First record of ‘climbing’ and ‘jumping’ by juvenile Galaxias truttaceus Valenciennes, 1846 (Galaxiidae) from south-western Australia
Paul G. Close A D , Tom J. Ryan A , David L. Morgan B , Stephen J. Beatty B and Craig S. Lawrence CA Centre of Excellence in Natural Resource Management, The University of Western Australia, PO Box 5771, Albany, WA 6330, Australia.
B Freshwater Fish Group and Fish Health Unit, School of Veterinary and Life Sciences, Murdoch University, Murdoch, WA 6150, Australia.
C Western Australian Fisheries and Marine Research Laboratories, Department of Fisheries, Hillarys, WA 6025, Australia.
D Corresponding author. Email: paul.close@uwa.edu.au
Australian Journal of Zoology 62(2) 175-179 https://doi.org/10.1071/ZO14004
Submitted: 4 February 2014 Accepted: 26 March 2014 Published: 17 April 2014
Abstract
Upstream migration of juvenile stages of temperate Australian amphidromous fish typically coincides with seasonally low river discharge when hydraulic (e.g. cascades) and physical (e.g. rock bars) barriers may be common. The ability to ‘climb’ or ‘jump’ may be expected to assist in negotiating low-flow barriers; however, it is presumed to be limited to a few native Australian freshwater fishes. Juvenile stages of Galaxias truttaceus Valenciennes, 1846 were observed ‘climbing’ and ‘jumping’ to successfully negotiate a low, vertical weir wall during their upstream recruitment migrations in south-western Australia. Based on this observation, we propose initial definitions for ‘climbing’ and ‘jumping’ to describe locomotory strategies employed by fishes to negotiate obstacles that would otherwise prevent free passage by normal swimming behaviour. Greater knowledge of the climbing, jumping and swimming performance, especially for small-bodied species and early life stages, will help improve the management of instream barriers for this critically endangered species and other freshwater fishes of southern Australia.
Additional keywords: amphidromy, definition, diadromous, fishway, instream barriers, locomotory, migration.
Fishes that undertake regular and predictable movements between freshwater and marine environments (i.e. diadromy: McDowall 1988), are a conspicuous component of coastal riverine fish communities in Australia; however, knowledge of their lifecycles and ecology is generally limited. The biology of at least half of the diadromous fishes in Australia is considered to be ‘mostly unknown’, and for 60% of these species this has been attributed to limited information on the biology of early life stages (Miles et al. 2013). In many cases, this has led to poor management decisions and consequently many of the Australian diadromous species are under increasing threat from a range of environmental impacts (Miles et al. 2013). In particular, the impact of instream barriers on migratory fish species is well documented, including the alteration of faunal assemblages and reduced ecosystem productivity (Harris 1984; Gehrke and Harris 2001; Lucas and Baras 2001; O’Hanley 2011). Instream barriers can also threaten the sustainability of individual populations by increasing predation rates, restricting the availability of habitats critical for spawning, feeding, or as nurseries and reducing overall recruitment success (Northcote 1998; Lucas and Baras 2001). As a result, fishways are commonly installed to enable fish passage past instream barriers for individual fish species, and also whole fish communities (Katopodis 1992; Mallen-Cooper 2000).
Upstream migration of juvenile life stages represents an important process in the life of amphidromous species (McDowall 1988). Amphidromy, a form of ‘diadromy’, is the regular, non-reproductive migration between freshwater and the sea, and typically involves spawning in freshwater habitats, development and growth of early life stages in marine habitats and the return-migration of juvenile life stages to freshwater habitats (McDowall 1988; Koehn and Crook 2013). In temperate systems with strongly seasonal river discharge, these upstream migrations typically occur between higher flow pulses in spring and autumn when river flows are relatively low (McDowall 1996; Morgan and Beatty 2006). While this seems logical, because early life stages may be expected to have comparatively weaker swimming ability (compared with adult stages), the timing of these migrations also coincides with periods when hydraulic (e.g. high-velocity riffles and cascades) and physical (e.g. rock-bars, log jams) barriers may be common (sensu Reinfelds et al. 2009). It may be reasonable to suspect, therefore, that there may be other locomotory strategies employed by migrating fish that could help overcome the increased prevalence of instream barriers during low-flow periods. While the relative swimming ability of some large-bodied Australian freshwater species (Mallen-Cooper 1994) and their life stages (Mallen-Cooper 1992; Lyon et al. 2008) have been documented, similar published information is generally lacking for most small-bodied Australian native freshwater fish species, with a few exceptions (e.g. Kilsby and Walker 2010, 2012).
The trout minnow, Galaxias truttaceus Valenciennes, 1846, is known from Western Australia, South Australia, Tasmania and Victoria, and King, Flinders and Clarke islands in Bass Strait (Allen et al. 2002). Although Western Australian populations of the species were originally described as a discrete taxon (Galaxias truttaceus hesperius Whitely, 1944), more recent studies synonymise G. truttaceus hesperius with G. truttaceus (Paxton et al. 2006). Notwithstanding this, G. truttaceus hesperius is listed Federally as ‘critically endangered’ under the Environment Protection and Biodiversity Conservation Act 1999, and ‘endangered’ by the Western Australian Government under the Wildlife Conservation Act 1950.
In Western Australia, the species is known from only three catchments located on the south coast (Morgan 2003; Colman 2010). Each of these populations is ‘landlocked’; however, they have retained a semiamphidromous life history (cf. McDowall 1988) and migrate between riverine and downstream lacustrine habitats (Morgan 2003; Morgan and Beatty 2006). For two of the known populations in Western Australia, lotic habitats supporting adult stages are typically short, low gradient with predominately low-flow velocity, while the third population exists in a river characterised by numerous rock bars separating extensive pool habitats.
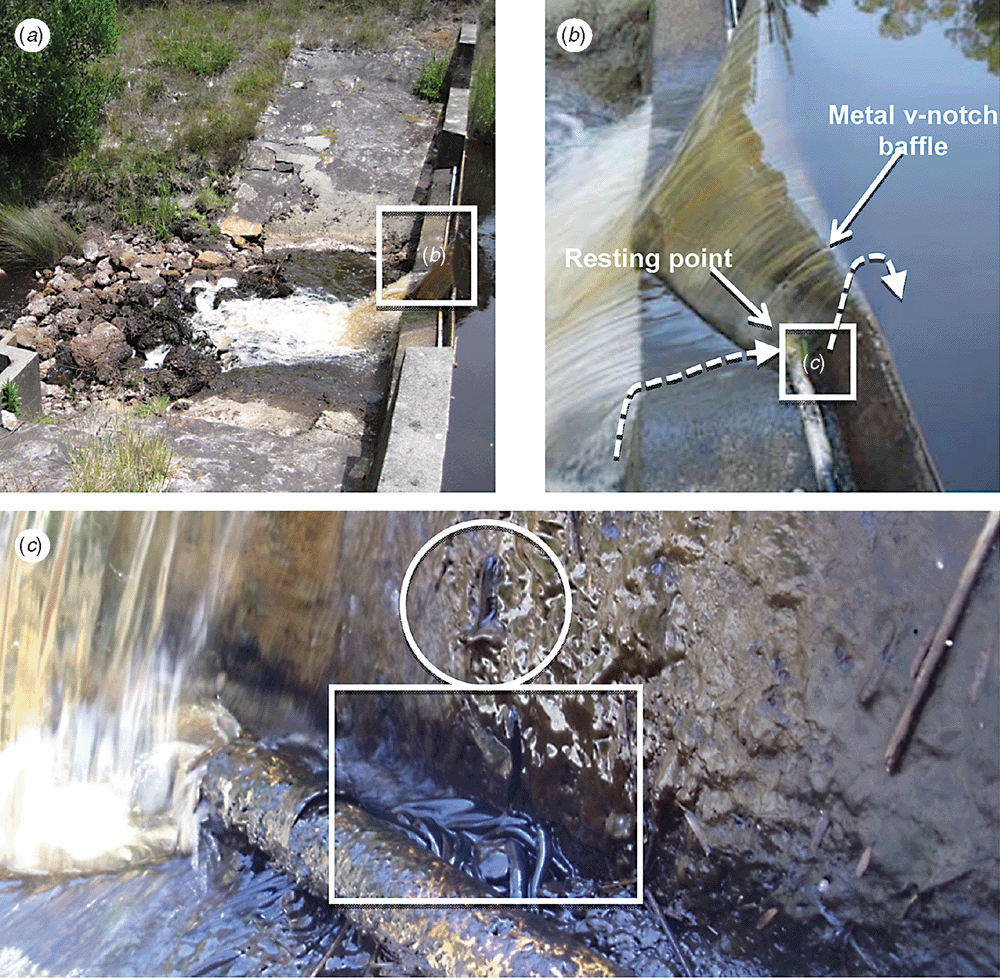
This paper reveals a previously undocumented climbing and jumping behaviour of G. truttaceus based on opportunistic observations of juvenile life stages successfully ascending a low-head vertical weir in the Goodga River during their upstream recruitment migration. The Goodga River weir (total height 1.5 m) is a typical V-notch concrete gauging structure comprising a vertical wall and a narrow angled step to which a partial-width metal baffle (~30 cm high) creates the V-notch construction (Fig. 1a) (see also Morgan and Beatty 2006). Prior to the construction of a vertical-slot fishway, this weir restricted the entire population of G. truttaceus to ~2 km of downstream riverine habitat (Morgan 2003).
Over two opportunistic, 20–30-min periods of observation in consecutive weeks of late October and early November 2013, a total of ~50 juvenile fish (40–45 mm standard length) were observed attempting to negotiate the Goodga River weir by ‘climbing’ vertically through the wetted margin or ‘splash zone’ (Fig. 1b). During this ascent, fish appeared to maintain connection with the substrate, using a modified swimming technique similar to the ‘wriggle’ movement described by Baker and Boubée (2006). Some individual fish were also observed to ‘rest’, mid-ascent, maintaining a stationary position on the vertical weir wall. Whether this ability is achieved through enhanced surface tension via modification to fin structures (e.g. McDowall 2003) remains unclear. Approximately 30–40% of these individuals successfully reached the narrow angled skirt below the metal V-notch baffle (Fig. 1b). The fate of those individuals unsuccessful in their attempt to ascend the vertical weir wall is unknown. On both occasions, up to 30 individual fish were observed congregated to the side of the flow and behind conduit piping that houses communication leads associated with the telemetered hydrographic station (Fig. 1c). These fish were exposed to the air, presumably for periods of minutes, but were kept moist with regular ‘water splash’. The fact that these individuals were capable of tolerating presumably short periods of exposure to air is interesting, although not unique in the Galaxiidae, which includes species capable of both long-term (e.g. aestivation) (Pusey 1989) and short-term (e.g. Magellan et al. 2014) exposure. Approximately 20 individual fish were observed attempting to ascend a height of 30 cm over the metal V-notch baffle (Fig. 1b, c), of which 15 (~75%) were successful. Those fish that successfully ascended the metal baffle employed the same ‘climbing’ technique described above; however, they did so with sufficient burst speed to achieve significant separation from the baffle and water surface (i.e. ‘jump’) and re-enter the water in the weir pool ~20 cm upstream from the metal baffle. Prior to reaching the weir wall, these fish also negotiated turbulent and high-velocity water created by the weir skirt (Fig. 1a). Over both periods of observation, all fish were observed attempting to negotiate wetted surfaces.
Based on the observations described here, we propose two definitions, ‘climbing’ and ‘jumping’, that describe locomotory techniques used by fish to negotiate obstacles (physical or hydraulic) that would otherwise prevent free passage by normal swimming behaviour. ‘Climbing’ by fish is defined as the ability to ascend a wetted or dry, vertical or near vertical obstacle using modified movement, behaviour and/or morphology (especially fin structure) while maintaining connection to the substratum and subject to full or partial exposure to air. This definition is consistent with observations by other researchers of fish climbing natural and artificial structures with vertical or high gradients often using behavioural and morphological modifications (McDowall 1993, 2003; Schoenfuss and Blob 2003; Blob et al. 2007; David et al. 2009). We also define ‘jumping’ by fish as the ability to achieve significant separation from the water or substrate (if exposed to air). This appears consistent with fish that have an ability to jump out of the water, e.g. propelled by a swimming burst, and also those species capable of ‘terrestrial’ jumping using tail-flip or axial bending behaviour (sensu Gibb et al. 2011).
The ability of G. truttaceus to ‘climb’ the vertical weir wall and to ‘jump’ contrasts with most other Australian native species and particularly their juvenile stages; less than 5% of ~205 native Australian freshwater species are thought to posses some ability to climb and jump (Allen et al. 2002). Within the Galaxiidae, Galaxias brevipinnis Günther, 1866 is known to have capabilities for climbing (McDowall 1996, 2003). Less definitive observations also exist for Galaxias olidus Günther, 1866 in high-altitude streams of south-eastern Australia (Green 2008). New Zealand galaxiids known to ‘climb’ include Galaxias fasciatus Gray, 1842, Galaxias postvectis Clarke, 1899 and the local form of G. brevipinnis (McDowall 1990, 2003; David et al. 2009). Moreover, David et al. (2009) demonstrated vertical climbing of G. fasciatus up a 0.5-m weir via polypropylene mussel spat ropes. Similar observations also exist for other Australian native species, including Gobiomorphus coxii (Krefft, 1864), Anguilla reinhardtii Steindachner, 1867 and Anguilla australis Richardson, 1841 (Beumer 1996; Larson and Hoese 1996), Geotria australis Gray, 1851 (Morgan et al. 1998) and members of the Sicydiine gobies (Donaldson et al. 2013). The ability of native Australian freshwater species to jump is largely unknown, although Stuart et al. (2006) noted that many large-bodied Australian native freshwater species may not. Whilst some small-bodied freshwater fish, e.g. Galaxias occidentalis Ogilby, 1899 (Morgan and Beatty 2008) and Nannatherina balstoni Regan, 1906 (Morgan 1993), have been observed jumping, this ability in other Australian freshwater species is poorly documented.
As many as 400 juvenile (young-of-year) G. truttaceus are known to use the Goodga River fishway per day during their upstream recruitment migration (Morgan and Beatty 2006). Those individuals reported here, successfully negotiating the vertical weir, may therefore represent only a small proportion of the total number of juvenile migrants. The reported absence of this species in habitats upstream of the weir before the opening of the fishway in 2003 (Morgan and Beatty 2006) suggests that, if individuals had successfully negotiated the weir historically, their numbers were insufficient to maintain a detectable population, and that it was the construction of the fishway that allowed for the establishment of a relatively abundant population (0.5 fish.1 m–2) upstream of the weir (Morgan and Beatty 2006).
It would seem reasonable to predict that juvenile stages of fish may be more capable of climbing given both their lower mass and also surface area upon which high-velocity water may impact. Both these factors may limit the ability of adults to ascend vertical structures (sensu Blob et al. 2007). Key considerations in the design of fish passage structures include both hydraulic and biological parameters (e.g. Rodríguez et al. 2006), the latter usually including the relative swimming ability of resident fish species such that water velocities do not exceed burst (<20 s), prolonged (20 s to 200 min) and sustained (>200 min) swimming capabilities (Beamish 1978). However, as demonstrated by our observations of G. truttaceus in south-western Australia (and observations by other researchers of climbing freshwater fish species in New Zealand), further consideration of the climbing and jumping capabilities is required for freshwater migratory species to improve our ability to prioritise mitigation works on instream barriers and help improve fishway design (sensu Baker and Boubée 2006; David et al. 2009; Doehring et al. 2011). If the management goal is to improve fish passage for accomplished climbers such as G. truttaceus in south-western Australia, design criteria for fish passage structures may be less complicated than previously thought.
Acknowledgements
We are grateful for the observations of Adam Pope, Rebecca Lester and Jan Barton and for the photographic images supplied by Judy Maughan. These observations were made as part of a broader study on the threatened freshwater fish of south-western Australia funded by the Western Australian Government’s State Natural Resource Management Program. All work was carried out under relevant permits: Department of Parks and Wildlife Licence to take fauna (SF009259) and Lawful Authority to access Conservation and Land Management areas, and Murdoch University Animal Ethics approval (RW2759/13).
References
Allen, G. R., Midgley, S. H., and Allen, M. (2002). ‘Field Guide to the Freshwater Fishes of Australia.’ (Western Australian Museum: Perth.)Baker, C. F., and Boubée, J. A. T. (2006). Upstream passage of inanga Galaxias maculatus and redfin bullies Gobiomorphus huttoni over artificial ramps. Journal of Fish Biology 69, 668–681.
| Upstream passage of inanga Galaxias maculatus and redfin bullies Gobiomorphus huttoni over artificial ramps.Crossref | GoogleScholarGoogle Scholar |
Beamish, F. W. H. (1978). Swimming capacity. In ‘Fish Physiology. Vol. VII’. (Eds W. S. Hoar and D. J. Randall.) pp. 101–187. (Academic Press: New York.)
Beumer, J. P. (1996). Family Anguillidae: freshwater eels. In ‘The Freshwater Fishes of South-eastern Australia’. (Ed. R. M. McDowall.) pp. 39–43. (Reed: Sydney.)
Blob, R. W., Wright, K. M., Becker, M., Maie, T., Iverson, T. J., Julius, M. L., and Schoenfuss, H. L. (2007). Ontogenetic change in novel functions: waterfall climbing in adult Hawaiian gobiid fishes. Journal of Zoology 273, 200–209.
| Ontogenetic change in novel functions: waterfall climbing in adult Hawaiian gobiid fishes.Crossref | GoogleScholarGoogle Scholar |
Colman, J. C. (2010). New records of Galaxias truttaceus (Galaxiidae) in the Kent River catchment, southwestern Australia. Journal of the Royal Society of Western Australia 93, 189–193.
David, B. O., Hamer, M. P., and Collier, K. J. (2009). Mussel spat ropes provide passage for banded kokopu (Galaxias fasciatus) in laboratory trials. New Zealand Journal of Marine and Freshwater Research 43, 883–888.
| Mussel spat ropes provide passage for banded kokopu (Galaxias fasciatus) in laboratory trials.Crossref | GoogleScholarGoogle Scholar |
Doehring, K., Young, R. G., and McIntosh, A. R. (2011). Factors affecting juvenile galaxiid fish passage at culverts. Marine and Freshwater Research 62, 38–45.
| Factors affecting juvenile galaxiid fish passage at culverts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtFGlsw%3D%3D&md5=66376e7ff72521fa2c2e7c2690472de9CAS |
Donaldson, J. A., Ebner, B. C., and Fulton, C. J. (2013). Flow velocity underpins microhabitat selection by gobies of the Australian Wet Tropics. Freshwater Biology 58, 1038–1051.
| Flow velocity underpins microhabitat selection by gobies of the Australian Wet Tropics.Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., and Harris, J. H. (2001). Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales, Australia. Regulated Rivers: Research & Management 17, 369–391.
| Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Gibb, A. C., Ashley-Ross, M. A., Pace, C. M., and Long, J. H. (2011). Fish out of water: terrestrial jumping by fully aquatic fishes. The Journal of Experimental Zoology 315A, 649–653.
| Fish out of water: terrestrial jumping by fully aquatic fishes.Crossref | GoogleScholarGoogle Scholar |
Green, K. (2008). Fragmented distribution of a rock climbing fish, the mountain galaxias Galaxias olidus, in the Snowy Mountains. Proceedings of the Linnean Society of New South Wales 129, 175–182.
Harris, J. H. (1984). Impoundment of coastal drainages of south-eastern Australia and a review of its relevance to fish migration. Australian Zoologist 21, 235–250.
Katopodis, C. (1992). ‘Introduction to fishway design.’ (Freshwater Institute, Department of Fisheries and Oceans: Winnipeg.)
Kilsby, N. N., and Walker, K. F. (2010). Linking the swimming ability of small freshwater fish to body form and ecological habit. Transactions of the Royal Society of South Australia 134, 89–96.
Kilsby, N. N., and Walker, K. F. (2012). Behaviour of two small pelagic and demersal fish species in diverse hydraulic environments. River Research and Applications 28, 543–553.
| Behaviour of two small pelagic and demersal fish species in diverse hydraulic environments.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Crook, D. A. (2013). Movements and migration. In ‘Ecology of Australian Freshwater Fishes’. (Eds P. Humphries and K. F. Walker.) (CSIRO Publishing: Melbourne.)
Larson, H. K., and Hoese, D. F. (1996). Family Gobiidae, subfamilies Eleotridinae and Butinae: gudgeons. In ‘The Freshwater Fishes of South-eastern Australia’. (Ed. R. M. McDowall.) pp. 200–219. (Reed: Sydney.)
Lucas, M. C., and Baras, E. (2001). ‘Migration of Freshwater Fishes.’ (Blackwell Science: Oxford.)
Lyon, J. P., Ryan, T. J., and Scroggie, M. P. (2008). Effects of temperature on the fast-start swimming performance of an Australian freshwater fish. Ecology of Freshwater Fish 17, 184–188.
| Effects of temperature on the fast-start swimming performance of an Australian freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Magellan, K., Pinchuck, S., and Swartz, E. R. (2014). Short and long-term strategies to facilitate aerial exposure in a galaxiid. Journal of Fish Biology 84, 748–758.
| Short and long-term strategies to facilitate aerial exposure in a galaxiid.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cvis1OlsQ%3D%3D&md5=e2c707da5f30ceafb651bdffa32405adCAS | 24502191PubMed |
Mallen-Cooper, M. (1992). Swimming ability of juvenile Australian bass, Macquaria novemaculeata (Steindachner), and juvenile barramundi, Lates calcarifer (Bloch), in an experimental vertical-slot fishway. Australian Journal of Marine and Freshwater Research 43, 823–834.
| Swimming ability of juvenile Australian bass, Macquaria novemaculeata (Steindachner), and juvenile barramundi, Lates calcarifer (Bloch), in an experimental vertical-slot fishway.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M. (1994). Swimming ability of adult golden perch, Macquaria ambigua (Percichthyidae), and adult silver perch, Bidyanus bidyanus (Teraponidae), in an experimental vertical-slot fishway. Australian Journal of Marine and Freshwater Research 45, 191–198.
| Swimming ability of adult golden perch, Macquaria ambigua (Percichthyidae), and adult silver perch, Bidyanus bidyanus (Teraponidae), in an experimental vertical-slot fishway.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M. (2000). Review of fish passage in NSW. NSW Fisheries, Sydney.
McDowall, R. M. (1988). ‘Diadromy in Fishes: Migrations Between Freshwater and Marine Environments.’ (Croom Helm: London.)
McDowall, R. M. (1990). ‘New Zealand Freshwater Fishes – A Natural History and Guide.’ (Heinemann Reed: Auckland.)
McDowall, R. M. (1993). Implications of diadromy for the structuring and modelling of riverine fish communities in New Zealand. New Zealand Journal of Marine and Freshwater Research 27, 453–462.
| Implications of diadromy for the structuring and modelling of riverine fish communities in New Zealand.Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (1996). ‘Freshwater Fishes of South-eastern Australia.’ (Reed Books: Sydney.)
McDowall, R. M. (2003). The key to climbing in the koaro. Water & Atmosphere 11, 16–17.
Miles, N. G., Walsh, C. T., Butler, G., Ueda, H., and West, R. J. (2013). Australian diadromous fishes – challenges and solutions for understanding migrations in the 21st century. Marine and Freshwater Research 65, 12–24.
| Australian diadromous fishes – challenges and solutions for understanding migrations in the 21st century.Crossref | GoogleScholarGoogle Scholar |
Morgan, D. L. (1993). The biology and ecology of Nannatherina balstoni Regan, 1906, in the D’Entrecasteaux National Park. B.Sc. (Honours) Thesis, Murdoch University, Perth.
Morgan, D. L. (2003). Distribution and biology of Galaxias truttaceus (Galaxiidae) in south-western Australia, including first evidence of parasitism of fishes in Western Australia by Ligula intestinalis (Cestoda). Environmental Biology of Fishes 66, 155–167.
| Distribution and biology of Galaxias truttaceus (Galaxiidae) in south-western Australia, including first evidence of parasitism of fishes in Western Australia by Ligula intestinalis (Cestoda).Crossref | GoogleScholarGoogle Scholar |
Morgan, D. L., and Beatty, S. J. (2006). Use of a vertical-slot fishway by galaxiids in Western Australia. Ecology Freshwater Fish 15, 500–509.
| Use of a vertical-slot fishway by galaxiids in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Morgan, D. L., and Beatty, S. J. (2008). Check structures and fish and crayfish fauna in the Harvey Irrigation Area. Murdoch University report to the Department of Water.
Morgan, D. L., Gill, H. S., and Potter, I. C. (1998). Distribution, identification and biology of freshwater fishes in southwestern Australia. Records of the Western Australian Museum , 1–97.
| 1:CAS:528:DyaK1cXmtVyhs78%3D&md5=fdc0336d5110fdb3b3c023d3d668822bCAS |
Northcote, T. G. (1998). Migratory behavior of fish and its significance to movement through riverine fish passage facilities. In ‘Fish Migration and Fish Bypasses’. (Eds M. Jungwirth, S. Schmutz and S. Weiss.) pp. 3–18. (Blackwell Science: Oxford.)
O’Hanley, J. R. (2011). Open rivers: barrier removal planning and restoration of free-flowing rivers. Journal of Environmental Management 92, 3112–3120.
| Open rivers: barrier removal planning and restoration of free-flowing rivers.Crossref | GoogleScholarGoogle Scholar | 21880412PubMed |
Paxton, J. R., Gates, J. E., Bray, D. J., and Hoese, D. F. (2006). Galaxiinae. Galaxias, native minnows, native trout. In ‘Zoological Catalogue of Australia’. (Eds P. L. Beesley, and A. Wells.) 35.2, 402–411.
Pusey, B. J. (1989). Aestivation in the teleost fish Lepidogalaxias salamandroides (Mees). Comparative Biochemistry and Physiology Part A: Physiology 92, 137–138.
| Aestivation in the teleost fish Lepidogalaxias salamandroides (Mees).Crossref | GoogleScholarGoogle Scholar |
Reinfelds, I., Lincoln-Smith, M., Haeusler, T., Ryan, D., and Growns, I. (2009). Hydraulic assessment of environmental flow regimes to facilitate fish passage through natural riffles: Shoalhaven River below Tallowa Dam, New South Wales, Australia. River Research and Applications 26, 589–604.
| Hydraulic assessment of environmental flow regimes to facilitate fish passage through natural riffles: Shoalhaven River below Tallowa Dam, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Rodríguez, T. T., Puertas Agudo, J., Mosquera, L. P., and Peña González, E. (2006). Evaluating vertical-slot fishway designs in terms of fish swimming capabilities. Ecological Engineering 27, 37–48.
| Evaluating vertical-slot fishway designs in terms of fish swimming capabilities.Crossref | GoogleScholarGoogle Scholar |
Schoenfuss, H. L., and Blob, R. W. (2003). Kinematics of waterfall climbing in Hawaiian freshwater fishes (Gobiidae): vertical propulsion at the aquatic–terrestrial interface. Journal of Zoology 261, 191–205.
| Kinematics of waterfall climbing in Hawaiian freshwater fishes (Gobiidae): vertical propulsion at the aquatic–terrestrial interface.Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., Williams, A., McKenzie, J., and Holt, T. (2006). Managing a migratory pest species: a selective trap for common carp. North American Journal of Fisheries Management 26, 888–893.
| Managing a migratory pest species: a selective trap for common carp.Crossref | GoogleScholarGoogle Scholar |