Predation of two common native frog species (Litoria ewingi and Crinia signifera) by freshwater invertebrates
Natasha J. Wilson A C , Jamie E. Seymour B and Craig R. Williams AA Sansom Institute for Health Research, School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, SA 5000, Australia.
B Queensland Tropical Health Alliance, James Cook University, Cairns, Qld 4878, Australia.
C Corresponding author. Email: natasha.wilson@mymail.unisa.edu.au
Australian Journal of Zoology 62(6) 483-490 https://doi.org/10.1071/ZO14026
Submitted: 11 April 2014 Accepted: 8 December 2014 Published: 15 January 2015
Abstract
The primary aim of this study was to identify aquatic invertebrate predators of amphibian eggs and tadpoles in an area of South Australia. The presence and abundance of aquatic invertebrates was monitored at four field sites for a period of 5–6 months; this revealed notonectids, freshwater crayfish and odonates to be amongst the most common invertebrate predator types. The ability of these predators to consume eggs and tadpoles of the native Australian frogs Litoria ewingi and Crinia signifera was then investigated. Freshwater crayfish (Cherax destructor) were the most prolific consumers of frog eggs and tadpoles. The notonectid Enithares woodwardi significantly impacted tadpole survivorship for both species while Anisops sp. was less successful at capturing and consuming these tadpoles. Caddisfly nymphs (Lectrides varians and Leptorussa darlingtoni) reduced egg survivorship but not to the same extent as C. destructor. Unlike some predators, which prey upon particular life stages, freshwater crayfish are large, polytrophic omnivores that can act as important predators of both anuran eggs and tadpoles. Given that predation is a key source of mortality in juveniles, identification of likely common predators is useful for understanding the regulation of amphibian populations.
Introduction
Preying on eggs and larvae, aquatic invertebrates can be an important contributor to amphibian mortality (Smith 1983; Alford 1999; Cruz et al. 2006). Despite this, invertebrate predators are typically less well studied than their vertebrate counterparts (Cabrera-Guzmán et al. 2012). Aquatic invertebrate predators are diverse and include several feeding modes: from those with biting and chewing mouth parts (e.g. odonate nymphs) to those with sucking mouthparts used to ingest internal body fluids (e.g. hemipterans). Several aquatic invertebrates are reported to prey on amphibian eggs and/or tadpoles; in particular, odonate naiads, aquatic hemipterans, beetles and freshwater crayfish are commonly reported predators (Axelsson et al. 1997; Alford 1999). However, limited data are available describing invertebrate predation of eggs and tadpoles for native amphibians in southern Australia.
In total, 27 frog species have been recorded in South Australia (Tyler and Walker 2011). The most abundant and common frogs recorded for the State are the common froglet (Crinia signifera), the spotted marsh frog (Limnodynastes tasmaniensis) and the southern brown tree frog (Litoria ewingi) (Walker 2003). Each of these species has a different mode of egg deposition which may in turn influence rates of predator encounter (Magnusson and Hero 1991; Stebbins and Cohen 1995). C. signifera lays eggs attached to submerged vegetation in small clusters of up to 30 eggs (Williamson 1988; Williamson and Bull 1995). L. tasmaniensis deposits spawn in foam nests on open water while the eggs of L. ewingi are typically attached in large clumps to the submerged tips of vegetation hanging into the water (Tyler and Walker 2011). All three species may co-occur in ponds in southern Australia (N. J. Wilson, pers. obs.) yet may have differing levels of vulnerability to aquatic invertebrate predators.
One population of C. signifera located at Bridgewater in the Adelaide Hills was previously studied extensively across three breeding seasons, identifying several invertebrate predators. The most abundant predators of C. signifera tadpoles included naiads of two dragonfly species (Aeshna brevistyla and Hemicordulia tau), larvae of the damselfly Austrolestes annulosus and the larvae of the dytiscid beetle Rhantus suturalis, which also acted as an egg predator (Williamson 1988; Williamson and Bull 1999). Both A. annulosus and R. suturalis were restricted to preying on small tadpoles (Williamson 1988). Although capable of taking larger tadpoles, both A. brevistyla and H. tau showed a preference for small tadpoles (Williamson 1988). The naiad of the dragonfly H. tau was also shown to prey on the tadpoles of L. tasmaniensis, L. ewingi and Pseudophryne bibronii (Richards and Bull 1990a, 1990b; Peterson et al. 1992). Caddisfly (Leptorussa darlingtoni) nymphs have been identified as potential predators of frog eggs in southern Australia, with reports that they fed on frog spawn when limited resources were available (Jackson 1984). Aside from these few accounts, little information exists on likely common invertebrate predators of immature frog stages in southern Australia.
Compared with the invertebrate predators of the rest of the country, invertebrate predators of northern Australia have received a little more attention. Specifically, researchers have investigated the capacity of invertebrate predators to consume the toxic eggs and tadpoles of the invasive cane toad, Rhinella marina (=Bufo marinus) (Crossland 1998; Crossland and Alford 1998; Cabrera-Guzmán et al. 2012). R. marina is toxic to many predators, owing to a suite of bufadienolides (cardiac glycosides) present in the eggs, larvae and the parotid glands of adult toads (Hayes et al. 2009). Since its introduction to Australia in 1935 the dispersal range of this invasive pest has continued to expand (Urban et al. 2007; Tyler and Knight 2009). Projections of the maximal distribution of R. marina have been modelled using existing distribution and bioclimatic data and suggest that the risk of colonisation extends to southern Australia (van Beurden 1981; Urban et al. 2007). Establishing which anuran predators are the most common is of interest when considering the potential risks posed to these predators with range expansion of R. marina.
While the impacts of this noxious amphibian have been well studied for many vertebrate predators (e.g. Phillips et al. 2003; Crossland et al. 2008; Letnic et al. 2008; Hagman et al. 2009; Greenlees and Shine 2011) less research has been conducted on invertebrate predators (Crossland and Alford 1998; Punzo and Lindstrom 2001; Ward-Fear et al. 2010; Cabrera-Guzmán et al. 2012). Previous investigation of invertebrate predators of R. marina has produced mixed results. Several invertebrate groups, including crayfish, beetles, odonates and nepids are reported to consume immature life stages of R. marina without ill affect (Crossland 1998; Cabrera-Guzmán et al. 2012). In contrast, other invertebrate predators such as leeches have succumbed to toxin ingestion and have died after feeding on immature life stages of R. marina (Crossland and Alford 1998). Further, some predators, such as dytiscid beetles and belostomatid bugs, can successfully prey upon particular life stages but ingestion of other developmental stages has been associated with mortality, suggesting ontogenetic variation in toxicity (Crossland 1998; Crossland and Alford 1998). If the invertebrate predators of native frog eggs and tadpoles are known, then we can study these species further to determine their susceptibility to toxins present in R. marina.
The primary aim of this study was to identify invertebrate predators of eggs and tadpoles of native amphibians. This was achieved by first identifying invertebrate predators abundant at field sites where frogs were present, and then determining the relative rate of egg/tadpole consumption by these putative predators in the laboratory. We sought to identify which predators may potentially play important roles in regulation of amphibian populations. This study is an initial step in identifying common invertebrate predators of native amphibians to inform further studies of these predators for their ability to consume toxic eggs and tadpoles of R. marina.
Methods
Study sites
Four field sites were assessed for the presence of frogs and putative invertebrate predators. Two sites were located within metropolitan Adelaide, South Australia, namely a groundpool at Globe Derby Park (34°47ʹ3.93ʹʹS, 138°35ʹ12.39ʹʹE), and an artificial watercourse situated within the grounds of the Grange Golf Club in Seaton (34°53ʹ4.80ʹʹS, 138°30ʹ3.06ʹʹE). Outside of the city, a wetland in Milang (35°24ʹ20.77ʹʹS, 138°58ʹ37.80ʹʹE) and, finally, what would become the focal site for numerous frog egg collections, a pond at Bridgewater (35°0ʹ13.24ʹʹS, 138°44ʹ55.62ʹʹE) in the Adelaide Hills, were also studied.
Seaton, Milang and Globe Derby Park were all sampled monthly in 2011 from August to December inclusive whereas the Bridgewater site was sampled from May to October in 2012. These sites were selected as they represent a diversity of common habitat types in southern Australia. A decision was made to include the Bridgewater site, which was sampled at a later date, after it was identified as a site of high frog abundance. The waterbodies surveyed in this study ranged in size from 160 to 4500 m2.
Establishing the presence of adult frogs
Audio strip transects adapted from Heyer et al. (1994) were used to count and identify calling adult males for each of the study sites. The total perimeter of each waterbody was sampled. One of us (NJW) stood quietly and stationary for 3 min at an initial sampling point and made note of calling frogs. Each additional sampling point was situated 25 m from the previous one, walking along the edge of the waterbody. Owing to the varying sizes of the sites, the number of sampling points ranged from just one at Seaton to 13 at Milang.
Evaluating presence and abundance of aquatic invertebrates, frog eggs and tadpoles
Tadpoles and aquatic invertebrates were sampled using a sweep net (260 × 180 mm frame, 0.3 mm mesh, Australian Entomological Supplies, Bangalow, NSW). For each location, a minimum of 10 sweeps, each 1 m in length, were performed following a protocol previously used by Gunzburger (2007). If additional species (i.e. not represented in any of the previous sweeps) were collected in the tenth sweep, additional sweeps were performed until the last 10% of sweeps netted no additional species (Heyer et al. 1994). The same researcher performed each sweep at all sites; sweep location was randomised using a random number table. Tadpole identification was assisted with a guide published by Anstis (2007).
All invertebrates collected in sweep nets were identified using Williams (1980). At a minimum, freshwater insects were classified to order. Due to the diversity and volume of organisms collected in field sampling, only invertebrates selected for predation trials have been identified to species level. Opera-house-style nets (700 mm L × 480 mm W × 190 mm H with two entry points that are both 75 mm in diameter) were baited with fresh meat and set for 16 h overnight to monitor waterbodies for crayfish.
The visual encounter technique was employed to search methodically for frog eggs at field sites. Each waterbody was searched along a series of transects that began at the margin and ran to the midpoint (or to a maximum depth of 45 cm). All frog eggs within 1 m of the transect were counted following Heyer et al. (1994).
Laboratory predation trials
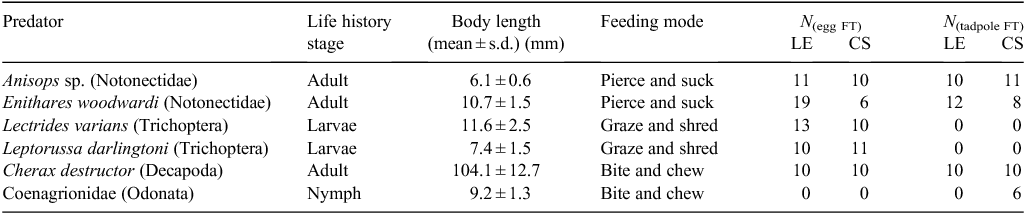
Feeding trials were conducted in the laboratory to establish consumption of frog eggs and tadpoles by a subset of the putative predators identified in field surveys. Some predators identified in field surveys were not present across the time frame that frog eggs and tadpoles were sampled (and thus not available for study) or were not present in sufficient abundance to perform a reasonable number of replicate feeding trials. Pilot trials conducted with the nepid Ranatra dispar, the dragonfly Adversaeschna brevistyla (Aeshnidae), adult coleopterans, and larval ephemeropterans suggest that these predators consume frog eggs and/or tadpoles. However, due to insufficient replication, these data are not presented. All frog eggs, tadpoles and predators (listed in Table 1) for laboratory trials were collected in the field. Field collections were transported to the laboratory in an insulated container and sorted immediately. Frog eggs and tadpoles were identified to species (Anstis 2007) and then staged according to Gosner (1960).
|
All predators were fasted for 24 h before onset of feeding trials. Predators were always tested in isolation in individual experimental tanks; the number of replicates performed for each predator treatment are listed in Table 1. Feeding trials ran for 72 h following introduction of prey. Each predator was offered either 10 live eggs (Gosner (1960) Stage 1–20) or 10 live tadpoles (Gosner Stage 21–26) and prey consumption was monitored across the 72-h period. Control trials (n = 6 for L. ewingi eggs, 11 with L. ewingi tadpoles, 7 for C. signifera eggs and 10 for C. signifera tadpoles) without predators were also conducted to determine baseline survivorship levels for eggs and tadpoles. Allocation of eggs and tadpoles to control and predator treatments was randomised.
All predators were tested in glass aquaria (100 mm L × 100 mm W × 200 mm H filled with 1 L aged tap water) except for crayfish, which were tested in 9-L containers (250 mm diameter) filled with 5 L of aged tap water. Trials were conducted in a temperature-controlled room set at 22°C with a 12 h light : dark cycle. Aquaria habitat was simplified and included a washed gravel substrate but no refuge.
As an extension of identifying invertebrates that prey on frog eggs and tadpoles, we also examined the rate of consumption to identify which predators could be considered major predators of frog eggs and/or tadpoles. We adopted the definition for a major predator as proposed by Portheault et al. (2007), that is, any predator consuming more than 50% of the eggs or tadpoles offered was considered to be a major predator. We considered that this would identify predators that may significantly affect survivorship of amphibian eggs and tadpoles in the field and thus may play important roles in population regulation.
Statistical analysis
Egg and tadpole survivorship in control and predator treatments was analysed with the Kruskal–Wallis test (owing to non-normally distributed data). Dunn’s Multiple Comparisons post hoc test was used to compare prey survivorship for each predator treatment to survivorship in the predator-free control (Zar 1999). If a predator significantly impacted egg or tadpole survivorship of both frog species we used the Mann–Whitney test to determine whether the feeding rate differed with C. signifera and L. ewingi prey.
We also used the Mann–Whitney test to determine whether predators that reached major predator status were significantly larger than those predators that consumed fewer than 50% of tadpoles in feeding trials. Predator size was determined by measuring body length (BL), which was defined as the distance between the anterior of the head and the posterior of the terminal abdominal segment. Crayfish were excluded from this analysis because they were more than nine times larger than all other tadpole predators studied and hence would heavily bias the data. Finally, an additional Mann–Whitney test was used to determine whether BL was significantly different between the two notonectid predators studied. Statistical significance was set at α = 0.05 for all tests performed.
Results
Putative invertebrate predator abundance
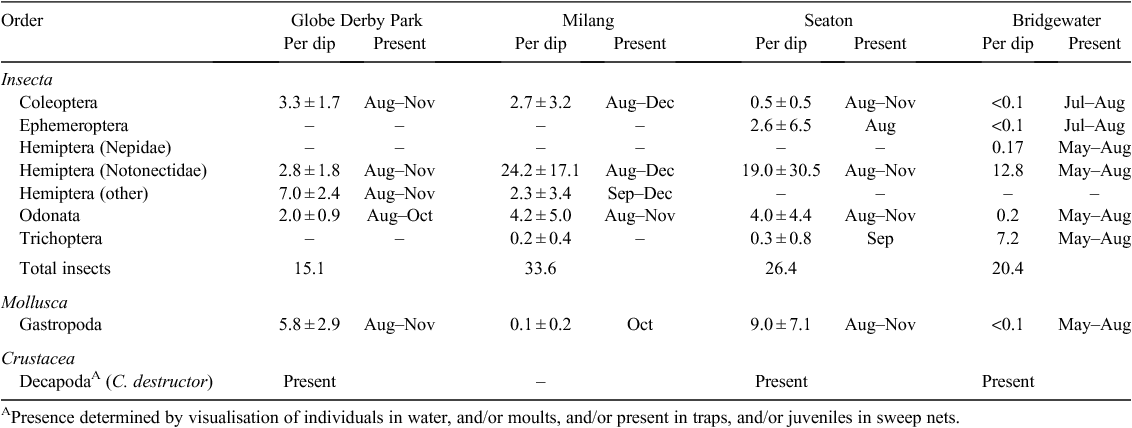
Notonectids and odonates were common to all waterbodies surveyed and ranked amongst the highest in terms of overall abundance compared with other predatory invertebrates (Table 2). Notonectid density was greatest at Milang, for which the mean catch (±s.e.m.) was 24.2 ± 17.1 individuals per dip. Caddisfly larvae were particularly common at Bridgewater and were also sampled on occasion at both Milang and Seaton. The common yabby (Cherax destructor) was detected at all sites except for Milang. Many of the invertebrates sampled in field site surveys were recognised by the researchers as strict herbivores and these species are not shown in Table 2.
|
Anuran field records
Three native species were recorded across the four field sites. C. signifera and L. tasmaniensis were detected at all sites at least once and L. ewingi was also recorded at the Bridgewater site (Table 3). Audio strip transects detected amphibians more frequently than did visual encounter egg surveys or tadpole dip netting, with adult frogs frequently detected while eggs and tadpoles were often absent (Table 3). The search time required to inspect each waterbody for eggs during the visual encounter surveys varied between sites, ranging from ~20 to 90 min depending on egg visibility. Egg visibility was lower in deep or turbid water and at densely vegetated sites, increasing the search time required to complete the survey.
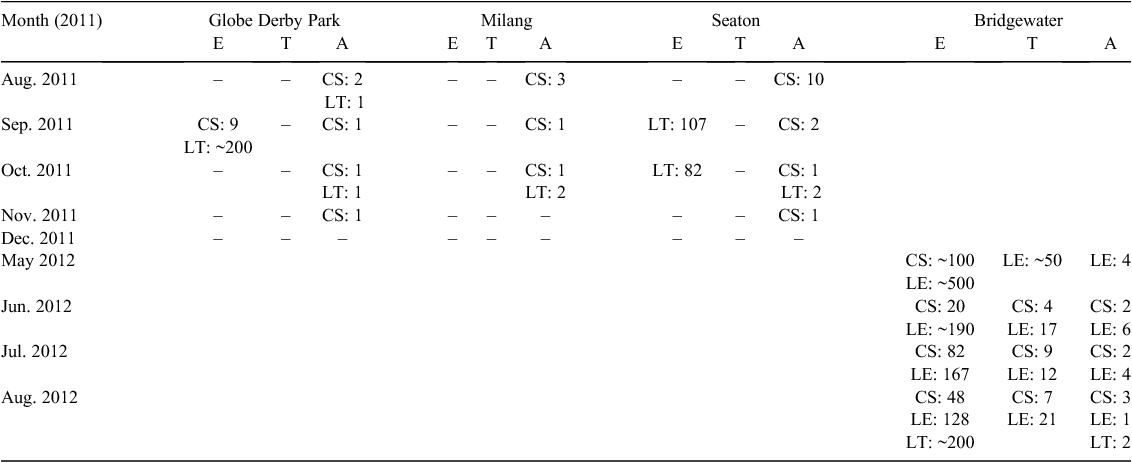
|
Consistent with the literature, L. ewingi eggs (mean clutch size ± s.d., 44.9 ± 32.3) were almost always found on the submerged tips of vegetation that was overhanging the pond, such as reeds/rushes (Tyler and Walker 2011). C. signifera attached eggs to submerged vegetation either individually or in small clumps (4.6 ± 4.8) while L. tasmaniensis deposited eggs in foam nests (91.0 ± 37.2). It was decided to focus on predator interactions with C. signifera and L. ewingi as the eggs and tadpoles of these frogs were by far the most abundant. Bridgewater became the focal field site for collection of both invertebrate predators and amphibian prey for laboratory trials.
Predation on amphibian eggs
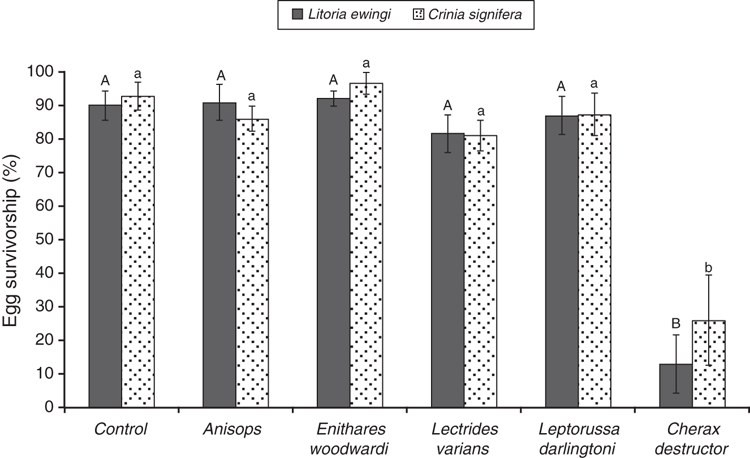
Notonectids (Anisops sp. and Enithares woodwardi) did not significantly affect egg survivorship for either species (Fig. 1). Although never reaching statistical significance, exposure of both L. ewingi and C. signifera eggs to the caddisfly larvae Lectrides varians and L. darlingtoni caused a 5–10% reduction in egg survivorship compared with controls (Fig. 1). The freshwater crayfish (C. destructor) was a major predator, significantly reducing egg survivorship of both species. Egg survivorship was reduced to 13% and 26% for L. ewingi (χ2 = 27.82, P = 0.001, Dunn’s Q = 3.15) and C. signifera (χ2 = 19.94, P = 0.001, Dunn’s Q = 3.21) respectively (Fig. 1). Prey identity was not a significant factor in rate of egg consumption by C. destructor (z = 0.76, P = 0.456).
Predation on tadpoles
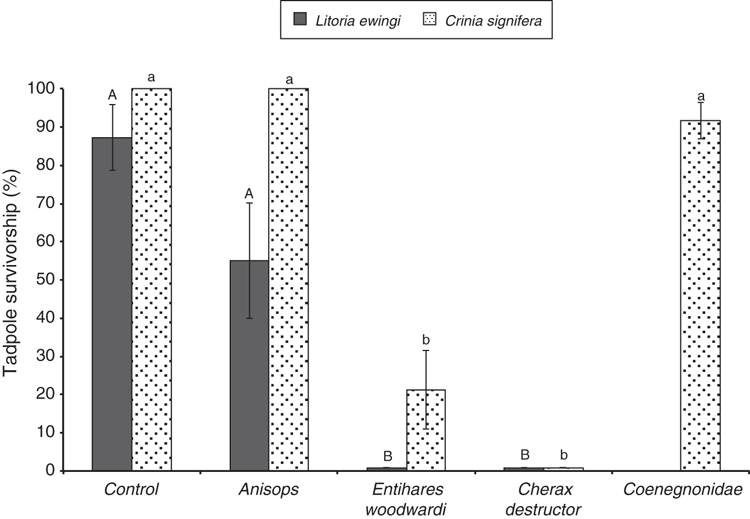
L. ewingi (mean ± s.d. SVL 3.3 ± 0.4 mm) and C. signifera (SVL 3.7 ± 0.7 mm) tadpoles were offered to the same notonectid and crayfish predator species that were offered eggs. Additionally, we also offered C. signifera tadpoles to coenagrionid damselfly nymphs. As depicted in Fig. 2, Anisops sp. was observed to consume some L. ewingi tadpoles, reducing survivorship to 86% compared with the control. However, Anisops sp. did not significantly impact survivorship of either anuran. In contrast, E. woodwardi was a major predator of tadpoles (χ2 = 32.11, P = 0.001, Dunn’s Q = 4.53). Enithares woodwardi consumed all available L. ewingi tadpoles in feeding trials. A significant reduction in C. signifera survivorship was also observed: just 21% of tadpoles survived exposure to E. woodwardi predators (χ2 = 40.84, P = 0.001, Dunn’s Q = 3.66) (Fig. 2). Prey identity did influence tadpole consumption rates by E. woodwardi: significantly more L. ewingi tadpoles were consumed than C. signifera tadpoles (z = 3.04, P = 0.002). C. destructor was again a major predator and always consumed all available tadpoles, regardless of prey species (L. ewingi: Dunn’s Q = 4.74; C. signifera: Dunn’s Q = 4.94). Coenagrionid damselfly nymphs consumed some C. signifera tadpoles but did not significantly reduce survivorship when compared with the control group. Surviving tadpoles in the damselfly treatment often sustained tail damage, indicating that they escaped following capture by a predator. Tadpoles in the control group were never observed to have any tail damage.
Influence of predator size
Predators that consumed more than 50% of offered tadpoles were significantly larger (as measured by BL) than predators that consumed less than 50% of tadpoles; this held true when crayfish were excluded from the analysis (z = 3.68, P = 0.002). There was a significant size difference between the two notonectid groups studied here: Enithares woodwardi were substantially larger than the Anisops predators (BL: 10.7 ± 1.5 and 6.1 ± 0.6 mm respectively) (Table 1) and this difference was statistically signficant (z = 8.88,P ≤ 0.001). This size difference was of interest given the predation rate differences between them.
Discussion
Field surveys of the study sites, which represented a range of habitat types from natural groundpools to artificially constructed wetlands, identified the presence of three frog species: C. signifera, L. ewingi and L. tasmaniensis. Frog eggs and tadpoles were most abundant at the Bridgewater site, which had one of the lowest total number of putative predators per dip in sweep surveys. In comparison, a high number of putative predators were recorded for Milang, where frog eggs and tadpoles were never detected. This result is tentatively suggestive that predator density may be an important predictor of egg and tadpole abundance for a given site. However, extrapolation from field data based on four sites should be considered cautiously.
Freshwater crayfish had the highest, per individual, consumption of amphibian eggs and tadpoles and were the only predators studied that preyed on both life stages. Crayfish are large invertebrates and have been identified by others (Momot 1995; Nyström et al. 1996) as keystone predators in freshwater environments, particularly in habitats lacking predatory fish. However, considering the high abundance of notonectids observed at field sites, and their size relative to crayfish, these aquatic hemipterans are also likely significant predators of young tadpoles/hatchlings in field settings. Collectively, notonectids may very well apply higher predation pressure than freshwater crayfish. Yet, unlike notonectids, freshwater crayfish are capable of preying on older, larger tadpoles (N. J. Wilson, unpubl. data). Comparison of the predation pressure exerted by crayfish and notonectids is beyond the scope of this paper and warrants further investigation.
Body length appeared to be an important determinant of tadpole predation success for notonectids. Enithares woodwardi was a major predator and significantly affected tadpole survivorship while the smaller Anisops notonectids were less successful despite observed attempts to strike tadpoles (N. J. Wilson, unpubl. data). The short BL of Anisops may limit their ability to overpower tadpole prey: size can be a limiting factor in predation success and has been described previously for notonectid predators (Cronin and Travis 1986; Streams 1994).
Notonectids did not prey on anuran eggs. This observation is consistent with the literature, which indicates that notonectids rely on vibration to detect and localise prey and thus mobility is a prerequisite of prey items (Murphey and Mendenhall 1973). Although they did not consume large quantities, caddisfly larvae appeared to have some impact on egg survivorship; this is a significant observation given that, to our knowledge, only one other report of consumption of amphibian eggs exists for Australian caddisflies (Jackson 1984). Survivorship of frog eggs in our feeding trials with L. darlingtoni scarcely deviated from the control, yet L. darlingtoni were previously reported to feed on frog eggs when food resources were limited (Jackson 1984). Given this, it is possible that consumption rates in feeding trials could increase with an extended fasting period. The lack of reports on caddisflies as amphibian predators within Australia is in contrast to the situation in North America and Europe, where caddisfly larvae are commonly reported to consume amphibian eggs (e.g. Murphy 1961; Stout et al. 1992; Majecki and Majecka 1996, 1998; Gall and Brodie 2011).
Damselfly nymphs did not prey significantly on tadpoles in this study. We suspect that the short BL of the damselfly nymphs used in feeding trials limited predation success. It has been previously noted that damselfly nymphs are successful at preying only upon small tadpoles (Williamson 1988). Tail injuries in surviving tadpoles were common (N. J. Wilson, unpubl. data) and suggest that damselfly nymphs were able to capture tadpoles but unable to overpower them.
The consumption rates reported here cannot reasonably be directly translated to estimates of predation in field settings and may better be thought of as an estimate of maximum rate of consumption. One limitation to this investigation is that predators were not provided with alternative prey. Provision of alternative prey may influence the consumption rates observed for these frog eggs and tadpoles. Predators do not typically feed indiscriminately and provision of alternative prey can influence consumption rates (Gunzburger and Travis 2005). Alternative prey, particularly in the form of macroinvertebrates, were present at the field sites surveyed and likely influence prey selection in the natural setting. One avenue of further study, which would further understanding of predator choice behaviour, would be to redesign the feeding trials as choice experiments and offer frog eggs or tadpoles in combination with an alternate prey that was abundant at field sites.
These feeding trials were conducted in small aquaria that lacked refugia and in no way mimic the habitat complexity seen in natural waterbodies. The simplistic nature of the laboratory set-up likely forces prey into closer contact with predators than would be encountered under normal circumstances in the field and probably increases capture rates (Alford 1999). Habitat structure, such as the presence of vegetation, has been shown to influence the outcome of predator–prey interactions. In one study, predation on tadpoles by dragonfly naiads and belostomatids was high in the absence of any vegetation (Tarr and Babbitt 2002). Another study demonstrated that tadpole survivorship was greater with high vegetation cover compared with lower levels of vegetation cover (Babbitt and Tanner 1997).
However, the provision of greater habitat complexity in this study may not have necessarily increased prey survival. Previous work has showed that provision of vegetation appears to offer no survival advantage against crayfish predators (Figiel and Semlitsch 1991). In some instances, vegetation has been shown to facilitate predation and thus reduce tadpole survivorship. Vegetation was reported to provide crayfish a framework for greater access to the upper regions of the water column as well as places to hide and wait for prey in one study (Davis et al. 2012).
Our research focus was to identify invertebrate predators of common and abundant tadpoles. In doing so, we aimed to identify predators that would likely encounter the eggs and tadpoles of the cane toad R. marina should the invasion front reach southern Australia. Although not specifically reporting on the same species investigated in this study, freshwater crayfish, odonate larvae and notonectids collected from within the present dispersal range of R. marina in northern Australia have been reported to consume immature developmental stages of R. marina (Crossland 1998; Crossland and Alford 1998). This suggests that, if encountered, the crayfish, notonectids and damselfly nymphs identified at field sites investigated in this study may attempt to prey on R. marina eggs and/or tadpoles. Future research could assess how these southern Australian predators fare against R. marina eggs and tadpoles in comparison to their northern (R. marina exposed) counterparts.
This work does not definitively describe the relative impact of different predators on amphibians. However, by identifying the most likely invertebrate predators and characterising their capacity for predation at an individual level, we have advanced our understanding of the role of such predators feeding on immature frogs in southern Australia. The freshwater crayfish (C. destructor) was a very efficient predator in laboratory trials, although it is not as numerous at field locations as notonectid hemipterans. Both may play important roles as regulators of amphibian assemblages. This study may have important implications for the design and management of wetland habitats and could inform conservation programs to assist with the protection of locally threatened frog species. An important future direction would be to research predation of the eggs and tadpoles of rarer frogs; these predators could have a significant impact on the survival of small or fragmented frog populations.
Acknowledgements
We thank David Doherty and Arbury Park Outdoor School for access to the Bridgewater site. We also thank the team at the Australian Water Quality Centre, Adelaide, for assistance with formal identification of invertebrates. Alice Wells (ABRS, Canberra) kindly provided Jean Jackson’s 1984 unpublished thesis. We also thank Marina Louter for assistance provided in the field. Finally, we thank two anonymous reviewers for their helpful and constructive comments; this feedback has been valuable and has helped strengthen the paper overall. This work was approved by the SA Pathology/CHN Animal Ethics Committee (permits 63/11 and 27/13).
References
Alford, R. A. (1999). Ecology: resource use, competition and predation. In ‘Tadpoles: The Biology of Anuran Larvae’. (Eds R. W. McDiarmid and R. Altig.) pp. 240–278. (The University of Chicago Press: Chicago.)Anstis, M. (2007). ‘Tadpoles of South-eastern Australia: a Guide with Keys.’ (New Holland Publishers: Sydney.)
Axelsson, E., Nyström, P., Sidenmark, J., and Brönmark, C. (1997). Crayfish predation on amphibian eggs and larvae. Amphibia-Reptilia 18, 217–228.
| Crayfish predation on amphibian eggs and larvae.Crossref | GoogleScholarGoogle Scholar |
Babbitt, K. J., and Tanner, G. W. (1997). Effects of cover and predator identity on predation of Hyla squirella tadpoles. Journal of Herpetology 31, 128–130.
| Effects of cover and predator identity on predation of Hyla squirella tadpoles.Crossref | GoogleScholarGoogle Scholar |
Cabrera-Guzmán, E., Crossland, M. R., and Shine, R. (2012). Predation on the eggs and larvae of invasive cane toads (Rhinella marina) by native aquatic invertebrates in tropical Australia. Biological Conservation 153, 1–9.
| Predation on the eggs and larvae of invasive cane toads (Rhinella marina) by native aquatic invertebrates in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Cronin, J. T., and Travis, J. (1986). Size-limited predation on larval Rana areolata (Anura: Ranidae) by two species of backswimmer (Insecta: Hemiptera: Notonectidae). Herpetologica 42, 171–174.
Crossland, M. R. (1998). Ontogenetic variation in toxicity of tadpoles of the introduced toad Bufo marinus to native Australian aquatic invertebrate predators. Herpetologica 54, 364–369.
Crossland, M. R., and Alford, R. A. (1998). Evaluation of the toxicity of eggs, hatchlings and tadpoles of the introduced toad Bufo marinus (Anura: Bufonidae) to native Australian aquatic predators. Australian Journal of Ecology 23, 129–137.
| Evaluation of the toxicity of eggs, hatchlings and tadpoles of the introduced toad Bufo marinus (Anura: Bufonidae) to native Australian aquatic predators.Crossref | GoogleScholarGoogle Scholar |
Crossland, M. R., Brown, G. P., Anstis, M., Shilton, C. M., and Shine, R. (2008). Mass mortality of native anuran tadpoles in tropical Australia due to the invasive cane toad (Bufo marinus). Biological Conservation 141, 2387–2394.
| Mass mortality of native anuran tadpoles in tropical Australia due to the invasive cane toad (Bufo marinus).Crossref | GoogleScholarGoogle Scholar |
Cruz, M. J., Pascoal, S., Tejedo, M., and Rebelo, R. (2006). Predation by an exotic crayfish, Procambarus clarkii, on natterjack toad, Bufo calamita, embryos: its role on the exclusion of this amphibian from its breeding ponds. Copeia 2006, 274–280.
| Predation by an exotic crayfish, Procambarus clarkii, on natterjack toad, Bufo calamita, embryos: its role on the exclusion of this amphibian from its breeding ponds.Crossref | GoogleScholarGoogle Scholar |
Davis, M. J., Purrenhage, J. L., and Boone, M. D. (2012). Elucidating predator–prey interactions using aquatic microcosms: complex effects of a crayfish predator, vegetation, and atrazine on tadpole survival and behavior. Journal of Herpetology 46, 527–534.
| Elucidating predator–prey interactions using aquatic microcosms: complex effects of a crayfish predator, vegetation, and atrazine on tadpole survival and behavior.Crossref | GoogleScholarGoogle Scholar |
Figiel, J. C. R., and Semlitsch, R. D. (1991). Effects of nonlethal injury and habitat complexity on predation in tadpole populations. Canadian Journal of Zoology 69, 830–834.
| Effects of nonlethal injury and habitat complexity on predation in tadpole populations.Crossref | GoogleScholarGoogle Scholar |
Gall, B. G., and Brodie, E. D. (2011). Survival and growth of the caddisfly Limnephilus flavastellus after predation on toxic eggs of the rough-skinned newt (Taricha granulosa). Canadian Journal of Zoology 89, 483–489.
| Survival and growth of the caddisfly Limnephilus flavastellus after predation on toxic eggs of the rough-skinned newt (Taricha granulosa).Crossref | GoogleScholarGoogle Scholar |
Gosner, K. (1960). A simplified table for staging anuran embryos and larvae with notes on identificaton. Herpetologica 16, 183–190.
Greenlees, M. J., and Shine, R. (2011). Impacts of eggs and tadpoles of the invasive cane toad (Bufo marinus) on aquatic predators in tropical Australia. Austral Ecology 36, 53–58.
| Impacts of eggs and tadpoles of the invasive cane toad (Bufo marinus) on aquatic predators in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Gunzburger, M. S. (2007). Evaluation of seven aquatic sampling methods for amphibians and other aquatic fauna. Applied Herpetology 4, 47–63.
| Evaluation of seven aquatic sampling methods for amphibians and other aquatic fauna.Crossref | GoogleScholarGoogle Scholar |
Gunzburger, M. S., and Travis, J. (2005). Critical literature review of the evidence for unpalatability of amphibian eggs and larvae. Journal of Herpetology 39, 547–571.
| Critical literature review of the evidence for unpalatability of amphibian eggs and larvae.Crossref | GoogleScholarGoogle Scholar |
Hagman, M., Phillips, B. L., and Shine, R. (2009). Fatal attraction: adaptations to prey on native frogs imperil snakes after invasion of toxic toads. Proceedings of the Royal Society B: Biological Sciences 276, 2813–2818.
| Fatal attraction: adaptations to prey on native frogs imperil snakes after invasion of toxic toads.Crossref | GoogleScholarGoogle Scholar | 19419984PubMed |
Hayes, R., Crossland, M., Hagman, M., Capon, R., and Shine, R. (2009). Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): toxin profiles and effects on predators. Journal of Chemical Ecology 35, 391–399.
| Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): toxin profiles and effects on predators.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlt1Kis74%3D&md5=5ce249a0fbdff88bbb6e205533a1d7b8CAS | 19263169PubMed |
Heyer, W. R., Donnelly, M. A., McDiarmid, R. W., and Hayek, L. A. F. M. S. (Eds) (1994). ‘Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians.’ Biological Diversity Handbook Series (Smithsonian Institution Press: Washington, DC.)
Jackson, J. E. (1984). Taxonomy, biology and case function of Lectrides varians Mosely and Leptorussa darlingtoni Banks larvae (Trichoptera: Leptoceridae). B.Sc.(Honours) Thesis, University of Adelaide.
Letnic, M., Webb, J. K., and Shine, R. (2008). Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia. Biological Conservation 141, 1773–1782.
| Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Magnusson, W., and Hero, J.-M. (1991). Predation and the evolution of complex oviposition behaviour in Amazon rainforest frogs. Oecologia 86, 310–318.
| Predation and the evolution of complex oviposition behaviour in Amazon rainforest frogs.Crossref | GoogleScholarGoogle Scholar |
Majecki, J., and Majecka, K. (1996). Predation of Oligotricha striata caddis larvae on amphibian eggs: effects of a high quality food on growth rate. Netherlands Journal of Aquatic Ecology 30, 21–25.
| Predation of Oligotricha striata caddis larvae on amphibian eggs: effects of a high quality food on growth rate.Crossref | GoogleScholarGoogle Scholar |
Majecki, J., and Majecka, K. (1998). Predation of Oligotricha striata (Trichoptera, Phryganeidae) larvae on amphibian eggs. Amphibia-Reptilia 19, 230–233.
| Predation of Oligotricha striata (Trichoptera, Phryganeidae) larvae on amphibian eggs.Crossref | GoogleScholarGoogle Scholar |
Momot, W. T. (1995). Redefining the role of crayfish in aquatic ecosystems. Reviews in Fisheries Science 3, 33–63.
| Redefining the role of crayfish in aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar |
Murphey, R. K., and Mendenhall, B. (1973). Localization of receptors controlling orientation to prey by the back swimmer Notonecta undulata. Journal of Comparative Physiology 84, 19–30.
| Localization of receptors controlling orientation to prey by the back swimmer Notonecta undulata.Crossref | GoogleScholarGoogle Scholar |
Murphy, T. D. (1961). Predation on the eggs of the salamander, Ambystoma maculatum, by caddisfly larvae. Copeia 1961, 495–496.
| Predation on the eggs of the salamander, Ambystoma maculatum, by caddisfly larvae.Crossref | GoogleScholarGoogle Scholar |
Nyström, P. E. R., Brönmark, C., and Granéli, W. (1996). Patterns in benthic food webs: a role for omnivorous crayfish? Freshwater Biology 36, 631–646.
| Patterns in benthic food webs: a role for omnivorous crayfish?Crossref | GoogleScholarGoogle Scholar |
Peterson, A. G., Bull, C. M., and Wheeler, L. M. (1992). Habitat choice and predator avoidance in tadpoles. Journal of Herpetology 26, 142–146.
| Habitat choice and predator avoidance in tadpoles.Crossref | GoogleScholarGoogle Scholar |
Phillips, B. L., Brown, G. P., and Shine, R. (2003). Assessing the potential impact of cane toads on Australian snakes. Conservation Biology 17, 1738–1747.
| Assessing the potential impact of cane toads on Australian snakes.Crossref | GoogleScholarGoogle Scholar |
Portheault, A., Diaz-Paniagua, C., and Gomez-Rodriguez, C. (2007). Predation on amphibian eggs and larvae in temporary ponds: the case of Bufo calamita in southwestern Spain. Revue d’Ecologie: La Terre et la Vie 62, 315–322.
Punzo, F., and Lindstrom, L. (2001). The toxicity of eggs of the giant toad, Bufo marinus to aquatic predators in a Florida retention pond. Journal of Herpetology 35, 693–697.
| The toxicity of eggs of the giant toad, Bufo marinus to aquatic predators in a Florida retention pond.Crossref | GoogleScholarGoogle Scholar |
Richards, S. J., and Bull, C. M. (1990a). Non-visual detection of anuran tadpoles by odonate larvae. Journal of Herpetology 24, 311–313.
| Non-visual detection of anuran tadpoles by odonate larvae.Crossref | GoogleScholarGoogle Scholar |
Richards, S., and Bull, C. (1990b). Size-limited predation on tadpoles of three Australian frogs. Copeia 1990, 1041–1046.
| Size-limited predation on tadpoles of three Australian frogs.Crossref | GoogleScholarGoogle Scholar |
Smith, D. C. (1983). Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) on Isle Royale, Michigan. Ecology 64, 501–510.
| Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) on Isle Royale, Michigan.Crossref | GoogleScholarGoogle Scholar |
Stebbins, R. C., and Cohen, N. W. (1995). ‘A Natural History of Amphibians.’ (Princeton University Press: Princeton, NJ.)
Stout, B. M., Stout, K. K., and Stihler, C. W. (1992). Predation by the caddisfly Banksiola dossuaria on egg masses of the spotted salamander Ambystoma maculatum. American Midland Naturalist 127, 368–372.
| Predation by the caddisfly Banksiola dossuaria on egg masses of the spotted salamander Ambystoma maculatum.Crossref | GoogleScholarGoogle Scholar |
Streams, F. A. (1994). Effect of prey size on attack components of the functional response by Notonecta undulata. Oecologia 98, 57–63.
| Effect of prey size on attack components of the functional response by Notonecta undulata.Crossref | GoogleScholarGoogle Scholar |
Tarr, T. L., and Babbitt, K. J. (2002). Effects of habitat complexity and predator identity on predation of Rana clamitans larvae. Amphibia-Reptilia 23, 13–20.
| Effects of habitat complexity and predator identity on predation of Rana clamitans larvae.Crossref | GoogleScholarGoogle Scholar |
Tyler, M. J., and Knight, F. (2009). ‘Field Guide to the Frogs of Australia.’ (CSIRO Publishing: Melbourne.)
Tyler, M. J., and Walker, S. J. (2011). ‘Frogs of South Australia.’ 3rd edn. (Michael Tyler and Associates: Adelaide.)
Urban, M. C., Phillips, B. L., Skelly, D. K., and Shine, R. (2007). The cane toad’s (Chaunus [Bufo] marinus) increasing ability to invade Australia is revealed by a dynamically updated range model. Proceedings of the Royal Society B: Biological Sciences 274, 1413–1419.
| The cane toad’s (Chaunus [Bufo] marinus) increasing ability to invade Australia is revealed by a dynamically updated range model.Crossref | GoogleScholarGoogle Scholar | 17389221PubMed |
van Beurden, E. K. (1981). Bioclimatic limits to the spread of Bufo marinus in Australia: a baseline. Proceedings of the Ecological Society of Australia 11, 143–149.
Walker, S. (2003). Frog Census 2002. Community monitoring of water quality and habitat condition in South Australia using frogs as indicators. Environment Protection Authority, Adelaide.
Ward-Fear, G., Brown, G. P., and Shine, R. (2010). Using a native predator (the meat ant, Iridomyrmex reburrus) to reduce the abundance of an invasive species (the cane toad, Bufo marinus) in tropical Australia. Journal of Applied Ecology 47, 273–280.
| Using a native predator (the meat ant, Iridomyrmex reburrus) to reduce the abundance of an invasive species (the cane toad, Bufo marinus) in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1980). ‘Australian Freshwater Life: the Invertebrates of Australian Inland Waters.’ 2nd edn. (Macmillan: Melbourne.)
Williamson, I. (1988). Ecology and life history variation in a population of the frog Ranidella signifera. Ph.D. Thesis, Flinders University, Adelaide.
Williamson, I., and Bull, C. M. (1995). Life-history variation in a population of the Australian frog Ranidella signifera: seasonal changes in clutch parameters. Copeia 1995, 105–113.
| Life-history variation in a population of the Australian frog Ranidella signifera: seasonal changes in clutch parameters.Crossref | GoogleScholarGoogle Scholar |
Williamson, I., and Bull, C. M. (1999). Population ecology of the Australian frog Crinia signifera: larvae. Wildlife Research 26, 81–99.
| Population ecology of the Australian frog Crinia signifera: larvae.Crossref | GoogleScholarGoogle Scholar |
Zar, J. H. (1999). ‘Biostatistical Analysis.’ 4th edn. (Prentice Hall: New Jersey.)