Optimum inclusion rate of barley in diets of meat chickens: an incremental and practical program
M. Toghyani A B * , S. P. Macelline A B , S. Greenhalgh A B , P. V. Chrystal A B , P. H. Selle
A B * , S. P. Macelline A B , S. Greenhalgh A B , P. V. Chrystal A B , P. H. Selle  B and S. Y. Liu A B
B and S. Y. Liu A B
A School of Life and Environmental Sciences, Faculty of Science, The University of Sydney, Sydney, NSW 2006, Australia.
B Poultry Research Foundation, The University of Sydney, Camden Campus, Camden, NSW 2570, Australia.
Animal Production Science 62(7) 645-660 https://doi.org/10.1071/AN21437
Submitted: 26 August 2021 Accepted: 4 February 2022 Published: 8 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Barley can be included in poultry diets as a cost-effective energy-contributing ingredient. However, its inclusion in meat chicken diets is limited because it is considered a viscous grain due to high crude fibre and soluble non-starch polysaccharide contents.
Aims: The study quantified the optimum inclusion rate of barley in meat chicken diets during different growing phases, using an incremental program.
Methods: Eight dietary treatments followed a 4 × 2 factorial arrangement, with three levels of barley inclusion to a wheat-based diet, and a nil-barley control, with or without β-glucanase supplementation. Barley was initially included at 0% (low), 7.5% (medium) and 15% (high) in starter diets (Days 1–9), scaling up by 7.5% for each level in grower (Days 9–21), finisher (Days 23–35) and withdrawal (Days 35–42) diets. Each diet was fed ad libitum to six replicate pens of 18 chicks. On Day 42, four birds per replicate pen were euthanised to determine carcass yield and collect digesta.
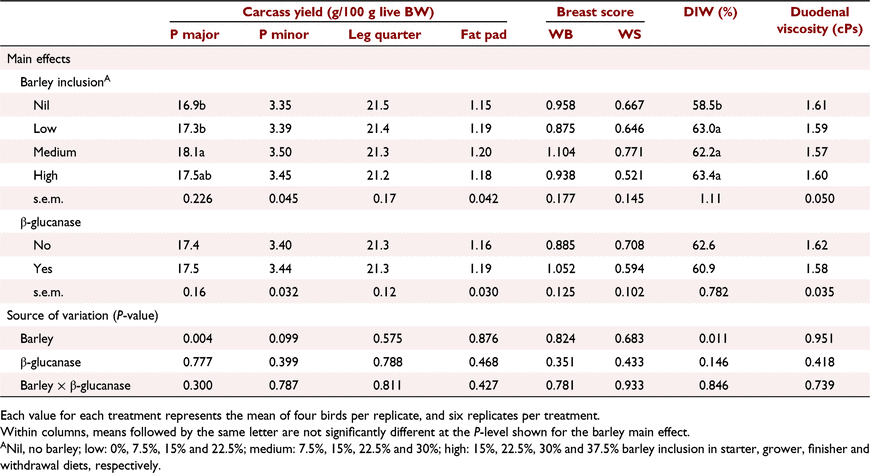
Key results: During the starter period, a significant (P < 0.05) barley × β-glucanase interaction resulted in lower bodyweight gain (8%) and higher feed conversion ratio (8.5 points) at 15% barley inclusion without β-glucanase, whereas performance was restored with β-glucanase supplementation. No treatment interaction was apparent on growth performance assessed over the entire production period (Days 1–42). Barley inclusion at medium and high levels increased bodyweight gain, and at all levels improved feed efficiency (P < 0.01) compared with the control. β-Glucanase improved (P < 0.05) feed efficiency. Highest (P < 0.01) breast meat yield was measured for diets with medium barley inclusion. There were no interactive or main effects on duodenal digesta viscosity. Barley inclusion increased distal ileal digesta water content by ∼8–10% (P < 0.05).
Conclusions: Incremental inclusion of barley from 15% in a starter diet, scaling up to 37.5% in a withdrawal diet, does not compromise growth performance or carcass yields in broiler chickens. β-Glucanase supplementation favours both bodyweight gain and feed efficiency. Medium level of barley inclusion favours breast meat yield.
Implications: Barley can be considered an economical grain to formulate cost-effective diets for broiler chickens. An incremental program is a practical approach to optimise barley inclusion rate.
Keywords: barley, β-glucanase, broiler chicks, carcass yield, feed cost, viscosity, white striping, woody breast.
Introduction
In any farming system (i.e. intensive, free range or organic rearing systems), the cost of chicken meat production largely depends on the feed cost, which represents ∼60–75% of total production cost. Dietary energy is the largest and most expensive constituent of meat chicken diets, often supplied by high inclusion of cereal grains. In Australia, wheat-based diets are commonly used in poultry production and are often supplemented with exogenous xylanase to mitigate the negative impact of wheat non-starch polysaccharides (NSP), mostly soluble arabinoxylans, on the bird’s growth performance.
Barley (Hordeum vulgare L.), ranked fourth in global grain production, can also be included in poultry diets as an energy-contributing ingredient. Diets igh in barley are commonly fed to swine and layer hens, but its inclusion in meat chicken diets is limited mainly because of its high fibre content, low energy (apparent metabolisable energy, AME) and high levels of soluble NSP (Jacob and Pescatore 2012). On a dry matter basis, barley contains 33.3% and 55.2% more crude fibre, and 46.5% and 95.0% more soluble NSP than wheat and maize, respectively (Choct 2006; Knudsen 2014). Large variations in the chemical and physical characteristics of barley exist even among similar types. Depending on cultivar, the concentration of soluble β-glucan in barley can be nearly 10 times higher than in wheat (Jacob and Pescatore 2014). Perera et al. (2019a) measured β-glucan contents of 6.86%, 3.85% and 0.77% in waxy-starch hull-less barley, normal-starch hulled barley and wheat, respectively. Barley has a lower and more variable (40–55%) starch concentration than maize (62–67%) or wheat (55–60%). When expressed as percentage of total crude protein (CP), the amino acid profile of barley is similar to that of maize or wheat; however, its protein and amino acid digestibility coefficients are considered lower (McNab and Shannon 1974).
High concentrations of soluble NSPs, particularly β-glucan, have long been identified as the main antinutritive factors in barley, responsible for increasing gut viscosity (White et al. 1983) and sticky droppings (Gohl et al. 1978), as well as impairing nutrient digestibility (Salih et al. 1991) in chickens. This eventually led to the development and use of commercial β-glucanases (McNab and Smithard 1992). Since then, supplementation of barley-based meat chicken diets with multi-carbohydrase enzymes targeting mainly the soluble NSP fraction has been comprehensively researched. β-Glucanase supplementation of diets high in barley has been shown to increase feed intake and weight gain, and improve flock uniformity, feed efficiency and nutrient utilisation (Hesselman and Åman 1986; Marquardt et al. 1994; Almirall et al. 1995; Bergh et al. 1999). Research has indicated that exogenous multi-carbohydrase, when added to high barley diets, is able to reduce digesta viscosity (Almirall et al. 1995; Józefiak et al. 2006), eliminate the nutrient-encapsulation effect of cell walls (Hesselman and Åman 1986; Bedford and Morgan 1996), and modify gut microbiota through the supply of prebiotic oligosaccharides (González-Ortiz et al. 2017; Bedford 2018). Nonetheless, application of NSPase in barley-based diets has not always generated consistent results, and wide variability in responses to enzyme supplementation has been reported (Bao et al. 2013; Karunaratne et al. 2021; Perera et al. 2021). Published literature on recommendations for barley inclusion, either with or without β-glucanases or other NSPase enzymes, has also been contradictory. This has resulted in a range of inclusion levels being recommended in broiler chicken diets from as little as 10% to >50%. The discrepancy in published data may be explained by the variations in the chemical and physical characteristics of barley (Izydorczyk et al. 2000), the stage of ripeness (Fuente et al. 1998), the birds’ age when barley has been introduced into the diet (Ayres et al. 2019), the source of nutrient composition data used for barley, the grain profile in the background diet, and the differences in enzyme cocktails tested.
Australia produces high-quality two-row spring-type barley, with annual production averaging ∼7.5 Mt/year (Grain Research and Development Corporation (GRDC) 2018). Most of the barley produced in Australia is exported, which makes the domestic barley market very volatile. Considering the market trends for Australian barley and the lack of updated literature, it is necessary to re-establish the optimum inclusion rate of barley in meat chicken diets, giving nutritionists confidence to formulate more cost-effective diets with a focus on maximum profitability. The present study was designed to quantify the optimum inclusion rate of barley for different growing phases of meat chickens, and to test the additional benefit on growth performance by supplementing β-glucanase in the presence of xylanase in diets based on wheat and barley. An initial industry report of the experimental work, described below, has been published online (AgriFutures Australia, Publication No. 21-053, May 2021).
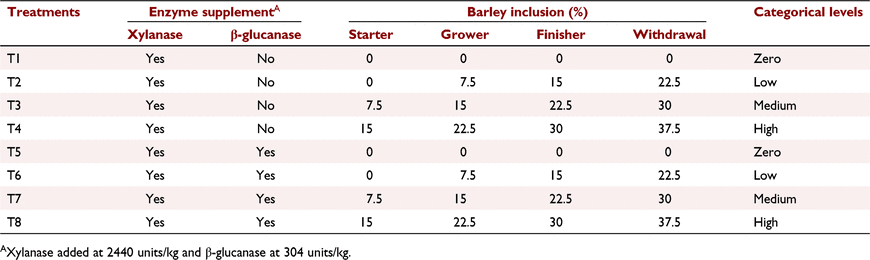
Materials and methods
Birds and experimental diets
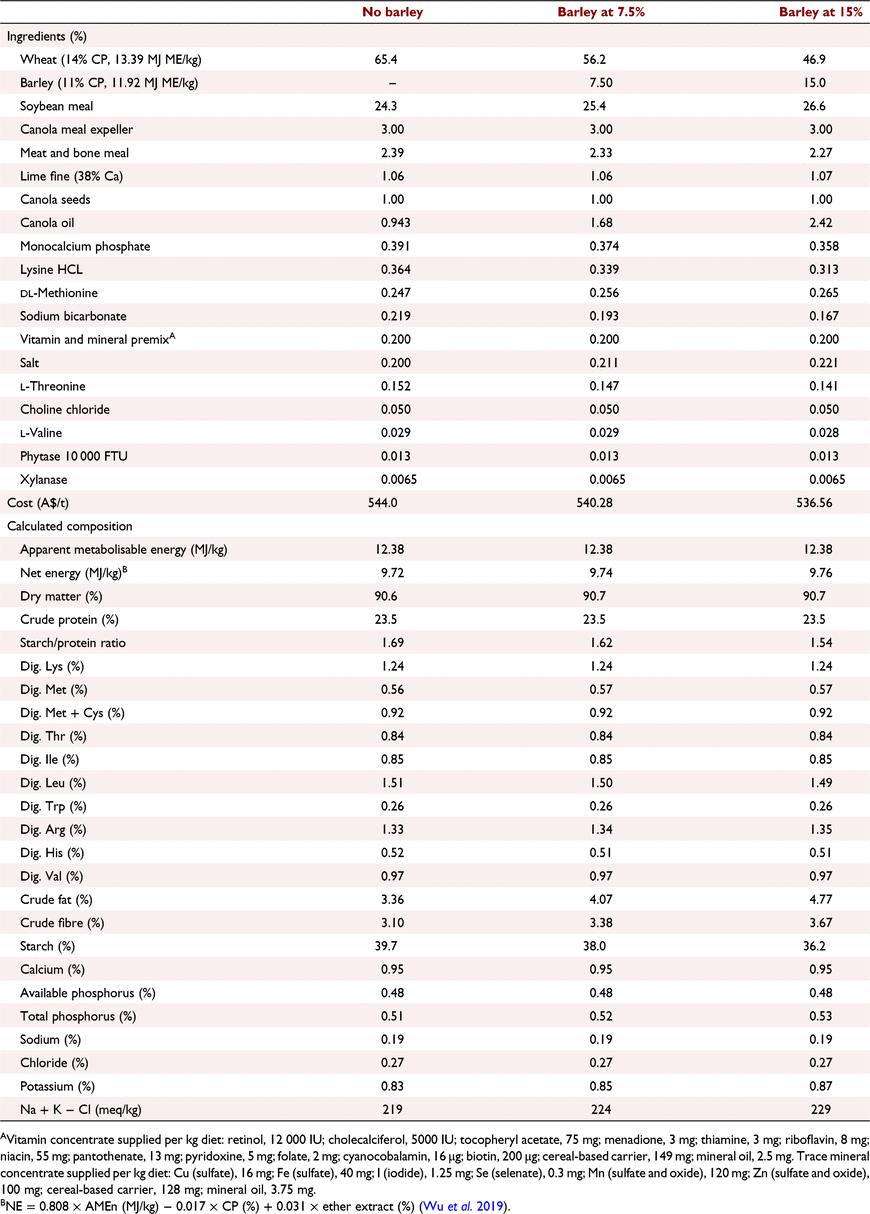
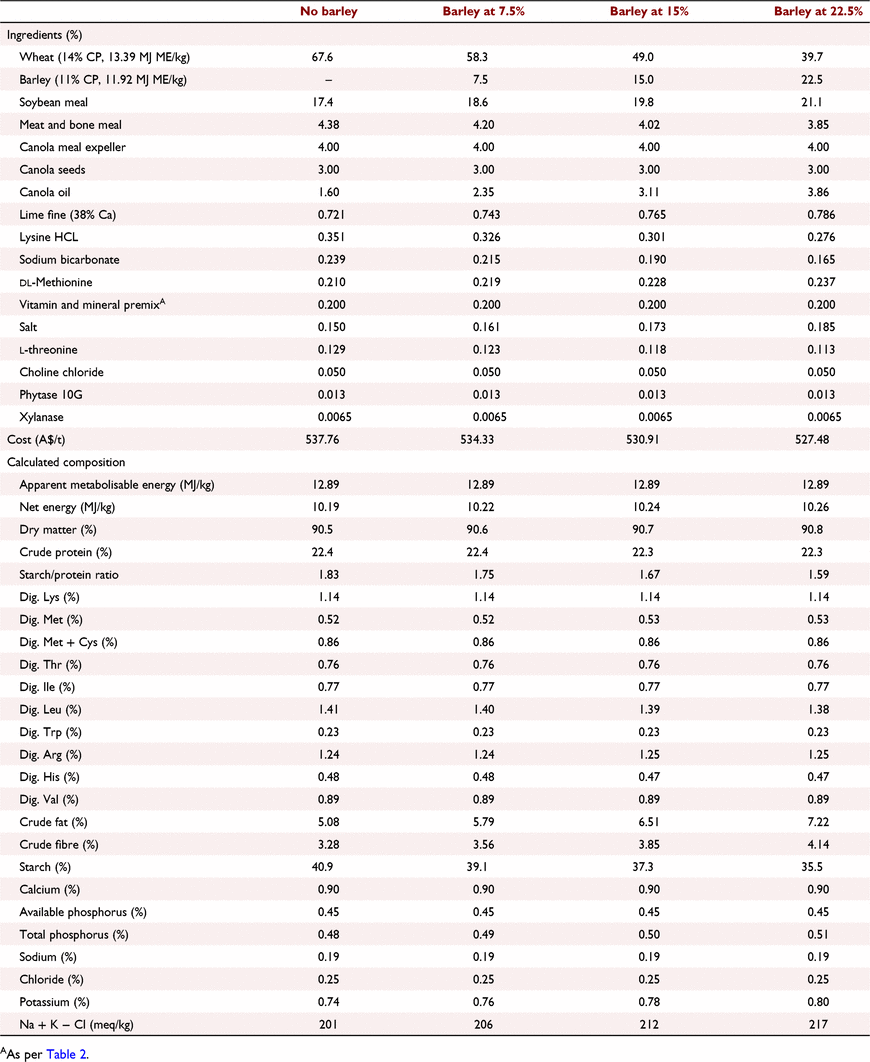
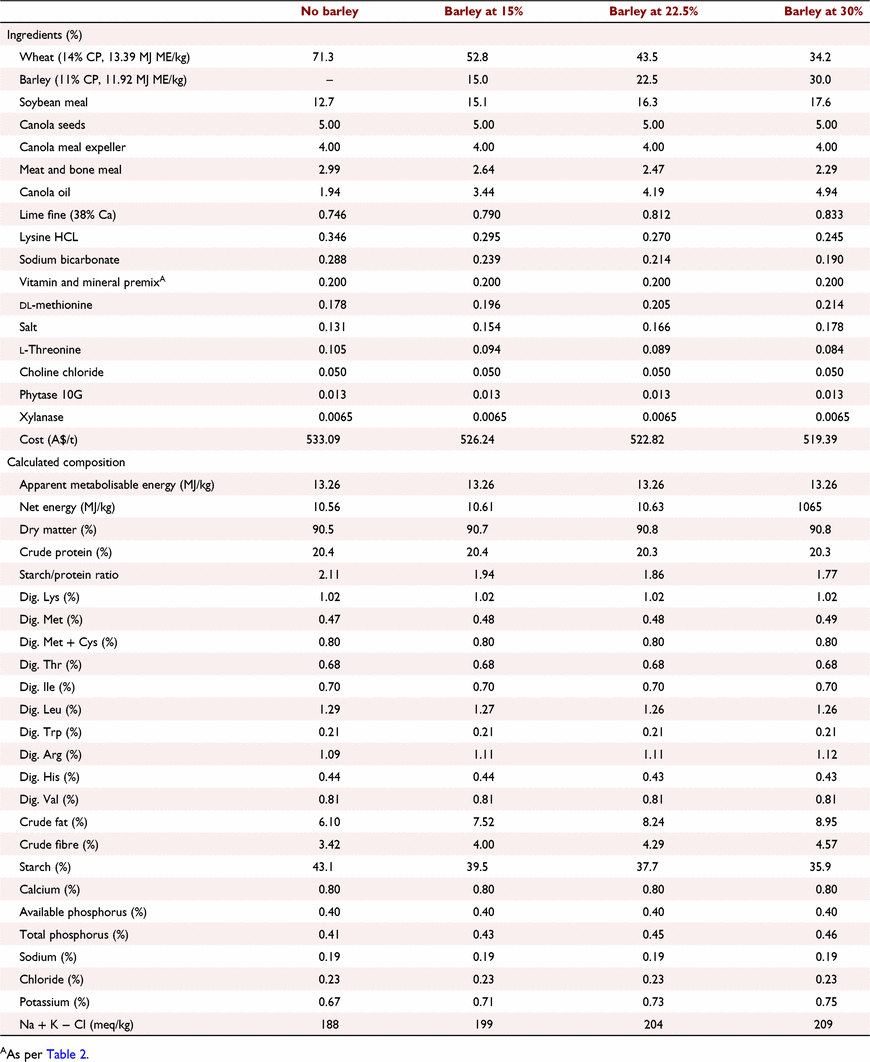
All experimental protocols and procedures for the study were reviewed and approved by the University of Sydney Animal Ethics Committee (2020/1776). In total, 864 1-day-old off-sex male Ross 308 chicks were obtained from a commercial hatchery (Goulburn, NSW, Australia). On arrival, birds were group weighed and assigned to their respective treatments into 48 floor pens. Each treatment was replicated six times with 18 birds per replicate. The feeding study consisted of eight dietary treatments designed as a 4 × 2 factorial arrangement, which included three incremental levels of barley inclusion (low, medium, and high) and a nil-barley (wheat-based only) control, and two concentrations of β-glucanase (0 or 304 unit/kg) supplementation. Barley was formulated into the wheat-based diet and scaled up over the experimental phases as follows: 0% (low), 7.5% (medium) and 15% (high) for the starter phase (Days 1–9); 7.5%, 15% and 22.5% for the grower phase (Days 9–23); 15%, 22.5% and 30% for the finisher phase (Days 23–35); and 22.5%, 30% and 37.5% for the withdrawal phase (Days 35–42) (Table 1). Therefore, the starter diets, having 0% barley at the low inclusion rate, were arranged as a 3 × 2 factorial with 12 replicate pens having no barley.
|
All diets were formulated to be iso-caloric (based on AME) and iso-nitrogenous, having a balance of digestible essential amino acids similar to breeder recommendations (Aviagen 2019; Tables 2–5). All diets had exogenous phytase (Axtra PHY 10 TPT; Danisco Animal Nutrition, Copenhagen, Denmark) added at 130 g/t to provide 1300 phytase units (FTU)/kg, with the matrix applied only for calcium, available phosphorus and sodium as per manufacturer recommendations. Diets without β-glucanase had xylanase (Danisco Xylanase; Danisco Animal Nutrition) added at 65 g/t, to provide 2440 units xylanase activity/kg. Diets with β-glucanase had a blend of xylanase and β-glucanase (Axtra XB, Danisco Animal Nutrition) added at 200 g/t, to provide 2440 units xylanase activity and 304 units β-glucanase activity/kg. Diets were steam-pelleted at a conditioning temperature of 80°C for 14 s. The pellet machine was equipped with a die ring with 4.0 mm holes and 38 mm thickness. The starter diets were further crumbled to maximise intake of feed. All diets were offered ad libitum. Pellet durability index (PDI) of all diets (excluding starter diets) were tested in triplicate, using the NHP 200 New Holman Automatic Pellet Tester (TekPro, Norfolk, UK).
|
|
|

|
Prior to diet formulation, representative subsamples of wheat, barley, soybean meal, meat and bone meal, canola meal and canola seed were analysed by near-infrared spectroscopy to predict proximate analysis, digestible amino acid concentrations and AME using AMINONIR PROX, NIR and NRG (Evonik Nutrition & Care, Hanua, Germany), respectively. Accordingly, the predicted AME values of 13.39 and 11.92 MJ/kg for wheat and barley, respectively, were used to formulate diets.
Parameters
Birds were weighed on a pen basis on Days 1, 9, 23, 35 and 42 to determine bodyweight (BW) and calculate BW gain (BWG). Feed intake (FI) was measured in similar intervals and used to calculate feed conversion ratio (FCR) for each phase. Mortality was recorded daily, and the BW of dead bird was used to correct FCR values. On Day 42, BW in the control group (no barley, no β-glucanase) was used to calculate BW-corrected FCR (FCRc) because there were treatment-associated differences in BW. This correction was achieved by considering that a 50 g difference in BW was equivalent to one point (0.01) in FCR.
On Day 42, a total of four birds per pen were randomly selected and euthanised for carcass analysis. Skinless breast meat (pectoral major and minor), leg quarter (thigh + drumstick), and abdominal fat pad were removed, weighed and calculated as a percentage of live BW. Breast major muscle were also visually examined and scored for the occurrence of woody breast (Fig. 1) and white striping (Fig. 2; Kuttappan et al. 2012).
|
|
|
|
The digesta contents of the duodenum loop were gently squeezed out, and collected into ice-cooled plastic containers to determine digesta viscosity. Distal ileal digesta from individual birds were also collected to measure dry matter and water content.
Chemical analysis
The diets and the digesta dry matter were analysed in duplicate (method 930.15; Association of Official Analytical Communities (AOAC) 2016). The nitrogen contents of raw ingredients and diet samples were determined on a 0.25-g sample in a combustion analyser (FP-2000 N analyser; LECO, St Joseph, MI, USA) using EDTA as a calibration standard, with CP being calculated by multiplying percentage N by a correction factor (6.25).
Starch concentration in wheat, barley and the diets was determined by a procedure based on dimethyl sulfoxide, α-amylase and amyloglucosidase as described by Mahasukhonthachat et al. (2010).
For viscosity analysis, the duodenal digesta samples were centrifuged at 12 000g for 10 min at 4°C, and a sample of supernatant (∼0.5 mL) was used to measure viscosity with a DVIII viscometer (Brookfield, Stoughton, MA, USA) at 25°C with a CP 40 cone. The shear rate ranged from 5 to 500 s−1, over which the samples did not exhibit shear thinning.
Statistical analyses
Data were checked for normality and then subjected to two-way analysis of variance, using the GLM procedure of JMP13.0.0 (SAS Institute, Cary, NC, USA), to assess the main effects of barley inclusion levels and β-glucanase supplementation, and their interaction. Each pen was considered an experimental unit and the values presented in the tables are means with pooled standard error of mean (s.e.m.). If a significant effect of treatment was detected, differences between treatments or main effects were separated by least square differences test. Significance was called at P < 0.05, and trends were indicated where P < 0.1.
Pearson correlation coefficients and associated significance were generated using PROC GLM of JMP to determine the relationship between FCR values and the analysed starch/protein ratios (ST/CP) and calculated net energy (NE) of the diets without β-glucanase supplementation.
Results
Enzyme activity, growth performance and pellet durability index
The diets were formulated to have a phytase activity of 1300 FTU/kg, and the phytase activity test revealed a recovery of minimum 94% (1220 FTU/kg) to a maximum of 107% (1390 FTU/kg) across different diets for each period.
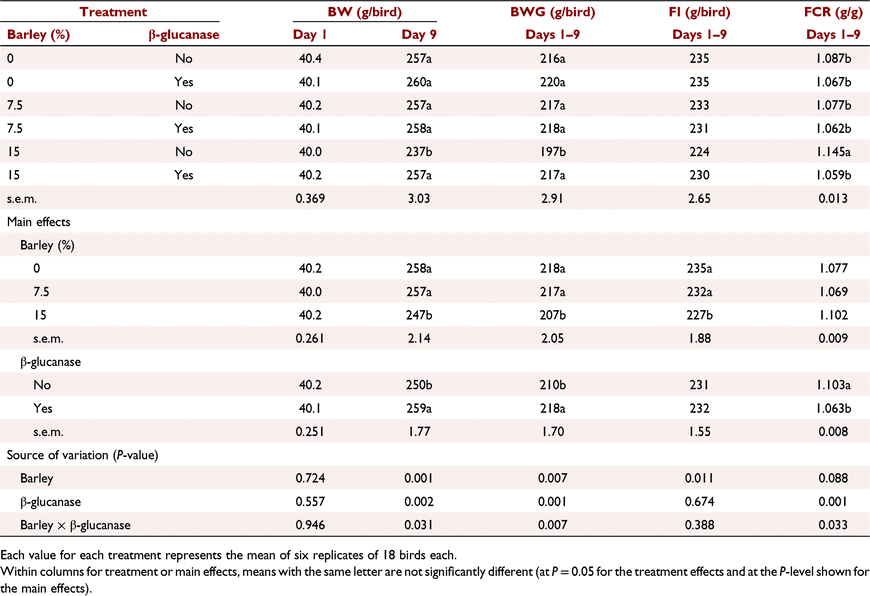
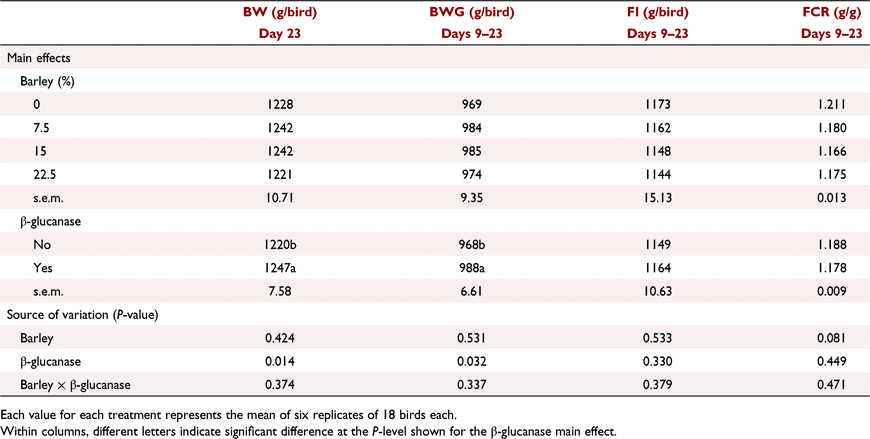
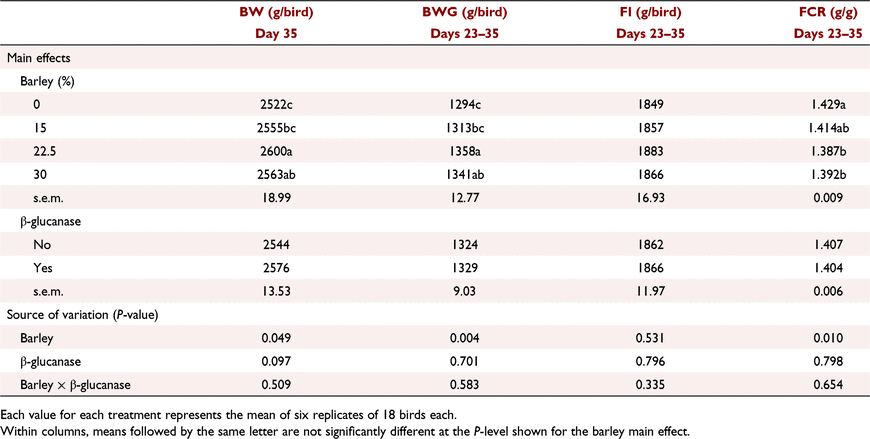
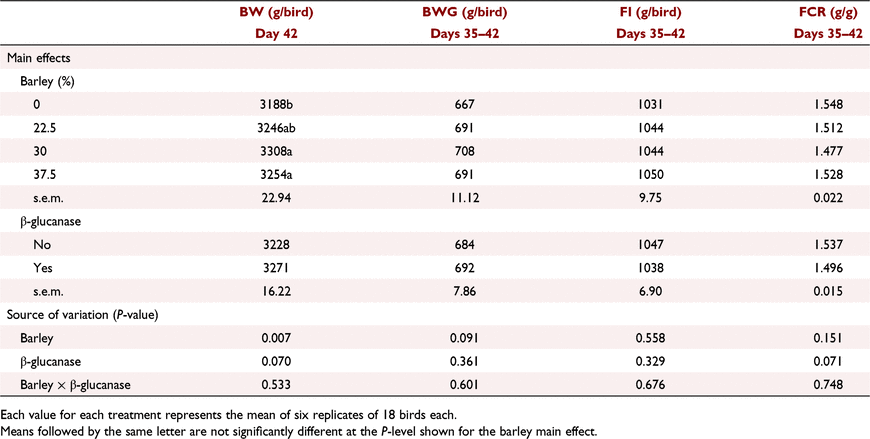
The interactive effects of barley inclusion and β-glucanase on the performance parameters over the starter period (Days 1–9) are presented in Table 6. Barley inclusion at 7.5% did not have a negative effect on BW or FI. Barley inclusion at 15% without added β-glucanase decreased BWG by ∼8%, and increased FCR by 8.6 points relative to control birds. However, β-glucanase supplementation restored this lower BWG and higher FCR at high barley inclusion, resulting in a significant (P < 0.05) barley × β-glucanase interaction for both BWG and FCR. Over the grower period (Days 9–23), barley inclusion had no effect on BW and FI but tended (P = 0.081) to improve FCR particularly when added at 15% (Table 7). Addition of β-glucanase increased (P < 0.05) BW by an average of 27 g/bird (2.2%). According to the data presented for the finisher period in Table 8, on Day 35, barley inclusion at 15% and 22.5% increased (P < 0.05) BW compared with no-barley diets. Although there was no significant effect of β-glucanase alone or in an interaction with barley inclusion, the enzyme numerically improved BW when barley was included at 15% (2517 vs 2597 g/bird) or 30% (2544 vs 2581 g/bird), but had no marked effect at 22.5% (2591 vs 2609 g/bird). Barley inclusion at 22.5% and 30%, regardless of β-glucanase addition, significantly (P < 0.01) improved FCR over the finisher period (Days 23–35). On Day 42, birds fed diets with barley at 30% and 37.5% recorded higher (P < 0.01) BW than those fed no-barley diets (Table 9). β-Glucanase supplementation, as a main effect, tended (P = 0.07) to improve final BW by an average of 43 g/bird. FI and FCR were not affected by barley or β-glucanase during the withdrawal period (Days 35–42; Table 9).
|
|
|
|
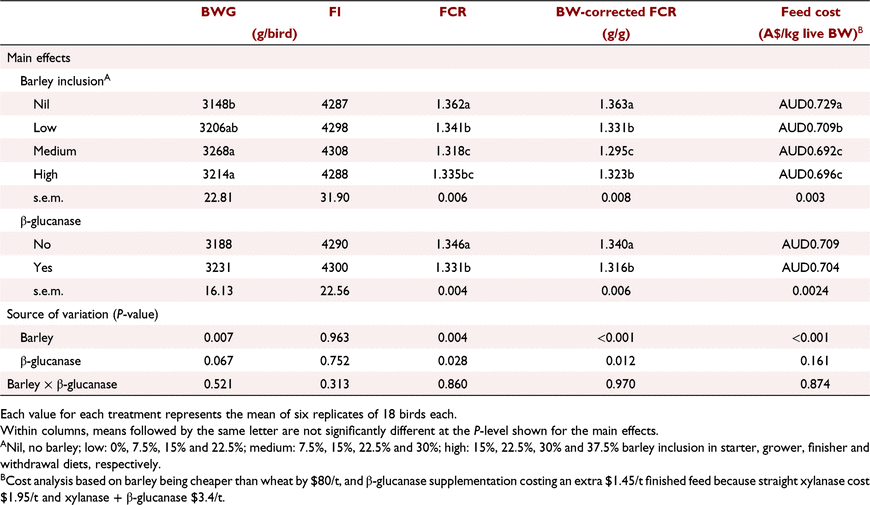
Table 10 summarises the effects of dietary treatments on performance parameters over the entire production period (Days 1–42). Birds fed diets with medium and high barley levels had higher (P < 0.01) BWG than birds offered no-barley diets. Addition of β-glucanase to the diets tended (P = 0.067) to improve BWG across all treatments. A greater response to β-glucanase in increasing BWG was observed in birds fed the low (3164 vs 3247 g/bird) and high (3178 vs 3249 g/bird) barley diets; however, these differences did not lead to a significant interaction between barley inclusion and β-glucanase supplementation. Barley inclusion at low, medium and high levels improved final FCR by 2.1, 4.4 and 2.7 points compared with no-barley diets (P < 0.01), and when FCR was corrected for BW, these improvements further increased to 3.2, 6.8 and 4.0 FCR points, respectively (P < 0.01). There was a statistically significant (P < 0.05) improvement in both FCR and BW-corrected FCR in response to β-glucanase inclusion over the entire production period.
|
On the basis of the data on feed cost per kg live BW (Table 10), the highest and lowest feed costs, respectively, were for birds fed the control (no-barley) diet without β-glucanase and the medium barley diet with β-glucanase ($0.734 vs $0.689, 6.4% lower).
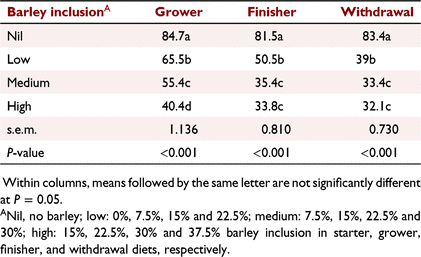
The introduction of barley to the diets negatively affected the pellet quality index (Table 11). Barley inclusion at low levels significantly reduced grower diet PDI by almost 30% (84.7 vs 65.5), which further dropped by 10 and 15 units at medium and high barley inclusion, respectively (P <<0.001). The PDI values in finisher diets dropped from 81.5 to 50.5 at low barley inclusion, and to ∼34 at medium and high barley inclusion (P < 0.001). Similarly, introduction of barley at the low level in withdrawal diets reduced PDI by >113% (83.4 vs 39.0), and at medium and high inclusion levels, dropped the PDI by almost 150% compared with the control (P < 0.001).
|
As expected, the barley used in this study had less starch (47.1%) than wheat (54.6%) and lower AME (11.92 vs 13.39 MJ/kg, NIR predictions). Thus, increasing barley inclusion decreased ST/CP ratio, and increased the calculated NE of the corresponding diets due to higher supplemental oil. FCR values, except for the starter period, were positively (P < 0.05) correlated with ST/CP ratios. There were also negative (P < 0.05) correlations between FCR values and dietary NE in grower, finisher and the entire production (Days 1–42) period (Table 12).

|
Carcass yield, digesta viscosity and water content
Barley inclusion at medium levels significantly (P < 0.01) increased breast major muscle yield and tended (P = 0.099) to increase breast tender yield. Leg quarter and fat pad percentages were not affected by barley inclusion or β-glucanase addition (P > 0.05). The woody breast and white striping scores (Table 13) suggest that the differences in grain source (barley vs wheat), ST/CP ratio and growth rate were not associated with the occurrence and severity of these modern myopathies (P > 0.05).
Barley, regardless of inclusion rate, increased (P < 0.05) distal ileal digesta water content by ∼8–10% compared with no-barley diets. β-Glucanase supplementation non-significantly (P = 0.146) decreased digesta water content by ∼4%. There was no effect of barley and β-glucanase alone or as an interaction on digesta viscosity measured at the duodenum.
Discussion
Barley inclusion at the highest levels without β-glucanase supplementation in the starter (15%) and grower (22.5%) phases suppressed BWG compared with the wheat-based control diet. However, birds were able to restore their growth performance later, and considering the entire production period, birds fed the barley diets, regardless of β-glucanase addition, had superior performance to the control birds. Similarly, Perera et al. (2019b) did not detect an interaction of an exogenous carbohydrase that had β-glucanase activity with barley inclusion level in broiler chicken diets with respect to productive traits. Based on performance results, the authors suggested an optimum inclusion of 28.3% barley in wheat-based diets. In another study, hulless barley at 30% inclusion did not compromise BWG and FCR, regardless of β-glucanase supplementation (Karunaratne et al. 2021). However, when barley inclusion level increased to 60%, it impaired productive traits, without any positive response to β-glucanase supplementation.
The introduction of barley changed the dynamic of the formulations. Barley contains less starch than wheat; hence, the inclusion of barley reduced dietary starch concentration but increased oil supplementation in order to formulate iso-energetic diets based on AME. This resulted in lower ST/CP ratio and higher calculated NE for the barley diets. Both ST/CP ratio and the NE content of the diets were significantly correlated with FCR values, except during the withdrawal period. Differences in dietary ST/CP ratios impact on starch–protein digestive dynamics in broiler chickens (Liu and Selle 2017), and a lower ST/CP has been shown to benefit FCR through greater intestinal uptake of amino acids relative to glucose (Selle and Liu 2019). Dietary fat has a lower heat increment and higher NE/AME ratio than protein and carbohydrates (starch), resulting in improved energy utilisation and muscle protein accretion (Wu et al. 2019). Thus, the higher calculated NE of the barley diets could also, to some extent, account for the superior performance of birds offered the barley diets. Furthermore, excess dietary energy or an imbalanced ratio of protein to energy enhances the portion of energy retained as fat, which is illustrated as higher fat-pad yield (Musigwa et al. 2021). The dietary treatments fed in this study, despite having different ST/CP ratios and NE content, did not affect the relative abdominal fat-pad measured on Day 42, implying that the barley AME used in the formulations was not underestimated.
The wheat used in this study was a high protein wheat (14.2% CP), and replacing that wheat with barley (11.3% CP) increased the soybean meal inclusion in the diets, resulting in more CP being supplied from soybean meal in barley diets. All diets within each phase were formulated to be iso-nitrogenous and balanced for the main essential amino acids (Lys, Met + Cys, Thr, Ile, His, Arg and Val). Nonetheless, on a dry matter basis, soybean protein has a better balance of non-essential amino acids than grain protein (Gorissen et al. 2018), and as such, the differences in soybean meal inclusion level and contribution into the diet’s protein pool could partly explain the better productive traits of birds fed the barley diets.
Overall, birds fed the diets with medium levels of barley (7.5%, 15%, 22.5% and 30% in starter, grower, finisher and withdrawal diets, respectively) gained the most BW and recorded the lowest FCR, and, independent of age, appeared to be less responsive to β-glucanase supplementation. Similarly, measurements of intestinal viscosity and rate of passage of digesta through the gut suggest that birds offered diets containing barley but without exogenous enzymes adapt to β-glucans, most probably through colonisation of β-glucanase-producing bacteria or by a physiological change in the secretions or in the anatomy of the gut (Salih et al. 1991). This may indicate the importance of conditioning the gut and exposing intestinal microbiota (priming microbiota) to any dietary changes at a young age.
The analysed in vitro viscosity of the barley used in this study was nearly 2.5 times higher than that of the wheat (24.75 vs 10.50 cPs). The viscosity values measured and reported for Australian barley (2019–20 harvest) are quite variable, ranging from as low as 10 to >700 cPs (S. Wilkinson, Feedworks internal report, unpubl. data, 2020). There are many factors that could contribute to this high degree of variability, such as barley cultivar, storge time since harvest, β-glucans, pectin, cellulose and hemicellulose content and their solubility (Fuente et al. 1998; Perera et al. 2019a, 2019b). Viscous digesta reduce the rate of diffusion and distribution of feed substrates in the gut, alter intestinal microbiota populations, and as such hamper digestive enzyme activity, thereby decreasing the absorption of nutrients (Bedford 1995). Hence, increased digesta viscosity in high barley diets has long been identified as the main antinutritive effect of barley for broiler chicks. In this regard, previous studies have shown that a multi-carbohydrase and/or β-glucanase supplementation of high barley diets decreases the viscosity of digesta (Perera et al. 2019a, 2019b; Karunaratne et al. 2021). However, in the present study, digesta viscosity was not affected by barley presence, β-glucanase supplementation, or two-way interactions of barley and the enzyme. Such results could be because of the low viscosity of the barley used in this study, and the age (Day 42) at which viscosity was measured. Strong correlations between in vitro dietary viscosity and in vivo proximal and distal jejunal intestinal viscosity were reported by Bedford and Classen (1993), indicating that the in vitro viscosity assay is reliable for predicting the in vivo viscosity. Similarly, Ayres et al. (2019) showed that both dietary in vitro viscosity and in vivo intestinal viscosity can correlate to broiler performance at a young age (up to Day 21). Philip et al. (1995) reported that the intestinal viscosity of birds fed a barley-based diet decreases with age, either because the birds adapt to the presence of β-glucans and β-glucanase in the diet or because the bigger size of the birds negates the adverse effects observed among young birds. As also evident from both the performance and digesta viscosity results in the present study, it appears that the nutritive value of barley increases with the bird’s age, and performance improvements in response to β-glucanase are less pronounced in adult birds (Salih et al. 1991).
As barley inclusion in the diets increased, the PDI decreased to the extent that barley at its highest level in finisher (30%) and withdrawal (37.5%) diets reduced PDIs by nearly 150% compared with no-barley control diets. The higher supplemental oil in barley diets, the fibrous nature of barley grains and its lower starch content should have resulted in less durable pellets (Muramatsu et al. 2015). Pellet quality influences broiler performance and behaviour, so that higher PDI improves feed consumption (Meinerz et al. 2001) and increases the bird’s resting time, which lowers energy expenditure for maintenance and increases the availability of NE for production (McKinney and Teeter 2004). In fact, 10% improvement in PDI represents ∼14 kcal (∼58.6 kJ) of effective caloric value in the feed. However, the low PDI values determined for barley diets in this study did not affect productive traits, because there was no correlation between PDI and feed intake, which suggests the reduction of PDI did not confound the growth performance response to barley inclusion in broiler diets.
Globally, the meat chicken industry is facing emerging quality issues with breast meat, including white striping (white striations parallel to muscle fibres) and woody breast (hardness of raw fillet) (Tijare et al. 2016). Although both white striping and woody breast have been characterised with myodegeneration and necrosis, fibrosis, lipidosis and regenerative changes, the occurrence of these modern myopathies has been associated with increased growth rate in birds (Kuttappan et al. 2016). The severity of white striping and woody breast can adversely affect consumer acceptance of raw cut-up parts and/or quality of further processed meat products, resulting in huge economic loss to the industry. Despite the higher growth rate and the superior FCR of birds fed barley diets, the results of carcass yield, white striping and woody breast scores obtained in here suggest that differences in diet grain source, dietary ST/CP ratio and NE do not impact on breast meat yield and myopathies.
Conclusions
The results obtained in the present study indicate that application of an incremental program is an effective approach to optimise barley inclusion in broiler chickens’ diets, especially when diets are formulated to similar digestible amino acids and metabolisable energy. A low-viscous barley can be included up to 7.5% in broiler chickens’ starter diets without compromising growth performance. At higher inclusion levels (15%) in the starter period, diets should be supplemented with β-glucanase in addition to xylanase to mitigate the performance loss (lower BW and higher FCR). Increasing barley levels to 22.5% in grower diets can slightly decrease growth rate but has no effect on feed efficiency. Stepping up barley levels to 30% in finisher and 37.5% in withdrawal diets did not compromise growth performance, and such high levels could improve both BWG and FCR. Post starter period, there was no statistically significant interaction of barley inclusion level and β-glucanase supplementation, and the enzyme, independent of barley level, improved feed efficiency and favoured gain in BW.
High barley inclusion increased digesta water content by ∼8–10%, which can lead to high litter moisture and, under commercial high-density rearing conditions, may cause wet litter issues if the extra moisture is not removed by increased ventilation. β-Glucanase supplementation of high barley diets reduced ∼30–40% of this increased moisture.
The price difference between wheat and barley, on a similar protein basis, and the source and cost of supplemental fat in the diet are the major factors determining the economics of barley usage and inclusion levels in broiler chickens’ diets.
Data availability
The datasets generated during and/or analysed during the study are available from the corresponding author on reasonable request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was funded by AgriFutures Australia, Meat Chicken Program (Project No. PRJ-012265).
Acknowledgements
The authors thank Ms Joy Gill, Mr Duwei Chen, Ms Kylie Warr and Mr Peter Bird from Poultry Research Foundation for their assistance with feed manufacturing, bird management, sample collection, and laboratory analyses.
References
Almirall M, Francesch M, Perez-Vendrell AM, Brufau J, Esteve-Garcia E (1995) The differences in intestinal viscosity produced by barley and beta-glucanase alter digesta enzyme activities and ileal nutrient digestibilities more in broiler chicks than in cocks. The Journal of Nutrition 125, 947–955.| The differences in intestinal viscosity produced by barley and beta-glucanase alter digesta enzyme activities and ileal nutrient digestibilities more in broiler chicks than in cocks.Crossref | GoogleScholarGoogle Scholar | 7536829PubMed |
Association of Official Analytical Communities (AOAC) (2016) ‘Official methods of analysis of AOAC International.’ 20th edn. (AOAC International: Rockville, MD, USA)
Aviagen (2019) Broiler Ross 308 Nutrition specifications. (Aviagen: Huntsville, AL, USA) Available at http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf
Ayres VE, Broomhead JN, Li X, Raab RM, Moritz JS (2019) Viscosity and growth response of broilers fed high fiber diets supplemented with a corn-produced recombinant carbohydrase. Journal of Applied Poultry Research 28, 826–836.
| Viscosity and growth response of broilers fed high fiber diets supplemented with a corn-produced recombinant carbohydrase.Crossref | GoogleScholarGoogle Scholar |
Bao YM, Romero LF, Cowieson AJ (2013) Functional patterns of exogenous enzymes in different feed ingredients. World’s Poultry Science Journal 69, 759–774.
| Functional patterns of exogenous enzymes in different feed ingredients.Crossref | GoogleScholarGoogle Scholar |
Bedford MR (1995) Mechanism of action and potential environmental benefits from the use of feed enzymes. Animal Feed Science and Technology 53, 145–155.
Bedford MR (2018) The evolution and application of enzymes in the animal feed industry: the role of data interpretation. British Poultry Science 59, 486–493.
| The evolution and application of enzymes in the animal feed industry: the role of data interpretation.Crossref | GoogleScholarGoogle Scholar | 29877713PubMed |
Bedford MR, Classen HL (1993) An in vitro assay for prediction of broiler intestinal viscosity and growth when fed rye-based diets in the presence of exogenous enzymes. Poultry Science 72, 137–143.
| An in vitro assay for prediction of broiler intestinal viscosity and growth when fed rye-based diets in the presence of exogenous enzymes.Crossref | GoogleScholarGoogle Scholar | 8426842PubMed |
Bedford MR, Morgan AJ (1996) The use of enzymes in poultry diets. World’s Poultry Science Journal 52, 61–68.
| The use of enzymes in poultry diets.Crossref | GoogleScholarGoogle Scholar |
Bergh MO, Razdan A, Åman P (1999) Nutritional influence of broiler chicken diets based on covered normal, waxy and high amylose barleys with or without enzyme supplementation. Animal Feed Science and Technology 78, 215–226.
| Nutritional influence of broiler chicken diets based on covered normal, waxy and high amylose barleys with or without enzyme supplementation.Crossref | GoogleScholarGoogle Scholar |
Choct M (2006) Enzymes for the feed industry: past, present and future. World’s Poultry Science Journal 62, 5–16.
| Enzymes for the feed industry: past, present and future.Crossref | GoogleScholarGoogle Scholar |
Fuente JM, Perez de Ayala P, Flores A, Villamide MJ (1998) Effect of storage time and dietary enzyme on the metabolizable energy and digesta viscosity of barley-based diets for poultry. Poultry Science 77, 90–97.
| Effect of storage time and dietary enzyme on the metabolizable energy and digesta viscosity of barley-based diets for poultry.Crossref | GoogleScholarGoogle Scholar | 9469757PubMed |
Gohl B, Aldén S, Elwinger K, Thomke S (1978) Influence of β-glucanase on feeding value of barley for poultry and moisture content of excreta. British Poultry Science 19, 41–47.
| Influence of β-glucanase on feeding value of barley for poultry and moisture content of excreta.Crossref | GoogleScholarGoogle Scholar |
González-Ortiz G, Kozłowski K, Drażbo A, Bedford MR (2017) Response of turkeys fed wheat–barley–rye based diets to xylanase supplementation. Animal Feed Science and Technology 229, 117–123.
| Response of turkeys fed wheat–barley–rye based diets to xylanase supplementation.Crossref | GoogleScholarGoogle Scholar |
Gorissen SHM, Crombag JJR, Senden JMG, Waterval WAH, Bierau J, Verdijk LB, van Loon LJC (2018) Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50, 1685–1695.
| Protein content and amino acid composition of commercially available plant-based protein isolates.Crossref | GoogleScholarGoogle Scholar | 30167963PubMed |
Grain Research and Development Corporation (GRDC) (2018) Barley.. Available at https://grdc.com.au/__data/assets/pdf_file/0030/369228/GrowNote-Barley-South-0-Introduction.pdf [Accessed 22 February 2022]
Hesselman K, Åman P (1986) The effect of β-glucanase on the utilization of starch and nitrogen by broiler chickens fed on barley of low- or high-viscosity. Animal Feed Science and Technology 15, 83–93.
| The effect of β-glucanase on the utilization of starch and nitrogen by broiler chickens fed on barley of low- or high-viscosity.Crossref | GoogleScholarGoogle Scholar |
Izydorczyk MS, Storsley J, Labossiere D, MacGregor AW, Rossnagel BG (2000) Variation in total and soluble β-glucan content in hulless barley: effects of thermal, physical, and enzymic treatments. Journal of Agricultural and Food Chemistry 48, 982–989.
| Variation in total and soluble β-glucan content in hulless barley: effects of thermal, physical, and enzymic treatments.Crossref | GoogleScholarGoogle Scholar | 10775338PubMed |
Jacob JP, Pescatore AJ (2012) Using barley in poultry diets: a review. Journal of Applied Poultry Research 21, 915–940.
| Using barley in poultry diets: a review.Crossref | GoogleScholarGoogle Scholar |
Jacob JP, Pescatore AJ (2014) Barley β-glucan in poultry diets. Annals of Translational Medicine 2, 20
| Barley β-glucan in poultry diets.Crossref | GoogleScholarGoogle Scholar | 25332996PubMed |
Józefiak D, Rutkowski A, Jensen BB, Engberg RM (2006) The effect of β-glucanase supplementation of barley- and oat-based diets on growth performance and fermentation in broiler chicken gastrointestinal tract. British Poultry Science 47, 57–64.
| The effect of β-glucanase supplementation of barley- and oat-based diets on growth performance and fermentation in broiler chicken gastrointestinal tract.Crossref | GoogleScholarGoogle Scholar | 16546798PubMed |
Karunaratne ND, Classen HL, Ames NP, Bedford MR, Newkirk RW (2021) Effects of hulless barley and exogenous beta-glucanase levels on ileal digesta soluble beta-glucan molecular weight, digestive tract characteristics, and performance of broiler chickens. Poultry Science 100, 100967
| Effects of hulless barley and exogenous beta-glucanase levels on ileal digesta soluble beta-glucan molecular weight, digestive tract characteristics, and performance of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 33652524PubMed |
Knudsen KEB (2014) Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poultry Science 93, 2380–2393.
| Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets.Crossref | GoogleScholarGoogle Scholar |
Kuttappan VA, Lee YS, Erf GF, Meullenet J-FC, McKee SR, Owens CM (2012) Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poultry Science 91, 1240–1247.
| Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping.Crossref | GoogleScholarGoogle Scholar | 22499884PubMed |
Kuttappan VA, Hargis BM, Owens CM (2016) White striping and woody breast myopathies in the modern poultry industry: a review. Poultry Science 95, 2724–2733.
| White striping and woody breast myopathies in the modern poultry industry: a review.Crossref | GoogleScholarGoogle Scholar | 27450434PubMed |
Liu SY, Selle PH (2017) Starch and protein digestive dynamics in low-protein diets supplemented with crystalline amino acids. Animal Production Science 57, 2250–2256.
| Starch and protein digestive dynamics in low-protein diets supplemented with crystalline amino acids.Crossref | GoogleScholarGoogle Scholar |
Mahasukhonthachat K, Sopade PA, Gidley MJ (2010) Kinetics of starch digestion and functional properties of twin-screw extruded sorghum. Journal of Cereal Science 51, 392–401.
| Kinetics of starch digestion and functional properties of twin-screw extruded sorghum.Crossref | GoogleScholarGoogle Scholar |
Marquardt RR, Boros D, Guenter W, Crow G (1994) The nutritive value of barley, rye, wheat and corn for young chicks as affected by use of a Trichoderma reesei enzyme preparation. Animal Feed Science and Technology 45, 363–378.
| The nutritive value of barley, rye, wheat and corn for young chicks as affected by use of a Trichoderma reesei enzyme preparation.Crossref | GoogleScholarGoogle Scholar |
McKinney LJ, Teeter RG (2004) Predicting effective caloric value of nonnutritive factors: I. Pellet quality and II. Prediction of consequential formulation dead zones. Poultry Science 83, 1165–1174.
| Predicting effective caloric value of nonnutritive factors: I. Pellet quality and II. Prediction of consequential formulation dead zones.Crossref | GoogleScholarGoogle Scholar | 15285508PubMed |
McNab JM, Shannon DWF (1974) The nutritive value of barley, maize, oats and wheat for poultry. British Poultry Science 15, 561–567.
| The nutritive value of barley, maize, oats and wheat for poultry.Crossref | GoogleScholarGoogle Scholar |
McNab JM, Smithard RR (1992) Barley β-glucan: an antinutritional factor in poultry feeding. Nutrition Research Reviews 5, 45–60.
| Barley β-glucan: an antinutritional factor in poultry feeding.Crossref | GoogleScholarGoogle Scholar | 19094312PubMed |
Meinerz C, Ribeiro AML, Penz AM, Kessler AdM (2001) Energy level and pelleting on performance and carcass yield of pair-fed broilers. Revista Brasileira de Zootecnia 30, 2026–2032.
| Energy level and pelleting on performance and carcass yield of pair-fed broilers.Crossref | GoogleScholarGoogle Scholar |
Muramatsu K, Massuquetto A, Dahlke F, Maiorka A (2015) Factors that affect pellet quality: a review. Journal of Agricultural Science and Technology 9, 717–722.
| Factors that affect pellet quality: a review.Crossref | GoogleScholarGoogle Scholar |
Musigwa S, Morgan N, Swick RA, Cozannet P, Kheravii SK, Wu S-B (2021) Multi-carbohydrase enzymes improve feed energy in broiler diets containing standard or low crude protein. Animal Nutrition 7, 496–505.
| Multi-carbohydrase enzymes improve feed energy in broiler diets containing standard or low crude protein.Crossref | GoogleScholarGoogle Scholar | 34258438PubMed |
Perera WNU, Abdollahi MR, Ravindran V, Zaefarian F, Wester TJ, Ravindran G (2019a) Nutritional evaluation of two barley cultivars, without and with carbohydrase supplementation, for broilers: metabolisable energy and standardised amino acid digestibility. British Poultry Science 60, 404–413.
| Nutritional evaluation of two barley cultivars, without and with carbohydrase supplementation, for broilers: metabolisable energy and standardised amino acid digestibility.Crossref | GoogleScholarGoogle Scholar | 30995865PubMed |
Perera WNU, Abdollahi MR, Zaefarian F, Wester TJ, Ravindran G, Ravindran V (2019b) Influence of inclusion level of barley in wheat-based diets and supplementation of carbohydrase on growth performance, nutrient utilisation and gut morphometry in broiler starters. British Poultry Science 60, 736–748.
| Influence of inclusion level of barley in wheat-based diets and supplementation of carbohydrase on growth performance, nutrient utilisation and gut morphometry in broiler starters.Crossref | GoogleScholarGoogle Scholar | 31267769PubMed |
Perera WNU, Abdollahi MR, Zaefarian F, Wester TJ, Ravindran V (2021) High steam conditioning temperatureduring the pelleting process impairs growth performance and nutrientutilization in broiler starters fed barley-based diets, regardless ofcarbohydrase supplementation. Poultry Science 100, 101166
Philip JS, Gilbert HJ, Smithard RR (1995) Growth, viscosity and beta-glucanase activity of intestinal fluid in broiler chickens fed on barley-based diets with or without exogenous beta-glucanase. British Poultry Science 36, 599–603.
| Growth, viscosity and beta-glucanase activity of intestinal fluid in broiler chickens fed on barley-based diets with or without exogenous beta-glucanase.Crossref | GoogleScholarGoogle Scholar | 8590092PubMed |
Salih ME, Classen HL, Campbell GL (1991) Response of chickens fed on hull-less barley to dietary β-glucanase at different ages. Animal Feed Science and Technology 33, 139–149.
| Response of chickens fed on hull-less barley to dietary β-glucanase at different ages.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Liu SY (2019) The relevance of starch and protein digestive dynamics in poultry. Journal of Applied Poultry Research 28, 531–545.
| The relevance of starch and protein digestive dynamics in poultry.Crossref | GoogleScholarGoogle Scholar |
Tijare VV, Yang FL, Kuttappan VA, Alvarado CZ, Coon CN, Owens CM (2016) Meat quality of broiler breast fillets with white striping and woody breast musclemyopathies. Poultry Science 95, 2167–2173.
White WB, Bird HR, Sunde ML, Marlett JA, Prentice NA, Burger WC (1983) Viscosity of β-d-glucan as a factor in the enzymatic improvement of barley for chicks. Poultry Science 62, 853–862.
| Viscosity of β-d-glucan as a factor in the enzymatic improvement of barley for chicks.Crossref | GoogleScholarGoogle Scholar |
Wu S-B, Swick RA, Noblet J, Rodgers N, Cadogan D, Choct M (2019) Net energy prediction and energy efficiency of feed for broiler chickens. Poultry Science 98, 1222–1234.
| Net energy prediction and energy efficiency of feed for broiler chickens.Crossref | GoogleScholarGoogle Scholar | 30265337PubMed |