Collateral damage: epiphytic orchids at risk from myrtle rust
Heidi Zimmer A * , Mark Clements A , Endymion Cooper A , David Jones B , Robert Makinson C , Katharina Nargar D and Kristy Stevenson E
A * , Mark Clements A , Endymion Cooper A , David Jones B , Robert Makinson C , Katharina Nargar D and Kristy Stevenson E
A
B
C
D
E
Abstract
Epiphytic orchids rely on the habitat provided by their plant hosts to survive. The naturalisation of Austropuccinia psidii (G. Winter) Beenken (the causal agent for myrtle rust) in Australia means that some of these plant hosts, from the family Myrtaceae, are at risk of serious decline. We aimed to identify orchid species that associate with myrtaceous host plants and determine which, if any, might be susceptible to loss of habitat as a result of myrtle rust. We reviewed species descriptions and herbarium records and identified 73 epiphytic orchid species that are commonly found growing on myrtaceous hosts. At least seven orchid species are predominantly reliant on myrtaceous hosts, are distributed predominantly in the myrtle rust zone, and have host species that are highly or extremely susceptible to myrtle rust. Four of these orchid species are already listed as threatened. The impact of myrtle rust is broader than causing decline of Myrtaceae species, with knock-on effects on other biota, including epiphytic orchids. Moreover, there is the potential for further impact on these orchids through fragmentation (e.g. affecting pollination) and interactive effects with fire. Increased effort is required to identify the relative frequency of myrtaceous and non-myrtaceous hosts for these epiphytic orchid species, especially in relation to the compound effects of myrtle rust and other perturbations, such as fire and climate change. Where this is not possible, ex situ conservation may be required.
Keywords: Austropuccinia psidii, conservation, Durabaculum, Myrtaceae, Orchidaceae, threatened species.
Introduction
Epiphytes are often characterised as plants that are not rooted in the ground, and that grow on other plants for support (Benzing 2008; Zotz 2016). True epiphytes do not contact with host vasculature – although they may benefit from their host, via organic matter decomposition and leaching (Benzing 2008). For water and nutrients, epiphytes are reliant on atmospheric inputs (rain, mist) and organic matter deposition (Benzing 2008). Epiphytes constitute approximately 10% of the world’s vascular plant species richness (Zotz et al. 2021), and more than half of all vascular epiphytes are from the Orchidaceae (Benzing 2004). Araceae, Bromeliacae and several fern families are also well represented in the world epiphytic flora (Benzing 1987).
In line with the dominance of Orchidaceae in the epiphytic flora, most of the world’s orchid species are epiphytic (c. 70%; Givnish et al. 2015). Epiphytic orchids mainly occur in tropical regions, whereas terrestrial orchids are more common in temperate regions (Gravendeel et al. 2004). Australia’s vascular epiphytic flora is dominated by orchids and ferns (c. 40% each; Sanger 2016). Australia has 283 species of epiphytic orchids, which comprise 14% of Australia’s total orchid species richness (Jones 2021). This is relatively low by global standards, but is unsurprising given the desert, semi-arid and temperate climates that predominate in Australia (Peel et al. 2007). Nevertheless, approximately 72% of Australia’s epiphytic orchids are endemic, including many range-restricted and threatened species (Jones 2021).
The drivers of host bias and specificity in epiphytic orchids remain poorly understood, including in Australia. Although there appear to be many specific host–epiphyte relationships, this may be because of the microenvironment provided by the host, via bark characteristics or tree architecture, rather than the host species per se (Wagner et al. 2015). Specific relationships may be formed to facilitate mycorrhizal relationships, but this too remains uncertain (Gowland et al. 2013; Wagner et al. 2015). For example, Ai et al. (2023), in a study of orchids in Myanmar, identified 20 species of epiphytic orchids that appear to be associated with specific host tree species, although the mechanism for this association remains unclear. More common than obligate/exclusive relationships are records of orchid–host species bias or preference (Laube and Zotz 2006; Gowland et al. 2013).
Members of the Myrtaceae, such as Eucalyptus spp., are structural dominants in many Australian vegetation communities (Leishman et al. 2017) and, as such, are frequent hosts for epiphytes in Australia. Myrtaceae is one of the world’s largest plant families, with 126 genera and approximately 6000 species of trees and shrubs (POWO 2023). Myrtaceae has a strong southern hemisphere or Gondwanan distribution, and has centres of diversity in Australia, South America and Southeast Asia (Vasconcelos et al. 2017). The most speciose genera in the Myrtaceae are Eugenia s.l. and Myrcia s.l. and well-known myrtaceous species include Metrosideros excelsa (pōhutukawa), Pimenta dioica (allspice), Psidium guajava (guava), and Syzygium aromaticum (clove).
Myrtle rust is a fungal disease caused by Austropuccinia psidii (G. Winter) Beenken, which affects plants in the Myrtaceae family (Beenken 2017). Myrtle rust originated in South America and was detected in New South Wales (NSW), Australia, in 2010 (Carnegie et al. 2016). It has since spread along most of Australia’s eastern seaboard, to the Top End of the Northern Territory and the far north-east of Western Australia, to Norfolk Island (Hansard, cited in Makinson 2018), and to Lord Howe Island initially in 2016 (subsequently eradicated, Makinson 2018), but with a new incursion in 2023 (ABC News 2023). To date, Australia has only one strain of A. psidii, known as the ‘pandemic strain’ (Stewart et al. 2017). Myrtlerust attacks new growth, causing progressive defoliation and habit distortion, with dieback and death in highly susceptible species (from genera including Gossia, Rhodamnia, Melaleuca; Carnegie et al. 2016; Makinson 2018). There is deep concern about the potential impact of myrtle rust in Australia, not only because of species declines and the importance of Myrtaceae in Australia’s natural environment, but also for its potential to impact Myrtaceae-based industries (e.g. cut flower, essential oil, forestry and nursery) and cause losses of future biological and genetic resources (Cannon 2011; Carnegie et al. 2016; Carnegie and Pegg 2018; Makinson 2018).
Epiphytes are physically dependent on their hosts (Zotz 2016). Potentially important host attributes, specifically bark characteristics, leaf and bark chemistry, tree architecture (diameter distribution of branches and leaf density), tree size at maturity and tree longevity (Wagner et al. 2015), may be influenced by myrtle rust. For example, myrtle rust-induced defoliation can cause changes to canopy transparency (Carnegie et al. 2016; Fernandez-Winzer et al. 2020) and hence could impact the light/shade and temperature of orchid microhabitat. Deposition of leaf litter may also cause large increases in organic matter and nutrients in the orchid microhabitat. In addition, host dieback is likely to result in changes to the bark characteristics, tree architecture and, if fatal, tree longevity.
The aim of this study was to review key literature and herbarium records to identify which Australian epiphytic orchids are associated with Myrtaceae species, and which orchids may be at risk of decline due to myrtle rust impacts on their host.
Methods
First we reviewed key literature, encompassing information on epiphytic orchids and myrtle rust on a national scale (i.e. Makinson 2018; Jones 2021) to identify general statements (cf. individual records) of hosts of epiphytic orchid species, identifying records mentioning orchids ‘growing on’ or ‘hosts’.
Next, we downloaded herbarium records from the Australasian Virtual Herbarium (AVH; https://avh.chah.org.au/) for 63 Australian epiphytic orchid genera totalling 21 741 records. We then searched these records for those that contained information about the host species (or genus) that the epiphytic orchid was growing on. Data were filtered by key search terms indicating a host/epiphyte relationship, such as ‘on’ or ‘host’ within the ‘habitat’ and ‘occurrence remarks’ fields, resulting in 7636 records. These records were then manually sorted into those that included a host genus and/or species, a total of 1562 records – the remainder of records were either not specific about host identity, or described a non-plant substrate (e.g. rock). Records were then sorted in to those describing a myrtaceous or a non-myrtaceous host, 563 and 999 records respectively. We then further subset the orchid record data to include only species with three or more records with a specific host given. Myrtle rust susceptibility ratings for myrtaceous host species were from Pegg et al. (2014, 2018) and range from extremely susceptible to relatively tolerant. Myrtaceous host species without ratings in Pegg et al. (2014, 2018), or records where only host genus (not species) were given, were recorded as data deficient. To enable us to proceed with our identification orchids at risk from myrtle rust, despite uncertainties around host susceptibility, our classification system considers the percent of orchid records recorded on myrtaceous hosts, in addition to host susceptibility.
In order to identify the epiphytic orchid species most at risk from the impacts of myrtle rust, we divided the records into three groups: (1) epiphytic orchids that are predominantly or exclusively reliant on myrtaceous hosts, with at least one host rated as highly or extremely susceptible, and with the majority of the orchid distribution within the myrtle rust zone; (2) epiphytic orchids that are largely reliant on myrtaceous hosts, and with the majority of the orchid distribution within the myrtle rust zone; (3) epiphytic orchids for which myrtaceous species constitute the minority of hosts, but still with the majority of the orchid distribution in the myrtle rust zone (Table 1). Table 1 includes species names as given in the literature and herbarium records, with names currently accepted by the Australian Plant Census (https://biodiversity.org.au/nsl/) highlighted. We have included the orchid names outlined by Jones (2021) in the results section because they allow inference about smaller groups of orchid species (often with similar ecology) that are, in other taxonomies, incorporated within larger and more diverse genera.
Orchid species | Myrtaceous host list (literature review) | Myrtaceous host list (AVH data) | N records with specific hosts | % myrtaceous hosts | At least one host is rated and has rating wholly or partly in HS or ES | Orchid distribution wholly or substantially in myrtle rust zone | Threatened species listing status (jurisdiction: category) | |
|---|---|---|---|---|---|---|---|---|
Group 1 – Orchids that are: • Predominantly (or entirely) reliant on myrtaceous hosts (>80% records), • With majority of distribution in myrtle rust zone, and • With at least one host rated highly or extremely susceptible. | ||||||||
Durabaculum canaliculatum (syn. Dendrobium canaliculatum*) | Melaleuca viridiflora* (HS) (1,2) | Corymbia sp., Melaleuca sp., Melaleuca dealbata* (DD), Melaleuca leucadendra* (RT-HS), Melaleuca quinqinervia* (RT-ES), Melaleuca saligna* (MS), Melaleuca stenostachya* (DD), Melaleuca viridiflora* (HS) | 31 | 100% | Yes | Yes | ||
Durabaculum carronii (syn. Dendrobium carronii*) | Melaleuca viridiflora* (HS) (1,2) | Melaleuca sp., Melaleuca viridiflora* (HS), Lophostemon suaveolens* (RT) (syn. Tristania suaveolens) | 7 | 86% | Yes | Yes | EPBC Act: VU | |
Durabaculum fellowsii (syn. Dendrobium fellowsii*) | Melaleuca spp. (1) | Corymbia abergiana* (DD) (syn. Eucalyptus abergiana), Eucalyptus crebra* (DD) (syn. Eucalyptus drepanophylla), Eucalyptus sp., Leptospermum sp., Lophostemon sp., Syncarpia sp. | 8 | 100% | DD | Yes | Qld: VU | |
Durabaculum foelschei (syn. Dendrobium foelschei*) | Melaleuca spp. (1,2) | Melaleuca acacioides* (DD), Melaleuca cajuputi* (DD), Melaleuca sp., Melaleuca leucadendra* (RT-HS), Melaleuca viridiflora* (HS) | 18 | 94% | Yes | Yes | ||
Durabaculum johannis (syn. Dendrobium johannis*) | Melaleuca spp. (1,2) | Corymbia sp. (as Bloodwood), Eucalyptus chlorophylla (DD), Leptospermum sp., Lophostemon suaveolens* (RT), Melaleuca diosmifolia (DD) (syn. Melaleuca foliosa, Melaleuca leucadendra* (RT-HS), Melaleuca sp., Melaleuca stenostachya* (DD), Melaleuca viridiflora* (HS). | 43 | 98% | Yes | Yes | EPBC Act: VU | |
Durabaculum tattonianum (syn. Dendrobium tattonianum*) | Melaleuca spp. (1), Melaleuca viridiflora* (HS) (2) | – | LR | Yes | Yes | |||
Thelychiton melaleucaphilus (syn. Dendrobium melaleucaphilum*) | Paperbark Melaleucas, particularly Melaleuca styphelioides* (DD) (1,2); Archirhodomyrtus beckleri* (HS), Backhousia leptopetala* (syn. Choricarpia leptopetala (DD) (2) | Backhousia myrtifolia* (RT-MS), Melaleuca sp., Melaleuca styphelioides* (DD) | 16 | 81% | Yes | Yes | NSW: EN | |
Group 2 – Orchids which are • Largely reliant on myrtaceous hosts (>50% herbarium records) •With majority of distribution in myrtle rust zone | ||||||||
Cadetia maideniana* | Syzygium bamagense* (MS), Xanthostemon chrysanthus* (RT-MS) | 7 | 100% | No | Yes | |||
Cadetia taylorii * | Leptospermum wooroonooran* (DD), Xanthostemon chrysanthus* (RT-MS) | 5 | 80% | No/DD | Yes | |||
Cestichis reflexa (syn. Liparis reflexa*) | Tristaniopsis laurina* (RT), Backhousia sp. | 4 | 100% | No/DD | Yes | |||
Cymbidium canaliculatum* | Corymbia spp. Eucalyptus spp. Angophora spp. (1) | Corymbia abergiana* (DD), Corymbia bella* (DD), Corymbia bleeseri* (DD), Corymbia citriodora* (DD), Corymbia confertiflora* (DD), Corymbia foelscheana* (DD), Corymbia papuana* (DD), Corymbia pocillum* (DD), Corymbia sp., Eucalyptus bigalerita* (DD), Eucalyptus caleyi* (DD), Eucalyptus camaldulensis* (DD), Eucalyptus cambageana* (DD), Eucalyptus clavigera (syn. Corymbia clavigera*) (DD), Eucalyptus conica* (DD), Eucalyptus cullenii* (DD), Eucalyptus leptophleba* (DD), Eucalyptus microtheca* (DD), Eucalyptus paniculata* (DD), Eucalyptus platyphylla* (DD), Eucalyptus polycarpa (DD) (syn. Corymbia polycarpa*), Eucalyptus populnea* (DD), Eucalyptus sp., Eucalyptus tectifica* (DD), Melaleuca cajuputi* (DD), Melaleuca sp. | 89 | 85% | DD | Yes | ||
Cymbidium suave* | Eucalyptus spp., Melaleuca spp. (1) | Eucalyptus sp. (as Ironbark), Eucalyptus sp., Eucalyptus maculata (DD) (syn. Corymbia maculata*), Eucalyptus crebra* (DD) (syn. Eucalyptus drepanophylla), Corymbia sp. (as Bloodwood). | 16 | 56% | DD | Yes | ||
Durabaculum trilamellatum (syn. Dendrobium semifuscum) | Melaleuca spp. (1,2) | – | LR | DD | Yes | |||
Grastidium tozerense* | Melaleuca sp., Tristaniopsis sp. | 3 | 67% | DD | Yes | Qld: VU | ||
Luisia atacta* | Syzygium forte* (RT) (syn. Syzygium rubiginosum), | 4 | 75% | No/DD | Yes | |||
Oxysepala shepherdii (syn. Bulbophyllum shepherdii*) | Backhousia myrtifolia* (RT-MS), Backhousia sp., Melaleuca styphelioides* (DD) | 7 | 57% | No/DD | Yes | |||
Peristeranthus hillii* | Melaleuca styphelioides* (DD), Syzygium luehmannii* (MS) | 5 | 60% | No/DD | Yes | NSW: VU | ||
Plectorrhiza beckleri* | Callistemon sp. (1), myrtaceous trees in rainforest and other vegetation (2) | Syzygium sp., Tristaniopsis sp., Tristaniopsis laurina* (RT) | 4 | 75% | No/DD | Yes | ||
Plectorrhiza tridentata* | Backhousia myrtifolia* (RT-MS), Backhousia sp., Syzygium smithii* (RT-MS), Melaleuca styphelioides* (DD), Syncarpia glomulifera* (DD), Tristaniopsis laurina* (RT) (syn. Tristania laurina), Tristaniopsis sp. | 38 | 53% | No/DD | Yes | |||
Pomatocalpa macphersonii* | Melaleuca sp. | 3 | 67% | DD | Yes | |||
Sarcochilus hillii* (including Sarcochilus minutiflos) | Backhousia myrtifolia* (RT-MS) (1); Backhousia sciadophora (2) | Backhousia myrtifolia* (RT-MS), Backhousia sp., Callistemon viminalis* (MS-HS), Tristaniopsis laurina* (RT), Tristaniopsis sp. | 26 | 65% | DD/No | Yes | ||
Stilbophyllum toressae (syn. Dockrillia toressae*) | Callistemon viminalis* (MS-HS), Leptospermum sp. | 3 | 67% | Yes | Yes | |||
Thelychiton moorei (syn. Dendrobium moorei*) | Leptospermum polygalifolium* (DD) | 3 | 67% | DD | Yes (Lord Howe Island) | |||
Thelychiton tetragonus (syn. Dendrobium tetragonum*) | Backhousia myrtifolia* (RT-MS), Tristaniopsis laurina* (RT) (1) | Backhousia myrtifolia* (RT), Melaleuca sp., Melaleuca styphelioides* (DD), Syzygium australe* (RT-MS) | 22 | 59% | No/DD | Yes | ||
Trachyrhizum agrostophyllum (syn. Dendrobium agrostophyllum*) | Callistemon spp., Leptospermum spp. (1) | Leptospermum amboinense* (DD), Lophostemon suaveolens* (RT), Syncarpia sp. (possible syn. Metrosideros sp.), Syncarpia glomulifera* (DD) | 8 | 75% | No/DD | Yes | ||
Tropilis aemula (syn. Dendrobium aemulum*) | Eucalyptus crebra* (DD) (1,2), Eucalyptus paniculata* (DD) (2) | Backhousia myrtifolia* (RT-MS), Eucalyptus sp. (Ironbark), (syn. Eucalyptus drepanophylla (DD) (syn. Eucalyptus crebra*), Eucalyptus fergusonii (DD) (syn. Eucalyptus paniculata*), Eucalyptus siderophloia* (DD), Lophostemon confertus* (DD) (syn. Tristania conferta), Tristaniopsis sp. | 75 | 75% | No/DD | Yes | ||
Tropilis eungellensis | Eucalyptus drepanophylla (syn. Eucalyptus crebra*) (DD) (1,2) | – | LR | DD | Yes | |||
Tropilis radiata | Lophostemon confertus* (DD) (1,2) | – | LR | DD | Yes | |||
Group 3 – Orchids which • Are recorded as having myrtaceous hosts (<50% records) • With majority of distribution in myrtle rust zone | ||||||||
Acriopsis emarginata* | Melaleuca spp., Eucalyptus robusta* (DD) (1) | – | LR | DD | Yes | |||
Adelopetalum exiguum (syn. Bulbophyllum exiguum*) | Backhousia myrtifolia* (RT-MS), Metrosideros sp. | 25 | 24% | No/DD | Yes | |||
Adelopetalum lilianiae (syn. Bulbophyllum lilianiae*) | Backhousia sp. | 4 | 25% | DD | Yes | |||
Australorchis monophyla (syn. Dendrobium monophylum*) | Melaleuca sp. | 10 | 10% | DD | Yes | |||
Blepharochilum macphersonii (syn. Bulbophyllum macphersonii*) | Syzygium sp. | 5 | 20% | DD | Yes | |||
Bulbophyllum pygmaeum | Metrosideros excelsa* (DD), Metrosideros robusta (DD), Metrosideros sp. | 14 | 29% | DD | New Zealand | |||
Coelandria smillieae (syn. Dendrobium smillieae*) | Corymbia sp., Eucalyptus sp. (Ironbark), Melaleuca sp. or Leptospermum sp. (as tea tree), Syzygium bamagense* (MS) | 11 | 36% | No/DD | Yes | |||
Cymbidium madidum* | Melaleuca sp. | 10 | 30% | DD | Yes | |||
Dockrillia calamiformis* | Syzygium tierneyanum* (RT) | 4 | 25% | No | Yes | |||
Dockrillia bowmanii* | Leptospermum sp., Lophostemon confertus* (DD), Melaleuca styphelioides* (DD) | 17 | 47% | DD | Yes | |||
Dockrillia cucumerina* | Backhousia myrtifolia* (RT-MS) (1) | Backhousia myrtifolia* (RT-MS) | 17 | 6% | No | Yes | ||
Dockrillia dolichophylla* | Lophostemon confertus* (DD) | 6 | 17 | DD | Yes | |||
Dockrillia linguiformis* | Backhousia myrtifolia* (RT-MS), Corymbia tessellaris* (DD), Leptospermum wooroonooran* (DD), Lophostemon confertus* (DD) | 28 | 39% | No/DD | Yes | |||
Dockrillia pugioniformis* | Backhousia myrtifolia* (RT-MS), Backhousia sp., Melaleuca acacioides* (DD) | 20 | 20% | No/DD | Yes | |||
Dockrillia rigida* | Melaleuca spp. (1) | Melaleuca acacioides* (DD), Syzygium bamagense* (MS) | 6 | 33% | No/DD | Yes | ||
Dockrillia schoenina* | Backhousia sciadophora* (RT), Melaleuca styphelioides* (DD) | 10 | 40% | No/DD | Yes | |||
Dockrillia sulphurea* | Welchiodendron sp. | 5 | 40% | DD | Yes | |||
Dockrillia teretifolia* | Melaleuca sp., Melaleuca styphelioides* (DD), Syzygium tierneyanum* (RT) | 47 | 6% | No/DD | Yes | |||
Drymoanthus adversus | Leptospermum scoparium* (DD), Metrosideros sp. | 18 | 11% | DD | New Zealand | |||
Durabaculum bigibbum (syn. Dendrobium bigibbum*). These records likely include Durabaculum phalaenopsis, which is a separate species (Jones 2021), but not differentiated in the herbarium records). | Melaleuca spp. (1) | Corymbia clarksoniana* (DD), Eucalyptus sp., Eugenia reinwardtiana* (ES), Lophostemon sp., Melaleuca leucadendra* (RT-HS), Melaleuca sp. | 29 | 45% | Yes | Yes | EPBC Act: VU | |
Durabaculum dicuphum (syn. Dendrobium dicuphum*) | Melaleuca spp. (1) | Eucalyptus tectifica* (DD) (syn. Eucalyptus spenceriana), Melaleuca acacioides* (DD), Melaleuca cajuputi* (DD), Melaleuca leucadendra* (RT-HS), Melaleuca nervosa* (HS), Melaleuca sp., Syzygium minutuliflorum* (RT), Tristaniopsis sp., Xanthostemon eucalyptoides* (DD), Welchiodendron sp. | 66 | 42% | Yes | Yes | ||
Flickingeria nativitatis* | Syzygium nervosum* (HS) | – | LR | Yes | No | |||
Grastidium baileyi* | Xanthostemon sp. | 4 | 25% | DD | Yes | |||
Oberonia complanata* | Callistemon sp., Leptospermum sp., Melaleuca styphelioides* (DD) | 11 | 45% | DD | Yes | |||
Oberonia palmicola* | Backhousia myrtifolia* (RT-MS), Melaleuca sp. | 7 | 29% | No/DD | Yes | |||
Oberonia titania* | Melaleuca alternifolia* (DD), Backhousia myrtifolia* (RT-MS) | 8 | 38% | No/DD | Yes | |||
Octarrhena pusilla* | Eugenia sp. | 5 | 40 | DD | Yes | |||
Oxysepala schilleriana (syn. Bulbophyllum schillerianum*) | Syzygium australe* (RT-MS), Syzygium sp. | 6 | 50% | No/DD | Yes | |||
Oxysepala wadsworthii (syn. Bulbophyllum wadsworthii*) | Leptospermum wooroonooran* (DD) | 4 | 25% | DD | Yes | |||
Plectorrhiza purpurata* | Leptospermum polygalifolium* (1) | Backhousia myrtifolia* (RT-MS), Leptospermum polygalifolium* (DD) | 6 | 33% | No/DD | Yes | ||
Rhinerrhiza divitiflora* | Backhousia sciadophora* (RT) (2) | – | LR | No | Yes | |||
Sarcochilus australis* | Tristaniopsis laurina* (RT), Backhousia myrtifolia* (RT-MS) (1); Tristania spp. (2). | Backhousia myrtifolia* (RT-MS), Baeckia virgata (DD) (syn. Sannantha virgata), Callistemon sieberi* (HS), Leptospermum polygalifolium* (DD), Tristaniopsis laurina* (RT) | 31 | 45% | Yes | Unclear | ||
Sarcochilus dilatatus* | Backhousia myrtifolia* (RT-MS), Syzygium sp. | 7 | 29% | No/DD | Yes | |||
Sarcochilus falcatus* | Austromyrtus spp., Backhousia spp., Syzygium spp. Lophostemon suaveolens* (RT), Tristaniopsis laurina* (RT), Lophostemon confertus* (DD) (1); Backhousia sciadophora* (RT) (2) | Backhousia myrtifolia* (RT-MS), Backhousia sp. (DD) | 23 | 13% | No/DD | Yes | ||
Sarcochilus olivaceus* | Myrtles (2) | – | LR | Yes | ||||
Sarcochilus parviflorus* | Myrtles (2) | Backhousia myrtifolia* (RT-MS), Syzygium sp. | 10 | 30% | DD/No | Yes | ||
Sarcochilus spathulatus* | Myrtaceae (2) | Syzygium smithii* (RT-MS) | 4 | 25% | DD | Yes | ||
Sarcochilus weinthalii* | Acmena brachyandra (syn. Syzygium ingens*) (DD) | 3 | 33 | DD | Yes | EPBC Act: VU | ||
Schoenorchis micrantha* | Neofabricia myrtifolia* (RT-MS) | 6 | 17% | No | Yes | |||
Serpenticaulis johnsonii (syn. Bulbophyllum johnsonii*) | Syzygium sp. | 6 | 33% | DD | Yes | |||
Sestochilos baileyi (syn. Bulbophyllum baileyi*) | Backhousia hughesii* (MS), Lophostemon sp. | 17 | 41% | DD | Yes | |||
Taeniophyllum baumei* | Austromyrtus sp., Melaleuca brassii (DD) (syn. Asteromyrtus brassii*), Tristaniopsis sp. | 8 | 50% | DD | Yes | |||
Taeniophyllum muelleri* | Callistemon sp., Callistemon salignus* (RT), Callistemon viminalis* (MS-HS), Lophostemon laurina (RT) (syn. Tristaniopsis laurina*), Melaleuca sp., Melaleuca styphelioides* (DD), Syzygium australe* (RT-MS), Tristaniopsis sp. | 37 | 43% | Yes | Yes | |||
Thelychiton adae (syn. Dendrobium adae*) | Xanthostemon graniticus* (DD) | 11 | 18% | DD | Yes | |||
Tropilis callitrophilis (syn. Dendrobium callitrophilum*) | Gossia spp., Rhodamnia spp., Rhodomyrtus spp. (1) | Austromyrtus sp. | 16 | 38% | DD | Yes | EPBC Act: VU | |
Asterisks (*) denote species names that are listed on the Australian Plant Census. Literature review sources are denoted as (1) Jones (2021) and (2) Makinson (2018). N records with specific hosts is the number of records (from the Australasian Virtual Herbarium, AVH) for the species that give host specific information, % myrtaceous hosts is the percent of records mentioning a specific myrtaceous host. Where hosts are mentioned only in the literature review (LR) cf. herbarium records, we note LR in the percent myrtaceous hosts column. Susceptibility ratings are according to Pegg et al. (2014, 2018). The myrtle rust zone is from Makinson (2018). Conservation status information was sourced from http://www.environment.gov.au/cgi-bin/sprat/public/sprat.pl on 1 March 2023.
Abbreviations: RT, relatively tolerant; MS, moderately susceptible; HS, highly susceptible; ES, extremely susceptible; DD, data deficient. VU, vulnerable; EN, endangered, CR, critically endangered.
Results
The literature review (i.e. Makinson 2018; Jones 2021) revealed 32 epiphytic orchid species with myrtaceous hosts. Through analysis of herbarium records, an additional 41 epiphytic orchid species were characterised by having ≥3 records mentioning a specific host, with at least one record growing on a myrtaceous host.
In total we identified 73 orchid species partially to predominantly utilising myrtaceous hosts (Fig. 1). Of these, nine are currently listed as threatened under national or state/territory biodiversity conservation legislation (others may be eligible but have not yet been assessed) (Table 1).
Crossplot showing the associations among myrtaceous host species (x axis) and epiphytic orchid genera (y axis). Circles indicate the number of species from each genus recorded as having an epiphyte–host relationship. The first column shows the total number of species in each orchid genus that occur in Australia. The second column shows the total number of species with an association with at least one myrtaceous host in each orchid genus that occur in Australia.
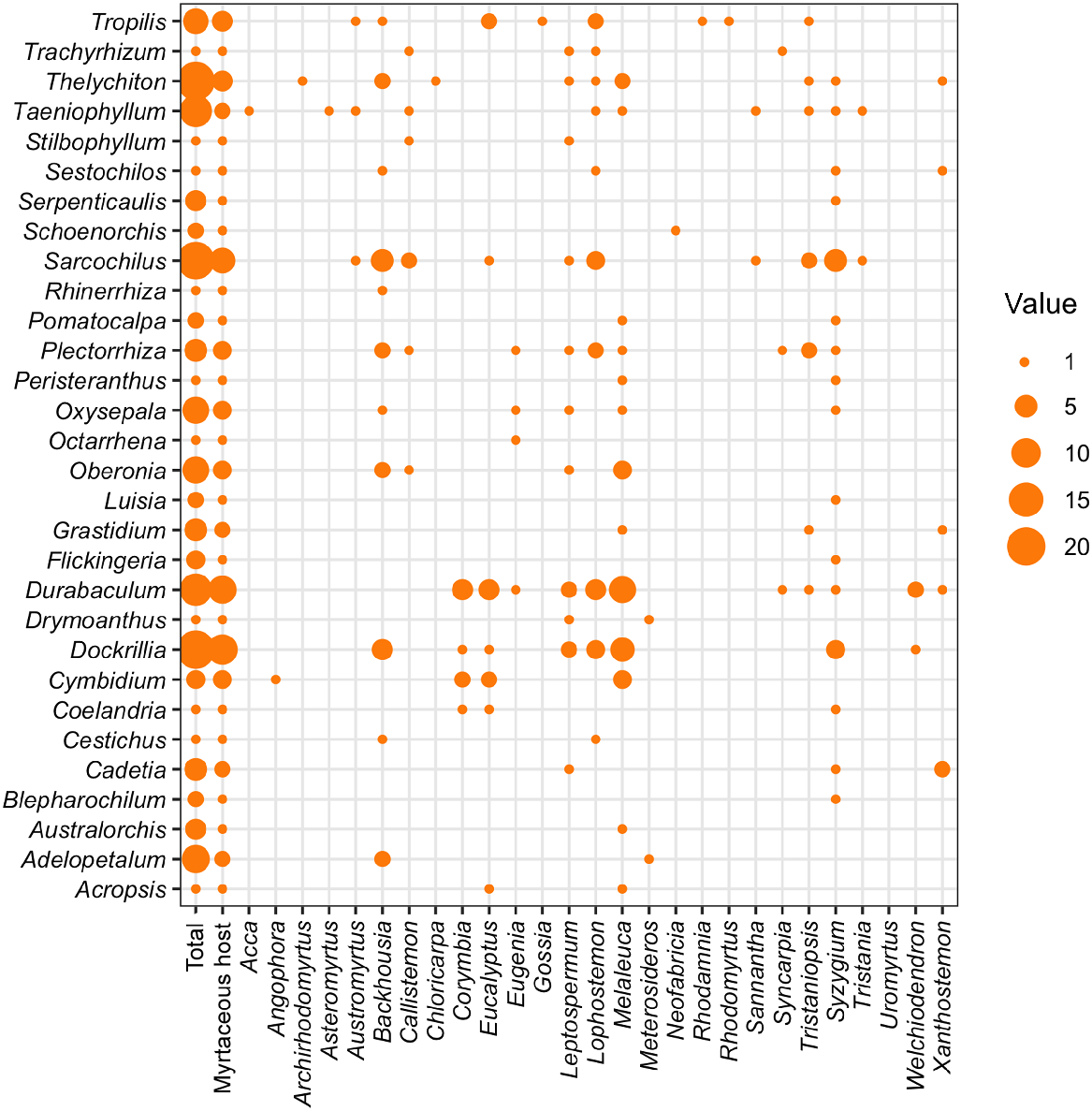
Group 1 is composed of species at highest risk from myrtle rust impacts and was dominated by orchid species from the Dendrobium s.l. alliance, which is one of the largest groups of epiphytic orchids in the world (approximately 1600 species; POWO 2023). Specifically, Group 1 included orchid species from the genus Durabaculum, commonly referred to as antelope orchids. These were predominantly hosted by Melaleuca (paperbark) shrub/tree species (including M. viridiflora). Group 1 also included the orchid Thelychiton melaleucaphilus (Fig. 2) hosted by Melaleuca shrub/tree species and the small shrub/tree Archirhodomyrtus beckleri.
Thelychiton melaleucaphilus attached to tree (likely Gossia hillii) killed by repeated myrtle rust infection. Photo: Kristy Stevenson.

Group 2 was similarly dominated by orchid species from the Dendrobium s.l. alliance. These included orchid species from the genus Cadetia, which typically occur as small compact clumps or spreading patches, and have short fleshy pseudobulbs and creeping rhizomes. Cadetia species were hosted by tall rainforest trees including Syzygium bamagense and Xanthostemon chrysanthus. Group 2 also included Durabaculum species that were mainly hosted by Melaleuca species, and Trachyrhizum agrostophyllum (a clumping orchid with grass-like leaves) largely found on small shrubby myrtaceous Callistemon and Leptospermum species. There were also orchids from the genus Tropilis, which were hosted by tall rainforest Myrtaceae, from genera including Eucalyptus and Lophostemon. From the Vanda orchid alliance (orchids which are characterised by having a single main stem from which leaves and flowers are produced and a lack of pseudobulbs), there was Plectorrhiza beckleri, which was hosted by Myrtaceae shrubs and trees including Callistemon, Syzygium and Tristaniopsis species, and Sarcochilus hillii (common name: myrtle orchid) on Backhousia.
Group 3 contained 45 species, including from the Bulbophyllum s.l. alliance, another of the world’s largest epiphytic orchid groups (approximately 2500 species; POWO 2023): Adelopetalum exiguum and Adelopetalum lilaniae, both tiny orchids commonly hosted by Backhousia tree and shrub species. From the Dendrobium s.l. alliance, Group 3 included orchids from the genus Dockrillia (commonly referred to as pencil orchids), which were frequently hosted by Backhousia, Melaleuca and Syzygium species. Tropilis callitrophilis was often associated with shrubby myrtles, which are highly susceptible to myrtle rust, including Austromyrtus, Gossia, Rhodamnia and Rhodomyrtus species. Flickingeria nativitatis, endemic to Christmas Island where myrtle rust is not present, was associated with the small-to-medium rainforest tree Syzygium nervosum. From the Vanda alliance, Plectorrhiza orchid species were associated with myrtaceous trees and shrub species from the genera Backhousia, Leptospermum, Melaleuca and Tristaniopsis. Sarcochilus species were often hosted by Austromyrtus, Backhousia, Lophostemon and Tristaniopsis. Taeniophyllum species were also commonly found on Austromyrtus, Callistemon and Melaleuca species.
Discussion
Myrtle rust has the potential to have catastrophic impacts on the Australian environment, with dozens of Myrtaceae species identified as being at risk of extinction, and vast knowledge gaps remaining (Fensham et al. 2020; Fensham and Radford-Smith 2021), including the potential for secondary declines or extinctions of associated biota. Approximately one quarter of Australia’s epiphytic orchids have myrtaceous hosts, reflecting the dominance of myrtaceous species in Australia’s vegetation. The fact that three-quarters of Australian epiphytic orchids were not recorded on myrtaceous hosts is likely related to the abundance of non-myrtaceous hosts available in the rainforests where epiphytes proliferate – such as in the rainforests of the Wet Tropic region in north-east Queensland and the Gondwana Rainforests of northern NSW and south-east Queensland (Australian ‘epiphyte hotspots’ sensuWallace 1983).
Orchids with myrtaceous hosts, at risk from the impacts of myrtle rust, were spread across 30 genera (Fig. 1). The number of at-risk orchid species in a genus was sometimes small (one or two); however, in some cases these orchid species are the sole representatives of that genus in Australia (e.g. Peristeranthus hillii) – thus their loss would be significant in terms of phylogenetic distinctiveness. At the other end of the scale is the genus Durabaculum, which has 13 species in Australia, of which at least seven species appear to predominantly use myrtaceous hosts. A key limitation of this study was the absence of myrtle rust susceptibility ratings for many myrtaceous host species (Table 1). With the exception of five species, every orchid in Table 1 (i.e. 68 species in total) has at least one myrtaceous host of unknown susceptibility, either because only the host genus was given, or the susceptibility of host species has not been published. Myrtaceous host species that are recognised as being data deficient must not be assumed to be tolerant, and placement of orchid species into risk categories is likely to change as research into myrtle rust progresses. The orchid species we highlight as being the most at risk (Group 1) have at least one myrtaceous host rated as highly or extremely susceptible and, as such, placement in Group 1 may be considered conservative. A second limitation of this study is that we used host names as given in the herbarium records: misidentification is possible, especially as these taxa were not the focus of the record.
Epiphytic orchid species that have a range of host species that includes both myrtle-rust-susceptible species and species not susceptible to myrtle rust (including non-Myrtaceae species), may also be at risk of decline. Loss of a proportion of an orchid population (i.e. individuals on myrtaceous hosts) may result in population fragmentation or thinning, with long-term impacts on population dynamics, through the impacts on pollinators, and therefore pollination, seed set and recruitment. In a study of Catasetum viridiflavum, an epiphytic orchid in Panama pollinated by euglossine bees, Murren (2002) showed that fruit set was lower in island populations compared to mainland populations, separated by 100–500 m, highlighting the potential impact of fragmentation on pollinator effectiveness, even over hundreds of metres. Epiphytic orchid species with hosts including both myrtaceous and non-myrtaceous species are theoretically at an advantage, as they can escape the myrtle rust threat by shifting to non-myrtaceous hosts. However, the potential impact of competition for the habitat on non-myrtaceous host trees, especially from epiphytic species already occupying these habitats, may be limiting. Moreover, surrounding environment, including habitat fragmentation, cohesion and edge length can influence the composition of orchid communities, through impacts on resource availability, including for pollinators, fungal symbionts, as well as for light and water (Martín-Forés et al. 2022). The impact of global change is also hypothesised to be severe for orchids, both directly, impacting their growth, flowering, and survival, and indirectly, through impacts on fungal symbionts, pollinators, and habitat (Gale et al. 2018) which includes impacts on host tree ranges, phenology and/or survival (Thuiller et al. 2008).
Epiphytic orchids were also identified as among those species disproportionately affected by the Australia’s 2019–2020 bushfires, especially those orchid species that rely on recolonisation by dispersal from unburnt areas, that are (obligately) epiphytic and lack a seedbank (e.g. Dockrillia and Plectorrhiza; Godfree et al. 2021). In 2019–2020, 24.3-33.8 million hectares was burnt in Australia (Binskin et al. 2020), including large areas between East Gippsland in Victoria to north-east NSW and within 200 km of the coast (NSW Government 2022). The largest overlap between fire-recovering forest and myrtle rust current/predicted distribution (Makinson 2018) appears to be the North Coast region of NSW, which includes the distributions of range-restricted epiphytic orchids, which use myrtaceous hosts, including Plectorrhiza purpurata. Moreover, the negative impacts of fire may be amplified as myrtle rust preferentially impacts new growth, such as the epicormic and basal resprouts produced by many myrtaceous species (e.g. Eucalyptus spp.) after fire (Pegg et al. 2020). Myrtle rust may also increase mortality of seedlings of myrtaceous species that germinate in nutrient-rich ash beds after fire (e.g. Leptospermum spp.) – thereby reducing the future populations of these host species. An increase in dead and dying trees, as a result of myrtle rust, may also increase fuel loads, increasing fire severity and leading to further negative impacts on remaining host trees – this effect was described for Phytophthora ramorum in California, where coarse woody debris and standing deadwood were significantly higher in infected stands (Cobb et al. 2012; Shaw et al. 2017).
Targeted field surveys of the at-risk orchids identified by this study are needed to confirm levels of fidelity to myrtaceous hosts, and the size class for effective epiphyte colonisation. Where non-myrtaceous hosts are identified, the potential for transition to these hosts could be investigated. This may include translocation of orchids and/or planting of non-Myrtaceae hosts to fill gaps left by myrtaceous species. Further research is needed to develop methods for ex situ cultivation (and/or germplasm conservation) of at-risk epiphytic orchid species, prior to any large-scale declines. Knowledge of propagation/cultivation methods for Australian epiphytic orchids is limited, often focused on growing horticulturally attractive species (Teixeira da Silva et al. 2015).
Further research is needed to refine our knowledge of the impacts of myrtle rust – direct, indirect and interactions. The indirect impacts on biodiversity caused by losses of tree hosts were emphasised by Mitchell et al. (2022), who showed that the loss of Fraxinus excelsior and Quercus petraea/robur, threatened by a range of pests and pathogens, would impact 512 associated species, across multiple taxonomic groups. Some studies predict knock-on effects from tree pathogens on entire food webs, for example, Phytophthora alni impacting riparian Alnus spp. trees, resulting in changes to litter inputs, shading and streambank stability potentially leading to impacts on microbes, invertebrates, amphibians and fish (Bjelke et al. 2016). There is a need for similar ecosystem-scale studies on myrtle rust.
Conclusion
This study highlights the potential impact of myrtle rust on non-myrtaceous species, in particular epiphytic orchids – including epiphytic orchids that have both myrtaceous and non-myrtaceous hosts, via impacts on fragmentation and pollination. Interactions between fire and myrtle rust are a further potential stressor for these species. Although this study focuses on epiphytic orchids, a suite of other epiphytic species may also rely on myrtaceous hosts, including non-vascular plants and parasitic plants, such as mistletoes (Makinson 2018). These plants must not be overlooked in conservation planning and risk assessment around myrtle rust.
Data availability
The data that support this study are available from the Atlas of Living Australia at https://doi.org/10.26197/ala.b0ea4986-4b23-40d8-beba-ec41c121e7f9, https://doi.org/10.26197/ala.38eb43c8-617a-412b-a6b4-cbcb8d5afac6, https://doi.org/10.26197/ala.9d9bb22f-1d49-42f5-95d7-44fb5317ead4 and https://doi.org/10.26197/ala.7581d22c-f6f4-47a5-bd3a-1cb6295abb29.
References
ABC News (2023) Most of Lord Howe Island off limits to visitors due to ‘highly infectious’ plant disease myrtle rust. Australian Broadcasting Commission. Available at https://www.abc.net.au/news/2023-03-16/lord-howe-island-partly-closed-due-to-myrtle-rust-nsw/102098242 [Accessed 30 March 2023]
Ai Y-Y, Liu Q, Hu H-X, Shen T, Mo Y-X, Wu X-F, Li J-L, Dossa GGO, Song L (2023) Terrestrial and epiphytic orchids exhibit different diversity and distribution patterns along an elevation gradient of Mt. Victoria, Myanmar. Global Ecology and Conservation 42, e02408.
| Crossref | Google Scholar |
Beenken L (2017) Austropuccinia: a new genus name for the myrtle rust Puccinia psidii placed within the redefined family Sphaerophragmiaceae (Pucciniales). Phytotaxa 297, 53-61.
| Crossref | Google Scholar |
Benzing DH (1987) Vascular epiphytism: taxonomic participation and adaptive diversity. Annals of the Missouri Botanical Garden 74, 183-204.
| Crossref | Google Scholar |
Bjelke U, Boberg J, Oliva J, Tattersdill K, McKie BG (2016) Dieback of riparian alder caused by the Phytophthora alni complex: projected consequences for stream ecosystems. Freshwater Biology 61, 565-579.
| Crossref | Google Scholar |
Carnegie AJ, Pegg GS (2018) Lessons from the incursion of myrtle rust in Australia. Annual Review of Phytopathology 56, 457-478.
| Crossref | Google Scholar | PubMed |
Carnegie AJ, Kathuria A, Pegg GS, Entwistle P, Nagel M, Giblin FR (2016) Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia. Biological Invasions 18, 127-144.
| Crossref | Google Scholar |
Cobb RC, Chan MN, Meentemeyer RK, Rizzo DM (2012) Common factors drive disease and coarse woody debris dynamics in forests impacted by sudden oak death. Ecosystems 15, 242-255.
| Crossref | Google Scholar |
Fensham RJ, Radford-Smith J (2021) Unprecedented extinction of tree species by fungal disease. Biological Conservation 261, 109276.
| Crossref | Google Scholar |
Fensham RJ, Carnegie AJ, Laffineur B, Makinson RO, Pegg GS, Wills J (2020) Imminent extinction of Australian Myrtaceae by fungal disease. Trends in Ecology & Evolution 35(7), 554-557.
| Crossref | Google Scholar | PubMed |
Fernandez-Winzer L, Berthon KA, Entwistle P, Manea A, Winzer N, Pegg GS, Carnegie AJ, Leishman MR (2020) Direct and indirect community effects of the invasive plant pathogen Austropuccinia psidii (myrtle rust) in eastern Australian rainforests. Biological Invasions 22, 2357-2369.
| Crossref | Google Scholar |
Gale SW, Fischer GA, Cribb PJ, Fay MF (2018) Orchid conservation: bridging the gap between science and practice. Botanical Journal of the Linnean Society 186(4), 425-434.
| Crossref | Google Scholar |
Givnish TJ, Spalink D, Ames M, Lyon SP, Hunter SJ, Zuluaga A, IIes WJD, Clements MA, Arroyo MTK, Leebens-Mack J, Endara L, Kriebel R, Neubig KM, Whitten WM, Williams NH, Cameron KM (2015) Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proceedings of the Royal Society B: Biological Sciences 282(1814), 20151553.
| Crossref | Google Scholar |
Godfree RC, Knerr N, Encinas-Viso F, Albrecht D, Bush D, Christine Cargill D, Clements M, Gueidan C, Guja LK, Harwood T, Joseph L, Lepschi B, Nargar K, Schmidt-Lebuhn A, Broadhurst LM (2021) Implications of the 2019–2020 megafires for the biogeography and conservation of Australian vegetation. Nature Communications 12, 1023.
| Crossref | Google Scholar | PubMed |
Gowland KM, van der Merwe MM, Linde CC, Clements MA, Nicotra AB (2013) The host bias of three epiphytic Aeridinae orchid species is reflected, but not explained, by mycorrhizal fungal associations. American Journal of Botany 100, 764-777.
| Crossref | Google Scholar | PubMed |
Gravendeel B, Smithson A, Slik FJW, Schuiteman A (2004) Epiphytism and pollinator specialization: drivers for orchid diversity? Philosophical Transactions of the Royal Society. Series B: Biological Sciences 359(1450), 1523-1535.
| Crossref | Google Scholar | PubMed |
Laube S, Zotz G (2006) Neither host-specific nor random: vascular epiphytes on three tree species in a Panamanian lowland forest. Annals of Botany 97(6), 1103-1114.
| Crossref | Google Scholar | PubMed |
Martín-Forés I, Bywaters SL, Sparrow B, Guerin GR (2022) Simultaneous effect of habitat remnancy, exotic species, and anthropogenic disturbance on orchid diversity in South Australia. Conservation Science and Practice 4(4), e12652.
| Crossref | Google Scholar |
Mitchell RJ, Bellamy PE, Broome A, Ellis CJ, Hewison RL, Iason GR, Littlewood NA, Newey S, Pozsgai G, Ray D, Stockan JA, Stokes V, Taylor AFS (2022) Cumulative impact assessments of multiple host species loss from plant diseases show disproportionate reductions in associated biodiversity. Journal of Ecology 110, 221-231.
| Crossref | Google Scholar |
Murren CJ (2002) Effects of habitat fragmentation on pollination: pollinators, pollinia viability and reproductive success. Journal of Ecology 90, 100-107.
| Crossref | Google Scholar |
NSW Government (2022) NSW fire and the environment 2019–20 summary. Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Parks-reserves-and-protected-areas/Fire/fire-and-the-environment-2019-20-summary-200108.pdf
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences 11, 1633-1644.
| Crossref | Google Scholar |
Pegg GS, Giblin FR, McTaggart AR, Guymer GP, Taylor H, Ireland KB, Shivas RG, Perry S (2014) Puccinia psidii in Queensland, Australia: disease symptoms, distribution and impact. Plant Pathology 63, 1005-1021.
| Crossref | Google Scholar |
Pegg G, Carnegie A, Giblin F, Perry S (2018) Final report – managing myrtle rust in Australia CRC2063. Plant Biosecurity Cooperative Research Centre, Bruce, ACT. Available at http://www.pbcrc.com.au/publications/pbcrc2206
Pegg GS, Entwistle P, Giblin FR, Carnegie AJ (2020) Fire and rust – the impact of Austropuccinia psidii (myrtle rust) on regeneration of Myrtaceae in coastal heath following wildfire. Southern Forests: A Journal of Forest Science 82, 280-291.
| Crossref | Google Scholar |
POWO (2023) Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available at http://www.plantsoftheworldonline.org/ [Accessed 17 September 2023]
Sanger JC (2016) The distribution of epiphytes over environmental and habitat gradients in tropical and subtropical Australia. PhD Thesis, University of Tasmania, Hobart, Australia. Available at https://eprints.utas.edu.au/23443/7/Sanger_whole_thesis_ex_pub_mat.pdf [Accessed 30 March 2023]
Shaw DC, Woolley T, Kelsey RG, McPherson BA, Westlind D, Wood DL, Peterson EK (2017) Surface fuels in recent Phytophthora ramorum created gaps and adjacent intact Quercus agrifolia forests, East Bay Regional Parks, California, USA. Forest Ecology and Management 384, 331-338.
| Crossref | Google Scholar |
Stewart JE, Ross-Davis AL, Graca RN, Alfenas AC, Peever TL, Hanna JW, Uchida JY, Hauff RD, Kadooka CY, Kim M-S, Cannon PG, Namba S, Simeto S, Pérez CA, Rayamajhi MB, Lodge DJ, Arguedas M, Medel-Ortiz R, López-Ramirez MA, Tennant P, Glen M, Machado PS, McTaggart AR, Carnegie AJ, Klopfenstein NB (2017) Genetic diversity of the myrtle rust pathogen (Austropuccinia psidii) in the Americas and Hawaii: global implications for invasive threat assessments. Forest Pathology 48, e12378.
| Crossref | Google Scholar |
Teixeira da Silva JA, Tsavkelova EA, Ng TB, Parthibhan S, Dobránszki J, Cardoso JC, Rao MV, Zeng S (2015) Asymbiotic in vitro seed propagation of Dendrobium. Plant Cell Reports 34, 1685-1706.
| Crossref | Google Scholar | PubMed |
Thuiller W, Albert C, Araújo MB, Berry PM, Cabeza M, Guisan A, Hickler T, Midgley GF, Paterson J, Schurr FM, Sykes MT, Zimmermann NE (2008) Predicting global change impacts on plant species’ distributions: future challenges. Perspectives in Plant Ecology, Evolution and Systematics 9(3–4), 137-152.
| Crossref | Google Scholar |
Vasconcelos TNC, Proença CEB, Ahmad B, Aguilar DS, Aguilar R, Amorim BS, Campbell K, Costa IR, De-Carvalho PS, Faria JEQ, Giaretta A, Kooij PW, Lima DF, Mazine FF, Peguero B, Prenner G, Santos MF, Soewarto J, Wingler A, Lucas EJ (2017) Myrteae phylogeny, calibration, biogeography and diversification patterns: increased understanding in the most species rich tribe of Myrtaceae. Molecular Phylogenetics and Evolution 109, 113-137.
| Crossref | Google Scholar |
Wagner K, Mendieta-Leiva G, Zotz G (2015) Host specificity in vascular epiphytes: a review of methodology, empirical evidence and potential mechanisms. AoB Plants 7, plu092.
| Crossref | Google Scholar |
Wallace BJ (1983) The Australian vascular epiphytes: flora and ecology. PhD Thesis, University of New England, NSW, Australia. Available at https://rune.une.edu.au/web/bitstream/1959.11/23348/6/open/SOURCE05.pdf [Accessed 4 May 2023]
Zotz G, Weigelt P, Kessler M, Kreft H, Taylor A (2021) EpiList 1.0: a global checklist of vascular epiphytes. Ecology 102, e03326.
| Crossref | Google Scholar | PubMed |


