas in this region are at the western limits of their geographic range, and form a trailing-edge population. There was a marked decline in the area of occupancy and in population size since the mid 1990s, although their extent of occurrence, e.g. the geographic range, contracted only slightly. Their area of occupancy contracted mainly to the most optimal habitat along creeks and rivers, with little use of habitat away from water courses. The decline coincided with a period of 4–5 years where there was a combination of low summer rainfall and an above average number of very hot days, which mimic the predicted direction of climate change in the region and are known to affect koala survival (Gordon et al. 1988; Gordon et al. 1990; Ellis et al. 2010). The changes in distribution (both the extent of occurrence and the area of occupancy) reflect the koalas’ response to alterations in their environment and ability to disperse in south-west Queensland.
Extreme events, such as drought and increased temperature, can affect mortality rates, breeding success and dispersal of wildlife. For example, Adams (2010) found that higher temperatures, and particularly severe drought, significantly reduced reproductive output in bats in western North America. In Australia, one day over 42°C killed thousands of flying foxes (Pteropus spp), with greater mortality among young and adult females (Welbergen et al. 2008). Bolger et al. (2005) found that drought years resulted in low food availability for insectivorous passerine birds in California, leading to very low breeding success rates. Albright et al. (2010) modelled the impacts of drought on North American birds and found that abundance, richness and species composition of avian communities were affected in different ways and at different magnitudes, with the greatest negative impacts on species in the semiarid Great Plains. They also suggested that there could be stronger effects for prolonged or more frequent droughts (Albright et al. 2010). Our study supports these effects. We found the most likely causes of the decline in numbers and area of occupancy of the koala in south-west Queensland were climate extremes, particularly the combination of low summer rainfall and very hot days, i.e. heatwaves. Reports from landholders, based on the community survey and subsequent discussions, allowed the view to be formed that local disappearances of koalas occurred around 2002–2003 across most of the region, and around 2006 in the north-east of the region. Although we cannot unequivocally state that the drought caused the koala population declines, increased mortality due to low rainfall and/or heatwaves has been found in other koala research. In the very hot, dry summer of 1979–1980, 63% of koalas died along on the Mungallala Creek in the eastern part of the Mulgalands (Gordon et al. 1988). Over this period, the nearby town of Bollon recorded 31 days over 40°C and summer rainfall of 154 mm (see Fig. 3 for comparison with recent weather records). A resurvey of sites in Springsure, central Queensland, in 2009 found that, since 1995, koalas had disappeared at two out of four sites, and densities were much decreased in the other two (Ellis et al. 2010). Similarly, Lunney et al. (in press) estimated that ~25% of koala population around Gunnedah in north-west New South Wales perished in November–December 2009 during the heatwaves in a long-running drought. At a larger scale, Gordon et al. (1990) found that in northern and western Queensland variability in rainfall, and its effect on food sources, was probably the most important factor affecting koala populations. Adams-Hosking et al. (2011) modelled a significant eastward contraction in the koala’s distribution in Queensland and New South Wales under projected climate change scenarios. They concluded that in arid and semiarid regions, such as south-west Queensland, climate change is likely to compound the impacts of habitat loss, resulting in significant contractions in the distribution of this species. Our study provides empirical evidence supporting this prediction.
Understanding the impacts of climate change on species will be of great importance in biodiversity conservation over the coming decades (Parmesan 2006). The ability of species to adapt to climate change will depend on several factors, including: (i) dispersal ability; (ii) phenotypic plasticity; (iii) evolutionary adaptability; and (iv) physiological tolerance (Williams et al. 2008). Range shifts in response to higher temperatures have been seen in several species, generally either polewards or to higher elevations (Tidemann 1999; Hughes 2003; Thomas et al. 2006; Thuiller et al. 2008; Gibson et al. 2009; Thomas 2010). Some species are showing signs of physiological adaptation to climate change, either through phenotypic plasticity or behavioural change (Parmesan 2006; Fuller et al. 2010). For example, migratory species are changing their departure and arrival times (Green and Pickering 2002; Gordo et al. 2005) and breeding seasons are commencing earlier (Crick et al. 1997).
Thermal tolerance has been identified as one of the key limitations for physiological adaptation to climate change (Fuller et al. 2010). There is evidence that koalas can adapt their behaviour and physiology to deal with heat to some extent (Krockenberger 2003; Ellis et al. 2010). However, Clifton et al. (2007) found that in coastal communities, the physiological tolerance of koalas seemed be affected by hot night-time temperatures and humidity, which reduced evaporative heat loss. Bioclimatic modelling carried out by Adams-Hosking et al. (2011) indicates that the maximum summer temperatures that are physiologically acceptable for koalas are ~37.7°C. This is supported by Degabriele and Dawson (1979) who found that evaporative water loss in koalas more than doubled and the respiratory rate rose from a mean of 25.9 to 231.3 breaths per minute when air temperatures increased from 30°C to 40°C. If these temperatures are exceeded on a regular basis, physiological stress will lead to increased koala mortality. Thus, this study raises significant implications for the effect of climate change for koalas in south-west Queensland, and for other wildlife in the region. In Australia, the mean annual temperature has increased by 0.9°C since 1901 (Hennessy et al. 2008). Since 1950, Queensland has the greatest rise in mean annual temperature in Australia (1.2°C) and the greatest decline in total annual rainfall (–107 mm). By 2040, exceptionally hot years are predicted to occur every 2 years, affecting 60% of the state (Hennessy et al. 2008). Climate change predictions from the Queensland Government report that, in Charleville, the number of days over 35°C will nearly double to 130 days per annum by 2070, and while there is not a consensus on whether average annual rainfall will increase or decrease, the majority of predictions point to a decrease in rainfall, with increased variability and the likelihood of more frequent droughts (DERM 2009). The drought and exceptionally hot years that occurred between 2001 and 2007 mimicked these climate change predictions.
Climate change will also affect terrestrial vegetation and thus habitat quality and resource availability for many species (Hughes 2003). Changes in foliar chemistry due to elevated CO2 are predicted to significantly affect arboreal folivores, including koalas (Kanowski 2001; Hughes 2003; Moore et al. 2004; Lunney et al. in press). For example, DeGabriel et al. (2009) found that the reproductive success of common brushtail possums was reduced when leaves contained a high tannin content, which lowers the nitrogen available to folivores. Lawler et al. (1997) found higher CO2 levels affected the carbon : nitrogen ratio in forest red gum (Eucalyptus tereticornis), which in turn increased levels of plant secondary metabolites (PSMs). Herbivorous beetle larvae feeding on these leaves showed increased mortality rates and reduced body size. Higher CO2 levels also decreased the amount of nitrogen allocated to leaf protein and increased that allocated to the production of toxic plant defences, reducing the nutritional quality of leaves (Gleadow et al. 1998). Both studies found the effects enhanced when nitrogen availability was limited. In addition to changes in leaf chemistry, if drought conditions are severe enough, particularly when combined with extremely hot days, leaf fall and tree die back can lead to population crashes, such as those seen at Mungallala Creek and Springsure (Gordon et al. 1988; Ellis et al. 2010).
Habitat loss and fragmentation are also important drivers of changes in species distribution and numbers (Fahrig 2003; Wiegand et al. 2005). Habitat loss and fragmentation were identified as one of the main causes of extinction of woodland birds in Australia, related to poor dispersal to isolated patches, increasing nest predation, changes in habitat quality and resource availability, and increasing interspecific completion from aggressive bird species (Ford 2011). Brown et al. (2008) found that reptiles in Victoria, Australia, had declined significantly with loss of habitat and changes in woodland structure, with declines in species richness even in remaining large patches. For howler monkeys in the Central and South American Neotropics, loss of habitat was more important than fragmentation on distribution and abundance, probably because howler monkeys are highly mobile and resistant to initial habitat disturbance (Arroyo-Rodriguez and Dias 2010). Loss of habitat has been identified as one of the most significant threats to koalas in New South Wales (Reed and Lunney 1990) and south-east Queensland (Seabrook et al. 2003; McAlpine et al. 2006b). Although in Queensland, broad-scale clearing of remnant vegetation has been regulated since 2006, some clearing is still permitted for drought fodder, infrastructure and urban development, and non-remnant vegetation continues to be cleared (McGrath 2004;/2005). In a study of koalas in south-east Queensland, the total area of forest, larger forest patches and smaller distances between patches were important predictors of koala presence (McAlpine et al. 2006b). Land clearance and habitat fragmentation are likely to have affected koala distribution and numbers, particularly in Sample Zone (SZ) 6 and 9 in the east of the region (Table 3 and see Fig. 1 for locations). Between 1995 and 2008, SZ 9 lost 28% of high quality river red gum and coolabah habitats to clearing, and nearly 25% of poplar box habitat, while SZ 6 lost 15% of riverine and coolabah vegetation and 21% of poplar box habitat. The contraction of koalas across the region to riverine habitats suggests that animals had vacated less-favourable poplar box habitats over the drought, but the clearing of key riverine habitat would almost certainly have affected the number of koalas.
Management of trailing-edge populations during climate change will be challenging because, even if greenhouse gases are significantly reduced, there are likely to be lag effects on species. There are already delays between habitat clearance and loss of species, leading to an extinction debt (Tilman et al. 1994; Mouquet et al. 2011), and the extinction debt may contribute to the finding of Anderson et al. (2009) that changes in trailing-edge populations happen more slowly than those in leading-edge expansion. The interaction of habitat loss, fragmentation, and climate extremes will increase pressure on some populations and species, particularly in isolated trailing-edge populations where dispersal ability is limited (Travis 2003; Thomas et al. 2004). For instance, Piessens et al. (2009) found that for populations of Cupido minimus, a specialist herbivore butterfly, extinction probabilities were significantly higher for small populations in fragmented habitat during and after the hot summer in 2003 in Europe. Williams et al. (2008) suggested that the first step towards conserving species in a changing climate is to understand the relative vulnerability of the species and implement known conservation techniques in potential refuge areas, including increasing habitat amount and connectivity. However, we also need to adopt a dynamic and proactive approach to conservation to deal with spatial changes in species distribution and ecosystem components (Williams et al. 2008). For many trailing-edge populations, variations in local microclimates may make the difference between survival and extinction (Fuller et al. 2010). For example, research on European mussels indicates that populations with low genetic diversity at the southern or trailing edge of their range are less adapted to heat stress than central populations (Pearson et al. 2009). However, cold upwelling explained the presence of relict populations of barnacles in Europe, indicating that microclimatic influences can override broad climate change (Wethey and Woodin 2008). Local microclimatic variations may explain occasional koala sightings in the Grey Ranges in the south-west of the study area. Here, the local climate and habitat could be sufficiently different from those along the Bulloo River to allow koalas to persist. Further surveys would be required in this region to offer a more definitive explanation.
For koalas in south-west Queensland, the identification of potential refugia through continued monitoring of population change at the edges of the range will allow us to assess which local populations are resilient to climate change, and therefore where to concentrate management actions. In 2009, koalas were found almost exclusively along creek lines where river red gum and coolabah were present. There was little evidence of koalas at high densities in residual landscapes, as found by Sullivan et al. (2004) in the mid 1990s. This demonstrates that riverine habitats are critical refugia in the region and their role as core habitat will become more important as climate change leads to a greater incidence of hot, dry conditions. This finding agrees with other research in semiarid western Queensland. Along Mungallala Creek in the south-east of the SWNRM region, Gordon et al. (1988) found that koalas survived in riparian vegetation and died in more marginal habitats away from the creek during a very hot dry summer. In the Desert Uplands bioregion north of our study area, Munks et al. (1996) found that the greatest densities of koalas were along creeks, with strong relationships with proximity to water and leaf moisture. However, in Springsure, in central Queensland, the areas worst affected by drought were along creeks, where forest red gums and carbeen (Corymbia tessellaris) suffered extensive tree death, while koalas survived off creeks (Ellis et al. 2010). Ellis et al. (2010) reported similar results at Blair Athol in central Queensland, where plains vegetation comprising popular box and narrow-leaved ironbark (E. crebra) have continued to support koala populations and are the preferred food trees at the site. It appears that the key refuge habitats where koalas survive in times of environmental stress vary among locations, and this is provisionally linked to both moisture availability and soil nutrients, which in turn affect the balance between leaf nutrients and leaf toxins (Creagh 1992; Cork and Braithwaite 1996; Bryan 1997; Moore and Foley 2000; Moore et al. 2004). Although there will continue to be years when numbers will expand and koalas will disperse into less optimal habitat, in south-west Queensland the maintenance of core habitat along creek lines with permanent waterholes will become increasingly critical in the coming decades.
Approach and limitations
There are some differences between this study and that by Sullivan that affect direct comparison in some sample zones and in habitat units 9 and 10. First, the region surveyed by Sullivan and the region of this study are not identical and although estimates of koala abundance in 2009 are limited to the overlap zone, Sullivan’s koala estimates are for a larger area. The area covered by SZ 2 in 2009 is smaller than in 1995, and did not include the region around Idalia National Park, where Sullivan et al. (2004) found koalas in HU9 and HU10 vegetation. We found no fresh pellets in HU9 or HU10 in SZ 2, but we sampled a much smaller area of these two habitat units and this reduced our chances of finding fresh pellets. Overall, we had proportionally fewer sites in HU9 and 10 than in Sullivan et al. (2004). This may affect the comparison between the two studies, as their population estimates were high for these two habitats (Table 3). We found no fresh pellets in HU10, so no density estimates could be calculated, and we estimated low densities from fresh pellets in HU9. Our conclusion is that the koala population in these habitats markedly decreased during the drought, despite the difference in sampling effort. The area covered by SZ 6 and SZ 9 was slightly larger in Sullivan’s study (Fig. 1). Fresh pellets were found by Sullivan at two sites on one property in SZ 6 that was not in the overlap zone. The sites with fresh pellets in SZ 8 were close to the boundary with SZ 9 and the different estimates in these two zones may reflect a slightly different boundary position between the two survey periods, rather than a significant change in numbers. Finally, there appears to be a discrepancy in koala abundances in HU1 and HU2 between the 1995 and 2009 figures, with our HU2 showing higher numbers. This may reflect a difference in the habitat unit assigned to each site. Notwithstanding these minor differences in the two survey methods, the field data and analysis reflect a real decline in koala numbers since 1995 in south-west Queensland. Our results show that climate change and land clearing are key concerns for the long-term survival of koalas in this region.
Acknowledgements
This project is funded by the Australian Research Council, the Australian Koala Foundation and South West NRM through LP0882090. Data on land clearing were supplied by the Remote Sensing Centre of the Queensland Department of Environment and Resource Management. Field surveys were carried out by Rebecca Condon, Virginia Seymour and Nicole Davies, with help from Leonie Seabrook and several volunteers. We would like to thank the staff of South West NRM and all landholders who participated in the community survey and who allow us to carry out faecal pellet surveys on their land. Their knowledge, help and hospitality were much appreciated. The approved protocol for this project was obtained from the Animal Ethics Committee at The University of Queensland (GPA/603/08/ARC) and the Scientific Purposes Permit WISP05343008 was obtained from the Environmental Protection Agency (now the Department of Environment and Resource Management).
References
Adams, R. A. (2010). Bat reproduction declines when conditions mimic climate change projections for western North America. Ecology 91, 2437–2445.| Bat reproduction declines when conditions mimic climate change projections for western North America.Crossref | GoogleScholarGoogle Scholar |
Adams-Hosking, C., Grantham, H. S., Rhodes, J. R., McAlpine, C. A., and Moss, P. T. (2011). Modelling climate change-induced shifts in the distribution of the koala. Wildlife Research 38, 122–130.
| Modelling climate change-induced shifts in the distribution of the koala.Crossref | GoogleScholarGoogle Scholar |
Albright, T. P., Pidgeon, A. M., Rittenhouse, C. D., Clayton, M. K., Flather, C. H., Culbert, P. D., Wardlow, B. D., and Radeloff, V. C. (2010). Effects of drought on avian community structure. Global Change Biology 16, 2158–2170.
| Effects of drought on avian community structure.Crossref | GoogleScholarGoogle Scholar |
Anderson, B. J., Akçakaya, H. R., Araújo, M. B., Fordham, D. A., Martinez-Meyer, E., Thuiller, W., and Brook, B. W. (2009). Dynamics of range margins for metapopulations under climate change. Proceedings Biological Sciences 276, 1415–1420.
| Dynamics of range margins for metapopulations under climate change.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M3js1Kqtw%3D%3D&md5=e8ffbc2813427d411f742cdd0e019a53CAS |
Andrén, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportion of suitable habitat – a review. Oikos 71, 355–366.
| Effects of habitat fragmentation on birds and mammals in landscapes with different proportion of suitable habitat – a review.Crossref | GoogleScholarGoogle Scholar |
Arroyo-Rodriguez, V., and Dias, P. A. D. (2010). Effects of habitat fragmentation and disturbance on howler monkeys: a review. American Journal of Primatology 72, 1–16.
| Effects of habitat fragmentation and disturbance on howler monkeys: a review.Crossref | GoogleScholarGoogle Scholar |
Barnes, R. F. W. (2001). How reliable are dung counts for estimating elephant numbers. African Journal of Ecology 39, 1–9.
Beeton, R. J. S., Buckley, K. I., Jones, G. J., Morgan, D., Reichelt, R. E., and Trewin, D. (2006). ‘Australia State of the Environment 2006.’ (Department of the Environment and Heritage: Canberra)
Bennett, A., Ratcliffe, P., Jones, E., Mansfield, H., and Sands, R. (2005). Other mammals. In ‘Handbook of Biodiversity Methods: Survey, Evaluation and Monitoring.’ (Eds D. Hill, M. Fasham, G. Tucker, M. Shewry and P. Shaw.) pp. 450–471. (Cambridge University Press: Cambridge)
Bolger, D. T., Patten, M. A., and Bostock, D. C. (2005). Avian reproductive failure in response to an extreme climatic event. Oecologia 142, 398–406.
| Avian reproductive failure in response to an extreme climatic event.Crossref | GoogleScholarGoogle Scholar |
Brown, J. H., Stevens, G. C., and Kaufman, D. M. (1996). The geographic range: size, shape, boundaries, and internal structure. Annual Review of Ecology and Systematics 27, 597–623.
| The geographic range: size, shape, boundaries, and internal structure.Crossref | GoogleScholarGoogle Scholar |
Brown, G. W., Bennett, A. F., and Potts, J. M. (2008). Regional faunal decline - reptile occurrence in fragmented rural landscapes of south-eastern Australia. Wildlife Research 35, 8–18.
| Regional faunal decline - reptile occurrence in fragmented rural landscapes of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Bryan, B. A. (1997). A generic method for identifying regional koala habitat using GIS. Australian Geographical Studies 35, 125–139.
| A generic method for identifying regional koala habitat using GIS.Crossref | GoogleScholarGoogle Scholar |
Campbell, P., Prentice, R., and McRae, P. (1979). Report on the 1977 koala survey. Wildlife in Australia 16, 2–6.
Caughley, G., Grice, D., Barker, R., and Brown, B. (1988). The edge of the range. Journal of Animal Ecology 57, 771–785.
| The edge of the range.Crossref | GoogleScholarGoogle Scholar |
Clifton, D. I., Ellis, W. A. H., Melzer, A., and Tucker, G. (2007). Water turnover and the northern range of the koala (Phascolarctos cinereus). Australian Mammalogy 29, 85–88.
| Water turnover and the northern range of the koala (Phascolarctos cinereus).Crossref | GoogleScholarGoogle Scholar |
Cogger, H., Ford, H., Johnson, C., Holman, J., and Butler, D. (2003). ‘Impacts of Land Clearing on Australian Wildlife in Queensland.’ (WWF Australia: Brisbane)
Cork, S. J., and Braithwaite, L. W. (1996). Resource availability, eucalypt chemical defences, and habitat quality for leaf-eating marsupials. In ‘Koalas: Research for Management.’ (Ed. G. Gordon.) pp. 9–16. (World Koala Research Inc.: Brisbane)
Creagh, C. (1992). Soil clues to koala country. Ecos 73, 11–13.
Crick, H. Q., Dudley, C., and Glue, D. E. (1997). UK birds are laying eggs earlier. Nature 388, 526.
| UK birds are laying eggs earlier.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlt1arsbs%3D&md5=5c42ac27843ccc2aeb934088ce3f226bCAS |
DeGabriel, J. L., Moore, B. D., Foley, W. J., and Johnson, C. N. (2009). The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology 90, 711–719.
| The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal.Crossref | GoogleScholarGoogle Scholar |
Degabriele, R., and Dawson, T. J. (1979). Metabolism and heat balance in an arboreal marsupial, the koala (Phascolarctos cinereus). Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 134, 293–301.
| Metabolism and heat balance in an arboreal marsupial, the koala (Phascolarctos cinereus).Crossref | GoogleScholarGoogle Scholar |
DERM (2009). ‘ClimateQ: Toward a Greener Queensland.’ (Department of Environment and Resource Management: Brisbane)
Dique, D. S., Thompson, J., Preece, H. J., Penfold, G. C., de Villiers, D. L., and Leslie, R. S. (2003). Koala mortality on roads in south-east Queensland: the koala speed-zone trial. Wildlife Research 30, 419–426.
| Koala mortality on roads in south-east Queensland: the koala speed-zone trial.Crossref | GoogleScholarGoogle Scholar |
Dique, D. S., Preece, H. J., Thompson, J., and de Villiers, D. L. (2004). Determining the distribution and abundance of a regional koala population in south-east Queensland for conservation management. Wildlife Research 31, 109–117.
| Determining the distribution and abundance of a regional koala population in south-east Queensland for conservation management.Crossref | GoogleScholarGoogle Scholar |
DNRW (2008). ‘Land Cover Change in Queensland 2006–07: a Statewide Landcover and Trees Study (SLATS) report.’ (Department of Natural Resources and Water: Brisbane)
Efron, B., and Tibshirani, R. (1986). Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science 1, 54–75.
| Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy.Crossref | GoogleScholarGoogle Scholar |
Ellis, W., Melzer, A., Carrick, F. N., and Hasegawa, M. (2002). Tree use, diet and home range of the koala (Phascolarctos cinereus) at Blair Athol, central Queensland. Wildlife Research 29, 303–311.
| Tree use, diet and home range of the koala (Phascolarctos cinereus) at Blair Athol, central Queensland.Crossref | GoogleScholarGoogle Scholar |
Ellis, W., Melzer, A., Clifton, I. D., and Carrick, F. (2010). Climate change and the koala Phascolarctos cinereus: water and energy. Australian Zoologist 35, 369–376.
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Annual Review of Ecology Evolution and Systematics 34, 487–515.
| Effects of habitat fragmentation on biodiversity.Crossref | GoogleScholarGoogle Scholar |
FitzGibbon, S. I., and Jones, D. N. (2006). A community-based wildlife survey: the knowledge and attitudes of residents of suburban Brisbane, with a focus on bandicoots. Wildlife Research 33, 233–241.
| A community-based wildlife survey: the knowledge and attitudes of residents of suburban Brisbane, with a focus on bandicoots.Crossref | GoogleScholarGoogle Scholar |
Ford, H. A. (2011). The causes of decline of birds of eucalypt woodlands: advances in our knowledge over the last 10 years. Emu 111, 1–9.
| The causes of decline of birds of eucalypt woodlands: advances in our knowledge over the last 10 years.Crossref | GoogleScholarGoogle Scholar |
Fuller, A., Dawson, T., Helmuth, B., Hetem, R. S., Mitchell, D., and Maloney, S. K. (2010). Physiological mechanisms in coping with climate change. Physiological and Biochemical Zoology 83, 713–720.
| Physiological mechanisms in coping with climate change.Crossref | GoogleScholarGoogle Scholar |
Gaston, K. J. (2009). Geographic range limits of species. Proceedings. Biological Sciences 276, 1391–1393.
| Geographic range limits of species.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M3js1KqsA%3D%3D&md5=31accfce042227af3452a80bd2a8fc06CAS |
Gaston, K. J., and Fuller, R. A. (2009). The sizes of species’ geographic ranges. Journal of Applied Ecology 46, 1–9.
| The sizes of species’ geographic ranges.Crossref | GoogleScholarGoogle Scholar |
Gibson, S. Y., Van Der Marel, R. C., and Starzomski, B. M. (2009). Climate change and conservation of leading-edge peripheral populations. Conservation Biology 23, 1369–1373.
| Climate change and conservation of leading-edge peripheral populations.Crossref | GoogleScholarGoogle Scholar |
Gleadow, R. M., Foley, W. J.,, and Woodrow, I. E. (1998). Enhanced CO2 alters the relationship between photosynthsis and defence in cyanogenic Eucalyptus cladoclayx F. Muell. Plant, Cell & Environment 21, 12–22.
| Enhanced CO2 alters the relationship between photosynthsis and defence in cyanogenic Eucalyptus cladoclayx F. Muell.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXitlCgsL0%3D&md5=6b0ede4efaee77bfc85f84123e78831aCAS |
Gordo, O., Brotons, L., Rerrer, X., and Comas, P. (2005). Do changes in climate patterns in wintering areas affect the timing of the spring arrival of trans-Saharan migrant birds? Global Change Biology 11, 12–21.
| Do changes in climate patterns in wintering areas affect the timing of the spring arrival of trans-Saharan migrant birds?Crossref | GoogleScholarGoogle Scholar |
Gordon, G., Brown, A. S., and Pulsford, T. (1988). A koala (Phascolarctos cinereus Goldfuss) population crash during drought and heatwave conditions in south-western Queensland. Australian Journal of Ecology 13, 451–461.
| A koala (Phascolarctos cinereus Goldfuss) population crash during drought and heatwave conditions in south-western Queensland.Crossref | GoogleScholarGoogle Scholar |
Gordon, G., McGreevy, D. G., and Lawrie, B. (1990). Koala populations in Queensland: major limiting factors. In ‘Biology of the Koala.’ (Eds A. K. Lee, K. A. Handasyde and G. D. Sanson.) pp. 85–95. (Surrey Beatty & Sons: Sydney)
Gordon, G., Hrdina, F., and Patterson, R. (2006). Decline in the distribution of the koala Phascolarctos cinereus in Queensland. Australian Zoologist 33, 345–358.
Green, K., and Pickering, C. M. (2002). A potential scenario for manmmal and bird diversity in the Snowy Mountains of Australia in relation to climate change. In ‘Mountain Biodiversity: A Global Assessment.’ (Eds K. C. and E. M. Spehn.) pp. 241–249. (Parthenon Publishing: London)
Hampe, A., and Petit, R. J. (2005). Conserving biodiversity under climate change: the rear edge matters. Ecology Letters 8, 461–467.
| Conserving biodiversity under climate change: the rear edge matters.Crossref | GoogleScholarGoogle Scholar |
Hanger, J., and Loader, J. (2009). Infectious disease in koalas: implications for conservation. In ‘Koala Conservation Conference. ‘ (Friends of the Koala: Lismore)
Hennessy, K., Fawcett, R., Kirono, D., Mpelasoka, F., Jones, D., Bathols, J., Whetton, P., Stafford Smith, M., Howden, M., Mitchell, C., and Plummer, N. (2008). ‘An assessment of the impact of climate change on the nature and frequency of exceptional climatic events.’ (Bureau of Meteorology and CSIRO: Canberra)
Hughes, L. (2003). Climate change and Australia: trends, projections and impacts. Austral Ecology 28, 423–443.
| Climate change and Australia: trends, projections and impacts.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. N., and Jarman, P. J. (1987). Macropod studies at Wallaby Creek. VI. A validation of the use of dung-pellet counts for measuring absolute densities of populations of macropodids. Australian Wildlife Research 14, 139–145.
| Macropod studies at Wallaby Creek. VI. A validation of the use of dung-pellet counts for measuring absolute densities of populations of macropodids.Crossref | GoogleScholarGoogle Scholar |
Kanowski, J. (2001). Effects of elevated CO2 on the foliar chemistry of seedlings of two rainforest trees from north-east Australia: implications for folivorous marsupials. Austral Ecology 26, 165–172.
| Effects of elevated CO2 on the foliar chemistry of seedlings of two rainforest trees from north-east Australia: implications for folivorous marsupials.Crossref | GoogleScholarGoogle Scholar |
Kikkawa, J., and Walter, M. (1968). Report on the koala survey, 1967. Wildlife in Australia 5, 100–103.
Krockenberger, A. (2003). Meeting the energy demands of reproduction in female koalas, Phascolartos cinereus: evidence for energetic compensation. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 173, 531–540.
| Meeting the energy demands of reproduction in female koalas, Phascolartos cinereus: evidence for energetic compensation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3sznvFOhsA%3D%3D&md5=bad5c10ca3034668b97ef931d843a78eCAS |
Latham, J. B., Staines, W., and Gorman, M. L. (1996). The relative densities of red (Cervus elephus) and roe (Capreolus capreolus) deer and their relationship in Scottish plantation forests. Journal of Zoology 240, 285–299.
| The relative densities of red (Cervus elephus) and roe (Capreolus capreolus) deer and their relationship in Scottish plantation forests.Crossref | GoogleScholarGoogle Scholar |
Lawler, I. R., Foley, W. J., Woodrow, I. E., and Cork, S. J. (1997). The effects of elevated CO2 atmospheres on the nutritional quality of Eucalyptus foliage and its interaction with soil nutrient and light availablity. Oecologia 109, 59–68.
| The effects of elevated CO2 atmospheres on the nutritional quality of Eucalyptus foliage and its interaction with soil nutrient and light availablity.Crossref | GoogleScholarGoogle Scholar |
Lunney, D., and Matthews, A. (2001). The contribution of the community to defining the distribution of a vulnerable species, the spotted-tailed quoll, Dasyurus maculatus. Wildlife Research 28, 537–545.
| The contribution of the community to defining the distribution of a vulnerable species, the spotted-tailed quoll, Dasyurus maculatus.Crossref | GoogleScholarGoogle Scholar |
Lunney, D., Gresser, S. M., O’Neill, L. E., Matthews, A., and Rhodes, J. (2007). The impact of fire and dogs on koalas at Port Stephens, New South Wales, using population viability analysis. Pacific Conservation Biology 13, 189–201.
Lunney, D., Crowther, M. S., Shannon, I., and Bryant, J. V. (2009). Combining a map-based public survey with an estimation of site occupancy to determine the recent and changing distribution of the koala in New South Wales. Wildlife Research 36, 262–273.
| Combining a map-based public survey with an estimation of site occupancy to determine the recent and changing distribution of the koala in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Lunney, D., Crowther, M. S., Wallis, I., Foley, W. J., Lemon, J., Wheeler, R., Madani, G., Orscheg, C., Griffith, J., Krockenberger, M., Retamales, M., and Stalenberg, E. (in press). Koala populations and climate change: a case for adapting a successful restoration strategy on the Liverpool Plains, north-west NSW. In ‘Wildlife and Climate Change: Towards Robust Conservation Strategies for Australian Fauna.’ (Eds D. Lunney and P. Hutchings.). (Royal Zoological Society of NSW: Mosman, NSW)
Martin, R., and Handasyde, K. A. (1999). ‘The Koala: A Natural History, Conservation and Management.’ (University of New South Wales Press: Sydney)
Mayle, B. A. (1996). Progress in predictive management of deer populations in British woodlands. Forest Ecology and Management 88, 187–198.
| Progress in predictive management of deer populations in British woodlands.Crossref | GoogleScholarGoogle Scholar |
McAlpine, C. A., Bowen, M. E., Callaghan, J. G., Lunney, D., Rhodes, J. R., Mitchell, D. L., Pullar, D. V., and Possingham, H. P. (2006a). Testing alternative models for the conservation of koalas in fragmented rural-urban landscapes. Austral Ecology 31, 529–544.
| Testing alternative models for the conservation of koalas in fragmented rural-urban landscapes.Crossref | GoogleScholarGoogle Scholar |
McAlpine, C. A., Rhodes, J. R., Callaghan, J. G., Bowen, M. E., Lunney, D., Mitchell, D. L., Pullar, D. V., and Possingham, H. P. (2006b). The importance of forest area and configuration relative to local habitat factors for conserving forest mammals: a case study of koalas in Queensland. Biological Conservation 132, 153–165.
| The importance of forest area and configuration relative to local habitat factors for conserving forest mammals: a case study of koalas in Queensland.Crossref | GoogleScholarGoogle Scholar |
McGrath, C. (2004/2005). Queensland’s new vegetation management regime. Queensland Environmental Practice Reporter 10, 27–38.
Melzer, A., and Lamb, D. (1994). Low density populations of the koala (Phascolarctos cinereus) in Central Queensland. Proceedings of the Royal Society of Queensland 104, 89–93.
Melzer, A., and Lamb, D. (1996). Habitat utilisation by a central Queenland koala colony. In ‘Koalas: Research for Management.’ (Ed. G. Gordon.) pp. 17–22. (World Koala Research Inc.: Brisbane)
Melzer, A., Carrick, F., Menkhorst, P., Lunney, D., and St John, B. (2000). Overview, critical assessment, and conservation implications of koala distribution and abundance. Conservation Biology 14, 619–628.
| Overview, critical assessment, and conservation implications of koala distribution and abundance.Crossref | GoogleScholarGoogle Scholar |
Moore, B. D., and Foley, W. J. (2000). A review of feeding and diet selection in koalas (Phascolarctos cinereus). Australian Journal of Zoology 48, 317–333.
| A review of feeding and diet selection in koalas (Phascolarctos cinereus).Crossref | GoogleScholarGoogle Scholar |
Moore, B. D., Wallis, I. R., Marsh, K. J., and Foley, W. J. (2004). The role of nutrition in the conservation of the marsupial folivores of eucalypt forests. In ‘Conservation of Australia’s Forest Fauna’. 2nd edn. (Ed. D. Lunney.) pp. 549–575. (Royal Zoological Society of New South Wales: Mosman, NSW.)
Mouquet, N., Matthiessen, B., Miller, T., and Gonzalez, A. (2011). Extinction debt in source-sink metacommunities. PLoS ONE 6, e17567.
| Extinction debt in source-sink metacommunities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFGku70%3D&md5=cfe4ee9288b2f12fa9fcb512d03c8178CAS |
Munks, S. A., Corkrey, R., and Foley, W. J. (1996). Characteristics of arboreal marsupial habitat in the semi-arid woodlands of northern Queensland. Wildlife Research 23, 185–195.
| Characteristics of arboreal marsupial habitat in the semi-arid woodlands of northern Queensland.Crossref | GoogleScholarGoogle Scholar |
Otley, H. M. (2001). The use of a community-based survey to determine the distribution of the Platypus Ornithorhynchus anatinus in the Huon River catchment, southern Tasmania. Australian Zoologist 31, 632–641.
Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics 37, 637–669.
| Ecological and evolutionary responses to recent climate change.Crossref | GoogleScholarGoogle Scholar |
Parmesan, C., and Yohe, G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42.
| A globally coherent fingerprint of climate change impacts across natural systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXoslM%3D&md5=7522d6adcc7be1348b894ecab713a896CAS |
Patterson, R. (1996). The distribution of koalas in Queensland – 1986 to 1989. In ‘Koalas: Research for Management. Proceedings of the Brisbane Koala Symposium’. (Ed. G. Gordon.) pp. 75–81. (World Koala Research Inc.: Brisbane.)
Pearson, G. A., Lago-Leston, A., and Mota, C. (2009). Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. Journal of Ecology 97, 450–462.
| Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations.Crossref | GoogleScholarGoogle Scholar |
Phillips, B. (1990). ‘Koalas: the little Australians we’d hate to lose.’ (Australian Government Publishing Service: Canberra.)
Phillips, S. (2000). Population trends and the koala conservation debate. Conservation Biology 14, 650–659.
| Population trends and the koala conservation debate.Crossref | GoogleScholarGoogle Scholar |
Phillips, S., and Callaghan, J. (2000). ‘The spot assessment technique for determining the significance of habitat utilisation by koalas (Phascolarctos cinereus).’ (Australian Koala Foundation: Brisbane)
Piessens, K., Adriaens, D., Jacquemyn, H., and Honnay, O. (2009). Synergistic effects of an extreme weather event and habitat fragmentation on a specialised insect herbivore. Oecologia 159, 117–126.
| Synergistic effects of an extreme weather event and habitat fragmentation on a specialised insect herbivore.Crossref | GoogleScholarGoogle Scholar |
Queensland Herbarium (2005). ‘Vegetation Communities and Regional Ecosystems Survey and Mapping Version 5.0.’ (Environmental Protection Agency: Brisbane)
Reed, P., and Lunney, D. (1990). Habitat loss: the key problem for the long-term survival of koalas in New South Wales. In ‘Koala summit: managing koalas in New South Wales.’ (Eds D. Lunney, C. A. Urquhart and D. Reed.) pp. 9–31. (New South Wales National Parks and Wildlife Service: Hurstville)
Reed, P. C., Lunney, D., and Walker, P. (1990). A 1986–1987 survey of the koala Phascolarctos cinereus (Goldfuss) in New South Wales and an ecological interpretation of its distribution. In ‘Biology of the Koala.’ (Eds A. K. Lee, K. A. Handasyde and G. D. Sanson.) pp. 55–74. (Surrey Beatty & Sons: Sydney)
Rhodes, J. R., Tyre, A. J., Jonzen, N., McAlpine, C. A., and Possingham, H. P. (2006). Optimising presence/absence surveys for detecting population trends. The Journal of Wildlife Management 70, 8–18.
| Optimising presence/absence surveys for detecting population trends.Crossref | GoogleScholarGoogle Scholar |
Rhodes, J. R., Lunney, D., Moon, C., Matthews, A., and McAlpine, C. A. (2011). The consequences of using indirect signs that decay to determine species’ occupancy. Ecography 34, 141–150.
| The consequences of using indirect signs that decay to determine species’ occupancy.Crossref | GoogleScholarGoogle Scholar |
Sattler, P. S., and Williams, R. D. (1999). ‘The Conservation Status of Queensland’s Bioregional Ecosystems.’ (Environmental Protection Agency and Queensland National Parks Association: Brisbane)
Seabrook, L., McAlpine, C., Phinn, S., Callaghan, J., and Mitchell, D. (2003). Landscape legacies: Koala habitat change in Noosa Shire, South-east Queensland. Australian Zoologist 32, 446–461.
Sullivan, B. J. (2000). Estimating the abundance of broadscale, low density populations: koalas in the mulgalands of south-west Queensland. School of Natural and Rural Systems Management, The University of Queensland, Brisbane.
Sullivan, B. J., Baxter, G. S., and Lisle, A. T. (2002). Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-west Queensland. I. Faecal pellet sampling protocol. Wildlife Research 29, 455–462.
| Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-west Queensland. I. Faecal pellet sampling protocol.Crossref | GoogleScholarGoogle Scholar |
Sullivan, B. J., Norris, W. M., and Baxter, G.S. (2003a). Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-west Queensland. II. Distribution and diet. Wildlife Research 30, 331–338.
| Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-west Queensland. II. Distribution and diet.Crossref | GoogleScholarGoogle Scholar |
Sullivan, B. J., Baxter, G. S., and Lisle, A. T. (2003b). Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-west Queensland. III. Broad-scale patterns of habitat use. Wildlife Research 30, 583–591.
| Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-west Queensland. III. Broad-scale patterns of habitat use.Crossref | GoogleScholarGoogle Scholar |
Sullivan, B. J., Baxter, G. S., Lisle, A. T., Pahl, L., and Norris, W. M. (2004). Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-west Queensland. IV. Abundance and conservation status. Wildlife Research 31, 19–29.
| Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-west Queensland. IV. Abundance and conservation status.Crossref | GoogleScholarGoogle Scholar |
Telfer, W. R., Griffiths, A. D., and Bowman, D. M. J. S. (2006). Scats can reveal the presence and habitat use of cryptic rock-dwelling macropods. Australian Journal of Zoology 54, 325–334.
| Scats can reveal the presence and habitat use of cryptic rock-dwelling macropods.Crossref | GoogleScholarGoogle Scholar |
Thomas, C. D. (2010). Climate, climate change and range boundaries. Diversity & Distributions 16, 488–495.
| Climate, climate change and range boundaries.Crossref | GoogleScholarGoogle Scholar |
Thomas, C. D., Cameron, A., Green, R. E., Bakkenes, M., Beaumont, L. J., Collingham, Y. C., Erasmus, B. F. N., Ferreria de Siqueira, M., Grainger, A., Hannah, L., Hughes, L., Huntley, B., van Jaarsveld, A. S., Midgley, G. F., Miles, L., Ortega-Huerta, M. A., Peterson, A. T., Philips, O. L., and Williams, S. E. (2004). Extinction risk from climate change. Nature 427, 145–148.
| Extinction risk from climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFOgtQ%3D%3D&md5=53db09bf4c36c19c9ae3f5de0f1fb74dCAS |
Thomas, C. D., Franco, A. M. A., and Hill, J. K. (2006). Range retractions and extinction in the face of climate warming. Trends in Ecology & Evolution 21, 415–416.
| Range retractions and extinction in the face of climate warming.Crossref | GoogleScholarGoogle Scholar |
Thuiller, W., Albert, C., Araujo, M. B., Berry, P. M., Cabeza, M., Guisan, A., Hickler, T., Midgely, G. F., Paterson, J., Schurr, F. M., Sykes, M. T., and Zimmermann, N. E. (2008). Predicting global change impacts on plant species’ distributions: Future challenges. Perspectives in Plant Ecology, Evolution and Systematics 9, 137–152.
| Predicting global change impacts on plant species’ distributions: Future challenges.Crossref | GoogleScholarGoogle Scholar |
Tidemann, C. R. (1999). Biology and management of the grey-headed flying fox, Pterophus poliocephalus. Acta Chiropterologica 1, 151–164.
Tilman, D., Lehman, R. M., and Nowak, M. A. (1994). Habitat destruction and the extinction debt. Nature 371, 65–66.
| Habitat destruction and the extinction debt.Crossref | GoogleScholarGoogle Scholar |
Travis, J. M. J. (2003). Climate change and habitat destruction: a deadly anthropogenic cocktail. Proceedings. Biological Sciences 270, 467–473.
| Climate change and habitat destruction: a deadly anthropogenic cocktail.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s7ivVelsw%3D%3D&md5=9e1cd8ca87ef37b4578de66e06acfdabCAS |
Welbergen, J. A., Klose, S. M., Markus, N., and Eby, P. (2008). Climate change and the effects of temperature extremes on Australian flying-foxes. Proceedings. Biological Sciences 275, 419–425.
| Climate change and the effects of temperature extremes on Australian flying-foxes.Crossref | GoogleScholarGoogle Scholar |
Wethey, D. S., and Woodin, S. A. (2008). Ecological hindcasting of biogeographic responses to climate change in the European intertidal zone. Hydrobiologia 606, 139–151.
| Ecological hindcasting of biogeographic responses to climate change in the European intertidal zone.Crossref | GoogleScholarGoogle Scholar |
White, N. A., and Kunst, N. D. (1990). Aspects of the ecology of the koala in south-eastern Queensland. In ‘Biology of the Koala.’ (Eds A. K. Lee, K. A. Handasyde and G. D. Sanson.) pp. 109–116. (Surrey Beatty and Sons: Chipping Norton, NSW)
Wiegand, T., Revilla, E., and Moloney, K. A. (2005). Effects of habitat loss and fragmentation on population dynamics. Conservation Biology 19, 108–121.
| Effects of habitat loss and fragmentation on population dynamics.Crossref | GoogleScholarGoogle Scholar |
Williams, S. E., Shoo, L. P., Isaac, J. L., Hoffmann, A. A., and Langham, G. (2008). Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biology 6, e325.
| Towards an integrated framework for assessing the vulnerability of species to climate change.Crossref | GoogleScholarGoogle Scholar |
Wilson, G. J., and Delahay, R. J. (2001). A review of methods to estimate the abundance of terrestrial carnivores using field signs and observations. Wildlife Research 28, 151–164.
| A review of methods to estimate the abundance of terrestrial carnivores using field signs and observations.Crossref | GoogleScholarGoogle Scholar |
Wilson, R. J., Thomas, C. D., Fox, R., Roy, D. B., and Kunin, W. E. (2004). Spatial patterns in species distributions reveal biodiversity change. Nature 432, 393–396.
| Spatial patterns in species distributions reveal biodiversity change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpvVCjtrw%3D&md5=963ea5dd46134b75f2a341f374f634dfCAS |
Witt, G. B., and Pahl, L. (1994) Mulgaland communities of south-west Queensland as habitat for koalas. In ‘Ecological Research and Management in the Mulgalands‘. (Eds M. J. Page and T. S. Beutel.) pp. 91–95. (Lawes, Qld: Dept of Management Studies, The University of Queensland.)