Evaluating nutritional quality and methane production from fermentation of pasture forages grown under current and future climate conditions using near-infrared spectroscopy
Isabelle L. Kite A * , Sally A. Power A , Richard G. Meyer B , Sabrina A. Meurs B , Kristy L. Bailes B , Manjunatha H. Chandregowda A and Ben D. Moore A
A * , Sally A. Power A , Richard G. Meyer B , Sabrina A. Meurs B , Kristy L. Bailes B , Manjunatha H. Chandregowda A and Ben D. Moore A
A
B
Abstract
Improved pasture nutritional quality and reduced enteric methane production under current and projected climate conditions are essential for the meat and livestock industry. Legumes and herbs could modify the ruminal fermentation process, owing to their high nutritional quality and plant secondary compound content.
This study aimed to evaluate the effects of climate stress on the nutritional quality and enteric methane (CH4) production associated with temperate legumes (Medicago sativa, Onobrychis viciifolia, and Biserrula pelecinus), a tropical legume (Desmanthus virgatus) and a temperate herb (Cichorium intybus).
Plants were grown in large polytunnels at Western Sydney University’s Pastures and Climate Extremes field facility, receiving simulated wet (La Niña) or dry (El Niño) rainfall regimes, under ambient or elevated (+3°C) temperatures. Samples were collected in multiple seasons, freeze dried and analysed using near-infrared spectroscopy to determine nutritional quality and associated methane production, and these results were validated in vitro.
B. pelecinus reduced CH4 production (−85%) and C. intybus provided reduced CH4 emissions intensity. Elevated temperature and lower rainfall (Dry) treatments resulted in lower CH4 production from B. pelecinus, D. virgatus and C. intybus.
Reductions in predicted CH4 production from fermentation were observed in multiple species under lower rainfall and elevated temperature treatments, including in the species with the lowest overall in vitro CH4 production, B. pelecinus.
The benefits of using legumes and herbs to reduce pasture-based CH4 emissions and improved nutritional quality are likely to be influenced by future changes in temperature and rainfall regime.
Keywords: climate change, elevated temperatures, enteric methane, methanogenesis, pasture nutritional quality, rainfall, temperate forage, tropical forage.
Introduction
The global food system contributes 21–37% of total net anthropogenic greenhouse-gas emissions (GHG; CO2 equivalents) (Rosenzweig et al. 2020) and methane (CH4) accounts for 35% of the food system emissions (Crippa et al. 2021). Enteric CH4 emissions from ruminants can reach 250–500 L per animal daily (Johnson and Johnson 1995), which scales up globally to 7,100,000 Gg CO2-eq per annum, or 14.5% of all anthropogenic GHG emissions (Gerber et al. 2013).
CH4 has a global warming potential approximately 80 times that of carbon dioxide (CO2) over the 20 years following its release (Forster et al. 2021), but has a shorter atmospheric lifetime of only 10–12 years (Dlugokencky et al. 2011). As a result, reducing CH4 emissions has the potential to rapidly slow down the current rate of warming and increase the likelihood of achieving climate mitigation targets (Ripple et al. 2014; Collins et al. 2018; United Nations Environment Programme and Climate and Clean Air Coalition 2021).
Climate models predict that mean surface temperatures will increase by 2.5–4.0°C by the end of the century (IPCC 2023). In south-eastern Australia, winter/spring rainfall has been declining and prolonged drier periods, interspersed with short-term, heavier rainfall events are becoming more frequent (CSIRO and Bureau of Meteorology 2024). Australia’s climate is also strongly influenced by medium-term global climate drivers including the El Niño Southern Oscillation (ENSO) (Torrence and Webster 1999; McPhaden et al. 2006; Collins et al. 2010) and Indian Ocean Dipole (IOD) (Pepler et al. 2014; Reddy et al. 2021). Global climate change may affect these processes and potentially lead to an amplification of extreme ENSO events (Cai et al. 2014; Chung and Power 2016; Chen et al. 2017; Lieber et al. 2024). Changes in forage quality under projected climate conditions could further exacerbate or mitigate climate change through altered enteric CH4 emissions. Indeed, it has been estimated that declines in nutritional quality could increase CH4 production by 0.9% under a 1°C temperature increase (Lee et al. 2017).
CH4 production is affected by the type and nutritional quality of ruminant diets (Archimède et al. 2011; Beauchemin et al. 2020; Smith et al. 2022) because the rumen microbial community is strongly influenced by diet (Henderson et al. 2015). Improvements in nutritional quality are associated with increases in digestibility and daily feed intake, which increases production efficiency (Hristov et al. 2013) and reduces CH4 emissions intensity per unit of milk (Knapp et al. 2014) or meat (Beauchemin et al. 2020) produced. Shifts in carbohydrate content, particularly reductions in structural carbohydrates and increases in water-soluble carbohydrates, can reduce CH4 yield by increasing the rate of passage through the rumen and increasing propionate production (Pacheco et al. 2014), as well as by altering fermentation parameters such as pH, leading to inhibition of methanogens (Sun et al. 2022).
There is increasing interest in the opportunities and management considerations involved in adoption of multi-species swards into Australian grazing systems (Thomson and Albornoz 2023) and many legume cultivars have been developed that could support this (Nichols et al. 2012). Multi-species swards including grasses, legumes and/or herbs offer many benefits, such as reducing the need for nitrogen fertiliser while maintaining productivity (Jaramillo et al. 2021) and increasing resilience to climate stresses such as drought (Lüscher et al. 2022). Legume–grass mixtures can provide higher biomass production (Nyfeler et al. 2009; Sturludóttir et al. 2014) and nitrogen content (Nyfeler et al. 2011) than do grass monocultures. The inclusion of potentially anti-methanogenic legumes and herbs into grass-based pastures may reduce overall enteric CH4 emissions and the emissions intensity of meat and livestock production, as a result of both their nutritional quality and secondary chemistry (Ku-Vera et al. 2020; Badgery et al. 2023).
The forage species selected in this study were a subset of those grown in a long-term climate manipulation experiment on two-species pasture mixtures. They have previously been observed to reduce in vitro CH4 production and represent contrasts in life cycles (annual vs perennial), climates-of-origin (temperate vs tropical) and functional groups (legume vs herb). Biserrula pelecinus is an annual, temperature legume, which has been shown to provide high nutritional quality (Hackney et al. 2021; Mcgrath et al. 2021). It also contains plant secondary compounds (PSCs), including saponins, flavonoids and phenolics (Ghamkhar et al. 2018; Latif et al. 2020), and has been shown to inhibit CH4 production in vitro (Banik et al. 2013a, 2013b,2016). The temperate, perennial legume Onobrychis viciifolia contains condensed tannins (Malisch et al. 2016; Tava et al. 2022), is highly palatable (Scharenberg et al. 2007; Maughan et al. 2014) and has potential to reduce CH4 production (Hatew et al. 2016). Desmanthus virgatus is a tropical, annual legume that contains condensed and hydrolysable tannins (Vandermeulen et al. 2018) and has shown some capacity to reduce CH4 production, particularly compared with low-quality tropical grass diets (Suybeng et al. 2020). Research on Desmanthus spp. has predominantly occurred under tropical conditions; however, tropical forage species may become more relevant to temperate regions of Australia under future climate conditions. Finally, Cichorium intybus is a highly nutritious herb (Cranston et al. 2015), also known to contain PSCs such as condensed tannins (Scharenberg et al. 2007; Abbas et al. 2015), which have been associated with lower rumen CH4 emissions (Waghorn et al. 2002; Ramírez-Restrepo and Barry 2005).
Very few studies have looked at the indirect effects of climate extremes on the effectiveness of legumes and herbs in suppression of CH4 production from ruminal fermentation. However, climate stresses such as drought, flooding and increased temperatures have been shown to induce changes in plant morphology and biochemistry that result in differences in their ruminal fermentation characteristics (Riede et al. 2019; Hart et al. 2022). Such climate-driven shifts in plant quality may therefore lead to increases in enteric CH4 emissions under future climates, representing a positive feedback loop that would further exacerbate climate change (Lee et al. 2017). The objective of this study was to evaluate the effects of reduced rainfall and elevated temperature on the nutritional quality and predicted in vitro CH4 production from potential methanogenesis-inhibiting forages by using near-infrared spectroscopy (NIRS). Near-infrared spectroscopy models are frequently used to predict forage nutritional quality (Norman et al. 2015; Parrini et al. 2019; Catunda et al. 2022a) and, more recently, to predict in vitro CH4 production and other fermentation products (Grieder et al. 2011; Doublet et al. 2013; Ghilardelli et al. 2022), allowing for rapid, non-destructive and consistent analysis of large datasets. We hypothesised (H1) that simulated climate extremes would reduce the nutritional quality of the studied forage species, and that (H2), the NIRS-predicted CH4 production associated with their digestion would be increased in line with these nutritional changes.
Materials and methods
Site description
This study was conducted from the Australian spring (October 2023) to autumn (March 2024) at the Pastures and Climate Extremes (PACE) experimental field facility on the Hawkesbury Campus of Western Sydney University, Richmond, New South Wales, Australia (33°36′40″S, 150°44′43″E). The site has a mean annual rainfall of 727 ± 36 (s.e.) mm with high interannual variability (Australian Government Bureau of Meteorology, Richmond – UWS Hawkesbury Station 1992–2021, Site number-067021). In January (summer), the average daily minimum and maximum temperatures are 17°C and 29°C, and in July (winter), they are 3°C and 17°C (Bureau of Meteorology 2024a). The soil is a Blackendon Sand with a sandy loam texture (81% sand, 6% silt, 11% clay), with a water-holding capacity of 20–22%, approximately 1.8% organic matter, and in the top 15 cm, a pH of 5.7 (Chandregowda et al. 2023).
The PACE experimental field facility includes six polytunnel shelters made from galvanised steel frames and covered with a 250-μm single-layer polyethylene film (Solarweave, GALE Pacific Pty Ltd, Australia) to intercept all ambient rainfall. Each shelter measures 48 m long × 8 m wide and has a maximum height of 4.6 m, with open ends that are oriented on a south-west–north-east axis. Each shelter has combinations of pasture species pairs, growing in 2 m × 2 m subplots. Species are randomly assigned to plots/subplots receiving rainfall and warming treatments in a fully factorial design. Further details of the PACE facility can be found in Churchill et al. (2022).
Root barriers (VercanTM, textured high-density polyethylene, Argosee Greenhouse Technology Pty Ltd, Australia) were installed between plots to a depth of 90 cm between plots and 30 cm between subplots, minimising root intrusion and ensuring hydrological isolation. There are aboveground buffer zones of 1 m between plots and 0.5 m between subplots. In 2022, the top 5 cm of surface soil was replaced with a homogeneous, low-carbon soil to reduce heterogeneity among the plots.
Treatments and experimental design
The year-round rainfall treatments simulated extreme wet (La Niña) and dry (El Niño) conditions for the local area in south-eastern Australia, in line with predictions of increasingly common and more extreme rainfall regimes under future climates (CSIRO and Bureau of Meteorology 2024). By using 30 years of rainfall data (1992–2021) from the Bureau of Meteorology (Richmond, NSW, Station 067105), we identified the five wettest and driest years, which had mean annual rainfall totals of 1060 mm (Wet) and 570 mm (Dry) (Bureau of Meteorology 2024b). Representative irrigation schedules were generated by selecting two wet years (1999 and 2021) and two dry years (1994 and 2019), categorising rainfall events by size (<2 mm, 2–5 mm, 5–10 mm, 10–20 mm and >20 mm) for each season (Fig. 1). Events under 2 mm were removed and redistributed to larger events within the same season, to simulate the shift towards heavier, short-duration rainfall events (CSIRO and Bureau of Meteorology 2024). Rainfall was applied at the plot level by using a computer-controlled spray irrigation system.
Monthly rainfall (Dry and Wet treatments) and daily mean surface temperatures (°C) of ambient (aT) and elevated (eT) plots at the PACE facility.
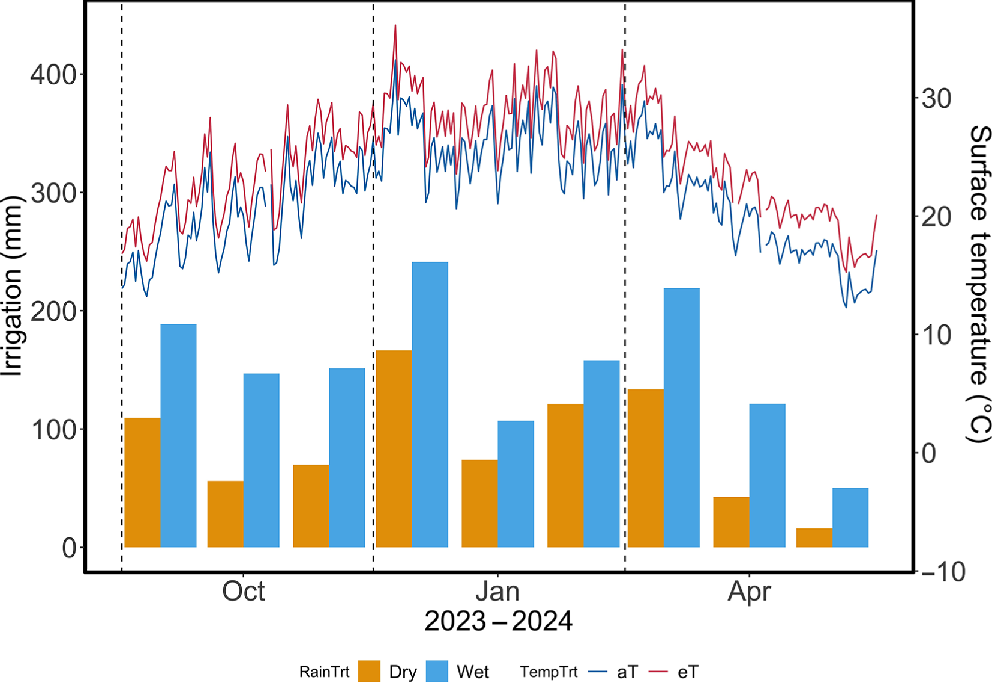
By the end of this century, the region is expected to warm by 2.5−4°C (Pearce et al. 2007; Sherwood et al. 2020; IPCC 2023). The warming treatment consisted of a year-round increase of +3°C (relative to ambient) in plot surface temperatures (Fig. 1). This was achieved using infrared heating arrays comprising eight 1000 W ceramic heaters (FTE 1000 W, Ceramicx, Ireland) installed 1.4 m above the ground and angled to uniformly heat the plot. Heater power was adjusted every minute via pulse-width modulation, controlled by a data logger (CR 1000, Campbell Scientific) with a proportional-integral-derivative algorithm. Target temperatures were regulated by feedback from IR sensors (SI-100, Apogee Instruments, Logan, UT, USA) mounted at 3.8 m, recording surface temperatures at 5-min intervals. Environmental conditions were monitored continuously in each shelter; a detailed overview is available in Churchill et al. (2022). Warming and rainfall treatments commenced in December 2022.
Species selection and sample collection
Two temperate legumes (Biserrula pelecinus and Onobrychis viciifolia), a temperate herb (Cichorium intybus) and a tropical legume (Desmanthus virgatus) were compared with a traditional temperate pasture legume, Medicago sativa (Table 1). M. sativa is widely used as forage, hay and silage globally (Bouton 2012) and is associated with, on average, slightly lower methane production than is perennial ryegrass (Banik et al. 2013a). Species were grown in two-species plots with suitable companion species of the same latitudinal origin (temperate or tropical) and complimentary growth cycles, to simulate a multi-species pasture environment. Samples were planted in experimental plots (n = 24) in factorial combination (n = 6 per warming × rainfall treatment). Species were sampled during their peak growth period (~30 cm height; Clements et al. 2003) in all plots with adequate growth (Table 1).
| Species | Plot type | Companion species | Origin | Lifecycle | Number of samples | |
|---|---|---|---|---|---|---|
| Medicago sativa (Cultivar-Sardi 7) | Med – Pha | Phalaris aquatic (Cultivar-Holdfast GT) | Temperate | Perennial | Spring: 24 Summer: 24 | |
| Biserrula pelecinus (Cultivar-Casbah) | Bis – Chic | Cichorium intybus (Cultivar-Puna 2) | Temperate | Annual | Spring: 21 | |
| Cichorium intybus (Cultivar-Puna 2) | Bis – Chic | Biserrula pelecinus (Cultivar-Casbah) | Temperate | Perennial | Summer: 24 Autumn: 24 | |
| Desmanthus virgatus (Cultivar-JCU2) | Des – Dig | Digitaria eriantha (Cultivar-Premier) | Tropical | Perennial | Spring: 23 Summer: 21 | |
| Onobrychis viciifolia (Cultivar-Othello) | Ono – Dac | Dactylis glomerata (Cultivar-Currie) | Temperate | Perennial | Spring: 19 Summer: 19 |
Nutritional quality
Each legume and herb species was sampled during peak growth (~30-cm height; Clements et al. 2003) in spring and summer (or in summer and autumn for C. intybus). Samples were frozen at −20°C, freeze-dried, and ground to pass through a 1-mm screen (Cyclone mill Twister; Retsch, Haan, Germany). Near-infrared spectroscopy (NIRS) was used to determine neutral detergent fibre (NDF), acid detergent fibre (ADF), crude protein (CP), dry organic-matter digestibility (DOMD), metabolisable energy (ME) and water-soluble carbohydrates (WSC) with a Bruker multi-purpose analyser (Bruker Optik, Ettlingen, Germany) and OPUS software package (Bruker Optik, OPUS ver. 9.0, https://bruker.com/en/products-and-solutions/infrared-and-raman/opus-spectroscopy-software.html, 2024). Calibrations were developed by the NSW Department of Primary Industries and Regional Development Feed Quality Service (NSW DPIRD FQS). A random 10% subsample was also analysed using wet chemistry to validate and ensure accuracy of the NIRS models used.
The NDF and ADF of samples used in the NIRS calibrations were measured using the methods of Van Soest et al. (1991) with an ANKOM fiber analyser (ANKOM Technology Corporation, Fairport, NY, USA). Crude protein was determined using the equation CP = N × 6.25 from nitrogen content measured by the Dumas combustion method (AFIA 2011). Dry organic-matter digestibility (DOMD) was assessed via pepsin–cellulase organic-matter digestibility and metabolisable energy, calculated as ME = 0.203 × DOMD − 3.001 (AFIA 2011). Water-soluble carbohydrates were measured by water extraction by using the alkaline ferricyanide method (AFIA 2011).
Prediction of total gas and CH4 production by using near-infrared spectroscopy
Total gas and CH4 production were predicted for all samples (n = 199; Table 1) by using NIRS. Spectra were collected on a Bruker MPA FT-NIR instrument, (Ettlingen, Germany) across a range of 12,500−3600 cm−1 (800−2770 nm) by using a resolution of 8 cm−1. In total, 32 scans were taken and averaged per sample. NIRS calibration models were previously developed from a diverse set of 343 freeze-dried forages, including all of the species covered in this study and validated using an independent set of 70 samples (Table 2).
| NIRS model | Number of calibration samples | Calibration R2 | Number of validation samples | SECV | RPD | |
|---|---|---|---|---|---|---|
| NDF | 950 | 0.930 | 981 | 3.9 | 3.8 | |
| ADF | 964 | 0.919 | 962 | 2.4 | 3.4 | |
| CP | 1622 | 0.981 | 1622 | 1.1 | 7.1 | |
| DOMD | 1114 | 0.934 | 1137 | 3.1 | 3.8 | |
| WSC | 1464 | 0.920 | 1489 | 1.9 | 3.5 | |
| CH4 production | 343 | 0.853 | 70 | 3.6 | 2.0 |
Validation R2 refers to the independent subset of samples measured for in vitro fermentation (n = 70).
SECV, standard error of calibration of cross-validation; RPD, ratio of performance to deviation, a measure of calibration quality.
Total gas and CH4 production values used to develop the NIRS calibrations were measured using 48-h in vitro fermentations as described in Li et al. (2025). For this study, one sample per species, season and treatment combination was randomly selected (n = 36) and in vitro fermentations were performed using the same method, to validate the NIRS predictions (Supplementary Table S1). Rumen fluid was collected from three fistulated cattle fed on a standardised diet of lucerne and oaten hay cubes (Multicube, NSW, Australia) at the Animal Nutrition Unit, Wagga Wagga Agricultural Institute. The fluid was transferred to pre-heated containers (39°C) and taken to the laboratory where half was directly strained through coarse muslin layers and fine cotton and the rest was homogenised, then strained through muslin cloth. Rumen fluid was mixed with a buffer solution at a 1:4 ratio and adjusted to pH 6.88. Buffer solution (4 L) contained NH4HCO3 4 g, NaHCO3 35 g, Na2HPO4 5.7 g, KH2PO4 6.2 g, MgSO4 · 7H2O 0.6 g, CaCl2 · 2H2O 0.066 g, MnCl2 · 4H2O 0.05 g, CoCl2 · 6H2O 0.005 g, FeCl3 · 6H2O 0.04 g, Cysteine · HCl 0.128 g, 1 N NaOH 0.8 mL, Na2S · 9H2O 0.128 g, resazurin 0.005 g, and trypticase 10 g.
The incubation was conducted in 250-mL glass bottles by using an Ankom RF Gas Production System (Ankom Technology, Macedon, NY, USA). Each run included one blank and three standards with known in vivo digestibility values. Bottles contained 1.00 ± 0.02 g of plant sample and 120 mL of buffer/rumen mixture (headspace volume = 190 mL). The headspace was flushed with nitrogen to ensure anaerobic conditions and bottles were fitted with an Ankom Gas Production Module to measure gas pressure at 10-min intervals. Bottles were incubated in a shaking water bath at 39°C, with automatic venting at 3 psi to prevent excessive pressure build-up.
Total gas production was measured throughout the incubation from headspace gas pressure and headspace gas composition was determined at the end of the 48-h incubation. At the completion of the fermentation, the gas sample was collected by inserting a syringe through a rubber septum in a sampling port located on the side of each fermentation jar, withdrawing 20 mL and then transferring this gas to a pre-evacuated 10-mL vacutainer. Gas samples were analysed for CH4 within 7 days, and typically within 2 days following the end of the incubation, on an SRI 8610 MG#5 gas chromatograph fitted with thermo-conductivity detector (TCD) and using a 2-m Hayesep D column, and a 1.5-m 5A molecular sieve column running high-purity helium as carrier gas. Samples of three standard species, including biserrula (B. pelecinus), white clover (Trifolium repens) and Sudan grass (Sorghum × drummondii), were analysed with each batch. Gas yield was corrected for a batch blank average, which was the amount of gas produced with the rumen fluid/artificial saliva mix incubated in the same batch. Methane yield for the entire fermentation period was expressed as mL/g dry matter at standard temperature and pressure.
Calculations
Gas pressure was converted to moles of gas produced according to the formula , where n is the gas produced (mol), p is the pressure (kPa), V is the headspace volume (L), T is the temperature (K), and R is the gas constant (8.314472 L × k × Pa / (K × mol)). This was converted to millilitres of gas produced by using the formula: Gas produced (mL) = n × 22.4 × 1000. CH4 production was also analysed as megajoules of metabolisable energy per kilogram of DM, to determine differences in CH4 emissions intensity relative to a nutritional metric strongly associated with meat and livestock production from forage systems.
Statistical analysis
All statistical analyses were performed using R (ver. 4.4.1; R Core Team 2024). Rainfall and warming treatments and their interactions were analysed with linear-mixed effects models by using the ‘lme4’ package (ver. 1.1-35.5, https://github.com/lme4; Bates et al. 2015), with rainfall and warming treatments as fixed factors and shelter as a random factor. P-values were obtained using the ANOVA function in the ‘car’ package (ver. 3.1-3, https://CRAN.R-project.org/package=car; Fox and Weisberg 2019). Post hoc pairwise comparisons among treatment combinations for each species per season were performed with the ‘emmeans’ package (ver. 1.10.5, https://rvlenth.github.io/emmeans; Lenth 2017). Whenever required, we applied natural logarithm, square root or Box–Cox transformations to meet normality assumptions.
Results
Nutritional quality under warming and altered precipitation regimes
All nutritional metrics varied significantly among species (P < 0.0001) and seasons (P < 0.0001), and for all except crude protein (CP) and water-soluble carbohydrates (WSC) there were significant interactions between species and seasons (P < 0.0001). The elevated temperature treatment resulted in significantly lower NDF and ADF for O. viciifolia in summer (Table 3), than the ambient temperature treatment. Acid detergent fibre was significantly higher in B. pelecinus (Table 3) grown under the low-rainfall treatment and D. virgatus and C. intybus grown under elevated temperature (Table 3).
| Species | Season | Treatment | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aT-W | aT-D | eT-W | eT-D | Rainfall | Temperature | Rainfall × temperature | |||
| Neutral detergent fibre (% DM) | |||||||||
| M. sativa | Spring | 52.0 ± 1.7 | 52.7 ± 1.0 | 52.1 ± 0.8 | 52.4 ± 1.1 | 0.667 | 0.940 | 0.864 | |
| Summer | 47.7 ± 2.4 | 47.4 ± 0.4 | 46.7 ± 0.7 | 47.2 ± 2.0 | 0.848 | 0.580 | 0.896 | ||
| B. pelecinus | Spring | 43.7 ±0.9 | 44.8 ± 1.0 | 43.4 ± 1.3 | 46.6 ± 0.8 | 0.063 | 0.460 | 0.302 | |
| D. virgatus | Spring | 34.7 ± 3.3 | 43.7 ± 2.7 | 39.5 ± 2.2 | 37.8 ± 2.7 | 0.237 | 0.657 | 0.059 | |
| Summer | 42.2 ± 0.5 | 42.3 ± 1.0 | 43.8 ± 1.2 | 44.8 ± 1.7 | 0.649 | 0.128 | 0.704 | ||
| O. viciifolia | Spring | 41.3 ± 1.0 | 40.9 ± 1.8 | 40.2 ± 0.2 | 43.5 ± 1.9 | 0.455 | 0.834 | 0.220 | |
| Summer | 38.7 ± 2.1 | 39.8 ± 3.0 | 31.0 ± 2.8 | 31.0 ± 1.7 | 0.867 | 0.004 | 0.901 | ||
| C. intybus | Summer | 27.7 ± 0.6 | 26.8 ± 0.6 | 27.3 ± 0.4 | 28.3 ± 0.7 | 0.976 | 0.274 | 0.073 | |
| Autumn | 18.1 ± 0.3 | 18.1 ± 0.4 | 18.3 ± 0.4 | 18.7 ± 0.3 | 0.493 | 0.299 | 0.646 | ||
| Acid detergent fibre (% DM) | |||||||||
| M. sativa | Spring | 37.7 ± 1.5 | 38.8 ± 0.7 | 37.6 ± 0.6 | 38.2 ± 0.7 | 0.344 | 0.711 | 0.789 | |
| Summer | 33.0 ± 0.8 | 33.2 ± 0.2 | 33.1 ± 0.4 | 32.7 ± 0.3 | 0.998 | 0.702 | 0.405 | ||
| B. pelecinus | Spring | 25.0 ± 0.8 | 26.3 ± 0.7 | 25.2 ± 0.8 | 27.5 ± 0.9 | 0.040 | 0.305 | 0.493 | |
| D. virgatus | Spring | 20.5 ± 2.5 | 25.9 ± 2.0 | 23.2 ± 1.1 | 22.2 ± 1.6 | 0.291 | 0.709 | 0.101 | |
| Summer | 25.4 ± 0.2 | 25.6 ± 0.8 | 27.6 ± 1.0 | 28.4 ± 1.4 | 0.621 | 0.026 | 0.822 | ||
| O. viciifolia | Spring | 31.3 ± 0.9 | 31.9 ± 1.5 | 31.0 ± 0.3 | 33.6 ± 1.8 | 0.318 | 0.609 | 0.450 | |
| Summer | 29.5 ± 1.5 | 29.8 ± 2.1 | 23.8 ± 1.9 | 23.4 ± 1.5 | 0.874 | 0.003 | 0.822 | ||
| C. intybus | Summer | 20.1 ± 0.3 | 19.3 ± 0.2 | 20.1 ± 0.3 | 20.7 ± 0.2 | 0.676 | 0.005 | 0.004 | |
| Autumn | 13.6 ± 0.3 | 13.8 ± 0.3 | 13.9 ± 0.3 | 13.8 ± 0.2 | 0.929 | 0.614 | 0.534 | ||
| Crude protein (% DM) | |||||||||
| M. sativa | Spring | 18.1 ± 1.6 | 17.4 ± 1.2 | 16.5 ± 1.3 | 17.8 ± 1.4 | 0.637 | 0.496 | 0.269 | |
| Summer | 19.9 ± 0.6 | 20.9 ± 1.3 | 21.7 ± 0.7 | 20.8 ± 0.9 | 0.978 | 0.251 | 0.207 | ||
| B. pelecinus | Spring | 15.0 ± 0.6 | 14.5 ± 0.4 | 14.9 ± 0.3 | 14.4 ± 0.2 | 0.278 | 0.644 | 0.857 | |
| D. virgatus | Spring | 15.0 ± 2.0 | 13.7 ± 1.3 | 15.3 ± 0.7 | 16.1 ± 1.1 | 0.878 | 0.236 | 0.472 | |
| Summer | 17.8 ± 1.1 | 17.1 ± 0.7 | 16.9 ± 0.6 | 17.5 ± 0.8 | 0.992 | 0.667 | 0.419 | ||
| O. viciifolia | Spring | 10.5 ± 0.7 | 11.1 ± 1.0 | 10.9 ± 0.8 | 9.3 ± 0.7 | 0.810 | 0.644 | 0.242 | |
| Summer | 12.0 ± 0.8 | 12.5 ± 1.4 | 15.2 ± 1.1 | 17.5 ± 1.0 | 0.154 | 0.001 | 0.553 | ||
| C. intybus | Summer | 12.9 ± 0.8 | 13.8 ± 0.3 | 12.9 ± 0.7 | 16 ± 1.0 | 0.017 | 0.181 | 0.160 | |
| Autumn | 14.8 ± 0.4 | 15.7 ± 0.5 | 16.9 ± 0.7 | 18.5 ± 0.4 | 0.029 | 0.0003 | 0.532 | ||
| Water-soluble carbohydrates (% DM) | |||||||||
| M. sativa | Spring | 5.6 ± 0.2 | 5.8 ± 0.3 | 5.3 ± 0.2 | 5.8 ± 0.3 | 0.236 | 0.516 | 0.478 | |
| Summer | 5.4 ± 0.2 | 5.6 ± 0.3 | 5.4 ± 0.3 | 5.9 ± 0.2 | 0.156 | 0.480 | 0.611 | ||
| B. pelecinus | Spring | 9.3 ± 0.5 | 9.0 ± 0.2 | 9.4 ± 0.7 | 8.9 ± 0.3 | 0.448 | 0.713 | 0.765 | |
| D. virgatus | Spring | 4.2 ± 0.3 | 4.9 ± 0.2 | 3.9 ± 0.2 | 5.1 ± 0.3 | 0.002 | 0.971 | 0.281 | |
| Summer | 4.6 ± 0.3 | 4.9 ± 0.3 | 4.2 ± 0.4 | 5.1 ± 0.4 | 0.049 | 0.430 | 0.321 | ||
| O. viciifolia | Spring | 6.9 ± 0.3 | 5.1 ± 0.5 | 7.2 ± 1.1 | 6.9 ± 0.9 | 0.107 | 0.151 | 0.486 | |
| Summer | 6.2 ± 0.7 | 5.8 ± 1.0 | 8.8 ± 0.5 | 8.4 ± 0.6 | 0.447 | 0.003 | 0.898 | ||
| C. intybus | Summer | 6.8 ± 0.3 | 8.0 ± 0.6 | 8.1 ± 0.9 | 6.6 ± 0.5 | 0.807 | 0.935 | 0.038 | |
| Autumn | 13.5 ± 0.4 | 12.0 ± 0.6 | 10.6 ± 0.6 | 9.9 ± 0.5 | 0.056 | 0.0002 | 0.426 | ||
| Dry organic matter digestibility (% DM) | |||||||||
| M. sativa | Spring | 62.2 ± 1.5 | 61.0 ± 1.0 | 61.8 ± 0.6 | 61.1 ± 0.8 | 0.326 | 0.893 | 0.803 | |
| Summer | 60.3 ± 3.3 | 63.9 ± 0.6 | 63.3 ± 0.4 | 60.2 ± 3.5 | 0.862 | 0.844 | 0.075 | ||
| B. pelecinus | Spring | 66.0 ± 0.8 | 65.5 ± 0.5 | 66.4 ± 0.8 | 64.5 ± 0.8 | 0.107 | 0.427 | 0.264 | |
| D. virgatus | Spring | 62.0 ± 2.7 | 60.8 ± 3.4 | 62.7 ± 1.7 | 62.8 ± 1.4 | 0.723 | 0.488 | 0.631 | |
| Summer | 52.1 ± 0.8 | 51.5 ± 0.7 | 50.4 ± 0.6 | 50.1 ± 0.8 | 0.440 | 0.031 | 0.793 | ||
| O. viciifolia | Spring | 58.9 ± 0.5 | 56.5 ± 0.7 | 58.5 ± 0.8 | 57.6 ± 1.1 | 0.012 | 0.907 | 0.566 | |
| Summer | 52.8 ± 1.2 | 51.3 ± 1.8 | 57.6 ± 1.2 | 58.5 ± 2.1 | 0.820 | 0.003 | 0.603 | ||
| C. intybus | Summer | 63.0 ± 0.9 | 63.6 ± 0.6 | 63.2 ± 0.5 | 63.1 ± 0.4 | 0.611 | 0.803 | 0.482 | |
| Autumn | 77.7 ± 0.3 | 77.1 ± 0.3 | 76.2 ± 0.4 | 76.1 ± 0.3 | 0.269 | 0.002 | 0.439 | ||
| Metabolisable energy (MJ/kg DM) | |||||||||
| M. sativa | Spring | 9.6 ± 0.3 | 9.3 ± 0.2 | 9.5 ± 0.1 | 9.4 ± 0.2 | 0.320 | 0.866 | 0.737 | |
| Summer | 9.2 ± 0.7 | 10.0 ± 0.1 | 9.9 ± 0.1 | 9.2 ± 0.7 | 0.858 | 0.857 | 0.070 | ||
| B. pelecinus | Spring | 10.4 ± 0.2 | 10.3 ± 0.1 | 10.5 ± 0.2 | 10.0 ± 0.2 | 0.091 | 0.451 | 0.254 | |
| D. virgatus | Spring | 9.6 ± 0.5 | 9.4 ± 0.7 | 9.7 ± 0.3 | 9.8 ± 0.3 | 0.722 | 0.431 | 0.631 | |
| Summer | 7.6 ± 0.2 | 7.4 ± 0.1 | 7.2 ± 0.1 | 7.2 ± 0.2 | 0.527 | 0.029 | 0.702 | ||
| O. viciifolia | Spring | 8.9 ± 0.1 | 8.4 ± 0.1 | 8.8 ± 0.2 | 8.6 ± 0.2 | 0.012 | 0.914 | 0.601 | |
| Summer | 7.7 ± 0.2 | 7.4 ± 0.4 | 8.6 ± 0.2 | 8.8 ± 0.4 | 0.800 | 0.003 | 0.604 | ||
| C. intybus | Summer | 9.8 ± 0.2 | 9.9 ± 0.1 | 9.8 ± 0.1 | 9.8 ± 0.1 | 0.434 | 0.881 | 0.541 | |
| Autumn | 12.8 ± 0.1 | 12.6 ± 0.1 | 12.5 ± 0.1 | 12.45 ± 0.1 | 0.268 | 0.004 | 0.372 | ||
Values are means ± s.e. Bold font represents statistical signficance (P < 0.05). aT-W, ambient temperature, Wet; aT-D, ambient temperature, Dry; eT-W, elevated temperature, Wet; eT-D, elevated temperature, Dry.
Crude protein was significantly higher in C. intybus under the low-rainfall treatment, than under the high-rainfall treatment, during both summer and autumn, but decreased under elevated temperature in summertime (Table 3). The elevated-temperature treatment also resulted in significantly decreased CP in O. viciifolia during summer. Spring and summer WSC were significantly higher in D. virgatus in the low-rainfall than high-rainfall treatment. Furthermore, the elevated-temperature treatment resulted in significantly higher WSC in O. viciifolia in summer, but a significantly lower WSC in C. intybus during autumn (Table 3) than in the ambient temperature treatment.
Springtime DOMD and ME were significantly lower in O. viciifolia grown under the low-rainfall treatment; values were also lower under elevated than ambient temperature for D. virgatus (summer) and C. intybus (autumn), but were significantly increased by warming in O. viciifolia (summer) (Table 3).
Effects of climate treatments on total gas production
Total gas production varied significantly among species (P < 0.0001) and seasons (P < 0.0001), and there were significant (P < 0.0001) species × season interactions. The low-rainfall treatment resulted in a significant reduction in total gas production from D. virgatus (spring), O. viciifolia (spring) and C. intybus (autumn) (Supplementary Fig. S1). Total gas production was significantly lower under elevated temperature for B. pelecinus (spring), D. virgatus (spring) and C. intybus (autumn), and there was also a significant treatment interaction for C. intybus, with the lowest gas production being evident under the combination of elevated-temperature and low-rainfall treatments (eT-D).
Effects of climate treatments on CH4 production
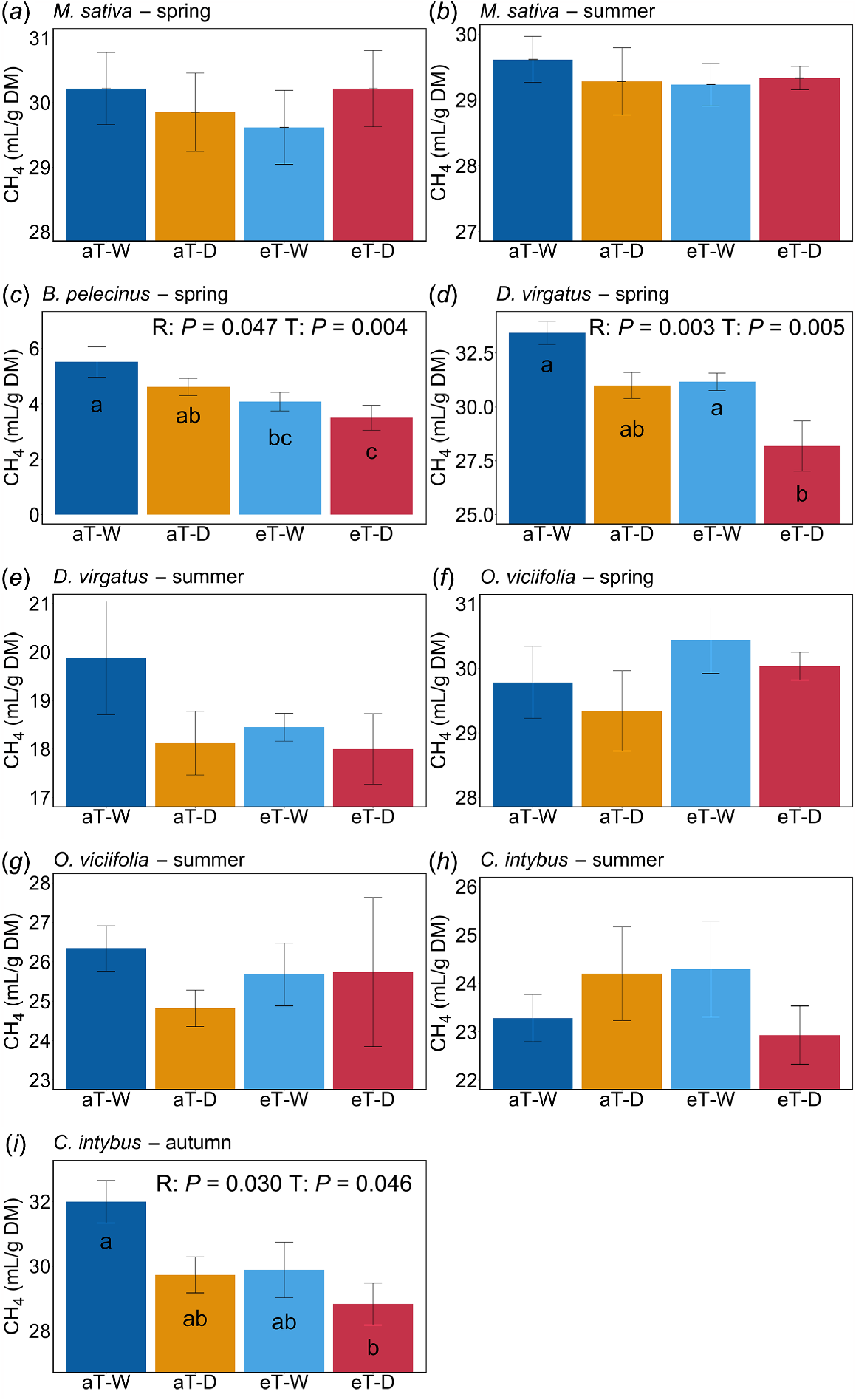
Methane (CH4) production varied significantly among species (P < 0.0001) and seasons (P < 0.0001). There was also a significant (P < 0.0001) species × season interaction. The low-rainfall and elevated-temperature treatments resulted in significant reductions in CH4 production from B. pelecinus (spring) (Fig. 2c), D. virgatus (spring) (Fig. 2d) and C. intybus (autumn) (Fig. 2i).
NIRS-predicted in vitro methane (CH4) production in response to factorial rainfall and temperature treatments from M. sativa (a and b), B. pelecinus (c), D. virgatus (d and e), O. viciifolia (f and g) and C. intybus (h and i). Different letters on bar plots indicate significant treatment differences by post hoc pairwise comparisons. Treatment abbreviations are as described in Table 2. R, rainfall treatment. T, remperature treatment.
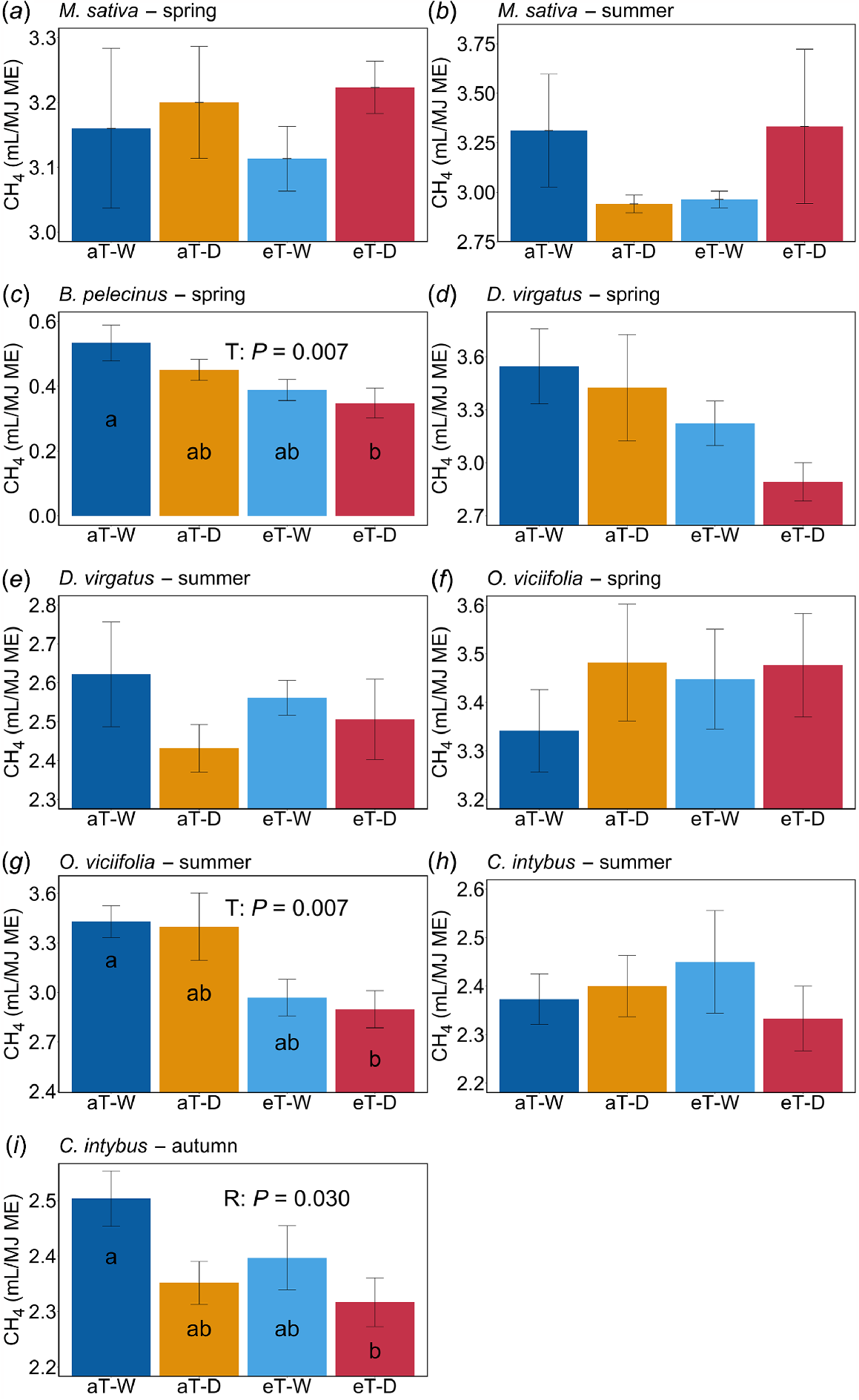
Treatment effects on CH4 production varied when considered per megajoule of metabolisable energy provided by the forage (CH4/MJ). The low-rainfall treatment resulted in a significantly lower CH4/MJ from C. intybus (autumn), than did the high-rainfall treatment. The elevated-temperature treatment was associated with significantly lower CH4/MJ from B. pelecinus (spring) and O. viciifolia (summer) (Fig. 3), than was the ambient-temperature treatment.
(a–i) NIRS-predicted in vitro methane (CH4) production, per unit of metabolisable energy content, in response to factorial rainfall and temperature treatments from M. sativa (a and b), B. pelecinus (c), D. virgatus (d and e), O. viciifolia (f and g) and C. intybus (h and i). Different letters on bar plots indicate significant treatment differences by post hoc pairwise comparisons. Climate treatment abbreviations are as described in Table 2 and Fig. 2.
Discussion
In this study, we evaluated the individual and combined effects of warming and rainfall extremes on NIRS predictions of nutritional quality and CH4 production associated with fermentation of a range of forage species to determine their potential to reduce CH4 emissions from pasture grazing under current and future climate conditions. The effects of the climate treatments differed by species and seasons, highlighting the need to consider the role of species’ phenology and seasonality when evaluating the effects of climate on associated CH4 emissions. NIRS models were effective at rapidly assessing the differences in nutritional quality and predicted CH4 production associated with the consumption of forages by ruminants.
Plant exposure to climate stress, i.e. elevated temperature, reduced rainfall and the combination of both treatments, progressively reduced the predicted in vitro CH4 production, per unit of forage mass, associated with three of our study species, i.e. B. pelecinus, D. virgatus and C. intybus, in at least one of the seasons tested. Interestingly, in the case of B. pelecinus and D. virgatus, this cannot readily be explained by the observed nutritional changes at that time. We speculate that plants grown under more extreme climates may have had higher levels of anti-methanogenic PSCs. For B. pelecinus, a clear relationship is emerging between its anti-methanogenic capabilities and its saponins (Ghamkhar et al. 2018; Li et al. 2025), as well as other PSCs (Latif et al. 2020). Desmanthus spp. contain hydrolysable and condensed tannins, which may reduce CH4 production (Vandermeulen et al. 2018) and could also be responsive to climate conditions, both in overall quantity and molecular composition, as has been observed in other plants (Top et al. 2017; Gourlay et al. 2022). PSCs are responsive to environmental conditions (Moore et al. 2014) and can be increased or decreased by abiotic stresses such as increased temperatures, elevated CO2, UV and drought (Robinson et al. 2012; Kumari et al. 2022; Zandalinas et al. 2022; Ahmad et al. 2023; Trush and Pal’ove-Balang 2023). Additionally, B. pelecinus and D. virgatus grown under climate stress were less fermentable, as both species also had a concomitant decrease in total gas production under warming and reduced rainfall. Reductions in the fermentation may be related to nutritional changes not apparent within our results; for example, a shift in ADF content to less degradable fibres and a higher degree of lignification (Tiemann et al. 2008) or an increase in other indigestible components such as silica (Villalba and Burritt 2015) or calcium oxalate (Han et al. 2015). This may have resulted in lower fermentation of CH4 but also lower quantities of other fermentation end-products. The effects of drought stress on forage growth have been previously observed to reduce organic-matter degradation and overall digestibility, resulting in reduced CH4 production, for other temperate forages (Riede et al. 2019; Hart et al. 2022). However, unlike in our study, Hart et al. (2022) did not observe a significant effect of elevated growing temperature on subsequent ruminal CH4 production from forages. Further research is required to understand the influence of climate stress on the PSCs in B. pelecinus and D. virgatus as well as the broader fermentation parameters associated with ruminant digestion of forage grown under varying climate conditions.
Of note, because B. pelecinus is an annual legume, samples were collected only during spring, following the peak of its growth. Banik et al. (2019) has shown that the inhibition of CH4 production associated with B. pelecinus is maintained across growth stages and post-harvesting as well as in regrowth under glasshouse conditions. Under field conditions in our study, regrowth in the summer was too limited for analysis. Despite this species being associated with the greatest inhibition of CH4 production during fermentation in our study, its low and highly seasonal biomass production may limit its ability to reduce enteric emissions throughout the livestock lifecycle in many regions.
The effects of the low-rainfall treatment on temperate legumes were evident because reductions in nutritional quality in B. pelecinus (increased ADF) and O. viciifolia (reduced DOMD and ME) were observed. Reported effects of reduced rainfall and drought on forage nutritional quality in the literature have been inconsistent; this may be due to variations in the drought intensity used and differences among plants and in local climatic conditions among studies (Sollenberger and Kohmann 2024). Previous studies have also observed that the nutritional quality of temperate legumes is sensitive to severe reductions in rainfall (Liu et al. 2018; Catunda et al. 2022b). Plant responses to drought can include increased cell wall allocation and lignification (Malavasi et al. 2016; Song et al. 2019; Yang et al. 2021; Blaschek et al. 2024), as well as reduced biomass production and leaf:stem ratio (Catunda et al. 2022b), all of which can contribute to higher ADF content and lower digestibility.
There were some instances where some aspects of nutritional quality were higher under climate stress; this included C. intybus, which had higher CP content under reduced rainfall in both of the seasons studied. This may be attributable to a reduction in plant growth (Grant et al. 2014) resulting in a lower growth dilution and therefore a greater concentration of CP; increases in CP content have been reported in a meta-analysis of forage responses to drought (Dumont et al. 2015). Increases in WSCs under low rainfall were also observed for D. virgatus and C. intybus (the latter only at ambient temperature), findings that have previously been reported in forage legumes (Küchenmeister et al. 2013) and herbs (Du et al. 2020) under drought. Water soluble carbohydrates, as well as other non-structural carbohydrates such as starch, act as a reserve of carbohydrates and their accumulation under drought is likely to be due to a disruption in plant growth and decreased development of structural fibres (De Roover et al. 2000; Fulkerson and Donaghy 2001; Martínez-Vilalta et al. 2016; Kagan 2022), as well as relating to their role in maintaining physiological and osmotic regulation of biochemical processes (Martínez-Vilalta et al. 2016; Gaur et al. 2022). These improvements in nutritional quality resulted in a lower CH4 production associated with C. intybus grown under climate stress, but this may come at the expense of biomass production and long-term plant resilience. Also related to its nutritional quality, C. intybus provided the second lowest CH4 emissions intensity, second only to B. pelecinus, which was associated with a near-total suppression of CH4 production. Production efficiency can be increased by improving digestibility and voluntary intake, and by suppressing enteric CH4 production through differences in nutritional composition and PSCs (Hristov et al. 2013). Our results support the proposition that C. intybus and other highly digestible forages could improve the feed efficiency of ruminant grazing systems, resulting in lowered CH4 emissions intensity of the meat and livestock industry (Hegarty et al. 2010; Hyland et al. 2016).
Elevated temperatures have been shown to negatively affect nutritional quality of many forage plants (Newman et al. 2005; Habermann et al. 2021; Rogers et al. 2022); this was also observed in our study for D. virgatus and C. intybus. During summer, elevated temperatures resulted in an increased ADF content of D. virgatus, as well as lower DOMD and ME. The higher ADF content, and the resulting lower DOMD and ME, may be related to changes in the formation of plant cell walls, which can be caused by heat stress, including increased synthesis of lignin and structural changes in the lignin produced (Le Gall et al. 2015), as well as accelerated senescence (Catunda et al. 2022b; Zhang et al. 2024). In contrast, elevated temperatures resulted in improved summer nutritional quality of O. viciifolia, with lower NDF and ADF contents and increased CP, WSC, DOMD and ME. This is likely to be due to a large reduction in biomass production in warmed plots, as growth of this species is known to be limited by high temperatures (Kallenbach et al. 1996). In general, increasing temperatures are likely to be detrimental to legume nutritional quality, unless harvesting or grazing frequency is increased to account for faster plant maturation and to provide more nutritious, vegetative material (Thivierge et al. 2016). Temperate legumes such as O. viciifolia may also require further breeding efforts to produce more heat-resilient cultivars.
Although a range of nutritional differences was observed as a result of the reduced rainfall and elevated temperature treatments, these did not directly result in differences in in vitro CH4 production in many cases. It may be the case that the reductions in nutritional quality, although significant, were not large enough to induce a significant difference in ruminal fermentation. Despite reductions in nutritional quality, such as higher fibre content, being shown to generally increase CH4 production (Eugène et al. 2014), CH4 production in the rumen is influenced by many factors and it is known that differences in nutritional quality and PSCs can play opposing roles (e.g. lower nutritional quality but higher PSCs) resulting in comparable CH4 production values (Suybeng et al. 2021).
Conclusions
This study demonstrated the significant impacts that climate stresses, such as elevated temperatures and reduced rainfall, can have on both the nutritional quality and the CH4 production from ruminal fermentation of a range of potentially anti-methanogenic pasture species. A key finding was that increased temperatures and reduced rainfall decreased NIRS-predicted in vitro CH4 production from three of the study species but that this did not directly relate to measured differences in nutritional quality. This was contrary to our hypothesis that climate stresses would reduce nutritional quality and result in higher CH4 production during fermentation. Further research is required to understand how climate treatments affect the other end-products of ruminant digestion, such as volatile fatty acids, to determine whether the observed species-specific and climate-driven reductions in CH4 observed would have knock-on compromising effects on livestock productivity. Reducing enteric CH4 emissions is an urgent target for the agricultural industry and it is clear that effects of climate stresses and environmental conditions on forage growth, digestibility and associated CH4 emissions must be considered.
Data availability
The data that support this study will be available upon reasonable request to the corresponding author.
Declaration of funding
This project was supported by funding from the Meat and Livestock Australia’s Donor Company (P.PSH.2009 and P.PSH.2014).
Acknowledgements
We thank Burhan Amiji, Craig Barton, Chick Chilby, Nor Azizah Kusai, Ephrem Ngendahimana, Awais Shakoor, Pankaj Tiwari and Nick Wright-Osment for their valuable assistance in the field. We thank NSW Department of Primary Industries and Regional Development Feed Quality Service (NSW DPIRD FQS) for laboratory support with nutritional quality analysis.
References
Abbas ZK, Saggu S, Sakeran MI, Zidan N, Rehman H, Ansari AA (2015) Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi Journal of Biological Sciences 22(3), 322-326.
| Crossref | Google Scholar | PubMed |
Ahmad S, Belwal V, Punia SS, Ram M, Dalip , Rajput SS, Kunwar R, Meena MK, Gupta D, Kumawat GL, Hussain T, Mohamed HI (2023) Role of plant secondary metabolites and phytohormones in drought tolerance: a review. Gesunde Pflanzen 75, 729-746.
| Crossref | Google Scholar |
Archimède H, Eugène M, Marie Magdeleine C, Boval M, Martin C, Morgavi DP, Lecomte P, Doreau M (2011) Comparison of methane production between C3 and C4 grasses and legumes. Animal Feed Science and Technology 166–167, 59-64.
| Crossref | Google Scholar |
Badgery W, Li G, Simmons A, Wood J, Smith R, Peck D, Ingram L, Durmic Z, Cowie A, Humphries A, Hutton P, Winslow E, Vercoe P, Eckard R (2023) Reducing enteric methane of ruminants in Australian grazing systems – a review of the role for temperate legumes and herbs. Crop & Pasture Science 74, 661-679.
| Crossref | Google Scholar |
Banik BK, Durmic Z, Erskine W, Ghamkhar K, Revell C (2013a) In vitro ruminal fermentation characteristics and methane production differ in selected key pasture species in Australia. Crop & Pasture Science 64(9), 935-942.
| Crossref | Google Scholar |
Banik BK, Durmic Z, Erskine W, Nichols P, Ghamkhar K, Vercoe P (2013b) Variability of in vitro ruminal fermentation and methanogenic potential in the pasture legume biserrula (Biserrula pelecinus L.). Crop & Pasture Science 64(4), 409-416.
| Crossref | Google Scholar |
Banik BK, Durmic Z, Erskine W, Revell CK, Vadhanabhuti J, McSweeney CS, Padmanabha J, Flematti GR, Algreiby AA, Vercoe PE (2016) Bioactive fractions from the pasture legume Biserrula pelecinus L. have an anti-methanogenic effect against key rumen methanogens. Anaerobe 39, 173-182.
| Crossref | Google Scholar | PubMed |
Banik BK, Durmic Z, Erskine W, Revell CK (2019) Anti-methanogenic advantage of biserrula (Biserrula pelecinus) over subterranean clover (Trifolium subterraneum) from in vitro fermentation is maintained across growth stages and cutting treatments. Crop & Pasture Science 70(3), 263-272.
| Crossref | Google Scholar |
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1-48.
| Crossref | Google Scholar |
Beauchemin KA, Ungerfeld EM, Eckard RJ, Wang M (2020) Review: fifty years of research on rumen methanogenesis: lessons learned and future challenges for mitigation. Animal 14(1), s2-s16.
| Crossref | Google Scholar | PubMed |
Blaschek L, Serk H, Pesquet E (2024) Functional complexity on a cellular scale: why in situ analyses are indispensable for our understanding of lignified tissues. Journal of Agricultural and Food Chemistry 72(24), 13552-13560.
| Crossref | Google Scholar |
Bouton JH (2012) An overview of the role of lucerne (Medicago sativa L.) in pastoral agriculture. Crop & Pasture Science 63(9), 734-738.
| Crossref | Google Scholar |
Bureau of Meteorology (2024a) Climate Statistics for Australian Locations: Summary Statistics RICHMMOND - UWS HAWKESBURY. Available at http://www.bom.gov.au/climate/avergaes/tables/cw_067021.shtml [accessed 2 December 2024]
Bureau of Meteorology (2024b) Climate Statistics for Australian Locations: Summary Statistics RICHMOND RAAF. Available at https://bom.gov.au/climate/averages/tables/cw_067105shtml [accessed 2 December 2024]
Cai W, Borlace S, Lengaigne M, Van Rensch P, Collins M, Vecchi G, Timmermann A, Santoso A, McPhaden MJ, Wu L, England MH, Wang G, Guilyardi E, Jin F-F (2014) Increasing frequency of extreme El Niño events due to greenhouse warming. Nature Climate Change 4, 111-116.
| Crossref | Google Scholar |
Catunda KLM, Churchill AC, Power SA, Moore BD (2022a) Near infrared spectroscopy calibration strategies to predict multiple nutritional parameters of pasture species from different functional groups. Journal of Near Infrared Spectroscopy 30(5), 254-263.
| Crossref | Google Scholar |
Catunda KLM, Churchill AC, Zhang H, Power SA, Moore BD (2022b) Short-term drought is a stronger driver of plant morphology and nutritional composition than warming in two common pasture species. Journal of Agronomy and Crop Science 208(6), 841-852.
| Crossref | Google Scholar |
Chandregowda MH, Tjoelker MG, Pendall E, Zhang H, Churchill AC, Power SA (2023) Belowground carbon allocation, root trait plasticity, and productivity during drought and warming in a pasture grass. Journal of Experimental Botany 74(6), 2127-2145.
| Crossref | Google Scholar | PubMed |
Chen L, Li T, Yu Y, Behera SK (2017) A possible explanation for the divergent projection of ENSO amplitude change under global warming. Climate Dynamics 49, 3799-3811.
| Crossref | Google Scholar |
Chung CTY, Power SB (2016) Modelled impact of global warming on ENSO-driven precipitation changes in the tropical Pacific. Climate Dynamics 47, 1303-1323.
| Crossref | Google Scholar |
Churchill AC, Zhang H, Fuller KJ, Amiji B, Anderson IC, Barton CVM, Carrillo Y, Catunda KLM, Chandregowda MH, Igwenagu C, Jacob V, Kim GW, Macdonald CA, Medlyn BE, Moore BD, Pendall E, Plett JM, Post AK, Powell JR, Tissue DT, Tjoelker MG, Power SA (2022) Pastures and climate extremes: impacts of cool season warming and drought on the productivity of key pasture species in a field experiment. Frontiers in Plant Science 13, 836968.
| Crossref | Google Scholar | PubMed |
Clements B, Ayres L, Langford C, McGarva L, Simpson P, Hennessy G, Keys M, Upjohn B, Leech F (2003) ‘The grazier’s guide to pastures: sowing and managing profitable pastures in the central and southern tablelands, monaro and upper south west slopes of New South Wales.’ (NSW Agriculture: Orange, NSW, Australia)
Collins M, An S-I, Cai W, Ganachaud A, Guilyardi E, Jin F-F, Jochum M, Lengaigne M, Power S, Timmermann A, Vecchi G, Wittenberg A (2010) The impact of global warming on the tropical Pacific Ocean and El Niño. Nature Geoscience 3, 391-397.
| Crossref | Google Scholar |
Collins WJ, Webber CP, Cox PM, Huntingford C, Lowe J, Sitch S, Chadburn SE, Comyn-Platt E, Harper AB, Hayman G, Powell T (2018) Increased importance of methane reduction for a 1.5 degree target. Environmental Research Letters 13(5), 054003.
| Crossref | Google Scholar |
Cranston LM, Kenyon PR, Morris ST, Kemp PD (2015) A review of the use of chicory, plantain, red clover and white clover in a sward mix for increased sheep and beef production. Journal of New Zealand Grasslands 77, 89-94.
| Crossref | Google Scholar |
Crippa M, Solazzo E, Guizzardi D, Monforti-Ferrario F, Tubiello FN, Leip A (2021) Food systems are responsible for a third of global anthropogenic GHG emissions. Nature Food 2, 198-209.
| Crossref | Google Scholar | PubMed |
CSIRO and Bureau of Meteorology (2024) State of the Climate 2024. (Government of Australia) Available at https://bom.gov.au/state-of-the-climate/
De Roover J, Vandenbranden K, Van Laere A, Van den Ende W (2000) Drought induces fructan synthesis and 1-SST (sucrose: sucrose fructosyltransferase) in roots and leaves of chicory seedlings (Cichorium intybus L.). Planta 210, 808-814.
| Crossref | Google Scholar | PubMed |
Dlugokencky EJ, Nisbet EG, Fisher R, Lowry D (2011) Global atmospheric methane: budget, changes and dangers. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 369, 2058-2072.
| Crossref | Google Scholar |
Doublet J, Boulanger A, Ponthieux A, Laroche C, Poitrenaud M, Cacho Rivero JA (2013) Predicting the biochemical methane potential of wide range of organic substrates by near infrared spectroscopy. Bioresource Technology 128, 252-258.
| Crossref | Google Scholar | PubMed |
Du Y, Lu R, Xia J (2020) Impacts of global environmental change drivers on non-structural carbohydrates in terrestrial plants. Functional Ecology 34(8), 1525-1536.
| Crossref | Google Scholar |
Dumont B, Andueza D, Niderkorn V, Lüscher A, Porqueddu C, Picon-Cochard C (2015) A meta-analysis of climate change effects on forage quality in grasslands: specificities of mountain and Mediterranean areas. Grass and Forage Science 70(2), 239-254.
| Crossref | Google Scholar |
Eugène M, Archimède H, Giger-Reverdin S, Doreau M, Sauvant D (2014) Effect of feeding forages on enteric methane emissions from ruminants: a meta-analysis. In ‘Joint ISNH/ISRP International Conference 2014: Harnessing the ecology and physiology of herbivores’, September 2014, Canberra, ACT, Australia. p. 30. (Proceedings of the Australian Society of Animal Production)
Forster P, Storelvmo T, Armour K, Collins W, Dufresne J-L, Frame D, Lunt D, Mauritsen T, Palmer M, Watanabe M, Wild M, Zhang H (2021) Chapter 7: the Earth’s energy budget, climate feedbacks, and climate sensitivity. Climate change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change.
Fox J, Weisberg S (2019) ‘An R companion to applied regression.’ 3rd edn. (Sage: Thousand Oaks, CA, USA) Available at https://www.john-fox.ca/Companion/
Fulkerson WJ, Donaghy DJ (2001) Plant-Soluble carbohydrate reserves and senescence - key criteria for developing an effective grazing management system for ryegrass-based pastures: a review. Australian Journal of Experimental Agriculture 41(2), 261-275.
| Crossref | Google Scholar |
Gaur A, Sharma D, Sheoran S, Chahal S, Chaudhary K, Singh G, Singh GP (2022) Role of water soluble carbohydrates in improving drought stress tolerance in wheat: an overview. Journal of Cereal Research 13,.
| Crossref | Google Scholar |
Ghamkhar K, Rochfort S, Banik BK, Revell C (2018) Candidate metabolites for methane mitigation in the forage legume biserrula. Agronomy for Sustainable Development 38, 30.
| Crossref | Google Scholar |
Ghilardelli F, Ferronato G, Gallo A (2022) Near-infrared calibration models for estimating volatile fatty acids and methane production from in vitro rumen fermentation of different total mixed rations. JDS Communications 3(1), 19-25.
| Crossref | Google Scholar | PubMed |
Gourlay G, Hawkins BJ, Albert A, Schnitzler J-P, Peter Constabel C (2022) Condensed tannins as antioxidants that protect poplar against oxidative stress from drought and UV-B. Plant, Cell & Environment 45(2), 362-377.
| Crossref | Google Scholar | PubMed |
Grant K, Kreyling J, Dienstbach LFH, Beierkuhnlein C, Jentsch A (2014) Water stress due to increased intra-annual precipitation variability reduced forage yield but raised forage quality of a temperate grassland. Agriculture, Ecosystems & Environment 186, 11-22.
| Crossref | Google Scholar |
Grieder C, Mittweg G, Dhillon BS, Montes JM, Orsini E, Melchinger AE (2011) Determination of methane fermentation yield and its kinetics by near infrared spectroscopy and chemical composition in maize. Journal of Near Infrared Spectroscopy 19(6), 463-477.
| Crossref | Google Scholar |
Habermann E, Dias de Oliveira EA, Delvecchio G, Belisário R, Barreto RF, Viciedo DO, Rossingnoli NO, de Pinho Costa KA, de Mello Prado R, Gonzalez-Meler M, Martinez CA (2021) How does leaf physiological acclimation impact forage production and quality of a warmed managed pasture of Stylosanthes capitata under different conditions of soil water availability? Science of The Total Environment 759, 143505.
| Crossref | Google Scholar | PubMed |
Hackney B, Rodham C, Dyce G, Piltz J (2021) Pasture legumes differ in herbage production and quality throughout spring, impacting their potential role in fodder conservation and animal production. Grass and Forage Science 76(1), 116-133.
| Crossref | Google Scholar |
Han H, Segal AM, Seifter JL, Dwyer JT (2015) Nutritional management of kidney stones (Nephrolithiasis). Clinical Nutrition Research 4(3), 137-152.
| Crossref | Google Scholar | PubMed |
Hart EH, Christofides SR, Davies TE, Rees Stevens P, Creevey CJ, Müller CT, Rogers HJ, Kingston-Smith AH (2022) Forage grass growth under future climate change scenarios affects fermentation and ruminant efficiency. Scientific Reports 12, 4454.
| Crossref | Google Scholar | PubMed |
Hatew B, Stringano E, Mueller-Harvey I, Hendriks WH, Carbonero CH, Smith LMJ, Pellikaan WF (2016) Impact of variation in structure of condensed tannins from sainfoin (Onobrychis viciifolia) on in vitro ruminal methane production and fermentation characteristics. Journal of Animal Physiology and Animal Nutrition 100(2), 348-360.
| Crossref | Google Scholar | PubMed |
Hegarty RS, Alcock D, Robinson DL, Goopy JP, Vercoe PE (2010) Nutritional and flock management options to reduce methane output and methane per unit product from sheep enterprises. Animal Production Science 50(12), 1026-1033.
| Crossref | Google Scholar |
Henderson G, Cox F, Ganesh S, Jonker A, Young W, Global Rumen Census Collaborators, Janssen PH (2015) Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Scientific Reports 5, 14567.
| Crossref | Google Scholar |
Hristov AN, Oh J, Firkins JL, Dijkstra J, Kebreab E, Waghorn G, Makkar HPS, Adesogan AT, Yang W, Lee C, Gerber PJ, Henderson B, Tricarico JM (2013) SPECIAL TOPICS – mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. Journal of Animal Science 91(11), 5045-5069.
| Crossref | Google Scholar | PubMed |
Hyland JJ, Styles D, Jones DL, Williams AP (2016) Improving livestock production efficiencies presents a major opportunity to reduce sectoral greenhouse gas emissions. Agricultural Systems 147, 123-131.
| Crossref | Google Scholar |
IPCC (2023) Climate change 2023: synthesis report. Contribution of working groups I, II and III to the sixth assessment report of the intergovernmentall panel on climate change. IPCC, Geneva, Switzerland. Available at https://doi.org/10.59327/IPCC/AR6-9789291691647
Jaramillo DM, Sheridan H, Soder K, Dubeux JCB, Jr (2021) Enhancing the sustainability of temperate pasture systems through more diverse swards. Agronomy 11(10), 1912.
| Crossref | Google Scholar |
Johnson KA, Johnson DE (1995) Methane emissions from cattle. Journal of Animal Science 73(8), 2483-2492.
| Crossref | Google Scholar | PubMed |
Kagan IA (2022) Water- and ethanol-soluble carbohydrates of temperate grass pastures: a review of factors affecting concentration and composition. Journal of Equine Veterinary Science 110, 103866.
| Crossref | Google Scholar | PubMed |
Kallenbach RL, Matches AG, Mahan JR (1996) Sainfoin regrowth declines as metabolic rate increases with temperature. Crop Science 36(1), 91-97.
| Crossref | Google Scholar |
Knapp JR, Laur GL, Vadas PA, Weiss WP, Tricarico JM (2014) Invited review: enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. Journal of Dairy Science 97(6), 3231-3261.
| Crossref | Google Scholar | PubMed |
Ku-Vera JC, Jiménez-Ocampo R, Valencia-Salazar SS, Montoya-Flores MD, Molina-Botero IC, Arango J, Gómez-Bravo CA, Aguilar-Pérez CF, Solorio-Sánchez FJ (2020) Role of secondary plant metabolites on enteric methane mitigation in ruminants. Frontiers in Veterinary Science 7, 584.
| Crossref | Google Scholar |
Küchenmeister K, Küchenmeister F, Kayser M, Wrage-Mönnig N, Isselstein J (2013) Influence of drought stress on nutritive value of perennial forage legumes. International Journal of Plant Production 7(4), 693-710.
| Google Scholar |
Kumari A, Lakshmi GA, Krishna GK, Patni B, Prakash S, Bhattacharyya M, Singh SK, Verma KK (2022) Climate change and its impact on crops: a comprehensive investigation for sustainable agriculture. Agronomy 12(12), 3008.
| Crossref | Google Scholar |
Latif S, Weston PA, Barrow RA, Gurusinghe S, Piltz JW, Weston LA (2020) Metabolic profiling provides unique insights to accumulation and biosynthesis of key secondary metabolites in annual pasture legumes of Mediterranean origin. Metabolites 10(7), 267.
| Crossref | Google Scholar | PubMed |
Le Gall H, Philippe F, Domon J-M, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants 4(1), 112-166.
| Crossref | Google Scholar |
Lee MA, Davis AP, Chagunda MGG, Manning P (2017) Forage quality declines with rising temperatures, with implications for livestock production and methane emissions. Biogeosciences 14, 1403-1417.
| Crossref | Google Scholar |
Lenth R (2017) emmeans: Estimated Marginal Means, aka Least-Squares Means. Available at https://doi.org/10.32614/CRAN.package.emmeans
Li GD, Newell MT, Boschma SP, Meyer R, Wood JA, Badgery WB, Hayes RC (2025) Agronomic performance, herbage quality, methane yield and methane emission potential of pasture mixtures. Crop & Pasture Science 76(4), CP24356.
| Crossref | Google Scholar |
Lieber R, Brown J, King A, Freund M (2024) Historical and future asymmetry of ENSO teleconnections with extremes. Journal of Climate 37(22), 5909-5924.
| Crossref | Google Scholar |
Liu Y, Wu Q, Ge G, Han G, Jia Y (2018) Influence of drought stress on afalfa yields and nutritional composition. BMC Plant Biology 18, 13.
| Crossref | Google Scholar | PubMed |
Lüscher A, Barkaoui K, Finn JA, Suter D, Suter M, Volaire F (2022) Using plant diversity to reduce vulnerability and increase drought resilience of permanent and sown productive grasslands. Grass and Forage Science 77(4), 235-246.
| Crossref | Google Scholar |
Malavasi UC, Davis AS, Malavasi MdM (2016) Lignin in woody plants under water stress: a review. Floresta e Ambiente 23(4), 589-597.
| Crossref | Google Scholar |
Malisch CS, Salminen J-P, Kölliker R, Engström MT, Suter D, Studer B, Lüscher A (2016) Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. Journal of Agricultural and Food Chemistry 64(49), 9307-9316.
| Crossref | Google Scholar | PubMed |
Martínez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F (2016) Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecological Monographs 86(4), 495-516.
| Crossref | Google Scholar |
Maughan B, Provenza FD, Tansawat R, Maughan C, Martini S, Ward R, Clemensen A, Song X, Cornforth D, Villalba JJ (2014) Importance of grass-legume choices on cattle grazing behavior, performance, and meat characteristics. Journal of Animal Science 92(5), 2309-2324.
| Crossref | Google Scholar | PubMed |
McGrath SR, Sandral GA, Sundermann L, Quinn JC, Weston LA, Friend MA (2021) Liveweight and carcass characteristics of white dorper and crossbred lambs grazing lucerne, subterranean clover, biserrula or a choice of subterranean clover plus biserrula in southern Australia. Animal Production Science 61(11), 1151-1159.
| Crossref | Google Scholar |
McPhaden MJ, Zebiak SE, Glantz MH (2006) ENSO as an integrating concept in earth science. Science 314(5806), 1740-1745.
| Crossref | Google Scholar | PubMed |
Moore BD, Andrew RL, Külheim C, Foley WJ (2014) Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytologist 201(3), 733-750.
| Crossref | Google Scholar | PubMed |
Newman YC, Sollenberger LE, Boote KJ, Allen LH, Jr, Vu JCV, Hall MB (2005) Temperature and carbon dioxide effects on nutritive value of rhizoma peanut herbage. Crop Science 45(1), 316-321.
| Crossref | Google Scholar |
Nichols PGH, Revell CK, Humphries AW, Howie JH, Hall EJ, Sandral GA, Ghamkhar K, Harris CA (2012) Temperate pasture legumes in Australia—their history, current use, and future prospects. Crop & Pasture Science 63(9), 691-725.
| Crossref | Google Scholar |
Nyfeler D, Huguenin-Elie O, Suter M, Frossard E, Connolly J, Lüscher A (2009) Strong mixture effects among four species in fertilized agricultural grassland led to persistent and consistent transgressive overyielding. Journal of Applied Ecology 46(3), 683-691.
| Crossref | Google Scholar |
Nyfeler D, Huguenin-Elie O, Suter M, Frossard E, Lüscher A (2011) Grass–legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agriculture, Ecosystems & Environment 140(1–2), 155-163.
| Crossref | Google Scholar |
Pacheco D, Waghorn G, Janssen PH (2014) Decreasing methane emissions from ruminants grazing forages: a fit with productive and financial realities? Animal Production Science 54(9), 1141-1154.
| Crossref | Google Scholar |
Parrini S, Acciaioli A, Franci O, Pugliese C, Bozzi R (2019) Near infrared spectroscopy technology for prediction of chemical composition of natural fresh pastures. Journal of Applied Animal Research 47(1), 514-520.
| Crossref | Google Scholar |
Pearce KB, Holper PN, Hopkins M, Bouma WJ, Whetton PH, Hennessy KJ, Power SB (2007) Climate change in Australia: technical report 2007. CSIRO Research Publications Repository. Available at https://publications.csiro.au/rpr/pub?list=BRO&pid=procite:6002444c-8fbc-4373-bb5a-bedeb935da66
Pepler A, Timbal B, Rakich C, Coutts-Smith A (2014) Indian ocean dipole overrides ENSO’s influence on cool season rainfall across the eastern seaboard of Australia. Journal of Climate 27(10), 3816-3826.
| Crossref | Google Scholar |
R Core Team (2024) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Ramírez-Restrepo CA, Barry TN (2005) Alternative temperate forages containing secondary compounds for improving sustainable productivity in grazing ruminants. Animal Feed Science and Technology 120(3–4), 179-201.
| Crossref | Google Scholar |
Reddy PJ, Perkins-Kirkpatrick SE, Sharples JJ (2021) Interactive influence of ENSO and IOD on contiguous heatwaves in Australia. Environmental Research Letters 17, 014004.
| Crossref | Google Scholar |
Riede S, Lindig C, Abel H, Tonn B, Isselstein J, Breves G (2019) Effects of drought-stressed temperate forage legumes on the degradation and the rumen microbial community in vitro. Journal of Animal Physiology and Animal Nutrition 103(2), 436-446.
| Crossref | Google Scholar | PubMed |
Ripple WJ, Smith P, Haberl H, Montzka SA, McAlpine C, Boucher DH (2014) Ruminants, climate change and climate policy. Nature Climate Change 4, 2-5.
| Crossref | Google Scholar |
Robinson EA, Ryan GD, Newman JA (2012) A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytologist 194(2), 321-336.
| Crossref | Google Scholar | PubMed |
Rogers ME, Lawson AR, Giri K, Williams Y, Garner JB, Marett LC, Wales WJ, Jacobs JL (2022) Effects of extreme summer heat events on nutritive characteristics of dairy pastures in northern Victoria, Australia. Animal Production Science 62, 736-742.
| Crossref | Google Scholar |
Rosenzweig C, Mbow C, Barioni LG, Benton TG, Herrero M, Krishnapillai M, Liwenga ET, Pradhan P, Rivera-Ferre MG, Sapkota T, Tubiello FN, Xu Y, Mencos Contreras E, Portugal-Pereira J (2020) Climate change responses benefit from a global food system approach. Nature Food 1(2), 94-97.
| Crossref | Google Scholar |
Scharenberg A, Arrigo Y, Gutzwiller A, Soliva CR, Wyss U, Kreuzer M, Dohme F (2007) Palatability in sheep and in vitro nutritional value of dried and ensiled sainfoin (Onobrychis viciifolia) birdsfoot trefoil (Lotus corniculatus), and chicory (Cichorium intybus). Archives of Animal Nutrition 61(6), 481-496.
| Crossref | Google Scholar | PubMed |
Sherwood SC, Webb MJ, Annan JD, Armour KC, Forster PM, Hargreaves JC, Hegerl G, Klein SA, Marvel KD, Rohling EJ, Watanabe M, Andrews T, Braconnot P, Bretherton CS, Foster GL, Hausfather Z, Von der Heydt AS, Knutti R, Mauritsen T, Norris JR, Proistosescu C, Rugenstein M, Schmidt GA, Tokarska KB, Zelinka MD (2020) An assessment of earth’s climate sensitivity using multiple lines of evidence. Reviews of Geophysics 58(4), e2019RG000678.
| Crossref | Google Scholar |
Smith PE, Kelly AK, Kenny DA, Waters SM (2022) Enteric methane research and mitigation strategies for pastoral-based beef cattle production systems. Frontiers in Veterinary Science 9, 958340.
| Crossref | Google Scholar |
Sollenberger LE, Kohmann MM (2024) Forage legume responses to climate change factors. Crop Science 64(5), 2419-2432.
| Crossref | Google Scholar |
Song Y, Lv J, Ma Z, Dong W (2019) The mechanism of alfalfa (Medicago sativa L.) response to abiotic stress. Plant Growth Regulation 89, 239-249.
| Crossref | Google Scholar |
Sturludóttir E, Brophy C, Bélanger G, Gustavsson A-M, Jørgensen M, Lunnan T, Helgadóttir Á (2014) Benefits of mixing grasses and legumes for herbage yield and nutritive value in northern Europe and Canada. Grass and Forage Science 69(2), 229-240.
| Crossref | Google Scholar |
Sun X, Cheng L, Jonker A, Munidasa S, Pacheco D (2022) A review: plant carbohydrate types—the potential impact on ruminant methane emissions. Frontiers in Veterinary Science 9, 880115.
| Crossref | Google Scholar | PubMed |
Suybeng B, Charmley E, Gardiner CP, Malau-Aduli BS, Malau-Aduli AEO (2020) Supplementing northern Australian beef cattle with Desmanthus tropical legume reduces in-vivo methane emissions. Animals 10(11), 2097.
| Crossref | Google Scholar | PubMed |
Suybeng B, Mwangi FW, McSweeney CS, Charmley E, Gardiner CP, Malau-Aduli BS, Malau-Aduli AEO (2021) Response to climate change: evaluation of methane emissions in northern Australian beef cattle on a high quality diet supplemented with desmanthus using open-circuit respiration chambers and greenfeed emission monitoring systems. Biology 10(9), 943.
| Crossref | Google Scholar | PubMed |
Tava A, Biazzi E, Ronga D, Pecetti L, Avato P (2022) Biologically active compounds from forage plants. Phytochemistry Reviews 21, 471-501.
| Crossref | Google Scholar |
Thivierge M-N, Jégo G, Bélanger G, Bertrand A, Tremblay GF, Rotz CA, Qian B (2016) Predicted yield and nutritive value of an alfalfa–timothy mixture under climate change and elevated atmospheric carbon dioxide. Agronomy Journal 108(2), 585-603.
| Crossref | Google Scholar |
Thomson AL, Albornoz RI (2023) Multispecies forages in the Australian dairy feedbase: is there a biological business case? Animal Production Science 63(18), 1958-1969.
| Crossref | Google Scholar |
Tiemann TT, Lascano CE, Kreuzer M, Hess HD (2008) The ruminal degradability of fibre explains part of the low nutritional value and reduced methanogenesis in highly tanniniferous tropical legumes. Journal of the Science of Food and Agriculture 88(10), 1794-1803.
| Crossref | Google Scholar |
Top SM, Preston CM, Dukes JS, Tharayil N (2017) Climate influences the content and chemical composition of foliar tannins in green and senesced tissues of Quercus rubra. Frontiers in Plant Science 8, 423.
| Crossref | Google Scholar |
Torrence C, Webster PJ (1999) Interdecadal changes in the ENSO–monsoon system. Journal of Climate 12(8), 2679-2690.
| Crossref | Google Scholar |
Trush K, Pal’ove-Balang P (2023) Biosynthesis and role of isoflavonoids in legumes under different environmental conditions. Plant Stress 8, 100153.
| Crossref | Google Scholar |
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74(10), 3583-3597.
| Crossref | Google Scholar | PubMed |
Vandermeulen S, Singh S, Ramírez-Restrepo CA, Kinley RD, Gardiner CP, Holtum JAM, Hannah I, Bindelle J (2018) In vitro assessment of ruminal fermentation, digestibility and methane production of three species of Desmanthus for application in northern Australian grazing systems. Crop & Pasture Science 69(8), 797-807.
| Crossref | Google Scholar |
Villalba JJ, Burritt EA (2015) Intake of medusahead by sheep: influence of supplements, silica and individual animal variation. Invasive Plant Science and Management 8(2), 151-159.
| Crossref | Google Scholar |
Waghorn GC, Tavendale MH, Woodfield DR (2002) Methanogenesis from forages fed to sheep. Proceedings of the New Zealand Grassland Association 64, 167-171.
| Crossref | Google Scholar |
Yang X, Lu M, Wang Y, Wang Y, Liu Z, Chen S (2021) Response mechanism of plants to drought stress. Horticulturae 7(3), 50.
| Crossref | Google Scholar |
Zandalinas SI, Balfagón D, Gómez-Cadenas A, Mittler R (2022) Plant responses to climate change: metabolic changes under combined abiotic stresses. Journal of Experimental Botany 73(11), 3339-3354.
| Crossref | Google Scholar |
Zhang K, Xie H, Wen J, Zhang J, Wang Z-Y, Xu B, Chai M (2024) Leaf senescence in forage and turf grass: progress and prospects. Grass Research 4(1), e004.
| Crossref | Google Scholar |


