Influence of crop and stubble management practices on blackleg disease of canola (Brassica napus) in Australia
Susan J. Sprague A * , Steve J. Marcroft B , Kurt Lindbeck C , Elizabeth M. Sheedy B and Angela P. Van de Wouw
A * , Steve J. Marcroft B , Kurt Lindbeck C , Elizabeth M. Sheedy B and Angela P. Van de Wouw  D
D
A
B
C
D
Abstract
Australian canola (Brassica napus) growers have shifted towards earlier sowing and flowering times in addition to the widespread adoption of stubble conservation practices.
This study determined the consequent impact of these crop and stubble management practices on blackleg crown canker and upper canopy infection caused by the stubble-borne pathogen Leptosphaeria maculans.
Ascospore maturation and release were measured across seasons in commercial canola crops in south-eastern Australia on standing/erect and lying/knocked down stubble. We determined the severity of blackleg crown canker and upper canopy infection in crops with early or late sowing and flowering windows exposed to different stubble treatments.
Ascospore maturation was delayed and ascospore release was 10-fold lower in standing stubble in the first season post-harvest. In the second season when standing stubble was knocked down, total ascospore production was up to 4-fold higher than in the first season. Stubble orientation had no effect on blackleg disease severity. The main driver of infection at vulnerable crop stages was determined by sowing and flowering times such that disease severity was reduced by 35–76% and 98% in early sown and later flowering crops, respectively.
Canola grown in short rotations or intensively within a landscape will be exposed to high blackleg inoculum loads from retained stubble. Revision of current blackleg recommendations are required to account for modern crop management practices.
Innovative blackleg control strategies are urgently required to safeguard host genetic resistance and fungicide efficacy in modern farming systems to ensure sustainable intensification of canola production in Australia and globally.
Keywords: ascospore release, crop residue, cultural practices, disease management, epidemiology, pathogen evolution, Phoma lingam, Plenodomus lingam.
Introduction
Canola or oilseed rape (Brassica napus) is an important grain crop globally for use as a food-grade oil, stock feed and more recently, as a biofuel. The largest canola production occurs in Canada, China, India, and Australia. Canola production in Australia increased from 2.31 million tonnes in 2018–2019 to 7.93 million tonnes in 2022–2023 (AOF 2024) driven by intensification in existing canola production regions and spread into lower rainfall regions (Slinger et al. 2016; Zhang et al. 2016). In Australia, blackleg disease, caused by the fungus Leptosphaeria maculans, is the main biotic constraint limiting production and is considered a serious threat globally. Infected canola residue (stubble) harbours L. maculans between seasons with sexual ascospores released from stubble following rainfall events (Hammond et al. 1985; West et al. 2001). Ascospores germinate and enter a susceptible plant through stomata or wounds to cause disease on all plant tissues including cotyledons, leaves, stems, branches, flowers, and pods of the canola plant (West et al. 2001; Sprague et al. 2018). Infection of the cotyledons and leaves during the vegetative period of canola growth leads to crown canker at the end of the growing season. In this scenario, the fungus grows from infected cotyledons and leaves through the petiole and into the stem. Colonisation of the stem vascular tissue restricts water and nutrient uptake thereby reducing yield and in severe situations, even plant death (Hammond et al. 1985; Sprague et al. 2009). More recently in Australia, L. maculans infection at later stages of B. napus development (including on flowers, upper stems and upper branches), termed upper canopy infection, has become prevalent (Sprague et al. 2018). Upper canopy infection limits yield by the abortion of infected flowers and reduced pod and seed set likely due to colonisation of the vascular tissue of the upper main stem and branches (Sprague et al. 2018).
Recent changes in canola crop management practices in Australia have largely been in response to the effects of climate change on rainfall patterns and extreme weather events (Verdon-Kidd et al. 2014; CSIRO 2020). Timely sowing and flowering windows are critical to optimise canola yield potential (Kirkegaard et al. 2016; Lilley et al. 2019; Van de Wouw et al. 2021a). Earlier sowing increases grain yield potential, oil content and water use efficiency (Kirkegaard et al. 2016), whilst timely flowering can increase grain yield by up to 20% (Lilley et al. 2019). In a survey of Australian canola producers, sowing and flowering times in 2018 were an average of 3 weeks earlier than in 2000 (Van de Wouw et al. 2021a). The adoption of earlier sowing is underpinned by genetic improvements such as herbicide tolerance and hybrid vigour as well as technological advances (e.g. satellite-guided sowing equipment) which minimise moisture loss (Hunt et al. 2019). Early sown canola is more likely to avoid the onset of ascospore maturation which is dependent on rainfall (McGee and Emmett 1977; Bondad et al. 2025a) and develops rapidly past the vulnerable early seedling stage (cotyledon to 5th leaf) in which infection with L. maculans leads to severe crown canker. In contrast, earlier flowering crops are now exposed to ascospores released during rainfall events which coincide with conditions more conducive to infection (Sprague et al. 2018; Van de Wouw et al. 2021a). A decline in blackleg crown canker severity since 2010 and increased prevalence of upper canopy infection indicate that altered patterns of crop development in relation to the timing of L. maculans ascospore maturation and infection events are consistent with predicted impacts on blackleg disease epidemics but these have not been widely tested experimentally (Sprague et al. 2018; Van de Wouw et al. 2021a).
Blackleg disease can be controlled by cultural practices that reduce exposure to inoculum, such as physical distancing, crop rotation, or removal of stubble (Marcroft et al. 2003; Marcroft et al. 2004). Adoption of reduced tillage practices in which stubble is retained to protect the soil from erosion and to enhance soil moisture conservation is widespread in Australia. Stubble is retained intact from harvest through to planting of the subsequent crop on 57% of crop area and retained but managed so that is not standing on 13% of the crop area with stubble on only 14% of crop area mechanically incorporated into the soil (Umbers 2016). No-till or zero-till sowing strategies (practices whereby less than 30% of soil is disturbed) represented 75% of the cropping area in Australia in 2016, up from only 25% in 2001 (Umbers 2016) resulting in persistence of stubble across seasons. Technical advances in machinery have also resulted in the conservation of higher volumes of stubble. Satellite-guidance allows the planting of new crops between the stubble rows of the previous crop and stripper front headers, which harvest only the seed heads and can reduce harvesting costs by up to 40% due to improved efficiency, leave higher standing stubble biomass (Condon et al. 2023). These management and technological changes have resulted in standing canola stubble that can persist until the subsequent canola crop in the rotation is sown, most notably in tighter canola-wheat (Triticum aestivum)-canola rotations. Compared to lying stubble, standing stubble releases fewer ascospores in the first season post-harvest and the maturation of ascospores is delayed (McCredden et al. 2018; Bondad et al. 2025a).
The objectives of this study were to: (1) extend the work of McCredden et al. (2018) and Bondad et al. (2025a) and investigate ascospore release patterns of canola stubble in differing orientations at a broader range of sites and across multiple seasons; and (2) determine the impact of stubble management and sowing/flowering times on crown canker and upper canopy blackleg disease severity outcomes in a field setting.
Materials and methods
Five canola (Brassica napus L.) paddocks on commercial farms practicing stubble conservation were identified in canola-producing regions of Victoria (Vic) and southern New South Wales (NSW) (Table 1). Canola stubble was collected monthly from each paddock during the first cropping season following the canola crop (April–November). At the Horsham, Laharum, Vectis, and Wagga Wagga sites, 20 pieces of standing and 20 pieces of lying stubble were collected each month whilst 60 pieces were collected for each stubble orientation for the Greenethorpe site. Ascospores released from the stubble samples were quantified as described below.
| Year | Location | January–March rainfall (LTM)A | GSR (LTM GSR)A | Year post-harvest | Stubble orientation | Segment | Total ascospores/g stubble for the growing seasonB | Proportion of total ascospores | |
|---|---|---|---|---|---|---|---|---|---|
| 2018 | Greenethorpe, NSW | 68 | 238 | 1 | Lying | 0–5 cm | 5433b | 65.8 | |
| (135) | (402) | 15–20 cm | 298a | 3.6 | |||||
| Standing | 0–5 cm | 2518ab | 30.5 | ||||||
| 15–20 cm | 4a | 0.1 | |||||||
| 2018 | Wagga Wagga, NSW | 83 | 265 | 1 | Lying | 0–5 cm | 1359ab | 80.1 | |
| (128) | (399) | 15–20 cm | 69a | 4.1 | |||||
| Standing | 0–5 cm | 268a | 15.8 | ||||||
| 15–20 cm | 0a | 0.0 | |||||||
| 2018 | Horsham, Vic | 33 | 222 | 1 | Lying | 0–5 cm | 34,815e | 67.9 | |
| (69) | (323) | 15–20 cm | 6074bc | 11.8 | |||||
| Standing | 0–5 cm | 9907c | 19.3 | ||||||
| 15–20 cm | 497a | 1.0 | |||||||
| 2018 | Laharum, Vic | 50 | 237 | 1 | Lying | 0–5 cm | 44,735f | 67.7 | |
| (113) | (700) | 15–20 cm | 9504c | 14.4 | |||||
| Standing | 0–5 cm | 10,234c | 15.5 | ||||||
| 15–20 cm | 1621ab | 2.5 | |||||||
| 2019 | Vectis, Vic | 21 | 277 | 1 | Lying | 0–5 cm | 11,834c | 54.4 | |
| (71) | (337) | 15–20 cm | 7391bc | 34.0 | |||||
| Standing | 0–5 cm | 1935ab | 8.9 | ||||||
| 15–20 cm | 600a | 2.8 | |||||||
| 2019 | Horsham, Vic | 19.3 | 292.4 | 2 | Lying-lying | 0–5 cm | 64,650h | 30.7 | |
| 15–20 cm | 48,231f | 22.9 | |||||||
| (69.4) | (321.6) | Standing-lying | 0–5 cm | 53,452g | 25.4 | ||||
| 15–20 cm | 29,382d | 14.0 | |||||||
| Standing-standing | 0–5 cm | 13,691c | 6.5 | ||||||
| 15–20 cm | 1041a | 0.5 | |||||||
| 2020 | Vectis, Vic | 91 | 309 | 2 | Lying-lying | 0–5 cm | 25,868d | 56.4 | |
| 15–20 cm | 4684b | 10.2 | |||||||
| (71) | (337) | Standing-lying | 0–5 cm | 12,471c | 27.2 | ||||
| 15–20 cm | 279a | 0.6 | |||||||
| Standing-standing | 0–5 cm | 2019ab | 4.4 | ||||||
| 15–20 cm | 544a | 1.2 |
At the Horsham (2018 and 2019) and Vectis (2019 and 2020) sites, ascospore release continued to be measured monthly from canola stubble during the second growing season post-harvest. In the first season post-harvest, ascospores were quantified from stubble collected from the paddock at monthly intervals. Prior to harvesting operations at the end of the first season, canola stubble in standing and lying orientations was collected from the paddock to ensure that stubble was not disturbed during the harvest and sowing operations. Collected stubble was maintained in the appropriate orientation at Riverside, Victoria, and subsequently collected at monthly intervals for ascospore quantification during the second growing season post-harvest. Collected stubble was kept either standing or lying using soil beds with similar soil type to the paddock from where the stubble was originally collected. The standing stubble was placed either in the ground and supported by wire mesh to represent stubble that was standing for two seasons (standing-standing stubble), or on the ground to represent stubble that was standing in Year 1 and then knocked into a lying position in Year 2 (standing-lying stubble). Lying stubble from Year 1 was placed on the ground to represent 2 years of lying orientation (lying-lying stubble).
Daily rainfall data for each site were taken from the nearest Australian Bureau of Meteorology station and the long-term mean (LTM) calculated using all records available for each weather station (BOM 2024) (Table 1).
Ascospore liberation
Ascospores were quantified from the stubble samples using a Burkard spore liberator (Hirst and Stedman 1962). To ensure consistency between timepoints and stubble orientation, stubble pieces were prepared by removing attached roots and cutting two 5 cm sections from the stem: one from the base of the stem to 5 cm above (0–5 cm, lower) and one between 15 and 20 cm above the stem base (15–20 cm, upper). Prior to liberation, stubble was primed by placing it at 100% humidity for 24 h, followed by air-drying for 12 h, and then immersing in water for 1 min immediately prior to loading the stubble in the liberator to trigger ascospore release (McCredden et al. 2018). Ascospores were liberated for 1 h and captured onto glass microscope slides as previously described (McCredden et al. 2018). For each stubble orientation and stem section (upper and lower), five pieces of stubble were placed in a single chamber that was repeated in four replicate chambers for all sites except Greenethorpe, which used 15 pieces per chamber. Liberation within the chambers was randomised for stubble orientation, section, and site.
Following liberation, stubble from each chamber was oven-dried (70°C for 48 h) and weighed. Discharged ascospores were counted as previously described (McCredden et al. 2018). Briefly, this involved removing ascospores from the glass slide, suspending the ascospores in 1 mL of Turpentine (to dissolve the Vaseline-Paraffin wax adhesive used on the glass slide), followed by counting using a haemocytometer.
Total ascospore release was calculated by summing the monthly ascospore release for both stubble segments (0–5 cm and 15–20 cm). The average weight of the stubble was determined for each stubble type (1-year-old lying, 1-year-old standing, 2-year-old lying, 2-year-old standing). The total ascospore release for each stubble type was calculated by multiplying the total spores/g for each stubble type across the growing season for the experiment (May–October) and the weight of each stubble load (Table 2).
| Site | Stubble type | kg/ha | Percentage of stubble load | Total spores/g of stubble | Total spores released per ha | Percentage of total spore release | |
|---|---|---|---|---|---|---|---|
| Greenethorpe (NSW) | Lying | 1079 | 73.0 | 4664 | 5.03E + 09 | 90.9 | |
| Standing | 399 | 27.0 | 1266 | 5.06E + 08 | 9.1 | ||
| Wagga Wagga (NSW) | Lying | 1601 | 87.9 | 1427 | 2.29E + 09 | 97.5 | |
| Standing | 220 | 12.1 | 268 | 5.90E + 07 | 2.5 | ||
| Horsham (Vic) | Lying | 1675 | 73.3 | 40,888 | 6.85E + 10 | 91.5 | |
| Standing | 610 | 26.7 | 10,404 | 6.35E + 09 | 8.5 | ||
| Laharum (Vic) | Lying | 951 | 75.1 | 19,225 | 1.83E + 10 | 95.8 | |
| Standing | 315 | 24.9 | 2535 | 7.99E + 08 | 4.2 |
Stubble biomass
In 2018, main stem sections of stubble were collected from four sites to determine the proportion of stems in lying or standing orientation after sowing (Table 1). At each site, stubble was collected along a ‘W’ transect across the paddock with five quadrats (50 cm × 50 cm) collected approximately 20 m apart along each arm of the W transect and combined into a single replicate (1.25 m2) such that four replicates covering a total area of 5 m2 were collected for each paddock. At each location along the transect, all stem stubble was collected within the quadrat and separated into lying or standing stubble. Roots attached to any standing stubble was removed as these were below the soil surface. All samples were oven dried (70°C for 48 h) and weighed to determine stubble biomass (kg/ha) of stubble in each orientation.
Field experiment to determine stubble and crop management on blackleg disease severity
In March 2021, both 1-year-old and 2-year-old stubble was collected from commercial canola paddocks near Horsham, Victoria, to conduct a field experiment to determine the impact of crop and stubble management practices on blackleg disease severity. The 1–year-old stubble (standing and lying) was collected from one paddock, with 2-year-old stubble (standing and lying) collected from a second paddock of the same canola cultivar (Pioneer 43Y92CL) located nearby on the same property. The 2-year-old lying stubble was a combination of stubble that was knocked down in the first season post-harvest (lying-lying) and knocked down during the harvest process in 2020 (standing-lying) as there was no way to determine when stubble was knocked down.
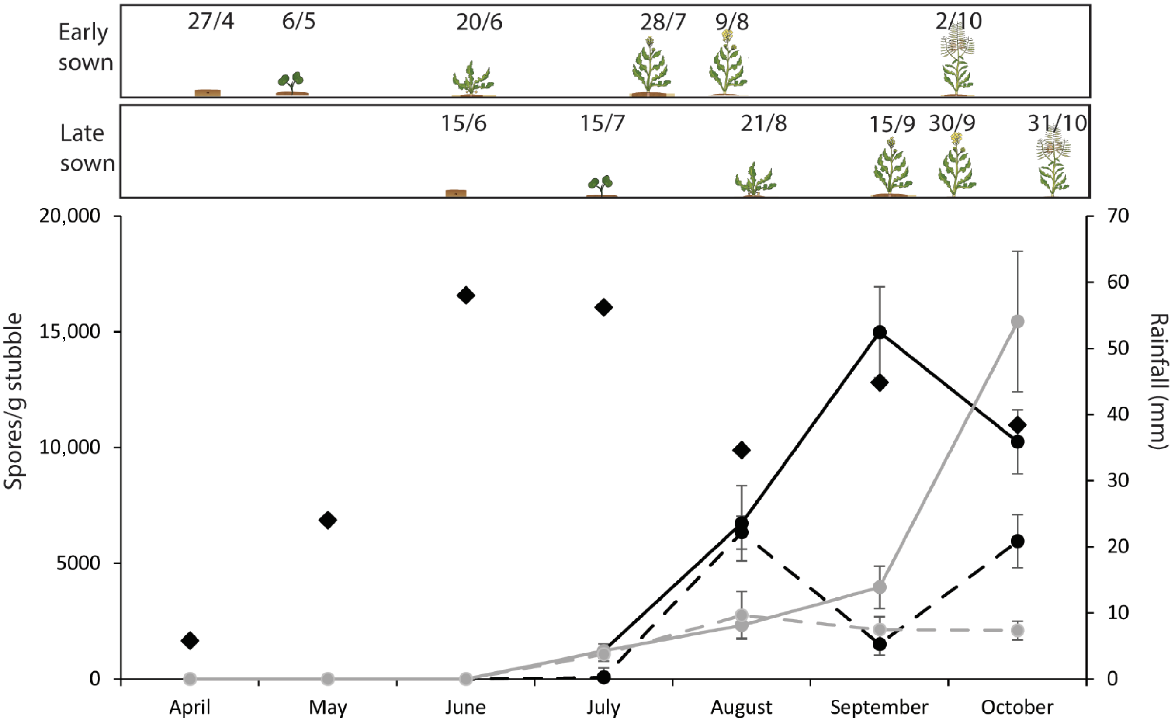
In April 2021, the 1- and 2-year old standing and lying stubble was used to establish experimental plots (2 m × 2 m) in the field at Riverside, Victoria to determine the impact of sowing and flowering time, stubble orientation, stubble age, and stubble load on crown canker and upper canopy infection (Fig. 1). Stubble treatments consisted of 1- or 2-year-old stubble of different orientation (standing or lying) and stubble load (high or low). Plots were isolated from each other by at least 30 m to limit ascospore dispersal between plots (Fig. 1a). Standing stubble treatments were established by placing individual pieces of stubble into holes made by a drill, whilst stubble in the lying treatments was placed directly onto the soil surface. The high and low stubble loads consisted of 400 and 40 stems, respectively, spread evenly within the 2-m × 2-m plot (Fig. 1). An additional area was established for each stubble type (1-year-old lying stubble, 1-year-old standing stubble, 2-year-old lying or 2-year-old standing stubble) adjacent to the plots with monthly collections between May and October to quantify ascospores, as described above.
Plot set up for testing the impact of sowing time, stubble orientation and stubble load on disease severity. (a) Graphical representation of the plot set up (not to scale); each plot contained a specific type of stubble and stubble load. Within each plot are eight replicate pots (each containing four plants small squares) sown either early, to capture upper canopy infection symptoms, or sown late to capture crown canker symptoms. Each plot is isolated by at least 30 m to prevent cross-infection. Stubble was set up as lying (b–e) or standing (f–i) with high (b, c, f, g) or low (d, e, h, i) stubble load (b, d, f, h). Plants were sown in pots on two dates (27 April 2021 and 15 June 2021) to promote upper canopy infection (early sown, advanced plants in figure) and crown canker (late sown, not yet germinated in the figure) blackleg disease.

Pots of canola cultivar BASF3000TR sown on 27 April or 15 June were positioned within each of the stubble treatments (Fig. 1). This cultivar is a fast-developing spring type susceptible to blackleg with a resistance rating of moderately susceptible-susceptible (MS-S) (GRDC 2025). The sowing date treatments generated differences in plant development during the period of blackleg ascospore release. Infection of the cotyledons and early true leaves leads to more severe crown canker such that plants sown in June were at the cotyledon to 4-leaf stage during the peak ascospore shower window (generally July through August). In contrast, infection of the flowers, upper stem and upper branches lead to upper canopy infection such that plants sown in April were at the elongation and flowering growth stages during the peak ascospore shower window (Fig. 1c, e, g, i). Each sowing date treatment consisted of eight replicate pots, each containing four plants (total 32 plants). Within each of the stubble plots, the pots sown at each time were not randomised due to the impacts of shading on the June-sown plants. Plots were protected to prevent damage from rabbits and birds, and insect pests were controlled as required. Diseases other than blackleg were absent from the experiment. Crop development was monitored for the key crop phenological stages (sowing, start of stem elongation, start of flowering and maturity).
Blackleg crown canker and upper canopy infection severity was measured on each individual plant at physiological maturity. Crown canker severity was measured by cutting plants at the crown to determine the proportion of internal infection (blackening), as previously described (Marcroft et al. 2012). The incidence and severity of upper canopy infection was determined as the proportion of branches with a blackleg lesion and the length of all external lesions on the plant at least 5 cm above the crown, respectively.
Statistical analysis of data
The relationship between rainfall and ascospore release was determined using Kendall’s Tau rank correlation. The main effects and interactions of location (Greenethorpe, Horsham, Laharum, Wagga Wagga or Vectis), stubble age (1- or 2-year old), stubble orientation (lying, standing, standing-lying and standing-standing), and segment (0–5 cm or 15–20 cm) on spore release patterns were evaluated using ANOVA and mean separations done using least significant differences (l.s.d.) tests. The main effects and interactions of stubble age (1- or 2-year old), stubble load (high or low), sowing date, and stubble orientation (lying or standing) on blackleg disease severity parameters (internal infection, stem lesion length, and branch infection) were evaluated using ANOVA. Statistically significant differences in means of each parameter tested were determined using Bonferroni multiple comparisons to determine least significant differences. Blackleg disease severity and ascospore release data were root transformed prior to analysis. Outputs of the statistical analyses are in Supplementary tables. All statistical analyses were carried out in Genstat ver. 22.
Results
Ascospore release from lying and standing stubble
Ascospore release from 1-year-old lying or standing stubble was measured at five locations in 2018 and 2019 (Fig. 2, Table 1). All sites received significantly lower annual rainfall than the LTM, with the reduction ranging from 44.5% at Laharum (2018) to 76% at Horsham (2018) (see Supplementary Table S1). April was dry at all sites with <5 mm rainfall recorded, and rainfall remained below the LTM throughout the growing season at all sites, except for Wagga Wagga in 2018 which recorded 95 mm, well above the average. The average total ascospores/g of stubble was 14,910/g but differed significantly between sites (P < 0.001, Table S2) ranging from 1696 spores/g stubble at Wagga Wagga in 2018 to 66,094 spores/g stubble at Laharum in 2018 (Table 1). Total ascospore load across the growing season at Wagga Wagga was <5% of the site averages despite the location receiving the second highest GSR (265 mm) although 95 mm of this was recorded in November (Fig. 2, Table S1). The highest ascospore load was at Laharum which produced 66,094 spores/g of stubble, despite having <50% of the LTM rainfall. There was no correlation between growing season rainfall (April–November) or pre-season rainfall (January–March) and total ascospore production from 1-year-old standing and lying stubble. However, March rainfall was highly correlated with total ascospore release (correlation 0.98, probability <0.0017).
Total monthly rainfall (black diamonds) and patterns of ascospore release from standing (grey line) or lying (black line) stubble collected in the first season post-harvest from commercial canola paddocks in Victoria (Vic) and New South Wales (NSW) in 2018 and 2019. Lying 1-year-old stubble produced at least 70% of the total ascospores compared to the 1-year-old standing stubble (Table 1). Ascospore release generally occurs earlier in lying compared to standing stubble. Ascospore data are presented as mean ± s.e.
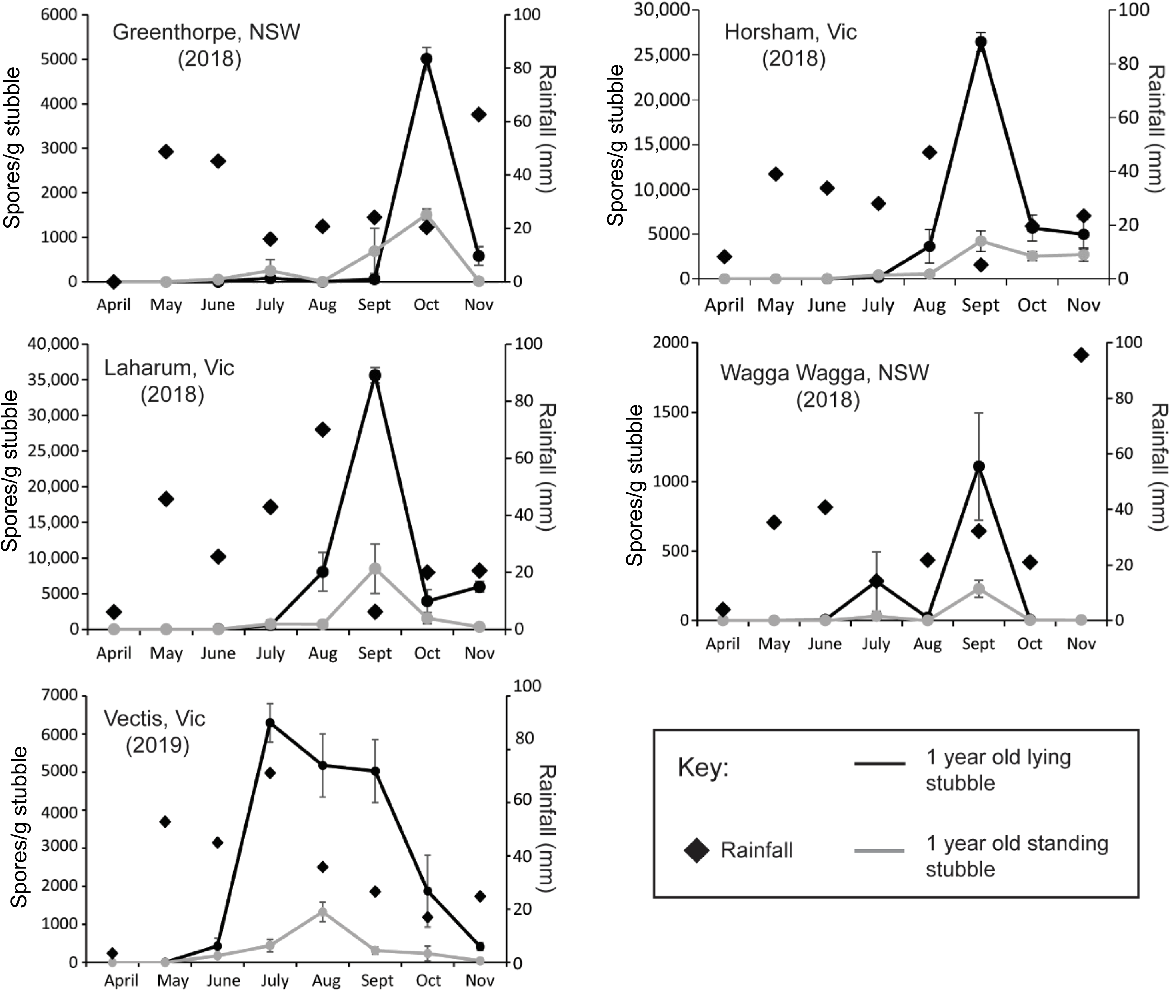
Stubble orientation (standing or lying), stubble age (1- or 2-year old) and stubble segment (lower and upper sections) all significantly influenced the quantity of ascospores released (P < 0.001, Table S2). Significant interactions were primarily due to differences in ascospore release between locations as the interactions for orientation × segment, age × segment, and orientation × age × segment were not significant (Table S2). For 1-year-old stubble across all sites, ≥70% of the total ascospores were released from lying stubble compared to standing stubble (Table 1, Fig. 2) and ≥76% were released from the lower stem segment for both standing and lying stubble (Table 1, Table S3). In general, the onset of ascospore release was later for the upper segment than for the lower segment and this was independent of stubble orientation (Table S3).
The start and peak of ascospore release differed amongst sites and years (Fig. 2). Ascospore release commenced in June at Vectis but was delayed until September at Greenethorpe. The timing of ascospore peaks were more consistent between sites, occurring in September at Laharum, Wagga Wagga, and Horsham while the peak occurred in October at Greenethorpe. At Vectis, the release was more prolonged with high ascospore numbers measured each month from July to September. At all sites except Greenethorpe, ascospore release began earlier in lying stubble than in standing stubble but the timing of peak ascospore release was similar irrespective of stubble orientation (Fig. 2).
The biomass of standing and lying stems in Year 1 post-harvest was measured in four paddocks in 2018. The stubble biomass ranged from 1266 kg/ha (Laharum) to 2285 kg/ha (Horsham) with an average of 1713 kg/ha. Lying stubble accounted for between 73 and 88% of the total stubble biomass, contributing 91–98% of the total ascospores released per ha. At Greenethorpe, where all canola stubble biomass was measured, 46% was lying stubble, 17% was standing stubble and 37% was header debris indicating that header debris can be a significant component of the stubble remaining in the paddock post-harvest. In comparison, lying and standing stubble biomass was 73% and 27% of total stubble biomass at Greenethorpe, respectively, when the header debris was excluded.
Ascospore release across seasons
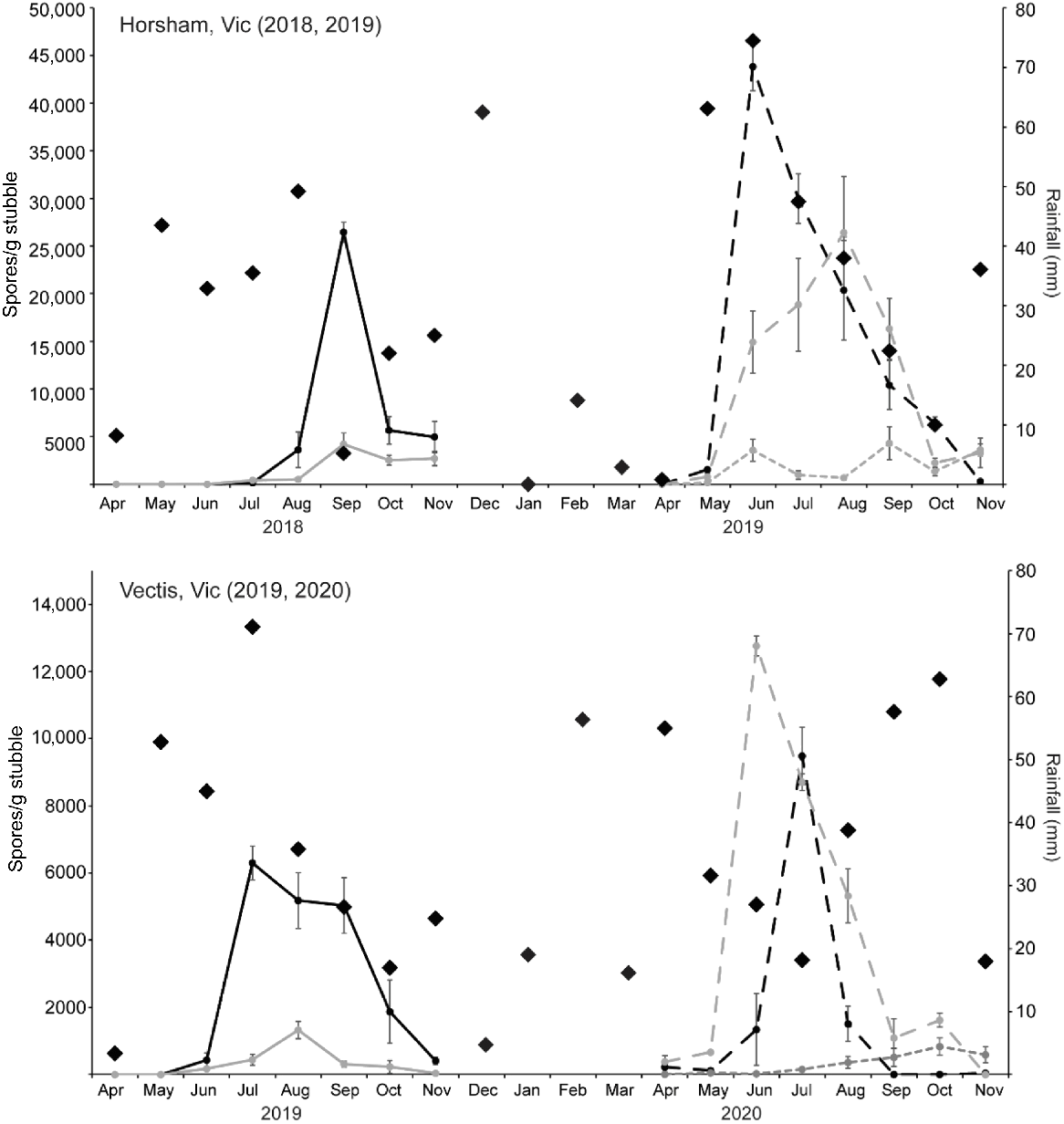
At the Horsham and Vectis sites, ascospore release was measured in Year 2 post-harvest to determine the effect of knocking down standing stubble into a lying position in the second season. Total ascospore production from standing and lying stubble combined at Vectis doubled in Year 2 post-harvest, a significant increase from 21,761 ascospores/g in Year 1 to 45,865 ascospores/g in Year 2 (Fig. 3, Tables S2 and S3). At Horsham, total ascospore production was four times higher in Year 2, increasing from 51,293 ascospores/g to 210,446 ascospores/g. In Year 2 post-harvest, the standing-lying stubble (i.e. stubble that was standing in Year 1 and then knocked down in Year 2), produced 39% of total ascospores at Horsham and 67% of total ascospores at Vectis (Table 1, Fig. 3). At Vectis, the standing-lying stubble peaked earlier than the lying-lying stubble but the opposite trend was observed at Horsham (Fig. 3). At both sites, standing-standing stubble (i.e. stubble remaining standing for both years) produced <10% of the total ascospores in Year 2 (Table 1, Fig. 3).
Total monthly rainfall (black diamond) and the pattern of ascospore release from stubble collected from the same paddock for two consecutive field seasons at Horsham (2018, 2019) and Vectis (2019, 2020). Ascospore release patterns were determined from lying (black line) and standing (grey line) stubble in Year 1 post-harvest and from lying-lying (black dashed line), standing-standing (grey dotted line), and standing-lying (grey dashed line) stubble in Year 2 post-harvest. In Year 2, stubble that was standing at the end of the first season but knocked down at the start of the second season (standing-lying) released similar levels of ascospores as 2-year-old stubble that was lying in both years (lying-lying). Stubble left standing across both years (standing-standing) released few ascospores. Ascospore data are presented as mean ± s.e.
Impact of crop development, stubble orientation, and stubble load on disease severity
The impact of crop and stubble management practices on the severity of blackleg crown canker and upper canopy infection was investigated in a field experiment. Total ascospore release was greatest for the 1-year-old lying stubble (4.69E + 07) and lowest for the 2-year-old standing-standing stubble (1.82E + 06). In the 1-year-old stubble, standing stubble produced ~30% fewer ascospores compared to the lying stubble. In comparison, the standing-standing stubble produced ~15% fewer ascospores than the lying-lying in the second year (Table 3). Ascospore release commenced in July although relatively few ascospores were released (Fig. 4). Ascospores increased in subsequent months and continued at relatively high levels across all stubble treatments until the final measurement in October. Indeed, ascospore release peaked in October for the standing stubble. The June-sown plants were at the open cotyledon stage in mid-July when ascospore release commenced and were at the 4–6 leaf growth stage on 20 August. In contrast, the April-sown plants were elongating in mid-July with the first flowers present in mid-August and 30% bloom at the end of August. For June-sown plants, first flower occurred at the start of October and 30% bloom was in mid-October.
| Stubble parameters | Blackleg disease severity A | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stubble age | Stubble orientation | Stubble B load | Internal infection (%) | Stem lesion length (cm) | Branch infection (%) | Total spore release B | ||||
| Early sown | Late sown | Early sown | Late sown | Early sown | Late sown | |||||
| 1-year-old | Lying | High | 49.8c | 85.6ab | 81.0a | 1.7c | 56.7a | 4.5d | 4.69E + 07 | |
| Low | 24.4de | 80.6ab | 8.3c | 0.3c | 27.2b | 3.0d | ||||
| Standing | High | 54.4c | 84.7ab | 67.6a | 0.4c | 69.1a | 10.9cd | 3.24E + 07 | ||
| Low | 21.3e | 85.9ab | 34.5b | 1.9c | 31.6b | 4.8d | ||||
| 2-year-old | Lying-lying | High | 36.6d | 80.6ab | 9.6c | 1.3c | 32.1b | 2.2d | 2.11E + 07 | |
| Low | 37.5d | 75.9b | 7.0c | 0.0c | 17.4c | 3.6d | ||||
| Standing-standing | High | 34.7de | 92.5a | 20.4b | 2.2c | 26.0b | 8.0d | 1.82E + 07 | ||
| Low | 32.5de | 89.4ab | 6.7c | 1.9c | 14.2cd | 4.9d | ||||
Ascospore release was measured from each stubble type. Early sown, 27 April; late sown, 15 June.
Total monthly rainfall (black diamonds) and ascospore release patterns from 1-year-old lying (black line), 1-year-old standing (grey line), 2-year-old lying (black dashed line), and 2-year-old standing (grey dashed line) stubble from commercial canola paddocks collected in 2021. The stubble was used to infect a field trial to investigate different blackleg disease severity parameters (Table 3). Ascospore data are presented as mean ± s.e.
Overall, the April-sown plants were flowering and the June-sown plants were at the early vegetative stage during the highest periods of ascospore release resulting in high levels of blackleg upper canopy and crown canker disease, respectively (Table 3). There were significant main effects and interactions of sowing time, stubble load, and stubble age on crown canker severity but no effect of stubble orientation (Table S4). Overall, crown canker severity was significantly lower in the April-sown than in the June-sown plants but was similar in the June-sown plants irrespective of stubble load or age with severity ranging from 76% to 93% (Table 3). In the April-sown plants, crown canker was greatest in the 1-year-old high stubble load treatments (52%) and declined to 23% with low stubble density (Table 3). In the 2-year-old stubble treatments, the mean crown canker severity was 35% with no significant effect of stubble load.
Sowing time, stubble load, and stubble age all had a significant impact on upper canopy infection incidence and severity (Tables 3 and S4). As expected, April-sown plants had significantly greater upper canopy infection (both stem lesion length and incidence of branch infection) compared to the June-sown plants, which had low levels of disease with no significant differences between treatments. The high stubble loads generally resulted in greater upper canopy infection severity and incidence in April-sown plants, irrespective of stubble age, except for infection incidence in the lying-lying stubble. In general, stubble orientation resulted in some small differences in upper canopy stem lesion severity but only at the low stubble load such that standing stubble caused significantly larger stem lesions than the lying stubble in both 1-year-old (34.5 cm vs 8.3 cm) and 2-year-old (20.4 cm vs 9.6 cm) stubble treatments.
Discussion
Stubble conservation practices have been widely adopted by Australian growers in response to increasing climate variability with stubble commonly retained in a standing position. In this study, we demonstrated that there is an associated change in epidemiology of L. maculans with the retention of undisturbed canola stubble. Consistent with previous studies by McCredden et al. (2018) and Bondad et al. (2025a), L. maculans ascospore maturation was consistently delayed and 75% fewer ascospores were produced from standing canola stubble compared to lying stubble in the first season post-harvest. In the second season post-harvest, the timing of ascospore maturity and total ascospore production was similar or greater in the stubble that was knocked down in the second season (standing-lying) compared to that which was lying for two seasons (lying-lying). Over the two seasons, ascospore release was minimal from 2-year-old standing stubble (standing-standing).
In this study, ascospores matured earlier on the lower stem segment than the upper segment in the first season post-harvest, irrespective of stubble orientation. This difference may be due to the lower stem segments retaining moisture more effectively than the upper segments, possibly influenced by anatomical, structural and/or chemical differences. Alternatively, ascospore development may initiate prior to plant senescence as infection and colonisation at the crown occurs during vegetative growth while infections on the upper stem are initiated later in the season after stem elongation. The Sporacle Ezy model (Salam et al. 2003; Salam et al. 2007) simulated ascospore maturation based on temperature and moisture thresholds with calibration required for different canola-growing environments globally. Recently, the inclusion of hydro-thermal time by Bondad et al. (2025a) improved the reliability of ascospore maturation predictions for canola-growing regions of Australia and Europe without the requirement for environmental calibration. Bondad et al. (2025a) demonstrated that ascospores at the base of standing stubble mature at 0.91 the rate of lying stubble, hypothesising that larger and more prolonged rainfall events are required to wet up standing stubble, which also tends to dry faster and therefore has shorter periods of wetness compared to lying stubble. These findings highlight the importance of moisture availability in the development of ascospores. However, both the timing of ascospore maturation in relation to plant development stage and ascospore load determine epidemic severity. In our study, total ascospore load correlated strongly with rainfall in March but not with in-season rainfall, although the relatively small number of sites (n = 5) limits broader conclusions. Previous studies have shown that pre-season rainfall, host genotype, crown canker severity, and in-season nitrogen application, all influence the production of pseudothecia (Bousset et al. 2018). There is a clear need for further research on the drivers of inoculum development and seasonal ascospore load to inform current seasonal blackleg disease risks and identify responses to climate changes or agricultural practices.
The emergence of blackleg upper canopy disease in Australia has been linked to altered L. maculans epidemiology due to the widespread adoption of stubble conservation in Australia (Sprague et al. 2018). Ascospore release from standing stubble was delayed in the first season post-harvest but peak release generally coincided with that from lying stubble; however, standing stubble contributed <10% of total ascospore production during the growing season despite accounting for 30% of total stubble load. In the second season, peak ascospore production in standing stubble that was knocked down (standing-lying) occurred either earlier (Vectis) or later (Horsham) leading to a wider window of potential infection, although stubble loads were not measured. Severity was reduced by up to 98% (e.g. 1-year-old stubble, high stubble load – stem lesion length reduced from 81 cm to 1.7 cm) in plants starting to flower in mid-September compared to late July despite later flowering coinciding with peak ascospore release. This supports earlier studies, which identified high disease severity in early flowering crops indicating that the adoption of earlier flowering windows to maximise yield potential is the primary driver of upper canopy disease (Sprague et al. 2018). Intensification of canola production and the persistence of high inoculum loads across multiple seasons also likely contribute to the widespread emergence of upper canopy infection. Disease severity of other stubble borne pathogens, such as crown rot (Fusarium pseudograminearum) and Septoria tritici blotch (Zymoseptoria tritici) of wheat, have increased in prevalence and severity due the adoption of stubble conservation methods (Simpfendorfer et al. 2020). Management strategies to mitigate crown rot infection include longer rotations between cereal crops to allow degradation of stubble, lower crop harvest height to reduce inoculum build-up post-harvest as the fungus saprophytically colonises cereal residue from infected crowns, and inter-row sowing of new crops to limit direct contact with infected stubble (Verrell et al. 2017; Petronaitis et al. 2022).
Similarly to upper canopy blackleg, crop management practices had a significant impact on crown canker severity. Whilst the effect of sowing time on crown canker severity was not as significant as early flowering on upper canopy infection, reductions in canker severity were between 36 and 75%. Interestingly, the national average for crown canker severity in Australia declined from 47.1% in 2010 to 32.2% in 2019, a trend that is consistent with shifts to earlier sowing over the same period (Van de Wouw et al. 2021a). These results indicate that early sown crops may avoid blackleg infection during the vulnerable early seedling stage as crops develop rapidly during early autumn when soil and air temperatures are warm (Whish et al. 2020). The use of fungicides applied to seed and fertiliser at sowing and foliar sprays to control blackleg crown canker are common in Australia with sustained or even increased use despite lower disease risk with earlier sowing and the availability of high levels of genetic resistance (Van de Wouw et al. 2021a). Indeed, purchased canola seed is often pre-treated prior to sale irrespective of the level of genetic resistance, or applied by growers to seed of retained open-pollinated varieties (S. Marcroft, pers. comm.). The results from the current study suggest that fungicide applications at the seedling stage to protect against crown canker should be based on regional and seasonal risk, rather than as a blanket rule, with significant potential for integration of digital decision tools. Near real-time paddock-level risk could be informed by a combination of proximity to previous years stubble identified via satellite imagery (Crop ID technology), crop simulation models to inform vulnerable developmental stages and ascospore maturation forecasting supported by automated spore traps (e.g. BioScout) such that fungicide application occurs only when high-risk scenarios are identified. With the increased detection of fungicide resistance both in Australia and internationally (Van de Wouw et al. 2021b; King et al. 2024; Scanlan et al. 2024), the use of such warning systems would reduce over-reliance on fungicides and reduce selection pressure towards fungicide resistance.
In addition to genetic resistance and fungicides, cultural practices such as tillage, crop rotation and isolation from the previous year’s stubble can influence blackleg disease (Turkington et al. 2000; West et al. 2001; Van de Wouw et al. 2016). In Australia, current cultural recommendations are based on studies undertaken in early 2000s under different farming systems in which stubble was cultivated into the soil or burnt, canola area was lower as were stubble loads as open-pollinated cultivars were grown rather than currently available higher biomass hybrid cultivars (Marcroft et al. 2003; Marcroft et al. 2004). In these studies, the majority of ascospores were released during the first year post-harvest with a 30-fold reduction in spore release in the second year as 90% of stubble was broken down within the first year post-harvest such that an isolation of 500 m from the previous crop was sufficient to control crown canker severity. This contrasts with the current study under modern farming practices where ascospore production in the second season was similar, or even higher, than in the first year, suggesting that canola grown in tight canola-wheat-canola rotations is exposed to high inoculum loads. In addition to greater ascospore loads in the second year under the current farming practices, canola production has intensified and stubble persists across multiple growing seasons with inter-row sowing and zero/minimum tillage practices, again increasing disease inoculum. Similarly in Canada, low levels of canola stubble and blackleg disease were present at the end of a 4-year canola rotation irrespective of tillage practice (Turkington et al. 2000) but blackleg disease was higher in zero tillage systems when canola was grown in tighter rotations (Guo et al. 2005). The role of inoculum load on disease and consequent yield outcomes is a complex interaction between multiple factors including environmental conditions, the level of host resistance, timing of infection in relation to crop growth stage, and pathogen genotype (Bondad et al. 2025b) such that a reduced inoculum load may not reliably lead to lower disease severity and improved yields. An additional consideration of high stubble loads is the large effective population size it supports which promotes pathogen adaptation to host genetic resistance, fungicides and changes in climate.
While Australian canola growers currently have high levels of genetic resistance and effective fungicides to control blackleg, it is evident that these pillars of control are under significant threat due to the persistence of high inoculum loads on stubble. Updated cultural strategies for modern farming systems are required as are next-gen strategies that incorporate forecasted and real-time data streams to support the sustainable management of blackleg disease. The integration of pest/disease constraints into agronomic and farming systems research to account for these potential impacts is sorely required to ensure ongoing realisation of productivity gains. Lastly, novel strategies or treatments that reduce inoculum production and carryover are required for blackleg and other stubble-borne diseases that threaten the viability of conservation agriculture methods in Australia and worldwide.
Declaration of funding
This research was funded by the Australian Grains Research and Development Corporation (UM00051) and supported by the CSIRO, University of Melbourne, and the Department of Primary Industries and Regional Development (NSW).
Acknowledgements
The authors thank growers in Victoria and New South Wales for their support of this research and access to their properties. We also thank John Graham (CSIRO) for technical support.
References
AOF (2024) Crop reports for the 2018/19 and 2022/23 seasons. Australian Oilseeds Federation. Available at https://australianoilseeds.com/
BOM (2024) Bureau of Meteorology. Available at http://www.bom.gov.au/
Bondad JJ, Whish JPM, Sprague SJ, Van de Wouw AP, Barry KM, Harrison MT (2025a) Modelling crop management and environmental effects on the development of Leptosphaeria maculans pseudothecia. European Journal of Plant Pathology 171, 431-443.
| Crossref | Google Scholar |
Bondad JJ, Whish JPM, Sprague SJ, Maher R, Barry KM, Harrison MT (2025b) Environmental and management determinants of blackleg crown canker disease (Leptosphaeria maculans) of canola (Brassica napus). Australasian Plant Pathology 54, 13-24.
| Crossref | Google Scholar |
Bousset L, Ermel M, Lebreton L (2018) The full life cycle of Leptosphaeria maculans completed on inoculated oilseed rape incubated under controlled conditions. Plant Pathology 67(6), 1321-1328.
| Crossref | Google Scholar |
Condon G, Condon K, Swan T, Verburg K, Holding J, Thompson H, Broster J, Eberbach P (2023) Fallow water impacts when harvesting with a draper front versus a stripper front in southern NSW. In ‘GRDC updates 2023’. 25 July 2023. (Grains Research and Development Corporation) Available at https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2023/07/fallow-water-impacts-when-harvesting-with-a-draper-front-versus-a-stripper-front-in-southern-nsw
GRDC (2025) Blackleg management guide. GRDC. Available at https://grdc.com.au/resources-and-publications/all-publications/factsheets/2025/blackleg-management-guide
Guo XW, Fernando WGD, Entz M (2005) Effects of crop rotation and tillage on blackleg disease of canola. Canadian Journal of Plant Pathology 27(1), 53-57.
| Crossref | Google Scholar |
Hammond KE, Lewis BG, Musa TM (1985) A systemic pathway in the infection of oilseed rape plants by Leptosphaeria maculans. Plant Pathology 34(4), 557-565.
| Crossref | Google Scholar |
Hirst JM, Stedman OJ (1962) The epidemiology of apple scab (Venturia inaequalis (Cke.) Wint.) II. Observations on the liberation of ascospores. Annual of Applied Biology 50(3), 525-550.
| Crossref | Google Scholar |
Hunt JR, Lilley JM, Trevaskis B, et al. (2019) Early sowing systems can boost Australian wheat yields despite recent climate change. Nature Climate Change 9, 244-247.
| Crossref | Google Scholar |
King KM, Barr L, Bousquet L, Glaab A, Canning G, Ritchie F, Kildea S, Fraaije BA, West JS (2024) Evolution of decreased sensitivity to azole fungicides in western European populations of Plenodomus lingam (Phoma stem canker on oilseed rape). Plant Pathology 73(6), 1517-1532.
| Crossref | Google Scholar |
Kirkegaard JA, Lilley JM, Brill RD, Sprague SJ, Fettell NA, Pengilley GC (2016) Re-evaluating sowing time of spring canola (Brassica napus L.) in south-eastern Australia – how early is too early? Crop & Pasture Science 67(4), 381-396.
| Crossref | Google Scholar |
Lilley JM, Flohr BM, Whish JPM, Farre I, Kirkegaard JA (2019) Defining optimal sowing and flowering periods for canola in Australia. Field Crops Research 235, 118-128.
| Crossref | Google Scholar |
Marcroft SJ, Sprague SJ, Pymer SJ, Salisbury P, Howlett BJ (2003) Factors affecting production of inoculum of the blackleg fungus (Leptosphaeria maculans) in south-eastern Australia. Australian Journal of Experimental Agriculture 43(10), 1231-1236.
| Crossref | Google Scholar |
Marcroft SJ, Sprague SJ, Pymer SJ, Salisbury PA, Howlett BJ (2004) Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in south-eastern Australia. Australian Journal of Experimental Agriculture 44(6), 601-606.
| Crossref | Google Scholar |
Marcroft SJ, Van de Wouw AP, Salisbury PA, Potter TD, Howlett BJ (2012) Effect of rotation of canola (Brassica napus) cultivars with different complements of blackleg resistance genes on disease severity. Plant Pathology 61(5), 934-944.
| Crossref | Google Scholar |
McCredden J, Cowley RB, Marcroft SJ, Van de Wouw AP (2018) Changes in farming practices impact on spore release patterns of the blackleg pathogen, Leptosphaeria maculans. Crop & Pasture Science 69(1), 1-8.
| Crossref | Google Scholar |
McGee DC, Emmett RW (1977) Black leg (Leptosphaeria maculans (Desm.) Ces. et de Not.) of rapeseed in Victoria: crop losses and factors which affect disease severity. Australian Journal of Agricultural Research 28(1), 47-51.
| Crossref | Google Scholar |
Petronaitis T, Forknall T, Simpfendorfer S, Backhouse D, Flavel R (2022) Stubble trouble! Moisture, pathogen fitness and cereal type drive colonisation of cereal stubble by three fungal pathogens. Australasian Plant Pathology 51(3), 363-368.
| Crossref | Google Scholar |
Salam MU, Khangura RK, Diggle AJ, Barbetti MJ (2003) Blackleg sporacle: a model for predicting onset of pseudothecia maturity and seasonal ascospore showers in relation to blackleg of canola. Phytopathology 93(9), 1073-1081.
| Crossref | Google Scholar | PubMed |
Salam MU, Fitt BDL, Aubertot J-N, Diggle AJ, Huang YJ, Barbetti MJ, Gladders P, Jedryczka M, Khangura RK, Wratten N, Fernando WGD, Penaud A, Pinochet X, Sivasithamparam K (2007) Two weather-based models for predicting the onset of seasonal release of ascospores of Leptosphaeria maculans or L. biglobosa. Plant Pathology 56(3), 412-423.
| Crossref | Google Scholar |
Scanlan JL, Idnurm A, Van de Wouw AP (2024) Genome-wide mapping in an international isolate collection identifies a transcontinental erg11/CYP51 promoter insertion associated with fungicide resistance in Leptosphaeria maculans. Plant Pathology 73(6), 1506-1516.
| Crossref | Google Scholar |
Simpfendorfer S, McKay A, Ophel-Keller K (2020) New approaches to crop disease management in conservation agriculture. In ‘Australian agriculutre in 2020: from conservation to automation’. (Eds J Pratley, JA Kirkegaard) pp. 173–188. (Agronomy Australia and Charlse Sturt University: Wagga Wagga, NSW)
Slinger D, Potter TD, Light KA, Moore T (2016) ‘Canola in Australia: 21st century progress.’ (New South Wales) Available at https://australianoilseeds.com/wp-content/uploads/2024/04/Canola_in_Australia_21st_century_progress_web_300.pdf
Sprague SJ, Kirkegaard JA, Howlett BJ, Graham J (2009) Effect of root rot and stem canker caused by Leptosphaeria maculans on yield of Brassica napus and measures for control in the field. Crop & Pasture Science 61(1), 50-58.
| Crossref | Google Scholar |
Sprague SJ, Marcroft SJ, Lindbeck KD, Ware AH, Khangura RK, Van de Wouw AP (2018) Detection, prevalence and severity of upper canopy infection on mature Brassica napus plants caused by Leptosphaeria maculans. Crop & Pasture Science 69(1), 65-78.
| Crossref | Google Scholar |
Turkington TK, Clayton GW, Klein-Gebbinck H, Woods DL (2000) Residue decomposition and blackleg of canola: influence of tillage practices. Canadian Journal of Plant Pathology 22(2), 150-154.
| Crossref | Google Scholar |
Umbers A (2016) Farm practices survey report 2016. Grains Research and Development Corporation, Canberra, ACT, Australia. Available at https://grdc.com.au/resources-and-publications/all-publications/publications/2018/farm-practices-survey-report-2016
Van de Wouw AP, Marcroft SJ, Howlett BJ (2016) Blackleg disease of canola in Australia. Crop & Pasture Science 67, 273-282.
| Crossref | Google Scholar |
Van de Wouw AP, Marcroft SJ, Sprague SJ, Scanlan JL, Vesk PA, Idnurm A (2021a) Epidemiology and management of blackleg of canola in response to changing farming practices in Australia. Australasian Plant Pathology 50, 137-149.
| Crossref | Google Scholar |
Van de Wouw AP, Scanlan JL, Marcroft SJ, Smith AJ, Sheedy EM, Perndt NW, Harrison CE, Forsyth LM, Idnurm A (2021b) Fungicide sensitivity and resistance in the blackleg fungus, Leptosphaeria maculans, across canola growing regions in Australia. Crop & Pasture Science 72(12), 994-1007.
| Crossref | Google Scholar |
Verdon-Kidd DC, Kiem AS, Moran R (2014) Links between the Big Dry in Australia and hemispheric multi-decadal climate variability – implications for water resource management. Hydrology and Earth System Sciences 18, 2235-2256.
| Crossref | Google Scholar |
Verrell AG, Simpfendorfer S, Moore KJ (2017) Effect of row placement, stubble management and ground engaging tool on crown rot and grain yield in a no-till continuous wheat sequence. Soil and Tillage Research 165, 16-22.
| Crossref | Google Scholar |
West JS, Kharbanda PD, Barbetti MJ, Fitt BDL (2001) Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathology 50(1), 10-27.
| Crossref | Google Scholar |
Whish JPM, Lilley JM, Morrison MJ, Cocks B, Bullock M (2020) Vernalisation in Australian spring canola explains variable flowering responses. Field Crops Research 258, 107968.
| Crossref | Google Scholar |
Zhang X, Peng G, Kutcher HR, Balesdent M-H, Delourme R, Fernando WGD (2016) Breakdown of Rlm3 resistance in the Brassica napusLeptosphaeria maculans pathosystem in western Canada. European Journal of Plant Pathology 145, 659-674.
| Crossref | Google Scholar |


