Reductions in fitness due to an endoparasitic plant are comparable to the impacts of hemiparasites
P. G. Ladd A * and M. E. Andrew A
A * and M. E. Andrew A
A
Abstract
Parasitic plants are widespread throughout the global flora and have diverse lifestyle strategies. In most cases these plants are detrimental to the host but may have some beneficial effects on the co-occurring plants in the sourrounding communities. Some have large macroscopic plant bodies and can photosynthesise, and are therefore able to produce some fixed carbon but do take water and nutrients from the host, especially if aerially attached. Very few species have vegetative parts completely enclosed in the host, having only reproductive structures externally displayed. Whether such internal parasites have as severe effects on the host as parasites with macroscopic plant bodies is unclear.
The endoparasite Pilostyles hamiltoniorum infests pea species (predominantly Daviesia species) in the south-west of Western Australia. We investigated the effect of this parasite on the vegetative growth and reproduction of Daviesia angulata in heathland vegetation.
Size, flowering and fruiting of parasitised and unparasitised host plants were recorded in three 6 × 30 m plots in a revegetated gravel pit in the Jurien Bay area of Western Australia.
A proportion of 21% of host plants was parasitised and these were significantly taller than unparasitised plants. These plants had 52% fewer flowers on average than unparasitised plants and subsequently far fewer fruits.
The reduction in reproductive output by this internal parasite was at least equal to or more severe than occurs in published examples of decreased productivity of other species parasitised by species with macroscopic plant morphology.
The reduced reproductive output of the host plants would be inimical to seed stores in the soil that this species relies on for regeneration after fires that commonly affect the vegetation in this region.
Keywords: Apodanthaceae, Daviesia, endoparasite, Fabaceae, holoparasite, Pilostyles, reduced flowering, reduced fruits.
Introduction
Parasitic plants constitute ~1% of angiosperm species and are distributed worldwide (Hatcher and Dunn 2011). These plants can have a serious impact on the plants that are parasitised (Marvier 1996) and sometimes even beneficial effects on surrounding communities (Heer et al. 2018). Many are pests of crop plants and can decrease the yield of crops significantly (Parker 2009). Most are hemiparasites, attaching to part of a host plant and obtain all water requirements and some other resources from that plant. However, most hemiparasites have chloroplasts in the leaves or stems and can make some photosynthate. The efficiency of photosynthesis for this group can vary widely and may depend on the host quality (Těšitel 2016). Taxa such as the aerial parasite Arceuthobium have proportionally less chlorophyll than the pine tree hosts and therefore derive most carbohydrates from the host. Heavy infestation decreases timber productivity and cone production, and may lead to death of the host (Worrall 2013). Adverse effects on hosts may vary depending on conditions or the parasite species involved. For example, the root parasite Rhianthus alectorolophus induced strong water stress in the host when water was limiting (Těšitel et al. 2015) as the shoots have a high transpiration rate. Removal of the root parasite Rhianthus angustifolius from herbfields resulted in a 24% increase in biomass of other plant species but removal of Pedicularis sylvatica, also a root parasite, had no effect (Demey et al. 2013).
The adverse effects of aerial parasites such as mistletoes are often clear but there are alternative views about the beneficial effects of the parasites. The flowers and fruits of some species are important food sources for pollinators and frugivores (Platt 1993). A study of a root hemiparasite found 50% higher bird species richness and a significantly higher biomass of invertebrates on the parasite than on a co-occurring non parasite of similar form (Watson et al. 2011). Canopy parasites (Viscum) have also been shown to structure plant communities by increasing nearby soil nutrient levels and facilitating colonisation and growth of zoochorus species that limited the influence of the dominant pine species (Mellado and Zamora 2017).
Parasites that have no chlorophyll are dependent on the host for fixed carbon – these are classified as holoparasites. Taxa in this group such as Orobanche cause crop losses in Egypt of up to 33% and up to 60% loss of yield in sunflowers in Greece while Striga causes losses from 12 to 20% in cereals in Africa (Parker 2009). In natural systems, the debilitating effects on host plants that are dominants of a community may allow other species that were adversely affected by competition from the dominant to become more prominent (Casadesus and Munne-Bosch 2021).
A very small group of holoparasites have vegetative tissue completely inside the host and only produce flowers (and subsequently fruits) outside the host, and are called endoparasites (Teixeira-Costa et al. 2021). Vegetative parasite tissues are completely contained in the host for taxa in Apodanthaceae, Cytinaceae and Mitrastemonaceae that mostly have very small flowers and Rafflesiaceae that can have very large flowers. Thus, unlike other parasites, these are unlikely to have any influence on nutrient cycling of litter or a great deal of influence on water relations of the host (see Teixeira-Costa et al. 2021). Unlike many holoparasites of annual hosts, endoparasites are likely to remain with the host for the entire lifespan of many years. Three species in the Apodanthaceae (genus Pilostyles) occur in pea plants in Western Australia.
Endoparasites with no macroscopic vegetative tissues would be expected to have less effect on host vigour and productivity than species with large vegetative plant bodies such as completely chlorophyll free taxa (e.g. Orobanche, Epifagus) or even species that have chlorophyll such as Nuytsia or aerial mistletoes such as Viscum or Loranthus. This has rarely been addressed.
Pilostyles hamiltoniorum Gardner parasitising Daviesia angulata Lindl. was studied in a revegetated gravel pit on Munbinea Road east of Jurien Bay (30°17′03.04″, 115°11′29.06″), Western Australia. The recruitment of the host plant was from stock-piled top soil spread across a disused gravel pit, the relatively level base of which was subsequently ripped. D. angulata is one of the most preferred hosts for P. hamiltoniorum out of five Daviesia species surveyed (Craig et al. 2024). The species is only distinctive by the parasite flowers that occur every year on ‘recently mature host stems some 10–20 cm from the shoot tips’ (Dell et al. 1982) and remain as dried up dark remains on all the old stem surfaces (Fig. 1). These flower remnants are the only evidence of the parasite and in general all main stems of the host have the flowers indicating almost 100% parasitism unlike macroscopic aerial parasites that can be variably abundant on a host. The host is an upright seeder shrub with sharply pungent phyllodes. Flowers are produced in the axils of phyllodes on new season growth and above where parasite flowers occur. The climate in the area is Mediterranean with mean annual rainfall of ~530 mm a−1. Rainfall before the sampling period of March/April was slightly lower than the long-term average but slightly higher over the sampling period of May/June (Bureau of Meteorology (BOM) 2025). The vegetation surrounding the gravel pit is heath with occasional emergent eucalypts on a sandy ironstone gravel formed over low nutrient content deep sand (see Turner and Laliberté 2015 for general soil nutrient information). Fires are an important influence on the vegetation and unlikely to have intervals of less than 15 years in the general area (Miller and Dixon 2014) but in a nature reserve near the study site the interval has been 37 years (PGL personal observation).
Daviesia angulata shrubs without (left) and with (right) the endoparasite Pilostyles hamiltoniorum. The yellow fruits on the left plant are the legumes of the host, while the small black protuberances on the lower branches of the shrub to the right are the old flowers of the parasite. Note that this parasitised plant is also infested by the vine Cassytha sp. (Photo credit: P. G. Ladd.).

The following questions about the host plants were addressed in this study.
Is there a difference in shoot length between parasitised and non-parasitised plants?
Is there a difference in phyllode length between parasitised and non-parasitised plants?
Does parasitism affect the nutrient content of parasitised plants?
Is there a difference in number of flowers produced by a parasitised and non-parasitised plant?
Is there a difference in number of fruits produced by a parasitised and non-parasitised plant?
Materials and methods
Parasitised and non-parasitised plants were measured in three 6 × 30 m quadrats. Plant location, height, two widths and presence or absence of host flowers were recorded for all plants. Flowering intensity was estimated on a four point scale (0 = no flowers; 1 = 1–10 flowers; 2 = 11–50 flowers; 3 = > 51 flowers). A t-test was used to confirm if parasitised and non-parasitised plants were of similar size and, following the finding of systematic differences, generalised linear models (GLMs) were used to evaluate the impacts of the parasite on flowering intensity after accounting for the size of the host plant. Canopy volume (log transformed), calculated from the height and width measurements assuming an ellipsoidal shape was found to be a better predictor of flowering than any individual dimension, and was used in all GLMs. Separate models were performed to test the effects of size and parasite status on the presence of flowering (flower intensity class 0 vs 1–3), and the occurrence of high (classes 0–1 vs 2–3) and very high (classes 0–2 vs 3) flowering intensity. All GLMs were fit using a binomial distribution and a logit link.
The impacts of the parasite on the host plant were evaluated in greater detail on samples of branches. A branch of ~4 mm diameter was collected from four parasitised and four non-parasitised plants to determine number and length of previous year’s shoots. Phyllodes from these shoots were removed, length measured, dried, ground in a grinding mill and analysed for calcium, potassium, magnesium, nitrogen and phosphorus using standard analytical methods. T-tests were used to evaluate the effect of parasite status on each response measured on this sample.
Five shoots with flowers were marked on eight parasitised and 10 non-parasitised plants haphazardly selected from plants that had flowers and collected when fruits were mature. Shoot length was measured, fruits were counted and, using a dissecting microscope, flower pedicels were counted (as unfertilised flowers abscised). Generalised linear mixed effects models (GLMMs) were used to determine the impact of the parasite on flowering and fruiting while controlling for individual level variation, using plant ID as a random grouping term. Additional covariates were shoot length in the model of flowering and flower abundance (i.e. number of flower pedicels) in models of fruiting. The model of flowering used flower abundance as the response variable, while three measures of fruiting were developed and evaluated: (1) presence of fruits, assessed over all shoots; (2) abundance of fruits on all shoots; and (3) abundance of fruits, evaluated over only the set of shoots that produced fruits. Flower abundance was included in the models of fruiting, therefore the latter two are comparable to evaluating the impacts of the parasite on flower to fruit conversion by all flowers and by all flowers that fruited, respectively. GLMMs of flower and fruit abundance used a Poisson distribution with a log link, while the model of fruit occurrence fit a binomial distribution with a logit link.
All GLM and GLMM analyses were performed in R ver. 4.2.2 (R Core Team 2022) using functions in the ‘stats’ (R Core Team 2022) and ‘MASS’ (Venables and Ripley 2002) packages. T-tests were performed in Excel. Model assumptions were evaluated visually.
Results
As the host species has episodic recruitment from seeds following disturbances, most plants would have become established at the same time shortly before 1993, when the gravel pit was revegetated. When plants would have first been parasitised is not known. Overall, 21% of plants in the sample quadrats were parasitised. There seemed to be no contagion in the distribution of parasitised plants with these scattered through non-parasitised plants.
Although parasitised plants on average tended to be taller than non-parasitised plants, the length of shoots on the branches of the four parasitised plants was significantly shorter (35.3 ± 1.59 mm, t = 9.61, P < 0.001) than on the four non-parasitised plants (66.5 ± 2.84 mm) but the number of shoots mm−1 diameter of the branch was similar (~7.2 ultimate shoots mm−1). There were 34 parasitised plants in the survey and 61 of the non-parsitised plants were in the same height range as the parasitised plants. Overall 48% of non-parasitised plants were smaller than the smallest parasitised plant. There was no significant difference between the phyllode lengths on any of the plants. Nutrient content of phyllodes of non-parasitised plants was slightly higher but not significantly so, than in parasitised plants (data not shown).
Almost all (94%) of the parasitised plants had flowers, while 63% of non-parasitised plants had flowers. However many of the non-parasitised plants were small due to immaturity or poor vigour. Examining only flowering individuals, the parasitised plants were significantly taller (mean 76 cm) than non-parasitised plants (51 cm, t = 5.57, P < 0.001) but there was no difference in plant width. These size differences complicate the patterns of flowering by parasite status.
Size was a strong predictor of all measures of flowering and flower intensity. After accounting for size, parasitism did not affect the occurrence of flowers but this did have a negative effect on the two higher flowering intensities (Fig. 2). This was more significant for the very high flowering intensity (P = 0.0002) than for high flowering intensity (P = 0.0064; Table 1).
Effects of plant size (canopy volume) on (a) the occurrence of flowering, and plant size and the presence of the endoparasite Pilostyles hamiltoniorum on the occurrence of (b) high and (c) very high flowering intensity in a field census of Daviesia angulata. Parasitised plants are plotted in red, and non-parasitised plants in green. All effects plotted are statistically significant (Table 1). When there was not a signficant effect of parasitism, the common effect of size is plotted in black. Large, dark points plot the average values of parasitised and non-parasitised plants, while small, light points plot the observed values. High and very high flowering intensity are defined as the presence of >10 flowers and >50 flowers, respectively. Canopy volume was calculated from the height, length and width of the shrub, assuming an ellipsoidal shape.
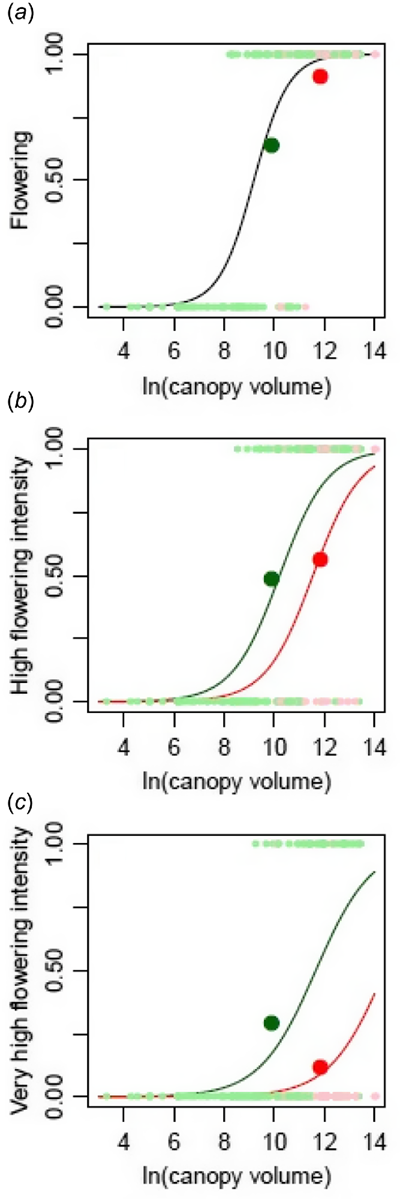
| Response | Term | Coef. | s.e. | z | P | |
|---|---|---|---|---|---|---|
| Flowering | Intercept | −13.23 | 2.44 | −5.43 | <0.0001 | |
| ln (canopy volume) | 1.44 | 0.26 | 5.62 | <0.0001 | ||
| Parasitised | −0.76 | 0.81 | −0.94 | 0.3480 | ||
| High flowering intensity | Intercept | −10.81 | 1.81 | −5.98 | <0.0001 | |
| ln (canopy volume) | 1.06 | 0.17 | 6.10 | <0.0001 | ||
| Parasitised | −1.41 | 0.52 | −2.73 | 0.0064 | ||
| Very high flowering intensity | Intercept | −10.41 | 1.95 | −5.33 | <0.0001 | |
| ln (canopy volume) | 0.89 | 0.17 | 5.12 | <0.0001 | ||
| Parasitised | −2.47 | 0.65 | −3.80 | 0.0001 |
All models evaluated a binomial response with a logit link, with flowering defined as the presence/absence of flowering, and high and very high flowering intensity defined as the presence of >10 flowers and >50 flowers respectively. Canopy volume was calculated from the height, length and width of the shrub, assuming an ellipsoidal shape. In all models, the non-parasitised treatment is the reference level represented by the intercept term.
Coef., coefficient; s.e., standard error.
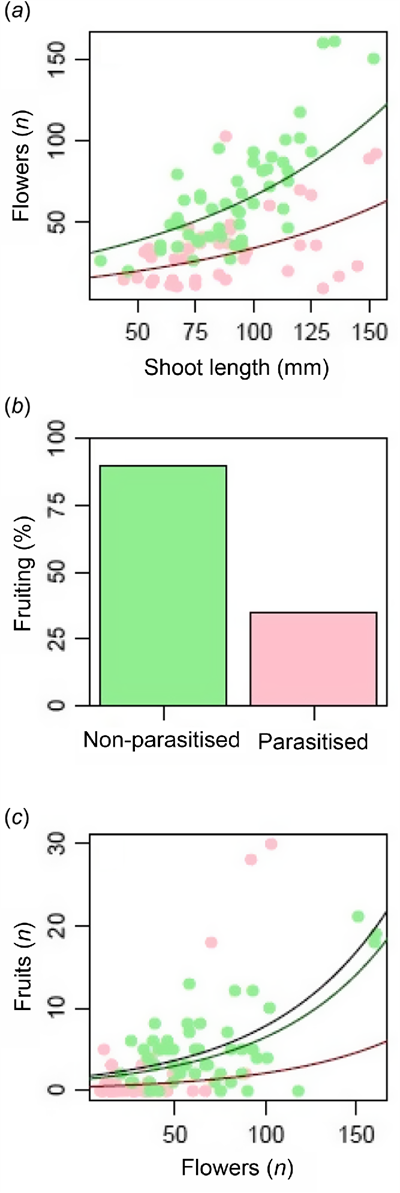
There were significantly more flowers on longer shoots and on shoots of non-parasitised vs parasitised plants for a given shoot length (Fig. 3a, Table 2). Most shoots on non-parasitised plants produced fruit, but a significantly lower proportion of shoots on parasitised plants did not (Table 2, Fig. 3b). There were significantly more fruit on shoots on non-parasitised plants compared with shoots on parasitised plants when analysing the full set of shoots (both with and without fruits). When shoots lacking fruits were excluded from the model, the effect of the parasite on fruit abundance became nonsignificant (Fig. 3c, Table 2).
Factors influencing (a) the abundance of flowers, and the (b) occurrence and (c) abundance of fruits on shoots of Daviesia angulata plants with and without the endoparasite Pilostyles hamiltoniorum. All effects plotted are statistically significant (Table 2). Parasitised plants are plotted in red and non-parasitised plants in green. Panel (c) presents the results of two models of the abundance of fruits that were performed on different subsets of shoots: in the analysis of all shoots, both the occurrence of the parasite (red vs green lines) and the number of flowers significantly affected the abundance of fruits. In the analysis of only shoots with >0 fruits (black line), only the number of flowers had a significant effect.
| Response | Term | Coef. | s.e. | d.f. | t | P | |
|---|---|---|---|---|---|---|---|
| n flowers | Intercept | 3.12 | 0.16 | 71 | 19.23 | <0.0001 | |
| Parasitised | −0.66 | 0.18 | 16 | −3.73 | 0.0018 | ||
| Shoot length (mm) | 0.01 | 0.00 | 71 | 9.02 | <0.0001 | ||
| Fruiting | Intercept | 2.01 | 1.22 | 71 | 1.64 | 0.1048 | |
| Parasitised | −3.52 | 1.25 | 16 | −2.82 | 0.0124 | ||
| n flowers | 0.02 | 0.01 | 71 | 1.45 | 0.1524 | ||
| n fruits | Intercept | 0.32 | 0.38 | 71 | 0.84 | 0.4045 | |
| Parasitised | −1.12 | 0.56 | 16 | −1.99 | 0.0642 | ||
| n flowers | 0.02 | 0.00 | 71 | 6.83 | <0.0001 | ||
| n fruits (non-zero) | Intercept | 0.53 | 0.24 | 42 | 2.22 | 0.0318 | |
| Parasitised | 0.15 | 0.33 | 14 | 0.44 | 0.6631 | ||
| n flowers | 0.02 | 0.00 | 42 | 6.87 | <0.0001 |
All models included plant identity as a random grouping variable. Models of abundance (n flowers, n fruits, n fruits (non-zero)) fit a Poisson distribution with a log link; models of occurrence (Fruiting) fit a binomial model with a logit link. The n fruits (non-zero) model analysed the set of all shoots that contained fruits (n = 59), while the remaining models analysed the full set of sampled shoots (n = 90). In all models, the non-parasitised treatment is the reference level represented by the intercept term.
Coef., coefficient; s.e., standard error; d.f., degrees of freedom.
Flower to fruit conversion in D. angulata at the site was very low with only ~8% of flowers on healthy plants producing fruit. The fitted models were used to predict the number of fruits for a standard shoot length of 100 mm. Although shoots with fruits had a similar productivity regardless of the parasite status (producing 0.07–0.08 fruits flower−1), parasitised plants produced far fewer fruits overall (0.8 vs 3.8 fruits 100 mm−1 of shoot) due to initially fewer flowers (34.1 vs 66.2 flowers 100 mm−1 of shoot) and fewer shoots with fruits (35% vs 90% of flowering shoots; Fig. 3b).
Discussion
In D. angulata at the study site, parasitised plants were on average taller than non-parasitised plants. This is a conundrum as parasitised plants had shorter shoots. However this is an artifact of the population structure. The population of D. angulata became established from the seed bank of topsoil that was spread over the base of the gravel pit for rehabilitation. There seemed to have been a short time after the plants became established when some plants were inoculated with parasite seeds. Pilostyles seeds are small and claimed to be dispersed by animals (Bellot and Renner 2013) based on little actual evidence except fruit morphological characteristics. The mechanism by which Pilostyles enters host plants is unclear but inoculation is considered to occur close to the base of the plant (Teixeira-Costa et al. 2021). In animals, parasite transmission is either density dependent or frequency dependent (Patterson and Ruckstuhl 2013) but in plants, frequency dependence is unlikely as adult plants cannot move to encounter the parasite. The lack of clustering of parasitised plants indicates that inoculation seemed not to have been continuous or smaller parasitised plants should also have been found close to larger plants but this was not the case. The Daviesia population continued to grow after initial establishment as interdisturbance recruitment does occur in species that are generally episodic recruiters such as D. angulata (e.g. Schmidberger and Ladd 2020) but no small plants were parasitised.
Most studies on the effect of parasites on hosts deal with productivity reduction in the host but we could not find any studies that specifically addressed reduction in flower production. Parasite load influences the damage to the host and a study on the effect of the hemiparasite Cassytha pubescens showed that parasitised plants of Ulex had significantly lower biomass and that the parasite damaged smaller more than larger host plants (Cirocco et al. 2020). Unlike mistletoes where parasite load can be determined by counting individuals on a host plant, this is not possible for Pilostyles. Parasitised plants retain evidence of old flowers along all old stems therefore the parasite filaments must be present throughout the cortex of all shoots. Determining whether there is only one parasite individual in a host plant can only be undertaken by molecular analysis of parasite tissues from different parts of a host (Bellot and Renner 2013 address this issue), not from external observation. As yield reduction in grain crops must be related to the reproductive parts, there seems to be little focus on whether the flowering or fruiting are the main impact. In D. angulata the main parasitic effect is the reduction in flowering and the consequential reduction in fruit.
There was low flower to fruit conversion in all plants that may be due to poor pollinator attention to the plants or low nutrient soils leading to poor productivity, although this aspect was not part of this study. The fruit production was much lower than in other similar pea species such as Dillwynia juniperina (47–70%, Gross 2001). Parasitism did result in 52% reduced productivity of flowers in parasitised plants and reduced fruit set. Parasite flowering and hence the main drain on host plant resources occurs in February/March (Thiele et al. 2008) while that of the host occurs in May/June. Host resources will therefore be depleted over the autumn period when rainfall in the region is very low and host growth only resumes with late autumn-winter rainfall. Thus the reduced flowering of the host may be the result of depleted carbohydrate resources due to parasite demand over the non-growing season. Host resources would begin to accumulate once rainfall increases beginning in late autumn, becoming available to support host fruit production. This is somewhat supported by the phyllode inorganic nutrient analyses that showed slightly higher (but not significant) nutrient content in non-parasitised than parasitised plants. The conversion rate of flowers to fruit in parasitised and non-parasitised plants only differed by a small amount, indicating that parasitised plants could still provide resources for the reduced number of flowers to produce fruit. In a study of another Pilostyles species, no adverse photosynthetic physiological effects of P. ingae on the associated Mimosa host were detected by Fernandes et al. (1998), although ∂13 was higher in parasitised than unparasitised plants but no other productivity measures were recorded.
The adverse effect of parasites that produce macroscopic plant bodies would be expected to be more severe on host plants than endoparasites that only have macroscopic reproductive organs. In the Orobanchaceae, Epifagus (Andrés-Hernández et al. 2024) and Orobanche lack chlorophyll and therefore need all the fixed carbon to be provided by the host. However, Striga does have some chlorophyll and is still a damaging parasite that causes loss of yield from 43 to 61% of cereal crops in northern Africa (Van Mourik et al. n.d.). Orobanche crenata reduced pea crop species biomass from 30 to 38% and caused 45% reduction in reproductive production compared to non-parasitised plants of field peas (Fernández-Aparicio et al. 2016). Our results of 52% reduction in flowers is considerably higher than that for field peas.
P. hamiltoniorum had a detrimental effect on reproduction of the host Daviesia angulata through much poorer flower and hence fruit production than in non-parasitised plants. This exacerbated an already poor fruit set by the species even if not harbouring the parasite. This would have a detrimental effect on the population health due to reduced seed input to the soil-stored seedbank as the species is a seeder and adults cannot survive fires that are periodic in the vegetation in which the species grows.
References
Andrés-Hernández AR, Ames-Martínez FN, Macada A, Teixeira-Costa L, Rodríguez-Ramíez EC (2024) Functional traits of Epifagus virginiana (Orobanchaceae) tubers as adaptations to the Mexican beech microenvironment. Flora 320, 152622.
| Crossref | Google Scholar |
Bellot S, Renner SS (2013) Pollination and mating systems of Apodanthaceae and the distribution of reproductive traits in parasitic angiosperms. American Journal of Botany 100(6), 1083-1094.
| Crossref | Google Scholar | PubMed |
Bureau of Meteorology (BOM) (2025) Rainfall at Jurien Bay Station. Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=136&p_display_type=dailyDataFile&p_startYear=2011&p_c=-16693899&p_stn_num=009131 [accessed April 2025]
Casadesus A, Munne-Bosch S (2021) Holoparasitic plant–host interactions and their impact on Mediterranean ecosystems. Plant Physiology 185(4), 1325-1338.
| Crossref | Google Scholar | PubMed |
Cirocco RM, Facelli JM, Watling JR (2020) The impact of a native hemiparasite on a major invasive shrub is affected by host size at time of infection. Journal of Experimental Botany 71(12), 3725-3734.
| Crossref | Google Scholar | PubMed |
Craig RJ, Pittway B, Wu T, Turner SR, Batley J (2024) Applying resource-selection functions to assess host preference in the endemic endoparasite Pilostyles hamiltoniorum (Apodanthaceae) and its principal host Daviesia (Fabaceae). Australian Journal of Botany 72, BT24026.
| Crossref | Google Scholar |
Dell B, Kuo J, Burbidge AH (1982) Anatomy of Pilostyles hamiltonii C. A. Gardner (Rafflesiaceae) in stems of Daviesia. Australian Journal of Botany 30(1), 1-9.
| Crossref | Google Scholar |
Demey A, Ameloot E, Staelens J, De Schrijver A, Verstraeten G, Boeckx P, Hermy M, Verheyen K (2013) Effects of two contrasting hemiparasitic plant species on biomass production and nitrogen availability. Oecologia 173, 293-303.
| Crossref | Google Scholar | PubMed |
Fernandes GW, De Mattos EA, Franco AC, Lüttge U, Ziegler H (1998) Influence of the parasite Pilostyles ingae (Rafflesiaceae) on some physiological parameters of the host plant, Mimosa naguirei (Mimosaceae). Botanica Acta 111(1), 51-54.
| Crossref | Google Scholar |
Fernández-Aparicio M, Flores F, Rubiales D (2016) The effect of Orobanche crenata infection severity in Faba bean, Field pea, and Grass pea productivity. Frontiers in Plant Science 7, 1409.
| Crossref | Google Scholar | PubMed |
Gross CL (2001) The effect of introduced honeybees on native bee visitation and fruit set in Dillwynia juniperina (Fabaceae) in a fragmented ecosystem. Biological Conservation 102(1), 89-95.
| Crossref | Google Scholar |
Heer N, Klimmek F, Zwahlen C, Fischer M, Hölzel N, Klaus VH, Kleinebacker T, Prati D, Boch S (2018) Hemiparasite-density effects on grassland plant diversity, composition and biomass. Perspectives in Plant Ecology, Evolution and Systematics 32, 22-29.
| Crossref | Google Scholar |
Marvier MA (1996) Parasitic plant-host interactions: plant performance and indirect effects on parasite-feeding herbivores. Ecology 77(5), 1398-1409.
| Crossref | Google Scholar |
Mellado A, Zamora R (2017) Parasites structuring ecological communities: the mistletoe footprint in Mediterranean pine forests. Functional Ecology 31(11), 2167-2176.
| Crossref | Google Scholar |
Parker C (2009) Observations on the current status of Orobanche and Striga problems worldwide. Pest Management Science 65(5), 453-459.
| Crossref | Google Scholar | PubMed |
Patterson JEH, Ruckstuhl KE (2013) Parasite infection and host group size: a meta-analytical review. Parasitology 140(7), 803-813.
| Crossref | Google Scholar | PubMed |
R Core Team (2022) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Schmidberger JW, Ladd PG (2020) Geographic distribution and the reproductive and demographic ecology of two congeneric seeder and resprouter tree species. Forest Ecology and Management 475, 118428.
| Crossref | Google Scholar |
Teixeira-Costa L, Davis CC, Ceccantini G (2021) Striking developmental convergence in angiosperm endoparasites. American Journal of Botany 108(5), 756-768.
| Crossref | Google Scholar | PubMed |
Těšitel J (2016) Functional biology of parasitic plants: a review. Plant Ecology and Evolution 149(1), 5-20.
| Crossref | Google Scholar |
Těšitel J, Těšitelová T, Fisher JP, Lepš J, Cameron DD (2015) Integrating ecology and physiology of root-hemiparasitic interaction: interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytologist 205(1), 350-360.
| Crossref | Google Scholar | PubMed |
Thiele KR, Wylie SJ, Maccarone L, Hollick P, McComb JA (2008) Pilostyles coccoidea (Apodanthaceae), a new species from Western Australia described from morphological and molecular evidence. Nuytsia 18, 273-284.
| Crossref | Google Scholar |
Turner BL, Laliberté E (2015) Soil development and nutrient availability along a 2 Million-year coastal dune chronosequence under species-rich Mediterranean shrubland in southwestern Australia. Ecosystems 18, 287-309.
| Crossref | Google Scholar |
Watson DM, McGregor HW, Spooner PG (2011) Hemiparasitic shrubs increase resource availability and multi-trophic diversity of eucalypt forest birds. Functional Ecology 25(4), 889-899.
| Crossref | Google Scholar |


