The tortoise and the hare? Post-fire regeneration in mixed Eucalyptus–Callitris forest
Ian D. Lunt A D , Heidi C. Zimmer B C and David C. Cheal BA Institute for Land, Water and Society, Charles Sturt University, PO Box 789, Albury, NSW 2640, Australia.
B Arthur Rylah Institute, Department of Sustainability and Environment, 123 Brown Street, Heidelberg, Vic. 3081, Australia.
C Present address: School of Biological Sciences, Faculty of Science, Monash University, Clayton, Vic. 3800, Australia.
D Corresponding author. Email: ilunt@csu.edu.au
Australian Journal of Botany 59(6) 575-581 https://doi.org/10.1071/BT11151
Submitted: 14 June 2011 Accepted: 11 August 2011 Published: 5 October 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Seedling regeneration after a high intensity wildfire was assessed in a mixed forest dominated by Eucalyptus species and Callitris endlicheri (Parl.) F.M. Bailey. Patterns were compared against the ‘slow seedling’ or ‘tortoise-and-hare’ theory of competitive interactions between gymnosperms and angiosperms. Browsing effects were documented using fenced plots, and seedling density, mortality and height were assessed over 6 years, from 2004–10. Consistent with expectations, Eucalyptus seedlings grew faster than Callitris seedlings in most situations. Callitris seedlings grew faster and produced seed cones sooner in plots with fewer Eucalyptus seedlings compared with plots with dense Eucalyptus seedlings. The local growth rates of Callitris seedlings were not associated with long-term site suitability for Callitris, as many plots with diminutive Callitris seedlings and dense Eucalyptus seedlings were dominated by Callitris trees before the 2003 fire. Contrary to expectations, few Callitris seedlings died during the 6-year period, so competition during the regeneration phase did not regulate co-existence. Strong drought tolerance and the ability to persist in dense, unthinned stands may enable Callitris to persist beneath dense Eucalyptus regeneration. Nevertheless, Callitris seedlings growing with dense Eucalyptus seedlings have a longer primary juvenile period than seedlings in sites with fewer seedling or adult eucalypts, which places these stands at greater risk of mortality in future fires and greater risk of browsing in the meantime.
Introduction
A principal goal of vegetation ecology is to understand the factors that influence vegetation patterns and dynamics. In 1989, William Bond developed a general model to interpret competitive interactions among two groups of seed plants: the gymnosperms and angiosperms. Bond’s (1989) ‘slow seedling’ or ‘tortoise-and-hare’ hypothesis stated that, because gymnosperm seedlings had lower growth rates than angiosperm seedlings due to architectural constraints, gymnosperms were largely restricted to low productivity ecosystems where they escaped competition from vigorous, fast-growing angiosperms. By contrast, in productive ecosystems, competition from angiosperms would suppress gymnosperm growth rates, leaving gymnosperm seedlings vulnerable to processes that cause size-dependent mortality, such as drought, herbivory and fire. In some instances, gymnosperm decline would be hastened if angiosperms promoted adverse disturbances, for example, where grasses fuelled fires that killed gymnosperm seedlings (Bond and Scott 2010). Cast in the language of Grime’s (2001) plant strategy scheme, gymnosperms were viewed by Bond (1989) as ‘stress tolerators’, poorly equipped to regenerate and persist in productive environments dominated by more ‘competitive’ angiosperms.
Our understanding of the physiological mechanisms underlying low growth rates of gymnosperm seedlings has since been refined (Becker et al. 1999; Becker 2000; Lusk et al. 2003; Brodribb et al. 2005), and Bond’s ‘slow seedling’ model may be viewed within a broader plant leaf economics spectrum, in which relative growth rate is related to leaf longevity and specific leaf area (Cornelissen et al. 1996; Reich et al. 1999; Wright et al. 2004). Gymnosperms characteristically have long-lived leaves of low specific leaf area, which lead to lower relative growth rate than many, but not all, angiosperms (Reich et al. 1999; Lusk et al. 2003).
The most widespread and abundant genus of gymnosperms in Australia is Callitris (Family Cupressaceae), which occurs in tropical, subtropical, arid, semiarid, Mediterranean and temperate regions (Bowman and Harris 1995). Callitris dynamics have received considerable attention from fire ecologists as Callitris are often killed by fire and usually do not resprout after being subjected to 100% leaf scorch (Lacey 1973; Bradstock and Cohn 2002; Russell-Smith 2006). Consequently, population dynamics are highly sensitive to changes in fire regimes (Bowman and Panton 1993; Bradstock et al. 2006; Prior et al. 2010). Surprisingly, Bond’s (1989) model has never been referred to in studies on Callitris ecology. This may be because few studies have documented interactions between Callitris and other woody species (e.g. Clayton-Greene 1981; Bowman et al. 1988; Clayton-Greene and Ashton 1990), even though Callitris occur in heathlands, woodlands and forests dominated by Eucalyptus and other woody plants (Bowman and Harris 1995). In savannah and arid woodlands, fire regimes and Callitris dynamics are regulated by dominant grasses rather than woody plants (Bowman et al. 1988; Bradstock and Cohn 2002). Indeed, we know of only one study (an unpublished seedling pot trial) that has documented competitive interactions between Callitris seedlings and other woody taxa (Clayton-Greene 1981).
This paucity of information raises the question, to what extent do interactions with woody angiosperms influence Callitris dynamics in productive forest ecosystems? Under Bond’s (1989) model, inter-specific interactions could influence the ability of non-resprouting Callitris to persist in ecosystems dominated by flammable, resprouting angiosperms. In this study, we attempt to address this issue by asking: to what extent are post-fire recruitment patterns in a mixed Callitris–Eucalyptus forest in a relatively high rainfall region (~850-mm mean annual rainfall) consistent with Bond’s slow seedling hypothesis? We discuss the implications of these patterns for species co-existence and fire management. Our study is observational rather than manipulative, which means that while we can assess whether patterns are consistent with Bond’s hypothesis, we cannot be definitive about the mechanisms that underlie observed patterns.
Materials and methods
Study area
The study was conducted in Chiltern-Mt Pilot National Park in north-eastern Victoria, Australia. Mean annual rainfall in the study area is ~850 mm, based on data from nearby Beechworth (960 mm) and Beechworth Woolshed rainfall gauges (770 mm; Bureau of Meteorology 2011). The underlying geology is Devonian granite and the topography is moderately to steeply undulating (Parks Victoria 2008). The area supports dry sclerophyll forests dominated by Eucalyptus macrorhyncha F.Muell. ex Benth., E. goniocalyx F.Muell., E. polyanthemos Schauer and E. blakelyi Maiden, with patches dominated by Callitris endlicheri. Most Callitris-dominated stands are small (<1 ha) and in close proximity to rocky outcrops (Watson 2004).
In January 2003, 7300 ha of the reserve burnt in a high intensity wildfire (Watson 2004). In July 2003, two, 10 × 10-m fenced plots were erected in each of three burnt Callitris stands near Mt Pilot to assess browsing impacts on Callitris seedlings. Post-fire vegetation monitoring began in autumn 2004. Twelve stands of Callitris were selected within a 2.2-km radius of the Mt Pilot summit (36°15′06′S, 146°40′13′E). All 12 sites were burnt at high intensity, as evidenced by complete mortality of pre-fire Callitris and resprouting of surviving Eucalyptus from basal coppice rather than from epicormic buds on trunks or branches. Six stands, including the three stands containing fenced plots, were dominated by mature C. endlicheri, presumed to have recruited in the late 1800s, with subdominant Eucalyptus. The other six stands were dominated by Eucalyptus above smaller Callitris, which were presumed to have recruited after 1950.
Permanently marked 100-m2 (10 × 10 m) plots were established at each site. Paired unfenced plots were established at the three fenced sites, giving four plots in total at these sites (plus an extra unfenced plot in one large stand), and two unfenced plots were established in the nine stands without fences. Pre-fire stand structure was estimated in autumn 2004 by measuring all dead and resprouting trees in each 100-m2 plot. Small saplings that were totally incinerated would not have been detected.
The height, number of stems, and girth over bark at breast height (GOBBH) of the largest stem was recorded annually for all coppicing Eucalyptus in each 100-m2 plot. For clarity, all post-fire seedling recruits are called ‘seedlings’ in this paper, even though many had grown tall (i.e. to ‘sapling’ size) by the end of the monitoring period. The species identity, height and GOBBH of all post-fire seedlings were assessed annually in 10 (in 2004) and 20 (2005–10) randomly placed 1-m2 subplots within each 100-m2 plot. These subplots were re-randomised each year. GOBBH was recorded as 0.1 cm if plants were <1.3 m tall. Sampling was conducted in autumn 2004–10, but only two sites were sampled in 2009, due to inclement weather. At Beechworth, annual rainfall was slightly above average from 2003 to 2005 (965–1215 mm/year), very low in 2006 (413 mm) and ~80% of average from 2007 to 2009 (755–790 mm; Bureau of Meteorology 2011).
Data analysis
Linear mixed models were used to examine:
-
The relationship between initial seedling density (including live and dead seedlings in 2004) and the pre-fire basal area of each genus;
-
The effects of fencing and time on the density and height of live seedlings of each genus; and
-
Associations between seedling height in 2010 and (a) the pre-fire basal area of each genus, (b) the density of competing seedlings in 2010, and (c) the basal area of resprouting Eucalyptus in 2010.
The influence of fencing on seedling heights and densities was assessed using data from the three sites with fenced and unfenced plots only. In each case, year, fencing treatment, basal area (at the plot scale), and seedling density in subplots were included as fixed effects, and nested sites, plots and subplots were incorporated as random effects. Where necessary, density, height and basal area data were log-transformed to meet the assumption of constant variance. F-tests were used to investigate the significance of individual model term. Analyses were undertaken in Genstat version 13 (VSN International 2010). In addition t-tests were used to compare: (1) the height of Eucalyptus and Callitris seedlings in 2010, (2) the proportion of seedlings in fenced and unfenced plots with evidence of browsing in 2010, and (3) the mean height of seedlings in fenced and unfenced plots in 2010. Few Callitris seedlings produced seed cones by 2010. Consequently, associations between the density of Callitris and Eucalyptus seedlings versus the number of Callitris seedlings bearing seed cones and the number of cones produced were analysed at the plot scale, using Spearman’s rank correlation coefficients.
Results
Seedling density and mortality
Callitris and Eucalyptus seedlings both regenerated at high density after the 2003 fire. On average, there were 5.8 Callitris seedlings/m2 and 2.8 Eucalyptus seedlings/m2 in 2004, 16 months after the fire. The initial, post-fire density of Callitris seedlings was significantly and positively associated with the pre-fire basal area of Callitris (P = 0.031). After accounting for the association of Callitris seedling density with Callitris pre-fire basal area, the association with Eucalyptus pre-fire basal area was not significant. By contrast, the initial density of Eucalyptus seedlings was not significantly associated with the pre-fire basal area of either genus (P = 0.333 and 0.717 for Eucalyptus and Callitris, respectively).
Mortality rates after the first monitoring event were extremely low for both genera. The mean density of live Callitris seedlings apparently declined from 5.2 to 3.7 seedlings/m2 from 2004 to 2010; however, this change was not statistically significant (at P < 0.05). The mean density of Callitris seedlings did not change significantly over time (P = 0.783) nor did it differ significantly across fencing treatments, when all plots (including all unfenced plots) were compared (P = 0.348). Similarly, when data from only the three sites with fenced plots were analysed, the density of Callitris seedlings was not significantly associated with year (P = 0.723), fencing (P = 0.392) nor the year*fencing interaction (P = 0.945; Fig. 1a).
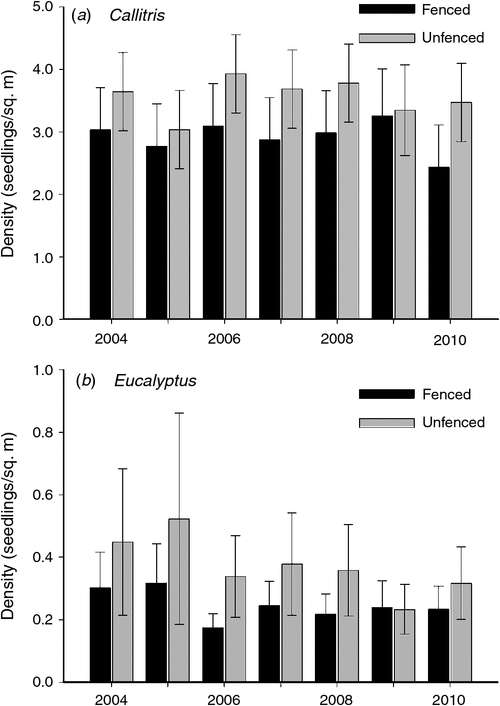
|
By contrast, there was a significant decline in the density of live Eucalyptus seedlings over the same period. Across all sites, mean (back-transformed) density declined from 2.8 to 1.2 seedlings/m2. When data from all plots were analysed, the density of Eucalyptus seedlings differed significantly among years (P < 0.001) but not among fencing treatments (P = 0.122) or with the year*fencing interaction (0.348). When data from only the three sites with fenced plots were analysed, the density of Eucalyptus seedlings was not significantly affected by year (P = 0.224), fencing (P = 0.428) nor year*fencing interaction (P = 0.947; Fig. 1b).
Seedling growth rates
Callitris seedlings were, on average, shorter than Eucalyptus seedlings throughout the survey period (Fig. 2). In 2010, the mean height of Eucalyptus seedlings was over twice that of Callitris seedlings (184 cf. 84 cm; t-test, P < 0.001), and the tallest Eucalyptus seedling was over twice as tall as the tallest Callitris seedling (max. height, 800 cf. 350 cm). In 2010, 30% of Callitris seedlings were <0.5 m tall.
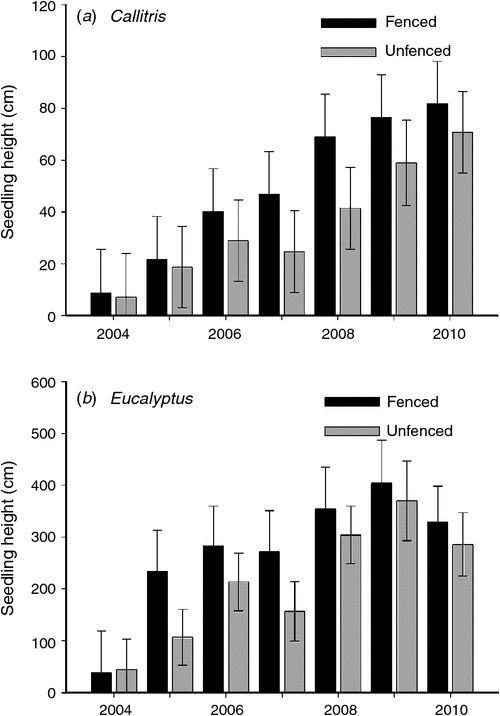
|
Fencing effects
In 2010, it appeared that more Callitris seedlings showed evidence of browsing in unfenced than fenced plots (25% cf. 2% at the plot scale) although this difference was not significant at the P = 0.05 level (paired t-test, P = 0.09). Callitris seedlings grew taller in fenced than unfenced plots (Fig. 2a). The mean (back-transformed) height of Callitris seedlings varied significantly with year (P < 0.001) and fencing treatment (P = 0.007) with no significant year*fencing interaction (P = 0.198). In 2010, the mean height of Callitris seedlings in fenced plots was 145 cm compared with 116 cm in unfenced plots (t-test, P < 0.001). By contrast, only 5% of all Eucalyptus seedlings surveyed in 2010 (in all fenced and unfenced plots) displayed evidence of browsing. No Eucalyptus seedlings were browsed within the three fenced plots in 2010. Consequently, the height of Eucalyptus seedlings varied significantly with year (P = 0.002) but there was no significant fencing effect (P = 0.747) or year*fencing treatment interaction (P = 0.558; Fig. 2b). In 2010, the mean height of fenced and unfenced Eucalyptus seedlings was 226 and 245 cm, respectively (t-test, P = 0.70).
Factors associated with seedling height
The linear mixed model showed that the height of Callitris seedlings in 2010 was significantly and positively associated with fencing (P = 0.025), and negatively associated with the density of Eucalyptus seedlings (P = 0.006). After accounting for these terms, there was no significant association with (a) the density of Callitris seedlings, (b) the basal area of coppicing Eucalyptus, nor (c) the pre-fire basal area of Callitris or Eucalyptus (Table 1). The lack of association between Callitris seedling heights and the pre-fire basal area of either genus suggests that Callitris seedlings neither grew better nor worse in plots dominated by either genus before the fire.
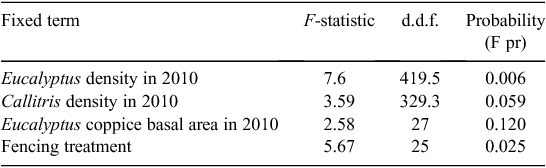
|
In contrast, the height of Eucalyptus seedlings in 2010 was significantly negatively associated with Eucalyptus seedling density (P < 0.001) and positively associated with Callitris seedling density (P = 0.002) and the pre-fire basal area of Callitris (P = 0.053; Table 2), which indicates that Eucalyptus seedlings grew faster where Callitris dominated before the fire.
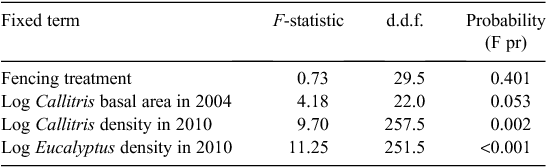
|
Seed cone production
In 2010, 7 years after the fire, 2% of Callitris seedlings possessed seed cones. Seed cones were almost entirely restricted to the tallest seedlings. Thus, 83% of cones were on seedlings taller than 150 cm (Fig. 3), and 23% of seedlings >2 m possessed seed cones (n = 100). The shortest seedling with seed cones was 140 cm tall. Cone production varied from <5% to almost 30% of Callitris seedlings at each plot.
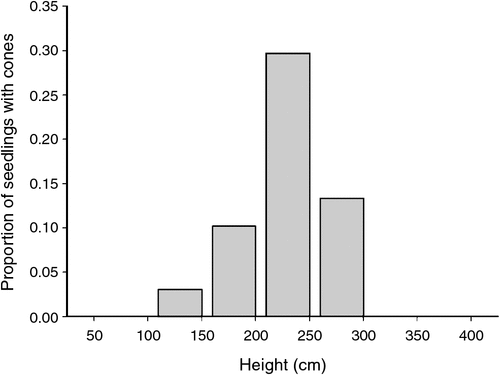
|
In 2010, there was no significant correlation between the number of Callitris seedlings in each plot and either the number of Callitris seedlings bearing seed cones (Spearman’s rank correlation coefficient, rho = –0.030, d.f. = 29, P = 0.874) or the number of seed cones produced (rho = –0.039, P = 0.833). However, there was a significant negative correlation between the number of Eucalyptus seedlings in each plot and the number of Callitris seedlings bearing seed cones (rho = –0.454, P = 0.010) and the number of seed cones produced (rho = –0.448, P = 0.012). The total number of Eucalyptus and Callitris seedlings in each plot was positively correlated (rho = 0.380, P = 0.0352). Thus, Callitris seedlings produced more seed cones in plots with fewer Eucalyptus seedlings.
Discussion
These results illustrate several patterns that are consistent with Bond’s (1989) slow seedling hypothesis: (1) Eucalyptus seedlings grew faster than Callitris seedlings; (2) Callitris seedlings grew faster, and produced seed cones sooner, in plots with fewer Eucalyptus seedlings; and (3) small Callitris seedlings growing beneath dense Eucalyptus seedlings remained vulnerable to browsing and burning for longer than tall Callitris seedlings in areas with fewer Eucalyptus seedlings. Spatial variations in the growth rate of Callitris seedlings were not related to the suitability of sites to support Callitris, as many patches with small Callitris and dense Eucalyptus seedlings were dominated by mature Callitris before the 2003 fire. However, contrary to Bond’s (1989) theory, few Callitris seedlings died, so competition during the regeneration phase did not regulate co-existence. We expand on each of these points in the discussion below.
Seedling growth rates
The lower growth rate of Callitris compared with Eucalyptus seedlings is consistent with the global trend for lower growth rates in gymnosperms than in sympatric angiosperms (Bond 1989; Reich et al. 1999; Lusk et al. 2003), and with pot and field trials in which planted Eucalyptus seedlings grew faster than Callitris seedlings (Clayton-Greene 1981; Allcock and Hik 2004). Apart from fencing, the factor most strongly associated with the growth rate of Callitris seedlings was the density of Eucalyptus seedlings. Callitris seedlings grew slowest where Eucalyptus seedlings were most abundant, and fastest where Eucalyptus seedlings were most sparse. Removal experiments are required to disentangle the effects of abiotic site factors and competition on Callitris growth rates. Nevertheless, the negative association suggests that competition from dense, tall Eucalyptus seedlings may have slowed the growth of smaller Callitris seedlings. The lack of a significant association between the growth rate of Callitris seedlings and the pre-fire basal area of Callitris indicates that sites with dense Eucalyptus seedlings and small Callitris seedlings were not unsuitable for Callitris growth and persistence; Callitris dominated some of these sites before the fire. In contrast to savanna ecosystems, grasses had very low cover and biomass at Mt Pilot, and grass competition is unlikely to have had a substantial impact on growth rates or survival of Callitris or Eucalyptus seedlings, except perhaps in the first year or two after fire.
Silvicultural trials have demonstrated that the growth rate of retained Callitris is enhanced when dense Callitris stands are thinned (Knott 1995; Ross et al. 2008). However, few studies have documented competitive interactions between Callitris and Eucalyptus. Clayton-Greene (1981) reported that Eucalyptus melliodora seedlings suppressed the growth of Callitris glaucophylla seedlings in a pot competition experiment. In a small thinning trial, Bowman et al. (1988) found that Callitris grew fastest in treatments with the lowest representation of co-occurring Eucalyptus and Callitris, but unfortunately their results do not clearly differentiate the competitive effects that were exerted by the two genera.
Browsing damage
Results from the fencing trial indicate that slow-growing Callitris seedlings were vulnerable to browsing, and that browsing magnified the height difference between the two genera. However, this set-back was temporary rather than persistent. As time progressed, the height difference between fenced and unfenced Callitris seedlings diminished, perhaps because taller, older plants were less accessible to browsing animals. Nevertheless, a high proportion of Callitris seedlings at Mt Pilot remain vulnerable to future damage by browsing animals and other disturbances.
The low level of mortality in grazed plots is likely to reflect low herbivore densities, as few large herbivores were observed in the first few years after the 2003 wildfire (I. Lunt, pers. obs.). In contrast, Mackenzie and Keith (2009) recorded high mortality of C. endlicheri seedlings in an area grazed by feral Cervus timorensis (Rusa Deer) in coastal New South Wales. Over 12 months, 98% of unprotected seedlings were browsed at least once, leading to a 59% decline in seedling density (Mackenzie and Keith 2009). Seedlings of the related species, C. glaucophylla, are known to be sensitive to repeated browsing by rabbits and sheep (Lacey 1972).
Seedling mortality
In contrast with Bond’s (1989) theory, Callitris populations did not decline markedly during the establishment period due to direct competition with angiosperms nor any other cause. We recorded no significant decline in the density of Callitris seedlings over 6 years, despite a high sampling intensity. By contrast, the density of Eucalyptus seedlings declined by 57% during the 6-year period, consistent with many studies that have documented self-thinning in Eucalyptus stands after fire (Florence 1996).
The stability in Callitris numbers was surprising, as small seedlings are usually highly susceptible to mortality arising from insufficient resources and disturbances, and the survey period included years with marked rainfall deficiencies. By contrast, Mackenzie and Keith (2009) found that the density of protected (ungrazed) C. endlicheri seedlings declined by 19% within a 12-month period, from 2 to 3 years after fire. Initial seedling densities may have been underestimated in our study, as sampling began in April 2004, 15 months after the fire. Nevertheless, greater mortality was expected over the following 6 years, especially given that Callitris seedlings were so much smaller than associated Eucalyptus seedlings. Post-fire rainfall at the nearby Beechworth weather station was slightly higher than the long-term average for the first 3 years after burning (2003–05), which would have assisted initial seedling establishment. However, rainfall in subsequent years was relatively low, with just 43% of mean annual rainfall in 2006 (413 mm) and ~80% of average from 2007 to 2009 (755–790 mm; Bureau of Meteorology 2011).
Callitris species are extremely drought tolerant (Attiwill and Clayton-Greene 1984; Zeppel and Eamus 2008; Brodribb et al. 2010), and saplings self-thin extremely slowly, forming dense ‘locked stands’ containing suppressed, slow-growing trees (Lacey 1973; Thompson and Eldridge 2005; Ross et al. 2008). Lacey (1973) reported that ‘in excess of … 125 000 [trees]/ha are commonly encountered over widespread areas’ in 20-year-old stands of C. glaucophylla. At the 100-m2 plot scale, the maximum density of C. endlicheri seedlings at Mt Pilot in 2010 was 13 050 seedlings/ha. In unburnt areas at Mt Pilot, stands suspected to be over 100 years old contain up to 50 live Callitris/100 m2 (5000 Callitris/ha) and exceed 60 m2/ha basal area (I. Lunt, unpubl. data). Thus, C. endlicheri can persist in dense stands for many decades, similar to C. glaucophylla. This ability to tolerate low resource levels, especially low levels of soil moisture (Brodribb et al. 2010), for lengthy periods, may allow C. endlicheri to persist in mixed forests containing dense Eucalyptus regrowth, provided that plants are not killed by fire or other disturbances.
Seed cone production
Callitris species are usually killed when subjected to 100% leaf scorch and do not resprout after fire or form a soil seed bank (Hawkins 1966; Stocker 1966; Bowman et al. 1988; Cohn et al. 2011). Consequently, populations are prone to extinction if high intensity fires occur before regenerating plants set seed (Keith 1996). Callitris populations at Mt Pilot will remain susceptible to fires for many years as only 2% of plants had formed seed cones within 7 years of the 2003 fire. A fire-free period of at least 15 years is considered necessary to maintain populations of Callitris species elsewhere (Price and Bowman 1994; Russell-Smith et al. 1998; Bradstock and Cohn 2002).
Our results show that the length of the primary juvenile period (or duration of ‘immaturity risk’) is size dependent, as fast-growing, tall seedlings produced seed cones earlier than smaller, slow-growing seedlings. Moreover, rates of plant growth and seed production were both negatively associated with the density of Eucalyptus seedlings. Callitris seedlings grew faster and produced seed cones earlier in plots with fewer Eucalyptus seedlings, regardless of the density of Callitris seedlings.
Thinning trails have shown that competition constrains plant growth and levels of seed production in Callitris (Lacey 1972; Knott 1995; Thompson and Eldridge 2005). However, little information is available on the effects of stocking levels on the primary juvenile period, other than the general observation that, ‘suppressed trees exhibit little flowering and therefore have poor seed production’ (Lacey 1972), and similar observations (Prober and Thiele 2004). However, competition has been shown to extend the primary juvenile period in other conifers, including Pinus species (Cremer 1992; Verkaik and Espelta 2006). Thus, while removal experiments are required before spatial variations in primary juvenile periods can unequivocally be attributed to competition from Eucalyptus seedlings, this interpretation is consistent with prior information (Lacey 1972, 1973; Clayton-Greene 1981; FCNSW 1988), and it appears highly likely that competition contributes at least partly to the observed pattern.
These patterns have implications for future fire management. Under all but extreme conditions, fires are more likely to kill small than large Callitris (Bowman et al. 1988; Prober and Thiele 2004; Cohn et al. 2011; Zimmer et al. 2011). Consequently, if managers aim to maintain Callitris populations, then fires of moderate to high intensity should be excluded from areas containing young Callitris and moderate to dense Eucalyptus regeneration for longer than areas containing young Callitris amid sparse Eucalyptus regeneration. High basal area of Callitris reduces fire intensity in mixed Eucalyptus–Callitris stands (Bowman and Wilson 1988; Cohn et al. 2011). Consequently, over the longer term, protection of Callitris populations may reduce forest flammability and potential fire intensity.
Conclusion
These results indicate that Bond’s (1989) model provides a useful framework for interpreting Callitris dynamics in mixed forests co-dominated by Eucalyptus. Australian ecologists have commonly interpreted Callitris dynamics in fire-prone ecosystems in relation to immaturity risk, or the risk of repeated fires occurring before regenerating plants set adequate seed (Bowman et al. 1988; Russell-Smith et al. 1998; Bradstock and Cohn 2002; Thompson and Eldridge 2005). This attribute is clearly important at Mt Pilot, as elsewhere. However, Bond’s (1989) model highlights that immaturity risk may be strongly influenced by competition from co-occurring species. By inhibiting plant growth and lengthening the primary juvenile period, competitors may extend the period of immaturity risk, and potentially reduce the habitability of productive environments for Callitris and other non-resprouters. The mechanism of this extended immaturity risk may include an extended opportunity for browsing to eliminate Callitris seedlings and for fires to burn (and hence kill) plants before adequate seed is set. Regardless of the physiological or anatomical mechanisms that control growth rates, the spatial pattern of slower Callitris growth in areas with dense Eucalyptus regeneration has implications for future fire management. To maintain Callitris populations, fire-free intervals should be longer in areas stocked with Callitris, particularly areas in which Callitris is a minor component of the flora and where dense Eucalyptus regeneration dominates the regrowing vegetation.
Acknowledgements
We extend our thanks to Brian Pritchard of Parks Victoria for field support; students in the subject, Vegetation and Disturbance Ecology, at Charles Sturt University, who collected the data on which this paper is based; and Graham Hepworth for his excellent statistical support. The drafting of this paper was assisted by funding from the Victorian Bushfire Reconstruction and Recovery Authority and supported by the Victorian Government Department of Sustainability and Environment.
References
Allcock KG, Hik DS (2004) Survival, growth, and escape from herbivory are determined by habitat and herbivore species for three Australian woodland plants. Oecologia 138, 231–241.| Survival, growth, and escape from herbivory are determined by habitat and herbivore species for three Australian woodland plants.Crossref | GoogleScholarGoogle Scholar |
Attiwill PM, Clayton-Greene KA (1984) Studies of gas exchange and development in a subhumid woodland. Journal of Ecology 72, 285–294.
| Studies of gas exchange and development in a subhumid woodland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXhvVyqtb8%3D&md5=10a08dd2c182f765e387aae4eb546537CAS |
Becker P (2000) Competition in the regeneration niche between conifers and angiosperms: Bond’s slow seedling hypothesis. Functional Ecology 14, 401–412.
| Competition in the regeneration niche between conifers and angiosperms: Bond’s slow seedling hypothesis.Crossref | GoogleScholarGoogle Scholar |
Becker P, Tyree MT, Tsuda M (1999) Hydraulic conductances of angiosperms versus conifers: similar transport sufficiency at the whole-plant level. Tree Physiology 19, 445–452.
Bond WJ (1989) The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence. Biological Journal of the Linnean Society. Linnean Society of London 36, 227–249.
| The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence.Crossref | GoogleScholarGoogle Scholar |
Bond WJ, Scott AC (2010) Fire and the spread of flowering plants in the Cretaceous. New Phytologist 188, 1137–1150.
| Fire and the spread of flowering plants in the Cretaceous.Crossref | GoogleScholarGoogle Scholar |
Bowman DMJS, Harris S (1995) Conifers of Australia’s dry forests and open woodlands. In ‘Ecology of the southern conifers’. (Eds NJ Enright, RS Hill) pp. 252–270. (Melbourne University Press: Melbourne)
Bowman DMJS, Panton WJ (1993) Decline of Callitris intratropica R.T. Baker & H.G. Smith in the Northern Territory: implications for pre- and post-European colonization fire regimes. Journal of Biogeography 20, 373–381.
| Decline of Callitris intratropica R.T. Baker & H.G. Smith in the Northern Territory: implications for pre- and post-European colonization fire regimes.Crossref | GoogleScholarGoogle Scholar |
Bowman DMJS, Wilson BA (1988) Fuel characteristics of coastal monsoon forests, Northern Territory, Australia. Journal of Biogeography 15, 807–817.
| Fuel characteristics of coastal monsoon forests, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Bowman DMJS, Wilson BA, Davis GW (1988) Response of Callitris intratropica R.T. Baker & H.G. Smith to fire protection, Murgenella, northern Australia. Australian Journal of Ecology 13, 147–159.
| Response of Callitris intratropica R.T. Baker & H.G. Smith to fire protection, Murgenella, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Cohn JS (2002) Demographic characteristics of mallee pine (Callitris verrucosa) in fire-prone mallee communities of central New South Wales. Australian Journal of Botany 50, 653–665.
| Demographic characteristics of mallee pine (Callitris verrucosa) in fire-prone mallee communities of central New South Wales.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Bedward M, Cohn JS (2006) The modelled effects of differing fire management strategies on the conifer Callitris verrucosa within semi-arid mallee vegetation in Australia. Journal of Applied Ecology 43, 281–292.
| The modelled effects of differing fire management strategies on the conifer Callitris verrucosa within semi-arid mallee vegetation in Australia.Crossref | GoogleScholarGoogle Scholar |
Brodribb TJ, Holbrook NM, Hill RS (2005) Seedling growth in conifers and angiosperms: impacts of contrasting xylem structure. Australian Journal of Botany 53, 749–755.
| Seedling growth in conifers and angiosperms: impacts of contrasting xylem structure.Crossref | GoogleScholarGoogle Scholar |
Brodribb TJ, Bowman D, Nichols S, Delzon S, Burlett R (2010) Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytologist 188, 533–542.
| Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2011) Climate statistics for Australian locations. summary statistics, Beechworth composite [82001]. Available at http://reg.bom.gov.au/climate/averages/tables/cw_082001.shtml. Accessed August 2011.
Clayton-Greene KA (1981) The autecology of Callitris columellaris F. Muell. and associated Eucalyptus spp. in south-eastern Australia. PhD thesis, University of Melbourne.
Clayton-Greene KA, Ashton DH (1990) The dynamics of Callitris columellaris/Eucalyptus albens communities along the Snowy River and its tributaries in south-eastern Australia. Australian Journal of Botany 38, 403–432.
| The dynamics of Callitris columellaris/Eucalyptus albens communities along the Snowy River and its tributaries in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Cohn JS, Lunt ID, Ross KA, Bradstock RA (2011) How do slow-growing, fire-sensitive conifers survive in flammable eucalypt woodlands? Journal of Vegetation Science 22, 425–435.
| How do slow-growing, fire-sensitive conifers survive in flammable eucalypt woodlands?Crossref | GoogleScholarGoogle Scholar |
Cornelissen JHC, Diez PC, Hunt R (1996) Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. Journal of Ecology 84, 755–765.
| Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types.Crossref | GoogleScholarGoogle Scholar |
Cremer KW (1992) Relations between reproductive growth and vegetative growth of Pinus radiata. Forest Ecology and Management 52, 179–199.
| Relations between reproductive growth and vegetative growth of Pinus radiata.Crossref | GoogleScholarGoogle Scholar |
Florence RG (1996) ‘Ecology and silviculture of eucalypt forests’. (CSIRO: Collingwood)
Forestry Commission of New South Wales (FCNSW) (1988) Notes on the silviculture of major NSW forest types. 10. Cypress pine types. Forestry Commission of New South Wales, Sydney.
Grime JP (2001) ‘Plant strategies, vegetation processes, and ecosystem properties’. (John Wiley & Sons: Chichester)
Hawkins PJ (1966) Seed production and litter fall studies of Callitris columellaris. Australian Forest Research 2, 3–16.
Keith D (1996) Fire-driven extinction of plant populations: a synthesis of theory and review of evidence from Australian vegetation. Proceedings of the Linnean Society of New South Wales 116, 37–78.
Knott J (1995) White cypress pine thinning trials of the Western Region. Research Paper No. 27, State Forests of New South Wales, Sydney, Australia.
Lacey CJ (1972) Factors influencing occurrence of cypress pine regeneration in New South Wales. Technical Report No. 21, Forestry Commission of NSW, Sydney, Australia.
Lacey CJ (1973) Silvicultural characteristics of white cypress pine. Research Note No. 26, Forestry Commission of NSW, Sydney, Australia.
Lusk CH, Wright I, Reich PB (2003) Photosynthetic differences contribute to competitive advantage of evergreen angiosperm trees over evergreen conifers in productive habitats. New Phytologist 160, 329–336.
| Photosynthetic differences contribute to competitive advantage of evergreen angiosperm trees over evergreen conifers in productive habitats.Crossref | GoogleScholarGoogle Scholar |
Mackenzie BDE, Keith DA (2009) Adaptive management in practice: conservation of a threatened plant population. Ecological Management & Restoration 10, S129–S135.
| Adaptive management in practice: conservation of a threatened plant population.Crossref | GoogleScholarGoogle Scholar |
Parks Victoria (2008) ‘Chiltern-Mt Pilot National Park management plan’. (Parks Victoria: Melbourne)
Price O, Bowman DMJS (1994) Fire-stick forestry: a matrix model in support of skilful fire management of Callitris intratropica R.T.Baker by north Australian aborigines. Journal of Biogeography 21, 573–580.
| Fire-stick forestry: a matrix model in support of skilful fire management of Callitris intratropica R.T.Baker by north Australian aborigines.Crossref | GoogleScholarGoogle Scholar |
Prior LD, Lee Z, Brock C, Williamson GJ, Bowman DMJS (2010) What limits the distribution and abundance of the native conifer Callitris glaucophylla (Cupressaceae) in the West MacDonnell Ranges, central Australia? Australian Journal of Botany 58, 554–564.
| What limits the distribution and abundance of the native conifer Callitris glaucophylla (Cupressaceae) in the West MacDonnell Ranges, central Australia?Crossref | GoogleScholarGoogle Scholar |
Prober SM, Thiele KR (2004) Fire recovery vegetation monitoring in White Box – White Cypress Pine woodlands of East Gippsland. Technical Report, Department of Sustainability and Environment, Victoria, Australia.
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80, 1955–1969.
| Generality of leaf trait relationships: a test across six biomes.Crossref | GoogleScholarGoogle Scholar |
Ross KA, Bedward M, Ellis MV, Deane A, Simpson CC, Bradstock RA (2008) Modelling the dynamics of white cypress pine Callitris glaucophylla woodlands in inland south-eastern Australia. Ecological Modelling 211, 11–24.
| Modelling the dynamics of white cypress pine Callitris glaucophylla woodlands in inland south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith J (2006) Recruitment dynamics of the long-lived obligate seeders Callitris intratropica (Cupressaceae) and Petraeomyrtus punicea (Myrtaceae). Australian Journal of Botany 54, 479–485.
| Recruitment dynamics of the long-lived obligate seeders Callitris intratropica (Cupressaceae) and Petraeomyrtus punicea (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Russell-Smith J, Ryan PG, Klessa D, Waight G, Harwood R (1998) Fire regimes, fire-sensitive vegetation, and fire management of the sandstone Arnhem Plateau, monsoonal northern Australia. Journal of Applied Ecology 35, 829–846.
| Fire regimes, fire-sensitive vegetation, and fire management of the sandstone Arnhem Plateau, monsoonal northern Australia.Crossref | GoogleScholarGoogle Scholar |
Stocker GC (1966) ‘Aspects of the seeding habit of Callitris intratropica’. (Department of National Development: Canberra)
Thompson WA, Eldridge DJ (2005) White cypress pine (Callitris glaucophylla): a review of its roles in landscape and ecological processes in eastern Australia. Australian Journal of Botany 53, 555–570.
| White cypress pine (Callitris glaucophylla): a review of its roles in landscape and ecological processes in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Verkaik I, Espelta JM (2006) Post-fire regeneration thinning, cone production, serotiny and regeneration age in Pinus halepensis. Forest Ecology and Management 231, 155–163.
| Post-fire regeneration thinning, cone production, serotiny and regeneration age in Pinus halepensis.Crossref | GoogleScholarGoogle Scholar |
VSN International (2010) ‘Genstat for Windows 13th Edition’. (VSN International, Hemel Hempstead, UK)
Watson C (2004) ‘Habitat modelling and effects of fire on Callitris endlicheri in the Chiltern-Mt Pilot National Park, Victoria’. (School of Environmental and Information Sciences, Charles Sturt University: Albury)
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428, 821–827.
| The worldwide leaf economics spectrum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjt1Crt74%3D&md5=fc332b26cb96990d6c0d9fc5f55da652CAS |
Zeppel M, Eamus D (2008) Coordination of leaf area, sapwood area and canopy conductance leads to species convergence of tree water use in a remnant evergreen woodland. Australian Journal of Botany 56, 97–108.
| Coordination of leaf area, sapwood area and canopy conductance leads to species convergence of tree water use in a remnant evergreen woodland.Crossref | GoogleScholarGoogle Scholar |
Zimmer H, Green P, Cheal D, Clarke MF (2011) Reconstructing Mallee fire history using Callitris verrucosa tree rings. Technical Report No. 215, Arthur Rylah Institute for Environmental Research, Heidelberg, Victoria, Australia.


