Environmental variables that influence platypus (Ornithorhynchus anatinus) eDNA detection: an insight into eDNA study design for platypus occupation
Breony Webb A * , Nakia Belmer A and Adrian Dickson A
A * , Nakia Belmer A and Adrian Dickson A
A
Abstract
Surveying platypus, Ornithorhynchus anatinus, occupancy patterns presents significant challenges because of their elusive nature and the often-inaccessible environments they inhabit. Traditional methods, such as observer sightings and mark–recapture, are labour-intensive and limited in spatio-temporal coverage. Recent advances in environmental DNA (eDNA) technology offer a promising alternative, allowing for broader and less invasive detection of aquatic species. This study investigates the use of eDNA for detecting platypuses across various environmental conditions in Kosciuszko National Park, New South Wales (NSW), focusing on how abiotic factors such as altitude, stream order, and seasonal variations may influence detection probabilities. Sampling occurred over four seasons from November 2021 to May 2023 at 46 sites, including high-altitude and remote locations. Results indicated that eDNA successfully detected platypuses in previously undocumented high-altitude sites of NSW and showed significant influences of stream order, altitude and seasonality on detection rates. This research highlighted the potential of eDNA to improve platypus distribution knowledge and emphasises the importance of considering environmental factors in monitoring. Future studies should refine eDNA protocols to enhance reliability across diverse habitats.
Keywords: Alpine streams, Australia, detection, eDNA, environmental conservation, monitoring, platypus, semi-aquatic, survey methods.
Introduction
Access to techniques that show species occupation over time and space is crucial for effective species management and conservation. Surveying freshwater mammals presents significant challenges because of the aquatic medium in which they forage, and the vast, frequently inaccessible environments they inhabit (Hood 2020). Unlike the detection of spraints in wild otters, Lutrinae (Reuther et al. 2000) and food middens by rakali, Hydromys chrysogaster (Triggs 1996; Sanders et al. 2024), the Australian freshwater platypus, Ornithorhynchus anatinus, lacks detection evidenced by vocalisations, footprints or food and scat remains (Triggs 1996). Therefore, the need for specialised equipment and techniques to track and monitor these animals adds to the complexity of conducting thorough, and accurate assessments (Seymour et al. 2021). When target species are elusive, nocturnal, or occur at low densities, low capture rates and inaccuracies in observer sightings can lead to costly and time-consuming survey efforts (Grant 2012; Griffiths et al. 2018). Because of these challenges, our understanding of some species’ occupation on a broader spatio-temporal scale is limited, particularly in remote and difficult to access locations (Seymour et al. 2021). Recent advances in environmental DNA (eDNA) technology have significantly enhanced our ability to detect and monitor aquatic species such as the platypus (Griffiths et al. 2018; Seymour et al. 2021). eDNA involves collecting water samples and analysing them for traces of genetic material shed by organisms, providing an alternative method for detection of species across larger spatial scales (Bohmann et al. 2014). In some wildlife monitoring areas, the use of eDNA has significantly improved our understanding of aquatic biodiversity and informed resource management and conservation decisions (Yang et al. 2021). The method generally provides significant advantages over traditional techniques, because it is non-invasive, rapid, inexpensive and can be sensitive to species in low density (Ficetola et al. 2008; Goldberg et al. 2011; Pilliod et al. 2013; Biggs et al. 2015; Smart et al. 2015). A limitation of the method is that it can be difficult to interpret results and ensure appropriate species-specific study design in the planning and development phase.
Although eDNA offers substantial advantages, it is not without limitations. The method provides presence–absence data but does not offer information on population size or density (Goldberg et al. 2011). Additionally, eDNA detection is influenced by various factors, including environmental conditions and the biology of the target species, which can complicate the interpretation of results (Barnes and Turner 2016). For instance, water body characteristics, including stream order and flow dynamics, significantly affect eDNA detection rates (Fremier et al. 2019). This is because eDNA concentration can be diluted in high-flow environments or fragmented in intermittent water bodies (Barnes and Turner 2016). Furthermore, the spatial arrangement of species-specific habitat plays a crucial role. Consequently, detection rates may be higher in regions with optimal habitat features compared with areas with suboptimal conditions (Rees et al. 2015). Temporal factors also affect eDNA detection. Seasonal changes influence a species’ behaviour and population size (Buxton et al. 2017), which in turn affects eDNA presence. Breeding seasons may alter movement patterns, population size and habitat use, potentially affecting the quantity of eDNA shed into the environment (Thalinger et al. 2019). Additionally, seasonal variations in water temperature, flow, and chemistry can influence eDNA persistence and degradation rates (Strickler et al. 2015). Cold temperatures and lower flow conditions typically slow down DNA degradation, potentially leading to higher detection probabilities during these periods (Pilliod et al. 2014). eDNA sampling is also influenced by abiotic environmental variables. Abiotic factors such as water temperature, pH, and turbidity affect eDNA stability and detection. Elevated temperatures and high UV exposure can accelerate DNA degradation, whereas high turbidity can interfere with the detection process by clogging filters and reducing DNA recovery (Stoeckle et al. 2017). Understanding the effects of these variables is crucial for optimising eDNA monitoring protocols and improving accuracy in species detection.
The platypus, a mostly nocturnal, semi-fossorial and semi-aquatic mammal of Australia’s eastern coast, thrives in productive and permanent freshwater environments (Grant 2007). Despite the platypus’s unique biology and ecological importance, understanding its spatio-temporal occupation on a large scale remains challenging (Grant 2007; Griffiths et al. 2018). They are considered cryptic in nature, foraging at night for macroinvertebrate prey species in the water benthos (Faragher et al. 1979; McLachlan-Troup et al. 2010; Marchant and Grant 2015; Hawke et al. 2022) and resting in hidden burrows in the banks during the day (Serena et al. 1998). In addition, population densities are considered to be low, ranging from 1.2 to 2.1 animals km−1 in urban environments of Melbourne (Serena 1994; Serena et al. 2014) to 2–3 animals km−1 on Kangaroo Island (Serena and Williams 1997). Previous research on platypus occupation by using traditional methods, such as observer sightings, mark–recapture, radio tracking, and acoustic tag detection, has largely been limited to easily accessible river sections or catchments with sufficient platypus numbers, and can be labour intensive (Grant 2007). Observer sightings are prone to inaccuracies, relying on daylight, and require proximity to water (Grant 2012). Mark–recapture often faces low recapture rates because of net avoidance behaviour (Grant 2004; Serena and Williams 2012; Griffiths et al. 2013), whereas acoustic and microchip tracking is limited by receiver placement (Bino et al. 2018). These methods generally offer incomplete insights into the full spatio-temporal range of platypus occupation (Grant 2007; Griffiths et al. 2018).
Recent platypus eDNA research has enabled broad-scale, cost-effective assessments of occupation (Lugg et al. 2017; McColl-Gausden et al. 2023). In Brisbane, Sydney, and Melbourne, long-term eDNA studies have been instrumental in identifying platypus occupancy and inferring their preferred habitats (Griffiths et al. 2018; Brunt et al. 2021; Webb et al. 2021). Although eDNA provides significant advantages for detecting platypus occupation compared with some traditional methods, its application can be affected by environmental conditions and platypus behaviour, complicating result interpretation (Barnes and Turner 2016). To our knowledge, there is limited formal guidance on interpreting eDNA results and designing studies that consider these challenges, while accurately revealing the broader spatio-temporal patterns of platypus occupation.
Kosciuszko National Park, in southern New South Wales (NSW), with its diverse, mostly pristine aquatic systems, offers a unique opportunity to study platypus eDNA detection across variable climatic and topographic conditions, including remote, high-altitude streams and large rivers. Significant gaps exist in platypus occupancy records within the Park and unlike previous studies focused on accessible areas, this research will address eDNA detection in more challenging environments.
This study specifically investigated the predictor effect of environmental variables on platypus detection and correlation with previous platypus records by using eDNA across Kosciuszko National Park, and aimed to address the following questions:
Can eDNA be used to detect platypus in high-altitude, remote waterways of Kosciuszko National Park?
Is there a relationship between platypus eDNA detection probability and abiotic factors such as stream order, altitude, and seasonality that may influence decisions on study design for eDNA?
Do study design features such as sampling effort or the occurrence of previous platypus records interact with platypus eDNA detection probability?
Methods
Study area
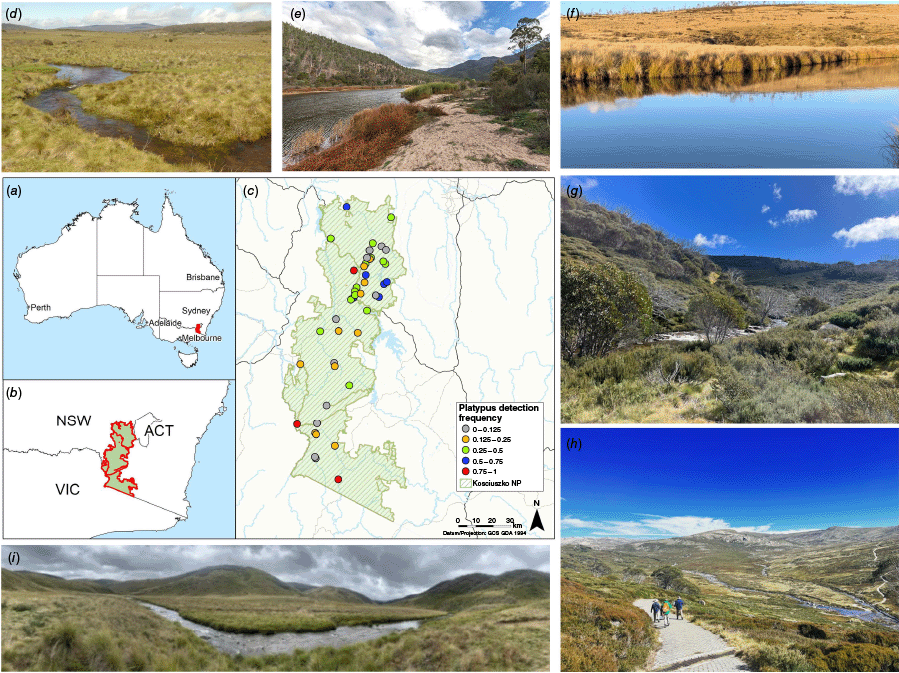
Kosciuszko National Park (KNP, the Park) is the largest National Park in New South Wales (NSW), at approximately 6900 km2. The Park is part of the greater Australian Alpine Bioregion in the Great Dividing Range and includes Australia’s highest mountain peak, Mount Kosciuszko at 2228 m ASL. Across the Park there is a large range in altitude (450–2228 m) and a corresponding range of mean air temperature (−3 to 25°C), which combine to produce the range and variability of seasonality, stream levels and vegetation types. In winter, the mountainous regions, >1300 m ASL are generally covered in snow. In early spring months, the snow melt drains into the catchments of the Snowy River, Murray River, and Murrumbidgee River (Fig. 1) and the upper catchments of these systems that are within the Park.
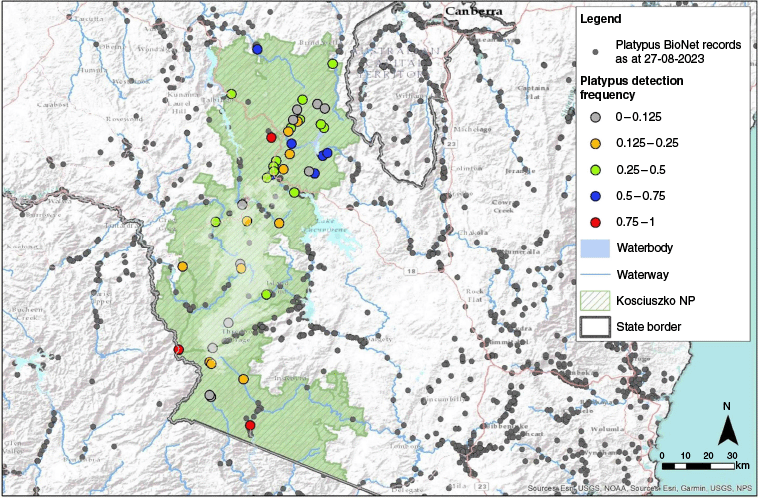
(a, b) Location of Kosciuszko National Park in NSW, Australia. (c) Map of study location in Kosciuszko National Park with 46 sites; coloured circles represent eDNA detection probability. (d–i) Location site photos of (d) Murrumbidgee River, Upper reaches, Peppercorn Trail, (e) Snowy River, Byadbo Wilderness Area, Running Waters campground, (f) Murrumbidgee River, Long Plain, (g) Geehi River, upstream of Valentine Falls, (h) Snowy River, upper reaches, Charlotte Pass, and (i) Tumut River, Round Mountain.
Study design
In total, 46 sites were sampled, with most sites pre-selected because of their inclusion in existing NSW National Parks and Wildlife Services (NPWS) monitoring programs that span the entire Park. Six additional sites were selected to increase spatial coverage, apply sampling stratification across the independent environmental variables (altitude and stream order) and provide information on platypus presence across areas of the park with no previous records (Fig. 1c). Altitude was taken at each site with a handheld GPS, with the approximate midpoint of the assessed stream reach being used. The Strahler stream order method (Strahler 1952) was used to define the stream networks. Site stream orders were referenced from the Water Management (General) Regulation 2018 hydroline spatial data 1.0 (NSW Government 2018).
Surveys were conducted over four separate seasonal sampling occasions during November 2021, May 2022, November 2022 and May 2023 (Table 1), with 106 samples collected across the Park (Fig. 1c). Late spring sampling occurred in November–December to avoid the dilution influence of melting snow on eDNA detection, and autumn sampling was conducted in April–May. Records of platypus occupancy were attained from NSW BioNet (NSW Environment and Heritage 2023) and data up to the 23rd August 2023 were included. A ‘yes’ status was given to those sites that had a past record within 7 km upstream or downstream of a sample location. This distance was based on an approximate average maximum home range of platypus in the region and NSW, plus a buffer for variation (Grant et al. 1992; Hawke et al. 2020) and non-independence of environmental samples within a moving medium (Stoeckle et al. 2017).
| Season | Number of sites | Altitude (m ASL) | Number of sites | Strahler stream order | Number of sites | Historical record | Number of sites | Sampling effort | Number of sites | |
|---|---|---|---|---|---|---|---|---|---|---|
| Spring 2021 | 16 | >1500 | 5 | 2–3 | 14 | Yes | 24 | 1 | 18 | |
| Autumn 2022 | 33 | 1250–1500 | 28 | 4–5 | 24 | No | 22 | 2 | 5 | |
| Spring 2022 | 23 | 1000–1250 | 6 | 6–8 | 8 | 3 | 8 | |||
| Autumn 2023 | 40 | <1000 | 7 | 4 | 15 |
eDNA sampling and sample analysis
At each site and survey period, water samples were collected in duplicate (one from the bank edge and another from the centre of the stream) to minimise the risk of false negative results in the analysis for eDNA and to account for incomplete DNA mixing in the stream. Depth of sampling was maintained at no more than 0.5 m below the surface because DNA has been shown to deplete at depth in aquatic sampling environments (Govindarajan et al. 2022). In a duplicate sample, if platypus is present it is expected to have >95% cumulative available probability (Lugg et al. 2017). In some sampling periods, sites were missed owing to site access or suboptimal sampling conditions that would compromise the results such as heavy rainfall in the few days leading up to sampling (>5 mm per day). Therefore, each site varied in sampling effort over the four periods (between 1 and 4 samples). Low turbidity of the waterways allowed relatively large volumes of water to be filtered on-site by pumping an average of 1.95 ± 0.34 L over the two samples per site through a 5 μm filter by using the Smith-root eDNA sampler or a vacuum pump. Large volumes increase the likelihood of detection if the target species is present to a point of saturation at volumes greater than 1 L (Mächler et al. 2016; Govindarajan et al. 2022). Although there was low variation in the large volume collected, correlation between detection result and volume was checked for any bias by calculating the point biserial correlation. Correlation was positive but very weak and insignificant (rpb = 0.06, P = 0.5).
Contamination was controlled by avoiding transfer of water or organic material among sites, and by using sterile equipment at each site. Smith-Root self-preserving filters were used for each sample with the Smith-Root eDNA backpack sampler and stored in a dark cool container for up to 3 weeks before being transported to the commercial laboratory EnviroDNA (Parkville, Victoria). DNA was isolated from the filters by using a commercial DNA extraction kit (Qiagen DNeasy Blood and Tissue Kit). To detect platypus DNA, a species-specific marker targeting a 57 base-pair segment of the mitochondrial cytochrome b (CytB) gene (Griffiths et al. 2014) was employed. Real-time quantitative polymerase chain reaction (qPCR) TaqMan assays were used to amplify and quantify the DNA. For each sample, three replicate qPCR assays were conducted, totalling six for each site, with both negative and positive controls being included in all assays. There was no evidence of contamination from field or laboratory procedures, as all controls returned negative results, confirming that the sampling and analysis protocols were reliable. Details of eDNA extraction and qPCR are presented in Appendix S1.
The high sensitivity of eDNA methods helps minimise the occurrence of false negatives (failing to detect a present species) (Thomsen et al. 2012; Biggs et al. 2015; Smart et al. 2015). However, false positives (detecting a species when it is not actually present) can arise from probe non-specificity, contamination from the field or external sources (such as water birds, anglers, or water flow), or PCR artifacts during high cycle replications (Ficetola et al. 2015). To minimise false positives, a site was considered positive for platypus presence only if at least two of the six qPCR assays detected the target DNA and sites with only one positive qPCR result were considered negative (Griffiths et al. 2018).
Statistical analysis
Generalised logistic regression (LR) mixed effects models were developed in R studio (ver. 4.4.0) using the package ‘lme4’ (ver. 1.1-37; https://CRAN.R-project.org/package=lme4; Bates et al. 2013) and the probability of detection for each of the model predictor values was calculated by the predict function in R package ‘ggeffects’ (ver. 2.3.0; https://CRAN.R-project.org/package=ggeffects; Lüdecke 2018) and plotted against independent variable of each model. This statistical approach allows for a nuanced understanding of the combined effects of environmental and sampling factors on eDNA detection. The selected variables and model combinations in this analysis were chosen to account for both environmental variation and sampling effort, which can influence the likelihood of platypus DNA detection. By including season, altitude, and stream order as mixed effect predictor variables, the model incorporates key environmental factors that could affect DNA presence across different sites in KNP. The number of surveys per site was included to account for sampling effort, addressing the variation in detection probability observed in previous studies (Lugg et al. 2017). The random effect of sampling period was added to account for the longitudinal nature of repeated sampling, ensuring that temporal autocorrelation did not bias results. To manage multicollinearity between stream order and altitude, these variables were not combined in the same model. Model performance was assessed using the goodness of fit by the Akaike information criterion (AIC), and fixed effect/s were assessed on the coefficient estimate and significance test (P < 0.05). Individual predictor effects were examined through post hoc analysis, providing a clear evaluation of how each factor influences detection probability. Before model analysis was performed, predictor variables were standardised.
To examine the relationship between platypus detection probability and previous historical records of platypus at each site, a tetrachoric correlation was calculated. The tetrachoric correlation was computed using R (ver. 4.4.0), employing the ‘tetrachoric’ function in the ‘psych’ package for R (ver. 2.5.6; https://CRAN.R-project.org/package=psych; Revelle 2024). The procedure involves estimating the covariance between the latent continuous variables, under the assumption that they follow a bivariate normal distribution.
Results
Can eDNA be used to detect platypus in high-altitude, remote waterways of Kosciuszko National Park?
Platypus was detected in waterways at high altitudes and low stream orders not previously recorded in NSW (Fig. 2). The highest positive detection site was Valentine Creek, a headwater tributary of the Geehi River, with the sampling site at approximately 1670 m ASL. Eight locations with a stream order of three represented the lowest stream order with positive platypus detection. Autumn produced more positive results than did spring at high altitude.
Does platypus eDNA detection probability vary with abiotic variables in the study design?
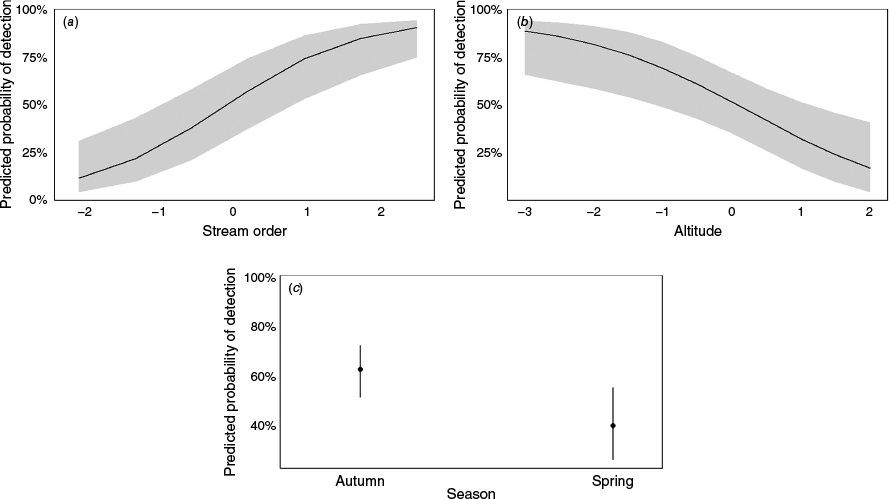
All the fixed effect abiotic variables in the model had a significant influence on the probability of platypus detection (Table 2). Stream order, as a single variable, had the strongest significant positive effect on the probability of detection (β = 1.10, P < 0.0001), followed by season (spring) and altitude (β = −0.96, P = 0.02 and β = −0.80, P = 0.003 respectively) (Fig. 3). Stream order as a single predictor variable of DNA detection also produced the best model fit, AIC = 137.3, followed by altitude and season, AIC = 146.1, and AIC = 153.9, respectively (Table 2).
| Parameter | AIC | d.f. | Coefficient (β) ± s.e. | P-value | |
|---|---|---|---|---|---|
| Stream order | 137.3 | 109 | 1.10 ± 0.28 | 0.0001 | |
| Altitude | 146.1 | 109 | −0.80 ± 0.27 | 0.003 | |
| Season (spring) | 153.9 | 109 | −0.96 ± 0.41 | 0.02 | |
| Stream order + Season (spring) | 134.9 | 109 | 1.1 ± 0.28 | 0.00009 | |
| −1.45 ± 0.57 | 0.01 | ||||
| Altitude + Season (spring) | 143.4 | 109 | −0.81 ± 0.27 | 0.002 | |
| −1.20 ± 0.45 | 0.008 |
Generalised LR mixed effects probability model of platypus detection for fixed environmental variables: (a) stream order, (b) altitude, and (c) season, by using eDNA sampling. The x-axis represents standardised fixed environmental variables, where the values have been transformed to have a mean of 0 and a standard deviation of 1. This transformation allows for easier comparison across variables with different scales. The y-axis shows the eDNA detection probability. Grey shaded area indicates the 95% confidence intervals.
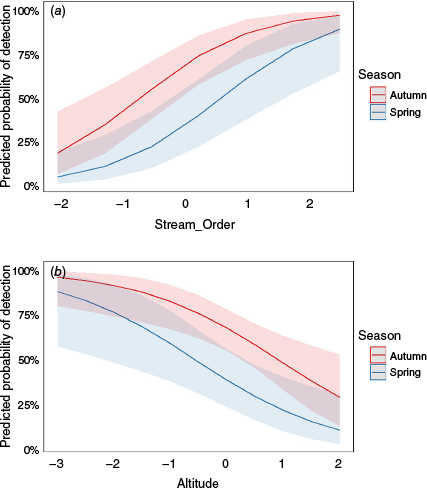
For multiple variables as fixed effects, the best fit model for predicting platypus DNA detection was stream order and season (spring) AIC = 134.9, where stream order had positive effect (β = 1.1) and spring season sampling had negative relationship (β = −1.45) with eDNA detection probability, with all predictor variables being significant (Table 2). As stream order increased, the probability of platypus detection increased in both sampling seasons, with autumn having a greater increase and with greater certainty in platypus detection as stream order increased compared with spring (Fig. 4a). Similarly, platypus detection was significantly more likely in autumn across all altitudes, with highest probability and greatest certainty at higher altitudes in autumn (Fig. 4b).
Generalised LR mixed effects probability model of platypus detection for fixed environmental variables in combined models: (a) stream order + season and (b) altitude + season, by using eDNA sampling. The x-axis represents standardised fixed environmental variables, where the values have been transformed to have a mean of 0 and a standard deviation of 1. This transformation allows for easier comparison across variables with different scales. The y-axis shows the eDNA detection probability. Grey shaded area indicates the 95% confidence intervals.
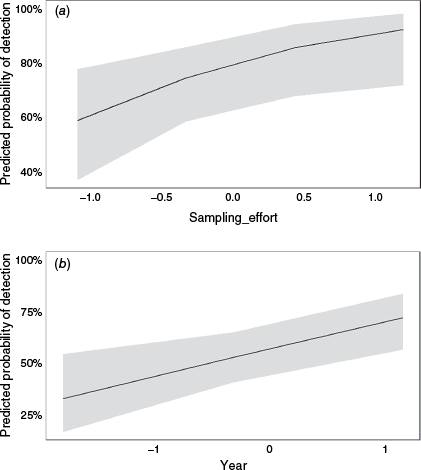
Do study design features such as sampling effort or the occurrence of previous records interact with platypus eDNA detection probability?
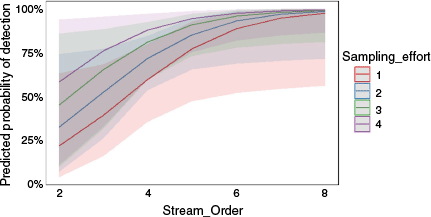
Over the 3 years of sampling from November 2021 to May 2023, there was a slight but significant increase in the probability of platypus detection (β = 0.47, P < 0.01), while accounting for the sampling period (Table 3, Fig. 5). In addition, a generalised logistic regression model on sampling effort found the more a site was sampled the higher the detection probability was (β = 0.72, P < 0.001) (Table 3, Fig. 5). Sampling effort and stream order combined to produce the best fit generalised regression model AIC = 45.7, where sampling effort had a positive and significant estimate coefficient (β = 0.83, P < 0.01) (Table 3). Sampling effort was most differential in its effect on platypus detection at lower stream orders and the model converged to 100% probability of detection at the highest stream order (eight; Snowy River in the Byadbo Wilderness area) regardless of the sampling effort (Fig. 6).
| Parameter | AIC | d.f. resid | Coefficient (β) ± s.e. | P | |
|---|---|---|---|---|---|
| Sampling effort | 48.8 | 43 | 0.72 ± 0.33 | 0.03 | |
| Year | 153.7 | 109 | 0.47 ± 0.20 | 0.02 | |
| Sampling effort + Stream order | 45.7 | 43 | 0.83 ± 0.41 | 0.04 | |
| 0.53 ± 0.34 | 0.12 |
Generalised LR (mixed effects; year only) probability model of platypus detection for abiotic variables in combined models: (a) sampling effort, and (b) year, by using eDNA sampling. The x-axis represents standardised fixed environmental variables, where the values have been transformed to have a mean of 0 and a standard deviation of 1. This transformation allows for easier comparison across variables with different scales. The y-axis shows the eDNA detection probability. Grey shaded area indicates the 95% confidence intervals.
Generalised LR mixed effects probability model of platypus detection for variables in a combined model; stream order and sampling effort, by using eDNA sampling. The x-axis represents actual stream order. The y-axis shows the eDNA detection probability. Grey shaded area indicates the 95% confidence intervals.
The tetrachoric correlation between previous historic records status of a site and platypus detection probability was calculated. The correlation was positive (r = 0.48), suggesting a moderate relationship between the two variables. This relationship was not statistically significant (P = 0.86), indicating that previous records are not a good basis for predicting occupation probability from eDNA detection. For example, in this study platypus was detected 17 times at sites with no previous records of platypus and not detected 20 times at sites with previous records of platypus.
Discussion
This study has shown that eDNA methods are a useful tool for defining environmental conditions associated with platypuses at a large scale in the Australian Alps. The ease of eDNA sample collection allows for a broader range of sites and environments, enhancing the spatio-temporal coverage of surveys compared with traditional methods. The results highlighted that accounting for spatially variable abiotic factors and ongoing sampling effort over time improves our understanding of spatio-temporal variations in platypus populations, which affects eDNA detection probability. How and why these factors may be interacting with survey design or indicate variation in platypus occupation or detection probability from seasonal movement behaviours is discussed below.
Platypus in Kosciusko National park in remote, and at high-altitude locations
During four sampling events, platypus DNA was detected across Kosciuszko National Park by using eDNA methods, including at locations where no prior records existed. Notably, detections occurred in remote areas at altitudes above 1600 m ASL and in the headwaters of the park’s northern and southern catchments. This provides new insights into platypus occupation in these remote areas. However, a clear negative trend in detection probability was observed with increasing altitude, especially during late spring. This trend aligns with earlier surveys from the 1980s and 1990s in the Thredbo River, which found platypuses to be less common or absent in upper reaches (Goldney 1998), while platypuses were observed more commonly in mid- to lowland reaches, with few in steep-gradient tributaries. Across the state border platypuses have been recorded at high elevation in the Victorian region of the Australian Alps. Recorded in the Atlas of Living Australia (ALA), a platypus was live-trapped in 1996 at Mount Hotham at 1687 m ASL, and in 2022 the ALA notes a juvenile rescued along a ridgeline at approximately 1602 m ASL. Perhaps, although it is rare, it is not unheard of to find platypuses at high altitudes in the Australian Alps.
In our study, three high-altitude tributaries, with steep gradients, all tested positive for platypus DNA in autumn 2023. This suggests that eDNA methods may offer advantages over traditional surveys in remote and challenging locations, providing a less invasive and labour-intensive means to detect platypuses, even with low abundances. However, the low detection probability at high altitudes observed in this study perhaps supports the expectation that low platypus abundance in these areas can result in lower eDNA detection rates.
What factors may influence seasonal, altitude and stream order variation in platypus detection via eDNA?
Over the four sampling periods, stream order significantly influenced platypus DNA detection probability, with platypus occupation remaining relatively localised at high stream order sites and those closer to dams, whereas detection probability was lower at low stream order sites. Although platypuses are known to favour permanent waterbodies, the impact of stream order on platypus detection has not been thoroughly investigated. Low detection rates in any method are often loosely attributed to low abundance (Griffiths et al. 2018) or sampling outside the species’ habitat niche. However, for eDNA, low detection can also result from various environmental factors, including heat, water chemistry, UV light, suspended solids, hydrology, and rate of DNA shedding by individual animals (Pilliod et al. 2014; Strickler et al. 2015; Barnes and Turner 2016; Stoeckle et al. 2017; Harrison et al. 2019). Furthermore, the retention of eDNA in a section of stream or river has been shown to be positively influenced by water transient retention time (e.g. presence of ponds, larger slow-moving channels, dams and lakes, backwaters and swamps) and, conversely, influenced negatively by increasing stream slope and substrate roughness (Fremier et al. 2019). Although this study did not quantify these factors, from observation these did vary at the chosen sample sites with catchment location. Generally, high stream order reaches are characterised by larger slow-moving channels and finer sediment as substrate, whereas low stream order reaches are headwaters that have higher sloped gradients and rougher substrate (Closs et al. 2004). Understanding how the various geomorphologic characteristics of a catchment interact with platypus eDNA retention time and subsequent detectability is an area needing further investigation because it could present bias in eDNA studies.
Furthermore, high altitude and proximity to snowlines can dilute eDNA in spring, potentially reducing detection probability at some sites. To mitigate this, sampling was scheduled for late spring and early summer to avoid these effects. The results indicated that, across two sampling seasons, platypus detection probability was only slightly higher in low stream order waterways during autumn, with significant overlap in confidence intervals between the two seasons. This overlap makes it challenging to distinguish between dilution effects and true platypus absence. Therefore, it is recommended that eDNA sampling in alpine catchments avoid periods during and immediately after snowmelt.
Detection probability was generally higher during autumn, with the highest results observed in lower catchment sites at high stream order and low altitude. Autumn sampling also yielded higher detection probabilities at high altitudes. In contrast, late spring detections were less likely and more localised to lower catchment sites, low altitudes, and high stream orders. Although there may be environmental factors influencing sampling methods that have yet to be considered, patterns of platypus activity and habitat preference could also explain the observed seasonal and stream order effects on detection probability. For instance, male platypuses exhibit spatial-temporal patterns influenced by breeding activity (Grant 2007). For example,in Victoria, male platypuses expand their home ranges and establish exclusive territories before and during the breeding season (Gust and Handasyde 1995). Furthermore, autumn coincides with the emergence and dispersal of first-year juveniles (Grant et al. 2004; Serena and Williams 2012, 2013). In NSW, long-term mark–recapture studies have shown a low recapture rate of male juveniles (86%) within their original trapping locations (Grant 2004). Recent acoustic monitoring in NSW and Victorian Alpine regions indicated peak movement and range expansion from April to June before the breeding season (Bino et al. 2018; Hawke et al. 2021). In Tasmania, where reproductive events may occur 2 months later than in mainland populations, camera-trap surveys have shown increased platypus activity during late November, coinciding with breeding activity (Roberts and Serena 2024). Territorial and patrolling behaviour by dominant males could also drive juvenile and subadult males to disperse from their natal areas (Thomas et al. 2019; Hawke et al. 2021). This increased mobility could contribute to greater DNA dispersion in late autumn. In contrast, female platypuses spend less time in the water during egg incubation (~10 days in early spring and late summer) (Holland and Jackson 2002; Hawkins and Battaglia 2009; Thomas et al. 2020), likely reducing DNA detection probability. During early autumn, females may experience peak energetic demands from lactation, potentially increasing their time in water for foraging (Grant et al. 2004; Thomas et al. 2020).
Without specific knowledge of platypus demographics, which eDNA did not provide, attributing higher detection probability to a particular sex is challenging. Variations in platypus movement and reproductive timing across different Australian locations further complicate this. Nonetheless, juvenile recruitment from March to June could be a key factor contributing to higher detection during autumn sampling in April–May.
Upper catchment sites in this study, characterised by high altitude and low stream order, are often snow-covered in winter, presenting a harsh environment (Grigg et al. 1992). Snowmelt and increased stream discharge during spring–summer might limit platypus movement into these streams because of higher foraging energy expenditure (Grant 2007). However, during autumn, when stream levels decrease, these upper catchment systems may become more accessible and productive habitats for dispersing or subadult male displacement from lower catchment areas.
How can study design factors such as sampling effort affect platypus eDNA detections?
Sampling effort varied from one to four events per site, with sites sampled more frequently showing higher platypus DNA detection probabilities and upper catchment sites had a greater likelihood of at least one positive detection. Low detection rates at upper catchment locations in spring and summer may be due to reduced platypus movement patterns and environmental factors affecting eDNA detectability, but increased sampling in autumn could mitigate this. This underscores the importance of extending eDNA sampling beyond a single event to include multiple sample events across those targeted stages of the platypus breeding cycle to improve detection. This approach is particularly advantageous given that, unlike more invasive methods, eDNA sampling is non-invasive, and reduces the likelihood of detection issues caused by behaviours such as net avoidance seen in mark–recapture studies (Connolly and Obendorf 1998; Grant 1992; 2004; Griffiths et al. 2013).
Understanding the relationship between historical records with platypus detection via eDNA
The study showed that platypus eDNA detection probability was not significantly correlated with sites where platypuses had been previously recorded within a 7 km range. For example, platypus DNA was detected 17 times at sites with no previous records of platypus and not detected 20 times at sites with previous records. This may be due to many sample sites being in largely remote areas in which occupancy has never been assessed. Never-the-less, historical data on species presence serves as a valuable reference for establishing current occupancy and can guide methods for assessing species distribution. Databases such as BioNet and ALA provide baseline information and help track changes in population numbers (McClenachan et al. 2012; Bilney 2014). Although past records have been used to identify localised declines in platypus populations (e.g. Bino et al. 2020), such analyses may not be fully accurate without further investigation. Given that historical records have shaped our understanding of current platypus distribution, we propose that eDNA methods offer a cost-effective, efficient approach to reassessing current occupancy in these areas of decline, to broaden our understanding of their distribution, particularly in remote areas of their range.
In this study, locations with historical records in the BioNet database were considered within a 7 km radius from the reported coordinates. This buffer accounts for variations in home-range estimates from different studies, including 0.37–2.3 km upstream of Lake Jindabyne (Grant et al. 1992) and 0.24–15.93 km downstream of the Snowy River dam (Hawke et al. 2020). The former is comparable to the range reported in Victoria’s Badger Creek (0.33–2.28 km) (Serena 1994), and the latter aligns with the Winmera and Yarra River catchment in Victoria maximum range of 13.9 km (Serena and Williams 2013). Although average home ranges are typically smaller than 7 km, a larger buffer is recommended to accommodate the downstream transport of DNA. Additionally, when sampling in areas with high water velocities, ensuring adequate spatial coverage and sample independence should be considered.
Implications for future use of eDNA to detect and monitor platypus occupation
This research highlights how environmental factors, i.e stream order, altitude, seasonality, as well as study design elements such as sampling effort and prior platypus records, can interact with eDNA detection probability. Whereas some potential explanations for these influences have been proposed, determining the primary causal factors remain challenging. It is unclear whether sampling method limitations and environmental variability cause false negatives or whether these gradients reflect the true spatio-temporal patterns of platypus occupation. Therefore, eDNA studies on platypus should account for these limitations during the study design phase. Current research has yet to fully explore what eDNA can reveal about platypus spatio-temporal trends of occupation and distribution. We recommend that surveys of platypus and aquatic biota incorporate eDNA methods to better understand how environmental variables affect detection over time and space. Addressing these factors will enhance monitoring efforts and conservation strategies, leading to more effective management.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This work was completed by the NSW Government DCCEEW Estuaries and Catchments Team in partnership and funded by NSW National Parks and Wildlife Services as part of two separate waterway health monitoring projects.
Acknowledgements
We acknowledge the traditional custodians of the lands recognised as Kosciuszko National Park on which this research was performed on. It is a privilege to walk and work on the land of the Monaro-Ngarigo, Ngunnawal Wiradjuri and Wolgalu people. We pay our respects to ancestors and elders, past, present, and emerging. Thanks go to the NPWS Park Operations Projects team that made this project, and the parent projects that this work was completed for, possible, including Mel Schroder, Danielle Stokeld, Campbell Young and Marion Battishall among many other very helpful and knowledgeable people of the NPWS. Thanks go to Dr Tracey MacDonald and Jessica Bourner of the DCCEEW Water, Wetlands and Coastal Science Branch for their insightful reviews. Special thanks go to the DCCEEW Estuaries and Catchments Team members that assisted with the field and laboratory work of this project: Nuria Lahuerta Pineiro, Fabiola Silva, Valentina Hurtado McCormick and Adam Wethered. Special thanks go to Dr Peter Scanes, Dr Jaimie Potts and Dr Angus Ferguson for providing advice, inspiration, and motivation throughout the project.
References
Barnes, M. A., and Turner, C. R. (2016). The ecology of environmental DNA and implications for conservation genetics. Conservation Genetics 17, 1-17.
| Crossref | Google Scholar |
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2013) lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-37. Available at https://CRAN.R-project.org/package=lme4
Bilney, R. J. (2014). Poor historical data drive conservation complacency: the case of mammal decline in south‐eastern Australian forests. Austral Ecology 39, 875-886.
| Crossref | Google Scholar |
Bino, G., Kingsford, R. T., Grant, T., Taylor, M. D., and Vogelnest, L. (2018). Use of implanted acoustic tags to assess platypus movement behaviour across spatial and temporal scales. Scientific Reports 8, 5117.
| Crossref | Google Scholar | PubMed |
Bino, G., Kingsford, R. T., and Wintle, B. A. (2020). A stitch in time – Synergistic impacts to platypus metapopulation extinction risk. Biological Conservation 242, 108399.
| Crossref | Google Scholar |
Bohmann, K., Evans, A., Gilbert, M. T., Carvalho, G. R., Creer, S., Knapp, M., Yu, D. W., and de Bruyn, M. (2014). Environmental DNA for wildlife biology and biodiversity monitoring. Trends in Ecology & Evolution 29, 358-367.
| Crossref | Google Scholar | PubMed |
Biggs, J., Ewald, N., Valentini, A., Gaboriaud, C., Dejean, T., Griffiths, R. A., Foster, J., Wilkinson, J. W., Arnell, A., Williams, P., Dunn, F., and Brotherton, P. (2015). Using eDNA to develop a national citizen science-based monitoring programme for the great crested newt (Triturus cristatus). Biological Conservation 183, 19-28.
| Crossref | Google Scholar |
Brunt, T., Cecil, M., Griffiths, J., Adams-Hosking, C., and Murray, P. (2021). Where are the platypuses (Ornithorhynchus anatinus) now? A snapshot in time of their distribution in the Greater Brisbane region. Australian Mammalogy 43, 368-372.
| Crossref | Google Scholar |
Buxton, A. S., Groombridge, J. J., Zakaria, N. B., and Griffiths, R. A. (2017). Seasonal variation in environmental DNA in relation to population size and environmental factors. Scientific Reports 7(1), 46294.
| Crossref | Google Scholar | PubMed |
Connolly, J. H., and Obendorf, D. L. (1998). Distribution, capture and physical characteristics of the platypus (Ornithorhynchus anatinus) in Tasmania. Australian Mammalogy 20, 231-237.
| Crossref | Google Scholar |
Faragher, R. A., Grant, T. R., and Carrick, F. N. (1979). Food of the platypus (Ornithorhynchus anatinus) with notes on the food of brown trout (Salmo trutta) in the Shoalhaven River, NSW. Australian Journal of Ecology 4, 171-179.
| Crossref | Google Scholar |
Ficetola, G. F., Miaud, C., Pompanon, F., and Taberlet, P. (2008). Species detection using environmental DNA from water samples. Biology Letters: Population Genetics 4, 423-425.
| Crossref | Google Scholar | PubMed |
Ficetola, G. F., Pansu, J., Bonin, A., Coissac, E., Giguet-Covex, C., De Barba, M., Gielly, L., Lopes, C. M., Boyer, F., Pompanon, F., Rayé, G., and Taberlet, P. (2015). Replication levels, false presences and the estimation of the presence/absence from eDNA metabarcoding data. Molecular Ecology Resources 15, 543-556.
| Crossref | Google Scholar | PubMed |
Fremier, A. K., Strickler, K. M., Parzych, J., Powers, S., and Goldberg, C. S. (2019). Stream Transport and Retention of Environmental DNA Pulse Releases in Relation to Hydrogeomorphic Scaling Factors. Environmental Science & Technology 53, 6640-6649.
| Crossref | Google Scholar | PubMed |
Goldberg, C. S., Pilliod, D. S., Arkle, R. S., and Waits, L. P. (2011). Molecular Detection of Vertebrates in Stream Water: a Demonstration Using Rocky Mountain Tailed Frogs and Idaho Giant Salamanders. PLoS One 6(7), e22746.
| Crossref | Google Scholar | PubMed |
Goldney, D. (1998). The distribution and abundance of platypuses in the Thredbo River–Lake Jindabyne system. Australian Mammalogy 20, 303.
| Crossref | Google Scholar |
Govindarajan, A. F., McCartin, L., Adams, A., Allan, E., Belani, A., Francolini, R., Fujii, J., Gomez-Ibañez, D., Kukulya, A., Marin, F., Tradd, K., Yoerger, D. R., McDermott, J. M., and Herrera, S. (2022). Improved biodiversity detection using a large-volume environmental DNA sampler with in-situ filtration and implications for marine eDNA sampling strategies. Deep-Sea Research. Part I, Oceanographic Research Papers 189, 103871.
| Crossref | Google Scholar |
Grant, T. R. (1992). Captures, movements and dispersal of platypus, Ornithorhynchus anatinus, in the Shoalhaven river, New South Wales, with evaluation of capture and marking techniques. In ‘Platypus and Echidnas’. (Ed ML Augee) pp. 255–262. (Royal Zoological Society of New South Wales: Sydney, NSW, Australia)
Grant, T. (2004). Captures, capture mortality, age and sex ratios of platypuses, Ornithorhynchus anatinus, during studies over 30 years in the upper Shoalhaven river in New South Wales. Proceedings of the Linnean Society of New South Wales 125, 217-226.
| Google Scholar |
Grant, T. R., Griffiths, M., and Temple-Smith, P. D. (2004). Breeding in a free-ranging population of platypuses, Ornithorhynchus anatinus, in the upper Shoalhaven River, New South Wales – a 27 year study. Proceedings of the Linnean Society of New South Wales 125, 227-234.
| Google Scholar |
Griffiths, J., Kelly, T., and Weeks, A. (2013). Net-avoidance behaviour in platypuses. Australian Mammalogy 35, 245-247.
| Crossref | Google Scholar |
Griffiths, J., van Rooyen A., Benier, J-M., Coleman, R., and Weeks, A. (2018). Tracking platypus populations through genetic fingerprints. In ‘Proceedings of the 9th Australian Stream 1 Management Conference’, Hobart, Tas, Australia. Available at https://asnevents.s3.amazonaws.com/Abstrakt-FullPaper/51678/9ASM_Griffiths+et+al_paper.pdf
Grigg, G., Beard, L., Grant, T., and Augee, M. (1992). Body-temperature and diurnal activity patterns in the platypus (Ornithorhynchus anatinus) during winter. Australian Journal of Zoology 40, 135-142.
| Crossref | Google Scholar |
Gust, N., and Handasyde, K. (1995). Seasonal variation in the ranging behavior of the platypus (Ornithorhynchus anatinus) on the Goulburn River, Victoria. Australian Journal of Zoology 43, 193-208.
| Crossref | Google Scholar |
Harrison, J. B., Sunday, J. M., and Rogers, S. M. (2019). Predicting the fate of eDNA in the environment and implications for studying biodiversity. Proceedings of the Royal Society. B, Biological Sciences 286, 20191409.
| Crossref | Google Scholar | PubMed |
Hawke, T., Bino, G., Kingsford, R. T., Iervasi, D., Iervasi, K., and Taylor, M. D. (2020). Fine‐scale movements and interactions of platypuses, and the impact of an environmental flushing flow. Freshwater Biology 66, 177-188.
| Crossref | Google Scholar |
Hawke, T., Bino, G., Kingsford, R. T., Iervasi, D., Iervasi, K., and Taylor, M. D. (2021). Long-term movements and activity patterns of platypus on regulated rivers. Scientific Reports 11, 3590.
| Crossref | Google Scholar | PubMed |
Hawke, T., Bino, G., Shackleton, M. E., Ross, A. K., and Kingsford, R. T. (2022). Using DNA metabarcoding as a novel approach for analysis of platypus diet. Scientific Reports 12, 2247.
| Crossref | Google Scholar | PubMed |
Hawkins, M., and Battaglia, A. (2009). Breeding behaviour of the platypus (Ornithorhynchus anatinus) in captivity. Australian Journal of Zoology 57, 283-293.
| Crossref | Google Scholar |
Holland, N., and Jackson, S. M. (2002). Reproductive behaviour and food consumption associated with the captive breeding of platypus (Ornithorhynchus anatinus). Journal of Zoology 256, 279-288.
| Crossref | Google Scholar |
Lüdecke, D. (2018). ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. Journal of Open Source Software 3, 772.
| Crossref | Google Scholar |
Lugg, W. H., Griffiths, J., van Rooyen, A. R., Weeks, A. R., and Tingley, R. (2017). Optimal survey designs for environmental DNA sampling. Methods in Ecology and Evolution 9, 1049-1059.
| Crossref | Google Scholar |
Mächler, E., Deiner, K., Spahn, F., and Altermatt, F. (2016). Fishing in the Water: Effect of Sampled Water Volume on Environmental DNA-Based Detection of Macroinvertebrates. Environmental Science & Technology 50, 305-312.
| Crossref | Google Scholar | PubMed |
Marchant, R., and Grant, T. R. (2015). The productivity of the macro invertebrate prey of the platypus in the upper Shoalhaven River, New South Wales. Marine and Freshwater Research 66, 1128-1137.
| Crossref | Google Scholar |
McClenachan, L., Ferretti, F., and Baum, J. K. (2012). From archives to conservation: why historical data are needed to set baselines for marine animals and ecosystems. Conservation Letters 5, 349-359.
| Crossref | Google Scholar |
McColl-Gausden, E. F., Griffiths, J., Collins, L., Weeks, A. R., and Tingley, R. (2023). The power of eDNA sampling to investigate the impact of Australian mega-fires on platypus occupancy. Biological Conservation 286, 110219.
| Crossref | Google Scholar |
McLachlan-Troup, T. A., Dickman, C. R., and Grant, T. R. (2010). Diet and dietary selectivity of the platypus in relation to season, sex and macroinvertebrate assemblages. Journal of Zoology 280, 237-246.
| Crossref | Google Scholar |
NSW Environment and Heritage (2023). BioNet Atlas. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/biodiversity/nsw-bionet [accessed 23 August 2023]
NSW Government. (2018). Water Management (General) Regulation 2018 hydroline spatial data 1.0. Available at https://www.dpie.nsw.gov.au/water/our-work/licensing-and-trade/controlled-activity-approvals/waterfront-land-e-tool/hydro-line-spatial-data [accessed 27 October 2023]
Pilliod, D. S., Goldberg, C. S., Arkle, R. S., and Waits, L. P. (2013). Estimating occupancy and abundance of stream amphibians using environmental DNA from filtered water samples. Canadian Journal of Fisheries and Aquatic Sciences 70, 1123-1130.
| Crossref | Google Scholar |
Pilliod, D. S., Goldberg, C. S., Arkle, R. S., and Waits, L. P. (2014). Factors influencing detection of eDNA from a stream-dwelling amphibian. Molecular Ecology Resources 14, 109-116.
| Crossref | Google Scholar | PubMed |
Rees, H. C., Gough, K. C., Middleditch, D. J., Patmore, J. R., and Maddison, B. C. (2015). Applications and limitations of measuring environmental DNA as indicators of the presence of aquatic animals. Journal of Applied Ecology 52(4), 827-831.
| Crossref | Google Scholar |
Reuther, C., Dolch, D., and Green, S., et al. (2000). Surveying and monitoring distribution and population trends of the Eurasian otter (Lutra lutra): guidelines and evaluation of the standard method for surveys as recommended by the European Section of the IUCN/SSC Otter Specialist Group. (Gruppe Naturschutz)
Revelle, W. (2024). psych: Procedures for psychological, psychometric, and personality research (R package version 2.4.1) [Manual]. Northwestern University. Available at https://personality-project.org/r/psych-manual.pdf
Roberts, S., and Serena, M. (2024). Use of consolidated time-lapse camera imagery to detect and monitor platypus (Ornithorhynchus anatinus) activity. Australian Mammalogy 46, AM23045.
| Crossref | Google Scholar |
Sanders, E., Nimmo, D. G., Turner, J. M., Wassens, S., and Michael, D. R. (2024). Putting rakali in the spotlight: innovative methods for detecting an elusive semi-aquatic mammal. Wildlife Research 51, WR24002.
| Crossref | Google Scholar |
Serena, M. (1994). Use of time and space by platypus (Ornithorhynchus anatinus: Monotremata) along a Victorian stream. Journal of Zoology 232, 117-131.
| Crossref | Google Scholar |
Serena, M., and Williams, G. A. (1997). Population attributes of platypus (Ornithorhynchus anatinus) in Flinders Chase National Park, Kangaroo Island. The South Australian Naturalist 72, 28-34.
| Google Scholar |
Serena, M., and Williams, G. A. (2012). Effect of sex and age on temporal variation in the frequency and direction of platypus (Ornithorhychus anatinus) captures in fyke nets. Australian Mammalogy 34(1), 75-82.
| Crossref | Google Scholar |
Serena, M., and Williams, G. A. (2013). Movements and cumulative range size of the platypus (Ornithorhynchus anatinus) inferred from mark–recapture studies. Australian Journal of Zoology 60, 352-359.
| Crossref | Google Scholar |
Serena, M., Thomas, J. L., Williams, G. A., and Officer, R. C. E. (1998). Use of stream and river habitats by the platypus, Ornithorhynchus anatinus, in an urban fringe environment. Australian Journal of Zoology 46, 267-282.
| Crossref | Google Scholar |
Serena, M., Williams, G. A., Weeks, A. R., and Griffiths, J. (2014). Variation in platypus (Ornithorhynchus anatinus) life-history attributes and population trajectories in urban streams. Australian Journal of Zoology 62, 223-234.
| Crossref | Google Scholar |
Seymour, M., Edwards, F. K., Cosby, B. J., Bista, I., Scarlett, P. M., Brailsford, F. L., Glanville, H. C., de Bruyn, M., Carvalho, G. R., and Creer, S. (2021). Environmental DNA provides higher resolution assessment of riverine biodiversity and ecosystem function via spatio-temporal nestedness and turnover partitioning. Communications Biology 4(1), 512.
| Crossref | Google Scholar | PubMed |
Smart, A. S., Tingley, R., Weeks, A. R., Van Rooyen, A. R., and McCarthy, M. A. (2015). Environmental DNA sampling is more sensitive than a traditional survey technique for detecting an aquatic invader. Ecological Applications 25, 1944-1952.
| Crossref | Google Scholar | PubMed |
Stoeckle, B. C., Beggel, S., Cerwenka, A. F., Motivans, E., Kuehn, R., and Geist, J. (2017). A systematic approach to evaluate the influence of environmental conditions on eDNA detection success in aquatic ecosystems. PLoS One 12, e0189119.
| Crossref | Google Scholar | PubMed |
Strahler, A. N. (1952). Hypsometric (area-altitude) analysis of erosional topography. Geological Society of America Bulletin 62, 1117-1142.
| Crossref | Google Scholar |
Strickler, K. M., Fremier, A. K., and Goldberg, C. S. (2015). Quantifying effects of UV-B, temperature, and pH on eDNA degradation in aquatic microcosms. Biological Conservation 183, 85-92.
| Crossref | Google Scholar |
Thalinger, B., Wolf, E., Traugott, M., and Wanzenböck, J. (2019). Monitoring spawning migrations of potamodromous fish species via eDNA. Scientific Reports 9(1), 15388.
| Crossref | Google Scholar | PubMed |
Thomas, J. L., Parrott, M. L., Handasyde, K. A., and Temple-Smith, P. (2019). Burrow use by juvenile platypuses (Ornithorhynchus anatinus) in their natal home range. Journal of Mammalogy 100, 1182-1190.
| Crossref | Google Scholar |
Thomas, J. L., Parrott, M. L., Handasyde, K. A., and Temple-Smith, P. (2020). Maternal care of platypus nestlings (Ornithorhynchus anatinus). Australian Mammalogy 42, 283-292.
| Crossref | Google Scholar |
Thomsen, P. F., Kielgast, J., Iversen, L. L., Møller, P. R., Rasmussen, M., et al. (2012). Detection of a Diverse Marine Fish Fauna Using Environmental DNA from Seawater Samples. PLoS One 7, e41732.
| Crossref | Google Scholar | PubMed |
Webb, B., Morrison, K., Wright, I., and Ryan, M. (2021). The effect of water quality, macroinvertebrate assemblages and habitat suitability on the distribution of platypuses; a pilot study in Cattai catchment, northwest Sydney. In ‘Proceedings of the 10th Australian Stream Management Conference’. Available at https://asnevents.s3.amazonaws.com/Abstrakt-FullPaper/74342/10ASM+paper+Platypus+.pdf
Yang, J., Zhang, X., Jin, X., Seymour, M., Richter, C., Logares, R., Khim, J., and Klymus, K. (2021). Recent advances in environmental DNA‐based biodiversity assessment and conservation. Diversity and Distributions 27, 1876-1879.
| Crossref | Google Scholar |