Genetics and ecology of plant species occurring on the Banded Iron Formations in the Yilgarn, Western Australia
Margaret ByrneBiodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Locked Bag 104, Bentley Delivery Centre, WA 6983, Australia; and Department of Molecular and Life Sciences, Curtin University, Perth, WA, Australia. Email: margaret.byrne@dbca.wa.gov.au
Australian Journal of Botany 67(3) 165-171 https://doi.org/10.1071/BT19048
Submitted: 17 March 2019 Accepted: 15 April 2019 Published: 16 May 2019
Abstract
Banded Iron Formations (BIFs) are a distinctive feature in the Yilgarn craton of southern Western Australia occurring as geographically isolated ranges within a mosaic of alluvial clay soils interspersed with sandplains and occasional granite outcrops. They are prominent features across a flat, highly weathered plateau, forming unique geologically stable components in an unglaciated landscape. The topographic complexity of BIFs provides areas of key environmental heterogeneity in a subdued landscape, offering a mosaic of habitats and abundance of niche microhabitats that support unique plant communities with high species diversity including many narrowly endemic species and those with distributions centred on these banded iron formations. Genetic and ecological studies have been undertaken on several species that are endemic to, or have distributions centred on, the banded iron formations of the Yilgarn. These studies provide a basis for understanding the diversity and evolutionary history of the plant communities that occur in these diverse environments. This Special Issue brings together studies on several these species to complement studies already published, and this overview provides a summary of the genetics and ecology of 21 species that are restricted to, or have distributions centred on, BIFs. Many of these species have conservation status under national and state legislation and understanding of genetics and ecology of these species assists with conservation strategies. A range of genetic patterns was identified among these species making generalisations difficult and indicating analysis of individual species is required in order to provide information for conservation and management decisions.
Additional keywords: BIF, differentiation, ecology, genetic diversity, plants.
Introduction
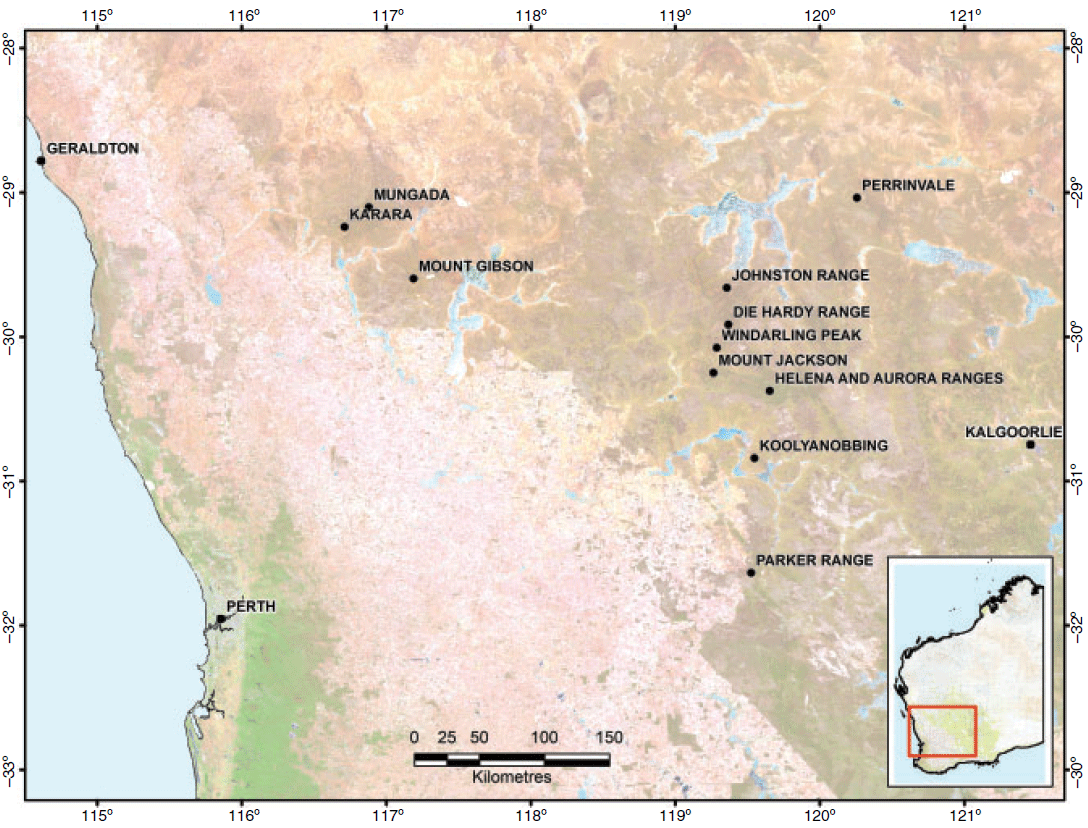
Inselbergs across the world harbour diverse plant communities with high levels of endemism and Banded Iron Formations (BIFs) are a particular type of inselberg associated with Archean cratons throughout the world (Klein 2005). Banded Iron Formations are a feature in the Yilgarn craton of southern Western Australia (Fig. 1), comprising banded iron talus slopes and areas of weathered duricrust (Hocking et al. 2007), and are associated with greenstones and granites that formed 2.6 to 3 billion years ago (Myers 1993). They occur along the boundary of the South-west Australia Floristic Region, a global biodiversity hotspot (Myers et al. 2000) that is well known for its high species richness and levels of local endemism (Hopper and Gioia 2004), and extend over 750 km into the inland arid zone with seasonality of rainfall changing from generally winter rainfall in the south to summer rainfall in the north (Gibson et al. 2012). These terrestrial islands occur as geographically isolated ranges within a mosaic of alluvial clay soils interspersed with sandplains and occasional granite outcrops. They are distinct elevated features across a flat, highly weathered plateau, forming unique geologically stable components in an unglaciated landscape. However, they are subdued in comparison to other ranges worldwide, with a maximum elevation of only 702 m above sea level and rising no more than 200 m above the surrounding plain.
|
The topographic complexity of BIFs provides areas of key environmental heterogeneity in a subdued landscape, providing a mosaic of habitats and abundance of niche microhabitats through variation in elevation, temperature, moisture and soil depth (Gibson et al. 2010, 2012) along with water-retaining depressions and south-facing slopes that may facilitate persistence of species during periods of aridity (Byrne et al. 2008; Yates et al. 2011). This diversity of habitats supports unique plant communities that are floristically distinct from those in the surrounding matrix of woodlands and shrublands, with high species diversity including many narrowly endemic species and those with distributions centred on these BIFs (Butcher et al. 2007b; Gibson et al. 2010, 2012). In addition, these stable geological formations allow for the development and persistence of diversity over historical timeframes (Gibson et al. 2010). There is high species turnover among BIF ranges that is not explained by geology or significant change in climate, and is likely a result of isolation over long time frames (Gibson et al. 2012). This species turnover and endemism is higher in the formations in the semi-arid region bordering the South-west Australian Floristic Region than those further inland in the arid zone (Gibson et al. 2010, 2012). The exceptionally high β diversity of BIFs of south-western Australia make them centres of plant diversity similar to other areas of specific substrate around the world, such as the ultramafic habitats of California (Safford et al. 2005), the granite outcrops of south-western Australia (Hopper et al. 1997) and the Piedmont (Shure 1999), the canga inselbergs of south-eastern Brazil (Jacobi et al. 2007; Jacobi and Carmo 2008), the limestone cedar glades of the south-eastern USA (Baskin and Baskin 2003; Estill and Cruzan 2001) and the Appalachian shale barrens (Braunschweig et al. 1999).
Genetic and ecological studies have been undertaken on several species that are endemic or have distributions centred on the BIFs of the Yilgarn, which provide a basis for understanding their diversity and the evolutionary history of this component of the plant communities that occur in these diverse environments. The species have been studied either to understand potential impacts of mining activities, or for their potential in restoration of disturbed sites after mining. This Special Issue brings together studies on several of these species to complement studies already published. This overview gives a brief summary of the genetics and ecology for 21 species where studies have been undertaken as published here and elsewhere (Box 1), and a summary of the overall patterns observed across these species.
| Box 1. Genetics and ecology of species occurring on BIF |
| Summary information from studies on the genetics and ecology of 21 species that occur on BIFs in the Yilgarn (Fig. 1) is provided here and further information is available in the cited references published in this special issue and elsewhere. Many of these species are recognised as rare and threatened under Western Australian legislation (https://www.dpaw.wa.gov.au/images/documents/plants-animals/threatened-species/Listings/flora_notice.pdf; accessed 3 March 2019), or are listed on a Priority List for those species considered to be rare and potentially threatened but for which there is insufficient information on which to make a formal assessment of conservation status (https://www.dpaw.wa.gov.au/images/documents/plants-animals/threatened-species/Listings/Conservation%20code%20definitions.pdf; accessed 3 March 2019). |
| Acacia adinophylla is recognised in Western Australia as a Priority 1 taxon (known from fewer than five locations, none of which are managed for conservation). It has a localised distribution across the Helena and Aurora Ranges, mostly found on the iron ridges but also occurs on lower slopes and adjacent plains, and is generally abundant where it occurs (Maslin 1999). Seed biology shows the presence of a soil seed bank with seed dormancy that responds to treatment with gibberellic acid and smoke (Stevens et al. 2010). A study of nuclear genetic diversity (Nevill and Wardell-Johnson 2017) showed moderate diversity with low genetic differentiation and little genetic structure, with a clinal distribution of diversity but no differentiation between plants on ridges versus slopes or plains. |
| Acacia karina is a tall shrub that is recognised as a Priority 1 taxon and has a regional distribution centred on BIFs in the Blue Hills/Mt Karara area, but also occurs on adjacent flats or low slopes. It is likely to be pollinated by generalist insects, and seed is likely to be dispersed by gravity and then by ants, birds or small mammals (Stone et al. 2003; Pascov et al. 2015). A study of nuclear genetic diversity (Funnekotter et al. 2019) showed high levels of diversity and generally low genetic differentiation among populations except for two isolated populations in the south-east of the distribution that were identified as a separate genetic cluster. Similarly, a high level of haplotype diversity was found in a study of cpDNA diversity, with no differentiation between BIF and non-BIF populations consistent with long-term persistence of both BIF and non-BIF populations (Funnekotter et al. 2019). |
| Acacia woodmaniorum is recognised as threatened under Western Australian legislation with a conservation status of Endangered. It has a localised distribution across several neighbouring BIFs in the Mungada/Mt Karara area occurring on steep slopes and in rock crevices and gullies, although generally abundant where it occurs. It is a low open shrub that is likely to be pollinated by generalist insects (Stone et al. 2003) and with seed dispersal by ants due to the presence of an aril (Millar et al. 2013). Genetic analysis (Millar et al. 2013) showed high diversity with moderate genetic structure that was distributed among four genetic clusters with geographical association with neighbouring ridges and some degree of admixture among clusters. The mating system is highly outcrossed and pollen dispersal is extensive and sufficient to counteract genetic drift, even in more isolated populations (Millar et al. 2014). |
| Banksia arborea occurs on several adjacent BIFs across an area of ~100 km2 in the Helena and Aurora Ranges, Die Hardy Range and Koolyanobbing area and is recognised as a Priority 4 taxon (not listed as rare or threatened but could be if circumstances change, or otherwise in need of monitoring). It has a patchy distribution occurring across the extent of the BIFs, and is a long lived tree that is considered to be pollinated by insects and birds, with gravity dispersed seeds (Wrigley and Fagg 1989). A study nuclear genetic diversity of B. arborea showed low genetic diversity and evidence of genetic differentiation among ranges, indicating limited pollen dispersal among ranges even among populations on a large range, the Die Hardy Range, where it is more common (Nistelberger et al. 2015b). A more complex pattern was observed in cpDNA with unexpected shared haplotypes among northern populations indicating common ancestry given the level of nuclear differentiation does not support genetic connectivity, and higher differentiation among southern ranges (Nistelberger et al. 2015b). |
| Darwinia masonii is restricted to the Mount Gibson – Extension Hill BIF complex and is recognised as Critically Endangered under Western Australian legislation and as Vulnerable under national legislation as. Study of seed biology showed a long-lived seed bank with seasonal dormancy, providing a means of persistence in an unpredictable and low rainfall environment (Miller et al. 2019). A nuclear genetic diversity study showed moderate genetic diversity similar to that in closely related species that occur in geographically nearby sandplains and granite rocks (Miller and Barrett 2019). |
| Grevillea georgeana is a small bird pollinated shrub that occurs in generally small populations on several BIFs in the Helena and Aurora, and Die Hardy Ranges area similar to B. arborea. It is recognised as a Priority 3 taxon (known from several locations and not under imminent threat). A nuclear genetic study showed similar results to that of B. arborea with moderate levels of genetic diversity and high genetic differentiation, indicating limited pollen dispersal among neighbouring populations despite bird pollination (Nistelberger et al. 2015a). Analysis of cpDNA showed contrasting patterns to B. arborea with high diversity and a large number of haplotypes on the large Die Hardy Range and neighbouring Mt Manning, and strong differentiation among ranges, except for those connected on a greenstone belt in the east of the distribution, indicating an evolutionary history of long-term isolation and persistence (Nistelberger et al. 2015a). |
| Grevillea globosa is a large insect pollinated shrub that has a regional distribution in the Midwest occurring on BIFs and on surrounding sandplain, and is recognised as Priority 3. A genetic analysis of the nuclear genome showed moderate levels of genetic diversity and low genetic differentiation (Millar et al. 2016). A cpDNA study showed relatively low haplotype diversity with no genetic structure due to a commonly distributed haplotype, and some signals of historical demographic expansion (Millar et al. 2016). Results suggest an unexpected high level of seed dispersal, possibly by ants, in addition to high levels of pollen dispersal. |
| Grevillea paradoxa is a large bird pollinated shrub and seeds with an elaiosome suggesting the possibility of ant dispersal. It is widely distributed across the Midwest occurring on a range of substrates but commonly occurring on slopes of BIFs. A nuclear genetic study showed moderate levels of diversity, with high genetic structure and evidence for three genetic clusters geographically distributed across the range (Millar et al. 2017). A cpDNA study showed high haplotype diversity with little genetic structure and two common haplotypes distributed across populations, with evidence of historical isolation of a population in the Murchison River gorge at Kalbarri (Millar et al. 2017). |
| Lepidosperma bungalbin has a conservation status of Vulnerable under Western Australian legislation, and is restricted to the upper slopes of the Helena and Aurora Ranges (Barrett 2007). A study by Nevill and Wardell-Johnson (2017) showed high nuclear genetic diversity with generally low genetic differentiation and little inbreeding; however, three genetic clusters were identified that correspond with geographic breaks in the distribution, indicating some genetic structure across the limited range of the species. |
| Lepidosperma costale is a species complex of wind pollinated sedges comprised of both diploid and tetraploid individuals as well as some triploid and pentaploids that occur on BIFs, granite outcrops and sandplain (Wallace et al. 2019). A nuclear genetic study showed high genetic diversity, with higher diversity in the diploids, but higher differentiation among tetraploids suggesting multiple origins of polyploidy (Wallace et al. 2019). Analysis of cpDNA also showed high haplotype diversity with little genetic structure and greater similarity among diploids and autopolyploids than allopolyploids (Wallace et al. 2019). |
| Lepidosperma gibsonii has a conservation status of Endangered under Western Australian legislation with. It is a clonal species that is restricted to a local area on a single BIF at Mt Gibson where it is locally common. Analysis of genetic diversity showed high diversity with no genetic structure across the range (Miller and Barrett 2019). Analysis of ecology and seed biology showed reliance on consecutive high rainfall years for growth, reproduction and recruitment (Miller et al. 2019). The species has a long-lived seed bank but also resprouts after fire thus maximising mechanisms for persistence in an arid environment with unpredictable rainfall (Miller et al. 2019). |
| Lepidosperma sp. Blue Hills is recognised as Priority 1 and is restricted to the Blue Hills BIF adjacent to the Mungada/Mt Karara BIFs. It shows moderate levels of genetic diversity and moderate genetic differentiation among populations (Barrett et al. 2008). |
| Lepidosperma sp. Mt Caudan is recognised as Priority 1. It has a restricted distribution occurring across BIFs in the Parker Range area, and shows extensive patterns of clonality within populations (Binks et al. 2015a). A nuclear genetic study showed high diversity and little genetic structure indicating reasonable levels of gene flow and connectivity among populations, although high levels of inbreeding were detected, most likely due to mating within clonal patches (Binks et al. 2015b). Analysis of cpDNA showed a high level of haplotype diversity with multiple shared haplotypes across populations indicating historical connectivity across populations and persistence over time (Binks et al. 2015b). |
| Lepidosperma sp. Parker Range is recognised as a Priority 1 taxon. It occurs as a few isolated populations across the Parker Range, and exhibits clonality but over lesser distances than that in L. sp. Mt Caudan (Binks et al. 2015a). A genetic study showed high nuclear genetic diversity with genetic differentiation across the populations and two genetic clusters of central and more peripheral populations, and high inbreeding most likely associated with clonality (Binks et al. 2015b). Analysis of cpDNA showed a moderate level of haplotype diversity with two common haplotypes that showed some geographic structure across populations (Binks et al. 2015b). |
| Melaleuca nematophylla is a large, probably insect pollinated shrub with small light seeds, likely wind dispersed. It has a widespread distribution in the Midwest occurring on various substrates but generally associated with slopes on BIFs. A nuclear genetic study showed high diversity with no genetic structure across the range (Millar et al. 2017). A cpDNA study showed high number of haplotypes but little phylogeographic structure with a common haplotype shared among populations in the central part of the range and another one in the south-eastern part of the range, with signals of long-term persistence over evolutionary history (Millar et al. 2017). Low pollen to seed ratios suggests seed dispersal is higher than might be expected in this environment. |
| Mirbelia sp. bursarioides is an insect pollinated shrub that has a regional distribution in the Midwest with occurrences centred on BIFs and granite outcrops. Assessment of contemporary nuclear genetic diversity showed high levels of diversity and some level of differentiation among populations, with signals of isolation by distance across the distribution (Millar et al. 2016). A cpDNA study showed also showed moderate levels of haplotype diversity with some sharing of haplotypes in adjacent populations and little phylogeographic structure (Millar et al. 2016). Low pollen to seed ratios and little phylogeographic structure imply an unexpected level of seed dispersal contributing to gene flow across the populations in addition to good levels of pollen dispersal. |
| Ricinocarpos brevis is recognised as Endangered under both Western Australian and national legislation. This woody shrub is likely pollinated by generalist insects and has a localised distribution across three ranges north of Southern Cross, two with restricted areas of occupancy and one with greater area of extent at Windarling Range. There are differences in occurrence among the populations as it occurs on southern rocky slopes at the Windarling and Perrinvale Ranges and on gentle southern and western slopes at the Johnston Range where the substrate and species assemblage are also different to that at the Windarling and Perrinvale Ranges (Krauss and Anthony 2019a). A nuclear genetic study (Krauss and Anthony 2019a) showed high genetic diversity and high genetic differentiation among populations on the different ranges, particularly at the Johnston Range that is also ecologically differentiated from the populations on the Windarling and Perrinvale Ranges. |
| Tetratheca aphylla subsp. aphylla is recognised as Vulnerable under both Western Australian and national legislation. It has a localised distribution on the Helena and Aurora Ranges where it occurs on cliff tops and steep slopes with skeletal soils and can be locally abundant (Yates et al. 2011). A nuclear genetic study showed high genetic diversity with low to moderate inbreeding, and low genetic differentiation across populations with association between genetic and geographic distance (Nevill and Wardell-Johnson 2017). Analysis of mating system parameters showed almost complete outcrossing (Butcher et al. 2011). |
| Tethratheca erubescens is recognised as Vulnerable under Western Australian legislation. It has a localised distribution being restricted to a very small geographical location on the south Koolyanobbing Range where it occurs in fissures on cliff faces (Yates et al. 2011). Modelling of species distribution and habitat features identified T. erubescens as occurring in the steepest and highest parts of the landscape with slope angle as an important aspect of suitable habitat (Miller 2015). As with other Tetratheca species, it has a specialised buzz pollination system and seed dispersal likely to be by ants (Krauss and Anthony 2019b). A genetic study (Krauss and Anthony 2019b) found low to moderate diversity but high genetic differentiation given the very restricted distribution, and genetic separation into two clusters consistent with presence on geographically separated ridges. |
| Tetratheca paynterae subsp. cremnobata is recognised Vulnerable under Western Australian legislation and Endangered under national legislation. It is restricted to cliffs on the Die Hardy Range (Yates et al. 2011) and analysis of genetic diversity showed moderate levels of diversity with high levels of genetic differentiation while analysis of mating system revealed close to complete outcrossing (Butcher et al. 2011). Seedling germination and survival is enhanced by winter rainfall (Yates et al. 2011). |
| Tetratheca paynterae subsp. paynterae is recognised Critically Endangered under Western Australian legislation and Endangered under national legislation. It is restricted to fissures on steep cliffs on a single BIF at Windarling (Yates et al. 2011), and as with other Tetratheca, the taxon is buzz pollinated by specialist insects, mainly bees (Ladd et al. 2019). A study on reproductive ecology showed flowering occurs primarily after rainfall, and generally in winter, and pollinator observations suggest the mating system is generally outcrossed (Ladd et al. 2019). Direct analysis of mating system showed high levels of outcrossing, although pollen dispersal is highly restricted and there were higher levels of inbreeding than in T. paynterae subsp. cremnobata and T. aphylla subsp. aphylla (Butcher et al. 2011). Most plants showed high seed set for an arid environment indicating generally effective pollination despite a specialised pollination syndrome (Ladd et al. 2019); however, seedling germination and survival is influenced by winter rainfall and observed recruitment is limited (Yates et al. 2011). Analysis of genetic diversity showed moderate levels of diversity, although higher than its sister taxon even though it is less abundant, with high levels of genetic differentiation over short geographic distances along the Windarling Range (Butcher et al. 2009) consistent with highly restricted pollen dispersal (Butcher et al. 2011). |
Patterns across BIF species
A recent review of genetics and ecology of the plant species of the Yilgarn BIFs has proposed that stochasticity and persistence are key determinants of evolutionary resilience of these species (Byrne et al. 2018). Phylogenetic studies have revealed high phylogenetic divergence among species from several genera. Phylogenetic analysis of Tetratheca shows the species occurring on BIFs are placed on long branches in multiple places in the phylogeny indicating that they have independent evolutionary history and do not represent allopatric replacement series across neighbouring BIFs (Butcher et al. 2007b). The exception to this is the sister taxa relationship between the two subspecies of T. paynterae that occur on adjacent BIFs (Butcher et al. 2007b). Similarly, species of Lepidosperma and Acacia occurring on BIFs occur in multiple places in their respective phylogenies (Barrett 2012; Williams 2011). In addition, species distribution modelling has shown that most species do not occupy all habitat that has been identified as being environmentally suitable (Robinson et al. 2019), suggesting current distributions have been influenced by stochastic events, with the exception of L. gibsonii that appears to occupy all available suitable habitat (Miller and Barrett 2019).
Phylogeographic studies show mixed levels of haplotype diversity with some species showing very high diversity and/or population specificity, indicating long-term persistence and isolation across the banded iron formations (e.g. Grevillea georgeana, Nistelberger et al. 2015a) while others showed high diversity with some haplotypes shared across populations (e.g. Lepidosperma sp. Parker Range, Binks et al. 2015b; Mirbelia sp. bursarioides, Millar et al. 2016; Melaleuca nematophylla, Millar et al. 2017; Acacia karina, Funnekotter et al. 2019), yet other showed low diversity and some sharing of haplotypes (Banksia arborea, Nistelberger et al. 2015b; Grevillea globosa, Millar et al. 2016). For species restricted to BIFs these patterns of haplotype sharing are generally not geographical, demonstrating the influence of long-term isolation, but for species with more widespread distributions and those occurring on multiple substrates, some geographical pattern is evident.
Nuclear genetic analyses also showed a range of genetic differentiation among populations, with some species showing high differentiation as expected from the isolation of geographically separated terrestrial islands at regional scale (e.g. Banksia arborea, Nistelberger et al. 2015b; Grevillea georgeana, Nistelberger et al. 2015a; Lepidosperma sp. Parker Range, Binks et al. 2015a), and others showing relatively little differentiation, due to having restricted and localised distribution (e.g. Acacia adinophylla, Nevill and Wardell-Johnson 2017), a widespread more connected distribution (Grevillea globosa, Millar et al. 2016) or being wind pollinated (e.g. Lepidosperma sp. Mt Caudan, Binks et al. 2015a; L. gibsonii, Miller and Barrett 2019). Interestingly, Tetratheca paynterae subsp. paynterae showed unexpectedly high differentiation across short geographical distances within BIFs due to highly restricted pollen dispersal (Butcher et al. 2009).
Analysis of genetic diversity within species shows generally moderate to high diversity despite the geographically restricted distributions of most species (Butcher et al. 2009; Miller and Barrett 2019; Millar et al. 2013, 2016; 2017; Binks et al. 2015a; Nistelberger et al. 2015a, 2015b; Nevill and Wardell-Johnson 2017; Funnekotter et al. 2019; Krauss and Anthony 2019a, 2019b). Some inbreeding was present within species but there was little evidence of bottlenecks. Assessment of life history traits does not provide any specific explanation for these patterns, and the levels of diversity are similar to other species with restricted distributions suggesting they are characteristic of species endemic to these island systems, highlighting that narrow endemism does not necessarily lead to low genetic diversity (Byrne et al. 2018). Landscape antiquity, climatic buffering and relatively large and stable populations are considered to contribute to the high level of genetic diversity observed within the species (Byrne et al. 2018).
Many of the species endemic to the Banded Iron formations of the Yilgarn have conservation status under national and state legislation (see Box 1). These formations are also prospective for iron ore mining leading to the need for a balance between conservation and development. The high levels of genetic diversity bode well for the ongoing persistence of populations of these species, providing them with the standing genetic variation to respond to changing environmental conditions. The high levels of genetic differentiation among populations, and lack of geographical structure to the differentiation, means that, in general, all populations make a contribution to genetic diversity within the species, and a conservation strategy for species with these genetic patterns would suggest conservation of all populations to maximise representation of genetic diversity across the species. In addition, the range of genetic patterns identified makes it difficult to make generalisations and analysis of individual species is required.
Mitigating impacts from development is challenging in these environments as many species occur in very shallow soil or in rock crevices. Translocations are difficult, and restoration of disturbed habitat in these harsh environments requires understating of these complex systems. It also provides many opportunities to further our understanding of the plant species that have persisted in these unique edaphic environments through evolutionary history.
Conflicts of Interest
The author declares no conflicts of interest.
Funding statement
This research did not receive any specific funding.
Acknowledgements
This Special Issue arose from a workshop on genetics and ecology of species on the banded iron formations of the Midwest. I thank the participants of the workshop for discussion on this topic, and the Department of Biodiversity, Conservation and Attractions, ARC Centre for Mine Site Restoration and Western Australian Biodiversity Science Institute for hosting the workshop. I thank Ben Miller, Melissa Millar, Carole Elliott, Paul Nevill and Philip Ladd for helpful comments on the manuscript, and Katherine Zdunic for preparation of Figure 1.
References
Barrett RL (2007) New species of Lepidosperma (Cyperaceae) associated with banded ironstone in southern Western Australia. Nuytsia 17, 37–60.Barrett RL (2012) Systematic studies in Cyperaceae tribe Schoeneae: Lepidosperma and allied genera. PhD Thesis, The University of Western Australia, Perth, Australia.
Barrett M, Wallace M, Barrett R, Chen Y, Krauss SL, Dixon K (2008) Clarification of the status of Lepidosperma sp. Blue Hills and genetic variation in the Lepidosperma costale complex. Report prepared by Botanic Gardens and Parks Authority (Kings Park and Botanic Garden) for Karara Mining Ltd, Perth, WA.
Baskin J, Baskin C (2003) The vascular flora of cedar glades of the southeastern United States and its phyto-geographical relationships. The Journal of the Torrey Botanical Society 130, 101–118.
| The vascular flora of cedar glades of the southeastern United States and its phyto-geographical relationships.Crossref | GoogleScholarGoogle Scholar |
Binks RM, Millar MA, Byrne M (2015a) Contrasting patterns of clonality and fine-scale genetic structure in two rare sedges with differing geographic distributions. Heredity 115, 235–242.
| Contrasting patterns of clonality and fine-scale genetic structure in two rare sedges with differing geographic distributions.Crossref | GoogleScholarGoogle Scholar | 25873148PubMed |
Binks RM, Millar MA, Byrne M (2015b) Not all rare species are the same: contrasting patterns of genetic diversity and population structure in two narrow endemic sedges. Biological Journal of the Linnean Society. Linnean Society of London 114, 873–886.
| Not all rare species are the same: contrasting patterns of genetic diversity and population structure in two narrow endemic sedges.Crossref | GoogleScholarGoogle Scholar |
Braunschweig S, Nilson E, Wieboldt T (1999) The mid-Appalachian shale barrens. In ‘Savannas, barrens, and rock outcrop plant communities of North America’. (Eds R Anderson, J Fralish, JM Baskin) pp. 83–98. (Cambridge University Press: New York)
Butcher R (2007) New taxa of ‘leafless’ Tetratheca (Elaeocarpaceae, formerly Tremandraceae) from Western Australia. Australian Systematic Botany 20, 139–160.
| New taxa of ‘leafless’ Tetratheca (Elaeocarpaceae, formerly Tremandraceae) from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Butcher PA, Bradbury D, Anthony JM, Krauss SL (2007a) An integrated research program focussed on practical outcomes for the ex situ and in situ conservation, restoration and translocation of the DRF Tetratheca paynterae subsp. paynterae. Report prepared by Botanic Gardens and Parks Authority (Kings Park and Botanic Garden) for Portman Iron Ore Limited, Perth, WA.
Butcher R, Byrne M, Crayn D (2007b) Evidence for convergent evolution among phylogenetically distant rare species of Tetratheca (Elaeocarpaceae, formerly Tremandraceae) from Western Australia. Australian Systematic Botany 20, 126–138.
| Evidence for convergent evolution among phylogenetically distant rare species of Tetratheca (Elaeocarpaceae, formerly Tremandraceae) from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Butcher PA, McNee SA, Krauss SL (2009) Genetic impacts of habitat loss on the rare ironstone endemic Tetratheca paynterae subsp. paynterae. Conservation Genetics 10, 1735–1746.
| Genetic impacts of habitat loss on the rare ironstone endemic Tetratheca paynterae subsp. paynterae.Crossref | GoogleScholarGoogle Scholar |
Butcher PA, Bradbury D, Krauss SL (2011) Limited pollen-mediated dispersal and partial self-incompatibility in the rare ironstone endemic Tetratheca paynterae subsp. paynterae increase the risks associated with habitat loss. Conservation Genetics 12, 1603–1618.
| Limited pollen-mediated dispersal and partial self-incompatibility in the rare ironstone endemic Tetratheca paynterae subsp. paynterae increase the risks associated with habitat loss.Crossref | GoogleScholarGoogle Scholar |
Byrne M, Yeates DK, Joseph L, Kearney M, Bowler J, Williams MA, Cooper S, Donnellan SC, Keogh JS, Leys R, Melville J, Murphy DJ, Porch N, Wyrwoll KH (2008) Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 18761619PubMed |
Byrne M, Krauss SL, Millar MA, Elliot CP, Coates DJ, Yates C, Binks R, Nevill P, Nistelberger H, Wardell-Johnson G, Robinson T, Butcher R, Barrett M, Gibson N (2018) Persistence and stochasticity are key determinants of genetic diversity in plants associated with banded iron formation inselbergs. Biological Reviews of the Cambridge Philosophical Society 94, 753–772.
| Persistence and stochasticity are key determinants of genetic diversity in plants associated with banded iron formation inselbergs.Crossref | GoogleScholarGoogle Scholar | 30479069PubMed |
Estill J, Cruzan M (2001) Phytogeography of rare plant specie endemic to the southeastern United States. Castanea 66, 3–23.
Funnekotter AV, Millar M, Krauss SL, Nevill PG (2019) Phylogeographic analyses of Acacia karina (Fabaceae) support long term persistence of populations both on and off banded ironstone formations. Australian Journal of Botany 67, 194–204.
Gibson N, Yates CJ, Dillon R (2010) Plant communities of the ironstone ranges of south western Australia: hotspots for plant diversity and mineral deposits. Biodiversity and Conservation 19, 3951–3962.
| Plant communities of the ironstone ranges of south western Australia: hotspots for plant diversity and mineral deposits.Crossref | GoogleScholarGoogle Scholar |
Gibson N, Meissner R, Markey AS, Thompson WA (2012) Patterns of plant diversity in ironstone ranges in arid south western Australia. Journal of Arid Ecology 77, 25–31.
| Patterns of plant diversity in ironstone ranges in arid south western Australia.Crossref | GoogleScholarGoogle Scholar |
Hocking RM, Langford RL, Thorne AM, Sanders AJ, Morris PA, Strong CA, Gozzard JR (2007) A classification system for regolith in Western Australia. Western Australia Geological Survey 2007, 19.
Hopper SD, Gioia P (2004) The southwest Australian floristic region: Evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology Evolution and Systematics 35, 623–650.
| The southwest Australian floristic region: Evolution and conservation of a global hot spot of biodiversity.Crossref | GoogleScholarGoogle Scholar |
Hopper SD, Brown AP, Marchant NG (1997) Plants of Western Australian granite outcrops. Journal of the Royal Society of Western Australia 80, 141–158.
Jacobi CM, Carmo FF (2008) The contribution of ironstone outcrops to plant diversity in the Iron Quadrangle, a threatened Brazilian landscape. Ambio 37, 324–326.
| The contribution of ironstone outcrops to plant diversity in the Iron Quadrangle, a threatened Brazilian landscape.Crossref | GoogleScholarGoogle Scholar | 18686515PubMed |
Jacobi CM, Carmo FF, Vincent RC, Stehmann JR (2007) Plant communities on ironstone outcrops: a diverse and endangered Brazilian ecosystem. Biodiversity and Conservation 16, 2185–2200.
| Plant communities on ironstone outcrops: a diverse and endangered Brazilian ecosystem.Crossref | GoogleScholarGoogle Scholar |
Klein C (2005) Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origins. The American Mineralogist 90, 1473–1499.
| Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origins.Crossref | GoogleScholarGoogle Scholar |
Krauss SL, Anthony J (2019a) Genetic impacts of habitat loss on the rare Banded Ironstone Formation endemic Ricinocarpus brevis (Euphorbiaceae). Australian Journal of Botany 67, 183–193.
Krauss SL, Anthony JM (2019b) The potential impact of mining on population genetic variation in the Banded Ironstone Formation endemic Tetratheca erubescens (Elaeocarpaceae). Australian Journal of Botany 67, 172–182.
Ladd PG, Yates CJ, Dillon R, Palmer R (2019) Pollination and reproductive ecology of Tetratheca species from isolated, arid habitats (BIFs) in Western Australia. Australian Journal of Botany 67, 248–255.
Maslin B (1999) Acacia adinophylla Maslin. Nuytsia 12, 318–320.
Millar MA, Coates DJ, Byrne M (2013) Genetic connectivity and diversity in inselberg populations of Acacia woodmaniorum, a rare endemic plant of the Yilgarn Craton Banded Iron Formations. Heredity 111, 437–444.
| Genetic connectivity and diversity in inselberg populations of Acacia woodmaniorum, a rare endemic plant of the Yilgarn Craton Banded Iron Formations.Crossref | GoogleScholarGoogle Scholar | 23860233PubMed |
Millar MA, Coates DJ, Byrne M (2014) Extensive long-distance pollen dispersal and highly outcrossed mating in historically small and disjunct populations of Acacia woodmaniorum (Fabaceae), a rare banded iron formation endemic. Annals of Botany 114, 961–971.
| Extensive long-distance pollen dispersal and highly outcrossed mating in historically small and disjunct populations of Acacia woodmaniorum (Fabaceae), a rare banded iron formation endemic.Crossref | GoogleScholarGoogle Scholar | 25100675PubMed |
Millar MA, Byrne M, Coates DJ, Roberts D (2016) Contrasting diversity and demographic signals in sympatric narrow range endemic shrubs of the south-west Western Australian semi-arid zone. Biological Journal of the Linnaean Society 118, 315–329.
| Contrasting diversity and demographic signals in sympatric narrow range endemic shrubs of the south-west Western Australian semi-arid zone.Crossref | GoogleScholarGoogle Scholar |
Millar MA, Byrne M, Coates DJ, Roberts D (2017) Comparative analysis indicates historical persistence and contrasting contemporary structure in sympatric woody perennials of semi-arid southwest Western Australia. Biological Journal of the Linnaean Society 120, 771–787.
| Comparative analysis indicates historical persistence and contrasting contemporary structure in sympatric woody perennials of semi-arid southwest Western Australia.Crossref | GoogleScholarGoogle Scholar |
Miller B (2015) Tetratheca erubescens habitat study. Report prepared by Botanic Gardens and Parks Authority (Kings Park and Botanic Garden) for Cliffs Asia Pacific Iron Ore Pty Ltd, Perth, WA.
Miller B, Barrett M (2019) Darwinia masonii and Lepidosperma gibsonii conservation and restoration research. An integrated research program into the ex situ and in situ conservation, restoration and translocation requirements of Darwinia masonii and Lepidosperma gibsonii, May 2007– June 2010. Report prepared by Botanic Gardens and Parks Authority (Kings Park and Botanic Garden) for Mount Gibson Mining Limited and Extension Hill Pty Ltd, Perth, WA.
Miller BP, Symons DR, Barrett MD (2019) Persistence of rare species depends on rare events: demography, fire response and phenology of two plant species endemic to a single semi arid Banded Iron Formation range in Western Australia. Australian Journal of Botany 67, 268–280.
Myers JS (1993) Precambrian history of the west Australian craton and adjacent orogens. Annual Review of Earth and Planetary Sciences 21, 453–485.
| Precambrian history of the west Australian craton and adjacent orogens.Crossref | GoogleScholarGoogle Scholar |
Myers N, Mittermeier RA, Mittermeier CG, da Fonesca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 10706275PubMed |
Nevill P, Wardell-Johnson G (2017) Assessment of population genetic variation and structure of priority and threatened taxa in the Helena-Aurora Range, south-western Australia. Report to Polaris Metals.
Nistelberger HM, Byrne M, Coates DJ, Roberts JD (2015a) Genetic drift drives evolution in the bird pollinated terrestrial island endemic Grevillea georgeana (Proteaceae). Botanical Journal of the Linnean Society 178, 155–168.
| Genetic drift drives evolution in the bird pollinated terrestrial island endemic Grevillea georgeana (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Nistelberger HM, Byrne M, Coates DJ, Roberts JD (2015b) Phylogeography and population differentiation in terrestrial island populations of Banksia arborea (Proteaceae). Biological Journal of the Linnean Society. Linnean Society of London 114, 860–872.
| Phylogeography and population differentiation in terrestrial island populations of Banksia arborea (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Pascov CM, Nevill PG, Elliott CP, Majer JD, Anthony JM, Krauss SL (2015) The critical role of ants in the extensive dispersal of Acacia seeds revealed by genetic parentage assignment. Oecologia 179, 1123–1134.
| The critical role of ants in the extensive dispersal of Acacia seeds revealed by genetic parentage assignment.Crossref | GoogleScholarGoogle Scholar | 26255273PubMed |
Robinson T, Di Vilgilio G, Temple-Smith D, Hesford J, Wardell-Johnson GW (2019) Characterisation of range-restriction amongst the rare flora of banded ironstone formation ranges in semi-arid southwestern Australia. Australian Journal of Botany 67, 234–247.
Safford HD, Viers JH, Harrison SP (2005) Serpentine endemism in the California flora: a database of serpentine affinity. Madrono 52, 222–257.
| Serpentine endemism in the California flora: a database of serpentine affinity.Crossref | GoogleScholarGoogle Scholar |
Shure D (1999) Granite outcrops of the southeastern United States. In ‘Savannas, barrens, and rock outcrop plant communities of North America’. (Eds R Anderson, J Fralish, JM Baskin) pp. 99–118. (Cambridge University Press: New York)
Stevens J, Symons D, Dixon K (2010) Progress report from BGPA Science to Polaris Metals NL and Process Minerals International, Mineral Resources Limited.
Stone GN, Raine NE, Prescott M, Willmer PG (2003) Pollination ecology of acacias (Fabaceae, Mimosoideae). Australian Systematic Botany 16, 103–118.
| Pollination ecology of acacias (Fabaceae, Mimosoideae).Crossref | GoogleScholarGoogle Scholar |
Wallace MJ, Krauss SL, Barrett MD (2019) Complex genetic relationships within and among cytotypes in the Lepidosperma costale species complex (Cyperaceae) on rocky outcrops in Western Australia. Australian Journal of Botany 67, 205–217.
Williams A (2011) Comparative phylogeography of two Acacia species associated with banded ironstone formations (BIFs): did BIF ranges act as refugia during climate change? Honours Thesis, The University of Western Australia, Perth, Australia.
Wrigley JW, Fagg M (1989) ‘Banksias, Waratahs and Grevilleas and all other plants in the Australian Proteaceae family.’ (Collins Publishers Australia: Sydney, NSW)
Yates C, Gibson N, Pettit NE, Dillon R, Palmer R (2011) The ecological relationships and demography of restricted ironstone endemic plant species: implications for conservation. Australian Journal of Botany 59, 692–700.
| The ecological relationships and demography of restricted ironstone endemic plant species: implications for conservation.Crossref | GoogleScholarGoogle Scholar |


