Water: the most important ‘molecular’ component of water stress tolerance research
Vincent Vadez A D , Jana Kholova A , Mainassara Zaman-Allah A B and Nouhoun Belko A CA International Crops Research Institute for the Semiarid Tropics (ICRISAT), Crop Physiology Laboratory, Patancheru 502 324 Andhra Pradesh, India.
B International Maize and Wheat Improvement Center (CIMMYT), PO Box MP 163 Mount Pleasant Harare, Zimbabwe.
C Centre d’Etude Régional pour l’Amélioration de l’Adaptation à la Sécheresse (CERAAS), BP 3320 Thiès-Escale, Sénégal.
D Corresponding author. Email: v.vadez@cgiar.org
This paper originates from a presentation at the ‘VI International Conference on Legume Genetics and Genomics (ICLGG)’ Hyderabad, India, 2–7 October 2012.
Functional Plant Biology 40(12) 1310-1322 https://doi.org/10.1071/FP13149
Submitted: 20 May 2013 Accepted: 20 September 2013 Published: 29 October 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Water deficit is the main yield-limiting factor across the Asian and African semiarid tropics and a basic consideration when developing crop cultivars for water-limited conditions is to ensure that crop water demand matches season water supply. Conventional breeding has contributed to the development of varieties that are better adapted to water stress, such as early maturing cultivars that match water supply and demand and then escape terminal water stress. However, an optimisation of this match is possible. Also, further progress in breeding varieties that cope with water stress is hampered by the typically large genotype × environment interactions in most field studies. Therefore, a more comprehensive approach is required to revitalise the development of materials that are adapted to water stress. In the past two decades, transgenic and candidate gene approaches have been proposed for improving crop productivity under water stress, but have had limited real success. The major drawback of these approaches has been their failure to consider realistic water limitations and their link to yield when designing biotechnological experiments. Although the genes are many, the plant traits contributing to crop adaptation to water limitation are few and revolve around the critical need to match water supply and demand. We focus here on the genetic aspects of this, although we acknowledge that crop management options also have a role to play. These traits are related in part to increased, better or more conservative uses of soil water. However, the traits themselves are highly dynamic during crop development: they interact with each other and with the environment. Hence, success in breeding cultivars that are more resilient under water stress requires an understanding of plant traits affecting yield under water deficit as well as an understanding of their mutual and environmental interactions. Given that the phenotypic evaluation of germplasm/breeding material is limited by the number of locations and years of testing, crop simulation modelling then becomes a powerful tool for navigating the complexity of biological systems, for predicting the effects on yield and for determining the probability of success of specific traits or trait combinations across water stress scenarios.
Additional keywords: hydraulics, lysimeters, roots, vapour pressure deficit.
Introduction
Water stress is the most important abiotic factor limiting crop yields worldwide. This is because of the photosynthesis-driven plant growth trade-off between carbon dioxide and water at the stomata level. Water stress occurs in all situations where plant water demand for growth is not met by water supply. Therefore, matching water supply to water demand is a key consideration to keep in mind when developing crop cultivars that are adapted to water stress. There has been a considerable quantity of research on water stress, but it appears that in many cases the essentiality of matching water supply and demand did not appear to be an important aspect. For example, cell protection homeostasis mechanisms (e.g. antioxidant enzyme, osmolytes) were shown to be beneficially involved in the water stress response by delaying death and improving survival under fairly unrealistic stress conditions (Bohnert and Shen 1998; Mittler 2002). Although considerable biotechnological research investments have been made to harness these properties (reviewed by Bhatnagar-Mathur et al. 2008), there has been no applied outcome thus far, most probably because plant survival has little economic value to farmers. Similarly, osmotic adjustment has long been viewed as a means of keeping stomata open and sustaining growth under water limited conditions (e.g. Blum et al. 1999), although keeping stomata open would also sustain water losses and could be detrimental in many situations. A review on osmotic adjustment later showed that this mechanism had virtually no effect on crop yield in most situations (Serraj and Sinclair 2002) like in chickpea (Turner et al. 2007) under water stress, with a few exceptions. Therefore, in this review we argue that research on water deficit must be indeed re-centred on water and we focus our attention on plant strategies that facilitate an economical yield under water-limited conditions. Such strategies revolve around two crucial aspects of the plant water budget: (i) the ability to capture more water; and (ii) the ability to conserve and use captured water more efficiently.
Several studies across crop species indicate that ‘superior’ structural root traits contribute to better performance of genotypes under water-limited conditions (e.g. Silim and Saxena 1993; Tuberosa et al. 2002; Matsui and Singh 2003; Ober et al. 2005; Sarker et al. 2005; Kashiwagi et al. 2006). Most of these studies assume that rooting traits relate to water uptake. However, the link between better rooting and plant performance lacks crucial information on how roots contribute to water extraction, with respect to quantity and timing, in ways that lead to higher grain yield. Therefore, although the assessment of roots provides valuable information on the potential of genotypes to cope with water limitation, this information remains static and sheds little light on the actual water contribution of the roots and on the timing of water uptake. In this review, we address the importance of looking at root functionality rather than structure or architecture, by measuring the volume of water taken up by roots, rather than roots themselves. We highlight the need to develop methods that allow a precise and dynamic measurement of water uptake at key times, together with relevant agronomic evaluations of plant materials, to critically assess relationships between water extraction patterns and grain yield.
The second part of this review relates to plant water conservation and efficient use. Biomass production is water use, thus, biomass production and water conservation are antagonistic features in nature. In other words, under water limitation, biomass production needs to match water availability in a way that allows plants to fulfil their life cycle (Passioura 2012). Here, we review the genetic options that plants have for optimising biomass production per unit of water use and to complete their life cycle. Because part of our current focus is on crops that grow in semiarid tropical conditions, we have a particular interest in water conservation mechanisms. However, we also discuss the trade-off between water conservation and biomass accumulation, and we address the need to maximise water utilisation from the soil profile so that no water remains available in the soil profile once the crop has matured. We note also that there are important management options that can reduce the water use of a crop canopy (e.g. alterations in nitrogen fertilisation or planting densities). These practices are also essential to improve crop productivity under water limitation and are the object of extensive recent reviews (Kirkegaard and Hunt 2010; Passioura and Angus 2010). In this paper we chose to focus only on the genetic aspects of plant water budget, although management aspects are briefly examined in the last section.
Manipulating these strategies towards genetic improvement is complicated by the fact that crop success under water-limited conditions is not dependent solely on capturing more water or conserving/using it better. Genotypes that are successful under water-limited conditions are those that are capable of both maximising biomass production while securing maximum partitioning to grains. Therefore, in this review, we advocate the need for tools and methods for comprehensive (rather than individual) assessment of these closely related strategies. Additionally, these strategies are over-ridden by the importance of plant phenology and crop duration, which, in turn, depend on the environment (and particularly on the weather). Thus, the third part of this review addresses the methodological requirements for undertaking such efforts. Crop simulation modelling is a powerful tool that helps predict how plant traits related to water capture and use/conservation interact with one another and how this interaction would lead to an increase in yield across the varying weather conditions in a given environment. Here also, we focus mostly on the modelling of the genetic aspects, although crop modelling also allows simulating the effect of management practices. In particular, we discuss the need for a plant breeding-based approach to crop improvement from an environmental perspective, and we postulate that future breeding is likely to become environment-specific, with the genetic gain of improved varieties driven by probability scenarios that are linked to weather conditions.
Root and water capture
Usual assumptions about roots under water-limited conditions
Roots are often viewed as the key to solving water stress issues, therefore, ‘structural root traits’ are often assimilated with ‘water stress tolerance traits’. A recent work supports the idea that roots will be the basis for a new green revolution (Gewin 2010). In a 2 year study of chickpea, Kashiwagi et al. (2006) showed that the root length density was indeed significantly correlated with higher seed yield under terminal water stress, although the 12 genotype experiment was strongly biased by one genotype with poor roots and good yield and one genotype with good roots and poor yield. However, in another experiment within the same study, the yield reduction caused by water stress was less severe, and there was no significant relationship between root length density and seed yield. Similar results were recently obtained in lentils, where root traits and grain yield were not significantly related in a rain-fed situation (Kumar et al. 2012). Ratnakumar and Vadez (2011) compared the root systems of 20 groundnut genotypes with variable yields under a range of water stress conditions and showed that the yield differences under stress were not related to differences in rooting depth or root length density at different depths. Zaman-Allah and colleagues (2011a) reached the same conclusion with twenty chickpea genotypes contrasting for terminal water stress tolerance. Nevertheless, the benefit of deeper root systems has been shown in other studies (Kirkegaard et al. 2007; Christopher et al. 2008; Hund et al. 2009). For example, a simulation study indicated that maize yields would increase if the root depth increased (Sinclair and Muchow 2001). Hammer et al. (2009) postulated that changes in root architecture contributed to maize yield increases in the USA These examples illustrate that roots may not be the answer to all water stress scenarios and that their contribution to yield increases in water-limited environments depends on the crop and the stress conditions (Palta et al. 2011). In summary, although there is no doubt that roots are important, their role in adapting to water stress might have been overstated. This may have occurred in part because roots are not akin to water, and it is too often assumed that additional roots lead to additional water extraction. Of course, water extraction is determined by both the water availability in the soil profile and the capacity of roots to access it. Therefore, although roots (deep, profuse at depth) are necessary structures for water extraction, they are by no means a sufficient condition for adapting to water stress.
Do differences in root length density and water uptake relate?
The fact that a deeper or more profuse rooting system does not always lead to increased yield under water stress conditions is indeed partially because roots and water extraction are not necessarily related. Some studies have shown that an increase in root length density (RLD) leads to additional water extraction (Passioura 1983; Monteith and Greenwood 1986; Lafolie et al. 1991; Hund et al. 2009; Vadez et al. 2013a). In contrast, other studies have shown poor relationships between water uptake and RLD across several cereals and legumes (Hamblin and Tennant 1987; Dardanelli et al. 1997; Katayama et al. 2000; Amato and Ritchie 2002; Ratnakumar and Vadez 2011; Zaman-Allah et al. 2011a). Therefore, whether RLD and water uptake are related is still a subject of debate. Part of the controversy is due to (i) the limited knowledge of the minimum RLD required to fully extract water from a given soil volume, (ii) the limited knowledge of how roots are distributed at a given soil depth, (iii) the fact that the soil profile depth may vary, and (iv) simply that the roots have reached layers where there is no water or limited water for uptake. Relatively few roots in deep layers would be sufficient to supply an ample amount of water to the plant when the topsoil is dry, provided that water is available at this depth (Gregory et al. 1978; Sharp and Davies 1985). Indeed, the development of a larger proportion of the root structure at depth has been shown to contribute to higher grain yield in peanut (Jongrungklang et al. 2011), more so than the RLD. Similarly, the extraction of more water in water stressed DREB1A transgenic peanut plants was related to higher root length density at depth (Vadez et al. 2013a). Thus, progress will be achieved by putting more focus on the distribution of roots in the soil profile, and an investigation of the role of roots in water stress adaptation may involve tomographic measurements of the root system in situ (e.g. Mooney et al. 2012) or an examination of root models that take into account rooting architecture (Lobet et al. 2011). However, measuring such a fine rooting differences at depth is a challenge, and although destructive measurements provide static data, they do not provide data on the quantities or kinetics of water extraction. Therefore, until there is a means of accessing root growth in situ and possibly under natural conditions, we argue that destructive root measurements provide only an indirect assessment of the potential of a given genotype to cope with water stress via its root system. Until then, the measurement of water uptake at different times during the cropping cycle might be an easier target for future research on the contribution of roots to water stress adaptation, as suggested previously (McIntyre et al. 1995; Dardanelli et al. 1997; Vadez et al. 2008; Zaman-Allah et al. 2011a; Vadez et al. 2013b).
The need for dynamic measurements of water extraction at key times
Thus far, we have discussed the need for a shift away from destructive root measurements and towards water uptake measurements to better understand differences in genotype adaptation to water deficit. Several authors have argued that the presence of small amounts of water during key crop stages such as the grain filling period would confer a major benefit (Ketring and Reid 1993; Christopher et al. 2008; van Oosterom et al. 2011). Unfortunately, it is difficult to measure water extraction precisely in the field, especially the small but key amounts that may be extracted during the grain filling period; however, a lysimetric method now exists in which a precise water extraction measurement is possible at all crop stages (e.g. Payne et al. 1992; Vadez et al. 2008). This method indeed confirms that the extraction of small amounts of water at key stages is critical and likely more informative than measuring total water extraction (Ratnakumar et al. 2009; Zaman-Allah et al. 2011a; Vadez et al. 2013b). Manschadi et al. (2006) also showed that each mm of water provided during grain filling would contribute a 55 kg ha–1 yield increase. Similar data were provided by Kirkegaard et al. (2007) in wheat (59 kg ha–1 yield increase mm–1 water), and these data are in line with those obtained from lysimeter experiments in pearl millet (Vadez et al. 2013b; 37–45 kg ha–1 yield increase mm–1 water). In chickpea, each additional mm of water would lead to a 40 kg ha–1 increase in grain yield, and the water extraction differences between tolerant and sensitive entries extrapolated to field conditions were only on the order of 25 mm (Zaman-Allah et al. 2011a).
The continued extraction of water at late crop stages would be possible if the roots continued to grow during these stages, as was shown earlier, especially under different conditions such as water stress (Chopart 1983; Hafner et al. 1993; Ketring and Reid 1993), thus, it might be valuable to screen for such a trait. Of course, the continued growth of the roots during the grain filling stage would be useful only if the soil profile were deep enough and had water available. Importantly, genotypes that extract more water during the grain filling period extract less water during the vegetative stage (e.g. Vadez et al. 2011b, 2013a; Zaman-Allah et al. 2011a). The continued extraction of water at later stages therefore appears to depend on water-saving mechanisms functioning at earlier stages, and water extraction by the root at key stages is a dynamic process that does not depend only on the root tapping more water at depth. This process is also the consequence of differences in the shoot water demand at earlier stages. The next section explores the reasons for the variations in this demand, wherein the important factors are canopy size and conductance. Therefore, we believe that an understanding of the role of root traits in water stress adaptation also involves an understanding of how traits interact at different levels of plant organisation (Kudoyarova et al. 2013) and also raises the question of how root and shoot traits are coordinated.
Are root and shoot growth under common or independent genetic control?
Under water limitation, larger, longer and deeper roots could confer a major benefit if water were available in the soil profile and if the roots were able to tap that water to substantially increase the water supply. However, enhanced root traits might provide little advantage to the plant if such characteristics are paralleled by a larger shoot that consumes more water. This raises the question as to whether root and shoot growth are under independent or coordinated genetic controls. Root and shoot growth is indeed closely coordinated (Jackson 1993; Palta et al. 2011), and abscisic acid (ABA) likely plays a major role in that regulation (Munns and Cramer 1996). It is, in fact, critical for plants to maintain a hydraulic integrity in the soil–root–xylem–leaf–atmosphere continuum to maintain water fluxes (Kudoyarova et al. 2013). A major quantitative trait locus (QTL) was identified for root length density and root depth in chickpea (Chandra et al. 2004), explaining ~35% of the phenotypic variation. However, this locus was also a QTL for shoot growth, explaining more than 50% of the shoot biomass. Recent work examining Arabidopsis thaliana showed that shoot and root growth are indeed under the same genetic control (Bouteillé et al. 2012; and references therein). These results suggest that any advantages conferred by bigger root systems in terms of additional water extraction might be offset by the presence of a larger shoot consuming the extra water (Palta et al. 2011). Two recent modelling studies showed exactly that: faster root growth generally led to faster soil water depletion, which subsequently led to yield penalties in soybean (Sinclair et al. 2010) and chickpea (Vadez et al. 2012).
In summary, although roots can play an important role in water stress adaptation, the focus on faster root growth and more profuse rooting risks the generation of plant types that exploit soil water quickly. However, faster root growth could still be important in specific conditions such as areas in which rainfall occurs over short durations, where slower root growth would not allow early maturity genotypes to extract all the water available from the soil profile (Vadez et al. 2012), or in areas where the soil depth is high and water is available at that depth (Sinclair et al. 2010).
Water use and conservation
Leaf canopy development
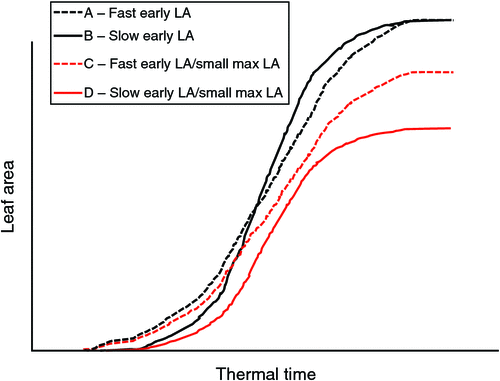
Plants use water to produce biomass for the development of leaf area. Because species or genotypes within species have different leaf areas, they have different water uses. Fig. 1 shows the typical sigmoid shape of the leaf area development curve as a function of thermal time. In the left part of the curve, the leaf area of two hypothetical genotypes, A and B, is represented. Genotype A shows faster leaf area development at earlier stages compared with genotype B, although both genotypes eventually reach a similar leaf area. For breeders, genotype A represents a high-vigour phenotype compared with B. The differences in area between the two curves of genotypes A and B represent the leaf area differences between the two genotypes. Assuming similar leaf conductance, these leaf area differences therefore represent differences in water use. Several genetic factors can contribute to these differences, including the rate of leaf appearance, or simply the size of individual leaves appearing at different stages. When the leaf area of the canopy reaches a hypothetical maximum, several situations can arise. Fig. 1 shows the case of genotypes A and C, which have similar initial kinetics of canopy development, but eventually differ in their maximum leaf areas. Genotypes A and D differ for both the initial rate of leaf area development and for the maximum leaf area. In addition to the genetic factors mentioned above that can contribute to these differences, the level of branching (or tillering for cereals) is important. Therefore, Fig. 1 illustrates four different hypothetical situations and is essentially a factorial of slow/fast canopy development with small/large canopies. Differences between the curves indicate differences in leaf area; however, at the same leaf conductance levels, these differences represent differences in water usage over the course of leaf area development. Therefore, every factor that affects how rapidly and how large the canopy develops is bound to affect how much water a given genotype will use before anthesis, and this would have significant implications in a water-limited context.
|
These theoretical differences are illustrated by several experimental results in different crops. Chickpea genotypes that were tolerant to terminal water stress conditions tended to have a smaller leaf canopy at the vegetative stage than sensitive genotypes (Zaman-Allah et al. 2011b). This partially explained the smaller plant water use at the vegetative stage in these genotypes (Zaman-Allah et al. 2011a). A similar observation was noted in peanut genotypes exposed to three different intermittent water stress treatments varying in intensity where the tolerance index was negatively related to the leaf area and the leaf dry weight, i.e. genotypes with a small canopy maintained their yield under water stress at levels closer to that of the fully irrigated control (Ratnakumar and Vadez 2011). Further, recent data have shown that the coefficients of an exponential function linking the node number on the main stem to the leaf area of plants (a function used in a family of robust legume crop models (Soltani and Sinclair 2011)) vary between genotypes and lead to different leaf area indices in peanut (O Halilou, TR Sinclair, F Hamidou, V Vadez, unpubl. data) and chickpea (V Vadez, R Wangari, P Murthy, unpubl. data). A set of contrasting cowpea genotypes, selected for differences in grain yield under terminal water stress across field conditions, also contrasted for leaf area at the time of flowering, with tolerant genotypes exhibiting less leaf area (Belko et al. 2012). Anyia and Herzog (2004) also showed that genotypes with larger leaf areas showed lower water stress avoidance. Similar examples can be found outside of legume crops. For example, in sorghum, hybrids with a higher leaf appearance rate showed reduced tillering, which led to both a reduced leaf area around anthesis and increased yield under water stress (van Oosterom et al. 2011). Of course, a smaller canopy would also restrict light capture and limit yield under certain conditions or crops (Sinclair and Muchow 2001).
Above, we noted that differences in the rate of leaf canopy development and in the maximum leaf area around anthesis. Some of these differences are genetic; however, environmental conditions are also known to play an important role in leaf area development through a combination of hydraulic and metabolic controls (Pantin et al. 2011, 2012; Kudoyarova et al. 2013). For example, maize leaf development varied among genotypes when plants were exposed to either a high vapour pressure deficit (VPD) or a low soil water potential (Reymond et al. 2003). Thus, genotypes that exhibit sensitivity to a high VPD with respect to their leaf area development would have smaller leaf areas than insensitive genotypes if grown under such conditions. It is indeed quite clear that leaf development is under hydraulic control (Munns and Cramer 1996; Tardieu et al. 2010; Pantin et al. 2012). Leaf development is also sensitive to soil drying, and the termination of leaf growth occurs before termination of transpiration (Sadras and Milroy 1996; Soltani et al. 2001; Reymond et al. 2003; Parent et al. 2009; Tardieu et al. 2010). Depending on the water stress scenario, limiting leaf development under water stress could limit productivity. QTLs for sustained leaf growth under a high VPD or soil drying were identified in maize, with beneficial alleles originating from water stress tolerant parents (Welcker et al. 2011). In other crops, as observed above, limiting leaf growth or decreasing it at higher soil water potentials could be beneficial (Lawlor and Tezara 2009; Ratnakumar and Vadez 2011). In any case, it will be important to harness the genetic determinants of leaf area development (both the inherent characteristics and the genetic responses to environmental conditions) to subsequently tailor cultivars with a trait makeup that fits specific conditions.
Leaf canopy conductance
Whereas limiting the size of the transpiring leaves is one way to control plant water losses, limiting the conductance of the leaf canopy is another point of control. Indeed, under similar environmental conditions, two genotypes with the same leaf area exhibiting differences in leaf canopy conductance would lose different amounts of water. For example, pearl millet genotypes varied with respect to leaf canopy conductance under fully irrigated conditions despite exhibiting a similar leaf area (Kholová et al. 2010a), and this appeared to be related to higher leaf ABA content in genotypes with low leaf canopy conductance (Kholová et al. 2010b). Similar results were obtained in chickpea (Zaman-Allah et al. 2011b) and cowpea (Belko et al. 2012), where the canopy conductance differed among genotypes. In the research reported by Kholová et al. (2010b), Zaman-Allah et al. (2011b) and Belko et al. (2012), the leaf canopy conductance was calculated as the ratio of gravimetric transpiration measurements at the whole-plant level divided by the leaf area and the time that plants were allowed to transpire (either an entire day or one-hour time periods across an entire day). Thus, it was ensured that the leaf area index of the plants was <1, such that there was a lack of (or limited) mutual shading of leaves. Leaf conductance measurements using gravimetric methods have a throughput that makes them suitable for breeding programs. These measurements were robust, and they were preferred over porometric measurements, which have several drawbacks (Turner 1991), including sampling (choice of leaf or choice of leaf section), time of sampling (possible changes in light or VPD conditions), and throughput. Porometric measurements would also not be able to cope with the possibility of stomatal patchiness (Buckley and Mott 2000). Using these methods, chickpea genotypes that were tolerant to terminal water stress were found to have a lower leaf canopy conductance at the vegetative stage and under fully irrigated conditions (Zaman-Allah et al. 2011b). Higher yielding chickpea genotypes also had a lower index of leaf canopy conductance, measured using canopy temperature data obtained from infrared images, than lower yielding genotypes, and this water saving feature led to a lower plant water use in tolerant genotypes at the vegetative stage (Zaman-Allah et al. 2011a). Similarly, in cowpea a majority of terminal water stress tolerant germplasms also had a lower leaf canopy conductance than sensitive germplasm (Belko et al. 2013). DREB1A groundnut, which has high transpiration efficiency (TE), also exhibited low stomatal conductance (Bhatnagar-Mathur et al. 2007). Transgenic tomatoes with high levels of ABA also showed reduced leaf conductance, which also led to a higher TE (Thompson et al. 2007). A similar situation was observed in wheat (Condon et al. 2002).
Stomatal opening is sensitive to the evaporative demand, and a high VPD reduces stomatal aperture to restrict water losses. This phenomenon has long been reported in different crop species (e.g. Squire 1979; Turner et al. 1984; Grantz 1990), and it ensures maximum transpiration at times of the day when the VPD crosses a threshold. However, genotypic variation has only recently been revealed in different species such as soybean (Fletcher et al. 2007; Sadok and Sinclair 2009), chickpea (Zaman-Allah et al. 2011b), cowpea (Belko et al. 2012), peanut (Devi et al. 2010) and cereals such as sorghum (Gholipoor et al. 2010) and pearl millet (Kholová et al. 2010b). Crop simulation analysis has shown that this maximum rate of transpiration would have a beneficial effect on yield and also that it would lead to water saving and a higher TE (Sinclair et al. 2005). In the case of cowpea, this trait was used to discriminate terminal water stress-tolerant from sensitive entries (Belko et al. 2012). More recently, a QTL for lower leaf canopy conductance under high VPD was identified in pearl millet (Kholová et al. 2012), and genotypes with VPD-responsive traits had higher yields under terminal water stress conditions. The method developed in that research was also designed in a way it can be used by a breeding program.
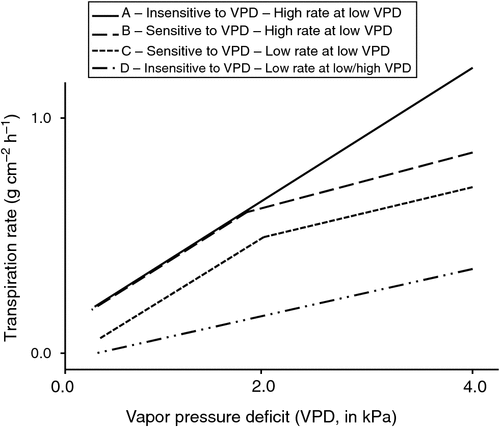
Fig. 2 synthesises the characteristics of leaf conductance that can affect plant water use: (i) high leaf conductance under low or moderate VPD conditions and insensitivity to the high VPD conditions in genotype A; (ii) high leaf conductance under low or moderate VPD conditions but sensitivity to the high VPD conditions in genotype B; (iii) low leaf canopy conductance under low or moderate VPD conditions and sensitivity to high VPD conditions in genotype C; and (iv) low leaf conductance under low VPD and a small positive slope for leaf conductance in response to VPD increase, leading to a low leaf canopy conductance at a high VPD but no VPD breakpoint in the case of genotype D. Genotypes A and D are illustrated by the chickpea genotypes ICC8058 and ICC14799 (Zaman-Allah et al. 2011b) and the cowpea genotypes UC-CB46 and IT93K-503-1 (Belko et al. 2013). Genotypes A and C are illustrated by the pearl millet genotypes H77/833-2 and PRLT-2/89-33 (Kholová et al. 2010b) and the cowpea genotypes IT82E-18 and IT93K-693-2 (Belko et al. 2013). Practically, genotype B saves more water compared with genotype A when the VPD is above the breakpoint value, whereas genotypes C and D save more water compared with genotype A under all VPD conditions. Therefore, genotypes C and D can be considered as ‘water savers’, and although this feature can be beneficial under water-limited conditions, it can also limit carbon fixation under moderate or no water limitation. However, Gilbert et al. (2011a, 2011b) have shown that slow wilting soybean, which displays the typical phenotype of genotypes B or C (Fig. 2), has a photosynthetic rate capable of compensating for a slight reduction in stomatal opening due to the sensitivity to high VPD. Indeed, the relationship between the photosynthetic rate and stomatal conductance has a logarithmic shape (Wong et al. 1979) such that above a certain stomatal conductance, any additional increase in stomatal conductance only leads to a marginal increase in the photosynthetic rate. In summary, the control of leaf water losses, especially under high VPD conditions, appears to be a major avenue for increasing the performance of crops grown under high VPD conditions, and this control mechanism likely leads to increases in TE.
|
Transpiration response to soil humidity
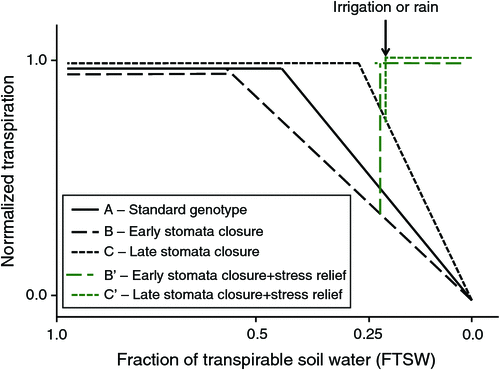
Upon progressive exposure to water deficit, stress-exposed plants reach a soil moisture level at which the root cannot support the full transpirational demand, and the plants must initiate stomatal closure to avoid shoot desiccation. It has long been believed that transpiration would start declining at a fraction of transpirable soil water (FTSW, i.e. the portion of the soil water that plants can take up to support transpiration) on the order of 30–40% regardless of plant species or genotypes within species (e.g. Sinclair and Ludlow 1986; Sinclair et al. 1998). The FTSW thresholds at which leaf expansion starts to decline are known to be higher than those at which transpiration declines; in other words, the leaf expansion processes are more sensitive than transpiration (Sadras and Milroy 1996). Fig. 3 represents the typical curve of the transpiration response of genotype A to soil drying, normalised to a fully irrigated control. This curve shows that the normalised transpiration rate (NTR) remains at a value of 1.0 until the FTSW has dropped below ~0.40 (i.e. 40% of the soil water available for transpiration remains). This also indicates that the leaf gas exchange of the stress-exposed plant is similar to that of a non-stressed plant until 60% of the soil moisture is depleted, i.e. there is no stress in terms of leaf gas exchange as long as the NTR remains at ~1.0. However, in more recent experiments, a large genotypic variation in these FTSW thresholds for transpiration decline was identified. For example, in soybean, differences in the FTSW thresholds were reported among different soil-applied manganese treatments (Vadez et al. 2000), and genetic differences were reported later (Sinclair et al. 2003). Genetic differences in these FTSW thresholds have been reported in groundnut (Devi et al. 2009; Leal-Bertioli et al. 2012), chickpea (Zaman-Allah et al. 2011b), cowpea (Belko et al. 2012) and other non-legume crops such as sorghum (Gholipoor et al. 2010) and pearl millet (Kholová et al. 2010a). This trait is important because genotypes with high FTSW thresholds begin to partially close their stomata at a relatively high soil water content and hence save water. A simulation study has shown that this trait would lead to a significant soybean yield increase in the USA, especially in years classified as dry (Sinclair et al. 2010), although the same trait has been reported to have only a limited impact on chickpea yields (Soltani et al. 2000).
|
Fig. 3 illustrates different potential cases of genotypic variation in FTSW thresholds. Genotype B has a high FTSW threshold value; therefore, it saves water early and has a ‘conservative’ behaviour with regard to plant water use. This phenotype could be advantageous under severe stress conditions such as long-terminal water stress or intermittent water stress with long gaps between rains and is illustrated by the peanut genotype ICGV86015 as described by Devi et al. (2009). Genotype C has a very low FTSW threshold, and its transpiration relative to a fully irrigated control drops at low soil moisture and declines rapidly thereafter. These characteristics are illustrated by the peanut genotypes TMV2 and ICGV86699 described by Devi et al. (2009) and the cowpea genotypes IT84S-2049, Mouride and Suvita 2, which are all terminal water stress tolerant (Belko et al. 2012). Such genotypes exhibit ‘opportunistic’ behaviour with regard to plant water use and would perform well under late terminal water stress or intermittent stress conditions with frequent relief from water stress by irrigation or rain. We recognise that a high FTSW threshold for transpiration decline would also bear negative consequences under intermittent stress conditions, in which there is frequent alleviation of stress and where genotypes with a high FTSW threshold would perform limited carbon fixation between the time when the FTSW threshold value is reached (~0.60 in Fig. 3) and relief of stress. The transpiration of genotype B’ illustrates such a case, whereas genotype C’, with a low FTSW threshold, would barely be affected by the stress before stress relief (Fig. 3).
Regulation of plant water loss: plant hydraulics and hormonal regulation
The sections above highlight the importance of canopy size, kinetics of canopy development, canopy conductance, environmental responses and combinations of these aspects in canopy water use. A considerable body of knowledge exists regarding the individual regulation of some of these factors; however, the co-ordination and integration of their regulation at the organ and plant level are very complex processes that are not yet understood.
Stomatal closure is partially under the control of ABA, which is produced in the roots and transported to the shoot where it targets stomatal guard cells (Zhang and Davies 1991; Tardieu et al. 2010). Evidence for the role of ABA in stomatal closure was obtained from partial root drying experiments, wherein half of the root system was partially dried, and the other half was kept fully irrigated. Although the leaf water potential of the plant exposed to the partial root drying treatment was similar to that of the fully irrigated control, its leaf conductance was reduced (Gowing et al. 1990). Several papers have reviewed the role of ABA in stomatal signalling (Zhang and Davies 1991; Davies et al. 2002; Buckley 2005). Part of the complication arises from the fact that ABA is also known to positively influence the hydraulic conductivity of the roots, which in turn influences the rate of transpiration under high VPD in tomato (Thompson et al. 2007). Comstock (2002) reviewed the possible role of both hydraulic and chemical signals on the control of stomata, providing evidence for both types of regulation. In fact, the question of whether signalling molecules control stomatal movements raises the question of how the rapid changes in stomatal closure can be explained (e.g. those recorded when transpiring plants were exposed to a gradient of increasing VPD conditions). The transpiration responses to VPD reported above indeed imply relatively rapid changes in transpiration, which is hypothesised to be hydraulic in nature (Sinclair et al. 2008; Kholová et al. 2010b). The hypothesis that a hydraulic signal controls stomatal aperture has indeed been proposed and offers an elegant and sensible model for the regulation of stomatal opening (e.g. Sperry et al. 2002). Indeed, it is accepted that some aspects of stomatal regulation are under hydraulic control, at least under a high VPD (Mott 2007).
A consensus has been reached regarding the hydraulic control of leaf development, and there is much evidence for hydraulic regulation (Salah and Tardieu 1996; Thompson et al. 2007; Ehlert et al. 2009; Pantin et al. 2011). However, leaf development is also influenced by ABA via several direct and indirect means (Tardieu et al. 2010), including its effect on root hydraulic conductance via the stimulation of aquaporin (AQP) transcription (Ehlert et al. 2009; Parent et al. 2009). A recent genetic analysis also showed that the sensitivity of leaf development to VPD is controlled by the same genomic regions that control the sensitivity to soil drying in ~75% of cases (Welcker et al. 2011). Given that the sensitivity of the leaf growth rate to VPD is under clear hydraulic control (Reymond et al. 2003; Sadok et al. 2007; Ehlert et al. 2009; Parent et al. 2009), this represents strong evidence that the leaf development response to soil drying is also under hydraulic control.
These examples illustrate the complexity of the regulation of two critical aspects of plant water use, i.e. leaf conductance and leaf development, and clearly show a central role for hydraulics in these processes. In summary, both hydraulic and chemical regulation appear to be playing a role in the control of stomatal aperture and in leaf development. We argue that hydraulic and chemical signalling need to be observed from two angles: first, in the short term control of stomatal aperture, where a hydraulic signal would likely have a prominent influence, although the ‘biochemical environment’ might be playing a role that is still not understood. For example, it was intriguing that pearl millet genotypes with contrasting VPD responses also contrasted with respect to their leaf ABA content (Kholová et al. 2010b). Several studies have also reported that ABA affects the level of AQP in different plant tissues, and this leads to differences in the hydraulic conductivity of tissues (Thompson et al. 2007; Ehlert et al. 2009). Therefore, even under the short-term time frame of stomatal aperture control, both hydraulic and chemical signalling are likely to be intertwined. Second, the long-term control of leaf water loss via the control of leaf development is under both biochemical and hydraulic control; however, it is becoming increasingly clear that the long-term growth environment eventually conditions a short-term leaf conductance response to VPD (Sermons et al. 2012; Schoppach and Sadok 2013).
What progress is needed in ‘water stress’ research?
The need to comprehensively understand root water uptake and shoot water loss/use
Summarising the sections above, we understand that under water limitation conditions plants have different means of either increasing access to water or optimising water use by rationing it for use during critical periods of the developmental cycle. These strategies are related to water input. In contrast, a plant loses water depending on the size of its canopy, the speed of its development, and its conductance (especially conductance under particular atmospheric conditions). These situations are related to water output. Water stress ‘tolerance’ therefore results from a complex combination of traits that influence supply and demand for water (Passioura 2012). The ability of a genotype to adapt to a particular water availability level eventually determines the level of ‘tolerance’ of that genotype. Therefore, to understand these complex interactions, methods are needed that can be used to analyse as many components as possible related to plant water balance such that a comprehensive understanding can be achieved. Recently, a lysimetric system has been developed (Vadez et al. 2008), in which plant water use can be monitored from a very early growth stage until maturity and highly relevant agronomic assessments can be performed in conditions that mimic field situations at least in terms of plant density and the soil and water volume available to each plant (Ratnakumar and Vadez 2011; Vadez et al. 2011a, 2011b, 2013b; Zaman-Allah et al. 2011a). In particular, this system allows the measurement of water extraction at key stages, especially the grain filling period, and it can be used to assess leaf conductance using the index of stomatal conductance (Zaman-Allah et al. 2011a).
The system also has the advantage of providing a means of stress response assessment via comparisons between the transpiration of water stressed plants and that of fully irrigated controls. For example, under terminal stress conditions, sorghum transpiration remained at the level of fully irrigated controls for approximately four weeks after stress imposition, after which the transpiration declined sharply (Vadez et al. 2011b). Similarly, in chickpea plants exposed to terminal water stress, the average transpiration of 20 genotypes remained at the level of fully irrigated controls for ~22 days after stress initiation (Zaman-Allah et al. 2011a). This finding provides better knowledge regarding the physiological stages of the plants that are affected by stress. In a recent study, the levels of antioxidant enzymes in water stress tolerant and water stress sensitive pearl millet genotypes were measured and were found to differ between genotypes. However, these differences were the consequence of the soil water status (FTSW) (Kholová and Vadez 2013). Further improvement of the system, which is currently ongoing, will allow for measurements of the kinetics of leaf canopy development in plants within which water uptake is also monitored. This will allow dynamic measurement of leaf area development together with the evolution of leaf canopy conductance over time.
Modelling as a tool to integrate the different water stress components
Once traits contributing to certain water stress patterns for any given crop have been identified, the evaluation of their effects remains difficult because several traits can play roles, and these traits are likely to interact with one another and with the environment. In summary, testing the effects of traits via experimental means is bound to be restricted to a few traits at a time and a few environmental and climatic scenarios. It is indeed becoming very clear that much of the complexity originates from the interaction among traits and from their interactions with the environment (Buckler et al. 2009; Schuster 2011). Therefore, a tool is needed that can artificially simulate the effect of a given trait across different layers of complexity. Crop models can serve to ‘integrate’ complex behavioural/developmental processes of plants that are all related through water need/use. Not all models are suitable for this purpose, and those that are suitable must be designed such that the algorithms that are part of the model structure reflect observable and quantifiable biological observations (Sinclair and Seligman 2000; Hammer et al. 2010). Only then can the model be sensitive to changes in the conditions and accurately predict effects.
A large body of convincing evidence has been gathered regarding the relevance of crop models in the guidance of breeding targets. Water use/conservation and water capture are composed of several ‘pieces’, and the effects of these pieces are more easily modelled than assessed. For example, using a robust crop model for chickpea, Soltani et al. (1999) showed that an early decline in leaf expansion and transpiration upon soil drying led to yield improvement under water stress conditions, although the yield improvements obtained were <5%. Although these two traits were discussed in earlier sections as potential key water saving traits, this example illustrates that under the geographical conditions in which the model was used, these traits had only limited interest, and making an investment in breeding them was not a priority. In other simulations with chickpea, a rapid root growth rate was shown to decrease yield by an average of 5%, whereas an increase in the depth of root water extraction by 20 cm increased yield by an average of 10%, therefore, among all the genetic traits tested, this trait conferred the largest yield benefit (Vadez et al. 2012). We note that this study also modelled the effect of management options and showed that a 40% yield improvement was derived from providing a 30 mm irrigation at the beginning of seed growth, which is in full agreement with previous results (Soltani et al. 2001). Therefore, these data show the efficacy of a model for comparing both genetic and management options. For example, crop simulation allowed the optimisation of sorghum planting density in a low rainfall environment (Hammer 2006). Simulations have been utilised for maize, in which different genetic traits of the maize cultivar were given values of sorghum cultivars. The results showed that increasing the depth of soil water access led to yield increases of ~20%, whereas the other traits either had no effect (e.g. early or a late decline of transpiration upon progressive water stress imposition) or a negative effect (e.g. the development of smaller leaves) (Sinclair and Muchow 2001). The modelling approach is powerful because it is now possible to simulate the effects of certain QTLs on yield based on the percentage effect of a given QTL on particular traits (Chapman et al. 2003; Welcker et al. 2007; Chenu et al. 2009). It should be noted that in these examples, the effects on yield are the results of single traits tested in isolation from others, whereas the use of a model allows for the assessment of trait combinations, which has been initiated recently (Vadez et al. 2013b).
‘Environment-specific’ breeding and probabilistic estimation of potential gains
The few examples provided in the last section illustrate the potential of crop models for predicting the value of specific traits. These examples also show that, depending on the regions targeted by the modelling exercise, the effects of traits on yield are often counterintuitive. Specifically, some traits can have either less of an effect than expected, a negative effect, or an unexpected effect. This illustrates the role of crop simulation as a critical pre-screening technique for the many traits that can be bred for and its role as a means of generating a yield-trait performance landscape (Messina et al. 2011). At the same time, it demonstrates the potential of crop simulation as a tool for deciphering the complexity of biological responses.
An important application of crop models is therefore to provide a geographical dimension for possible trait effects along with a stochastic measurement of the probability of success of a given trait in a given environment. This approach is still in its early stages, and we argue that it will have great potential for use in breeding programs. For example, the enormous benefit of the sensitivity of transpiration to high VPD on soybean yield in the USA has been recently shown (Sinclair et al. 2010). Moreover, the yield improvements were greater in the driest years, and there was no yield penalty in the wettest years. Finally, although the overall effect of this trait is highly beneficial, the model also provides the probability of trait success. In other words, although a given trait could have an overall positive effect on yield, the environmental variability is such that yield could be decreased in a substantial number of cases, whereas it would be increased in other cases. Unless there is a clear geographical zonation of these scenarios, a trait that would not lead to a majority of success cases would likely generate little interest among breeders for use in their program. Therefore, we argue that crop modelling will be increasingly used as a stochastic tool to predict trait effects and to assess the percentage of expected yield increase and the probability of trait success.
Engaging the breeding community
The different aspects covered in the present paper give a lot of insights on what traits potentially matter for water stress adaptation and on methods that can be applied by the breeding community to use these traits. We present traits but also trait responses to the environment, which also gives insight and possible explanations for the high levels of interactions between genotype and environment in the yield expression, i.e. one of the main challenge of breeding programs. The information presented in this paper offers direct possible applications by the breeding community. For instance, the gravimetric measurement of the transpiration rate under high VPD, a trait that leads to better adaptation to water stress in several crops and at least in some environment, can be measured at a fairly high throughput level (e.g. Kholová et al. 2012). The use of lysimetric measurements of the pattern of water extraction, or of long-term transpiration efficiency measurement, provides other examples of tools, methods and approach that breeding program can use. Moreover, we believe that the engagement of the breeding community needs to be around the approach. In the end, tools and methods are only parts of recipes which can only be as good as the approach has been. The examples herein give insights of the changes that are needed in the breeding approach. First, we talk about trait measurements. These imply new experimental setups that are not only fields, even well equipped fields, but specialised facilities towards specific measurements. These can be automatic setups to measure traits such as transpiration and leaf area, in which there is currently a technological revolution taking place. Second, we have seen the large influence of the environment on traits. Therefore, breeding is not only about understanding the genetic of the traits, but, in many cases, the genetics of the response of the trait to the environment. Recent work on maize leaf development gives us outstanding example of how an eco-physiological approach needs to be embraced by the breeding program (Welcker et al. 2011). Third, we provide examples of how crop modelling can assist the breeding program. Indeed increasingly, and especially for complex issues such as water stress, breeding programs will need to embrace a component of modelling to guide their target. Some companies and breeding program have already made this fully operational. Last, but not least, we believe that the engagement of the breeding community is about the integration of other disciplines in the breeding approach and process, namely physiology, genetics, and modelling, as far as this paper is concerned, but also of agronomy.
Conclusion
Plant adaptation to water deficit is not likely to depend on ‘water stress tolerance genes’ but rather, on both the inherent and adaptive characteristics that condition water supply and demand. The resulting ‘tolerance’ of a plant depends on the degree of fitness, i.e. how close the matching of water supply and demand is achieved in a given water stress scenario (Tardieu 2012). Among these characteristics, the speed and extent of canopy development, the water conductivity, and the sensitivity of plants to soil drying are important factors that influence plant fitness in a particular water stress environment. Here, we advocate that further progress in water stress research needs to place water at the centre of all considerations. Further progress will be made by harnessing the genetics of these traits, and particularly the genetics of ecophysiological responses to environmental cues. Given the complexity of the interactions between these characteristics and with the environment, crop modelling will become an increasingly critical tool for navigating the complexity of these numerous interactions. Breeding will then be no longer limited to field experimentation but will involve both precise phenotyping under specific conditions to target critical traits and utilisation of the simulation outputs to guide breeding targets. The research cited in this review also highlights the critical need for a close and equal collaboration between disciplines.
Acknowledgements
The senior author wishes to acknowledge support from the Bill and Melinda Gates Foundation through a grant to the Generation Challenge Program (Tropical Legume I project) and the Research Program on Climate Change, Agriculture and Food Security (CCAFS), which have supported some of the research presented in this review.
References
Amato M, Ritchie JT (2002) Spatial distribution of roots and water uptake of maize (Zea mays L.) as affected by soil structure. Crop Science 42, 773–780.| Spatial distribution of roots and water uptake of maize (Zea mays L.) as affected by soil structure.Crossref | GoogleScholarGoogle Scholar |
Anyia AO, Herzog H (2004) Water-use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought. European Journal of Agronomy 20, 327–339.
| Water-use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought.Crossref | GoogleScholarGoogle Scholar |
Belko N, Zaman-Allah M, Cisse N, Diop NN, Zombre G, Ehlers JD, Vadez V (2012) Lower soil moisture threshold for transpiration decline under water deficit correlates with lower canopy conductance and higher transpiration efficiency in drought tolerant cowpea. Functional Plant Biology 39, 306–322.
| Lower soil moisture threshold for transpiration decline under water deficit correlates with lower canopy conductance and higher transpiration efficiency in drought tolerant cowpea.Crossref | GoogleScholarGoogle Scholar |
Belko N, Zaman-Allah M, Diop NN, Cisse N, Zombre G, Ehlers JD, Vadez V (2013) Restriction of transpiration rate under high vapor pressure deficit and non-limiting water conditions is important for terminal drought tolerance in cowpea. Plant Biology 15, 304–316.
| Restriction of transpiration rate under high vapor pressure deficit and non-limiting water conditions is important for terminal drought tolerance in cowpea.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38fit1alsg%3D%3D&md5=6c2905df15b4e196aaee33f8b8a31b70CAS | 22823007PubMed |
Salah B-H, Tardieu F (1996) Quantitative analysis of the combined effects of temperature, evaporative demand and light on leaf elongation rate in well-watered field and laboratory-grown maize plants. Journal of Experimental Botany 47, 1689–1698.
| Quantitative analysis of the combined effects of temperature, evaporative demand and light on leaf elongation rate in well-watered field and laboratory-grown maize plants.Crossref | GoogleScholarGoogle Scholar |
Bhatnagar-Mathur P, Devi JM, Lavanya M, Reddy DS, Vadez V, Serraj R, Yamaguchi-Shinozaki K, Sharma KK (2007) Stress-inducible expression of At DREB1A in transgenic peanut (Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions. Plant Cell Reports 26, 2071–2082.
| Stress-inducible expression of At DREB1A in transgenic peanut (Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWrtr%2FI&md5=8b9804eb76ed6b4a824ab12e592e9fd6CAS | 17653723PubMed |
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Reports 27, 411–424.
| Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvFygt7k%3D&md5=14f15dcd942348fed3dd5fc474371accCAS | 18026957PubMed |
Blum A, Zhang JX, N’Guyen HT (1999) Consistent differences among wheat cultivars in osmotic adjustment and their relationship to plant production. Field Crops Research 64, 287–291.
| Consistent differences among wheat cultivars in osmotic adjustment and their relationship to plant production.Crossref | GoogleScholarGoogle Scholar |
Bohnert HJ, Shen B (1998) Transformation and compatible solutes. Scientia Horticulturae 78, 237–260.
| Transformation and compatible solutes.Crossref | GoogleScholarGoogle Scholar |
Bouteillé M, Rolland G, Balsera C, Loudet O, Muller B (2012) Disentangling the intertwined genetic bases of root and shoot growth in Arabidopsis. PLoS ONE 7, e32319
| Disentangling the intertwined genetic bases of root and shoot growth in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 22384215PubMed |
Buckler ES, Holland JB, Bradbury PJ, Acharya CB, Brown PJ, Browne C, Ersoz E, Flint-Garcia S, Garcia A, Glaubitz JC, Goodman MM, Harjes C, Guill K, Kroon DE, Larsson S, Lepak NK, Li H, Mitchell SE, Pressoir G, Peiffer JA, Oropeza Rosas M, Rocheford TR, Romay MC, Romero S, Salvo S, Sanchez Villeda H, da Silva HS, Sun Q, Tian F, Upadyayula N, Ware D, Yates H, Yu J, Zhang Z, Kresovich S, McMullen MD (2009) The genetic architecture of maize flowering time. Science 325, 714–718.
| The genetic architecture of maize flowering time.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptl2gu7k%3D&md5=29cb5a0d445e42c04745be87a2284a3eCAS | 19661422PubMed |
Buckley TN (2005) The control of stomata by water balance. New Phytologist 168, 275–292.
| The control of stomata by water balance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Smu7bI&md5=00245a1e868c3b96f4891c3b2c5e03d0CAS | 16219068PubMed |
Buckley TN, Mott KA (2000) Stomatal responses to non-local changes in PFD: evidence for long-distance hydraulic interactions. Plant, Cell & Environment 23, 301–309.
| Stomatal responses to non-local changes in PFD: evidence for long-distance hydraulic interactions.Crossref | GoogleScholarGoogle Scholar |
Chandra S, Buhariwalla HK, Kashiwagi J, Harikrishna S, Sridevi KR, Krishnamurthy L, Serraj R, Crouch JH (2004) Identifying QTL-linked markers in marker-deficient crops. In ‘Proceedings of the 4th international crop science congress’. (Ed T Fisher) (The Regional Institute Ltd.: Gosford, NSW)
Chapman S, Cooper M, Podlich D, Hammer G (2003) Evaluating plant breeding strategies by simulating gene action and dryland environment effects. Agronomy Journal 95, 99–113.
| Evaluating plant breeding strategies by simulating gene action and dryland environment effects.Crossref | GoogleScholarGoogle Scholar |
Chenu K, Chapman SC, Tardieu F, McLean G, Welcker C, Hammer GL (2009) Simulating the yield impacts of organ-level quantitative trait loci associated with drought response in maize – a ‘gene-to-phenotype’ modeling approach. Genetics 183, 1507–1523.
| Simulating the yield impacts of organ-level quantitative trait loci associated with drought response in maize – a ‘gene-to-phenotype’ modeling approach.Crossref | GoogleScholarGoogle Scholar | 19786622PubMed |
Chopart J (1983) Etude du systeme racinaire du mil (Pennisetum Typhoides) dans un sol sableux du sénégal. Agronomie Tropicale 38, 37–51.
Christopher JT, Manschadi AM, Hammer GL, Borrell AK (2008) Developmental and physiological traits associated with high yield and stay-green phenotype in wheat. Australian Journal of Agricultural Research 59, 354–364.
| Developmental and physiological traits associated with high yield and stay-green phenotype in wheat.Crossref | GoogleScholarGoogle Scholar |
Comstock JP (2002) Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. Journal of Experimental Botany 53, 195–200.
| Hydraulic and chemical signalling in the control of stomatal conductance and transpiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtlWkt7s%3D&md5=dad3c00381ed81472194c5291d493c35CAS | 11807122PubMed |
Condon AJ, Richards RA, Rebetzke GJ, Farquhar GD (2002) Improving intrinsic water use efficiency and crop yield. Crop Science 42, 122–131.
| Improving intrinsic water use efficiency and crop yield.Crossref | GoogleScholarGoogle Scholar |
Dardanelli JL, Bachmeier OA, Sereno R, Gil R (1997) Rooting depth and soil water extraction patterns of different crops in a silty loam Haplustoll. Field Crops Research 54, 29–38.
| Rooting depth and soil water extraction patterns of different crops in a silty loam Haplustoll.Crossref | GoogleScholarGoogle Scholar |
Davies WJ, Wilkinson S, Loveys B (2002) Stomatal control by chemical signaling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytologist 153, 449–460.
| Stomatal control by chemical signaling and the exploitation of this mechanism to increase water use efficiency in agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xit1Wgt7Y%3D&md5=239775fdfa040302f799744e5bfbad55CAS |
Devi JM, Sinclair TR, Vadez V, Krishnamurthy L (2009) Peanut genotypic variation in transpiration efficiency and decrease transpiration during progressive soil drying. Field Crops Research 114, 280–285.
| Peanut genotypic variation in transpiration efficiency and decrease transpiration during progressive soil drying.Crossref | GoogleScholarGoogle Scholar |
Devi JM, Sinclair TR, Vadez V (2010) Genotypic variation in peanut (Arachis hypogaea L.) for transpiration sensitivity to atmospheric vapor pressure deficit. Crop Science 50, 191–196.
| Genotypic variation in peanut (Arachis hypogaea L.) for transpiration sensitivity to atmospheric vapor pressure deficit.Crossref | GoogleScholarGoogle Scholar |
Ehlert C, Maurel C, Tardieu F, Simonneau T (2009) Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiology 150, 1093–1104.
| Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnsleiu78%3D&md5=5911a9ecbc3b5bd426ecdb8ecb7430d2CAS | 19369594PubMed |
Fletcher AL, Sinclair TR, Allen LH (2007) Transpiration responses to vapor pressure deficit in well watered ‘slow-wilting’ and commercial soybean. Environmental and Experimental Botany 61, 145–151.
| Transpiration responses to vapor pressure deficit in well watered ‘slow-wilting’ and commercial soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvF2qtb8%3D&md5=2f99c27966e6fa1cc682beb099cb9a41CAS |
Gewin V (2010) Food: an underground revolution. Nature 466, 552–553.
| Food: an underground revolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsFWku74%3D&md5=30bdb2772633cf60a60fb4402266bad6CAS | 20671689PubMed |
Gholipoor M, Prasad PVV, Mutava RN, Sinclair TR (2010) Genetic variability of transpiration response to vapor pressure deficit among sorghum genotypes. Field Crops Research 119, 85–90.
| Genetic variability of transpiration response to vapor pressure deficit among sorghum genotypes.Crossref | GoogleScholarGoogle Scholar |
Gilbert ME, Holbrook NM, Zwieniecki MA, Sadok W, Sinclair TR (2011a) Field confirmation of genetic variation in soybean transpiration response to vapor pressure deficit and photosynthetic compensation. Field Crops Research 124, 85–92.
| Field confirmation of genetic variation in soybean transpiration response to vapor pressure deficit and photosynthetic compensation.Crossref | GoogleScholarGoogle Scholar |
Gilbert ME, Zwieniecki MA, Holbrook NM (2011b) Independent variation in photosynthetic capacity and stomatal conductance leads to differences in intrinsic water use efficiency in 11 soybean genotypes before and during mild drought. Journal of Experimental Botany 62, 2875–2887.
| Independent variation in photosynthetic capacity and stomatal conductance leads to differences in intrinsic water use efficiency in 11 soybean genotypes before and during mild drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmsVyksbY%3D&md5=a1c1a4aed0b68ccde2dab026aaeff445CAS | 21339387PubMed |
Gowing DJ, Davies WJ, Jones HG (1990) A positive root-sourced signal as an indicator of soil drying in apple (Malus domestica Borkh.) Journal of Experimental Botany 41, 1535–1540.
| A positive root-sourced signal as an indicator of soil drying in apple (Malus domestica Borkh.)Crossref | GoogleScholarGoogle Scholar |
Grantz DA (1990) Plant response to atmospheric humidity. Plant, Cell & Environment 13, 667–679.
| Plant response to atmospheric humidity.Crossref | GoogleScholarGoogle Scholar |
Gregory PJ, McGowan M, Biscoe PV, Hunter B (1978) Water relations of winter wheat. 1. Growth of the root system. The Journal of Agricultural Science 91, 91–102.
| Water relations of winter wheat. 1. Growth of the root system.Crossref | GoogleScholarGoogle Scholar |
Hafner H, George E, Bationo A, Marschner H (1993) Effect of crop residues on root growth and phosphorus acquisition of pearl millet in an acid sandy soil in Niger. Plant and Soil 150, 117–127.
| Effect of crop residues on root growth and phosphorus acquisition of pearl millet in an acid sandy soil in Niger.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXkvFCmt74%3D&md5=b19e8d1aab008092d0cfad2ae9e49ef2CAS |
Hamblin AP, Tennant D (1987) Root length density and water uptake in cereals and grain legumes: how well are they correlated. Australian Journal of Agricultural Research 38, 513–527.
| Root length density and water uptake in cereals and grain legumes: how well are they correlated.Crossref | GoogleScholarGoogle Scholar |
Hammer GL (2006) Pathways to prosperity: breaking the yield barrier in sorghum. Agricultural Science 19, 16–22.
Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can changes in canopy and/or root systems architecture explain historical maize yield trends in the US corn belt? Crop Science 49, 299–312.
| Can changes in canopy and/or root systems architecture explain historical maize yield trends in the US corn belt?Crossref | GoogleScholarGoogle Scholar |
Hammer GL, van Oosterom E, McLean G, Chapman SC, Broad I, Harland P, Muchow RC (2010) Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops. Journal of Experimental Botany 61, 2185–2202.
| Adapting APSIM to model the physiology and genetics of complex adaptive traits in field crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVGnsr0%3D&md5=a147d22c9446ef9b06281b31bf2af1f3CAS | 20400531PubMed |
Hund A, Ruta N, Liedgens M (2009) Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance. Plant and Soil 318, 311–325.
| Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltVWrsLg%3D&md5=3024514055dcd0790f4007cb4a5ace61CAS |
Jackson MB (1993) Are plant hormones involved in root to shoot communication. Advances in Botanical Research 19, 103–187.
| Are plant hormones involved in root to shoot communication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXislCjtw%3D%3D&md5=593970eda5567b62c3fba3bd09e8be43CAS |
Jongrungklang N, Toomsan B, Vorasoot N, Jogloy S, Boote KJ, Hoogenboom G, Patanothai A (2011) Rooting traits of peanut genotypes with different yield responses to pre-flowering drought stress. Field Crops Research 120, 262–270.
| Rooting traits of peanut genotypes with different yield responses to pre-flowering drought stress.Crossref | GoogleScholarGoogle Scholar |
Kashiwagi J, Krishnamurthy L, Crouch JH, Serraj R (2006) Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crops Research 95, 171–181.
| Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress.Crossref | GoogleScholarGoogle Scholar |
Katayama K, Ito O, Adu-gyamfi JJ, Rao TP (2000) Analysis of relationship between root length density and water uptake by roots of five crops using minirhizotron in the semi-arid tropics. Japan Agricultural Research Quarterly 34, 81–86.
Ketring DL, Reid JL (1993) Growth of peanut roots under field conditions. Agronomy Journal 85, 80–85.
| Growth of peanut roots under field conditions.Crossref | GoogleScholarGoogle Scholar |
Kholová J, Vadez V (2013) Water extraction under terminal drought explains the genotypic differences in yield, not the anti-oxidants changes in leaves of pearl millet (Pennisetum glaucum (L.) R.Br.) Functional Plant Biology 40, 44–53.
| Water extraction under terminal drought explains the genotypic differences in yield, not the anti-oxidants changes in leaves of pearl millet (Pennisetum glaucum (L.) R.Br.)Crossref | GoogleScholarGoogle Scholar |
Kholová J, Hash CT, Kocŏvá M, Vadez V (2010a) Constitutive water conserving mechanisms are correlated with the terminal drought tolerance of pearl millet (Pennisetum americanum L.). Journal of Experimental Botany 61, 369–377.
| Constitutive water conserving mechanisms are correlated with the terminal drought tolerance of pearl millet (Pennisetum americanum L.).Crossref | GoogleScholarGoogle Scholar | 19861657PubMed |
Kholová J, Hash CT, Kumar LK, Yadav RS, Kocŏvá M, Vadez V (2010b) Terminal drought tolerant pearl millet (Pennisetum glaucum (L.) R.Br.) have high leaf ABA and limit transpiration at high vapor pressure deficit. Journal of Experimental Botany 61, 1431–1440.
| Terminal drought tolerant pearl millet (Pennisetum glaucum (L.) R.Br.) have high leaf ABA and limit transpiration at high vapor pressure deficit.Crossref | GoogleScholarGoogle Scholar | 20142425PubMed |
Kholová J, Nepolean T, Hash CT, Supriya A, Rajaram V, Senthilvel S, Kakkera A, Yadav RS, Vadez V (2012) Water saving traits co-map with a major terminal drought tolerance quantitative trait locus in pearl millet (Pennisetum glaucum (L.) R.Br.) Molecular Breeding 30, 1337–1353.
| Water saving traits co-map with a major terminal drought tolerance quantitative trait locus in pearl millet (Pennisetum glaucum (L.) R.Br.)Crossref | GoogleScholarGoogle Scholar |
Kirkegaard JA, Hunt JR (2010) Increasing productivity by matching farming system management and genotype in water-limited environments. Journal of Experimental Botany 61, 4129–4143.
| Increasing productivity by matching farming system management and genotype in water-limited environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlShtLnL&md5=f9877e2ee038c2127659598f6a995efcCAS | 20709725PubMed |
Kirkegaard JA, Lilley JM, Howe GN, Graham JM (2007) Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58, 303–315.
| Impact of subsoil water use on wheat yield.Crossref | GoogleScholarGoogle Scholar |
Kudoyarova GR, Kholodova VP, Veselov DS (2013) Current state of the problem of water relations in plants under water deficit. Russian Journal of Plant Physiology: a Comprehensive Russian Journal on Modern Phytophysiology 60, 165–175.
| Current state of the problem of water relations in plants under water deficit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXis1Cgurk%3D&md5=b1d77296475e7e66990b4d584274fd7cCAS |
Kumar N, Nandwal AS, Devi S, Sharma KD, Yadav A, Waldia RS (2012) Drought tolerance in chickpea as evaluated by root characteristics, plant water status, and membrane integrity and chlorophyll fluorescence techniques. Experimental Agriculture 48, 378–387.
| Drought tolerance in chickpea as evaluated by root characteristics, plant water status, and membrane integrity and chlorophyll fluorescence techniques.Crossref | GoogleScholarGoogle Scholar |
Lafolie F, Bruckier L, Tardieu F (1991) Modeling root water potential and soil-root water transport. 1. Model presentation. Soil Science Society of America Journal 55, 1203–1212.
| Modeling root water potential and soil-root water transport. 1. Model presentation.Crossref | GoogleScholarGoogle Scholar |
Lawlor DW, Tezara W (2009) Causes of decreased photosynthetic rate and metabolic capacity in water deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany 103, 561–579.
| Causes of decreased photosynthetic rate and metabolic capacity in water deficient leaf cells: a critical evaluation of mechanisms and integration of processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktVGnu7g%3D&md5=4893cb6c20217c69fee852580d5fde4eCAS | 19155221PubMed |
Leal-Bertioli SCM, Bertioli DJ, Guimarães PM, Pereira TD, Galhardo I, Silva JP, Brasileiro ACM, Vadez V, Oliveira RS, Silva PIT, Araújo ACG (2012) The effect of tetraploidization of wild Arachis on leaf anatomy and drought related traits. Environmental and Experimental Botany 84, 17–24.
| The effect of tetraploidization of wild Arachis on leaf anatomy and drought related traits.Crossref | GoogleScholarGoogle Scholar |
Lobet G, Pages L, Draye X (2011) A Novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiology 157, 29–39.
| A Novel image-analysis toolbox enabling quantitative analysis of root system architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Sit77P&md5=1a2e443f664bfda05bd36f59eff63013CAS | 21771915PubMed |
Manschadi AM, Christopher JT, Peter deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823–837.
| The role of root architectural traits in adaptation of wheat to water-limited environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XptVClsbY%3D&md5=fd2e4bfdcedcc102c14aea1ce4fc5e03CAS |
Matsui T, Singh BB (2003) Root characteristics in cowpea related to drought tolerance at the seedling stage. Experimental Agriculture 39, 29–38.
| Root characteristics in cowpea related to drought tolerance at the seedling stage.Crossref | GoogleScholarGoogle Scholar |
McIntyre BD, Riha SJ, Flower DJ (1995) Water uptake by pearl millet in a semi-arid environment. Field Crops Research 43, 67–76.
| Water uptake by pearl millet in a semi-arid environment.Crossref | GoogleScholarGoogle Scholar |
Messina C, Podlich D, Dong Z, Samples M, Cooper M (2011) Yield–trait performance landscapes: from theory to application in breeding maize for drought tolerance. Journal of Experimental Botany 62, 855–868.
| Yield–trait performance landscapes: from theory to application in breeding maize for drought tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVekurs%3D&md5=c235c8eb075e8873a3599caa7cd8ddd4CAS | 21041371PubMed |
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7, 405–410.
| Oxidative stress, antioxidants and stress tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XntVWnu7Y%3D&md5=6fc277d70817036dd692121577a2da70CAS | 12234732PubMed |
Monteith JL, Greenwood DJ (1986) How do crops manipulate water supply and demand? Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences 316, 245–259.
| How do crops manipulate water supply and demand?Crossref | GoogleScholarGoogle Scholar |
Mooney SJ, Pridmore TP, Helliwell J, Bennett MJ (2012) Developing x-ray computed tomography to non-invasively image 3-D root systems architecture in soil. Plant and Soil 352, 1–22.
| Developing x-ray computed tomography to non-invasively image 3-D root systems architecture in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjs1OlsL4%3D&md5=f05491539dbad289914969313f8bcd2dCAS |
Mott KA (2007) Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves. Plant, Cell & Environment 30, 1444–1449.
| Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1yhsrbO&md5=adc62657a1d9823faa21e4c0c3225a1dCAS |
Munns R, Cramer GR (1996) Is Coordination of leaf and root growth mediated by abscisic acid? Plant and Soil 185, 33–49.
| Is Coordination of leaf and root growth mediated by abscisic acid?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntlyjug%3D%3D&md5=a887425a2ad53a7eebbed3b9b3cd162eCAS |
Ober ES, Clark CJA, LeBloa M, Smith CHG (2005) Root growth, soil water extraction and drought tolerance in sugar beet. Aspects of Applied Biology 73, 213–220.
Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M (2011) Large root systems: are they useful in adapting wheat to dry environments. Functional Plant Biology 38, 347–354.
| Large root systems: are they useful in adapting wheat to dry environments.Crossref | GoogleScholarGoogle Scholar |
Pantin F, Simonneau T, Rolland G, Dauzat M, Muller B (2011) Control of leaf expansion: a developmental switch from metabolics to hydraulics. Plant Physiology 156, 803–815.
| Control of leaf expansion: a developmental switch from metabolics to hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvFWrtrs%3D&md5=5b74d1a9af1535d4058e11963c14627bCAS | 21474437PubMed |
Pantin F, Simonneau T, Muller B (2012) Coming of leaf age: control of growth by hydraulics and metabolics during leaf development. New Phytologist 196, 349–366.
| Coming of leaf age: control of growth by hydraulics and metabolics during leaf development.Crossref | GoogleScholarGoogle Scholar | 22924516PubMed |
Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F (2009) Drought and ABA effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiology 149, 2000–2012.
| Drought and ABA effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXks1Wnsrg%3D&md5=7d1f47857ae822680e33ff0e5b8d5400CAS | 19211703PubMed |
Passioura JB (1983) Roots and drought resistance. Agricultural Water Management 7, 265–280.
| Roots and drought resistance.Crossref | GoogleScholarGoogle Scholar |
Passioura JB (2012) Phenotyping for drought tolerance in grain crops: when is it useful to breeders. Functional Plant Biology 39, 851–859.
| Phenotyping for drought tolerance in grain crops: when is it useful to breeders.Crossref | GoogleScholarGoogle Scholar |
Passioura JB, Angus JF (2010) Improving productivity of crops in water-limited environments. Advances in Agronomy 106, 37–75.
| Improving productivity of crops in water-limited environments.Crossref | GoogleScholarGoogle Scholar |
Payne WA, Drew MC, Hossner LR, Lascano RJ, Onken AB, Wendt CW (1992) Soil phosphorus availability and pearl millet water use efficiency. Crop Science 32, 1010–1015.
| Soil phosphorus availability and pearl millet water use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXotVShsA%3D%3D&md5=2c4180a3ec885aea531b456610a56262CAS |
Ratnakumar P, Vadez V, Nigam SN, Krishnamurthy L (2009) Assessment of transpiration efficiency in peanut (Arachis hypogaea L.) under drought by lysimetric system. Plant Biology 11, 124–130.
| Assessment of transpiration efficiency in peanut (Arachis hypogaea L.) under drought by lysimetric system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlslajsrc%3D&md5=5604d0874b4d99c1bd091b570f8c14afCAS | 19778376PubMed |
Ratnakumar P, Vadez V (2011) Groundnut (Arachis hypogaea L.) genotypes tolerant to intermittent drought maintain a high harvest index and have small leaf canopy under stress. Functional Plant Biology 38, 1016–1023.
| Groundnut (Arachis hypogaea L.) genotypes tolerant to intermittent drought maintain a high harvest index and have small leaf canopy under stress.Crossref | GoogleScholarGoogle Scholar |
Reymond M, Muller B, Leonardi A, Charcosset A, Tardieu F (2003) Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of maize leaf growth to temperature and water deficit. Plant Physiology 131, 664–675.
| Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of maize leaf growth to temperature and water deficit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtlyjs7w%3D&md5=602d07781a27d53f5793cac3269f56a0CAS | 12586890PubMed |
Sadok W, Sinclair TR (2009) Genetic variability of transpiration response to vapor pressure deficit among soybean cultivars. Crop Science 49, 955–960.
| Genetic variability of transpiration response to vapor pressure deficit among soybean cultivars.Crossref | GoogleScholarGoogle Scholar |
Sadok W, Naudin P, Boussuge B, Muller B, Welcker C, Tardieu F (2007) Leaf growth rate per unit thermal time follows QTL-dependent daily patterns in hundreds of maize lines under naturally fluctuating conditions. Plant, Cell & Environment 30, 135–146.
| Leaf growth rate per unit thermal time follows QTL-dependent daily patterns in hundreds of maize lines under naturally fluctuating conditions.Crossref | GoogleScholarGoogle Scholar |
Sadras VO, Milroy SP (1996) Soil-water thresholds for the responses of leaf expansion and gas exchange: a review. Field Crops Research 47, 253–266.
| Soil-water thresholds for the responses of leaf expansion and gas exchange: a review.Crossref | GoogleScholarGoogle Scholar |
Sarker A, Erskine W, Singh M (2005) Variation in shoot and root characteristics and their association with drought tolerance in lentil landraces. Genetic Resources and Crop Evolution 52, 89–97.
| Variation in shoot and root characteristics and their association with drought tolerance in lentil landraces.Crossref | GoogleScholarGoogle Scholar |
Schoppach R, Sadok W (2013) Transpiration sensitivities to evaporative demand and leaf areas vary with night and day warming regimes among wheat genotypes. Functional Plant Biology 40, 708–718.
| Transpiration sensitivities to evaporative demand and leaf areas vary with night and day warming regimes among wheat genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSrtrrJ&md5=280522419f80b80eb23760b8019abbbdCAS |
Schuster I (2011) Marker-assisted selection for quantitative traits. Crop Breeding and Applied Biotechnology 11, 50–55.
| Marker-assisted selection for quantitative traits.Crossref | GoogleScholarGoogle Scholar |
Sermons SM, Seversike TM, Sinclair TR, Fiscus E, Rufty T (2012) Temperature influences the ability of tall fescue to control transpiration in response to atmospheric vapour pressure deficit. Functional Plant Biology 39, 979–986.
| Temperature influences the ability of tall fescue to control transpiration in response to atmospheric vapour pressure deficit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslKru7%2FL&md5=e1f6beffe9d7015978bff7c0eaee8ac7CAS |
Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant, Cell & Environment 25, 333–341.
| Osmolyte accumulation: can it really help increase crop yield under drought conditions?Crossref | GoogleScholarGoogle Scholar |
Sharp RE, Davies WJ (1985) Root growth and water uptake by maize plants in drying soil. Journal of Experimental Botany 36, 1441–1456.
| Root growth and water uptake by maize plants in drying soil.Crossref | GoogleScholarGoogle Scholar |
Silim SN, Saxena MC (1993) Adaptation of spring-sown chickpea to the mediterranean basin. I. Response to moisture supply. Field Crops Research 34, 121–136.
| Adaptation of spring-sown chickpea to the mediterranean basin. I. Response to moisture supply.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Ludlow MM (1986) Influence of soil water supply on the plant water balance of four tropical grain legumes. Australian Journal of Plant Physiology 13, 329–341.
| Influence of soil water supply on the plant water balance of four tropical grain legumes.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Muchow RC (2001) System analysis of plant traits to increase grain yield on limited water supplies. Agronomy Journal 93, 263–270.
| System analysis of plant traits to increase grain yield on limited water supplies.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Seligman N (2000) Criteria for publishing papers on crop modelling. Field Crops Research 68, 165–172.
| Criteria for publishing papers on crop modelling.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Hammond LC, Harrison J (1998) Extractable soil water and transpiration rate of soybean on sandy soils. Agronomy Journal 90, 363–368.
| Extractable soil water and transpiration rate of soybean on sandy soils.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Vadez V, Chenu K (2003) Ureide accumulation in response to Mn nutrition by eight soybean genotypes with N2 fixation tolerance to soil drying. Crop Science 43, 592–597.
| Ureide accumulation in response to Mn nutrition by eight soybean genotypes with N2 fixation tolerance to soil drying.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1erurw%3D&md5=09afdb25fcc0a8c933144da2d3b15865CAS |
Sinclair TR, Hammer GL, van Oosterom EJ (2005) Potential yield and water use efficiency benefits in sorghum from limited maximum transpiration rate. Functional Plant Biology 32, 945–952.
| Potential yield and water use efficiency benefits in sorghum from limited maximum transpiration rate.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Zwieniecki MA, Holbrook NM (2008) Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiologia Plantarum 132, 446–451.
| Low leaf hydraulic conductance associated with drought tolerance in soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksFWktb4%3D&md5=47d8b0752057897d1ff441203c1fa497CAS | 18333998PubMed |
Sinclair TR, Messina CD, Beatty A, Samples M (2010) Assessment across the United States of the benefits of altered soybean drought traits. Agronomy Journal 102, 475–482.
| Assessment across the United States of the benefits of altered soybean drought traits.Crossref | GoogleScholarGoogle Scholar |
Soltani A, Sinclair TR (2011) A simple model for chickpea development, growth and yield. Field Crops Research 124, 252–260.
| A simple model for chickpea development, growth and yield.Crossref | GoogleScholarGoogle Scholar |
Soltani A, Ghassemi-Golezani K, Khooie FR, Moghaddam M (1999) A simple model for chickpea growth and yield. Field Crops Research 62, 213–224.
| A simple model for chickpea growth and yield.Crossref | GoogleScholarGoogle Scholar |
Soltani A, Khooie FR, Ghassemi-Golezani K, Moghaddam M (2000) Thresholds for chickpea leaf expansion and transpiration response to soil water deficit. Field Crops Research 68, 205–210.
| Thresholds for chickpea leaf expansion and transpiration response to soil water deficit.Crossref | GoogleScholarGoogle Scholar |
Soltani A, Khooie FR, Ghassemi-Golezani K, Moghaddam M (2001) A simulation study of chickpea crop response to limited irrigation in a semiarid environment. Agricultural Water Management 49, 225–237.
| A simulation study of chickpea crop response to limited irrigation in a semiarid environment.Crossref | GoogleScholarGoogle Scholar |
Sperry JS, Hacke UG, Oren R, Comstock JP (2002) Water deficits and hydraulic limits to leaf water supply. Plant Cell and Environment 25, 251–263.
| Water deficits and hydraulic limits to leaf water supply.Crossref | GoogleScholarGoogle Scholar |
Squire GR (1979) The response of stomata of pearl millet (Pennisetum typhoides S. and H.) to atmospheric humidity. Journal of Experimental Botany 30, 925–933.
| The response of stomata of pearl millet (Pennisetum typhoides S. and H.) to atmospheric humidity.Crossref | GoogleScholarGoogle Scholar |
Tardieu F (2012) Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. Journal of Experimental Botany 63, 25–31.
| Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1yms73L&md5=999726b96ab3bc2946e769bd2a9341e7CAS | 21963615PubMed |
Tardieu F, Parent B, Simonneau T (2010) Control of leaf growth by abscisic acid: hydraulic or non-hydraulic processes? Plant, Cell & Environment 33, 636–647.
| Control of leaf growth by abscisic acid: hydraulic or non-hydraulic processes?Crossref | GoogleScholarGoogle Scholar |
Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRA, Black CR, Taylor IB (2007) Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiology 143, 1905–1917.
| Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFWjuro%3D&md5=9bd2537b73c2cf8082252d2dd5fcdd35CAS | 17277097PubMed |
Tuberosa R, Sanguineti MC, Landi P, Giuliani MM, Salvi S, Conti S (2002) Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Molecular Biology 48, 697–712.
| Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjsFWrt70%3D&md5=8d06e7a3e7ecdc6fe2869181e4c4ba40CAS | 11999844PubMed |
Turner NC (1991) Measurement and influence of environmental and plant factors on stomatal conductance in the field. Agricultural and Forest Meteorology 54, 137–154.
| Measurement and influence of environmental and plant factors on stomatal conductance in the field.Crossref | GoogleScholarGoogle Scholar |
Turner NC, Schulze ED, Gollan T (1984) The response of stomata and leaf gas exchange to vapour pressure deficits and soil water content. I. Species comparisons at high soil water contents. Oecologia 63, 338–342.
| The response of stomata and leaf gas exchange to vapour pressure deficits and soil water content. I. Species comparisons at high soil water contents.Crossref | GoogleScholarGoogle Scholar |
Turner NC, Abbo S, Berger JD, French RJ, Ludwig C, Mannur DM, Singh SJ, Yadav HS (2007) Osmotic adjustment in chickpea (Cicer arietinum L.) results in no yield benefit under terminal drought. Journal of Experimental Botany 58, 187–194.
| Osmotic adjustment in chickpea (Cicer arietinum L.) results in no yield benefit under terminal drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlOlt7s%3D&md5=5cf8bd51ade4324bd07d87e5b170bdd1CAS | 17088363PubMed |
Vadez V, Sinclair TR, Serraj R, Purcell LC (2000) Manganese application alleviates the water deficit-induced decline of N2 fixation. Plant, Cell & Environment 23, 497–505.
| Manganese application alleviates the water deficit-induced decline of N2 fixation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktVClsb8%3D&md5=d358a87e295d37266ef010300f85580eCAS |
Vadez V, Rao S, Kholova J, Krishnamurthy L, Kashiwagi J, Ratnakumar P, Sharma KK, Bhatnagar-Mathur P, Basu PS (2008) Roots research for legume tolerance to drought: quo vadis? Journal of Food Legumes 21, 77–85.
Vadez V, Deshpande SP, Kholova J, Hammer GL, Borrell AK, Talwar HS, Hash CT (2011a) Staygreen QTL effects on water extraction and transpiration efficiency in a lysimetric system: Influence of genetic background. Functional Plant Biology 38, 553–566.
| Staygreen QTL effects on water extraction and transpiration efficiency in a lysimetric system: Influence of genetic background.Crossref | GoogleScholarGoogle Scholar |
Vadez V, Krishnamurthy L, Hash CT, Upadhyaya HD, Borrell AK (2011b) Yield, transpiration efficiency, and water use variations and their relationships in the sorghum reference collection. Crop and Pasture Science 62, 645–655.
| Yield, transpiration efficiency, and water use variations and their relationships in the sorghum reference collection.Crossref | GoogleScholarGoogle Scholar |
Vadez V, Soltani A, Krishnamurthy L, Sinclair TR (2012) Modelling possible benefit of root related traits to enhance terminal drought adaption of chickpea. Field Crops Research 137, 108–115.
| Modelling possible benefit of root related traits to enhance terminal drought adaption of chickpea.Crossref | GoogleScholarGoogle Scholar |
Vadez V, Kholova J, Yadav RS, Hash CT (2013a) Small temporal differences in water uptake among varieties of pearl millet (Pennisetum glaucum (L.) R.Br.) are critical for grain yield under terminal drought. Plant and Soil
| Small temporal differences in water uptake among varieties of pearl millet (Pennisetum glaucum (L.) R.Br.) are critical for grain yield under terminal drought.Crossref | GoogleScholarGoogle Scholar | (In press).
Vadez V, Rao JS, Bhatnagar-Mathur P, Sharma KK (2013b) DREB1A promotes root development in deep soil layers and increases water extraction under water stress in groundnut. Plant Biology 15, 45–52.
| DREB1A promotes root development in deep soil layers and increases water extraction under water stress in groundnut.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjt1egt74%3D&md5=45a657f670addaf416ace3d29d4e9032CAS | 22672619PubMed |
van Oosterom EJ, Borrell AK, Deifel KS, Hammer GL (2011) Does increased leaf appearance rate enhance adaptation to postanthesis drought stress in sorghum? Crop Science 51, 2728–2740.
| Does increased leaf appearance rate enhance adaptation to postanthesis drought stress in sorghum?Crossref | GoogleScholarGoogle Scholar |
Welcker C, Boussuge B, Bencivenni C, Ribaut JM, Tardieu F (2007) Are source and sink strengths genetically linked in maize plants subjected to water deficit? A QTL study of the responses of leaf growth and of anthesis silking interval to water deficit. Journal of Experimental Botany 58, 339–349.
| Are source and sink strengths genetically linked in maize plants subjected to water deficit? A QTL study of the responses of leaf growth and of anthesis silking interval to water deficit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlOltL8%3D&md5=cb7c52be445f33b7dc23cd43410fe69eCAS | 17130185PubMed |
Welcker C, Sadok W, Dignat G, Renault M, Salvi S, Charcosset A, Tardieu F (2011) A common genetic determinism for sensitivities to soil water deficit and evaporative demand: meta-analysis of quantitative trait loci and introgression lines of maize. Plant Physiology 157, 718–729.
| A common genetic determinism for sensitivities to soil water deficit and evaporative demand: meta-analysis of quantitative trait loci and introgression lines of maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlahu7fM&md5=dad17b3f2808f5b160e75f5e0eee132dCAS | 21795581PubMed |
Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282, 424–426.
| Stomatal conductance correlates with photosynthetic capacity.Crossref | GoogleScholarGoogle Scholar |
Zaman-Allah M, Jenkinson D, Vadez V (2011a) A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. Journal of Experimental Botany 62, 4239–4252.
| A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVeit7jJ&md5=3e69da176cd45047bf294c59160fa392CAS | 21610017PubMed |
Zaman-Allah M, Jenkinson D, Vadez V (2011b) Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Functional Plant Biology 38, 270–281.
| Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use.Crossref | GoogleScholarGoogle Scholar |
Zhang J, Davies WJ (1991) Antitranspirant activity in the xylem sap of maize plants. Journal of Experimental Botany 42, 317–321.
| Antitranspirant activity in the xylem sap of maize plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXit1Gqt70%3D&md5=5427728be6b8508fe6d5a5e3e654b7eaCAS |


