Epigenetic memory and growth responses of the clonal plant Glechoma longituba to parental recurrent UV-B stress
Xiaoyin Zhang A , Cunxia Li A , Dan Tie A , Jiaxin Quan A , Ming Yue A and Xiao Liu A B
A B
A Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, Northwest University, Xi’an 710069, China.
B Corresponding author. Email: liuxiao@nwu.edu.cn
Functional Plant Biology 48(8) 827-838 https://doi.org/10.1071/FP20303
Submitted: 30 September 2020 Accepted: 10 March 2021 Published: 6 April 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
The responses of plants to recurrent stress may differ from their responses to a single stress event. In this study, we investigated whether clonal plants can remember past environments. Parental ramets of Glechoma longituba (Nakai) Kuprian were exposed to UV-B stress treatments either once or repeatedly (20 and 40 repetitions). Differences in DNA methylation levels and growth parameters among parents, offspring ramets and genets were analysed. Our results showed that UV-B stress reduced the DNA methylation level of parental ramets, and the reduction was enhanced by increasing the number of UV-B treatments. The epigenetic variation exhibited by recurrently stressed parents was maintained for a long time, but that of singly stressed parents was only short-term. Moreover, clonal plants responded to different UV-B stress treatments with different growth strategies. The one-time stress was a eustress that increased genet biomass by increasing offspring leaf allocation and defensive allocation in comparison to the older offspring. In contrast, recurring stress was a distress that reduced genet biomass, increased the biomass of storage stolons, and allocated more defensive substances to the younger ramets. This study demonstrated that the growth of offspring and genets was clearly affected by parental experience, and parental epigenetic memory and the transgenerational effect may play important roles in this effect.
Keywords: environmental stress, phenotypic plasticity, DNA methylation, stress memory, transgenerational effect.
Introduction
Plants often experience recurrent environmental stress throughout their lifespan, and their response to repeated stress events may be different from their response during their first encounter with the stress. These differences are considered to be attributable to the ‘stress memory’ of plants (Avramova 2015; Li et al. 2019). There is growing evidence that plants have the ability to remember their past environmental experience and retrieve information at a considerably later time (Chinnusamy and Zhu 2009; Ikeda 2012; Kim et al. 2012; Köhler et al. 2012; Saze et al. 2012; Song et al. 2013; Kinoshita and Seki 2014; Müller-Xing et al. 2014; Pascual et al. 2014). These memory capabilities of remembering and learning from past experience of exposure to biotic or abiotic stresses can be recorded through epigenetic mechanisms at the cellular level, and these epigenetic marks can be inherited as a pre-adaptation by subsequent generations as a form of the maternal effect, also called epigenetic memory. Thus, epigenetic memory serves as a key competence enabling organisms to better adapt to environmental circumstances (Ćuk et al. 2010; Verhoeven et al. 2010; Walter et al. 2011; Thellier and Lüttge 2013; Ding et al. 2014; Gagliano et al. 2014; Ramírez et al. 2015; Wang et al. 2015; Li and Liu 2016; de Freitas Guedes et al. 2018; Tombesi et al. 2018; Virlouvet et al. 2018).
Ultraviolet-B (UV-B, 280–315 nm) light is an intrinsic part of sunlight; although it comprises less than 1% of the total solar spectrum, UV-B light has a major effect on plant growth and development (Liu et al. 2015). Complex UV-B dose responses of plants have been demonstrated by many studies. High-dose UV-B radiation has a negative effect on plant growth and development, while low-dose radiation acts as a specific regulator for plants (Robson et al. 2015). More importantly, UV-B radiation reaching the Earth’s surface is increasingly due to depletion of the stratospheric ozone layer, which is one of the changes affecting the current climate-change pattern (Kataria et al. 2014). Moreover, the current ozone attenuation and increase in UV-B radiation are close to their peak, and it is unlikely that the ozone layer will return to the level of the 1980s by 2050 (McKenzie et al. 2011). Therefore, increased UV-B radiation is an important environmental factor affecting plant growth.
Our previous study indicated that increased UV-B radiation significantly influenced the physiological integration of photosynthetic processes, UV-absorbing compounds, and antioxidant enzymes between clonal ramet pairs (Li et al. 2011a, 2011b; Liu et al. 2015). These studies were all performed based on ramet pairs, and the variation between ramet pairs was clearly observed to affect the growth of clonal genets; however, the effect of UV-B radiation on clonal genets has not been reported. Moreover, current research on clonal plants has observed that because meiosis does not occur in asexual reproduction, clonal plants appear to have a greater ability than nonclonal plants to remember past environmental events. Furthermore, the variation and adaptive modifications induced by memory, especially by transgenerational epigenetic memory, may be an important mechanism contributing to the wide habitat occupation of clonal plants in various ecosystems (Latzel and Jitka 2010; Paszkowski and Grossniklaus 2011; Douhovnikoff and Dodd 2015; Latzel et al. 2016; Rendina González et al. 2016; Münzbergová and Hadincová 2017). Therefore, what is the role played by epigenetic memory in the response of clonal plants to UV-B stress? Although epigenetic modifications induced by UV-B radiation have been indicated in several nonclonal plants (Cloix and Jenkins 2008; Qüesta et al. 2010; Ohlsson et al. 2013; Rius et al. 2016; Pandey et al. 2019), the epigenetic mechanism governing the effect of UV-B radiation on clonal plant growth has not been elucidated, nor has parental ramets’ epigenetic memory and their transgenerational effect on offspring ramets.
In this study, parental ramets of clonal Glechoma longituba (Nakai) Kuprian were encountered with various recurrent UV-B stresses (one time, 20 times and 40 times), the epigenetic variation and the growth of ramets and genet were explored, and the following two hypotheses were presented: first, clonal plants could form epigenetic memory of their UV-B experience, and this memory might be transferred transgenerationally among clonal ramets; second, although only parental ramets were exposed to UV-B stress, the growth of the offspring and the whole clonal genet could be affected, and this effect might vary according to the recurrent frequency of stress.
Materials and methods
Plant material and propagation
Glechoma longituba (Nakai) Kuprian is a perennial clonal plant of the Labiatae family that is widely distributed and is observed in a variety of habitats, such as forests, roadsides, and creeks, in tropical, subtropical and temperate regions in China (Liu et al. 2015). This species produces long stolons with rooted ramets on its nodes and is commonly employed in clonal plant research due to its high phenotypic plasticity.
G. longituba used in our experiment was collected from Jiwozi in the Qinling Mountains, Shaanxi, China. The plant materials from the same genet were vegetatively propagated for at least 4 months in a greenhouse at the campus of North-west University in Xi’an (34.3°N, 108.9°E; 397 m a.s.l.) to reduce the impact of the previous environment. Next, healthy and similar-sized ramets were selected as parental ramets and transplanted to plastic pots (100 cm long × 40 cm wide × 20 cm high) for 41 days of growth. The peat soil, sand, and charcoal (1:1:1, v/v/v) were mixed and utilised as the culture soil of G. longituba. The culture conditions of the greenhouse were a 25/20°C day/night temperature cycle and a 12/12-h light/dark cycle, the light intensity was 300 μmol·m–2·s–1, and the relative humidity was maintained at 30%. Ramets were watered every 2 days to prevent water stress.
Experimental design
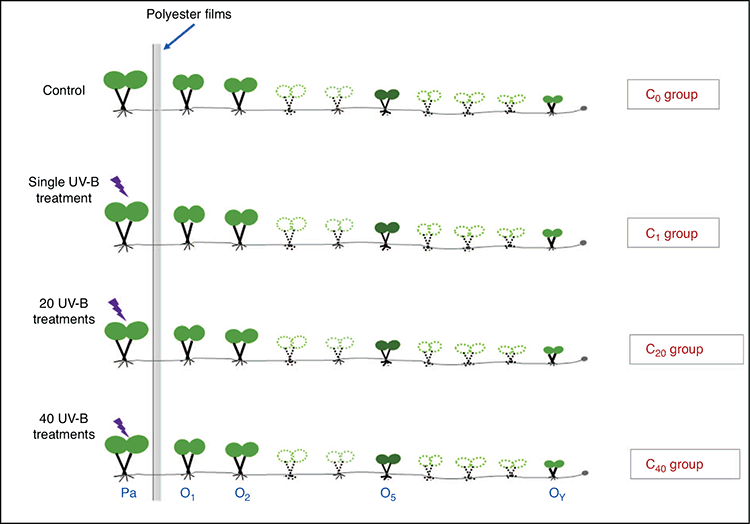
The experiment was begun with a single parental ramet. Forty-eight similar-sized parental ramets with an ~2-cm stolon were randomly divided into four groups and subsequently exposed to 0, 1, 20 or 40 UV-B stress treatments separately. Each treatment consisted of 8 h of continued UV-B radiation followed by 16 h of stress release. Therefore, there were four treatments involved in our study: the C0, C1, C20 and C40 groups (Fig. 1). C0 was the control treatment, parental and newborn offspring ramets grew under ambient light conditions, and no additional UV-B stress was added. C1 was single UV-B stress treatment, only parental ramets were exposed to 8 h UV-B radiation, and newborn offspring ramets grew under ambient light conditions without UV-B stress. C20 and C40 were 20 and 40 UV-B stress treatments, respectively, meaning that only parental ramets were exposed to 20 or 40 UV-B radiation treatments, each treatment was combined with 8 h UV-B stress and subsequent 16 h of stress release, and newborn offspring ramets grew under an ambient light condition without UV-B stress, thus there were 160 h and 320 h UV-B radiation in C20 and C40 group, respectively. Each treatment had 12 repetitions. During the experiments, only the parental ramets were irradiated with UV-B, and their newborn offspring ramets grew in an ambient greenhouse environment without additional UV-B treatment. Furthermore, the stolons among the parental ramets and their offspring ramets remained connected during the experiment. To avoid interception of scattered UV-B radiation by offspring ramets, polyester films (absorbing radiation below 320 nm, Grafix Plastics, Cleveland, OH, USA) were placed vertically between the parental ramets and their offspring ramets.
UV-B stress
UV-B stress was artificially supplied by square-wave UV-B fluorescent lamps (36 W, Beijing Lighting Research Institute, Beijing, China) by the procedure described in Liu et al. (2015). The maximum output wavelength of these lamps was 313 nm. The lamps were turned on for 8 h per day, from 0900 hours to 1700 hours. During the experiment, these lamps were wound with either 0.13-mm cellulose acetate film (transmission down to 290 nm, Grafix Plastics) for the supplemental UV-B radiation groups or with 0.13-mm polyester plastic film (absorbs radiation below 320 nm, Grafix Plastics) for the control group. The cellulose acetate and polyester plastic films were replaced every 5 days. The radiation level at the top of the plant canopy (8 µW cm–2) was adjusted by modifying the distance from the lamps to the canopy, and the UV-B level was measured with a UV radiometer (Handy, Beijing, China) every second day.
Measurement of parameters
After 41 days of growth, the longest primary stolon of G. longituba contained ~10 offspring ramets. Parents (represented by Pa) and some offspring ramets were collected for subsequent measurement of growth and epigenetic parameters. These offspring ramets were divided into three types according to their age: the elderly, middle-aged and youngest offspring ramets, which were represented by the first and second offspring ramets (represented by O1 and O2), the fifth offspring (represented by O5) and the ramet at the end of the stolon (represented by OY), respectively (Fig. 1).
Parameters of growth
At the end of the experiment, the whole genet of each treatment was harvested carefully. Leaf biomass and total biomass of the parental and abovementioned offspring ramets were analysed. Additionally, the leaf biomass, stolon biomass and total biomass of the genets of each treatment were measured. These samples were dried for 72 h at 80°C to a constant weight, and biomass was measured immediately with an electronic balance (Sartorius BT25S, Beijing, China). Next, leaf biomass allocation of different ramets (or genets) was calculated from leaf biomass divided by the total biomass of ramets (or genets), and stolon biomass allocation of the genets was calculated from stolon biomass divided by the total biomass of genets.
The fresh leaf of each ramet was collected for the measurement of specific leaf area (SLA) according to the method of Liu et al. (2015). Fresh leaves were scanned with a scanner (Perfection V19, EPSON, China), and leaf area was calculated with Motic software (Motic Images Plus 2.0. Ink, Motic, China). Next, the leaves were dried at 80°C for 72 h to a constant weight, and the dry mass was weighed using an electronic balance (Sartorius BT25S, Beijing, China). SLA was calculated as the ratio of leaf area to leaf dry mass.
UV-B-absorbing compounds concentration
The UV-B-absorbing compound content of fresh leaves was measured as described by Liu et al. (2015). Leaf discs were soaked in centrifuge tubes containing methanol, HCl and distilled H2O (79:1:20 volume) for 48 h in darkness. The total concentration of UV-B-absorbing compounds was estimated by measuring the absorbance at 300 nm with a multimode microplate reader (TECAN Infinite 200 PRO NanoQuant, Switzerland). The values obtained in this analysis, calculated based on the leaf area, were used as an index of the relative concentration of UV-B-absorbing compounds.
Methylation-sensitive amplification polymorphism (MSAP) analysis
Methylation alterations in cytosine modification of G. longituba were detected with the MSAP method. The MSAP approach enables extensive analyses of epigenetic variation for a high number of individuals to be performed based on the use of the isoschizomers HpaII and MspI. The two isoschizomers recognise and cleave the same tetranucleotide sequence 5′-CCGG but differ in their sensitivity to the methylation state of cytosine (Pérez-Figueroa 2013; Schulz et al. 2013). MSAP analyses have become an important tool employed in the field of ‘ecological epigenetics’, which studies epigenetic processes in an ecological context (Bossdorf et al. 2008).
In this study, the leaf samples were scrubbed gently with C2H5OH–H2O (3:1, v/v) to minimise contamination by microorganisms and subsequently dried in silica gel for the subsequent extraction of DNA. Total genomic DNA was extracted from 30 mg dry leaves using BioTeKe (Beijing, China), and the quality of the DNA was determined by electrophoresis in agarose gel (1%, w/v). DNA concentration and purity were examined with a spectrophotometer (BioSpec-nano, Shimadzu, Japan). Next, the DNA concentration was uniformly diluted to 100 ng μL–1.
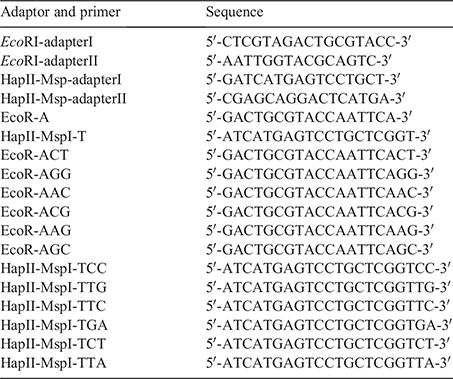
Genomic DNA was subjected to double-enzyme cleavage with the endonuclease combinations of 1 μL EcoRI + 1 μL HpaII (E+H) (NEB, USA) and 1 μL EcoRI + 2 μL MspI (E+M) (NEB, USA), and the digested ends were subsequently ligated with 1 μL HpaII-Msp-adaptor (50 pmol μL–1), 1 μL EcoRI adaptor (5 pmol μL–1) (Tsing Ke Biological Technology, China) and 0.5 μL T4 DNA ligase (TAKARA, Japan). Both the digestion and ligation reactions were performed in a final volume of 20 μL, and the quality of the reactions products was determined by electrophoresis in agarose gel (2%, w/v).
The 2 × Taq PCR MasterMix, preamplification and selective amplification primers used in the experiment were synthesised by Tsing Ke Biological Technology (China), and the sequences of adapters and primers are in Table 1. Both the preamplification and selective amplification reactions were performed in a final volume of 50 μL. A preamplification step was performed with EcoRl pre-selective primers and Hpall/Mspl pre-selective primers. The PCR mix contained 2 μL of ligated DNA, 21 μL of double distilled water, 1 μL of H-M pre-selective primer (10 μM), 1 μL of EcoRI pre-selective primer (10 μM), and 25 μL of 2 × Taq PCR MasterMix. The pre-amplification conditions were as follows: 72°C for 2 min; 94°C for 2 min; 20 cycles at 94°C for 30 s, 56°C for 30 s and 72°C for 1 min; and a final elongation step at 72°C for 10 min. The samples were subsequently examined by electrophoresis in agarose gel (1.5%, w/v).
|
The preamplification products were diluted 10 times as a selective amplification template. A selective amplification step was performed with 11 pairs of selective primer combinations, including the following: EcoRI-AAG/HpaII-TGA, EcoRI-AAG/HpaII-TTA, EcoRI-AAG/HpaII-TTG, EcoRI-ACT/HpaII-TCC, EcoRI-ACT/HpaII-TTG, EcoRI-AGG/HpaII-TTC, EcoRI-AGG/HpaII-TGA, EcoRI-AGG/HpaII-TCC, EcoRI-AGG/HpaII-TTG, EcoRI-ACG/HpaII-TTG, and EcoRI-AGC/HpaII-TCC. The PCR mix contained 1 μL of pre-amplified DNA, 22 μL of double distilled water, 1 μL of H-M selective primer (10 μM), 1 μL of EcoRl selective primer (10 μM), and 25 μL of 2 × Taq PCR MasterMix. The selective amplification conditions were as follows: 94°C for 2 min; 10 cycles at 94°C for 30 s; 65°C for 30 s and 72°C for 1 min (each cycle was decremented by 1°C); 23 cycles at 94°C for 30 s; 56°C for 30 s and 72°C for 1 min; and a final elongation step at 72°C for 10 min. The selective amplification samples were examined by electrophoresis in agarose gel (1.5%, w/v) and separated by 10% denaturing polyacrylamide gel electrophoresis (220 V, 4 h), and the gel was subjected to silver staining. Following staining of the gel, it was rinsed, developed, and photographed, and statistical analysis of the band was performed.
Statistical analyses
Growth traits and UV-B-absorbing compounds
Considering the slight difference among the initial biomass of parental ramets, analysis of covariance (ANCOVA) was performed to determine the effect of the parental UV-B environment on the growth traits (leaf area, leaf biomass, SLA, total biomass) of parental ramets and traits (total biomass, leaf biomass allocation, and stolon biomass allocation) of genets. Moreover, the differences in ramet biomass, SLA, leaf biomass and UV-B-absorbing compound content among different ramets (Pa, O1, O2, O5 and OY) in the same treatments were analysed through one-way analysis of variance (ANOVA). Duncan’s test was used to test the difference among treatments or ramets. All analyses were conducted with Statistica 10.0 software (Statsoft, USA). The confidence coefficient was set at P < 0.05. Analytical mapping was performed using Origin Pro 9.1 software (OriginLab, USA).
DNA methylation variation
Fragments of the MSAP products (~100–500 bp) in polyacrylamide gel were scored to analyse the DNA methylation variation. From the fragment presence/absence score matrix of both enzymatic reactions, the methylated state of every locus (5′-CCGG target) was assessed as follows: the presence of both EcoRI–HpaII and EcoRI–MspI products (1/1) denotes an unmethylated state; the presence of only one of the EcoRI–HpaII (1/0) or EcoRI–MspI (0/1) products represents methylated states (hemimethylated or internal methylation); and the absence of both EcoRI–HpaII and EcoRI–MspI products (0/0) was considered an uninformative state, as it could be caused by either fragment absence or hypermethylation (Salmon et al. 2008).
The effect of UV-B radiation on parental DNA methylation level and DNA methylation difference between parent and their offspring were tested using pairwise analyses of molecular variance (AMOVA), which estimates Phi-st as fixation index (an analogue of Fst for molecular data, Excoffier et al. 1992) by means of the ‘pegas’ package for R software (RStudio, New Zealand).
The binary matrix of MSAP data was analysed with the ‘msap’ package for R software. The percentages of unmethylated target DNA, hemimethylated target DNA, fully methylated target DNA and absent target DNA were calculated. The total methylation level (%) was calculated by dividing MSAP bands representing methylated 5′-CCGG sites (differential presence/absence of restricted fragments in HpaII and MspI assays) against the total number of scored bands (Liu et al. 2012).
Results
Effects of UV-B on the DNA methylation of the parental ramet
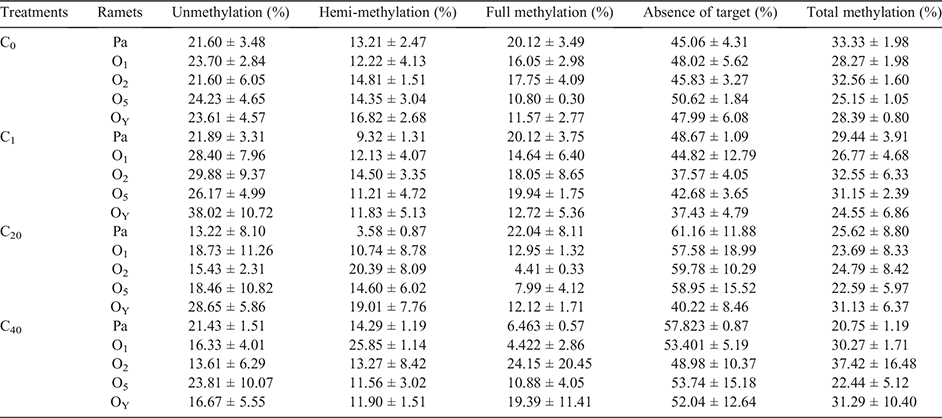
To investigate changes of DNA methylation by UV-B radiation, we tested total DNA methylation of parental and offspring ramets in different treatments by MSAP method. All fragments of the MSAP products (~100–500 bp) in the polyacrylamide gel were analysed with pairwise analysis. Table 2 was the results of parental ramets, compared with the C0 group, UV-B stress had a significant effect on parental DNA methylation levels (Table 2). Total DNA methylation level of parental ramets was reduced by direct UV-B stress, and the decrease was more significant with the increase in the number of stress events; thus, the total DNA methylation level of the C40 group was the lowest. The C20 group had the lowest unmethylated and hemimethylated levels, while the C40 group had the lowest fully methylated levels (Table 3).

|
Moreover, there was a significant difference in the DNA methylation level between the C1 and C40 groups (P < 0.05) and a marginally significant difference between the C1 and C20 groups (P = 0.0594), but there was no significant difference between the C20 and C40 groups (P = 0.1017) (Table 2).
Transgenerational effects of DNA methylation induced by UV-B stress
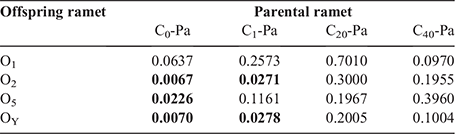
The DNA methylation percentages of parental and offspring ramets in different treatments are in Table 3. Pairwise analysis results indicated that there was a significant difference in DNA methylation levels between parental ramets and their offspring in the C0 and C1 groups; specifically, in the C0 group, a difference was observed between parental ramets (C0-Pa) and their offspring (O2, O5 and OY) (P < 0.05), except for the first offspring ramet (O1). In the C1 group, a difference was observed between the parental ramets (C1-Pa) and the O2 and OY groups. However, no significant difference was found between parental ramets and all their offspring ramets in the C20 and C40 treatments (P > 0.05) (Table 4).
|
Growth of parental ramets induced by direct UV-B stress
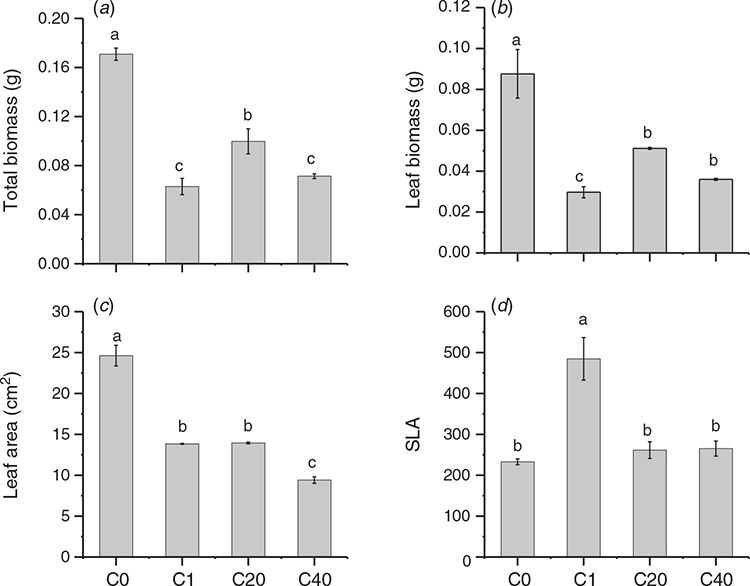
The effect of direct UV-B on the growth of parental ramets was analysed with total biomass, leaf biomass, leaf area and SLA of parent. The results displayed that UV-B significantly affected the growth traits of parental ramets (Fig. 2). The total biomass, leaf biomass and leaf area decreased in all UV-B stress groups, regardless of the number of stress events. The smallest leaf area and leaf biomass were observed in the C40 and C1 groups, respectively, while the highest SLA value was observed in the C1 group.
Changes in growth characteristics among ramets induced by UV-B stress in the parent
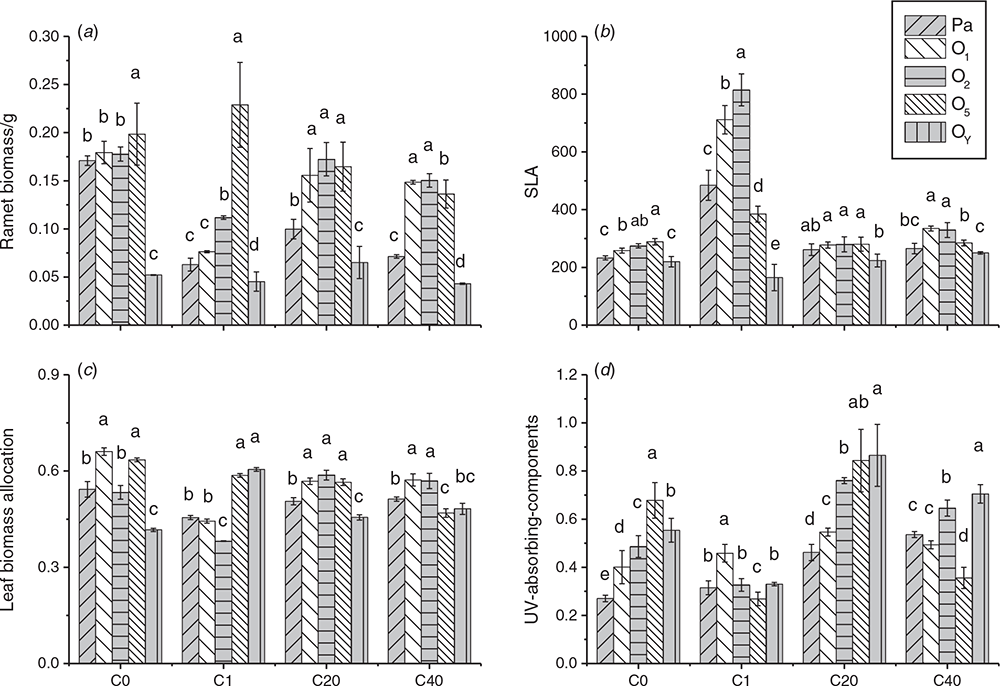
In order to analyse the effect of parental UV-B radiation on growth and defense of different ramets, changes in growth traits (biomass, leaf biomass allocation, SLA) and defensive substance (UV-B-absorbing compounds) content among parental ramets and their offspring in the longest primary stolon are depicted in Fig. 3. The maximum ramet biomass in both the C0 and C1 groups was observed at the O5 ramet, while that in the C20 group appeared in the O1, O2 and O5 ramets, and that in the C40 group appeared in the O1 and O2 ramets (Fig. 3a). Maximum leaf biomass allocation was exhibited by the O1 and O5 ramets in the C0 group and by the O5 and OY ramets in C1. In C20 and C40, maximum leaf allocation exhibited the same change as the total biomass of ramets (Fig. 3b). Moreover, maximum SLA of C0 appeared in O2 and O5 ramets, that of the C40 group was exhibited by the O1 and O2 ramets, but that of the C1 group was only in the O2 ramet, while each ramet in the C20 group had a similar SLA value (Fig. 3c).
Changes in the UV-B-absorbing compound content of parental ramets and their offspring in various treatments are in Fig. 3d. Clearly, the UV-B-absorbing compound content of the parental ramet improved with increasing stress, and the maximum content appeared in the C40 group. The comparison of the UV-B-absorbing compounds level of different ramets in each treatment indicated that the maximum value in the C0 group was exhibited by the O5 ramet; in the C1 group, it was the O1 ramet; in the C40 group, it was the OY ramet; and in the C20 group, it was the O5 and OY ramets.
Biomass allocation of clonal genets induced by parental UV-B stress
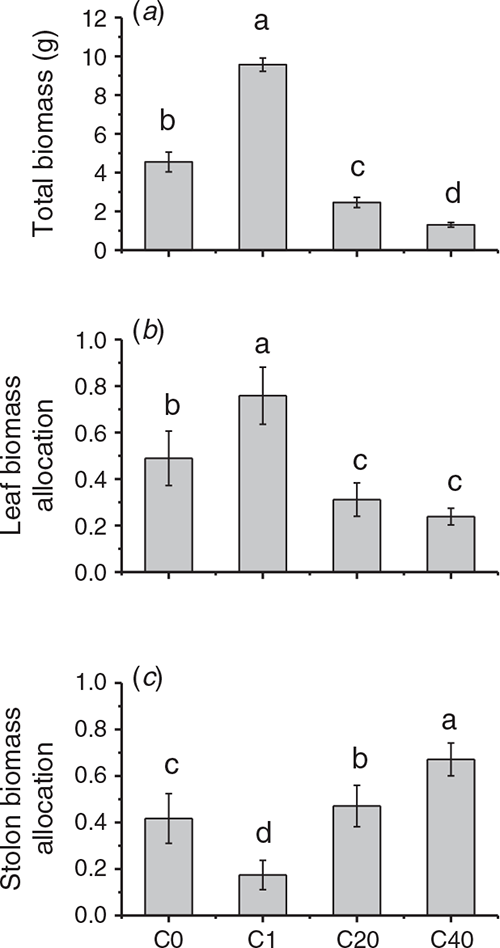
We also tested the effect of parental UV-B on biomass allocation of clonal genets, results showed that different UV-B radiation treatments affected the growth of the clonal genet in different ways (Fig. 4). One UV-B stress group (C1 group) exhibited a significant increase in genet total biomass, and this increase was primarily due to the increase in leaf allocation and the decrease in stem allocation. However, the total biomass of genet was clearly decreased in the C20 and C40 groups, which was due to the increase in stem allocation and reduction in leaf biomass allocation, and this change was most pronounced in the C40 group.
|
Discussion
Epigenetic memory and its transgenerational effects induced by recurrent UV-B stress
Absorption of UV-B radiation by DNA induces the formation of cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6–4) pyrimidone photoproducts (6–4PPs), and plants have various DNA repair systems to remove or tolerate these DNA lesions (Qüesta et al. 2013). Active DNA demethylation is a DNA repair-related mechanism; for example, decreased DNA methylation 1 (DDM1), a nucleosome remodeller involved in the maintenance of DNA methylation, and ROS1, a primary factor required for active DNA demethylation, have been determined to be involved in UV-B DNA damage repair. Furthermore, in mammals, nucleotide excision repair (NER) has been observed to contribute to active DNA demethylation at particular loci to regulate gene expression (Molinier 2017). In our study, the total DNA methylation level of parental ramets decreased significantly by direct UV-B radiation, even though the parent was encountering UV-B radiation for the first time. Decreased DNA methylation caused by UV-B stress was also reported in such species such as Picea abies L. (Norway spruce; Ohlsson et al. 2013), Zea mays L. (Sokolova et al. 2014), and Artemisia annua L. (Pandey and Pandey-Rai 2015). Similar to the findings reported by other groups, this decrease is probably related to the pathways used to repair UV-induced DNA lesions, which means that a new DNA strand is synthetised, and the associated epigenetic marks must be properly re-established for the accurate maintenance of the DNA methylome landscape after UV exposure (Molinier 2017; Graindorge et al. 2019).
Changes in DNA methylation caused by UV-B radiation can be considered as epigenetic memory, and many studies have reported that some forms of epigenetic memory can be inherited across generations, while others cannot (Chinnusamy and Zhu 2009). For clonal plants, every newborn ramet can be regarded as a new asexual offspring. In our study, the transgenerational effect of UV-B-induced epigenetic memory was related to the number of stress repetitions. a single stress induced short-term memory, epigenetic modification was exhibited only by the parent and their first offspring ramet and was reprogrammed in later generations. While multiple recurring stresses led to long-term memory, the modification of the parent was transferred to their last offspring. The effects of chronergy on epigenetic memory, including such phenomena as vernalisation, have also been reported in many studies, epigenetic variations in Arabidopsis thaliana L. caused by winter cold were maintained only in the current generation and were reprogrammed in the next generation. Moreover, in clonal white clover (Trifolium repens L.) transgenerational effects of epigenetic memory caused by drought stress were observed only in situations in which parental ramets experienced stress for a short period (González et al. 2016). Important in our study, the epigenetic variation among ramets caused by single UV-B stress was complex, and we interpreted this variation to be the combined effect of the transient transgenerational effect of epigenetic variation by single UV-B stress and clonal integration among ramets. For the existence of connected stolons, substances and information can be transferred among ramet nets; therefore, the growth and epigenetic variation of newborn offspring ramets were affected by both environments of their own and their connected elder ramets. For the transient epigenetic variation of the elder ramets, the effect of the offspring’s environment was dominant, and the methylation level of the following offspring was mainly affected by the environment of the offspring itself.
Research on transgenerational epigenetic inheritance has indicated that transgenerational effects could alter the behaviour of plants and may improve the adaptation of offspring to changing environments (Chinnusamy and Zhu 2009; Hauser et al. 2011; McIntyre and Strauss 2014; Preite et al. 2018). It has been hypothesised that the possible results of epigenetic memory and their transgenerational effect in clonal plants are pre-programming of offspring phenotypes for specific types of growth based on the previous interplay of the environment with the genet. This effect may significantly increase the overall success of the genet, particularly when environmental conditions are predictable (Douhovnikoff and Dodd 2015; Latzel et al. 2016; Münzbergová and Hadincová 2017). The changes in growth and defensive traits accompanied by low methylation induced by UV-B stress in our study are discussed below.
Changes in growth traits induced by recurrent UV-B stress in the parent
Growth of parental ramets
The change in DNA methylation level is regarded as an important regulatory mechanism for plants under various environmental stresses because it can lead to a significant change in some important traits (Liang et al. 2019). For example, the hypomethylation of mangrove individuals near salt marshes resulted in a more significant reduction in morphological traits (tree height, tree diameter, leaf width and leaf area) than the individuals located near rivers (Lira-Medeiros et al. 2010). Lower DNA methylation levels were induced in environments exhibiting coldness, salinity, aluminium toxicity, heavy metal, and UV-B stress conditions (Steward 2002; Choi and Sano 2007; Wang et al. 2011). In some studies, DNA demethylation was regarded as closely related to stress adaptability (Steward 2002; Wada et al. 2004; Liang et al. 2019); however, in other reports, increased DNA methylation levels were considered to be a stress-adaptive response (Yaish et al. 2018). In our research, the lower DNA methylation of parental ramets induced by direct UV-B stress was correlated with a decrease in their investment of the ramets in growth traits (leaf area, leaf biomass and total biomass) and with an increased investment in defensive substances (UV-B-absorbing compounds). These changes can be regarded as an adaptive reaction to UV-B stress. For instance, although the biomass of parental ramets was decreased, some strategies were beneficial to plants living in the UV-B environment, such as the decrease in leaf area and the increase in UV-B-absorbing compounds, and the change trend of these traits was intensified with increased number of UV-B stress events. Leaf area is very sensitive to the light environment, and UV-B radiation can influence leaf expansion and development by reducing cell division (Wargent et al. 2009). The reduction in leaf area can reduce the absorption of UV-B radiation to reduce damage, and this reduction increased significantly as the number of UV-B stress events increased in our study. The accumulation of UV-B-absorbing compounds is the most important protective response against UV-B stress, as these compounds can leach UV-B before it reaches sensitive molecules (Liu et al. 2015).
Growth of the offspring ramets
Our study showed that although only parental ramets were exposed to UV-B radiation, the growth and defence of clonal genet were affected. In all treatments, the maximum values of these traits all appeared in offspring ramets, rather than parental ramets, but the parental effect on offspring ramets varied under different UV-B conditions. If the parental ramet encountered 20 UV-B treatments, all the offspring ramets (except for the final ramet) had the same high level of SLA, leaf biomass and total biomass, and the youngest offspring ramets were excluded because they were not yet fully grown and could not have mature functional leaves. When parents encountered 40 UV-B treatments, the highest values of these parameters were assigned to the older offspring ramets, but in single UV-B treatment conditions, the maximum values of these growth indexes did not show regularity.
For defensive investment, the maximum content of UV-B-absorbing components was observed to the older ramet only after stress occurred, in the younger ramets in the control and higher-frequency stress environments, and even in the youngest offspring ramets under higher-frequency recurrent stress environments, although these ramets were still immature under that condition.
Growth of the genet
For the growth of the clonal genet, the total biomass decreased significantly if the parent was in the higher-frequency stress environments (C20 and C40). G. longituba responded to recurrent UV-B stress by distributing more biomass to the stem to store nutrients and decreasing the leaf biomass allocation to reduce the absorbance of UV-B light. In contrast, the genet biomass was increased if G. longituba was exposed to a single UV-B stress (C1). The genet increased the biomass allocation to leaves to capture more ambient light (Fig. 4). This result may seem puzzling, as it is contrary to the results obtained for recurring UV-B stress events. In fact, this opposite effect was closely related to the double effect of UV-B radiation, which means that exposure of plants to a stressor can cause reversible elastic eustress or a predominantly negative distress effect. In our study, the single UV-B stress can be regarded as a eustress, while repeated UV-B stress can be regarded as a distress. Eustress is an activating, stimulating stress that is a positive element in plant development; when a plant experiences mild, elastic eustress, its metabolism is adjusted, and the plant acclimates to the new environment (Kranner et al. 2010; Hideg et al. 2013). Thus, the total biomass of genets receiving a single UV-B treatment was increased, which was most likely related to the significant increase in SLA in almost every ramet and the improvement in leaf biomass allocation at the genet level. SLA is a trait that is positively correlated with the relative growth rate, and high SLA and leaf allocation cannot only capture more visible light in greenhouse conditions but can also obtain more UV-B light. Proper UV-B is an important regulator in plant growth and development, which is beneficial for the growth of plants in greenhouse conditions. Repeated UV-B stress acted as a severe stress that was regarded as ‘destructive stress’. Distressed plants produce elevated levels of reactive oxygen species (ROS), which may disrupt metabolic activities and influence plant growth and lead to a significant decrease in genet biomass in our study. Consequently, the strategies employed by clonal plants in various UV-B environments were different.
Overall, although only parental ramets were exposed to UV-B radiation, the growth behaviour of offspring ramets and genet was affected. One possible mechanism governing this effect was the modification of DNA methylation in parental ramets and their transgenerational effects. Epigenetic mechanisms have been proposed to be important in plasticity, enabling environmental exposure to shape future gene expression (Douhovnikoff and Dodd 2015). Epigenetic inheritance and its transgenerational effects may explain the heritable effects of environments on offspring phenotypes, which was proven by the modifications of DNA methylation and phenotype of Taraxacum officinale Weber ex Wigg. (Verhoeven et al. 2010; Verhoeven and van Gurp 2012), Alternanthera philoxeroides (Mart.) Griseb. (Dong et al. 2019) and T. repens (Rendina González et al. 2016, 2018). Furthermore, it was noted that if transgenerational effects are a significant component of the clonal lifestyle, it is possible that the growth and behaviour of an emerging clonal plant might be significantly changed by environments that had been experienced by the parental plant (Huber et al. 2014). Our study supported this possibility; that is, the transgenerational effect of epigenetic memory and phenotypic plasticity of the parental ramet’s UV-B experience ultimately influenced the behaviour of offspring and growth of the genet. For instance, the flavonoid (UV-B-absorbing compounds) biosynthetic pathway and related genes are overexpressed under UV-B exposure through DNA hypomethylation (Pandey et al. 2019). For G. longituba that were repeatedly exposed to UV-B in this study (C20 group), the reduced DNA methylation of parental ramets induced by UV-B was maintained until the final offspring, and the increase in UV-B-absorbing compounds in parental ramets was also maintained until the last offspring ramets, although they were not exposed to UV-B radiation. Although not all plasticity is adaptive, the fitness of plants may depend on adaptive phenotypic plasticity to adapt to predictable environmental variation and to minimise their risk in coping with unpredictable environmental variation (Willing et al. 2016).
Conclusion
The results of our experiments suggested that the parental ramets of clonal G. longituba could record their UV-B radiation experience and form epigenetic memory via a decrease in DNA methylation levels, even when they encountered UV-B stress for the first time. The degree of decrease in the methylation level increased gradually with an increasing number of repeated stress events. Moreover, the chronergy of epigenetic inheritance depended on the parental ramet’s stress experience. The epigenetic memory of a single UV-B was short-term, while that of recurrent stress was long-term. Furthermore, the UV-B stress of parents had a significant influence on the growth of their offspring and that of the genet, parental single or recurrent stress experience had different effects on the growth and defensive of the genet. Further, the growth allocation among offspring ramet was diverse under different frequency of recurrent stress. The mechanism underlying the effects of parental environments on offspring behaviours can be explained by the double effect of UV-B radiation, epigenetic memory of parents and its transgenerational effects.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was supported by the National Natural Science Foundation of China (31200249), and National Special Program on Basic Works for Science and Technology of China (2015FY1103003–6).
References
Avramova Z (2015) Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress‐response genes. The Plant Journal 83, 149–159.| Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress‐response genes.Crossref | GoogleScholarGoogle Scholar | 25788029PubMed |
Bossdorf O, Richards C, Pigliucci M (2008) Epigenetics for ecologists. Ecology Letters 11, 106–115.
Chinnusamy V, Zhu JK (2009) Epigenetic regulation of stress responses in plants. Current Opinion in Plant Biology 12, 133–139.
| Epigenetic regulation of stress responses in plants.Crossref | GoogleScholarGoogle Scholar | 19179104PubMed |
Choi CS, Sano H (2007) Abiotic-stress induces demethylation and transcriptional activation of a gene encoding a glycerophosphodiesterase-like protein in tobacco plants. Molecular Genetics and Genomics 277, 589–600.
| Abiotic-stress induces demethylation and transcriptional activation of a gene encoding a glycerophosphodiesterase-like protein in tobacco plants.Crossref | GoogleScholarGoogle Scholar | 17273870PubMed |
Cloix C, Jenkins GI (2008) Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin. Molecular Plant 1, 118–128.
| Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin.Crossref | GoogleScholarGoogle Scholar | 20031919PubMed |
Ćuk K, Gogala M, Tkalec M, Vidakovic’Cifrek Z (2010) Transgenerational stress memory in Arabidopsis thaliana (L.) heynh.: antioxidative enzymes and HSP70. Acta Botanica Croatica 69, 183–197.
de Freitas Guedes FA, Nobres P, Ferreira DCR, Menezes-Silva PE, Ribeiro-Alves M, Correa RL, DaMatta FM, Alves-Ferreira M (2018) Transcriptional memory contributes to drought tolerance in coffee (Coffea canephora) plants. Environmental and Experimental Botany 147, 220–233.
| Transcriptional memory contributes to drought tolerance in coffee (Coffea canephora) plants.Crossref | GoogleScholarGoogle Scholar |
Ding Y, Virlouvet L, Liu N, Riethoven JJ, Fromm M, Avramova Z (2014) Dehydration stress memory genes of Zea mays; comparison with Arabidopsis thaliana. BMC Plant Biology 14, 141
| Dehydration stress memory genes of Zea mays; comparison with Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 24885787PubMed |
Dong BC, Meng J, Yu FH (2019) Effects of parental light environment on growth and morphological responses of clonal offspring. Plant Biology 21, 1083–1089.
| Effects of parental light environment on growth and morphological responses of clonal offspring.Crossref | GoogleScholarGoogle Scholar | 31054216PubMed |
Douhovnikoff V, Dodd RS (2015) Epigenetics: a potential mechanism for clonal plant success. Plant Ecology 216, 227–233.
| Epigenetics: a potential mechanism for clonal plant success.Crossref | GoogleScholarGoogle Scholar |
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131, 479–491.
| Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data.Crossref | GoogleScholarGoogle Scholar | 1644282PubMed |
Gagliano M, Renton M, Depczynski M, Mancuso S (2014) Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 175, 63–72.
| Experience teaches plants to learn faster and forget slower in environments where it matters.Crossref | GoogleScholarGoogle Scholar | 24390479PubMed |
González APR, Chrtek J, Dobrev PI, Dumalasová V, Fehrer J, Mráz P, Latzel V (2016) Stress‐induced memory alters growth of clonal offspring of white clover (Trifolium repens). American Journal of Botany 103, 1567–1574.
| Stress‐induced memory alters growth of clonal offspring of white clover (Trifolium repens).Crossref | GoogleScholarGoogle Scholar |
Graindorge S, Cognat V, Berens PJT, Mutterer J, Molinier J (2019) Photodamage repair pathways contribute to the accurate maintenance of the DNA methylome landscape upon UV exposure. PLOS Genetics 15, e1008476
| Photodamage repair pathways contribute to the accurate maintenance of the DNA methylome landscape upon UV exposure.Crossref | GoogleScholarGoogle Scholar | 31738755PubMed |
Hauser MT, Aufsatz W, Jonak C, Luschnig C (2011) Transgenerational epigenetic inheritance in plants. Biochimica et Biophysica Acta 1809, 459–468.
| Transgenerational epigenetic inheritance in plants.Crossref | GoogleScholarGoogle Scholar | 21515434PubMed |
Hideg É, Jansen MAK, Strid K (2013) UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends in Plant Science 18, 107–115.
| UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates?Crossref | GoogleScholarGoogle Scholar | 23084465PubMed |
Huber H, Visser EJW, Clements G, Peters JL (2014) Flooding and fragment size interact to determine survival and regrowth after fragmentation in two stoloniferous Trifolium species. AoB Plants 6, plu024
| Flooding and fragment size interact to determine survival and regrowth after fragmentation in two stoloniferous Trifolium species.Crossref | GoogleScholarGoogle Scholar | 24887003PubMed |
Ikeda Y (2012) Plant imprinted genes identified by genome-wide approaches and their regulatory mechanisms. Plant & Cell Physiology 53, 809–816.
| Plant imprinted genes identified by genome-wide approaches and their regulatory mechanisms.Crossref | GoogleScholarGoogle Scholar |
Kataria S, Jajoo A, Guruprasad KN (2014) Impact of increasing ultraviolet-B (UV-B) radiation on photosynthetic processes. Journal of Photochemistry and Photobiology. B, Biology 137, 55–66.
| Impact of increasing ultraviolet-B (UV-B) radiation on photosynthetic processes.Crossref | GoogleScholarGoogle Scholar | 24725638PubMed |
Kim JM, To TK, Seki M (2012) An epigenetic integrator: new insights into genome regulation, environmental stress responses and developmental controls by HISTONE DEACETYLASE 6. Plant & Cell Physiology 53, 794–800.
| An epigenetic integrator: new insights into genome regulation, environmental stress responses and developmental controls by HISTONE DEACETYLASE 6.Crossref | GoogleScholarGoogle Scholar |
Kinoshita T, Seki M (2014) Epigenetic memory for stress response and adaptation in plants. Plant & Cell Physiology 55, 1859–1863.
| Epigenetic memory for stress response and adaptation in plants.Crossref | GoogleScholarGoogle Scholar |
Köhler C, Wolff P, Spillane C (2012) Epigenetic mechanisms underlying genomic imprinting in plants. Annual Review of Plant Biology 63, 331–352.
| Epigenetic mechanisms underlying genomic imprinting in plants.Crossref | GoogleScholarGoogle Scholar | 22404470PubMed |
Kranner I, Minibayeva FV, Beckett RP, Seal CE (2010) What is stress? Concepts, definitions, and applications in seed science. New Phytologist 188, 655–673.
| What is stress? Concepts, definitions, and applications in seed science.Crossref | GoogleScholarGoogle Scholar |
Latzel V, Jitka K (2010) Transgenerational plasticity in clonal plants. Evolutionary Ecology 24, 1537–1543.
| Transgenerational plasticity in clonal plants.Crossref | GoogleScholarGoogle Scholar |
Latzel V, Rendina González AP, Jonathan R (2016) Epigenetic memory as a basis for intelligent behavior in clonal plants. Frontiers in Plant Science 7, 1354
| Epigenetic memory as a basis for intelligent behavior in clonal plants.Crossref | GoogleScholarGoogle Scholar | 27630664PubMed |
Li X, Liu F (2016) Drought stress memory and drought stress tolerance in plants: biochemical and molecular basis. In ‘Drought Stress Tolerance in Plants, Volume 1.’ (Eds Hossain MA, Wani SH, Bhattacharjee S, Burritt DJ, Tran LSP) pp. 17–44. (Springer International Publishing: Switzerland)
Li Q, Liu X, Yue M, Tang WT, Meng QC (2011a) Response of physiological integration in Trifolium repens to heterogeneity of UV-B radiation. Flora 206, 712–719.
| Response of physiological integration in Trifolium repens to heterogeneity of UV-B radiation.Crossref | GoogleScholarGoogle Scholar |
Li Q, Liu X, Yue M, Zhang XF, Zhang RC (2011b) Effects of physiological integration on photosynthetic efficiency of Trifolium repens in response to heterogeneous UV-B radiation. Photosynthetica 49, 539–545.
| Effects of physiological integration on photosynthetic efficiency of Trifolium repens in response to heterogeneous UV-B radiation.Crossref | GoogleScholarGoogle Scholar |
Li P, Yang H, Wang L, Liu HJ, Huo HQ, Zhang CJ, Liu AZ, Zhu AD, Hu JY, Liu YJ, Liu L (2019) Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Frontiers in Genetics 10, 55
| Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice.Crossref | GoogleScholarGoogle Scholar | 30800142PubMed |
Liang X, Hou X, Li J, Han Y, Zhang YX, Feng NJ, Du J, Zhang WH, Zheng DF, Fang SM (2019) High-resolution DNA methylome reveals that demethylation enhances adaptability to continuous cropping comprehensive stress in soybean. BMC Plant Biology 19, 79
| High-resolution DNA methylome reveals that demethylation enhances adaptability to continuous cropping comprehensive stress in soybean.Crossref | GoogleScholarGoogle Scholar | 30777019PubMed |
Lira-Medeiros CF, Parisod C, Fernandes RA, Mata CS, Cardoso MA, Ferreira PCG (2010) Epigenetic variation in mangrove plants occurring in contrasting natural environment. PLoS One 5, e10326
| Epigenetic variation in mangrove plants occurring in contrasting natural environment.Crossref | GoogleScholarGoogle Scholar | 20436669PubMed |
Liu S, Sun K, Jiang T, Ho JP, Liu B, Feng J (2012) Natural epigenetic variation in the female great roundleaf bat (Hipposideros armiger) populations. Molecular Genetics and Genomics 287, 643–650.
| Natural epigenetic variation in the female great roundleaf bat (Hipposideros armiger) populations.Crossref | GoogleScholarGoogle Scholar | 22773086PubMed |
Liu X, Li Q, Yue M, Zhang X, Zhang R, Zhang B, Wang M (2015) Nitric oxide is involved in integration of UV-B absorbing compounds among parts of clonal plants under a heterogeneous UV-B environment. Physiologia Plantarum 155, 180–191.
| Nitric oxide is involved in integration of UV-B absorbing compounds among parts of clonal plants under a heterogeneous UV-B environment.Crossref | GoogleScholarGoogle Scholar | 25424287PubMed |
McIntyre PJ, Strauss SY (2014) Phenotypic and transgenerational plasticity promote local adaptation to sun and shade environments. Evolutionary Ecology 28, 229–246.
| Phenotypic and transgenerational plasticity promote local adaptation to sun and shade environments.Crossref | GoogleScholarGoogle Scholar |
McKenzie RL, Aucamp PJ, Bais AF, Bjorn LO, Ilyas M, Madronich S (2011) Ozone depletion and climate change: impacts on UV radiation. Photochemical & Photobiological Sciences 10, 182–198.
| Ozone depletion and climate change: impacts on UV radiation.Crossref | GoogleScholarGoogle Scholar |
Molinier J (2017) Genome and epigenome surveillance processes underlying UV exposure in plants. Genes 8, 316
| Genome and epigenome surveillance processes underlying UV exposure in plants.Crossref | GoogleScholarGoogle Scholar |
Müller-Xing R, Xing Q, Goodrich J (2014) Footprints of the sun: memory of UV and light stress in plants. Frontiers in Plant Science 5, 474
Münzbergová Z, Hadincová V (2017) Transgenerational plasticity as an important mechanism affecting response of clonal species to changing climate. Ecology and Evolution 7, 5236–5247.
| Transgenerational plasticity as an important mechanism affecting response of clonal species to changing climate.Crossref | GoogleScholarGoogle Scholar | 28770062PubMed |
Ohlsson AB, Segerfeldt P, Lindstrom A, Borg-Karlson AK, Berglund T (2013) UV-B exposure of indoor-grown Picea abies seedlings causes an epigenetic effect and selective emission of terpenes. Zeitschrift fur Naturforschung. C, Journal of Biosciences 68, 139–147.
Pandey N, Pandey-Rai S (2015) Deciphering UV-B-induced variation in DNA methylation pattern and its influence on regulation of DBR2 expression in Artemisia annua L. Planta 242, 869–879.
| Deciphering UV-B-induced variation in DNA methylation pattern and its influence on regulation of DBR2 expression in Artemisia annua L.Crossref | GoogleScholarGoogle Scholar | 25998525PubMed |
Pandey N, Goswami N, Tripathi D, Rai KK, Rai SK, Singh S, Pandey-Rai S (2019) Epigenetic control of UV-B-induced flavonoid accumulation in Artemisia annua L. Planta 249, 497–514.
| Epigenetic control of UV-B-induced flavonoid accumulation in Artemisia annua L.Crossref | GoogleScholarGoogle Scholar | 30267151PubMed |
Pascual J, Caňal MJ, Correia B, Escandon M, Hasbún R, Meijón M, Pinto G, Valledor L (2014) Can epigenetics help forest plants to adapt to climate change? In ‘Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications.’ (Eds Alvarez-Venegas R, De la Peña C, Casas-Mollano JA) pp. 125–146. (Springer International Publishing: Switzerland)
Paszkowski J, Grossniklaus U (2011) Selected aspects of transgenerational epigenetic inheritance and resetting in plants. Current Opinion in Plant Biology 14, 195–203.
| Selected aspects of transgenerational epigenetic inheritance and resetting in plants.Crossref | GoogleScholarGoogle Scholar | 21333585PubMed |
Pérez-Figueroa A (2013) Msap: a tool for the statistical analysis of methylation-sensitive amplified polymorphism data. Molecular Ecology Resources 13, 522–527.
| Msap: a tool for the statistical analysis of methylation-sensitive amplified polymorphism data.Crossref | GoogleScholarGoogle Scholar | 23311622PubMed |
Preite V, Oplaat C, Biere A, Kirschner J, van der Putten WH, Verhoeven KJF (2018) Increased transgenerational epigenetic variation, but not predictable epigenetic variants, after environmental exposure in two apomictic dandelion lineages. Ecology and Evolution 8, 3047–3059.
| Increased transgenerational epigenetic variation, but not predictable epigenetic variants, after environmental exposure in two apomictic dandelion lineages.Crossref | GoogleScholarGoogle Scholar | 29531716PubMed |
Qüesta JI, Walbot V, Casati P (2010) Mutator transposon activation after UV-B involves chromatin remodeling. Epigenetics 5, 352–363.
| Mutator transposon activation after UV-B involves chromatin remodeling.Crossref | GoogleScholarGoogle Scholar | 20421734PubMed |
Qüesta JI, Fina JP, Paula C (2013) DDM1 and ROS1 have a role in UV-B induced- and oxidative DNA damage in A. thaliana. Frontiers in Plant Science 4, 420
| DDM1 and ROS1 have a role in UV-B induced- and oxidative DNA damage in A. thaliana.Crossref | GoogleScholarGoogle Scholar | 24155752PubMed |
Ramírez DA, Rolando JL, Yactayo W, Monneveux P, Mares V, Quiroz R (2015) Improving potato drought tolerance through the induction of long-term water stress memory. Plant Science 238, 26–32.
| Improving potato drought tolerance through the induction of long-term water stress memory.Crossref | GoogleScholarGoogle Scholar | 26259171PubMed |
Rendina González AP, Chrtek J, Dobrev PI, Dumalasová V, Fehrer J, Mráz P, Latzel V (2016) Stress‐induced memory alters growth of clonal offspring of white clover (Trifolium repens). American Journal of Botany 103, 1567–1574.
| Stress‐induced memory alters growth of clonal offspring of white clover (Trifolium repens).Crossref | GoogleScholarGoogle Scholar |
Rendina González AP, Preite V, Verhoeven KJF, Latzel V (2018) Transgenerational effects and epigenetic memory in the clonal plant Trifolium repens. Frontiers in Plant Science 9, 1677
| Transgenerational effects and epigenetic memory in the clonal plant Trifolium repens.Crossref | GoogleScholarGoogle Scholar | 30524458PubMed |
Rius SP, Emiliani J, Casati P (2016) P1 epigenetic regulation in leaves of high altitude maize landraces: effect of UV-B radiation. Frontiers in Plant Science 7, 523
| P1 epigenetic regulation in leaves of high altitude maize landraces: effect of UV-B radiation.Crossref | GoogleScholarGoogle Scholar | 27148340PubMed |
Robson TM, Klem K, Urban O, Jansen MAK (2015) Re-interpreting plant morphological responses to UV-B radiation. Plant, Cell & Environment 38, 856–866.
| Re-interpreting plant morphological responses to UV-B radiation.Crossref | GoogleScholarGoogle Scholar |
Salmon A, Clotault J, Jenczewski E, Chable V, Manzanares-Dauleux MJ (2008) Brassica oleracea displays a high level of DNA methylation polymorphism. Plant Science 174, 61–70.
| Brassica oleracea displays a high level of DNA methylation polymorphism.Crossref | GoogleScholarGoogle Scholar |
Saze H, Tsugane K, Kanno T, Nishimura T (2012) DNA methylation in plants: relationship to small RNAs and histone modifications, and functions in transposon inactivation. Plant & Cell Physiology 53, 766–784.
| DNA methylation in plants: relationship to small RNAs and histone modifications, and functions in transposon inactivation.Crossref | GoogleScholarGoogle Scholar |
Schulz B, Eckstein LR, Durka W (2013) Scoring and analysis of methylation-sensitive amplification polymorphisms for epigenetic population studies. Molecular Ecology Resources 13, 642–653.
| Scoring and analysis of methylation-sensitive amplification polymorphisms for epigenetic population studies.Crossref | GoogleScholarGoogle Scholar | 23617735PubMed |
Sokolova D, Vengzhen G, Kravets A (2014) The effect of DNA methylation modification polymorphism of corn seeds on their germination rate, seedling resistance and adaptive capacity under UV-C exposure. American Journal of Plant Biology 1, 1–14.
Song J, Irwin J, Dean C (2013) Remembering the prolonged cold of winter. Current Biology 23, R807–R811.
| Remembering the prolonged cold of winter.Crossref | GoogleScholarGoogle Scholar | 24028964PubMed |
Steward N (2002) Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. The Journal of Biological Chemistry 277, 37741–37746.
| Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress.Crossref | GoogleScholarGoogle Scholar | 12124387PubMed |
Thellier M, Lüttge U (2013) Plant memory: a tentative model. Plant Biology 15, 1–12.
| Plant memory: a tentative model.Crossref | GoogleScholarGoogle Scholar | 23121044PubMed |
Tombesi S, Frioni T, Poni S, Palliotti A (2018) Effect of water stress “memory” on plant behavior during subsequent drought stress. Environmental and Experimental Botany 150, 106–114.
| Effect of water stress “memory” on plant behavior during subsequent drought stress.Crossref | GoogleScholarGoogle Scholar |
Verhoeven KJF, van Gurp TP (2012) Transgenerational effects of stress exposure on offspring phenotypes in apomictic dandelion. PLoS One 7, e38605
| Transgenerational effects of stress exposure on offspring phenotypes in apomictic dandelion.Crossref | GoogleScholarGoogle Scholar |
Verhoeven KJF, Jansen JJ, Biere DA (2010) Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytologist 185, 1108–1118.
| Stress-induced DNA methylation changes and their heritability in asexual dandelions.Crossref | GoogleScholarGoogle Scholar |
Virlouvet L, Avenson TJ, Qian D, Chi Z, Ning L, Michael F, Avramova Z, Russo SE (2018) Dehydration stress memory: gene networks linked to physiological responses during repeated stresses of Zea mays. Frontiers in Plant Science 9, 1058
| Dehydration stress memory: gene networks linked to physiological responses during repeated stresses of Zea mays.Crossref | GoogleScholarGoogle Scholar | 30087686PubMed |
Wada Y, Miyamoto K, Kusano T, Sano H (2004) Association between up-regulation of stress-responsive genes and hypomethylation of genomic DNA in tobacco plants. Molecular Genetics and Genomics 271, 658–666.
| Association between up-regulation of stress-responsive genes and hypomethylation of genomic DNA in tobacco plants.Crossref | GoogleScholarGoogle Scholar | 15148604PubMed |
Walter J, Nagy L, Hein R, Rascher U, Beierkuhnlein C, Willner E, Jentsch A (2011) Do plants remember drought? hints towards a drought-memory in grasses. Environmental and Experimental Botany 71, 34–40.
| Do plants remember drought? hints towards a drought-memory in grasses.Crossref | GoogleScholarGoogle Scholar |
Wang W, Zhao X, Pan Y, Zhu L, Fu B, Li Z (2011) DNA methylation changes detected by methylation-sensitive amplified polymorphism in two contrasting rice genotypes under salt stress. Journal of Genetics and Genomics 38, 419–424.
| DNA methylation changes detected by methylation-sensitive amplified polymorphism in two contrasting rice genotypes under salt stress.Crossref | GoogleScholarGoogle Scholar | 21930101PubMed |
Wang X, Vignjevic M, Liu F, Jacobsen S, Jiang D, Wollenweber B (2015) Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regulation 75, 677–687.
| Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Wargent JJ, Gegas VC, Jenkins GI, Doonan JH, Paul ND (2009) UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytologist 183, 315–326.
| UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation.Crossref | GoogleScholarGoogle Scholar |
Willing EM, Piofczyk T, Albert A, Winkler JB, Schneeberger K, Pecinka A (2016) UVR2 ensures transgenerational genome stability under simulated natural UV-B in Arabidopsis thaliana. Nature Communications 7, 13522
| UVR2 ensures transgenerational genome stability under simulated natural UV-B in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 27905394PubMed |
Yaish MW, Abbas AL, Ibtisam AH, Vishwas PH (2018) Genome-wide DNA methylation analysis in response to salinity in the model plant caliph medic (Medicago truncatula). BMC Genomics 19, 78
| Genome-wide DNA methylation analysis in response to salinity in the model plant caliph medic (Medicago truncatula).Crossref | GoogleScholarGoogle Scholar | 29361906PubMed |