Nesting habitat of the broad-shelled turtle (Chelodina expansa)
Kristen Petrov A , Heidi Stricker A , James U. Van Dyke A B E , Graham Stockfeld C , Peter West D and Ricky-John Spencer AA WildLab, School of Science and Health, Hawkesbury Institute, Western Sydney University, Locked Bag 1797, Penrith South DC, NSW 2751, Australia.
B Institute for Land, Water and Society, School of Environmental Sciences, Charles Sturt University, Albury–Wodonga Campus, Albury, NSW 2640, Australia.
C Turtles Australia Inc., 34 Ashlar Crescent, Blackburn, Vic. 3130, Australia.
D NSW Department of Primary Industries, 1447 Forest Road, Orange, NSW 2800, Australia.
E Corresponding author. Email: jvandyke@csu.edu.au
Australian Journal of Zoology 66(1) 4-14 https://doi.org/10.1071/ZO17061
Submitted: 21 September 2017 Accepted: 7 March 2018 Published: 3 April 2018
Abstract
Turtles have persisted for over 220 million years, despite facing threats at every life-history stage. In Australia, nest predation by introduced foxes has driven severe declines in some populations. Our project quantified the nesting habitat of the endangered broad-shelled turtle (Chelodina expansa) to facilitate protection of critical nesting grounds. We determined the nesting preferences of C. expansa at five distinct wetlands on the Murray River from 2011 to 2014. We identified environmental variables associated with nest sites in different habitats and compared those at nests and non-nest sites to determine nesting preferences. Kernel density estimates were used to identify important nesting grounds. Our study has important implications for conservation of C. expansa. Habitat preferences for nest sites of C. expansa are predictable both within and across sites, with females preferring to nest ~50 m from shore (~4 m elevation), in open habitat with little vegetation. Based on these habitat preferences, kernel density estimates showed that C. expansa may select the same nesting beaches in subsequent years. Fox depredation of nests (and nesting adults) drives turtle declines in Australia, so identifying nesting areas for protection is a first step in turtle conservation.
Additional keywords: conservation ecology, habitat preference, natural selection, reproduction.
Introduction
In turtles, embryo and hatchling success are significantly influenced by nest-site selection (Wilson 1998; Kolbe and Janzen 2002a; Spencer 2002a; Micheli-Campbell et al. 2013). Nest micro- and macrohabitats influence life-history traits, such as survival and growth (Wood and Bjorndal 2000; Kolbe and Janzen 2002a; Spencer 2002a; Micheli-Campbell et al. 2013). Selecting an appropriate nest site is thus fundamental for embryonic development and increasing offspring survival (Hughes and Brooks 2006; Tamplin and Cyr 2011). The effects of incubation temperature, in particular, pervade turtle life histories. Incubation temperature affects embryonic developmental rates, body size, performance, thermoregulation behaviour, and sex (Wood and Bjorndal 2000; Kolbe and Janzen 2002a; Dormer et al. 2016). Incubation temperature also affects long-term survival and age at maturity (Spencer 2002a; Spencer and Janzen 2010). Environmental conditions at potential nest sites can vary considerably and one variable, vegetation cover, is an important cue that females use to select nesting locations, because it directly affects the temperature of the nest (Janzen 1994; Wilson 1998; Janzen and Morjan 2001; Kolbe and Janzen 2002a).
Predation is another factor that may significantly influence nest-site selection. In general, nesting further away from shore decreases predation of freshwater turtle nests (Kolbe and Janzen 2002b; Marchand et al. 2002; Marchand and Litvaitis 2004) because nests are less clustered and predators cannot use linear search patterns to detect them (Robinson and Bider 1988; Marchand and Litvaitis 2004). However, nesting behaviour is also influenced by mothers’ perception of their own predation risk (Wood and Bjorndal 2000; Spencer 2002a), thus the ultimate position of a nest is likely to be a balance between maximising offspring success and minimising adult female mortality (Spencer and Thompson 2003).
In Australia, non-native species such as the European fox (Vulpes vulpes) have a significant impact on native freshwater turtle populations. The Murray River turtle (Emydura macquarii) and the common long-necked turtle (Chelodina longicollis), which inhabit the Murray River and adjoining waterways, are intensively depredated (nests and adults) by foxes (Thompson 1983; Spencer and Thompson 2005). Nest depredation rates are over 90% annually for both of these species (Thompson 1983; Spencer 2002a; Spencer et al. 2017). The broad-shelled turtle (Chelodina expansa) is sympatric with both E. macquarii and C. longicollis, and is listed as endangered in Victoria (e.g. DSE 2013). Chelodina expansa nests after rain and prefers to nest uphill, 30–300 m from the water’s edge (Booth 2010). However, C. expansa employs solitary nesting strategies and nest in autumn, unlike both E. macquarii and C. longicollis, which nest more communally in spring (Bowen et al. 2005). This solitary autumn nesting strategy may, to some degree, reduce nest predation rates of C. expansa (Spencer and Thompson 2005; Spencer et al. 2016). However, C. expansa embryos undergo diapause over the winter after oviposition and eggs are underground for up to 12 months before they hatch (Booth 2002). Given that incubation spans several seasons and is far longer than that of most other species of turtle, it is critical for females to select nest sites that both facilitate embryonic development and protect eggs from predators. Developing protocols to predict preferred nest sites of this cryptic endangered species is thus an important tool for its conservation.
Here, we determine the maternal nesting preferences of C. expansa in a range of natural and modified habitats on the Murray River, Victoria. We tested three aims over subsequent years: (1) in 2013 we determined the macro- and microhabitat characteristics of depredated C. expansa nests at Cockatoo Lagoon and compared these characteristics to non-nest locations at Cockatoo Lagoon; (2) in 2014, we determined how the locations of depredated C. expansa nests varied across multiple sites located along the Murray River, Victoria, by comparing macro- and microhabitat data collected at each nest, and (3) we determined whether populations of C. expansa nest at similar nesting beaches, within a site, across multiple years. Notably, our study focussed on the characteristics of nests depredated by invasive red foxes, because non-depredated nests are highly cryptic. Prior studies indicate that invasive foxes destroy 95% or more of all turtle nests present in the Murray catchment (Thompson 1983; Spencer and Thompson 2005; Spencer et al. 2016, 2017), so we assume that these depredated nests represent most of the nesting effort by C. expansa at our study sites.
Methods
Study species and sites
Chelodina expansa is the largest Australian chelid turtle (Goode 1967). It inhabits permanent water bodies along the Murray–Darling river system and coastal rivers of south-eastern Queensland, from the Logan–Albert drainage in the south to the Fitzroy drainage in the north. Offshore populations occur on Fraser, Moreton, and Stradbroke Islands, Queensland (Chessman 1988). C. expansa is a specialised predator and is solely carnivorous, predominantly consuming crustaceans, small fish, and aquatic insects (Chessman 1983). Clutch size is closely correlated with body size, and C. expansa delays maturity to 14–15 years to increase its reproductive output (Spencer 2002b).
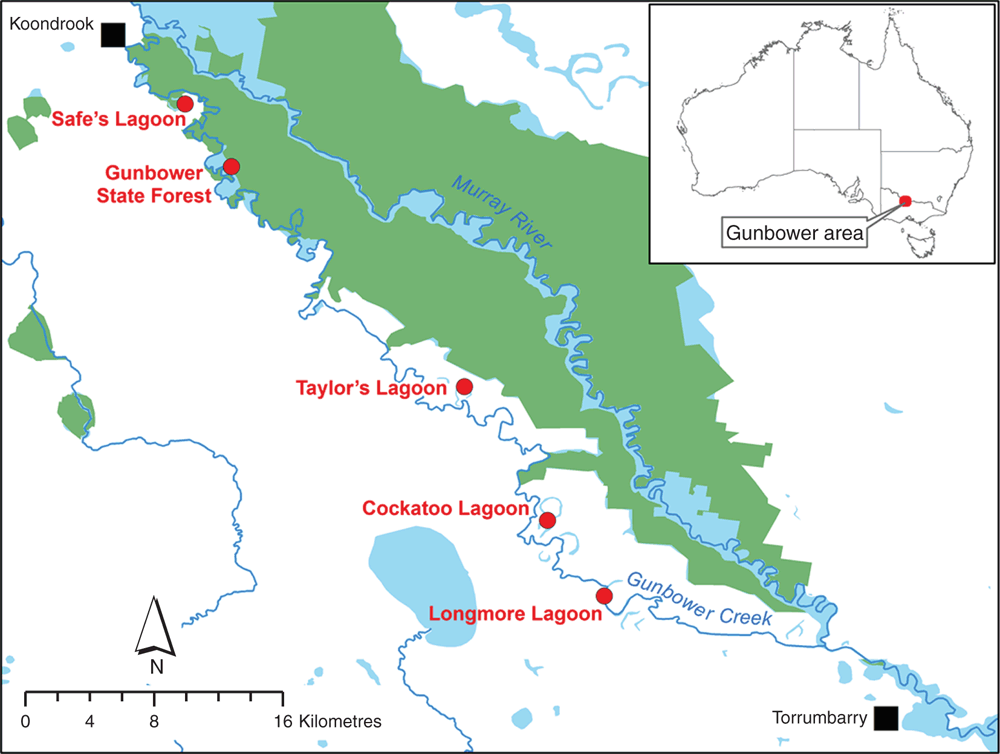
Our study sites were five separate wetlands located in Gunbower Forest on the Murray River floodplain near Gunbower and Cohuna, Victoria (Fig. 1). The Gunbower Forest wetlands are protected under the Convention on Wetlands of International Importance, i.e. Ramsar Convention (Department of Primary Industries 2013). The surrounding area is a mix of woodland and agricultural pastures. Data on C. expansa nesting have been collected at the Gunbower Lagoons of this study since 2011 (Turtles Australia Inc., pers comm.) and will be analysed as part of this current study.
Within-site comparison of C. expansa nest characteristics
In 2013 we determined the macro- and microhabitat characteristics of depredated C. expansa nests at Cockatoo Lagoon and compared these characteristics to non-nest locations at Cockatoo Lagoon. Cockatoo Lagoon is an oxbow of Gunbower Creek and is characterised by irrigated pasture, stubble sandhills and cattle grazing. Between 11 April and 10 May 2013 riparian zones of Cockatoo Lagoon were searched for depredated nests. Site surveys were undertaken during the known C. expansa nesting season, which occurs from March to June in Queensland populations (Booth 2010). We focussed our surveys on March–May due to previous reports of C. expansa nesting during this period at the Gunbower Lagoons (Turtle Australia Inc., pers comm.). Study sites were walked by a team of four people, with inspections undertaken across the various sites for up to 8 h per day, between 0800 and 1800 hours. Depredated turtle nests were identified as shallow, bare-dirt holes in the ground, ~12–15 cm deep, with scattered turtle eggshells nearby. C. expansa nests were specifically distinguished from nests of E. macquarii and C. longicollis through the presence of fresh eggshells, since C. expansa nests 4–6 months after the other species. Notably, the bare dirt hole and bright white eggshells of a depredated turtle nest contrast sharply with the adjacent ground, which is typically flat and brown-rust in colour, or covered with vegetation and/or leaf litter. Because all our surveys took place during or just after the nesting season, there is little opportunity for dirt, leaf litter, or vegetation to fill in the holes. Foxes are capable of destroying nests rapidly, including within 24 h of laying, and we have observed a single fox destroying 15 nests in just 5 h (Spencer et al. 2016). For these reasons, we argue that our ability to detect depredated nests is consistent across all habitat types and sites in our study. Latitude and longitude of nests were recorded with a Garmin GPSmap 62 Series, with an accuracy of 3 ± 1 m. Once recorded, nests were either filled or a marker stake was placed at the nest to avoid it being recorded twice.
During April–May 2013, macro- and microhabitat characteristics of nest sites were recorded and compared with non-nest sites at Cockatoo Lagoon. Non-nest sites were located 1–10 m, 11–25 m, 26–40 m, 41–60 m, 61–80 m, 81–100 m, 101–120 m before the nest, towards the water’s edge. Another category, 5 m beyond the nest, in the direction away from the water’s edge, was also sampled. Each category was not included if an individual nest was located closer than 120 m from shore. By using this approach, we assume that turtles would walk from the water to their nesting site in a straight line, and we acknowledge that our results may be biased as a consequence. However, it would be impossible to remove this bias without knowing the exact path each turtle took. Thus, we assume the impact of our bias here is similar across all nests included in our analysis due to our consistent approach.
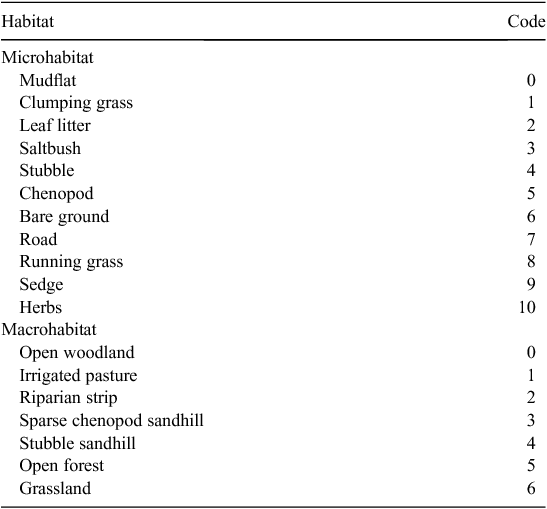
At each nest and non-nest site, we characterised the general macro- and microhabitat type (Table 1). Additional macro- and microhabitat characteristics of nest sites were then quantified and compared with measurements of the same variables for sites that were not selected by the nesting turtles. Macrohabitat variables measured were elevation and distance from the nest to the shore at each nest and non-nest site. Distances (m) to the shore were measured using a measuring wheel from the nest to the shore in a straight line. Elevation was measured using a laser level. Microhabitat variables that were measured were percentage coverages of vegetation, leaf litter and bare ground, percentage canopy cover, distance to the nearest vegetation that was less than 2 m tall, distance to the nearest vegetation that was greater than 2 m tall, and distance from the selected nest to the nearest nest and non-nest site. Percentage coverages of vegetation, leaf litter and bare ground were visually estimated after placing 1-m2 quadrats over each nest and non-nest point. We measured canopy cover above the nests and non-nest sites using a densitometer (Janzen 1994; Wilson 1998; Janzen and Morjan 2001). The densitometer has a convex mirror that reflects the overstorey vegetation. We held the densitometer over the nest and counted the number of squares covered by vegetation and multiplied this figure by 1.04 to obtain the percentage of the densitometer not covered by vegetation. This figure was then subtracted from 100% to obtain the canopy cover percentage. Distances (m) to the nearest shrub or tree, and nest, from each nest and non-nest site were measured in a straight line, using a measuring wheel. All variables measured were chosen because of their potential to impact nesting success, such as the thermal and hydric effects of canopy and/or ground cover, or the potential for nearness to the water level to indicate potential flooding risk.
|
Across-site comparisons of C. expansa nest characteristics
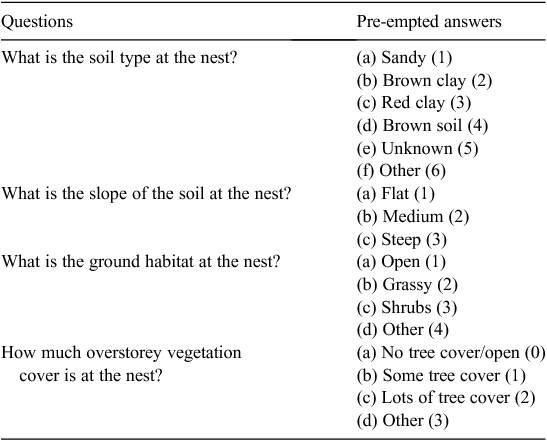
In March–April 2014, we determined how the locations of depredated C. expansa nests varied across multiple sites located along the Murray River, Victoria, by comparing macro- and microhabitat data collected at each depredated nest. We aimed to determine whether microhabitat nesting preferences of C. expansa differ across a larger area. Between 25 March and 22 April, riparian zones of Cockatoo Lagoon, Gunbower State Forest, Longmore Lagoon, Safe’s Lagoon, and Taylor’s Lagoon were searched for depredated nests. The main land practices at Cockatoo Lagoon, Longmore Lagoon, Safe’s Lagoon, and Taylor’s Lagoon are irrigated pasture and cattle grazing. Gunbower State Forest is characterised by red gum (Eucalyptus camaldulensis) forest. Sites were walked by a team of three people, with inspection undertaken across the various sites for up to 8 h per day, between 0800 and 1800 hours. At each nest, TurtleSAT, a Citizen Science tool that utilises the GPS function of mobile phones, was used to record nest location, date and time observed and micro- and macrohabitat nest data (TurtleSAT 2014). TurtleSAT is valuable for identifying hotspots of adult mortality (e.g. road deaths), as well as identify nesting grounds, and can be applied to any turtle species in Australia. TurtleSAT asks the user a series of questions relating to nest characteristics (see Table 2). The user answers the questions from a list of pre-empted answers, each of which was assigned a categorical code for analysis (Table 2). Nest data were also imported into Google Earth™ and qualitatively characterised based on the macrohabitat (Table 3). Distance to water, nearest tree and riparian zone of each nest were also determined by drawing a straight line from the nest to each variable using the ruler tool in Google Earth™. We acknowledge that mobile phone GPS locations may be accurate to only 5–10 m, but we assume that this inaccuracy applied randomly to all of the locations uploaded to TurtleSAT such that any significant differences that we detected in our analyses are real.
|

|
Data analyses
Microhabitat variables of nesting and non-nesting areas were compared using Analysis of Similarity (ANOSIM) and SIMPER analysis in PRIMER software (Clarke and Warwick 2001). The same analysis was used to compare nesting habitats among wetlands in 2014.
Latitude and longitude data from all nests found during April–May of 2013 and March–April of 2014, as well as data collected in an identical manner in 2011–12 (Turtles Australia Inc., pers comm.) were imported into ArcMAP (ESRI, ver. 10). To determine whether populations of C. expansa nest at similar nesting beaches within a site, across multiple years, we collated data from 2011–14 and used Spatial analysis (kernel density estimates, KDE). KDE calculate the density of nests within an area and overlay data from consecutive years to determine nesting hotspots within the study sites. Statistically, the kernel density technique is a better hot spot identifier than cluster analysis techniques (Shahrabi and Pelot 2009). Kernel density methods create a continuous surface to represent density variability over the entire study area, not just in certain clusters as in other hot spot techniques (Shahrabi and Pelot 2009). Kernel density methods allow density surfaces of incidents and activities to be easily compared (Shahrabi and Pelot 2009). We have chosen to show only KDE analysis from Cockatoo and Safe’s Lagoons in our results due to high densities of C. expansa nests over the 2011–14 period.
Results
Nesting preferences
Chelodina expansa nesting occurred any time from mid-March to late April and was often triggered by rain. During our study, all nesting events we observed occurred between 1200 and 1800 hours. Nests were generally clustered along elevated sections of the bank of the wetlands or on adjacent sandhills.
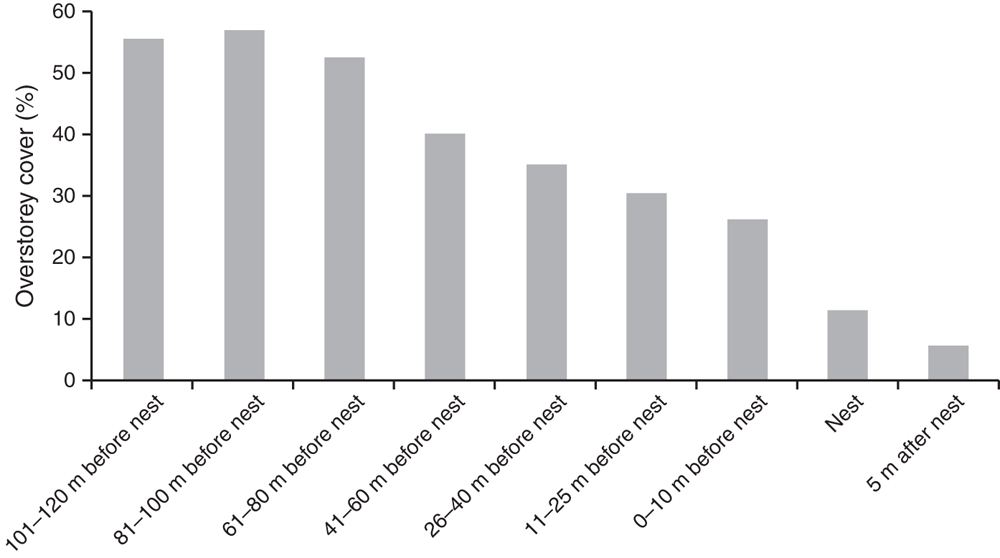
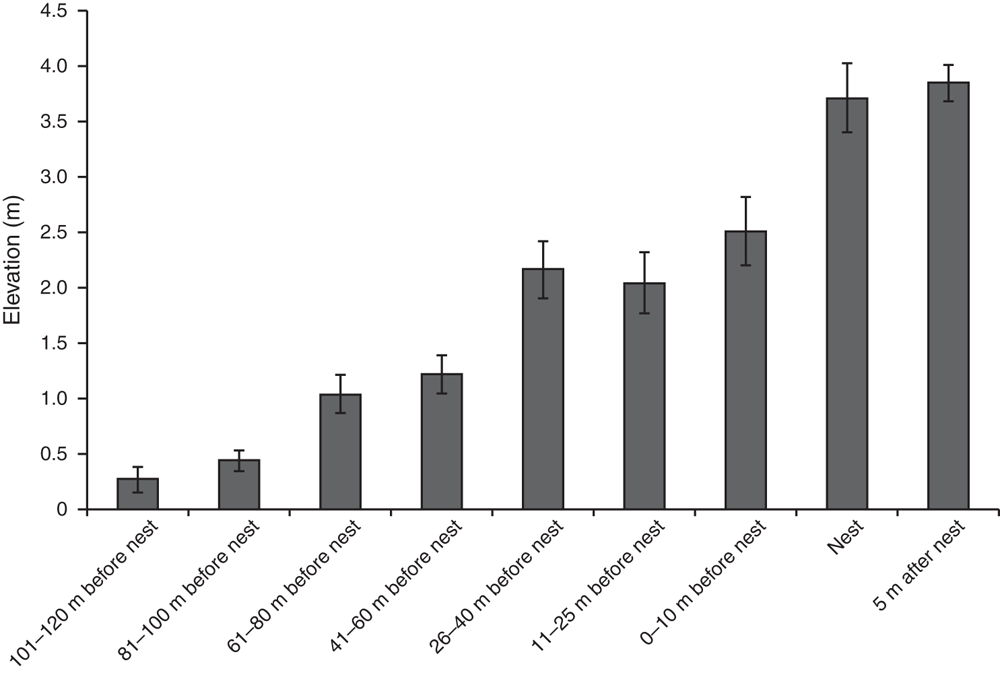
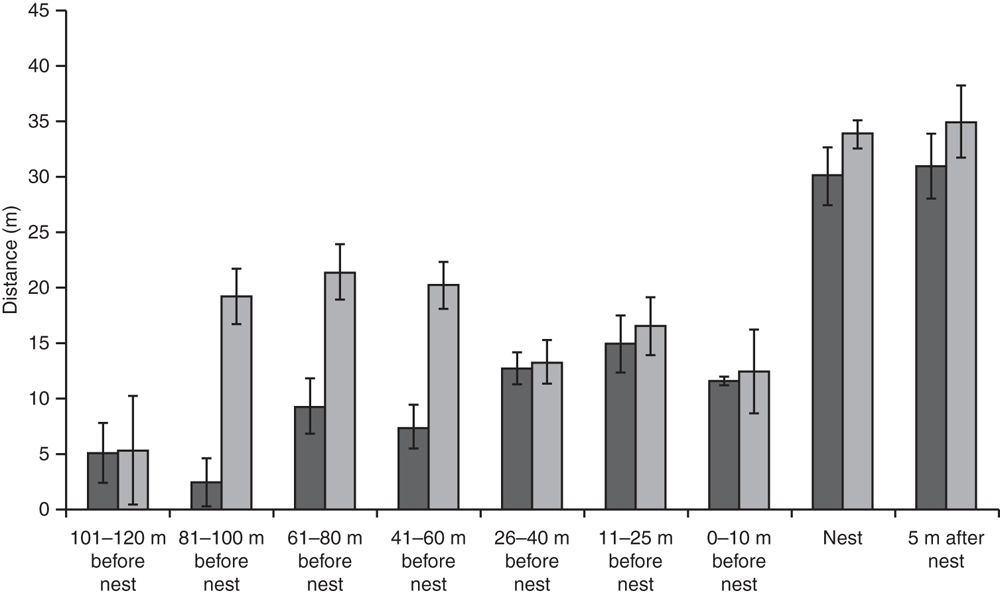
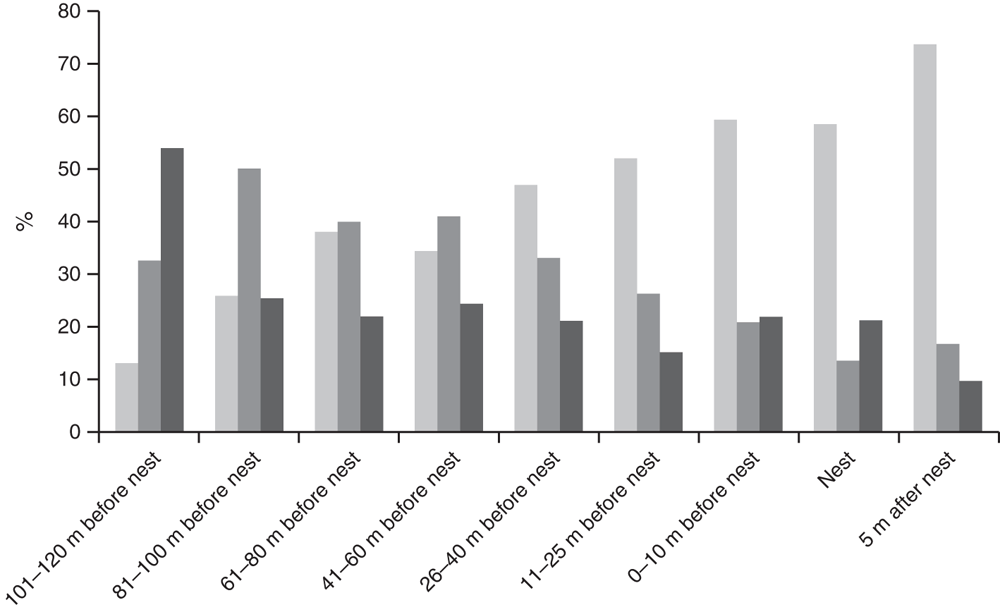
In 2013 habitat variables of nest sites were significantly different from those of areas that turtles might traverse before nesting, and from those beyond the nesting site (Global R = 0.337, P = 0.001). As female C. expansa moved farther from the water to nest, the elevation of the nest increased (Fig. 2). Nest sites were significantly farther from trees and shrubs compared with areas ≥10 m closer to water (Fig. 3). Thus, habitat variables related to tree and shrub cover, such as canopy cover, were significantly reduced over nests compared with at non-nesting sites (Fig. 4). Overstorey cover at the nest (10%) was generally twice that of 5 m beyond the nest (5%) and the average distance to trees was ~10 m further. Bare ground also consistently increased as distance from water increased, such that nests usually had little grass or leaf litter cover (Fig. 5).
|
|
|
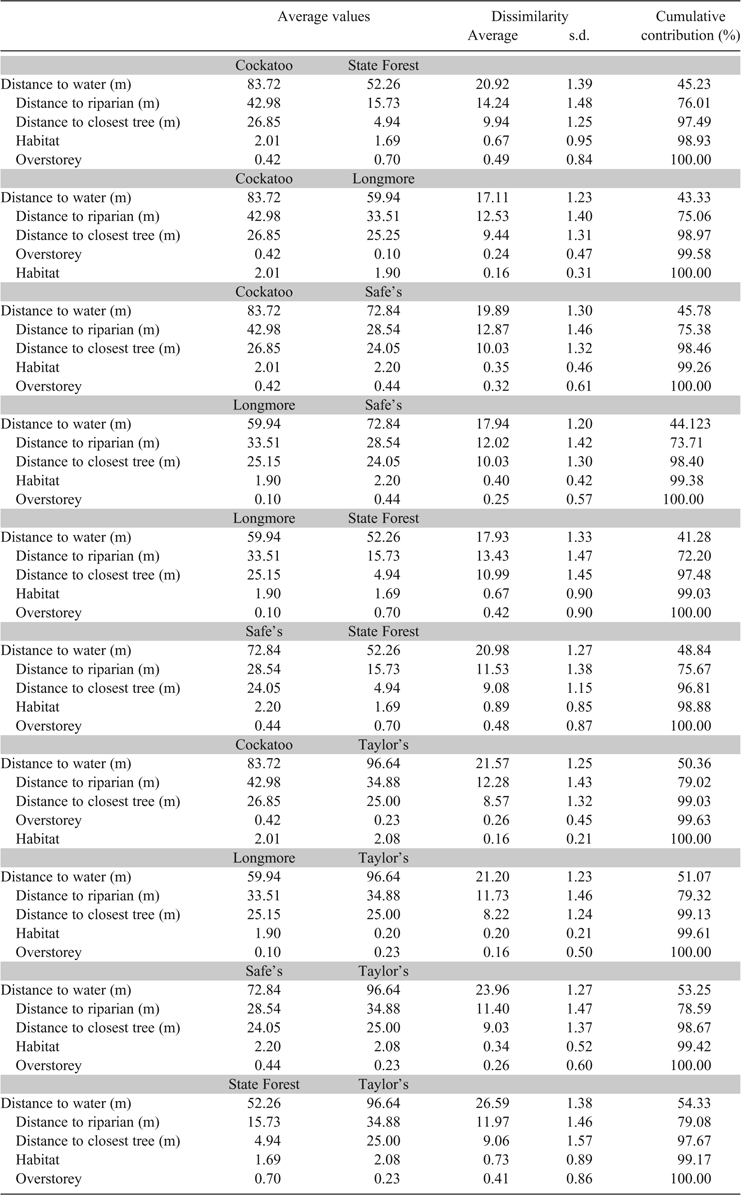
In 2014, 197 depredated nests were recorded across all sites. Habitat variables at nesting locations differed among sites (Global R = 0.098, P = 0.001) with nest sites at Gunbower State Forest differing from the pasture-based sites, Cockatoo, Longmore, Safe’s and Taylor’s Lagoons (Table 4). Nests were located in a narrow range, 50–100 m from the water and in relatively open areas. Nests were generally clumped, with most nests located 10–80 m from another nest. Nests were located well away from trees, although in Gunbower State Forest, where tree densities were higher than at other sites, nests were placed closer to trees (Table 4). The main difference between Gunbower State Forest and the other pasture-based wetlands was related to distance to trees and riparian zones (Table 4). Gunbower State Forest had fewer open areas and higher densities of trees, so nests were closer to shore and trees than at Cockatoo, Longmore, Safe’s and Taylor’s Lagoons (Table 4).
|
Nesting preferences were consistent across wetlands, despite wetland differences in surrounding habitat type. Hotspot analysis using KDE showed that the same nesting beaches were consistently used from 2011 to 2014 (Fig. 6). The habitats of these nesting beaches were similar despite wetlands being located in broad habitat types of woodland forest to highly modified pastures (P > 0.05) (Fig. 3).
Discussion
Freshwater turtle populations are in crisis. In total, 63% of the assessed species and ~42% of all known species are considered threatened (Baillie et al. 2004). Australian turtles are not immune; with 69–91% of Murray River turtle populations having declined over the last 40 years (Chessman 2011). The results from our study have several important implications for the conservation and management of the endangered C. expansa. We found that populations of C. expansa have preferred nesting areas, which allows for targeted management of nesting grounds. Habitat preferences for nesting sites of C. expansa are predictable, with females preferring to nest ~50 m from shore, in open habitat of low vegetation stubble (Fig. 7). Predictable nesting requirements allow for identification of potential nesting grounds in a region. The timing of nesting is also predictable, coinciding with rain in March/April, although some nesting may occur outside of this period (Booth 2010; Cann and Sadlier 2017). Most environmental and conservation management agencies have access to a suite of environmental and geological databases that could be utilised for GIS spatial modelling of potential nest locations in their region, using our data. For example, multivariate spatial interpolation modelling (e.g. CoKriging) combines spatial data (e.g. elevation) with variables of interest to make a single map based on correlates of all included variables (Krivoruchko 2011).

|
Preference for nesting in open areas is common in freshwater turtles, primarily because the thermal properties of open nests enhance hatchling developmental rates (Janzen 1994; Wilson 1998; Janzen and Morjan 2001; Kolbe and Janzen 2002a). The construction of nests in open microhabitats maximises hatchling survival partly because of the reduction in the incubation period (Spencer and Thompson 2003; Micheli-Campbell et al. 2013). Maternal selection of open nest sites allows eggs to reach optimal temperatures more rapidly (Wilson 1998) and facilitates the synchronous breaking of secondary diapause developmental stage (Booth 2002). However the long diapause experienced by C. expansa potentially increases susceptibility of the egg to bacterial and fungal infections. Ground with minimal vegetation also aids in nest cavity construction and receives higher levels of sunlight than does herbaceous cover (Flitz and Mullin 2006).
Elevation and distance to water are important characteristics that affect nesting. Nest sites at lower elevations are more likely to be inundated by flooding (Spencer and Thompson 2003), while those at higher elevations can increase the risk of hatchling disorientation (Warner and Mitchell 2013) and predation on adults (Spencer and Thompson 2003; Zare et al. 2012). Maternal preference of higher elevated sites (Fig. 7) may be an adaptation to flooding associated with winter and spring rainfall patterns. Because distance from the water’s edge and elevation are correlated, it is assumed that C. expansa selects a nest site based on elevation in order to avoid inundation. In our 2013 study two nests <10 m from shore were inundated with water from winter flooding.
Nesting behaviour and location may also offer some relief from predation. None of the nests in this study were found intact without the female present, which may reflect actual predation rates or be a function of sampling bias due to cryptic nature of intact nests. Chelodina expansa populations do not appear to be declining at rates similar to those of E. macquarii and C. longicollis on the Murray River (Chessman 2011), but broad-shelled turtles occur at much lower densities than the other two turtle species. Chelodina expansa nests during autumn, six months out of phase with both E. macquarii and C. longicollis (Cann 1998). Its nesting biology is also different. Individual C. expansa nest in response to rain (Booth 2010), but not all females respond to the same rain events (Bowen et al. 2005). Thus, the nesting season occurs over an ~4–6-week period in March–April but can extend from March to June, with some C. expansa nesting as late as November (Booth 2010). Nest densities of C. expansa are generally lower than those of the other two species because their population numbers are lower. Female C. expansa also nest far from shore, which scatters their nests widely.
Although natal homing is rarely observed and documented in freshwater turtles (Micheli-Campbell et al. 2013), the consistent use of nesting hotspots by C. expansa may indicate natal homing. Nest-site selection is highly heritable (Valenzuela and Janzen 2001; McGaugh et al. 2010) and closely related females nest close to one another (Freedberg et al. 2005). Unfortunately, this study largely identified nests after they were destroyed by foxes. Further research that identifies individuals is required to determine individual plasticity of nesting behaviour over several years, let alone the complexity of multigenerational natal homing.
Despite consistency in microhabitat selection by C. expansa, the mother’s perceived risk of predation can alter nest-site selection (Spencer 2002a). High nest densities close to shore provide more linear search patterns and facilitate detection by foxes (Robinson and Bider 1988; Marchand et al. 2002; Marchand and Litvaitis 2004). Soil disturbance and maternal cloacal secretions increase predation by foxes that use both visual and olfactory cues to detect nests (Spencer 2002a). In agricultural pasture areas, nests were clumped in distinct nesting areas, with most nests 0–30 m away from one another. In most cases, nest predation rates are highest during the first 24 h and up to five days after oviposition (Robinson and Bider 1988; Spencer 2002a; Wirsing et al. 2012), thus highlighting the importance of management strategies mitigating fox predation before and during oviposition.
Although populations of C. expansa appear stable, populations of sympatric species have declined by up to 91% in some areas (Chessman 2011). Foxes pose the greatest threat to freshwater turtle populations in southern Australia, but current management strategies to reduce their numbers have been largely ineffective (Spencer and Thompson 2005; Spencer et al. 2016). Our study significantly aids targeted management of likely nesting grounds on the Murray River. Once nesting areas are identified in a region, more dynamic and integrated strategies of fox mitigation can be implemented, because turtle populations and nesting grounds are often discrete and can be micromanaged. Currently, the primary strategy for reducing fox activity (if any control is implemented) is to run lethal baiting transects along fence lines or established paths, rather than specifically targeting nesting areas. However, foxes are extremely efficient at detecting nests and although transect baiting may result in some patchy reductions in fox activity, even a small number of foxes in an area can destroy most of the turtle nests (Spencer et al. 2016, 2017).
In conclusion, our study is a major step forward for conservation of C. expansa because it demonstrates a model for nest-site selection by C. expansa that can be used to identify critical nesting grounds for the species. The life history of freshwater turtles involves high but fluctuating rates of egg and juvenile mortality, balanced by extreme iteroparity (i.e. long-lived, highly fecund), in which threats to adult survival are low (Gibbons and Semlitsch 1982; Shine and Iverson 1995). The life history of C. expansa may differ from the life history of other freshwater turtles (i.e. E. macquarii) in that C. expansa matures later and has lower adult survival. C. expansa, however, appears to have lower rates of nest predation and higher rates of juvenile survival (Spencer and Thompson 2005), but these observations on C. expansa life history are limited to populations in the Albury region only. If we assume that C. expansa experiences the same risk of extinction as its sympatric species (E. macquarii and C. longicollis) then the mortality of eggs and young has increased, primarily because of predation by foxes (Thompson 1983), and adult mortality is increasing (Spencer and Thompson 2005). A major key for conserving C. expansa is to assess population numbers and nest predation rates along the Murray River and implement management strategies to reduce nest predation rates in locations that can be identified using our data (Spencer et al. 2017). Reducing nest predation rates may involve ‘mosaic’ fox management strategies where specific nesting areas are the focus for intensive and integrated fox control every 2–6 years (Spencer et al. 2017). Tools such as TurtleSAT, which are developed to identify areas of nest predation and high adult turtle mortality, are also applicable to the conservation of other species.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was funded by North Central Catchment Management Authority of Victoria and the Australian Research Council (LP140100011). TurtleSAT was funded by The Field Naturalists Society of South Australia, Fish Fuel Co., and the Barbara Hardy Institute of the University of South Australia. Support for TurtleSAT was provided by the Invasive Animals Cooperative Research Centre, NSW Department of Primary Industries, University of Sydney and University of Western Sydney. We thank T. Gibson, L. Rogers, A. Martins and J. James from the North Central Catchment Management Authority. We thank M. Laing and F. McDougall from the University of Western Sydney. We thank S. McDonald (Charles Sturt University) for helping develop the map in Fig. 1. Most of this work could not have happened without the help of Turtles Australia volunteers – True Citizen Scientists. We thank two anonymous reviewers for their comments on the manuscript.
References
Baillie, J. E. M., Hilton-Taylor, C., and Stuart, S. N. (2004). ‘2004 IUCN Red List of Threatened Species™. A Global Species Assessment.’ (IUCN: Gland, Switzerland and Cambridge, UK.)Booth, D. T. (2002). The breaking of diapause in embryonic broad-shelled river turtles (Chelodina expansa). Journal of Herpetology 36, 304–307.
| The breaking of diapause in embryonic broad-shelled river turtles (Chelodina expansa).Crossref | GoogleScholarGoogle Scholar |
Booth, D. T. (2010). The natural history of nesting in two Australian freshwater turtles. Australian Zoologist 35, 198–203.
| The natural history of nesting in two Australian freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Bowen, K. D., Spencer, R.-J., and Janzen, F. J. (2005). A comparative study of environmental factors that affect nesting in Australian and North American freshwater turtles. Journal of Zoology 267, 397–404.
| A comparative study of environmental factors that affect nesting in Australian and North American freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Cann, J. (1998). ‘Australian Freshwater Turtles.’ (Beaumont Publishing: Singapore.)
Cann, J., and Sadlier, R. (2017). ‘Freshwater Turtles of Australia.’ (CSIRO Publishing: Melbourne.)
Chessman, B. C. (1983). Observations on the diet of the broad-shelled turtle Chelodina expansa Gray (Testudines: Chelidae). Australian Wildlife Research 10, 169–172.
| Observations on the diet of the broad-shelled turtle Chelodina expansa Gray (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1988). Habitat preferences of freshwater turtles in the Murray Valley, Victoria and New South Wales. Australian Wildlife Research 15, 485–491.
| Habitat preferences of freshwater turtles in the Murray Valley, Victoria and New South Wales.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (2011). Declines of freshwater turtles associated with climatic drying in Australia’s Murray–Darling Basin. Wildlife Research 38, 664–671.
| Declines of freshwater turtles associated with climatic drying in Australia’s Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Clarke, K. R., and Warwick, R. M. (2001). ‘Change in Marine Communities: An Approach to Statistical Analysis and Interpretation.’ 2nd edn. (PRIMER-E: Plymouth, UK.)
Department of Primary Industries (2013). Gunbower Forest Ramsar Site Boundary Description Technical Report. Department of Environment and Primary Industries, Melbourne.
Dormer, J, Old, J. M., Van Dyke, J. U., and Spencer, R.-J. (2016). Incubation temperature affects development order of morphological features and staging criteria in turtle embryos. Journal of Zoology 299, 284–294.
Flitz, B. A., and Mullin, S. J. (2006). Nest-site selection in the eastern box turtle, Terrapene carolina carolinaxi, in Illinois. Chelonian Conservation and Biology 5, 309–312.
| Nest-site selection in the eastern box turtle, Terrapene carolina carolinaxi, in Illinois.Crossref | GoogleScholarGoogle Scholar |
Freedberg, S., Ewart, M. A., Ridenhour, B. J., Neiman, M., and Nelson, C. E. (2005). Nesting fidelity and molecular evidence for natal homing in the freshwater turtle, Graptemys kohnii. Proceedings of the Royal Society B: Biological Sciences 272, 1345–1350.
| Nesting fidelity and molecular evidence for natal homing in the freshwater turtle, Graptemys kohnii.Crossref | GoogleScholarGoogle Scholar |
Gibbons, J. W., and Semlitsch, R. D. (1982). Survivorship and longevity of a long-lived vertebrate species: how long do turtles live? The Journal of Animal Ecology 51, 523–527.
Goode, J. (1967). ‘Freshwater Tortoises of Australia and New Guinea (in the Family Chelidae).’ (Lansdowne: Melbourne.)
Hughes, E. J., and Brooks, R. J. (2006). The good mother: does nest-site selection constitute parental investment in turtles? Canadian Journal of Zoology 84, 1545–1554.
| The good mother: does nest-site selection constitute parental investment in turtles?Crossref | GoogleScholarGoogle Scholar |
Janzen, F. J. (1994). Vegetational cover predicts the sex ratio of hatchling turtles in natural nests. Ecology 75, 1593–1599.
| Vegetational cover predicts the sex ratio of hatchling turtles in natural nests.Crossref | GoogleScholarGoogle Scholar |
Janzen, F. J., and Morjan, C. L. (2001). Repeatability of microenvironment-specific nesting behaviour in a turtle with environmental sex determination. Animal Behaviour 62, 73–82.
| Repeatability of microenvironment-specific nesting behaviour in a turtle with environmental sex determination.Crossref | GoogleScholarGoogle Scholar |
Kolbe, J. J., and Janzen, F. J. (2002a). Impact of nest-site selection on nest success and nest temperature in natural and disturbed habitats. Ecology 83, 269–281.
| Impact of nest-site selection on nest success and nest temperature in natural and disturbed habitats.Crossref | GoogleScholarGoogle Scholar |
Kolbe, J. J., and Janzen, F. J. (2002b). Spatial and temporal dynamics of turtle nest predation: edge effects. Oikos 99, 538–544.
| Spatial and temporal dynamics of turtle nest predation: edge effects.Crossref | GoogleScholarGoogle Scholar |
Krivoruchko, K. (2011). ‘Spatial Statistical Data Analysis for GIS Users.’ (ESRI Press.)
Marchand, M. N., and Litvaitis, J. A. (2004). Effects of landscape composition, habitat features, and nest distribution on predation rates of simulated turtle nests. Biological Conservation 117, 243–251.
| Effects of landscape composition, habitat features, and nest distribution on predation rates of simulated turtle nests.Crossref | GoogleScholarGoogle Scholar |
Marchand, M. N., Litvaitis, J. A., Maier, T. J., and DeGraaf, R. M. (2002). Use of artificial nests to investigate predation on freshwater turtle nests. Wildlife Society Bulletin 30, 1092–1098.
McGaugh, S. E., Schwanz, L. E., Bowden, R. M., Gonzalez, J. E., and Janzen, F. J. (2010). Inheritance of nesting behaviour across natural environmental variation in a turtle with temperature-dependent sex determination. Proceedings of the Royal Society B: Biological Sciences 277, 1219–1226.
| Inheritance of nesting behaviour across natural environmental variation in a turtle with temperature-dependent sex determination.Crossref | GoogleScholarGoogle Scholar |
Micheli-Campbell, M. A., Baumgartl, T., Booth, D. T., Campbell, H. A., Connell, M., and Franklin, C. E. (2013). Selectivity and repeated use of nesting sites in a freshwater turtle. Herpetologica 69, 383–396.
| Selectivity and repeated use of nesting sites in a freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Robinson, C., and Bider, J. R. (1988). Nesting synchrony: a strategy to decrease predation of snapping turtle (Chelydra serpentine) nests. Journal of Herpetology 22, 470–473.
| Nesting synchrony: a strategy to decrease predation of snapping turtle (Chelydra serpentine) nests.Crossref | GoogleScholarGoogle Scholar |
Shahrabi, J., and Pelot, R. (2009). Kernel Density Analysis of maritime fishing traffic and incidents in Canadian Atlantic waters. Journal of Applied Sciences 9, 415–426.
| Kernel Density Analysis of maritime fishing traffic and incidents in Canadian Atlantic waters.Crossref | GoogleScholarGoogle Scholar |
Shine, R, and Iverson, J. B. (1995). Patterns of survival, growth and maturation in turtles. Oikos 72, 343–348.
Spencer, R.-J. (2002a). Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles. Ecology 83, 2136–2144.
| Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J. (2002b). Growth patterns of two widely distributed freshwater turtles and a comparison of common methods used to estimate age. Australian Journal of Zoology 50, 477–490.
| Growth patterns of two widely distributed freshwater turtles and a comparison of common methods used to estimate age.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., and Janzen, F. J. (2010). Demographic consequences of adaptive growth and the ramifications for conservation of long-lived organisms. Biological Conservation 143, 1951–1959.
| Demographic consequences of adaptive growth and the ramifications for conservation of long-lived organisms.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., and Thompson, M. B. (2003). The significance of predation in nest site selection of turtles: an experimental consideration of macro- and microhabitat preferences. Oikos 102, 592–600.
| The significance of predation in nest site selection of turtles: an experimental consideration of macro- and microhabitat preferences.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., and Thompson, M. B. (2005). Experimental analysis of the impact of foxes on freshwater turtle populations. Conservation Biology 19, 845–854.
| Experimental analysis of the impact of foxes on freshwater turtle populations.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., Van Dyke, J. U., and Thompson, M. B. (2016). The ethological trap: functional and numerical responses of highly efficient invasive predators driving prey extinctions. Ecological Applications 26, 1969–1983.
| The ethological trap: functional and numerical responses of highly efficient invasive predators driving prey extinctions.Crossref | GoogleScholarGoogle Scholar |
Spencer, R.-J., Van Dyke, J. U., and Thompson, M. B. (2017). Critically evaluating best management practices for preventing freshwater turtle extinctions. Conservation Biology 31, 1340–1349.
| Critically evaluating best management practices for preventing freshwater turtle extinctions.Crossref | GoogleScholarGoogle Scholar |
Tamplin, J. W., and Cyr, A. B. (2011). Effects of acclimation and egg-incubation temperature on selected temperature by hatchling western painted turtles (Chrysemys picta bellii). Journal of Thermal Biology 36, 507–514.
| Effects of acclimation and egg-incubation temperature on selected temperature by hatchling western painted turtles (Chrysemys picta bellii).Crossref | GoogleScholarGoogle Scholar |
DSE (2013). Advisory list of threatened vertebrate fauna in Victoria. Department of Sustainability and Environment, Victorian Government, Melbourne.
Thompson, M. B. (1983). Populations of the Murray river tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes. Australian Wildlife Research 10, 363–371.
| Populations of the Murray river tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
TurtleSAT (2014). About this project. TurtleSat, 25 June 2014. Available at: http://www.turtlesat.org.au/turtlesat/pagecontent.aspx?page=turtle_aboutproject
Valenzuela, N., and Janzen, F. J. (2001). Nest-site philopatry and the evolution of temperature-dependent sex determination. Evolutionary Ecology Research 3, 779–794.
Warner, D. A., and Mitchell, T. S. (2013). Does maternal oviposition site influence offspring dispersal to suitable habitat? Oecologia 172, 679–688.
| Does maternal oviposition site influence offspring dispersal to suitable habitat?Crossref | GoogleScholarGoogle Scholar |
Wilson, D. (1998). Nest-site selection: microhabitat variation and its effects on the survival of turtle embryos. Ecology 79, 1884–1892.
| Nest-site selection: microhabitat variation and its effects on the survival of turtle embryos.Crossref | GoogleScholarGoogle Scholar |
Wirsing, A. J., Phillips, J. R., Obbard, M. E., and Murray, D. L. (2012). Incidental nest predation in freshwater turtles: inter- and intraspecific differences in vulnerability are explained by relative crypsis. Oecologia 168, 977–988.
| Incidental nest predation in freshwater turtles: inter- and intraspecific differences in vulnerability are explained by relative crypsis.Crossref | GoogleScholarGoogle Scholar |
Wood, D. W., and Bjorndal, K. A. (2000). Relation of temperature, moisture, salinity and slope to nest selection in loggerhead sea turtles. Copeia , 119–128.
| Relation of temperature, moisture, salinity and slope to nest selection in loggerhead sea turtles.Crossref | GoogleScholarGoogle Scholar |
Zare, R., Vaghefi, M. E., and Kamel, S. J. (2012). Nest location and clutch success of the Hawksbill sea turtle (Eretmochelys imbricata) at Shidver Island, Iran. Chelonian Conservation and Biology 11, 229–234.
| Nest location and clutch success of the Hawksbill sea turtle (Eretmochelys imbricata) at Shidver Island, Iran.Crossref | GoogleScholarGoogle Scholar |