Habitat distributions of 12 co-occurring wallaby grasses (Rytidosperma spp., Poaceae) and their response to a transition from pastoral to conservation land use
S. McIntyre A B * , W. J. Müller
A B * , W. J. Müller  A and Jon Lewis
A and Jon Lewis  B
B
A CSIRO Land and Water, GPO Box 1700, Canberra, ACT 2601, Australia.
B Australian National University, Fenner School of Environment and Society, Canberra, ACT 0200, Australia.
Australian Journal of Botany 70(2) 131-145 https://doi.org/10.1071/BT21100
Submitted: 18 August 2021 Accepted: 6 December 2021 Published: 3 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The extent and abundance of Rytidosperma Steud. species in mixed woodland, forest and derived grassland was examined over a 15-year period following removal of long-term sheep grazing. Ground-layer vegetation in 73 permanent plots was surveyed five times between 2005 and 2020 in a 50-ha paddock on the southern tablelands of New South Wales. Sites were stratified over the slope positions and micro-habitats represented at the site. Of the 12 Rytidosperma species recorded, only R. pallidum was morphologically and ecologically distinct in the field. The remaining 11 species, termed ‘cryptic Rytidosperma’, were assessed using a novel sampling method developed for this group. Rytidosperma pallida was the only species strongly associated with sclerophyll forest habitat. The 11 other species varied in their habitat preferences but, as a group, were most dominant and persistent on upper slopes. Over the 15 years, the relative abundance of R. pilosum, R. erianthum, R. monticola, R. carphoides and R. caespitosum declined as annual exotics, native sub-shrubs and low-palatability graminoids became more dominant. But only one species (R. pilosum) significantly declined in overall frequency of presence (constancy) in the plots. The changes observed suggest that when pastoral land is converted to conservation management, reduced grazing pressure could affect the abundance of Rytidosperma species important to other species, such as the threatened moth Synemon plana.
Keywords: Austrodanthonia, Danthonia, eucalypt grassy woodland, grazing, Joycea pallida, macropod, R. auriculatum, R. laeve, R. monticola, R. penicillatum, R. racemosum, R. setaceum, R. tenuius.
Introduction
Species of Rytidosperma Steud. (wallaby grasses) are unusual among temperate native grasses because of their persistence, palatability and productivity under livestock grazing, making them of great importance to the pastoral industry in south-eastern Australia (Whalley et al. 1978; Garden et al. 2001). Moreover, some species are tolerant of fertilisation (Whalley et al. 1978), a rare feature in the native ground flora (Dorrough et al. 2011), and can produce quantities of high-quality forage (e.g. Archer and Robinson 1988; Robinson and Archer 1988). In the New South Wales (NSW) southern tablelands, Rytidosperma is an important naturally occurring component of sown and fertilised pastures, and is particularly associated with sedimentary lithology and tolerant of acid soils (Munnich et al. 1991; Robinson et al. 1993). Pastures dominated by Rytidosperma can be stable under a range of livestock grazing regimes (Garden et al. 2000) and appear to have recolonised less stable sown pastures on the NSW central and southern tablelands where the genus is the most common dominant in pastures, and is strongly associated with sheep grazing (Garden et al. 2001).
Beyond its economic significance to livestock industries, the genus has relevance to conservation. The threatened golden sun moth (Synemon plana Walker) has been observed to be positively associated with occurrence (Richter et al. 2013), and amount and species number (Kutt et al. 2016), of Rytidosperma. O’Dwyer and Attiwill (1999) found S. plana to be associated with four species in particular, whose roots were thought to be consumed by larvae of the moths. It is therefore important to understand the specific ecological characteristics of these species, as changes to management may affect existing habitat for golden sun moths.
There is widespread occurrence of reticulation in the sub-family Danthonioideae, explained by past hybridisation (Pirie et al. 2009), which results in a phylogenetic network rather than a more easily interpreted phylogenetic tree. Identification to species requires microscopic examination, but identifications are not always straightforward because the genus has a wide range of cytotypes, and continues to undergo both inter- and intra-specific hybridisation between cytotypes (Brock and Brown 1961; Waters et al. 2010). As a result, field researchers face major obstacles when conducting vegetation surveys when Rytidosperma is present. Understandably, vegetation surveys not specifically targeting this group tend to identify Rytidosperma only to genus at the plot level (e.g. Scott and Whalley 1982; Dowling et al. 1996; McIntyre 2008; Bryant et al. 2017), even though multiple taxa co-occur (Munnich et al. 1991; Waters et al. 2009). As wallaby grasses are broadly understood to be grazing tolerant and an important component of commercially grazed pastures, a transition to conservation management could result in changes to the component species. Moreover, as species of Rytidosperma vary in their ecological tolerances (Scott and Whalley 1982), some differences in response could be expected.
This paper documents changes in the extent and relative abundance of Rytidosperma species over 15 years, after a transition from long-term commercial sheep grazing to macropod grazing, in a heterogeneous paddock of 50 ha. More specifically we aim to:
-
further our understanding of the varying ecological characteristics within the genus through an investigation of their microhabitat preferences;
-
analyse the response of the species present to the removal of commercial livestock grazing;
-
interpret our observations in the context of the ecology of all Rytidosperma species in the region;
-
consider any implications for conservation management of the patterns and changes observed.
Materials and methods
Site characteristics and history
The study site is located on undulating hills and minor flats of the Yass River valley, on the southern tablelands of New South Wales. Permanent plots were established across a 50 ha paddock (centre 34°58′30′S, 149°12′23′E), covering an altitudinal range of 50 m (585–635 m). The soil parent materials are Ordovician sediments (Jenkins 2000) and the clay–loam soils are acidic (pH 4–5) and highly erodible. The mean annual temperature is 20°C (maximum) and 6.5°C (minimum). Annual rainfall averages 644 mm, with monthly averages ranging from 45 mm (in May, June, July) to 66 mm (November). Extremely wet or dry conditions may be experienced in any month of the year and frosts are frequent in winter. The site has a 200-year history of livestock grazing. Stocking in the 25 years before the first vegetation assessment (2005) was 100 wethers, reduced to 65 from 2000 to 2004 during the Millennium Drought. Although this stocking rate of 2 dry sheep equivalent (DSE) ha−1 is mid-range carrying capacity for native grassland in the region (Langford et al. 2004), one-third of the paddock was dry sclerophyll forest, which suggests higher grazing pressures.
Over the 50 ha, the vegetation comprises approximately equal amounts of Tablelands Sclerophyll Forest, Tablelands Grassy Woodland (Keith 2004) and grassland–open woodland mosaic derived from tree clearing. Although there is evidence of past ringbarking, most of the open areas resulted from pasture development in 1972–1974. Trees were bulldozed into windrows and burnt. The areas were chisel-ploughed, superphosphate was applied and sown to Trifolium spp. The entire site was burnt by wildfire in 1975. There have been no further pasture inputs, and soil sampling in 2006 indicated that available soil phosphorus had returned to ‘native’ levels (Colwell P, 5 mg kg−1; see McIntyre 2008). Conversion from pastoral use to conservation management was initiated at the end of 2004 with the permanent removal of all livestock. The site has supported populations of macropods both before and after removal of livestock. During the study, the site was grazed by macropods, which move freely within and through numerous points on the boundary fence. These were, in the order of decreasing abundance, Macropus giganteus, M. rufogriseus and Wallabia bicolor. Hares were also present in very low numbers, with little grazing impact evident. The macropods were regarded as largely uncontrolled, there being no effective culling or known predators apart from the fox (Vulpes vulpes L.). The grazing regime after the removal of livestock was more selective, because the wild herbivores were unconstrained by fencing. This resulted in spatially variable grazing pressure, evident from the patchiness of sward height throughout the site (as described in McIntyre and Tongway 2005). The upper slopes were the most severely grazed part of the landscape before, and throughout, the study, because this is where sheep had previously camped and where kangaroos persistently congregated during the observation period. Pre-existing grazing lawns persisted more strongly here as a consequence. Rainfall variation resulted in grazing pressure also varying in time, although even at the lowest point of the 2017–2019 drought, grassland on the upper slopes supported an average biomass in the order of 1500 kg ha−1.
Survey design
To monitor floristic changes, 73 permanent plots (5 × 6 m) were established and the first assessment was undertaken in spring 2005, 12 months after the removal of sheep. All plots were marked at two corners with sturdy metal stakes and were able to be accurately re-located in later surveys.
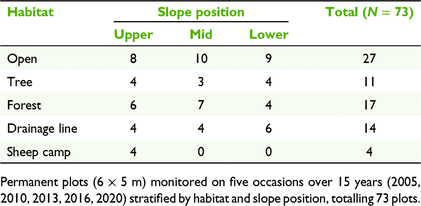
Plot locations were stratified to sample the range of environments over the site. These were categorised in terms of habitat and slope position (Table 1). All but the ‘Forest’ category were habitats that were in grassy woodland or grassland derived from grassy woodland:
-
1. Forest – in sclerophyll forest with continuous tree canopy characterised by Eucalyptus rossii and E. mannifera;
-
2. Open – cleared grassy woodland away from tree canopies;
-
3. Tree – under a well-developed tree canopy in grassy woodland;
For Habitats 1–3, upper slopes included hill crests and shoulders, lower slopes included break of slope and flats, whereas mid slopes were intermediate locations.
-
4. Sheep camp – these were physically equivalent to upper slope ‘Tree’ habitat but carried a nutrient legacy from their previous use by sheep as locations for habitual resting, resulting in dung and urine accumulation;
-
5. Drainage line – intermittent watercourses (first- and second-order) with scour ponds (Eyles 1977) and incised sections. Slope position for drainage lines was determined from the overall altitude at the study site as follows: upper (first-order drainage lines, >615 m), mid (first-order, 600–615 m) and lower (second-order drainage line, <600 m).
|
Assessments took place in late spring–early summer and were timed to allow for the flowering of cool-season grasses and Rytidosperma spp. The earliest commencement date was 20 November and latest finishing date 10 December. We avoided sampling in years when rainfall was low over the winter–spring growing period so as to maximise apparency. Comparing annual rainfall totals with the long-term mean indicates slightly above-average rainfall over the 15-year observation period (Fig. 1). Variability in rainfall was high; observations started in the Millennium Drought, which broke 5 years later with a La Niña event in 2010–2011, and average to wet conditions continued until the short severe drought of 2017–2019. In summary, we observed emergence from two severe droughts with an intervening wet period of 7 years, including the record-breaking wet winter–spring of 2016, when 1.5 times the average rainfall was recorded.
|
|
Sampling and nomenclature
In the initial survey (2005), aspect was recorded, and ground surface cover was estimated in each plot according to the following categories: cryptogam, litter, rock, bare ground. Litter depth was measured at four fixed locations in each plot.
Full floristic surveys were conducted, recording the presence of all taxa (see notes below relating to Rytidosperma) by using the ranking system of Mannetje and Haydock (1963). Using this method, each species (or species combination) was ranked according to its relative contribution to the total biomass (including attached litter). The ranks were converted to proportional contribution using the following geometric series:
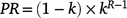
where R is the rank order and k is the parameter, which was set at 0.3, as determined for pasture by Scott (1986). Species ranked 1 (70%), 2 (21%) and 3 (6%) are referred to as the dominants in a plot. On average, the top-three ranked species account for 97% of the total biomass in grassy vegetation (Scott 1986). Ranking was recorded down to 7. Species ranked 8 and below were given a nominal relative abundance of 0.01%. This ranking method gives a measure of relative abundance, represented as a percentage of the total sward biomass. All surveys were conducted by S. McIntyre and J. Lewis.
Taxonomic changes during the observation period
At the time of the first survey in 2005, Joycea pallida was taxonomically separate from other wallaby grasses (then Austrodanthonia spp.) and was morphologically distinct in the field. Accordingly, in recording the rankings, we assessed the relative amount of biomass of the following two entities: (1) Joycea pallida and (2) Austrodanthonia spp. (as described above). Linder et al. (2010) placed Joycea pallida and all Austrodanthonia species into Rytidosperma. In this paper, we have addressed the potential confusion this name change has made for communication, by using the current name for Joycea pallida as R. pallidum. Being unidentifiable to species level in the field, the collective entity assessed during surveys as Austrodanthonia spp. is referred to here as ‘cryptic Rytidosperma’.
Elucidation of the species of ‘cryptic Rytidosperma’
In addition to its biomass ranking, we sampled the available ‘cryptic Rytidosperma’ in each plot by collecting one inflorescence randomly from 10 plants. All plants were sampled if there were fewer than 10 plants flowering. There is no vegetative reproduction in these species and the tussocks are distinct. Plants were identified to species in the laboratory through microscopic assessment of floret morphology and indumentum by using the Austrodanthonia key and descriptions of Linder (2005). In total, 1959 individual plants were identified to species in this way. At the plot level, the percentage biomass of ‘cryptic Rytidosperma’ was allocated to the individual species in the proportion to which they were represented in the inflorescence sample from each plot.
Data analysis
The following 12 species were recorded in the surveys: Rytidosperma auriculatum (J.M.Black) Connor & Edgar, R. caespitosum (Gaudich.) Connor & Edgar, R. carphoides (F.Muell. ex Benth.) Connor & Edgar, R. erianthum (Lindl.) Connor & Edgar, R. laeve (Vickery) Connor & Edgar, R. monticola (Vickery) Connor & Edgar, R. pallidum (R.Br.) A.M.Humphreys & H.P.Linder, R. penicillatum (Labill.) Connor & Edgar, R. pilosum (R.Br.) Connor & Edgar, R. racemosum (R.Br.) Connor & Edgar, R. setaceum (R.Br.) Connor & Edgar, and R. tenuius (Steud.) A.Hansen & Sunding. Although distinct examples of R. erianthum and R. monticola were identified, and the former was more frequent, there were enough intermediate forms to require a category combining both species for the analyses (‘R. erianthum–R. monticola’).
2005 habitat analyses
Analyses of the floristic data were initially performed on the calculated percentage biomass for the 2005 survey of ‘cryptic Rytidosperma’ and each individual Rytidosperma species (but with R. erianthum and R. monticola combined). These species or species groupings fell into the following three categories for analysis:
-
‘Cryptic Rytidosperma’, R. erianthum–R. monticola, R. pilosum – forward stepwise linear regression of percentage biomass in the 73 plots, examining the effects of plot environment (habitat and slope position), aspect, ground cover (estimated percentage cover of cryptogam, litter, rock, bare ground) and litter depth (average of five points in each plot), and interactions between all these categories and measurements.
-
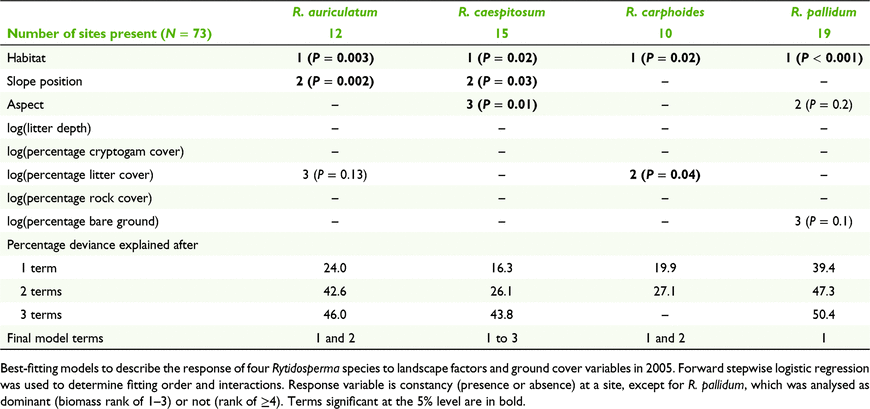
R. auriculatum, R. caespitosum, R. carphoides, R. pallidum – forward stepwise logistic regression of presence or absence (also referred to as constancy) in the 73 plots, examining the effects of the same variables listed in (1).
-
R. laeve, R. penicillatum, R. racemosum, R. setaceum, R. tenuius – tabulation of numbers of occurrences in the 73 sites as their frequencies were too low for formal analysis.
For all the variables in (1), the data were log-transformed, ln(1 + percentage biomass), before analysis. In (2), as the distribution of R. pallidum across the 73 plots was bimodal (i.e. present in large to moderate amounts, or in small amounts or absent), the binary response analysed was dominant (biomass rank of 1–3) or not (rank of ≥4).
Analyses over time
Over the five survey times, analyses of percentage biomass and constancy of Rytidosperma were performed for each of the taxonomic entities listed in (1) and (2) above. These analyses were restricted to those plots where the entity being analysed was present on at least one of the survey times. This meant that not all habitat and slope position combinations were represented, so habitat and slope were collapsed into a single ‘environment’ factor. For percentage biomass, split plot in time repeated-measures analyses of variance were performed to compare environments, years and their interaction. As previously, the data were log-transformed before analysis. For constancy, two-way ANOVAs were performed on numbers of plots where the species was present for each environment in each year, to determine whether there was a significant change over time after adjusting for environment differences.
Results
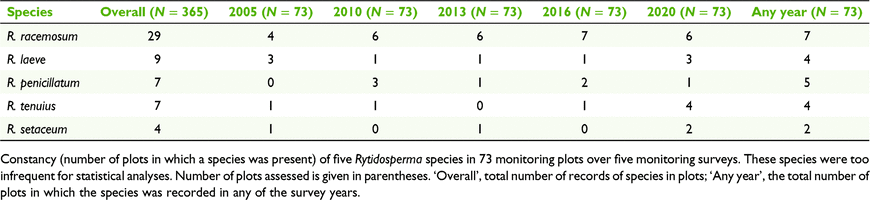
Of the 365 plot assessments (5 surveys × 73 plots), 79% recorded ‘cryptic Rytidosperma’ being present and 34% recorded the presence of R. pallidum. Of the 73 plots, 63 had ‘cryptic Rytidosperma’ recorded in any of the years and the range was from 61 (in 2005) to 56 (in 2013, 2016, 2020). Rytidosperma pallidum was recorded in 27 of the plots in any of the years, with a range from 26 (in 2010) to 24 (in 2020). Up to five species of Rytidosperma co-occurred in a plot. Records of the species that were too infrequent to analyse statistically are summarised in Table 2.
|
Environmental patterns in 2005
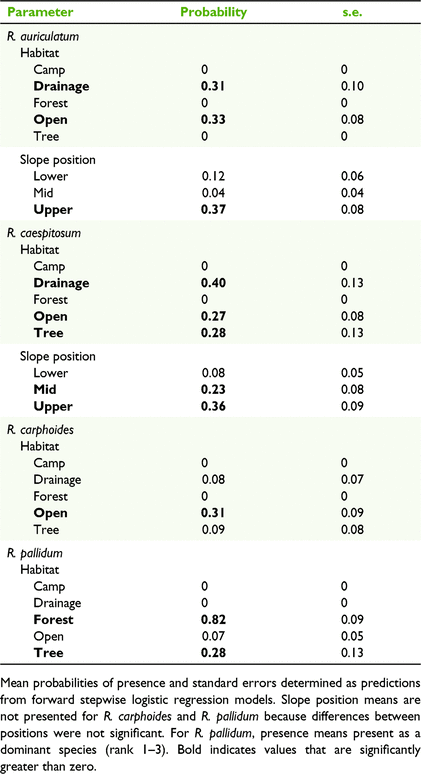
Forward stepwise regression showed habitat, then slope position, to be the best explanatory variables for the seven taxonomic entities analysed, and these two terms accounted for 25–50% the variance (for total biomass) or deviance (for constancy) (Tables 3, 4; Supplementary Table S1). Rytidosperma pallidum was significantly linked to sclerophyll forest and tree canopies, and there was little overlap in distribution between this and other species (Tables 4, 5). Cryptic Rytidosperma was strongly dominant on the upper slope plots, as was the common R. erianthum–R. monticola entity (Fig. 2). The other abundant species was R. pilosum, which had a more even distribution across slope positions, and tree and open plots (Fig. 2).

|
|
|
Among the moderately common taxa for which only constancy was analysed, open plots had a high likelihood of presence, with either no slope position preference (R. carphoides), upper slopes (R. auriculatum) or mid and upper slopes preferred (R. caespitosum) (Tables 4, 5). The latter species was as likely to occur under trees, or in drainage lines, as it was in the open.
Most taxa were recorded in drainage lines, but only on upper slopes (Table 4, Fig. 2), where the plots often included both wet areas and very dry microsites on the top of incised, eroded banks. Only one species, R. racemosum, was important on sheep camps, although R. pilosum was present in small amounts. The only species entirely restricted to one habitat type was R. penicillatum, which was recorded in five mid- and lower-drainage line plots.
Aspect was marginally significant for ‘cryptic Rytidosperma’, for which north-, east- and south-facing slopes were preferred, but only significant for one species, R. caespitosum, which was almost entirely restricted to eastern and southern aspects.
Ground cover attributes were significant in accounting for variability in occurrence for three of the seven taxa (or groups) analysed (Tables 3, 4), although much less important than habitat or slope position. R. carphoides was negatively associated with litter cover, consistent with its preference for open sites, and generally absent from beneath tree canopies. ‘Cryptic Rytidosperma’ and R. erianthum–R. monticola were positively associated with rock cover, which is consistent with their preference for upper slopes.
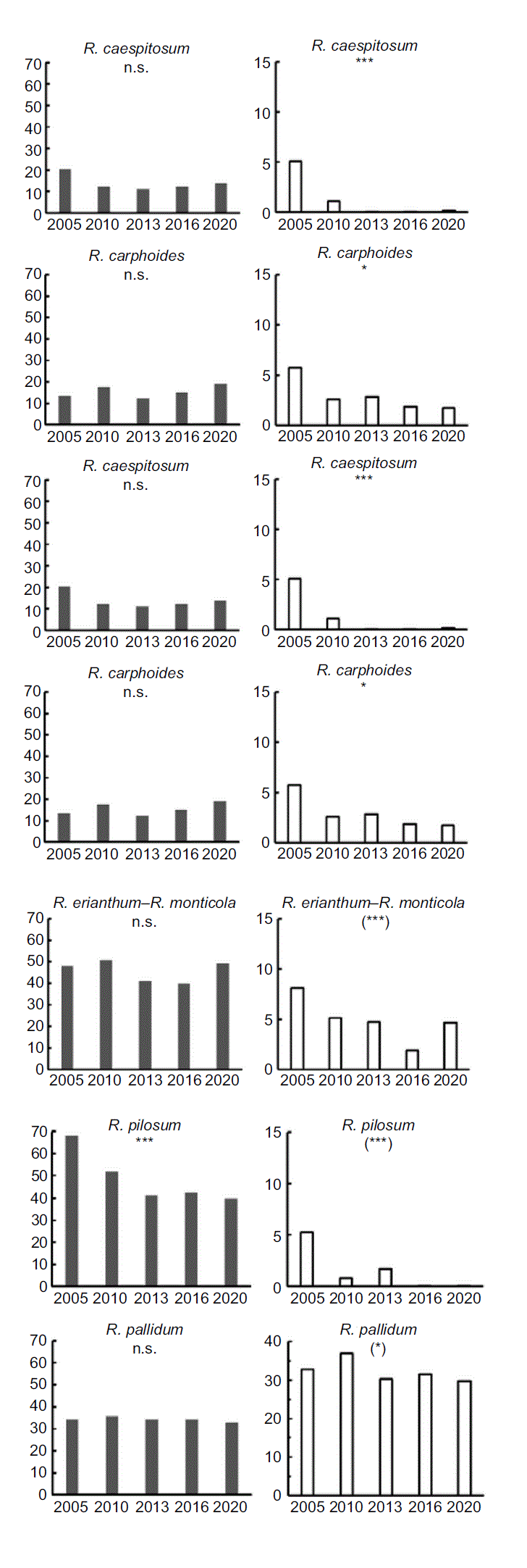
Changes over time
Rytidosperma pallidum
The distinctiveness of R. pallidum is the large size of the coarse, unpalatable tussocks, which are rarely grazed. Over the 15 years, this species maintained its constancy and dominance in sclerophyll forest plots on all slope positions (Fig. 3), but where it occurred under individual tree canopies, it was more dominant during the wet years (2010–2016) and was significantly reduced after droughts (i.e. in 2005 and 2020; data not shown).
‘Cryptic Rytidosperma’
The ‘cryptic Rytidosperma’ group similarly maintained its constancy, but representation in the sward declined progressively (Fig. 3). There were significant interactions with habitat and slope position. In open plots on upper slopes, progressive decline as a percentage of biomass occurred during the wet years, with some recovery in 2020 following three drought years. In other favoured habitats, ‘cryptic Rytidosperma’ declined after 2005, and did not recover in 2020 (e.g. upper-slope tree and drainage lines, mid slopes open and drainage lines, and lower slopes open). On camps, ‘cryptic Rytidosperma’ persisted for at least 8 years, but percentage biomass was very low in 2016 and 2020.
Common Rytidosperma species
Of the five other Rytidosperma entities analysed, constancy was maintained in four (R. auriculatum, R. caespitosum, R. carphoides, R. erianthum–R. monticola), whereas relative abundance was maintained only in R. auriculatum. The other three declined significantly over time (Fig. 3). One species, R. pilosum, declined in both constancy and dominance, most dramatically after the first survey in 2005 (Fig. 3). There was greater persistence on upper slopes (open) and lower slopes (open and tree), but not in any mid-slope habitats.
Infrequent species
Although no analyses could be performed on five species, the data suggest increasing presence of R. tenuius and persistence of the other four species (Table 2).
Replacement dominants
‘Cryptic Rytidosperma’ declined in dominance ranking in 74% of the plots where it was initially a dominant (i.e. a biomass ranking of 1, 2 or 3) in 2005. It did not become more dominant in any plot (Table S2). Dominance was greatest in the open grassland on the upper slopes, where R. erianthum–R. monticola, R. auriculatum, R. carphoides and R. pilosum formed short swards. These areas also tended to retain these species as a dominant over the 15 years (Fig. 4), with the exception of R. pilosum, which had disappeared from the upper slopes by 2020.
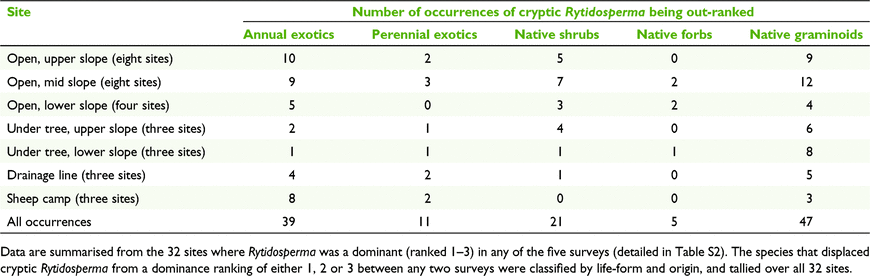
Where dominance was lost, ‘cryptic Rytidosperma’ was most frequently displaced or outgrown by three life-forms (Table S2, 6):
-
Native perennial graminoids – e.g. Lomandra filiformis (21 plots), Aristida ramosa (5 plots),
-
Annual exotics – e.g. Trifolium spp. (8 plots), Briza maxima (7 plots), Vulpia spp. (5 plots), Hypochoeris glabra (4 plots), Aira spp. (4 plots).
-
Native shrubs – e.g. Melichrus urceolatus (7 plots), various Fabaceae (12 plots).
|
Although the ‘cryptic Rytidosperma’ species tended to retain their constancy in the plots, there is evidence of decline that is both relative (as assessed by ranking) and absolute. There was an obvious reduction in plant numbers observed when sampling in the field, consistent with the reduced number of flowering plants available for sampling (Table 7), particularly in 2013 (the driest sampling year) and 2016 (the wettest sampling year, Fig. 1).

|
Discussion
Regional representation
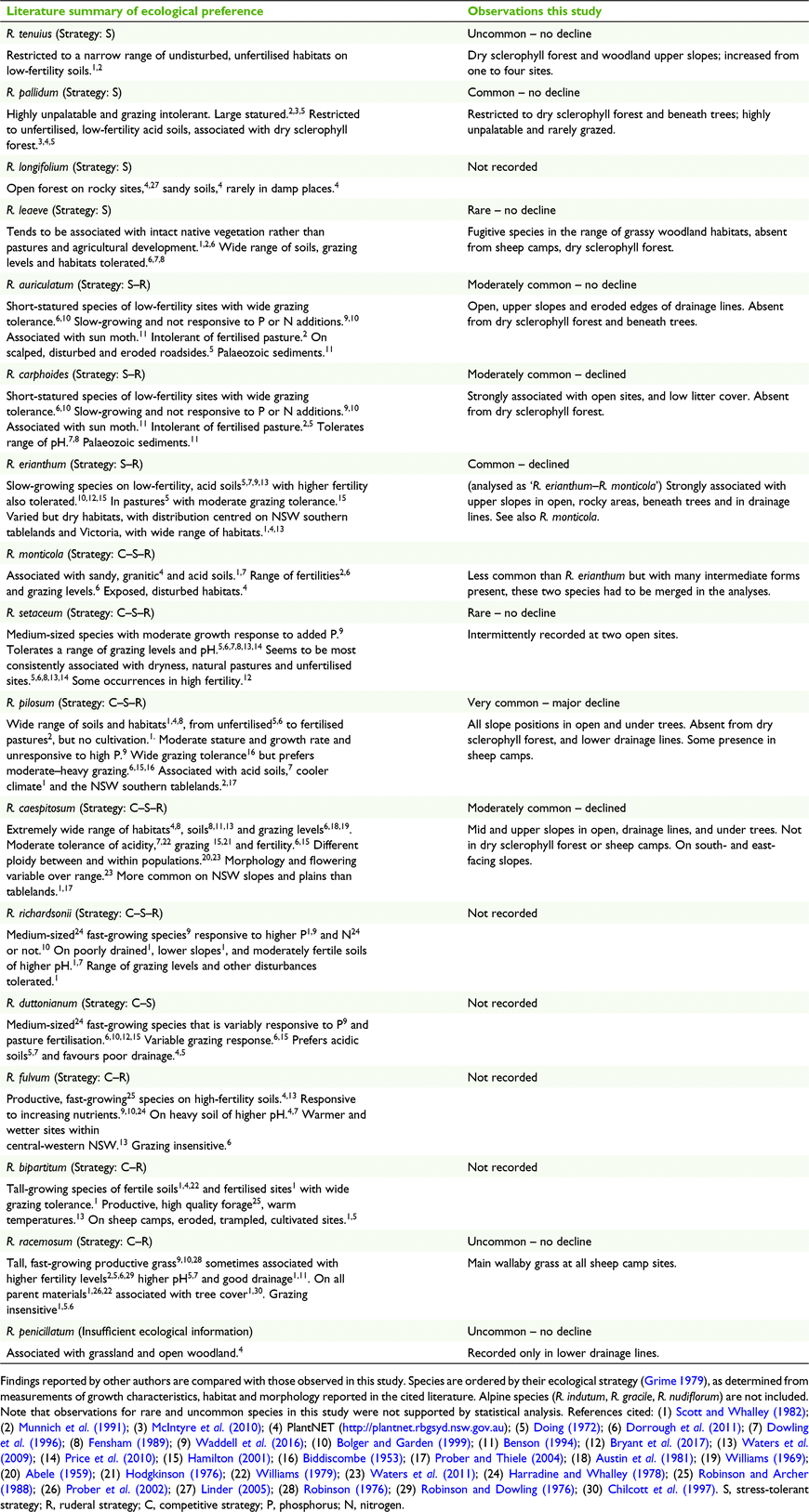
Of the 17 lowland Rytidosperma species occurring on the southern tablelands, 12 were identified in this study (Table 8). These are the same 12 species recorded in a survey of 1300 ha with a similar management history, i.e. long-term livestock grazing with limited pasture improvement (McIntyre et al. 2010). These two sites are 20 km distant and share vegetation types (a mosaic of southern tableland grassy woodland and sclerophyll forest). In a survey of 68 natural and improved pastures in an adjacent district centred 50 km NE of the study site, Munnich et al. (1991) recorded nine species of Rytidosperma, representing a subset of those reported here. Relative abundances were comparable to those at our study site, with the exception of our common R. erianthum not being recorded by Munnich et al., whereas R. monticola was frequent. Difficulties delineating these species have been experienced by other researchers in the region (D. L. Garden, pers. comm.) and it is possible that R. erianthum material was differently interpreted by Munnich et al.
|
Ecological differentiation within the genus
Within the study site, the variables ‘habitat’ and ‘slope position’ represented a readily discernible categorisation of the landscape, integrating environmental (soil attributes, soil moisture) and biotic factors (nutrients relocated by livestock, eucalypt presence and identity). On average, one or both of these variables accounted for 38% of the variation in the grasses analysed (range 20–58%; Tables 3, 4). The strongest differentiation was between the sclerophyll forest (dominated by R. pallidum on all slope positions) and the remaining habitats (camp, drainage, open, tree). The latter habitats represented variations within the grassy woodland vegetation type, and supported most of the ‘cryptic Rytidosperma’ populations. Within the grassy formation, we detected subtle patterns of habitat preference among individual species.
Aspect was significant for the ‘cryptic Rytidosperma’ group, and one species (R. caespitosum) demonstrated a preference for protected eastern- and southern-facing slopes. Few ground-cover variables proved important once habitat and slope position were taken into account. Open sites on upper slopes had the highest proportion of rock cover and lowest litter cover, but ‘cryptic Rytidosperma’ and ‘R. erianthum–R. monticola’ were positively associated with rock cover within those sites. Although R. carphoides differed from the group broadly in not being significantly associated with upper slopes, it was linked to the low litter cover that is characteristic of the open upper slope habitats. On the whole, tree canopies were not a favoured habitat, although on the less protected upper slopes, ‘cryptic Rytidosperma’ was as abundant under the widely scattered trees as in the open (Fig. 2). Only R. caespitosum and R. pilosum tolerated tree canopies on the mid and or lower slopes (Table 5, Fig. 2).
Synthesis of published observations of Rytidosperma growth rates and habitat through the C–S–R strategy framework (Grime 1979; Table 8) suggests that the species of the southern tablelands cover one primary strategy, stress tolerance (e.g. R. pallidum), as well as the four secondary strategies (S–R, C–R, C–S, C–S–R). Competitive ruderals (C–R) are fast-growing, grazing-tolerant species of productive habitats and were absent from the study site, with the exception of R. racemosum, which was uncommon, and largely restricted to remnant sheep camps. The species hypothesised to have the S–R and C–S–R strategies, based on published information of stature, growth rates, grazing tolerance and habitat productivity, were the most common species in our study (Table 8). These largely matched our own data, in which the S–R species occupied the most stressed sites (open, upper slopes, low litter cover, rocky), and the putative C–S–R species extended further into the more productive mid and lower slopes. Three of the southern tableland species ‘missing’ from our survey are reported in the literature to be associated with fertile soils of generally high pH (R. fulvum, R. bipartitum and R. richardsonii; Table 8), a sufficient reason for their absence from the predominantly infertile, acidic soils of the study site.
Decline and persistence
The relatively heavy sheep grazing on the study site in the 25 years preceding livestock removal would have been favourable to Rytidosperma generally (Langford et al. 2004; Dorrough et al. 2011), as reflected in the dominance of ‘cryptic Rytidosperma’ in the first 2005 survey, when it was ranked 1, 2 or 3 in 52% of the grassy woodland sites. By 2020, this proportion was reduced to 12%. This loss of dominance was also evident as significant declines in percentage of total biomass for ‘cryptic Rytidosperma’, R. caespitosum, R. carphoides, R. pilosum and ‘R. erianthum–R. monticola’. Nonetheless, presence in the plots (constancy) did not decline, except in the case of R. pilosum (Fig. 3). A ranking of less than three indicates an average representation of less than 3% of the total biomass in grassy vegetation (Scott 1986), so that regardless of the variation in total biomass over the survey period, these changes in rankings represent proportionately very large changes in abundance. That the populations in the study declined over the 15 years was very evident from ongoing and frequent field observations, and was supported by the reduced availability of flowering individuals in the 2013 and 2016 surveys (Table 8). We attribute the proportional decline (percentage total biomass) to the growth of native shrubs and native perennial graminoids, and pulses of annual exotic growth in wet seasons (as noted by Langford et al. 2004), and conclude that these were having a competitive effect on the reproductive output of Rytidosperma.
When macropod-only grazing replaced sheep and macropod grazing after 2004, the grazing pressure became lighter and the following broad changes could be expected: reduced trampling (Albon et al. 2007), a reduction in the pool of rapidly recycled nutrients (Ruess and McNaughton 1987), and a shift in species abundance in favour of more stress tolerance and its related conservative leaf economics, expressed as higher leaf dry matter content or reduced specific leaf area (McIntyre 2008; Pierce et al. 2017). These trends were evident in the reduced dominance of ‘cryptic Rytidosperma’ with S–R and C–S–R strategies, the lack of decline in the stress tolerators (S strategy, including R. pallidum) and the increasing dominance of species with high leaf dry matter content (LDMC), most notably Lomandra filiformis, Aristida ramosa, Melichrus urceolata, and Fabaceae sub-shrubs (see McIntyre 2008). These represent typical strategies for the site overall, whereas local-scale factors such as disturbance, fine-scale soil patterns, and biotic interactions enable a range of species with different traits to co-exist (Bruelheide et al. 2018). For example, the competitive ruderal species R. racemosum persisted in sheep camps, where historical nutrient inputs of sheep dung and urine remained high.
Implications for conservation
The removal of livestock in the interests of biodiversity conservation has resulted in the increased representation of native shrubs and graminoids in the ground layer at the study site. A wider survey of multiple sites under different land uses in the district suggests that this is a general response that could be expected if fertiliser inputs cease and grazing is removed (McIntyre 2008). This increase in structural diversity of the ground layer both reflects and promotes more varied grazing intensities spatially, which has benefits for both faunal and plant diversity (McIntyre et al. 2003; Howland et al. 2014, 2016).
With their tolerance for grazing and drought, Rytidosperma spp. overall appear robust and able to persist in the wider landscape, providing existing natural, low-input pastures are not replaced by cultivation and heavy fertiliser use (e.g. see McIntyre 2008). In that sense, their decline under conservation land use may not be an issue. However, we have shown that conservation management may disadvantage some short-statured species with a grazing requirement, including species with which the endangered golden sun moth has been associated (R. erianthum, R. auriculatum, R, carphoides, R. setaceum; O’Dwyer and Attiwill 1999). In our study, these species occurred on open sites in the upper slopes, the habitat in which Rytidosperma dominance was best retained. We attribute this to the preferential use of this part of the landscape by eastern grey kangaroos who have largely maintained these areas as grazing lawns over the 15 years (Fig. 4). In other conservation settings, grass, tree and shrub encroachment may have a greater impact on these short-statured species when livestock are removed. Managers aiming to conserve golden sun moth populations in reserves need to be aware of the grazing requirements of the food plants that nurture the moth larvae, and manage total grazing appropriately, whether it be macropod, livestock or feral species.
Two species reported in our study (R. tenuius and R. penicillatum; Table 8) are of unknown conservation status. Despite wide geographical ranges, they are not widely reported in the literature (Table 8) and appear to have somewhat restricted habitats. Without random field collection and subsequent microscopic examination, they would not have been detected at the study site or recorded in the surveys. Our method of sampling inflorescences, and determining the proportions of the different species present, could be applied to any method in which Rytidosperma abundance is collectively assessed. It is based on the assumption that flowering activity is proportionate to the abundance of that species which, while not ideal, represents a significant advance on the more common practice of grouping several disparate species into a single category.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors thank Denys Garden and David Mallinson for assistance with plant identifications.
References
Abele K (1959) Cytological studies in the genus Danthonia. Transactions of the Royal Society of South Australia 82, 163–173.Albon SD, Brewer MJ, O’Brien S, Nolan AJ, Cope D (2007) Quantifying the grazing impacts associated with different herbivores on rangelands. Journal of Applied Ecology 44, 1176–1187.
| Quantifying the grazing impacts associated with different herbivores on rangelands.Crossref | GoogleScholarGoogle Scholar |
Archer KA, Robinson GG (1988) Agronomic potential of native grass species on the northern tablelands of New-South Wales. II. Nutritive value. Australian Journal of Agricultural Research 39, 425–436.
| Agronomic potential of native grass species on the northern tablelands of New-South Wales. II. Nutritive value.Crossref | GoogleScholarGoogle Scholar |
Austin MP, Williams OB, Belbin L (1981) Grassland dynamics under sheep grazing in an Australian Mediterranean type climate. Vegetatio 46–47, 201–211.
| Grassland dynamics under sheep grazing in an Australian Mediterranean type climate.Crossref | GoogleScholarGoogle Scholar |
Benson JS (1994) The native grasslands of the Monaro region: Southern Tablelands of NSW. Cunninghamia 3, 609–650.
Biddiscombe EF (1953) A survey of the natural pastures of the Trangie District, New South Wales, with particular reference to the grazing factor. Australian Journal of Agricultural Research 4, 1–28.
| A survey of the natural pastures of the Trangie District, New South Wales, with particular reference to the grazing factor.Crossref | GoogleScholarGoogle Scholar |
Bolger TP, Garden DL (1999) Nutrient responses of wallaby grass (Danthonia spp.) from the New South Wales Tablelands. In ‘People and Rangelands: building the future. Proceedings of the VIth international rangeland congress’, 17–23 July 1999, Townsville, Qld, Australia. (Eds DJ Eldridge, D Freudenberger) pp. 269–271. (International Rangeland Society: Townsville, Qld, Australia)
Brock RD, Brown JAM (1961) Cytotaxonomy of Australian Danthonia. Australian Journal of Botany 9, 62–91.
| Cytotaxonomy of Australian Danthonia.Crossref | GoogleScholarGoogle Scholar |
Bruelheide H, Dengler J, Purschke O, et al. (2018) Global trait–environment relationships of plant communities. Nature Ecology & Evolution 2, 1906–1917.
| Global trait–environment relationships of plant communities.Crossref | GoogleScholarGoogle Scholar |
Bryant D, Bruce MJ, Sinclair SJ (2017) Observations of responses to re-introducing fire in a Basalt Plains grassland after the removal of grazing: Implications for restoration. Ecological Management & Restoration 18, 239–245.
| Observations of responses to re-introducing fire in a Basalt Plains grassland after the removal of grazing: Implications for restoration.Crossref | GoogleScholarGoogle Scholar |
Chilcott C, Reid NCH, King K (1997) Impact of trees on the diversity of pasture species and soil biota in grazed landscapes on the Northern Tablelands, NSW. In ‘Conservation outside nature reserves’. (Eds P Hale, D Lamb) pp. 378–386. (Centre for Conservation Biology, University of Queensland)
Doing H (1972) Botanical composition of pasture and weed communities in the Southern Tableland region, south-eastern Australia. CSIRO Division of Plant Industry technical paper no. 30, Melbourne.
Dorrough J, McIntyre S, Scroggie MP (2011) Individual plant species responses to phosphorus and livestock grazing. Australian Journal of Botany 59, 670–681.
| Individual plant species responses to phosphorus and livestock grazing.Crossref | GoogleScholarGoogle Scholar |
Dowling PM, Garden DL, Eddy DA, Pickering DI (1996) Effect of soil pH on the distribution of Danthonia species on the tablelands of central and southern New South Wales. New Zealand Journal of Agricultural Research 39, 619–621.
| Effect of soil pH on the distribution of Danthonia species on the tablelands of central and southern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Eyles RJ (1977) Changes in drainage networks since 1820, Southern Tablelands, N.S.W. The Australian Geographer 13, 377–386.
| Changes in drainage networks since 1820, Southern Tablelands, N.S.W.Crossref | GoogleScholarGoogle Scholar |
Fensham RJ (1989) The pre-european vegetation of the Midlands, Tasmania: a floristic and historical analysis of vegetation patterns. Journal of Biogeography 16, 29–45.
| The pre-european vegetation of the Midlands, Tasmania: a floristic and historical analysis of vegetation patterns.Crossref | GoogleScholarGoogle Scholar |
Garden DL, Dowling PM, Eddy DA, Nicol HI (2001) The influence of climate, soil, and management on the composition of native grass pastures on the central, southern, and Monaro tablelands of New South Wales. Australian Journal of Agricultural Research 52, 925–936.
| The influence of climate, soil, and management on the composition of native grass pastures on the central, southern, and Monaro tablelands of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Garden DL, Lodge GM, Friend DA, Dowling PM, Orchard BA (2000) Effects of grazing management on botanical composition of native grass-based pastures in temperate south-east Australia. Australian Journal of Experimental Agriculture 40, 225–245.
| Effects of grazing management on botanical composition of native grass-based pastures in temperate south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Grime JP (1979) ‘Plant strategies and vegetation processes.’ (Wiley: Chichester, UK)
Hamilton SD (2001) Impacts of agricultural land use on the floristics, diversity and life-form composition of a temperate grassy woodland. Pacific Conservation Biology 7, 169–184.
| Impacts of agricultural land use on the floristics, diversity and life-form composition of a temperate grassy woodland.Crossref | GoogleScholarGoogle Scholar |
Harradine AR, Whalley RDB (1978) Nitrogen responses of seedlings of Aristida ramosa R.Br. and Danthonia DC. spp. Australian Journal of Agricultural Research 29, 759–772.
| Nitrogen responses of seedlings of Aristida ramosa R.Br. and Danthonia DC. spp.Crossref | GoogleScholarGoogle Scholar |
Hodgkinson KC (1976) The effects of frequency and extent of defoliation, summer irrigation, and fertilizer on the production and survival of Danthonia caespitosa Gaud. Australian Journal of Agricultural Research 27, 755–767.
| The effects of frequency and extent of defoliation, summer irrigation, and fertilizer on the production and survival of Danthonia caespitosa Gaud.Crossref | GoogleScholarGoogle Scholar |
Howland B, Stojanovic D, Gordon IJ, Manning AD, Fletcher D, Lindenmayer DB (2014) Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands. PLoS ONE 9, e105966
| Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands.Crossref | GoogleScholarGoogle Scholar | 25501680PubMed |
Howland BWA, Stojanovic D, Gordon IJ, Radford J, Manning AD, Lindenmayer DB (2016) Birds of a feather flock together: using trait-groups to understand the effect of macropod grazing on birds in grassy habitats. Biological Conservation 194, 89–99.
| Birds of a feather flock together: using trait-groups to understand the effect of macropod grazing on birds in grassy habitats.Crossref | GoogleScholarGoogle Scholar |
Jenkins BR (2000) ‘Soil landscapes of the Canberra 1:100 000 sheet.’ (Department of Land and Water Conservation: Sydney, NSW, Australia)
Keith DA (2004) ‘Ocean shores to desert dunes: the native vegetation of the ACT and New South Wales.’ (Department of Environment and Conservation: Sydney, NSW, Australia)
Kutt AS, Dalton K, Wills TJ (2016) Identification of reliable predictors of golden sun moth Synemon plana habitat over multiple survey years can benefit conservation, restoration and surveys for new populations. Journal of Insect Conservation 20, 691–699.
| Identification of reliable predictors of golden sun moth Synemon plana habitat over multiple survey years can benefit conservation, restoration and surveys for new populations.Crossref | GoogleScholarGoogle Scholar |
Langford CM, Simpson PC, Garden DL, Eddy DA, Keys MJ, Rehwinkel R, Johnston WH (2004) ‘Managing native pastures for agriculture and conservation.’ (New South Wales Department of Primary Industries)
Linder HP (2005) Austrodanthonia, Poaceae 3. In ‘Flora of Australia. Volume 44B—Poaceae 3’. (Ed. K Mallett) pp. 45–63. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Linder PH, Baeza M, Barker NP, et al. (2010) A generic classification of the Danthonioideae (Poaceae). Annals of the Missouri Botanical Garden 97, 306–364.
| A generic classification of the Danthonioideae (Poaceae).Crossref | GoogleScholarGoogle Scholar |
Mannetje L, Haydock KP (1963) The dry-weight-rank method for the botanical analysis of pasture. Journal of the British Grassland Society 18, 268–275.
| The dry-weight-rank method for the botanical analysis of pasture.Crossref | GoogleScholarGoogle Scholar |
McIntyre S (2008) The role of plant leaf attributes in linking land use to ecosystem function and values in temperate grassy vegetation. Agriculture, Ecosystems & Environment 128, 251–258.
| The role of plant leaf attributes in linking land use to ecosystem function and values in temperate grassy vegetation.Crossref | GoogleScholarGoogle Scholar |
McIntyre S, Heard KM, Martin TG (2003) The relative importance of cattle grazing in subtropical grasslands: does it reduce or enhance plant biodiversity? Journal of Applied Ecology 40, 445–457.
| The relative importance of cattle grazing in subtropical grasslands: does it reduce or enhance plant biodiversity?Crossref | GoogleScholarGoogle Scholar |
McIntyre S, Stol J, Harvey J, Nicholls AO, Campbell M, Reid A, Manning AD, Lindenmayer D (2010) Biomass and floristic patterns in the ground layer vegetation of box-gum grassy eucalypt woodland in Goorooyarroo and Mulligans Flat Nature Reserves, Australian Capital Territory. Cunninghamia 11, 319–357.
McIntyre S, Tongway D (2005) Grassland structure in native pastures: links to soil surface condition. Ecological Management & Restoration 6, 43–50.
| Grassland structure in native pastures: links to soil surface condition.Crossref | GoogleScholarGoogle Scholar |
Munnich DJ, Simpson PC, Nicol HI (1991) A survey of native grasses in the Goulburn District and factors influencing their abundance. The Rangeland Journal 13, 118–129.
| A survey of native grasses in the Goulburn District and factors influencing their abundance.Crossref | GoogleScholarGoogle Scholar |
O’Dwyer C, Attiwill PM (1999) A comparative study of habitats of the Golden Sun Moth Synemon plana Walker (Lepidoptera: Castniidae): implications for restoration. Biological Conservation 89, 131–141.
| A comparative study of habitats of the Golden Sun Moth Synemon plana Walker (Lepidoptera: Castniidae): implications for restoration.Crossref | GoogleScholarGoogle Scholar |
Pierce S, Negreiros D, Cerabolini BEL, et al. (2017) A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Functional Ecology 31, 444–457.
| A global method for calculating plant CSR ecological strategies applied across biomes world-wide.Crossref | GoogleScholarGoogle Scholar |
Pirie MD, Humphreys AM, Barker NP, Linder HP (2009) Reticulation, data combination, and inferring evolutionary history: an example from Danthonioideae (Poaceae). Systematic Biology 58, 612–628.
| Reticulation, data combination, and inferring evolutionary history: an example from Danthonioideae (Poaceae).Crossref | GoogleScholarGoogle Scholar | 20525613PubMed |
Price JN, Wong NK, Morgan JW (2010) Recovery of understorey vegetation after release from a long history of sheep grazing in a herb-rich woodland. Austral Ecology 35, 505–514.
| Recovery of understorey vegetation after release from a long history of sheep grazing in a herb-rich woodland.Crossref | GoogleScholarGoogle Scholar |
Prober SM, Lunt ID, Thiele KR (2002) Determining reference conditions for management and restoration of temperate grassy woodlands: relationships among trees, topsoils and understorey flora in little-grazed remnants. Australian Journal of Botany 50, 687–697.
| Determining reference conditions for management and restoration of temperate grassy woodlands: relationships among trees, topsoils and understorey flora in little-grazed remnants.Crossref | GoogleScholarGoogle Scholar |
Prober SM, Thiele KR (2004) Floristic patterns along an east-west gradient in grassy box woodlands of Central New South Wales. Cunninghamia 8, 306–325.
Richter A, Osborne W, Hnatiuk S, Rowell A (2013) Moths in fragments: insights into the biology and ecology of the Australian endangered golden sun moth Synemon plana (Lepidoptera: Castniidae) in natural temperate and exotic grassland remnants. Journal of Insect Conservation 17, 1093–1104.
| Moths in fragments: insights into the biology and ecology of the Australian endangered golden sun moth Synemon plana (Lepidoptera: Castniidae) in natural temperate and exotic grassland remnants.Crossref | GoogleScholarGoogle Scholar |
Robinson GG (1976) Productivity and response to nitrogen fertilizer of the native grass Danthonia racemosa (wallaby grass). Australian Rangeland Journal 1, 49–52.
| Productivity and response to nitrogen fertilizer of the native grass Danthonia racemosa (wallaby grass).Crossref | GoogleScholarGoogle Scholar |
Robinson GG, Archer KA (1988) Agronomic potential of native grass species on the Northern Tablelands of New South Wales. I. Growth and herbage production. Australian Journal of Agricultural Research 39, 415–423.
| Agronomic potential of native grass species on the Northern Tablelands of New South Wales. I. Growth and herbage production.Crossref | GoogleScholarGoogle Scholar |
Robinson GG, Dowling PM (1976) Management of natural pastures on the Northern Tablelands of New South Wales – a survey. Australian Rangeland Journal 1, 70–74.
| Management of natural pastures on the Northern Tablelands of New South Wales – a survey.Crossref | GoogleScholarGoogle Scholar |
Robinson JB, Munnich DJ, Simpson PC, Orchard PW (1993) Pasture associations and their relation to environment and agronomy in the Goulburn District. Australian Journal of Botany 41, 627–636.
| Pasture associations and their relation to environment and agronomy in the Goulburn District.Crossref | GoogleScholarGoogle Scholar |
Ruess RW, McNaughton SJ (1987) Grazing and the dynamics of nutrient and energy regulated microbial processes in the Serengeti grasslands. Oikos 49, 101–110.
| Grazing and the dynamics of nutrient and energy regulated microbial processes in the Serengeti grasslands.Crossref | GoogleScholarGoogle Scholar |
Scott AW, Whalley RDB (1982) The distribution and abundance of species of Danthonia DC on the New England Tablelands (Australia). Australian Journal of Ecology 7, 239–248.
| The distribution and abundance of species of Danthonia DC on the New England Tablelands (Australia).Crossref | GoogleScholarGoogle Scholar |
Scott D (1986) Coefficients for the dry-weight rank method of botanical analysis of pasture. Grass and Forage Science 41, 319–321.
| Coefficients for the dry-weight rank method of botanical analysis of pasture.Crossref | GoogleScholarGoogle Scholar |
Waddell HA, Simpson RJ, Henderson B, Ryan MH, Lambers H, Garden DL, Richardson AE (2016) Differential growth response of Rytidosperma species (wallaby grass) to phosphorus application and its implications for grassland management. Grass and Forage Science 71, 245–258.
| Differential growth response of Rytidosperma species (wallaby grass) to phosphorus application and its implications for grassland management.Crossref | GoogleScholarGoogle Scholar |
Waters C, Melville G, Coates D, Virgona J, Young A, Hacker R (2011) Variation in morphological traits among and within populations of Austrodanthonia caespitosa (Gaudich.) H.P.Linder and four related species. Australian Journal of Botany 59, 324–335.
| Variation in morphological traits among and within populations of Austrodanthonia caespitosa (Gaudich.) H.P.Linder and four related species.Crossref | GoogleScholarGoogle Scholar |
Waters C, Murray BG, Melville G, Coates D, Young A, Virgona J (2010) Polyploidy and possible implications for the evolutionary history of some Australian Danthonieae. Australian Journal of Botany 58, 23–34.
| Polyploidy and possible implications for the evolutionary history of some Australian Danthonieae.Crossref | GoogleScholarGoogle Scholar |
Waters CM, Melville G, Jacobs S (2009) Association of five Austrodanthonia species (family Poaceae) with large and small scale environmental features in central western New South Wales. Cunninghamia 11, 65–80.
Whalley RDB, Robinson GG, Taylor JA (1978) General effects of management and grazing by domestic livestock on the rangelands of the Northern Tablelands of New South Wales. Australian Rangeland Journal 1, 174–190.
| General effects of management and grazing by domestic livestock on the rangelands of the Northern Tablelands of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Williams AR (1979) ‘A survey of natural pastures in the North-West Slopes of New South Wales.’ Technical Bulletin number 22. p. 36. (Department of Agriculture, New South Wales: Sydney, NSW, Australia)
Williams OB (1969) Studies in the ecology of the Riverine Plain V. Plant density response of species in a Danthonia caespitosa grassland to 16 years of grazing by Merino sheep. Australian Journal of Botany 17, 255–268.
| Studies in the ecology of the Riverine Plain V. Plant density response of species in a Danthonia caespitosa grassland to 16 years of grazing by Merino sheep.Crossref | GoogleScholarGoogle Scholar |