Inflated population density of island antechinus: a case of allochthonous marine inputs leading to increased food availability?
M. G. Sale A B C and J. P. Y. Arnould AA School of Life and Environmental Sciences, Deakin University, Burwood, Vic. 3125, Australia.
B Coffey Environments, 126 Trennery Crescent, Abbotsford, Vic. 3067, Australia.
C Corresponding author. Email: michael.g.sale@gmail.com
Australian Journal of Zoology 60(5) 343-351 https://doi.org/10.1071/ZO12073
Submitted: 30 July 2012 Accepted: 27 February 2013 Published: 4 April 2013
Abstract
Resource availability and other processes that affect maintenance, growth and decline of animal populations are central to ecology and conservation. This study quantified features indicative of population fitness and the availability of food resources for island and mainland populations of an insectivorous marsupial, the swamp antechinus (Antechinus minimus). The aim of the study was to test the hypothesis that colonial seabirds increase productivity of island habitats, ultimately providing greater food resources. The study found that antechinus biomass density was 4–13 times greater on the island site compared with the mainland site and was associated with higher recapture rates, suggesting that more individuals were surviving on the island during spring and summer months. An index of antechinus food availability (abundance and biomass of invertebrates) was also higher on the island site. Island antechinus also accessed marine food subsidies, in the form of seabird carrion, during the energetically demanding post-weaning growth period in spring and summer. Furthermore, based on soil nutrient and stable isotope analyses, there was strong evidence of nutrient enrichment from marine sources in the island ecosystem, commonly linked to increased productivity. Therefore, greater antechinus biomass and abundance on offshore islands are likely to be, in part, due to greater survival caused by higher availability of food resources.
Additional keywords: dynamics, ecology, productivity, seabirds, small mammal, stable isotope.
Introduction
The relative effects of resource availability, its partitioning and other processes that affect the maintenance, growth and decline of populations are central to ecology and conservation. Both resource availability and consumer density are considered to be a strong function of primary productivity. Generally, areas of high primary productivity provide sufficient resources to support large populations of consumers, whereas in areas of low primary productivity there are fewer resources and therefore fewer consumers (Persson et al. 1996).
Transported energy and nutrients from one ecosystem have been shown to increase the productivity of adjacent ecosystems (Polis et al. 1997; Spiller et al. 2010). A common instance where such divergent productivity occurs is between islands and neighbouring mainland habitats (Polis and Hurd 1996). Consumers living in terrestrial island habitats are known to take advantage of allochthonous resources that originate in more productive aquatic ones, and these inputs may subsidise consumer populations, permitting higher population densities than could be supported by terrestrial resources alone (Anderson and Polis 1999; Stapp and Polis 2003; Barrett et al. 2005; Pafilis et al. 2009). However, island consumers also generally have fewer competitors and relaxed predation (Adler and Levins 1994) and it has been proposed that insular vertebrate populations may increase to such an extent that food may become more limiting than on the mainland (Case 1978).
Colonial seabirds and seals are powerful vectors of marine nutrients and can significantly increase the productivity of island ecosystems (Gillham 1960; Polis et al. 1997; Farina et al. 2003; Caut et al. 2012). Seabirds, in particular, provide nutrients, in the form of guano, discarded prey, egg remains and carcasses, which can increase greatly the concentration of nutrients in the soil and plant productivity of colony areas (Gillham 1960; Smith 1978; Anderson and Polis 1999; Bancroft et al. 2005; Fukami et al. 2006; Jones 2010). In turn, this can lead to an increase in the availability of food resources for terrestrial vertebrates such as lizards (Markwell and Daugherty 2002; Barrett et al. 2005; Pafilis et al. 2009) and small mammals (Drever et al. 2000; Stapp 2002; Stapp and Polis 2003; Wolfe et al. 2004).
The swamp antechinus (Antechinus minumus), a small (30–80 g) insectivorous marsupial, exhibits divergent interpopulation features between island and mainland sites (Sale et al. 2008, 2009). Populations on the mainland of Australia occur at low densities (typically below 10 animals ha–1) in coastal localities from Wilsons Promontory in Victoria to Robe in South Australia (Menkhorst 1995; Bachmann and van Weenen 2001; Wilson et al. 2001). These contrast with high-density populations (often exceeding 100 animals ha–1) that occur on small islands in Bass Strait (Wainer 1976; Sale et al. 2006). Greater survival due to higher food availability on these islands has been proposed as one factor contributing to these large differences in population density (Sale et al. 2006). However, resource availability for the swamp antechinus has not been calculated nor have comparisons of population features indicative of a resource-rich environment been made (e.g. survival, seasonal mass) for island and mainland habitats.
This study tested the hypothesis that colonial seabirds increase productivity on Kanowna Island, providing greater food resources and ultimately influencing the insular population of the swamp antechinus. The three main aims of the study were: (1) to investigate features indicative of a subsidised population, such as population size, minimum survival, recruitment, animal movements and seasonal body mass; (2) to quantify invertebrate resource availability and small mammal biomass to assess resource availability and consumer density at each site; and (3) to examine whether nutrient enrichment from marine sources occurs on the island using soil nutrient testing and stable isotope analysis.
Materials and methods
Study sites
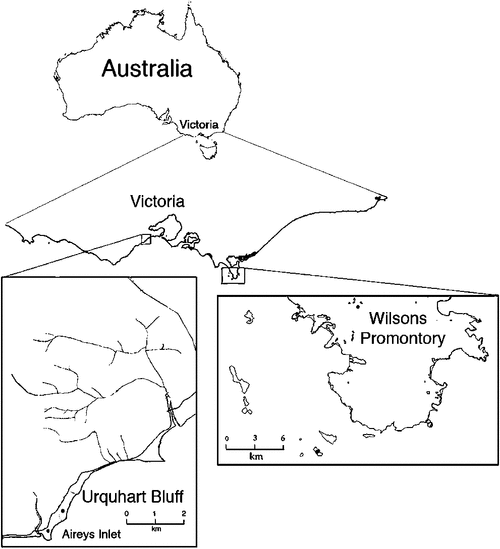
Two sites (one mainland and one island) were used (Fig. 1). The mainland site was on a coastal plateau (Urquharts Bluff) in the eastern Otway Ranges, ~100 km south-west of Melbourne, Victoria (38°26′S, 144°08′E). Surface sediments (Angahook Formation) at this site consist of shallow marine to coastal plain deposits of sandstone, conglomerate, shale, basalt and pyroclastic outcropping in cliff areas, likely to be formed during the Quaternary period. The soils are texture contrast Chromosols and Sodosols and gradational texture Dermosols. The plateau contains a diverse mosaic of vegetation communities consisting of low heathlands dominated by mid-storey species, including Australian oak (Eucalyptus obliqua), prickly tea-tree (Leptospermum continentale) and understorey species, such as thatch saw-sedge (Gahnia radula) and heath tea-tree (L. myrsinoides). Mean annual rainfall for the region is 600–800 mm and drought conditions were apparent during the period of the study. The site has not been burnt since the Ash Wednesday fires in 1983. Although the site was located within 400 m of the ocean, there was no history of nesting seabirds at the site.
|
The island site was on Kanowna Island (part of the Anser Island Group) located in northern Bass Strait 5 km south-west of Wilsons Promontory (39°09′S, 146°18′E). The geology of the site consists of Palaeozoic granites and deep Quaternary sand deposits with resulting coarse-grained sandy soils with varying amounts of incorporated organic matter. Vegetation consists predominantly of short tussock grassland (Poa poiformis) with smaller patches of low heath (<2 m high) dominated by coastal tea-tree (L. laevigatum), coastal beard heath (Leucopogon parviflorus), seaberry saltbush (Rhagodia baccata) and white correa (Correa alba). The island experiences maritime conditions and is frequently exposed to strong south-easterly winds. Mean annual rainfall at the nearest weather station, Wilsons Promontory Lighthouse (#085096, Bureau of Meteorology) since 1873, was 1052 mm; however, orographic effects of the mainland range mean that rainfall on the island is considerably lower. Annual rainfall in the years 2003, 2005, 2006 and 2007 was below average, and 2004 received slightly above average rainfall. Over 100 000 pairs of burrow-nesting seabirds are present on Kanowna Island from November to March each year, together with a breeding colony of 15 000 fur seals (Kirkwood et al. 2010).
Animal capture and handling
Elliott aluminium traps (100 × 100 × 300 mm) (Elliott Scientific, Upwey, Victoria, Australia), baited with a mixture of rolled oats, peanut butter and golden syrup, were used to capture animals. Each site (~1.5 ha) consisted of a similar trapping design of 100–150 traps positioned 10–15 m apart in a rectangular pattern. Captured animals were marked using a pattern of nicks along the ear margin for identification, weighed (±0.5 g) and measured (±0.1 mm). Trapping was conducted at four life-history stages: before breeding (autumn, April to June), during lactation (winter, August to September), during weaning (spring, November to December), and during the growth period (summer, January to March). Field data collected from 2005 to 2007 were supplemented with data on body mass and population features incorporated from previous work (Sale et al. 2006, 2008; B. A. Wilson, unpubl. data).
The direct enumeration of the number of individuals captured was used to obtain estimates of seasonal population size of the swamp antechinus (and other small mammals). Direct counts of individuals are acknowledged as underestimates but have been found to be relatively robust indices when comparing populations within species (Hilborn et al. 1976; Slade and Blair 2000). Minimum survival, defined as the proportion of animals that were recaptured in the following season, was calculated for the duration of the study. During spring, minimum survival of juveniles was estimated as the proportion of juveniles captured from the total number of pouch young counted during winter. Available data (2001–07 for the mainland and 2003–07 for the island) were pooled for both sites. This was undertaken to reduce erroneous estimates of survival based on small numbers of animals on the mainland and to increase the likelihood of sound site-specific estimates of survival. Recruitment was assessed seasonally over this same period by comparing the proportion of new individuals (both male and female) to the number of resident (marked) individuals.
Given that the biomass of animals per unit area more accurately reflects total energy requirements of the population rather than animal numbers only, the total biomass of small and medium-sized mammals (both insectivorous and herbivorous) was estimated at both sites. This was calculated seasonally at each site as the mean total mass of all individuals present (per hectare) for each species. Although herbivorous small mammals are unlikely to compete directly for food resources with the insectivorous swamp antechinus, they were included in the analysis as they may compete for habitat (e.g. nest sites).
The average distance between recapture locations can be used as an index of animal movement and home-range size (Slade and Russell 1998), which may be influenced by resource availability. Therefore the average distance between recapture locations of individuals was compared for both sites between 2005 and 2007 for both males and females.
A linear mixed model was used to compared body mass using site, gender and season as main factors with interactions between these factors also performed. Two sample t-tests were used to compare average distance between recapture locations, as well as the frequency of occurrence of, and the number of individuals within, different arthropod groups, for the island and mainland habitats.
Soil nutrient analysis and prey availability
Soil nutrient levels were tested from the A horizon (the top 10 cm of surface soil) during November 2005 at each site. Approximately 250 g of soil was collected from 15 soil cores (10 cm deep × 2.5 cm diameters) at random points within a 5 m × 5 m quadrat and combined into a composite sample. Ammonium and nitrate N concentrations were measured using a Lachat Flow Injection Analyser (Pro-Tech Group, Coolum Beach, Queensland, Australia) (Searle 1984). Available phosphorus and potassium were calculated using the Colwell method (Rayment and Higginson 1992). Extractable sulfur was extracted in 0.1 m KCl at 40°C and measured using inductively coupled plasma spectrometry (Blair et al. 1991). The percentage of organic carbon in the soil was measured using the techniques outlined by Walkley and Black (1934). Electrical conductivity, and pH in calcium chloride and in water were measured using the methods of Rayment and Higginson (1992). Comparisons of soil nutrient concentrations between the island and mainland sites were undertaken using Mann–Whitney U-tests.
Pitfall sampling is commonly used to estimate invertebrate abundance and potential food availability for insectivorous mammals (Stratham 1982; Gilfillan 2001; Miller et al. 2003; Allison et al. 2006). This method provides a useful index of food availability for the swamp antechinus, since surface-active arthropods contribute most to their diet (Allison et al. 2006; Sale et al. 2006). Pitfall traps (15–20) were set 10 m apart within each site during each season. Traps consisted of embedded 250-mL cylindrical plastic containers with their rims sitting flush to the surface soil. A plywood lid was placed 40 mm above each trap to minimise disturbance by larger animals. Each trap contained a solution of 50 mL ethanol and 70% glycol. Pitfall traps were set over four nights and captured invertebrates were transferred into vials containing 70% ethanol. Individual arthropods from pitfall traps were counted and sorted to Order then oven-dried at 50°C. The overall dry biomass of each pitfall trap was weighed to the nearest ±0.01 g.
Because pitfall sampling measures only the abundance of active surface-dwelling invertebrates, there are limitations in using pitfall sampling in isolation (Greenslade 1964; Luff 1975). Soil sampling was also undertaken to estimate the relative abundance of ground-dwelling invertebrates at each site. Soil samples, consisting of two soil volumes, measuring 250 mm × 250 mm to a depth of 100 mm, were collected and sieved onto a white sheet, and the invertebrates collected and stored in ethanol. Because the island site is a nesting habitat for numerous burrowing seabird species, repeated soil sampling for ground-dwelling invertebrates was not undertaken to avoid disturbing the site and damaging nesting burrows. Consequently, comparisons of the number of ground-dwelling invertebrates between sites were limited to the spring of 2005. Invertebrates occupying the shoots and roots of tussock grasses were not sampled to minimise disturbance to the site.
Pooled measures of invertebrate availability (biomass and abundance) were used as seasonal estimates of total food availability. Data for the arthropod biomass and the number of invertebrates per pitfall trap were log-transformed because of the variances between groups. Transformed data were compared using two-way analysis of variance (ANOVA) for comparisons of site and season. Seasonal comparisons of mean invertebrate biomass between sites (island and mainland) were undertaken using l.s.d. (least significant difference) mean separation tests (Steel and Torrie 1960). Two-sample t-tests were used to compare the frequency of occurrence and abundance of arthropod groups at each site.
Stable isotope analysis
The stable isotope ratios 13C/12C and 15N/14N (expressed as δ13C and δ15N) are widely used to investigate trophic relationships within ecosystems (Peterson and Fry 1987). In terrestrial systems, plants differ in δ13C depending on the photosynthetic pathway. C3 plants have relatively low δ13C (<–28%) compared with C4 and CAM plants (–12 to –13‰: Peterson and Fry 1987). δ13C based on marine phytoplankton (–19 to –24‰) are intermediate between the two terrestrial pathways (Mizutani and Wada 1988; Anderson and Polis 1998; Stapp et al. 1999). The presence of seabird guano-derived N can be clearly distinguished from terrestrial sources of N by the δ15N of plants and their consumers, because soils fertilised by seabird guano are enriched with δ15N (Stapp et al. 1999). Plant, arthropod and vertebrate tissues were collected on both the island and mainland sites to determine whether marine nutrients were being incorporated into the island food webs.
For each plant sample, at least three newly formed plant leaves were collected. Whole insects were also collected either by hand or using pitfall traps, and 30–70 mg of fur was plucked from the rump region of the swamp antechinus using forceps. Samples were immediately sealed in air-tight bags and frozen. Frozen plant and invertebrate samples for isotope analysis were oven-dried for 48 h at 60°C, and then finely ground. The animal fur was washed in a 2 : 1 chloroform : methanol solution to remove lipids, air-dried and then cut into fine fragments. Ratios of δ13C and δ15N were determined using a continuous-flow mass spectrometer with an Anca-Sl preparation system. Isotopic signatures are reported as the ratio of the sample to that of a known standard:
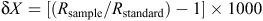
where X is the heavy isotope of interest and R is the ratio of the heavy to light isotope. Comparisons of δ13C and δ15N values between sites were undertaken using two-sample t-tests and the non-parametric Kruskal–Wallis test.
Isotopic values obtained from the consumer’s tissue can be used to reveal temporal shifts in their diets. This is because the isotopic ratios in the consumer’s tissue are related to those of its food source in a predictable manner. Metabolically inactive tissues such as hair do not reabsorb or turnover nutrients. Therefore, their stable isotope ratios reflect the diet of individuals during the limited period when the tissue was formed (Roth 2002; Mizukami et al. 2005). Therefore, the δ13C and δ15N of the fur of the swamp antechinus collected from the island in early spring and in the autumn were compared to determine whether a dietary shift to include seabird products occurred. On the basis of the moulting patterns of the closely related dusky and agile antechinus (A.swainsonii and A.agilis) (Leeson and Wallis 1986), it was predicted the swamp antechinus would have two moults, a spring (early) moult and summer (late) moult. Hence, hair samples collected during early spring would have grown when seabirds were absent, and represented diet during winter, while hair collected during autumn would have grown during the summer, when seabird products were a potential food source for the antechinus.
All data were analysed using SPSS 14.0 and 17.0 (SPSS, Inc., Chicago, IL, USA). Mean data are presented with standard errors and differences, significant at P ≤ 0.05.
Results
Population characteristics
The seasonal numbers of individuals were far greater in the island habitat than the mainland (Table 1). The mean number of individuals was lowest during winter, when only females were present, for the island and mainland respectively (19.0 ± 4.7 versus 7.0 ± 2.8). The island population had the highest number of individuals during summer (99.7 ± 21.1), whereas the highest number of individuals at the mainland site occurred during autumn (14.1 ± 3.1).

|
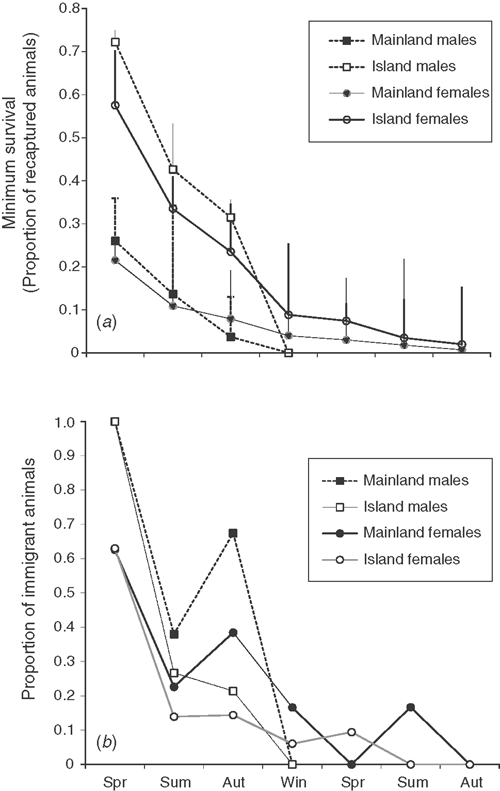
Minimum seasonal survival differed between the sites, with at least twice as many island individuals being recaptured after birth until breeding in comparison to those on the mainland (Fig. 2a). Disappearances of females are more likely to represent mortality than dispersal. Consequently, the difference in recapture rates between the sites is likely to indicate greater survival of females and lower dispersal (and potentially greater survival) of males on the island site.
|
The relative proportion of immigrant (new) individuals showed similar patterns, with a large influx of new animals entering the population during spring. However, during the summer and autumn the mainland population had a greater influx of new male and female individuals, relative to the island (Fig. 2b). This was particularly apparent for island males, for which new individuals represented less than 25% during summer and autumn compared with 38% and 67% during summer and autumn for mainland males. The average distance between recapture locations differed significantly between sites, with mainland animals moving significantly greater distances. This was consistent for both males (56.9 ± 8.8 versus 25.6 ± 1.9; t113 = 5.14, P < 0.001) and females (48.6 ± 3.5 versus 20.7 ± 1.8; t148 = 7.56, P < 0.001).
Overall body mass did not differ significantly between island and mainland sites (P = 0.12). However, there were significant differences between sex (males heavier than females; P < 0.001) and season (adding mass over time; P < 0.001). There was also a significant interaction of site × season (P = 0.02), and of most relevance to this study was the significant interaction between site × season × sex (P = 0.02). In spring, the body masses of males and females were similar between sites (27.6 ± 0.5 versus 31.9 ± 1.7 and 23.8 ± 0.8 versus 25.9 ± 1.7). However, during summer both males and females were heavier on the island, with males showing the greatest difference (48.1 ± 0.6 versus 39.7 ± 3.8 and 35.5 ± 0.9 versus 30.0 ± 2.5, respectively). By autumn body mass was similar between the sites for males and females (57.4 ± 0.8 versus 57.8 ± 1.7 and 34.1 ± 0.8 versus 34.7 ± 1.6, respectively). There was also considerable interannual variation of seasonal body mass for both males and females on the mainland, with mean seasonal mass often varying more than 30% between years. The degree of interannual variation was far lower on the island site, with seasonal means varying less than 15%.
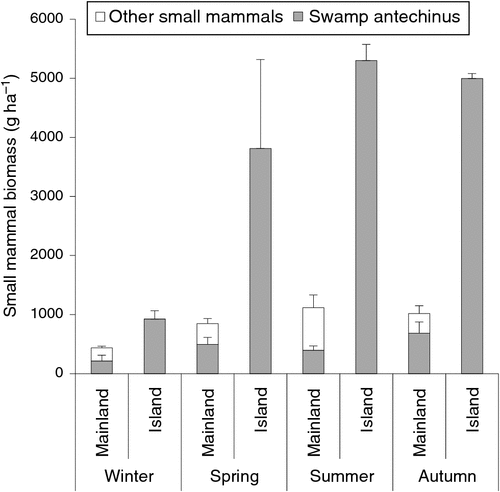
Whereas the swamp antechinus was the only terrestrial mammal present on the island site, bush rats (Rattus fuscipes), swamp rats (R. lutreolus), white-footed dunnarts (Sminthopsis leucopus) and house mice (Mus domesticus) were present at the mainland site. Despite the lower species diversity on Kanowna Island, the biomass (g ha–1) of the swamp antechinus was 2–4 times greater than the biomass for small to medium-sized terrestrial mammals and 4–13 times the biomass of the swamp antechinus compared with the mainland site (Fig. 3).
|
Prey availability and allochthonous marine inputs
The mean numerical abundances of invertebrates (individuals captured per pitfall trap), grouped by Order, on the island site was significantly greater (P < 0.05) for beetles (Coleoptera), earwigs (Dermatera), spiders (Araneae), cockroaches (Blattodea), flies (Diptera), mites (Acarina), caterpillars (Lepidoptera), worms (Oligochaeta) and grasshoppers (Orthoptera) than the mainland site. The only invertebrate category that was significantly more abundant on the mainland site was ants (Hymenoptera, Formicidae; P < 0.05). Although subsurface invertebrates were encountered in the soil samples, abundances were far lower compared with the catches of pitfall traps. Worms, spiders, beetle larvae and centipedes were the most frequent invertebrates in soil samples. More invertebrates were collected from the island soil in comparison to the soil from the mainland site (3.4 ± 0.8 versus 2.8 ± 0.7), although the difference was not significant (Mann–Whitney U-test, z = 0.7, P > 0.05).
Mean invertebrate biomass (all invertebrates pooled) collected in pitfall traps was significantly greater at the island site than at the mainland site, with more than twice the invertebrate biomass (0.27 ± 0.04 g pitfall–1 versus 0.13 ± 0.02 g pitfall–1: F1,110 = 18.1, P < 0.001). There was also a significant difference in invertebrate biomass between seasons (F3,110 = 12.5, P < 0.001), with greater biomass in spring compared with winter (Fig. 4a). However, a significant site × season interaction occurred for the invertebrate biomass (F3,110 = 2.7, P < 0.05). This interaction resulted from the almost three-fold higher invertebrate biomass collected in spring on the island compared with the mainland (0.49 ± 0.1 g pitfall–1 versus 0.17 ± 0.05 g pitfall–1: P < 0.05) whereas in summer there were no differences in invertebrate biomass between sites (0.28 ± 0.06 g pitfall–1 versus 0.23 ± 0.03 g pitfall–1: P > 0.05).
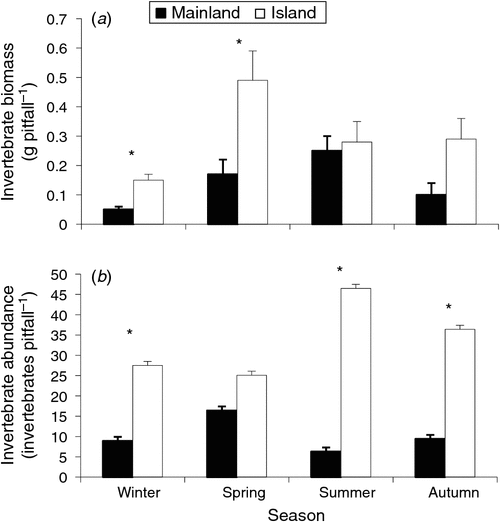
|
The number of invertebrate individuals (all invertebrate orders pooled) captured per pitfall trap differed significantly between sites (F1,110 = 6.1, P < 0.001), with more invertebrates captured on the island than on the mainland (31.6 ± 2.2 invertebrates pitfall–1 versus 9.9 ± 1.0 invertebrates pitfall–1). Unlike the biomass of arthropods, season had no significant impact on the number of invertebrates per pitfall (F3,110 = 0.3, P > 0.05). However, there was a significant site × season interaction (F3,110 = 7.7, P < 0.001) for invertebrate numbers (Fig. 4b). The basis for the interaction was the more than seven-fold increase in the number of invertebrates collected on the island in summer compared with the mainland (46.5 ± 4.0 versus 6.3 ± 2.0: P < 0.05), yet there were no significant differences in numbers between sites in the spring (25.1 ± 3.0 versus 16.4 ± 2.0: P > 0.05). Invertebrate numbers were also greater on the island in autumn (36.4 ± 4.0 versus 9.4 ± 2.0: P < 0.05) and in the winter (27.5 ± 2.0 versus 8.9 ± 2.0: P < 0.05).
Concentrations of nitrate-N, ammonium-N, phosphorus, potassium and sulfur in soil samples were 7–307 times greater on the island than the mainland (Table 2). For example, the concentration of ammonium-N in the island soil was 472.5 ± 70.6 mg kg–1, whereas the concentration on the mainland site was only 4.5 ± 0.4 mg kg–1 (Mann–Whitney U-test, z = 2.1, P < 0.05).
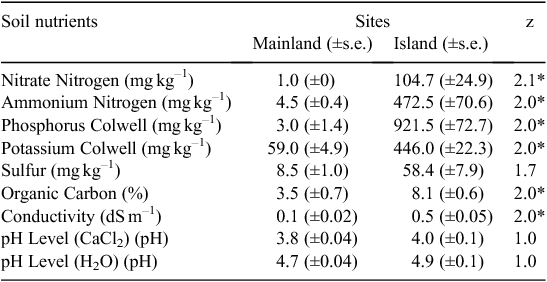
|
Stable isotope analysis of δ13C and δ15N indicated the presence of marine nutrients in the island terrestrial food web, which was absent from the mainland site, and that the marine nutrients are passed up the food chain. The δ13C of hair samples differed significantly (t50 = 4.9, P < 0.001) between sites (–21.1 ± 0.1‰ versus –22.2 ± 0.2‰ for the island and mainland respectively), while the δ15N values of island individuals reached extreme values compared with those on the mainland (23.5 ± 0.5‰ versus 5.3 ± 0.1‰: t50 = 17.7, P < 0.001). Similarly, the δ13C and δ15N values in whole arthropods collected from the island and mainland was also significantly different, with significant enrichment of δ13C (–23.3 ± 0.3‰ versus –25.2 ± 0.3‰: t34 = 3.7, P < 0.001) and δ15N (22.7 ± 0.8‰ versus 1.8 ± 0.9‰: t34 = 16.7, P < 0.001) (Fig. 5).
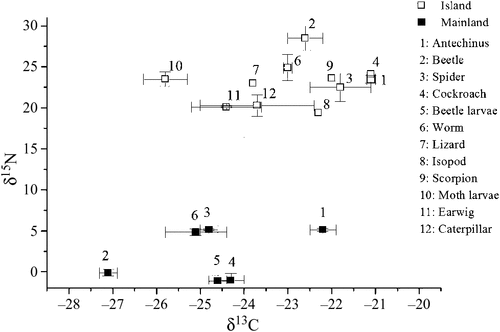
|
The δ13C values of antechinus fur did not differ significantly between spring and autumn (–21.1 ± 0.1 ± 0.8‰ versus –21.0 ± 0.1‰: t = 0.6, P > 0.05). However, δ15N values were significantly lower during autumn (22.4 ± 0.6‰ versus 24.7 ± 0.8‰: t = 2.1, P < 0.05), indicating a possible dietary shift between spring and autumn. During summer swamp antechinuses on the island site were seen feeding on seabird carcasses.
Discussion
The study found that a nutrient-rich island site with a greater abundance and biomass of invertebrates sustains a dense island population of the swamp antechinus, with 4–13 times the antechinus biomass of a mainland site. This supports the hypothesis that the island has greater productivity and available food resources for the swamp antechinus. MacArthur et al. (1972) coined the phrase ‘excess density compensation’ to describe such a situation, where the aggregate density of a given taxon is higher on islands than in equivalent mainland habitats. If mortality of Antechinus is the principal factor determining population size, because of a slow intrinsic rate of population increase, with annual breeding and associated male die-off (Wood 1970; Cockburn 1997), any factor that increases survival will increase the population density of Antechinus. Therefore higher survival and reduced emigration recorded in the island population are proposed to be the primary causes of the inflated population density.
High island population densities may be attributable to confounding site differences such as vegetation characteristics, soil type, rainfall or differing species interactions, such as predatory release (MacArthur et al. 1972). Indeed, high island population densities are often attributed to lower predation, resulting in reduced mortality relative to mainland populations (see reviews by Gliwicz 1980; Adler and Levins 1994). However, although introduced foxes (Vulpes vulpes) and cats (Felis catus) and native predators, such as snakes, are absent from the island habitat, avian predators such as brown falcons (Falco berigora) and Australian kestrels (F. cenchroides) were observed in greater numbers than on the mainland. The grassland habitat of the island affords little protective cover and avian predation was regularly observed, indicating that predatory release of antechinus was unlikely to occur in the island habitat. In addition, population density estimates of the swamp antechinus in the nearby heath habitat on Wilsons Promontory (<10 km from the island), were comparable to those for Urquhart Bluff (Sale and Arnould 2009). This habitat has a similar geological history and rainfall pattern to the island. Therefore confounding site differences are unlikely to be the primary cause for the divergent population densities of the swamp antechinus.
An important factor known to regulate population density is environmental resource availability (Hansen and Batzli 1978; Dickman 1989; Boutin 1990). In the present study, the biomass and numerical abundance of surface invertebrates was significantly higher on the island site, indicating that the island antechinus had more food available. In turn, more food could lead to higher survival and smaller movements of swamp antechinus on the island, resulting in higher population densities on the island.
Additional food resources for the antechinus on the island are likely to result, both directly and indirectly, from the presence of colonial seabirds on the island. Extensive research on seabird colonies has shown a significant positive effect on the productivity of island ecosystems (Smith 1978; Polis and Hurd 1996; Huxel and McCann 1998; Stapp and Polis 2003; Bancroft et al. 2005; Caut et al. 2012), including increased biomass/availability of invertebrates (e.g. Sánchez-Piñero and Polis 2000; Markwell and Daugherty 2002; Caut et al. 2012). In the present study, the concentrations of plant-available forms of nitrogen (nitrate-N and ammonium-N), phosphorus and potassium were up to 100 times higher in the island topsoil than in the nutrient-deficient soils in the mainland habitat. There was strong evidence that this nutrient enrichment was from seabirds, and these effects penetrated into areas adjacent to seabird colonies, perhaps via nutrient leaching. The δ15N values for terrestrial plants and invertebrates on the island greatly exceeded levels found in mainland samples and are indicative of marine-derived nitrogen (Mizutani and Wada 1988; Farina et al. 2003; Stapp and Polis 2003; Fukami et al. 2006; Caut et al. 2012).
The present study also indicates that antechinus benefited directly from seabirds during spring and summer, with the consumption of seabird carrion. This is supported by the significant change in δ15N values between winter and summer, indicating a potential dietary shift. Winter hair was more enriched in δ15N and is likely to indicate a guano–plant–insectivore pathway. In contrast, significantly lower δ15N values in antechinus fur during autumn may have resulted, in part, from the consumption of seabird tissues or eggs, which have comparatively lower δ15N values (e.g. 8–12 δ15N ‰: Minami et al. 1995), during the period of fur growth. The observations of island antechinuses consuming seabird carrion and the presence of feathers in antechinus scats during the fledging of seabird chicks, as determined by faecal analysis (Sale 2008), supports this proposition.
It has been proposed that with fewer competitors and relaxed predation, island populations increase to such an extent that food may become relatively more limiting for consumers on islands than on the mainland Case (1978). Palkovacs (2003) extended this prediction and proposed that high intraspecific competition on islands may eventually result in a reduction in growth. However, there was no evidence that the swamp antechinus on Kanowna Island was experiencing any food shortages as a result of intense intraspecific competition, with body mass and growth comparable to, or greater than, that of the mainland.
The unique life history of Antechinus means that lower food resources and increased predation risks associated with foraging in suboptimal environments may limit their capacity to survive. This is particularly evident when the spring flush of insect prey, which historically has been predictable, is interrupted by drought (Rhind 2002; Parrott et al. 2007; Sale et al. 2008). In the present study, there was considerable interannual variation in seasonal body mass of animals in the mainland habitat (particularly during spring and summer), but the island population had a more stable body mass. Potentially, marine inputs may subsidise and maintain a more stable spring and summer food supply between years, irrespective of climatic conditions. Seabirds also directly provide additional food resources (discarded prey items, broken eggs and carrion) during this energy-demanding growth period of the swamp antechinus. The fact that island individuals were significantly heavier than mainland individuals during summer supports this proposition.
Acknowledgements
We gratefully acknowledge Parks Victoria for logistical support; field assistance by M. Underwood, J. Sale, J. Egan, and K. Hicks; and funding by the Holsworth Wildlife Research Endowment, the Winifred Violet Scott Estate, the Australian Geographic Society, the M. A. Ingram Trust, the Wildlife Preservation Society of Australia, the Royal Zoological Society of New South Wales, and the Ecological Society of Australia. Work was completed under a Department of Sustainability and Environment Wildlife research permit (#10003288) and approval by the Deakin University Animal Welfare Committee (#A16/2005). We also thank two anonymous reviewers and P. Sale for their constructive comments.
References
Adler, G. H., and Levins, R. (1994). The island syndrome in rodent populations. The Quarterly Review of Biology 69, 473–490.| The island syndrome in rodent populations.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M7lvVCqtw%3D%3D&md5=326cf799c474356203961ae319215e7cCAS | 7855232PubMed |
Allison, L. M., Gibson, L. A., and Aberton, J. G. (2006). Dietary strategy of the swamp antechinus (Antechinus minimus maritimus) (Marsupialia: Dasyuridae) in coastal and inland heathland habitats. Wildlife Research 33, 67–76.
| Dietary strategy of the swamp antechinus (Antechinus minimus maritimus) (Marsupialia: Dasyuridae) in coastal and inland heathland habitats.Crossref | GoogleScholarGoogle Scholar |
Anderson, W. B., and Polis, G. A. (1998). Marine subsidies of island communities in the Gulf of California: evidence from stable carbon and nitrogen isotopes. Oikos 81, 75–80.
| Marine subsidies of island communities in the Gulf of California: evidence from stable carbon and nitrogen isotopes.Crossref | GoogleScholarGoogle Scholar |
Anderson, W. B., and Polis, G. A. (1999). Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118, 324–332.
| Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands.Crossref | GoogleScholarGoogle Scholar |
Bachmann, M. R., and van Weenen, J. (2001). ‘The Distribution and Status of the Swamp Antechinus Antechinus minimus maritimus (Marsupialia: Dasyuridae) in South Australia.’ (Nature Conservation Society of South Australia: Adelaide.)
Bancroft, W. J., Roberts, J. D., and Garkaklis, M. J. (2005). Burrowing seabirds drive decreased diversity and structural complexity, and increased productivity in insular-vegetation communities. Australian Journal of Botany 53, 231–241.
| Burrowing seabirds drive decreased diversity and structural complexity, and increased productivity in insular-vegetation communities.Crossref | GoogleScholarGoogle Scholar |
Barrett, K., Anderson, W. B., Wait, D. A., and Grismer, L. L. (2005). Marine subsidies alter the diet and abundance of insular and coastal lizard populations. Oikos 109, 145–153.
| Marine subsidies alter the diet and abundance of insular and coastal lizard populations.Crossref | GoogleScholarGoogle Scholar |
Blair, G. J., Chinoim, N., Lefroy, R. D. B., Anderson, G. C., and Crocker, G. J. (1991). A soil sulfur test for pastures and crops. Australian Journal of Soil Research 29, 619–626.
| A soil sulfur test for pastures and crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmsFGjtr4%3D&md5=d743f2c4b1584df1c91d3f5dbd3877c9CAS |
Boutin, S. (1990). Food supplementation experiments with terrestrial vertebrates – patterns, problems, and the future. Canadian Journal of Zoology 68, 203–220.
| Food supplementation experiments with terrestrial vertebrates – patterns, problems, and the future.Crossref | GoogleScholarGoogle Scholar |
Case, T. J. (1978). General explanation for insular body size trends in terrestrial vertebrates. Ecology 59, 1–18.
| General explanation for insular body size trends in terrestrial vertebrates.Crossref | GoogleScholarGoogle Scholar |
Caut, S., Angulo, E., Pisanu, B., Ruffino, L., Faulquier, L., Lorvelec, O., Chapuis, J., Pascal, M., Vidal, E., and Courchamp, F. (2012). Seabird modulations of isotopic nitrogen on islands. PLoS ONE 7, e39125.
| Seabird modulations of isotopic nitrogen on islands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xpt1Wntrs%3D&md5=b5a9846500f4b072c1730b19e311f744CAS | 22723945PubMed |
Cockburn, A. (1997). Living slow and dying young: senescence in marsupials. In ‘Marsupial Biology: Recent Research, New Perspectives’. (Eds N. R. Saunders and L. A. Hinds.) pp. 163–174. (University of New South Wales Press: Sydney.)
Dickman, C. R. (1989). Demographic responses of Antechinus stuartii (Marsupialia) to supplementary food. Australian Journal of Ecology 14, 387–398.
| Demographic responses of Antechinus stuartii (Marsupialia) to supplementary food.Crossref | GoogleScholarGoogle Scholar |
Drever, M. C., Blight, L. K., Hobson, K. A., and Bertram, D. F. (2000). Predation on seabird eggs by Keen’s mice (Peromyscus keeni): using stable isotopes to decipher the diet of a terrestrial omnivore on a remote offshore island. Canadian Journal of Zoology 78, 2010–2018.
Farina, J. M., Salazar, S., Wallem, K. P., Witman, J. D., and Ellis, J. C. (2003). Nutrient exchanges between marine and terrestrial ecosystems: the case of the Galapagos sea lion Zalophus wollebaecki. Journal of Animal Ecology 72, 873–887.
| Nutrient exchanges between marine and terrestrial ecosystems: the case of the Galapagos sea lion Zalophus wollebaecki.Crossref | GoogleScholarGoogle Scholar |
Fukami, T., Wardle, D. A., Bellingham, P. J., Mulder, C. P. H., Towns, D. R., Yeates, G. W., Bonner, K. I., Durrett, M. S., Grant‐Hoffman, M. N., and Williamson, W. M. (2006). Above‐ and below‐ground impacts of introduced predators in seabird‐dominated island ecosystems. Ecology Letters 9, 1299–1307.
| Above‐ and below‐ground impacts of introduced predators in seabird‐dominated island ecosystems.Crossref | GoogleScholarGoogle Scholar | 17118004PubMed |
Gilfillan, S. L. (2001). An ecological study of a population of Pseudantechinus macdonnellensis (Marsupialia: Dasyuridae) in central Australia. I. Invertebrate food supply, diet and reproductive strategy. Wildlife Research 28, 469–480.
| An ecological study of a population of Pseudantechinus macdonnellensis (Marsupialia: Dasyuridae) in central Australia. I. Invertebrate food supply, diet and reproductive strategy.Crossref | GoogleScholarGoogle Scholar |
Gillham, M. E. (1960). Destruction of indigenous heath vegetation in Victorian sea-bird colonies. Australian Journal of Botany 8, 277–317.
| Destruction of indigenous heath vegetation in Victorian sea-bird colonies.Crossref | GoogleScholarGoogle Scholar |
Gliwicz, J. (1980). Island populations of rodents – their organization and functioning. Biological Reviews of the Cambridge Philosophical Society 55, 109–138.
| Island populations of rodents – their organization and functioning.Crossref | GoogleScholarGoogle Scholar |
Greenslade, P. J. M. (1964). Pitfall trapping as a method for studying populations of Carabidae (Coleoptera). Journal of Animal Ecology 33, 301–310.
| Pitfall trapping as a method for studying populations of Carabidae (Coleoptera).Crossref | GoogleScholarGoogle Scholar |
Hansen, L., and Batzli, G. O. (1978). Influence of food availability on the white-footed mouse – populations in isolated woodlots. Canadian Journal of Zoology 56, 2530–2541.
| Influence of food availability on the white-footed mouse – populations in isolated woodlots.Crossref | GoogleScholarGoogle Scholar |
Hilborn, R., Redfield, J. A., and Krebs, C. J. (1976). Reliability of enumeration for mark and recapture census of voles. Canadian Journal of Zoology 54, 1019–1024.
| Reliability of enumeration for mark and recapture census of voles.Crossref | GoogleScholarGoogle Scholar |
Huxel, G. R., and McCann, K. (1998). Food web stability: the influence of trophic flows across habitats. American Naturalist 152, 460–469.
| Food web stability: the influence of trophic flows across habitats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cnit12gsg%3D%3D&md5=e70b2a5ce3a159ee40e54288c863cd48CAS | 18811452PubMed |
Jones, H. P. (2010). Prognosis for ecosystem recovery following rodent eradication and seabird restoration in an island archipelago. Ecological Applications 20, 1204–1216.
| Prognosis for ecosystem recovery following rodent eradication and seabird restoration in an island archipelago.Crossref | GoogleScholarGoogle Scholar | 20666244PubMed |
Kirkwood, R., Pemberton, D., Gales, R., Hoskins, A. J., Mitchell, T., Shaughnessy, P. D., and Arnould, J. P. Y. (2010). Continued population recovery by Australian fur seals. Marine and Freshwater Research 61, 695–701.
| Continued population recovery by Australian fur seals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvFKqur4%3D&md5=3ed876e9f5f9db4b7458415666e771d0CAS |
Leeson, R., and Wallis, R. (1986). Moult patterns in three species of small Victorian mammals. Australian Zoologist 23, 5–8.
Luff, M. L. (1975). Some features influencing the efficiency of pitfall traps. Oecologia 19, 345–357.
MacArthur, R. H., Diamond, J. M., and Karr, J. R. (1972). Density compensation in island faunas. Ecology 53, 330–342.
| Density compensation in island faunas.Crossref | GoogleScholarGoogle Scholar |
Markwell, T. J., and Daugherty, C. H. (2002). Invertebrate and lizard abundance is greater on seabird-inhabited islands than on seabird-free islands in the Marlborough Sounds, New Zealand. Ecoscience 9, 293–299.
Menkhorst, P. (1995). The swamp antechinus. In ‘Mammals of Victoria. Distribution, Ecology and Conservation’. pp. 43–44. (Oxford University Press: Melbourne.)
Miller, S., Bencini, R., Mills, H., and Moro, D. (2003). Food availability for the dibbler (Paratechinus apicalis) on Boullanger and Whitlock Islands, Western Australia. Wildlife Research 30, 649–654.
| Food availability for the dibbler (Paratechinus apicalis) on Boullanger and Whitlock Islands, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Minami, H., Minagawa, M., and Ogi, H. (1995). Changes in stable carbon and nitrogen isotope ratios in sooty and short-railed shearwaters during their northward migration. The Condor 97, 565–574.
| Changes in stable carbon and nitrogen isotope ratios in sooty and short-railed shearwaters during their northward migration.Crossref | GoogleScholarGoogle Scholar |
Mizukami, R. N., Goto, M., Izumiyama, S., Yoh, M., Ogura, N., and Hayashi, H. (2005). Temporal diet changes recorded by stable isotopes in Asiatic black bear (Ursus thibetanus) hair. Isotopes in Environmental and Health Studies 41, 87–94.
| Temporal diet changes recorded by stable isotopes in Asiatic black bear (Ursus thibetanus) hair.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtVWmsLw%3D&md5=a471f642b5ebb0b431ea3d57955794f8CAS | 15823859PubMed |
Mizutani, H., and Wada, E. (1988). Nitrogen and carbon isotope ratios in seabird rookeries and their ecological implications. Ecology 69, 340–349.
| Nitrogen and carbon isotope ratios in seabird rookeries and their ecological implications.Crossref | GoogleScholarGoogle Scholar |
Pafilis, P., Meiri, S., Foufopoulos, J., and Valakos, E. (2009). Intraspecific competition and high food availability are associated with insular gigantism in a lizard. Naturwissenschaften 96, 1107–1113.
| Intraspecific competition and high food availability are associated with insular gigantism in a lizard.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpvFaqsb0%3D&md5=061b7039a42e776a4c51155dea3b4626CAS | 19488731PubMed |
Palkovacs, E. P. (2003). Explaining adaptive shifts in body size on islands: a life history approach. Oikos 103, 37–44.
| Explaining adaptive shifts in body size on islands: a life history approach.Crossref | GoogleScholarGoogle Scholar |
Parrott, L. M., Ward, S. J., Temple-Smith, P. D., and Selwood, L. (2007). Effects of drought on weight, survival and breeding success of the agile antechinus (Antechinus agilis), dusky antechinus (A.swainsonii) and bush rats (Rattus fuscipes). Wildlife Research 34, 437–442.
| Effects of drought on weight, survival and breeding success of the agile antechinus (Antechinus agilis), dusky antechinus (A.swainsonii) and bush rats (Rattus fuscipes).Crossref | GoogleScholarGoogle Scholar |
Persson, L., Bengtsson, J., Menge, B. A., and Power, M. E. (1996). Productivity and consumer regulation – concepts, patterns and mechanism. In ‘Food Webs: Integration of Patterns and Dynamics’. (Eds G. A. Polis and K. Winemiller.) pp. 396–434. (Chapman Hall: New York.)
Peterson, B. J., and Fry, B. (1987). Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18, 293–320.
| Stable isotopes in ecosystem studies.Crossref | GoogleScholarGoogle Scholar |
Polis, G. A., and Hurd, S. D. (1996). Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. American Naturalist 147, 396–423.
| Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities.Crossref | GoogleScholarGoogle Scholar |
Polis, G. A., Anderson, W. B., and Holt, R. D. (1997). Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28, 289–316.
| Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs.Crossref | GoogleScholarGoogle Scholar |
Rayment, G. E., and Higginson, F. R. (1992). ‘Australian Laboratory Handbook of Soil and Water Chemical Methods.’ (Inkata Press: Melbourne.)
Rhind, S. G. (2002). Reproductive demographics among brush-tailed phascogales (Phascogale tapoatafa) in south-western Australia. Wildlife Research 29, 247–257.
| Reproductive demographics among brush-tailed phascogales (Phascogale tapoatafa) in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Roth, J. D. (2002). Temporal variability in arctic fox diet as reflected in stable-carbon isotopes; the importance of sea ice. Oecologia 133, 70–77.
| Temporal variability in arctic fox diet as reflected in stable-carbon isotopes; the importance of sea ice.Crossref | GoogleScholarGoogle Scholar |
Sale, M. G. (2008). Comparative ecology of island and mainland swamp antechinus populations. Ph.D. Thesis, Deakin University, Melbourne.
Sale, M. G., and Arnould, J. P. (2009). Spatial and temporal organization in the swamp antechinus: comparison between island and mainland populations. Journal of Mammalogy 90, 347–355.
| Spatial and temporal organization in the swamp antechinus: comparison between island and mainland populations.Crossref | GoogleScholarGoogle Scholar |
Sale, M. G., Ward, S. J., and Arnould, J. P. Y. (2006). Aspects of the ecology of swamp antechinus (Antechinus mininius maritimus) on a Bass Strait island. Wildlife Research 33, 215–221.
| Aspects of the ecology of swamp antechinus (Antechinus mininius maritimus) on a Bass Strait island.Crossref | GoogleScholarGoogle Scholar |
Sale, M. G., Wilson, B. A., and Arnould, J. P. Y. (2008). Factors influencing population dynamics in island and mainland populations of the swamp antechinus (Antechinus minimus). Australian Journal of Zoology 56, 187–194.
| Factors influencing population dynamics in island and mainland populations of the swamp antechinus (Antechinus minimus).Crossref | GoogleScholarGoogle Scholar |
Sale, M. G., Wilson, B. A., and Arnould, J. P. Y. (2009). Comparison of life-history characteristics of island and mainland populations of the swamp antechinus. Journal of Zoology 277, 119–125.
| Comparison of life-history characteristics of island and mainland populations of the swamp antechinus.Crossref | GoogleScholarGoogle Scholar |
Sánchez-Piñero, F., and Polis, G. A. (2000). Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81, 3117–3132.
Searle, P. L. (1984). The Bethelot or indophenol reaction and its use in the analytical chemistry of nitrogen. Analyst (London) 109, 549–568.
| The Bethelot or indophenol reaction and its use in the analytical chemistry of nitrogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXlsVartbk%3D&md5=e783715dec42e9f893a8716c19c63cbcCAS |
Slade, N. A., and Blair, M. (2000). An empirical test of using counts of individuals captured as indices of population size. Journal of Mammalogy 81, 1035–1045.
| An empirical test of using counts of individuals captured as indices of population size.Crossref | GoogleScholarGoogle Scholar |
Slade, N. A., and Russell, L. A. (1998). Distances as indices to movements and home-range size from trapping records of small mammals. Journal of Mammalogy 79, 346–351.
| Distances as indices to movements and home-range size from trapping records of small mammals.Crossref | GoogleScholarGoogle Scholar |
Smith, V. R. (1978). Animal–plant–soil nutrient relationships on Marion Island (Subantarctic). Oecologia 32, 239–253.
| Animal–plant–soil nutrient relationships on Marion Island (Subantarctic).Crossref | GoogleScholarGoogle Scholar |
Spiller, D. A., Piovia-Scott, J., Wright, A. N., Yang, L. H., Takimoto, G., Schoener, T. W., and Iwata, T. (2010). Marine subsidies have multiple effects on coastal food webs. Ecology 91, 1424–1434.
| Marine subsidies have multiple effects on coastal food webs.Crossref | GoogleScholarGoogle Scholar | 20503874PubMed |
Stapp, P. (2002). Stable isotopes reveal evidence of predation by ship rats on seabirds on the Shiant Islands, Scotland. Journal of Applied Ecology 39, 831–840.
| Stable isotopes reveal evidence of predation by ship rats on seabirds on the Shiant Islands, Scotland.Crossref | GoogleScholarGoogle Scholar |
Stapp, P., and Polis, G. A. (2003). Marine resources subsidize insular rodent populations in the Gulf of California, Mexico. Oecologia 134, 496–504.
| 12647121PubMed |
Stapp, P., Polis, G. A., and Pinero, F. S. (1999). Stable isotopes reveal strong marine and El Nino effects on island food webs. Nature 401, 467–469.
| Stable isotopes reveal strong marine and El Nino effects on island food webs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmslyru7o%3D&md5=d482f0d4123d0d4f865b69f465d4ec24CAS |
Steel, R. G. D., and Torrie, J. H. (1960). ‘Principles and Procedures of Statistics.’ (McGraw-Hill: New York.)
Stratham, H. L. (1982). Antechinus stuartii (Dasyuridae: Marsupialia) diet and food availability at Petroi, northwestern New South Wales. In ‘Carnivorous Marsupials’. (Ed. M. Archer.) pp. 151–163. (Royal Zoological Society of New South Wales: Sydney.)
Wainer, J. W. (1976). Studies of an island population of Antechinus minimus (Marsupialia, Dasyuridae). Australian Zoologist 19, 1–7.
Walkley, A., and Black, I. A. (1934). An examination of the Degtareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science 37, 29–38.
| An examination of the Degtareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaA2cXitlGmug%3D%3D&md5=ba87e7f6f19e8b159d36ce62eadd2dfdCAS |
Wilson, B. A., Aberton, J. G., and Reichl, T. (2001). Effects of fragmented habitat and fire on the distribution and ecology of the swamp antechinus (Antechinus minimus maritimus) in the eastern Otways, Victoria. Wildlife Research 28, 527–536.
| Effects of fragmented habitat and fire on the distribution and ecology of the swamp antechinus (Antechinus minimus maritimus) in the eastern Otways, Victoria.Crossref | GoogleScholarGoogle Scholar |
Wolfe, K. M., Mills, H. R., Garkaklis, M. J., and Bencini, R. (2004). Post-mating survival in a small marsupial is associated with nutrient inputs from seabirds. Ecology 85, 1740–1746.
| Post-mating survival in a small marsupial is associated with nutrient inputs from seabirds.Crossref | GoogleScholarGoogle Scholar |
Wood, D. H. (1970). An ecological study of Antechinus stuartii (Marsupialia) in a south-east Queensland rain forest. Australian Journal of Zoology 18, 185–207.
| An ecological study of Antechinus stuartii (Marsupialia) in a south-east Queensland rain forest.Crossref | GoogleScholarGoogle Scholar |


