Mechanistic understanding of iron toxicity tolerance in contrasting rice varieties from Africa: 2. Root oxidation ability and oxidative stress control
Dorothy A. Onyango A C D , Fredrickson Entila B , James Egdane B , Myrish Pacleb B , Meggy Lou Katimbang B , Mathew M. Dida C , Abdelbagi M. Ismail B and Khady N. Drame A
A C D , Fredrickson Entila B , James Egdane B , Myrish Pacleb B , Meggy Lou Katimbang B , Mathew M. Dida C , Abdelbagi M. Ismail B and Khady N. Drame A
A Africa Rice Center (AfricaRice), 01 BP 4029, Abidjan 01, Cote d’Ivoire.
B International Rice Research Institute (IRRI), DAPO Box 7777, Metro Manila, Philippines.
C Department of Applied Plant Sciences, Maseno University, Private bag, Maseno, Kenya.
D Corresponding author. Email: akinyidorothy@gmail.com
Functional Plant Biology 47(2) 145-155 https://doi.org/10.1071/FP19054
Submitted: 23 February 2019 Accepted: 28 September 2019 Published: 16 January 2020
Journal Compilation © CSIRO 2020 Open Access CC BY 4.0
Abstract
To enhance breeding efficiency for iron (Fe) toxicity tolerance and boost lowland rice production in sub-Saharan Africa, we have characterised the morphological, physiological and biochemical responses of contrasting rice varieties to excess iron. Here, we report the capacity of four varieties (CK801 and Suakoko8 (tolerant), Supa and IR64 (sensitive)) to oxidise iron in the rhizosphere and control iron-induced oxidative stress. The experiments were conducted in hydroponic conditions using modified Magnavaca nutrient solution and 300 ppm of ferrous iron (Fe2+) supplied in the form of FeSO4. Severe oxidative stress was observed in sensitive varieties as revealed by their high levels of lipid peroxidation. Histochemical and biochemical analyses showed that tolerant varieties exhibited a better development of the aerenchyma and greater oxygen release than the sensitive varieties in response to excess Fe. Both suberin and lignin deposits were observed in the root, stem and leaf tissues but with varying intensities depending on the variety. Under iron toxic conditions, tolerant varieties displayed increased superoxide dismutase (SOD), glutathione reductase (GR), peroxidase (POX) and ascorbate peroxidase (APX) activities in both the roots and shoots, whereas sensitive varieties showed increased APX and catalase (CAT) activities in the roots. This study had revealed also that Suakoko8 mainly uses root oxidation to exclude Fe2+ from its rhizosphere, and CK801 possesses a strong reactive oxygen species scavenging system, in addition to root oxidation ability. Key traits associated with these tolerance mechanisms such as a well-developed aerenchyma, radial oxygen loss restricted to the root cap as well as strong activation of antioxidative enzymes (SOD, GR, POX and APX) could be useful selection criteria in rice varietal improvement programs for enhanced Fe toxicity tolerance.
Additional keywords: abiotic stress, aerenchyma, antioxidants, iron oxidation control, radial oxygen loss, rice.
Introduction
Iron (Fe) toxicity is a complex nutrient disorder that greatly limits lowland rice production (Becker and Asch 2005; Sikirou et al. 2015; Onaga et al. 2016; Onyango et al. 2018), particularly in Africa where it was ranked as the second most important abiotic stress after drought in rice production areas (van Oort 2018). In response to the diversity of conditions under which Fe toxicity occurs (Becker and Asch 2005) and its seasonal and spatial variability (both in severity and duration), rice plants have developed a wide range of reactions to circumvent the harmful effects of Fe toxicity. These reactions can be classified into three main adaptation strategies: (1) exclusion of Fe2+ at the root level; (2) inclusion of Fe2+ in the root and shoot tissues but subsequent avoidance via internal compartmentation in less photosynthetically active tissues or storage in less reactive forms; and (3) inclusion of Fe2+ and tolerance to reactive oxygen species (ROS) formed during the Fenton’s reaction induced by Fe (Becker and Asch 2005; Engel et al. 2012; Onaga et al. 2016).
Mechanisms involved in root Fe exclusion include the oxidation of Fe2+ in the rhizosphere, which leads to low Fe2+ concentration in the growth medium and the exclusion of Fe at the root surface through root membrane selectivity to prevent iron from entering the root. Root oxidation ability is mediated by the transport of molecular oxygen (O2) from the aerial parts of the plant to the roots through the aerenchyma and its release in the rhizosphere. Hence, early and proper development of the aerenchyma tissue will enhance ventilation of roots through O2 diffusion from well aerated shoots. To minimise the loss of O2 to the surrounding environment, referred to as radial oxygen loss (ROL), and enhance longitudinal diffusion of O2 toward the root apex, some plant species like rice form an apoplastic barrier by accumulating suberin and/or lignin at the hypodermal/exodermal and endodermal cell walls (Colmer 2003a; Ismail 2018). Suberin is also believed to prevent the penetration of soil-derived toxins, such as reduced metal ions, into the roots by reducing cell wall permeability (Armstrong and Armstrong 2005; Greenway et al. 2006). The presence of a large number of fine lateral roots provides low resistance-pathway for root oxygen transportation and release into the rhizosphere. This has been shown to favour formation of root plaque as the result of the oxidisation of excess Fe2+ into a less toxic Fe3+ form (Kang et al. 2011; Wu et al. 2014; Onaga et al. 2016). Therefore, a balanced combination of good aerenchyma development, suberisation, lignification and a well-developed lateral root system is critical for the effectiveness of root oxidation mechanisms in response to Fe toxicity.
Excess Fe leads to enhanced generation of reactive oxygen species (ROS) in plants due to the strong catalytic power of Fe to generate highly reactive hydroxyl radicals (•OH) from its reaction with hydrogen peroxide (H2O2), called the Fenton’s reaction (Kao et al. 2001). ROS have been shown to be associated with serious damages in biological systems causing oxidative stress, which could include peroxidation of lipids, oxidation of proteins, damage to nucleic acids, enzyme inhibition and ultimately, cell death. To counteract oxidative stress, plants have elaborate defence systems of enzymatic and non-enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase, flavonoids, ascorbate (AsA), glutathione (GSH), phenolics and carotenoids, which, normally remove or detoxify ROS (Alscher et al. 2002). SOD constitutes the first line of defence in the oxidative stress response signal transduction pathway (Mittler et al. 2004) that converts superoxide into H2O2, which, in turn is detoxified by CAT or converted into H2O by APX in the ascorbate-glutathione cycle through the oxidation of reduced ascorbic acid (AsA). AsA is a primary metabolite involved in the regeneration of membrane-bound antioxidants (Hideg 1999) such as APX and glutathione reductase (GR) that scavenge hydroxyl radicals and singlet oxygen (Gill and Tuteja 2010). By scavenging the generated excess oxidants, the antioxidative systems of the cells keep ROS at a basal non-toxic level, thus preventing some of the deleterious effects of oxidative stress. Various studies have reported increased activity of the antioxidative systems in response to Fe toxicity and a relationship between increased tolerance of genotypes and high antioxidant capacity (Stein et al. 2014; Wu et al. 2014; Matthus et al. 2015). Because different adaptation strategies and mechanisms are required for each Fe toxicity scenario (Becker and Asch 2005), breeding rice varieties widely adapted to Fe toxic environments requires stacking of beneficial traits. Therefore, it is critical to fully characterise potential donors of Fe toxicity tolerance to guide optimal cross combinations to develop tolerant varieties and to accelerate genetic gains in affected soils.
We have previously reported differential morphological, physiological and metabolic responses of two tolerant rice varieties (CK801 and Suakoko8) and two sensitive varieties (IR64 and Supa) in response to Fe toxicity (Onyango et al. 2018). In some cases, different trends were observed within the same tolerance group, suggesting that the varieties tested employ different adaptation strategies to Fe toxicity. In this follow up study we performed a comparative assessment of the same four varieties for root oxidation ability and oxidative stress control in response to Fe toxicity. Through histochemical and biochemical analyses, key traits associated with Fe toxicity tolerance mechanisms have been determined and their relevance for breeding discussed.
Materials and methods
Plant material, growth conditions and sampling
Two tolerant (CK801 and Suakoko8) and two sensitive (Supa and IR64) rice varieties were evaluated in hydroponic experiments using two iron concentrations (non-stress and excess iron) as described by Onyango et al. (2018). Excess iron treatment was applied on 14-day-old seedlings and maintained for 16 days. For biochemical analyses, root and shoot samples were collected every 4 days, from the second week of plant growth (before the Fe treatment is applied) to the end of the experiment. The five sampling points were as follows: 1 = 0 day, just before excess Fe was applied; sampling point 2 to 5 = 4, 8, 12 and 16 days after treatment respectively. All samples were immediately snap-frozen in liquid nitrogen and transferred to –80°C until use. The samples were then ground to fine powder in liquid nitrogen before analyses.
Histochemical analyses
Main root, youngest fully expanded leaf and main stem of 28-day-old seedlings grown under excess Fe2+ were collected and conserved in 100 mL FAA (formalin, acetic acid and alcohol) (Johansen 1940) to fix the tissues. The fixative (FAA) was prepared using 50 mL of 95% ethanol, 5 mL of acetic acid, 10 mL of 37% formaldehyde, and 35 mL of distilled water. The samples were then kept at 4°C until further use. Thin cross-sections of the main root (root cap: 0–5 mm from root tip and root base: 15–20 mm from root tip or 5–10 mm from the shoot), shoot (3 mm from base), stem and leaf base were done manually and the formation of the aerenchyma was observed using a microscope equipped with a camera. The aerenchyma was quantified using GIMP 2.8 (Media Cybernetics) and the percentage of aerenchyma was calculated based on the total cross-sectional area of the tissue. For the observation of suberin and lignin deposits in the cell walls of the root sections (root cap and root base), stem and leaf blade, fixed tissue samples were hand-sliced into ~80 μm thick cross-sections and incubated in lactic acid saturated with chloral hydrate at 70°C for 1 h to clear the sections. To visualise suberin deposits, tissue cross-sections were stained with Sudan red 7B (Brundrett et al. 1991) and for lignin, they were stained for 5 min in phloroglucinol/hydrochloride (20 g phloroglucinol in 80 mL of 20% ethanol solution plus 20 mL of concentrated 12 N HCl) (Jensen 1962). Stained cross-sections were air-dried for 20 min at room temperature and then observed using a bifocal microscope equipped with a camera.
Qualitative determination of oxygen release in the rhizosphere
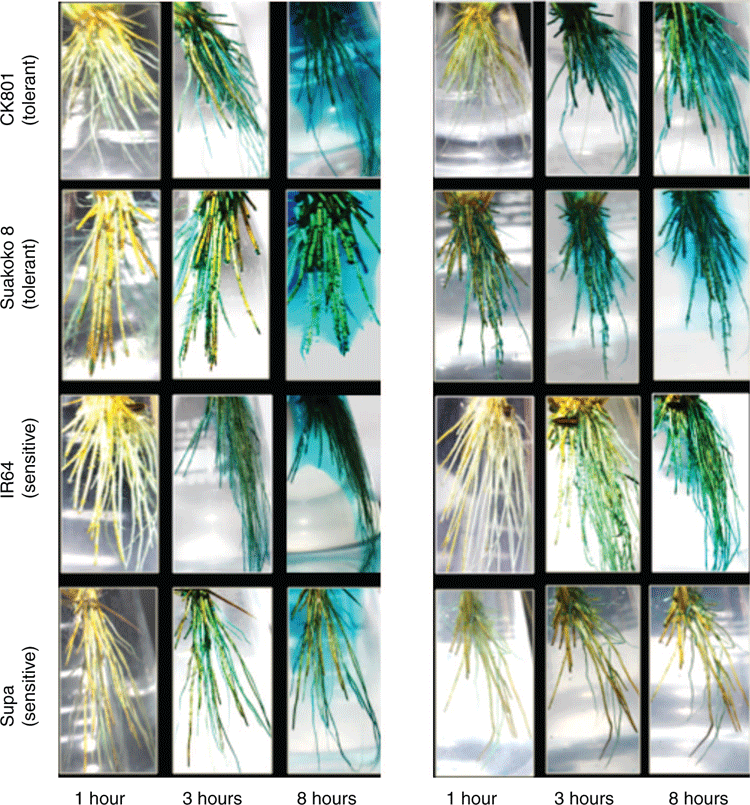
Oxygen gas released in the rhizosphere was assessed based on Armstrong and Armstrong (1988) with some modifications. After 10 days and 14 days of excess Fe2+ treatment (24- and 28-day-old plants), a single plant per variety and per replicate (three plants in total) was transferred from the hydroponic trays to transparent glass flasks containing 1 L of the nutrient solution used for plant growth with a low concentration agar (0.05% w/v) and 0.012 g of methylene blue. Prior to plants’ transfer, the solution in the flasks was reduced by adding 0.13 g of sodium dithionite following the reaction: MeB+ + 2H2O + S2O4–2 (blue colour solution) → MeBH + Cl– + 3H+ + 2SO3–2 (colourless solution) (Barton and Ollis 1979) and deoxygenated by flushing in O2-free nitrogen gas. The shoot base of the plants was fixed into a rubber lid tightly sealed on the ring of the flask so that only the roots were immersed in the O2-free medium and the shoot was out. Flushing of the solution with O2-free nitrogen gas was continued until the whole set up was complete. The flasks with the plants were kept in an aerated room at a constant temperature of 30°C for 24 h. The development of blue colour around the roots was observed every hour for 10 h during the day and captured with a camera. Differences in the intensity of the blue colour development were used to infer varietal differences in the rate of O2 diffusion from the shoots to the roots.
Measurement of lipid peroxidation
Lipid peroxidation was assessed by measuring malondialdehyde (MDA) concentration as described by Hodges et al. (1999). MDA was extracted from 200 mg of finely ground shoot and root samples (five time-points × four varieties × two Fe levels) mixed with 2.0 mL of extractant (4°C, 80 : 20 : 0.01 v/v/w, ethanol : water : trichloroacetic acid). The samples were homogenised and centrifuged at 4000g and 4°C for 15 min. The supernatant (0.5 mL) was transferred into a tube containing 0.5 mL of (+) TBA (thiobarbituric acid) reagent, and another 0.5 mL of the supernatant was transferred into a tube containing 0.5 mL (–) TBA reagent. The tubes were vortexed at maximum speed for 2 s and incubated at 95°C for 25 min. The reactions were stopped on ice. Absorbance was read at 440, 532, and 600 nm using a spectrophotometer (BMG SPECTROstar Nano). MDA concentration was calculated using the extinction coefficient of 157 mM–1 cm–1, as shown in the following equations:
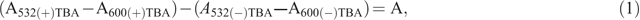

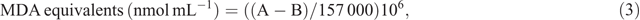
where MDA values are expressed as nmol mL–1 g FW–1.
Quantification of the total phenolic content (TPC)
TPC was measured following the protocol of Ainsworth and Gillespie (2007). The sample extracts remaining from the MDA quantification were used. Aliquots of 100 μL of samples or gallic acid standards were mixed with 200 μL of 10% (v/v) Folin–Ciocalteau phenol reagent, vortexed at maximum speed for 2 s and incubated at 25°C for 6 min in the dark. Then, 800 μL of 700 mM Na2CO3 was added and the mix was incubated at 25°C for 2 h in the dark. Absorbance values were read at 765 nm using a spectrophotometer (BMG SPECTROstar Nano) and total phenolic content was determined based on the standard curve of gallic acid. The results were expressed as gallic acid equivalents g FW–1.
Quantification of antioxidative enzymes activity
Crude protein extracts were prepared by thoroughly mixing frozen shoot and root samples (200 mg of finely ground tissue) with 2 mL of cold extraction buffer (100 mM HEPES-KOH, pH 8.0) followed by agitation at 4°C for 10 min and centrifugation at 10 000g, 4°C for 10 min (Tomlinson et al. 2004). Extracts were then dialysed for 24 h, with constant agitation at 4°C and changing the dialysate every 6 h. Purified samples were normalised to 10 μg protein mL–1 for all enzyme kinetics and analysis was done following Brand-Williams et al. (1995). SOD activities were quantified by mixing 185 µL of 50 mM potassium phosphate buffer (pH 7), 25 µL of 1 mM xanthine, 25 µL of 0.1 mM cytochrome C, 10 µL of purified sample extract, and 5 µL of 0.5 (U) xanthine oxidase per sample. Absorbance was measured at 550 nm using BMG SPECTROstar Nano. SOD activity (in units mg–1 protein) was calculated as: (slope of blank/slope of sample) – 1.
APX activity was determined using 200 µL of 50 mM potassium phosphate buffer (pH 7), 25 µL of 5 mM ascorbic acid, 10 µL of purified sample extract, and 15 µL of 10 mM H2O2 per sample. Absorbance was measured at 290 nm using BMG SPECTROstar Nano. Enzyme activity was calculated using the corresponding molar extinction coefficient of 2.8 mM–1 cm–1. CAT activity was determined using 230 µL of 0.1 M phosphate buffer (pH 7), 10 µL of purified sample extract, and 10 µL of 1 M H2O2 per sample. Absorbance was measured at 240 nm using BMG SPECTROstar Nano. Catalase activity was calculated using the molar extinction coefficient of 39.4 M–1 cm–1 based on H2O2 destroyed min–1 mg–1 protein. Glutathione reductase (GR) activities were determined using 225 µL of 0.2 M Tris–HCl buffer (pH 7.8), 10 µL of 5 mM NADPH, 10 µL of purified sample extract, and 5 µL of 0.05 mM oxidised glutathione per sample. Absorbance was measured at 340 nm using BMG SPECTROstar Nano. GR activity was calculated using the corresponding molar extinction coefficient of 6.22 mM–1 cm–1. Peroxidase (POX) activity was determined using 125 µL of 50 mM sodium acetate buffer (pH 7), 100 µL of 0.05% guaiacol, 10 µL of purified sample extract, and 15 µL of 10 mM H2O2 per sample. Absorbance was measured at 470 nm using BMG SPECTROstar Nano. POX activity was calculated using the molar extinction coefficient of 26.6 mM–1 cm–1.
Statistical analysis
Statistical analysis of the data was performed using the Statistical Tool for Agricultural Research (STAR) and R i386 ver. 3.2.2
Results
Varietal differences in the development of aerenchyma tissue
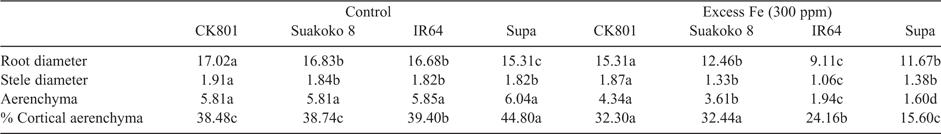
Observations on root cross-sections showed that under both control and Fe stress conditions, CK801 had the highest root and stele diameters whereas Suakoko8 had similar root and stele diameters as IR64 under control and Supa under Fe stress. All four varieties showed similar size of aerenchyma under control conditions but under Fe stress, the tolerant varieties (CK801 and Suakoko8) outperformed the sensitive ones (IR64 and Supa) (Table 1). In addition, the structure of the aerenchyma was better maintained in CK801 and Suakoko8 than in the sensitive varieties IR64 and Supa under stress (Fig. 1a).
Variation in ROL barriers and capability for oxygen release by roots
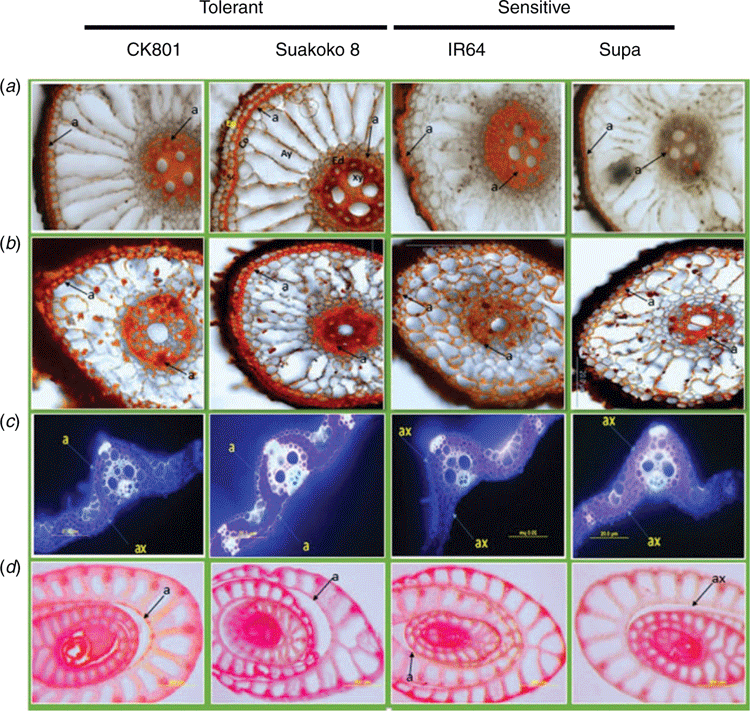
Deposits of suberin were visible both on the cross-sections from the root base (5–10 mm from the shoot base) and the root cap (0–5 mm from the root tip) of all the four varieties (Fig. 1a, b). Suberin deposits in the root cells were located at the endodermis as well as in the sclerenchyma (Fig. 1a). Observations of cross-sections revealed less suberin deposits in leaves than in roots. In Suakoko8, suberin deposits were visible on both the adaxial and abaxial leaf surfaces, whereas in CK801 suberin deposit was only observed on the adaxial surface. For the sensitive varieties, no suberisation was observed on both sides of the leaf (Fig. 1c). Although faint, suberin coloration could be detected in the cell walls of the stems of CK801, Suakoko8 and IR64 but not in Supa.
Microscopic observations of lignin deposits in the root cells showed more intense lignification in Suakoko8 than in CK801, and in the sensitive varieties IR64 and Supa (Fig. 2a, b). Besides, only Suakoko8 showed lignified tissues in the root cap (Fig. 2b) and lignin deposits in both the sclerenchyma and the endodermis of the root base and the root cap. In CK801 and IR64, lignin deposits were visible only in the root base and mostly in the sclerenchyma, with no lignin deposits in Supa (Fig. 2a, b). In leaf cross-sections, more intense lignification was observed in tolerant varieties than in the sensitive ones. CK801 and Supa showed lignification only on the abaxial side of the leaf whereas IR64 showed lignin deposits on the adaxial side and Suakoko8 on both sides of the leaf (Fig. 2c). Lignin accumulation was visible in the stem sections of all varieties (Fig. 2d).
Root oxygen release as assessed by the reduction/oxidation of methylene blue showed that among all the four varieties, Suakoko8 reacted faster and stronger (Fig. 3), and within one hour of incubation, a blue colour corresponding to oxidised methylene blue appeared around its roots. This was more noticeable on plants exposed to 14 days of excess Fe treatment compared with 10 days where the blue colour was localised on the root tips (Fig. 3). As the incubation time increases (from 1 to 8 h), oxygen release intensified in all four varieties and it formed a blue halo in the rhizosphere. However, on plants subjected to 14 days of excess Fe treatment, this blue halo was visible only with Suakoko8. For the other varieties, in particular CK801 and IR64, the blue colour was limited to the root system and did not diffuse to the rhizosphere. Among all the four varieties, Supa was the poorest in root oxygen release, especially after 14 days under Fe stress.
Variation in Fe-induced oxidative stress and its control
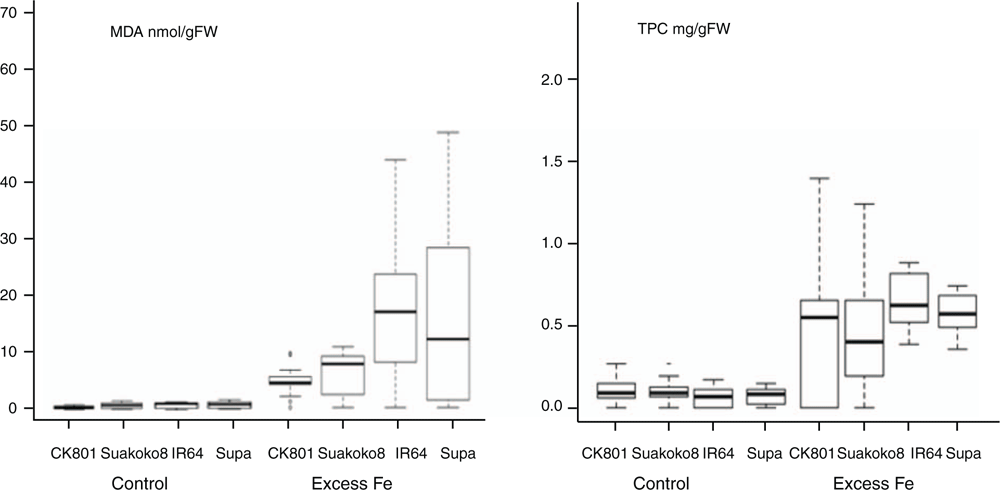
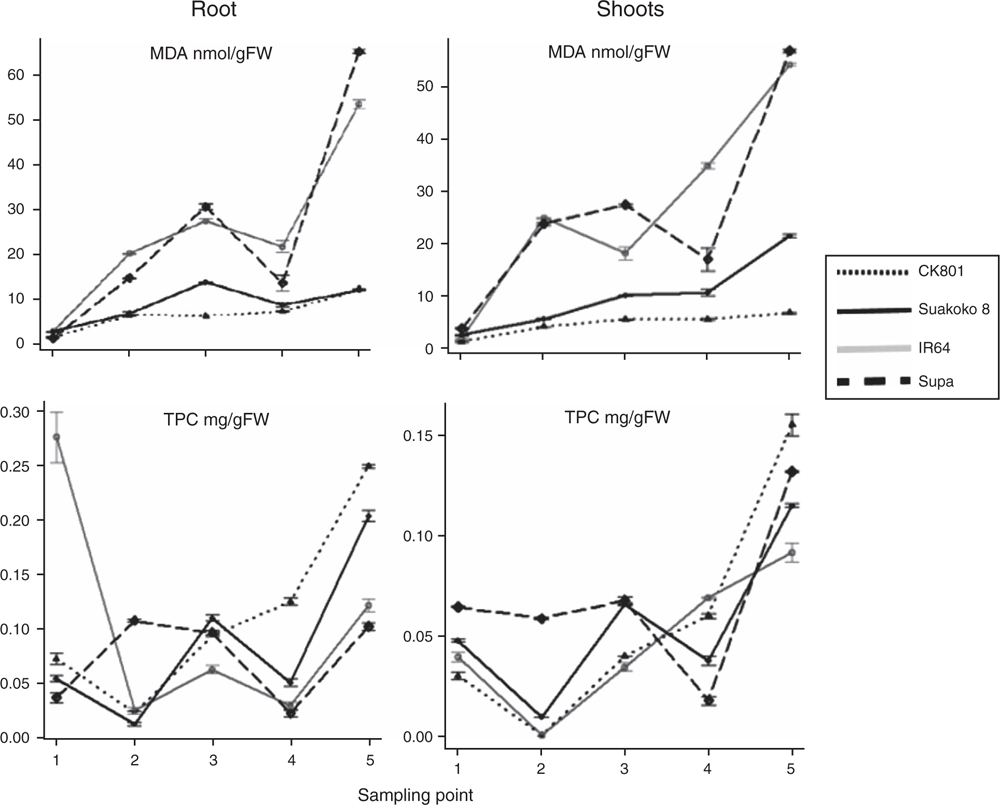
Oxidative stress was assessed through measurement of malondialdehyde (MDA) concentration, as the product of membrane lipid peroxidation. Higher concentrations of MDA were observed in the plants subjected to excess Fe treatment compared with plants grown under control conditions (Fig. 4). The least level of lipid peroxidation was observed in CK801, followed by Suakoko8; whereas the sensitive varieties IR64 and Supa showed significantly higher lipid peroxidation than the tolerant varieties. Similar concentration of MDA was observed in the roots and shoot of the four varieties and the variation in MDA concentration across varieties followed the same pattern in both tissues (Fig. 5). CK801 showed the lowest level of MDA, followed by Suakoko8 and, by far, by IR64 and Supa. Temporal variation in MDA in roots followed similar trend as in the shoots (Fig. 5). At sampling point 1, all varieties showed low MDA, but it increased at sampling points 2 and 3. At sampling point 4, a decrease was noted (except in CK801) followed by a sharp increase at sampling point 5. In the shoots, MDA was almost stable throughout the sampling period in CK801 and between sampling points 3 to 4 in Suakoko8. This was followed by a sharper increase at the 5th sampling point in Suakoko8 than in CK801. The sensitive varieties showed increasing levels of MDA across sampling points with a slight reduction at sampling point 4 in IR64 and at sampling point 3 in Supa (Fig. 5).
Oxidative stress control was assessed by quantifying total phenolic content (TPC) and relative activities of antioxidative enzymes. Low TPC concentrations were observed under control conditions (Fig. 4). With the excess Fe treatment, TPC levels increased significantly in all varieties; with highest concentrations in IR64 followed by Supa, CK801 and Suakoko8 (Fig. 4). TPC concentrations in roots were lower than in the shoots. In roots, CK801 recorded increasing levels of TPC from sampling point 2 to 5, whereas both Suakoko8 and IR64 showed alternate decrease and increase. Supa maintained similar TPC levels at sampling points 2 and 3 followed by a sharp decrease at sampling 4 and an increase at sampling 5. In shoots, CK801and IR64 had increasing levels of TPC between sampling points 2 to 5 (Fig. 5). Suakoko8 and Supa showed increasing levels of TPC between sampling point 2 to 3 and 4 to 5 with a decreasing level between sampling 3 and 4.
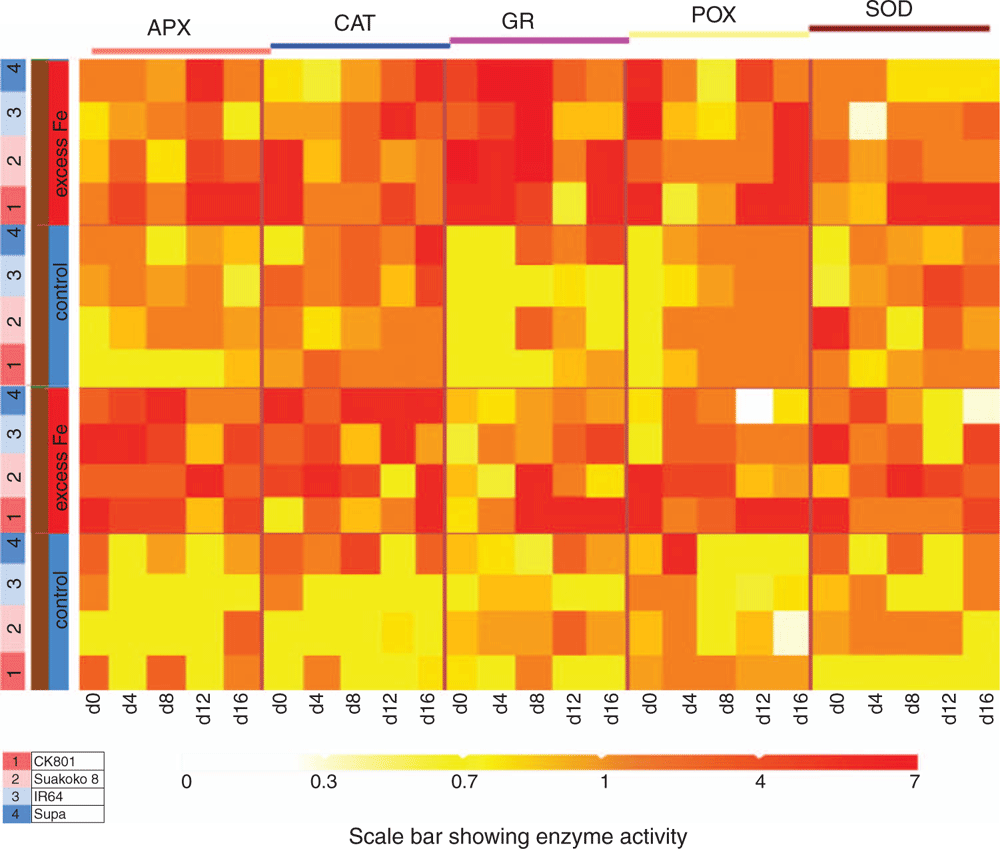
Activities of antioxidative enzymes (APX, CAT, GR, POX and SOD) varied across varieties, sampling points, tissues and Fe treatments (Fig. 6). Generally, higher enzymatic activities were recorded in plants subjected to excess Fe treatment than in control treatment, irrespective of the tissue. Under excess Fe conditions, high APX and CAT activities were recorded in the roots and high GR activity in the shoots (Fig. 6). Noticeable varietal differences in response to excess Fe were also observed. In shoots, activities of APX, POX and SOD were highest in CK801, whereas Suakoko8 showed the highest activity of GR. Similar high CAT activity was observed in CK801 and IR64 (Fig. 6). In the roots, IR64 showed the highest APX activity, closely followed by CK801. CK801 also showed the highest GR and POX activities. Supa recorded the highest CAT activity and Suakoko8 and CK801 showed similar high activity of SOD. It is worth noting that CK801 had in most cases maintained moderate to high activities of antioxidant enzymes at the late stage of the excess Fe treatment (sampling point 5; Fig. 6).
Discussion
Radial oxygen loss and root oxidation ability
Several studies have reported internal oxygen diffusion through the aerenchyma as a mechanism used by rice to tolerate anoxic environments of flooded soils and to maintain root growth and function (Garthwaite et al. 2008; Kotula et al. 2009; Shiono et al. 2011, 2014). Suberin and lignin deposits in both stem and root cells were suggested to form a barrier that prevents radial oxygen loss (ROL) along the stem and at the root base and thus, enhance oxygen transport to the root tips (Watanabe et al. 2013; Shiono et al. 2014). In the present study, we observed better root aerenchyma development in the tolerant varieties (CK801 and Suakoko8), in response to Fe toxicity, compared with the sensitive varieties (IR64 and Supa), suggesting better oxygen flow and aeration of roots of the tolerant varieties. Besides, suberin and lignin deposits observed in the inner and cortical root cells might have acted as barriers to ROL at the root base and enhanced oxygen flow to the active root tips. Suakoko8 seemed more efficient in establishing ROL barrier than the other varieties, as it had greater lignin and suberin deposits from the root base down to the root cap and both in the inner (endodermis) and cortical (sclerenchyma) cell layers of the roots. This is further demonstrated by its higher oxygen release in hydroponic media during the methylene blue assay. When subjected to excess Fe, Supa had poorly-developed root aerenchyma and limited ROL barrier, only on the cortical parts of the root base and released much less oxygen. Therefore, the concentration of oxygen that reaches its root tips may not be sufficiently high to effectively oxidise Fe2+ into Fe3+. Li et al. (2015) showed that root growth inhibition under Fe-toxic conditions occurs only when the root tip is in contact with toxic levels of Fe. Therefore, varieties like Suakoko8 that can efficiently oxidise Fe2+ in the vicinity of root tips can avoid Fe toxicity. Such varieties were called excluders (Becker and Asch 2005; Onaga et al. 2016) and can maintain lower uptake of Fe2+ by converting it into the less soluble forms at the active root tips.
Lipid peroxidation
MDA accumulation increased with excess Fe in all varieties. Enhanced lipid peroxidation under Fe toxicity has been reported previously in other rice varieties from Brazil and Africa (Kao et al. 2001; Souza-Santos et al. 2001; Majerus et al. 2007; Muller et al. 2015) and in other species, including the medicinal plant Bacopa monnieri (Sinha and Saxena 2006) and peanut (Shi et al. 2014). Overall, sensitive varieties showed higher concentrations of MDA than the tolerant varieties, an indication of higher lipid peroxidation as the result of oxidative stress. This observation is in accordance with the lower membrane stability found in sensitive varieties IR64 and Supa as opposed to the tolerant varieties, CK801 and Suakoko8 (Onyango et al. 2018). In addition to the disruption of membranes, oxidative stress alters important physiological and metabolic processes such as photosynthesis and synthesis of biomolecules (Mittler 2002; Gill and Tuteja 2010).
Increased production of oxidised polyphenol under Fe toxicity has been reported to cause leaf bronzing symptoms in rice (Tanaka et al. 1966; Yamauchi 1989). Hence, it is not surprising that the two sensitive varieties that were most affected by Fe-induced oxidative stress as depicted by their high leaf MDA concentration, were also reported to have high leaf bronzing scores (LBS) (Onyango et al. 2018). However, MDA levels and LBS are not necessarily indicative of the concentration of Fe in tissues. For instance, despite the high iron concentration recorded in the leaves of CK801 (Onyango et al. 2018), the variety maintained relatively low levels of MDA throughout the duration of the stress, suggesting internal mechanisms associated with mitigating the harmful effects of high tissue iron. Similar observations were made by Muller et al. (2015) who identified rice varieties with high tissue iron concentration but low MDA.
Antioxidant responses
Higher concentration of phenolic compounds was observed in all varieties subjected to excess Fe. In many studies it has been revealed that there is a direct relationship between antioxidant activity and phenolic content of plant extracts (Farrukh et al. 2006; Liu et al. 2008; Tung et al. 2011). According to Halliwell (2006), some phenolic compounds may bind pro-oxidant metals such as iron and copper, preventing the formation of free radicals from these pro-oxidants while maintaining their capacity to scavenge free radicals. Phenolic compounds are also known to suppress lipid peroxidation by recycling other antioxidants, such as α-tocopherol (Melissa and Enio 2011). In this study, sensitive varieties showed higher TPC (Fig. 4) than the tolerant varieties in which lipid peroxidation was lower. It is possible that TPC contributes to overcoming lipid peroxidation.
Overall, activities of all antioxidative enzymes (APX, CAT, GR, POX and SOD) increased when plants were subjected to excess Fe. This observation is in agreement with that of Saikia and Baruah (2012) and Bode et al. (1995) who reported, respectively, increased SOD and APX and SOD and POX activities in rice under iron toxicity stress. In contrast, Fang et al. (2001) reported a decrease in SOD activity in rice leaves subjected to excess Fe. As suggested by Wu et al. (2017), contradictory results regarding SOD activities could be explained by the existence of several SOD genes which respond differently to iron toxicity, probably due their sub-cellular localisation and the different co-factors required by SOD enzymes. In the present study, the tolerant variety CK801 maintained high activity of all antioxidative enzymes either in the shoots (CAT), the roots (GR) or in both tissues (APX, POX, SOD), suggesting a better control of Fe-induced oxidative stress than in the other varieties. Similar findings were reported by Stein et al. (2014) who observed increased GR, POX, and SOD activities in tolerant rice varieties subjected to Fe toxicity. SOD can scavenge ROS in all cellular compartments and reduce the highly toxic superoxide (O2–) into the less toxic hydrogen peroxide (H2O2) which in turn, can be detoxified by APX and CAT (Asada 1999, 2006; Mittler 2002; Moradi and Ismail 2007; Stein et al. 2014). Glutathione reductase (GR), catalyses the reduction of glutathione disulfide (GSSG) into glutathione (GSH), which is an important molecule in counteracting oxidative stress and maintaining reducing cellular environment (Mittler 2002; Gill et al. 2013). Hence, maintaining high activity of these enzymes will enhance ROS scavenging and mitigate the harmful effects of ROS.
Contrary to CK801, Suakoko8 showed increased activity of some but not all antioxidative enzymes under Fe toxicity and this increase was not maintained throughout the growth period. Although there are several H2O2-scavenging enzymes, one enzyme may not necessarily be a substitute for another one. Mittler (2002) indicated that since the affinities of APX (µM range) and CAT (mM range) for H2O2 are very different, APX might be responsible for the fine modulation of reactive oxygen intermediates (ROIs) for signalling, whereas CAT might be responsible for the removal of excess ROIs during stress. Therefore, it is critical for the survival of the plants, to strongly stimulate different enzymes to maximise the antioxidant response. Further, this reaction should occur at the very early stages of the stress as observed in CK801, before the control systems are overwhelmed with excess ROS.
Activities of SOD, GR, APX and POX decreased with the stress duration in Supa, either due to exhaustion or inhibition of new enzyme synthesis as suggested by Mittler (2002). The poor capacity of Supa to sustain activation of antioxidative enzymes may have contributed to its high lipid peroxidation and resulted in higher cellular damage than in the other varieties. In contrast to our findings, Stein et al. (2009) reported increased SOD activity in a sensitive rice cultivar grown in Fe-toxic field and this was attributed to a stress response rather than a tolerance mechanism.
Conclusion
Comparative assessment of root oxidation ability and oxidative stress control in four rice varieties contrasting in Fe toxicity tolerance showed that different mechanisms could contribute to tolerance of a particular genotype. We observed that both tolerant varieties, CK801 and Suakoko8, maintained better root aeration and rhizosphere oxidation, as well as ROS scavenging. However, Suakoko8 released more oxygen in the rhizosphere than CK801, whereas CK801 was better in upregulating the activities of antioxidative enzymes. Root aeration and the consequent rhizosphere oxidation, and oxidative stress control are likely genetically independent and could be combined using appropriate multi-parental crosses. This can be accomplished using CK801 and Suakoko8 as parental lines in breeding programs aiming at improving Fe toxicity tolerance in rice. Based on the present study, the key traits associated with adaption to high Fe soil conditions include well-developed aerenchyma, capacity for suberisation/lignification of both inner and cortical root cells beyond the root base to reduce ROL and high activity of antioxidative enzymes (in particular SOD, GR, POX and APX) for the effective control of oxidative stress. These traits could serve as selection criteria provided that high-throughput assays are developed. Alternatively, functional alleles of the genes controlling these traits could potentially be identified and developed into markers for breeding.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by the project ‘Stress Tolerant Rice for Africa and South Asia (STRASA)’ funded by The Bill and Melinda Gates Foundation and coordinated by IRRI and AfricaRice. The authors are grateful for the technical support provided by Nancy Sadiasa, Ricardo Eugenio, and Felix Waweru.
References
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nature Protocols 2, 875–877.| Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent.Crossref | GoogleScholarGoogle Scholar | 17446889PubMed |
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany 53, 1331–1341.
| Role of superoxide dismutases (SODs) in controlling oxidative stress in plants.Crossref | GoogleScholarGoogle Scholar | 11997379PubMed |
Armstrong J, Armstrong W (1988) Pragmites australis – a preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytologist 108, 373–382.
| Pragmites australis – a preliminary study of soil-oxidizing sites and internal gas transport pathways.Crossref | GoogleScholarGoogle Scholar |
Armstrong J, Armstrong W (2005) Rice: sulfide-induced barriers to root radial oxygen loss, Fe and water uptake, and lateral root emergence. Annals of Botany 96, 625–638.
| Rice: sulfide-induced barriers to root radial oxygen loss, Fe and water uptake, and lateral root emergence.Crossref | GoogleScholarGoogle Scholar | 16093271PubMed |
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50, 601–639.
| The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons.Crossref | GoogleScholarGoogle Scholar | 15012221PubMed |
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology 141, 391–396.
| Production and scavenging of reactive oxygen species in chloroplasts and their functions.Crossref | GoogleScholarGoogle Scholar | 16760493PubMed |
Barton DRM, Ollis WD (1979) ‘Comprehensive organic chemistry. Vol. 4.’ (Pergamon Press: Oxford, UK)
Becker M, Asch F (2005) Iron toxicity in rice – conditions and management concepts. Journal of Plant Nutrition and Soil Science 168, 558–573.
| Iron toxicity in rice – conditions and management concepts.Crossref | GoogleScholarGoogle Scholar |
Bode K, During O, Lüthje S, Nee HU, Bottler M (1995) The role of active oxygen in iron tolerance of rice (Oryza sativa L.). Protoplasma 184, 249–255.
| The role of active oxygen in iron tolerance of rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar |
Brand-Williams W, Cuvelier ME, Berste C (1995) Use of free radical method to evaluate antioxidant activity. Food Science and Technology 28, 25–30.
Brundrett MC, Kendrick B, Peterson CA (1991) Efficient lipid staining in plant material with Sudan Red 7B or floral yellow 088 in polyethylene glycol-glycerol. Biotechnic & Histochemistry 66, 111–116.
| Efficient lipid staining in plant material with Sudan Red 7B or floral yellow 088 in polyethylene glycol-glycerol.Crossref | GoogleScholarGoogle Scholar |
Colmer TD (2003a) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91, 301–309.
| Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar | 12509350PubMed |
Engel K, Asch F, Becker M (2012) Classification of rice genotypes based on their tolerance and the mechanisms of adaptation to conditions of iron toxicity. Journal of Plant Nutrition and Soil Science 175, 871–881.
| Classification of rice genotypes based on their tolerance and the mechanisms of adaptation to conditions of iron toxicity.Crossref | GoogleScholarGoogle Scholar |
Fang WC, Wang JW, Lin CC, Kao CH (2001) Iron induction of lipid peroxidation and effects on antioxidative enzyme activities in rice leaves. Plant Growth Regulation 35, 75–80.
| Iron induction of lipid peroxidation and effects on antioxidative enzyme activities in rice leaves.Crossref | GoogleScholarGoogle Scholar |
Farrukh A, Iqbal A, Zafar M (2006) Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turkish Journal of Biology 30, 177–183.
Garthwaite AJ, Armstrong W, Colmer TD (2008) Assessment of O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytologist 179, 405–416.
| Assessment of O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum.Crossref | GoogleScholarGoogle Scholar | 19086178PubMed |
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in plants. Plant Physiology and Biochemistry 48, 909–930.
| Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in plants.Crossref | GoogleScholarGoogle Scholar | 20870416PubMed |
Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I, Pereira E, Tuteja N (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiology and Biochemistry 70, 204–212.
| Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations.Crossref | GoogleScholarGoogle Scholar | 23792825PubMed |
Greenway H, Armstrong W, Colmer TD (2006) Conditions leading to high CO2 (>5 kPa) in waterlogged-flooded soils and possible effects on root growth and metabolism. Annals of Botany 98, 9–32.
| Conditions leading to high CO2 (>5 kPa) in waterlogged-flooded soils and possible effects on root growth and metabolism.Crossref | GoogleScholarGoogle Scholar | 16644893PubMed |
Halliwell B (2006) Reactive species and antioxidants redox biology is a fundamental theme of life aerobic life. Plant Physiology 141, 312–322.
| Reactive species and antioxidants redox biology is a fundamental theme of life aerobic life.Crossref | GoogleScholarGoogle Scholar | 16760481PubMed |
Hideg E (1999) Free radical production in photosynthesis under stress conditions. In ‘Handbook of plant and crop stress’. (Ed. M Pessarakli) pp. 911–930. (Marcel Dekker: New York)
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611.
| Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds.Crossref | GoogleScholarGoogle Scholar |
Ismail AM (2018) Submergence tolerance in rice: resolving a pervasive quandary. New Phytologist 218, 1298–1300.
| Submergence tolerance in rice: resolving a pervasive quandary.Crossref | GoogleScholarGoogle Scholar | 29738087PubMed |
Jensen WA (1962) ‘Botanical histochemistry: Principles and practice.’ (W.H Freeman: San Francisco, CA, USA)
Johansen DA (1940) ‘Plant microtechnique.’ (McGraw-Hill Book Co. Inc.: New York)
Kang DJ, Koichi F, Young-Jin S, Pisoot V, Ryuichi I (2011) Relationship of Fe-tolerance to morphological changes in roots in upland NERICA lines under Fe-treated hydroponic conditions. Crop Science 4, 311–315.
Kao CH, Fang WC, Wang JW, Lin CC (2001) Iron induction of lipid peroxidation and effects on antioxidative enzyme activities in rice leaves. Plant Growth Regulation 1, 75–80.
Kotula L, Ranathunge K, Schreiber L, Steudle E (2009) Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. Journal of Experimental Botany 60, 2155–2167.
| Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution.Crossref | GoogleScholarGoogle Scholar | 19443620PubMed |
Li G, Xu W, Kronzucker HJ, Shi W (2015) Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis. Journal of Experimental Botany 66, 2041–2054.
| Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 25711703PubMed |
Liu X, Zhao M, Wang J, Yang B, Jiang Y (2008) Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L) from six regions in China. Journal of Food Composition and Analysis 21, 219–228.
| Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L) from six regions in China.Crossref | GoogleScholarGoogle Scholar |
Majerus V, Bertin P, Lutts S (2007) Effects of iron toxicity on osmotic potential, osmolytes and polyamine concentration in the African rice (Oryza glaberrima Steud). Plant Science 173, 96–105.
| Effects of iron toxicity on osmotic potential, osmolytes and polyamine concentration in the African rice (Oryza glaberrima Steud).Crossref | GoogleScholarGoogle Scholar |
Matthus E, Wu LB, Ueda Y, Höller S, Becker M, Free M (2015) Loci, genes, and mechanisms associated with tolerance to ferrous iron toxicity in rice (Oryza sativa L.). Theoretical and Applied Genetics 128, 2085–2098.
| Loci, genes, and mechanisms associated with tolerance to ferrous iron toxicity in rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar | 26152574PubMed |
Melissa W, Enio M (2011) Phenolic compounds and antioxidant activity of rice. Brazilian Archives of Biology and Technology 54, 371–377.
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7, 405–410.
| Oxidative stress, antioxidants and stress tolerance.Crossref | GoogleScholarGoogle Scholar | 12234732PubMed |
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498.
| Reactive oxygen gene network of plants.Crossref | GoogleScholarGoogle Scholar | 15465684PubMed |
Moradi F, Ismail AM (2007) Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Annals of Botany 99, 1161–1173.
| Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice.Crossref | GoogleScholarGoogle Scholar | 17428832PubMed |
Muller C, Kuki KN, Pinheiro DT, de Souza LR, Silva AIS, Loureiro ME, Oliva MA, Almeida AM (2015) Differential physiological responses in rice upon exposure to excess distinct iron forms. Plant and Soil 391, 123–138.
| Differential physiological responses in rice upon exposure to excess distinct iron forms.Crossref | GoogleScholarGoogle Scholar |
Onaga G, Dramé KN, Ismail AM (2016) Understanding the regulation of iron nutrition: can it contribute to improving iron toxicity tolerance in rice? Journel of Functional Plant Biology 43, 709–726.
| Understanding the regulation of iron nutrition: can it contribute to improving iron toxicity tolerance in rice?Crossref | GoogleScholarGoogle Scholar |
Onyango DA, Entila F, Dida MM, Ismail AM, Drame KN (2018) Mechanistic understanding of iron toxicity tolerance in contrasting rice varieties from Africa: 1. Morpho-physiological and biochemical responses Journel of Functional Plant Biology 46, 93–105.
| Mechanistic understanding of iron toxicity tolerance in contrasting rice varieties from Africa: 1. Morpho-physiological and biochemical responsesCrossref | GoogleScholarGoogle Scholar |
Saikia T, Baruah KK (2012) Iron toxicity tolerance in rice (Oryza sativa) and its association with anti-oxidative enzyme activity. Journel of Crop Science 3, 90–94.
Shi G, Sun L, Wang X, Liu C (2014) Leaf responses to iron nutrition and low cadmium in peanut: anatomical properties in relation to gas exchange. Plant and Soil 375, 99–111.
| Leaf responses to iron nutrition and low cadmium in peanut: anatomical properties in relation to gas exchange.Crossref | GoogleScholarGoogle Scholar |
Shiono K, Ogawa S, Yamazaki S, Isoda H, Fujimura T, Nakazono M, Colmer TD (2011) Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Annals of Botany 107, 89–99.
| Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths.Crossref | GoogleScholarGoogle Scholar | 21097947PubMed |
Shiono K, Yamauchi T, Yamazaki S, Mohanty B, Malik AI, Nagamura Y, Nishizawa NK, Tsutsumi N, Colmer TD, Nakazono M (2014) Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). Journal of Experimental Botany 65, 4795–4806.
| Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa).Crossref | GoogleScholarGoogle Scholar | 24913626PubMed |
Sikirou M, Saito K, Achigan-Dako EG, Dramé KN, Ahanchédé A, Venuprasad R (2015) Genetic improvement of iron toxicity tolerance in rice – progress, challenges and prospects in West Africa. Plant Production Science 18, 423–434.
| Genetic improvement of iron toxicity tolerance in rice – progress, challenges and prospects in West Africa.Crossref | GoogleScholarGoogle Scholar |
Sinha S, Saxena R (2006) Effect of iron on lipid peroxidation, and enzymatic and non-enzymatic antioxidants and bacoside-A content in medicinal plant Bacopa monnieri L. Chemosphere 62, 1340–1350.
| Effect of iron on lipid peroxidation, and enzymatic and non-enzymatic antioxidants and bacoside-A content in medicinal plant Bacopa monnieri L.Crossref | GoogleScholarGoogle Scholar | 16219336PubMed |
Souza-Santos P, Ramos RS, Ferreira ST, Carvalho-Alves PC (2001) Iron-induced oxidative damage of corn root plasma membrane H+-ATPase. Biochimica et Biophysica Acta 1512, 357–366.
| Iron-induced oxidative damage of corn root plasma membrane H+-ATPase.Crossref | GoogleScholarGoogle Scholar | 11406113PubMed |
Stein RJ, Lopes SIG, Fett JP (2014) Iron toxicity in field-cultivated rice: contrasting tolerance mechanisms in distinct cultivars. Theoretical and Experimental Plant Physiology 26, 135–146.
| Iron toxicity in field-cultivated rice: contrasting tolerance mechanisms in distinct cultivars.Crossref | GoogleScholarGoogle Scholar |
Stein RJ, Duarte GL, Spohr MG, Lopes SIG, Fett JP (2009) Distinct physiological responses of two rice cultivars subjected to iron toxicity in the field. Annals of Applied Biology 154, 269–277.
| Distinct physiological responses of two rice cultivars subjected to iron toxicity in the field.Crossref | GoogleScholarGoogle Scholar |
Tanaka A, Loe R, Navasero SA (1966) Some mechanisms involved in the development of iron toxicity symptoms in the rice plant. Soil Science and Plant Nutrition 12, 32–38.
| Some mechanisms involved in the development of iron toxicity symptoms in the rice plant.Crossref | GoogleScholarGoogle Scholar |
Tomlinson KL, McHugh S, Labbe H, Grainger JL, Jame LE, Pomeroy KM, Mullin JW, Miller SS, Dennis DT, Miki BLA (2004) Evidence that the hexose-to-sucrose ratio does not control the switch to storage product accumulation in oilseeds: analysis of tobacco seed development and effects of overexpressing apoplastic invertase. Journal of Experimental Botany 55, 2291–2303.
| Evidence that the hexose-to-sucrose ratio does not control the switch to storage product accumulation in oilseeds: analysis of tobacco seed development and effects of overexpressing apoplastic invertase.Crossref | GoogleScholarGoogle Scholar | 15361535PubMed |
Tung YT, Cheng KC, Ho ST, Chen YL, Wu TL, Hung KC, Wu JH (2011) Comparison and characterization of the antioxidant potential of 3 wild grapes – Vitis thunbergii, V. flexuosa, and V. kelungeusis. Journal of Food Science 76, 701–706.
| Comparison and characterization of the antioxidant potential of 3 wild grapes – Vitis thunbergii, V. flexuosa, and V. kelungeusis.Crossref | GoogleScholarGoogle Scholar |
van Oort PAJ (2018) Mapping abiotic stresses for rice in Africa: drought, cold, iron toxicity, salinity and sodicity. Field Crops Research 219, 55–75.
| Mapping abiotic stresses for rice in Africa: drought, cold, iron toxicity, salinity and sodicity.Crossref | GoogleScholarGoogle Scholar | 29666552PubMed |
Watanabe K, Nishiuchi S, Kulichikhin K, Nakazono M (2013) Does suberin accumulation in plant roots contribute to waterlogging tolerance? Frontiers of Plant Science 4, 178
Wu L-B, Shhadi MY, Gregorio G, Matthus E, Becker M, Frei M (2014) Genetic and physiological analysis of tolerance to acute iron toxicity in rice. Rice 7, 8
| Genetic and physiological analysis of tolerance to acute iron toxicity in rice.Crossref | GoogleScholarGoogle Scholar | 24920973PubMed |
Wu L-B, Ueda Y, Lai S-K, Frei M (2017) Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.). Plant, Cell & Environment 40, 570–584.
| Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar |
Yamauchi M (1989) Rice bronzing in Nigeria caused by nutrient imbalances and its control by potassium sulfate application. Plant and Soil 117, 275–286.
| Rice bronzing in Nigeria caused by nutrient imbalances and its control by potassium sulfate application.Crossref | GoogleScholarGoogle Scholar |