A new method for separate evaluation of PSII with inactive oxygen evolving complex and active D1 by the pulse-amplitude modulated chlorophyll fluorometry
Masaru Kono A B , Sae Matsuzawa A , Takaya Noguchi A , Kazunori Miyata A , Riichi Oguchi A and Ichiro Terashima
A B , Sae Matsuzawa A , Takaya Noguchi A , Kazunori Miyata A , Riichi Oguchi A and Ichiro Terashima  A
A
A Department of Biological Sciences, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
B Corresponding author. Email: konom07@bs.s.u-tokyo.ac.jp
Functional Plant Biology - https://doi.org/10.1071/FP21073
Submitted: 6 March 2021 Accepted: 13 August 2021 Published online: 13 September 2021
Journal compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
A method that separately quantifies the PSII with inactive oxygen-evolving complex (OEC) and active D1 retaining the primary quinone acceptor (QA)-reducing activity from the PSII with damaged D1 in the leaf was developed using PAM fluorometry. It is necessary to fully reduce QA to obtain Fm, the maximum fluorescence. However, QA in PSII with inactive OEC and active D1 would not be fully reduced by a saturating flash. We used the acceptor-side inhibitor DCMU to fully reduce QA. Leaves of cucumber (Cucumis sativus L.) were chilled at 4°C in dark or illuminated with UV-A to selectively inactivate OEC. After these treatments, Fv/Fm, the maximum quantum yield, in the leaves vacuum-infiltrated with DCMU were greater than those in water-infiltrated leaves. In contrast, when the leaves were illuminated by red light to photodamage D1, Fv/Fm did not differ between DCMU- and water-infiltrated leaves. These results indicate relevance of the present evaluation of the fraction of PSII with inactive OEC and active D1. Several examinations in the laboratory and glasshouse showed that PSII with inactive OEC and active D1 was only rarely observed. The present simple method would serve as a useful tool to clarify the details of the PSII photoinhibition.
Keywords: Cucumis sativus, oxygen-evolving complex, pulse-amplitude modulated fluorometry, PAM, DCMU, Fv/Fm, PSII, photoinhibition.
Introduction
Light drives photosynthesis, but it also damages the photosynthetic apparatus. The loss of photosynthetic activity due to this damage is called photoinhibition. Although photoinhibition of PSI by the fluctuating light has been recently highlighted (Suorsa et al. 2012; Kono et al. 2014; Li et al. 2018; Roach et al. 2020), the main target of photoinhibition is PSII (Öquist et al. 1987; Tjus and Andersson 1993). PSII photoinhibition occurs in visible light (400–700 nm) and in ultraviolet (UV) light (220–400 nm), of which the latter is more effective (Jones and Kok 1966; Takahashi et al. 2010).
PSII, the type II reaction centre of the oxygenic photosynthetic organisms, is a redox enzyme, which is composed of dozens of polypeptides and several cofactors and contains a reaction centre and an oxygen-evolving complex (OEC). The cofactors involved in charge separation and water oxidation are coordinated by a pair of homologous polypeptides, D1 and D2, which are largely embedded in the thylakoid membrane. D1 protein provides most of the ligands to the Mn4CaO5 cluster (Lubitz et al. 2019). The water oxidation is catalysed by this cluster located on the luminal side. In the light, PSII reduces plastoquinone, using electrons released in the oxidation processes of H2O. The electrons from water, flow through the redox cofactors (TyrZ → P680 → pheophytin) in D1, and reduce the primary quinone acceptor, QA, bound to D2. Upon accepting two electrons via QA and two protons from the stroma, the secondary electron acceptor QB is released from PSII as a plastoquinol. In this way, electrons flow to the cytochrome b6/f complex, PSI, and eventually reduce NADP+ to NADPH (Tikhonov 2013).
Mechanisms for PSII inactivation have been controversial. There are two main hypotheses. The two-step hypothesis claims that the first step of photodamage is inactivation of OEC: Mn ions release from OEC upon absorption of light by Mn (III/IV) (Hakala et al. 2005; Ohnishi et al. 2005). Mn (III/IV) ions show high absorbance in UV and blue wavebands and thus the photoinhibitory quantum yields of these wavebands are higher than those at longer wavelengths (Jones and Kok 1966; Hakala et al. 2005; Ohnishi et al. 2005). According to the two-step hypothesis, the secondary damage site is D1, whereas this is the primary damage site according to the excess energy hypothesis (Demmig-Adams and Adams 1992). Although D1 performs several functions, here, we use ‘D1 damage’ to denote the loss of QA-reducing photochemical activity. Oguchi et al. (2009) showed that both mechanisms occur under rather mild physiological conditions (the mixed hypothesis). However, we still need to explore which of these mechanisms is relevant in nature. Since most of the biological mechanisms evolve by means of natural selection, ecological relevance of these mechanisms should be evaluated under natural conditions.
In the pulse-amplitude modulated (PAM) fluorometry, pulses of fluorescence excited by measuring beam pulses are monitored. By applying this technique, various PSII activities can be assessed non-invasively and thus the technique has been commonly used (Baker 2008). Based on the QA model, the quantum yield of chlorophyll fluorescence of PSII changes depending on the redox state of QA and the heat dissipation process such as non-photochemical quenching (NPQ, Schansker et al. 2014). When QA is oxidised, NPQ is fully relaxed in the dark, and the leaf is illuminated with a weak measuring beam, the fluorescence level remains minimal (F0). A saturating pulse (SP) given to the leaf pretreated in the dark reduces all QA in the functional PSII, resulting in the maximum fluorescence (Fm). Fv/Fm, where Fv = Fm – F0, has been used as an indicator of the maximum quantum yield of PSII photochemistry (Butler 1978).
Fv/Fm is widely used to assess the plant status in various situations (Maxwell et al. 1994), because any stress that causes damage to PSII (Long et al. 1994; Maxwell and Johnson 2000) or induction of the ‘sustained’ NPQ (Demmig-Adams and Adams 2006) results in the decrease in Fv/Fm. However, when the PAM fluorometory is used in photoinhibition studies, attention should be paid. Fv/Fm measured by the conventional way cannot differentiate between the OEC damage and D1 damage. If there are any PSII with inactive OEC and active D1, Fm induced by a SP would underestimate the QA-reducing activity of PSII, because QA in such PSII cannot be reduced by the SP due to the absence of electron supply from OEC. In the conventional measurement, reduction of the whole plastoquinone pool and QB by the SP is prerequisite for full reduction of QA. In the present study, we focussed on this point. By applying an electron donor, diphenyl-carbazide (DPC, Izawa 1980; Zavafer et al. 2015), which directly donates electrons to active D1 bypassing OEC, QA in active D1 would be reduced by the SP. DCMU, an inhibitor of the electron flow from QA to QB, would be also effective. In the present study, we examined whether we could distinguish the PSII with inactive OEC and active D1 from the PSII with inactive D1 by measuring Fv/Fm in the presence of the electron donor or inhibitor. We used cucumber, a chilling sensitive plant, because the previous studies have shown that the treatment of the leaves of this plant at 4°C in the dark selectively inactivates OEC (Margulies 1972; Kaniuga et al. 1978; Terashima et al. 1989; Shen et al. 1990; Higuchi et al. 2003). Our present results indicate that the conventional Fv/Fm certainly underestimated the QA-reducing activity in PSII with inactivated OEC. We also confirmed that the use of the chemicals enabled us to quantify the fraction of PSII with inactive OEC and active D1. This method would be very useful to analyse the PSII photoinhibition in nature through determination of the first step of photoinhibition.
Materials and methods
Plant materials
Seeds of cucumber (Cucumis sativus L. ‘Nanshin’) purchased from Takii and Co. were sown in vermiculite in 200 mL pots and supplied with deionised water. These pots were placed in a growth chamber, 14 h light/10 h dark cycle at an air temperature of 23°C for ~20 days. After germination, the seedlings were supplied with 0.1% Hyponex 6–10–5 (Hyponex Japan). Light was supplied by a bank of cool white fluorescent lamps (FPR96EX-N/A: Toshiba), and the photosynthetic photon flux density (PPFD) just above the plants was 200 μmol m–2 s–1. The first true leaves were used in all the experiments.
Inactivation of OEC
OEC was inactivated by a chilling treatment in the dark according to Terashima et al. (1989). Leaves were floated on ice-cold water in a plastic container placed on ice in a styrofoam box and kept in the dark in a cold room or in a refrigerator at 4°C for up to 48 h. By this treatment, OEC in cucumber is selectively inactivated whereas the D1 protein remains largely intact (Shen et al. 1990; Higuchi et al. 2003).
For photoinactivation of OEC, a UV-A lamp (LUV-16, AS ONE) peaked at 365 nm was used (for the spectrum, see Supplementary material Fig. S1). Leaves attached to the plants were illuminated with the UV-A lamp at a photon flux density of 50 μmol m–2 s–1 for 6 h at a room temperature of 23°C. A fan was used for keeping the leaves at the room temperature. The leaves were tied to the light source with threads so that they did not flutter. UV light was applied from either the adaxial or abaxial side of the leaves.
Photodamage to D1 protein
A square array of 36 LEDs peaked at 657 nm (red) or 446 nm (blue) covered with a transparent plastic plate (15 × 15 cm, ISLM150X150, CCS, for the spectra, see Fig. S1) was used. Leaf segments (1.5 × 1.5 cm2), which were kept in the dark for at least 30 min, were placed directly on the cover at just above the respective LEDs with their adaxial sides towards the LEDs at room temperature of 23°C for 30 min. A few drops of water were supplied to each leaf segment to avoid desiccation during the photoinhibitory treatment. The PPFD level at the leaf surface was 2000 μmol m–2 s–1.
Application of chemicals to the leaves
Leaf segments were vacuum-infiltrated with 1 mM diphenylcarbazide (DPC), 100 μM DCMU, or H2O. The chemicals were solved in dimethyl sulfoxide (DMSO). In the working solutions, the DMSO concentrations were kept less than 1% (v/v) (Fig. S2). A leaf segment (1.5 × 1.5 cm2) was submerged in either of these solutions in a 15-mL syringe, and the solution was infiltrated into the intercellular spaces by pulling and pushing a piston a few times by hand. After the infiltration, Fv/Fm was determined (Fig. S3). All these manipulations were conducted in dim light in a dark room. The optimal concentration of DCMU was determined by measuring the chlorophyll fluorescence inductions (Kautsky transient) in the presence of various concentrations of DCMU with a PAM-2500 at 23°C (Fig. S4). 1 mM was almost the maximum concentration for DPC in 1% DMSO solution.
To suppress repair of D1, we used lincomycin, an inhibitor of the 70S type protein synthesis. The leaf segment was infiltrated with 1 mM lincomycin solution using the 15-mL syringe as described above. After the infiltration, the leaf segment was softly sandwiched with two pieces of filter paper and kept for 30 min to eliminate the lincomycin solution from its intercellular spaces. Absence of the solution in the intercellular spaces was ensured by the loss of transparency of the leaf segment. Elimination of the solution was needed to avoid low O2 effects during the photoinhibitory treatment lasting typically for 30 min.
Measurements of chlorophyll fluorescence
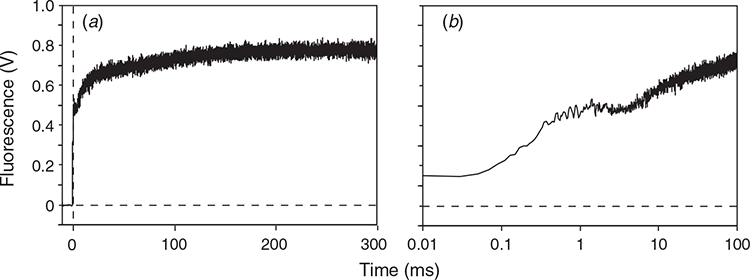
Before application of chemicals or water, the leaves or leaf segments were kept in the dark at least for 30 min. Chlorophyll fluorescence was measured using a PAM-2500 (Walz) in the room air at 23°C. A saturating pulse (SP) from the red LEDs (7000 μmol m−2 s−1 for 300 ms) was applied in the dark to reduce QA in the functional PSII and determine the maximum chlorophyll fluorescence level, Fm. The 300-ms SP was long enough to obtain Fm (Figs 1a, S5). The maximum quantum yield of PSII photochemistry in the dark, Fv/Fm, was calculated as (Fm – F0)/Fm (Butler 1978).
In this study, we paid special attention to the minimal fluorescence (F0), which is defined as a fluorescence level with oxidised QA in all PSII (Lazár 2006). In the presence of DCMU, F0 tends to be overestimated, because even a very weak measuring light reduces QA. Thus, we determined F0 level using the fast acquisition mode of PamWin-3 (maximum time resolution of 10 μs, Walz). In this mode, the measuring at a PPFD level of 0.1 μmol m–2 s–1 switched on 0.1 ms before the SP, and F0 was determined within 0.01–0.1 ms from the onset of the SP (for details, see ‘Results’ and Fig. 1). All these manipulations were made in dim light in a dark room.
Chlorophyll fluorescence measurement by a direct excitation method was performed using a PAR-FluoPen FP110/S portable fluorometer (Photon Systems Instruments). The polyphasic rise of the fluorescence transient curve (OJIP-transient) was measured based on Strasser et al. (2004). FJ, the fluorescence intensity at J-step at 2 ms in the OJIP-transient, was measured in addition to F0 and Fm, and another fluorescence parameter, (FJ – F0)/FJ, was calculated (Osmond et al. 2017).
Measurements of the Fv/Fm and DCIP photoreduction rate in thylakoid membranes
The leaf segments (~10 cm2) were ground in an ice-cold buffer containing 0.3 M sorbitol, 10 mM NaCl, 5 mM MgCl2, 0.1% (w/v) bovine serum albumin (BSA) and 40 mM HEPES-KOH (pH 7.0) with a Polytron homogeniser (Kinematica) at a line voltage of 3 for 5 s. The homogenate was filtered through a single layer of 20 µm nylon mesh and the filtrate was centrifuged at 1500g for 2 min at 4°C, and the pellet was resuspended in the same buffer but without BSA. These procedures were made in dim light.
Thylakoids were suspended in the same buffer without BSA but at pH of 7.5, at the chlorophyll concentration of 5 μM, and F0 and Fm were measured with a DUAL-PAM (Walz) in the fast acquisition mode operated by DualPam software. The measuring at 0.1 μmol m–2 s–1 switched on 0.1 ms before the SP, and F0 was determined within 0.01–0.1 ms from the onset of the SP. The chemicals were added to the thylakoid suspension. When present, the concentration of DPC, DCMU or 2,5-dibromo-6-isopropyl-3-methyl-1,4-benzoquinone (DBMIB) was 1 mM, 10 μM or 5 µM. All these procedures were made in dim light.
Photoreduction of 2,6-dichloroindophenol (DCIP) was measured with a spectrophotometer (Shimadzu UV200) with a custom-made cross illumination system, which illuminated a lateral side of the optical cuvette with a square optical fibre. Thylakoids were suspended in a buffer containing 0.3 M sorbitol, 10 mM NaCl, 5 mM MgCl2, and 40 mM MES-KOH (pH 6.5). The concentration of DCIP was 100 µM. When present, the concentration of DPC was 1 mM.
PSII photoinhibition by sunlight
Cucumber plants grown in the growth chamber for 17–22 days were transferred to a glasshouse on the rooftop of the department building. The leaf adaxial side was exposed to full sunlight for 3 h, during which, irradiance, air temperature and humidity were fluctuated from ~1050–1200 μmol m−2 s−1, ~38.0–40.5°C, and ~50–51%. After the exposure to sunlight, the leaves were kept in the dark for 30 min at 23°C, and F0 and Fm were determined after the infiltration of DCMU or water in the leaves and thylakoid membranes. For a spectrum of the sunlight, see Fig. S1.
Statistical analyses
ANOVA with the Dunnett test or Tukey-Kramer test and Student’s t-test were used with the Microsoft Excel 2016.
Results
Measurements of Fv/Fm in PSII with inactive OEC and active D1 in the leaves
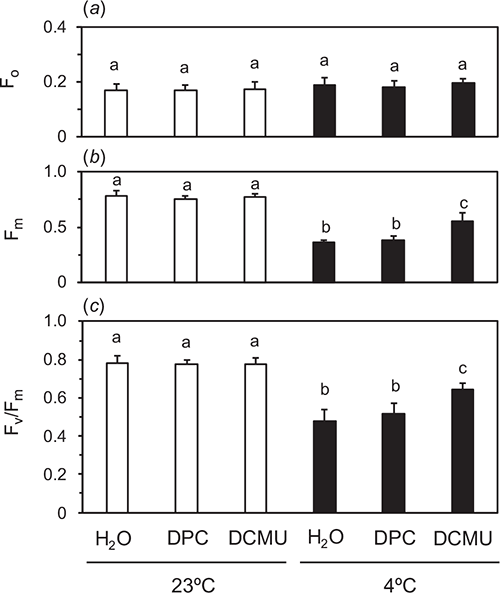
Cucumber leaves were chilled to selectively inactivate OEC at 4°C in the dark (dark-chilling treatment) for 48 h. We hypothesised that PSII with inactive OEC and active D1 could not reduce QA sufficiently during a SP and that addition of the electron donor to D1-TyrZ, DPC, or the acceptor-side inhibitor of PSII, DCMU, to the leaves would facilitate QA reduction by the SP. The chemicals were vacuum-infiltrated at 23°C in the dark after the dark treatment for 48 h. After the infiltration, F0 and Fm were measured in the fast acquisition mode (Fig. 1) without further dark incubation. Traces plotted against the normal scale (Fig. 1a) and log scale (Fig. 1b) are shown. Fv/Fm values in the leaves treated in the dark at room-temperature of 23°C (dark-RT treatment) for 48 h were 0.79 ± 0.027 regardless of the chemical treatments (white bars in Fig. 2c). Significant differences were not detected in Fm or F0, either (white bars in Fig. 2a, b).
|
The dark-chilling treatment for 48 h decreased Fv/Fm to 0.48 ± 0.060 in water-infiltrated leaves. Fv/Fm in DPC-infiltrated leaves was comparable to that of the water-infiltrated leaves, whereas Fv/Fm in DCMU-infiltrated leaves was greater and 0.64 ± 0.037 (Fig. 2). F0 after the dark-chilling treatment was not different from those after the dark-RT treatment for 48 h irrespective of the chemicals. In Fig. 2, the data using 1 mM DPC are shown. When DPC at the concentrations greater than 1 mM were used, Fv/Fm in the leaves did not differ from that at 1 mM DPC (data not shown). DCMU at 100 μM completely inhibited electron flows in PSII (Figs 2, S4).
To examine the cause of the decrease in Fv/Fm by the dark-chilling treatment for 48 h in DCMU-infiltrated leaves, we varied duration of the dark-chilling treatment from 6 to 48 h (Fig. S6). Fv/Fm in water-infiltrated leaves decreased with time. On the other hand, the extent of OEC inactivation assessed by the difference in Fv/Fm between water- and DCMU-infiltrated leaves increased with time. Fv/Fm in DCMU-infiltrated leaves after the dark-chilling for 6 h (0.66) was already lower than that before the treatment (0.79), and did not decrease further up to 48 h. To check a possibility that low temperature itself could exert some inhibitory effect on Fv/Fm in DCMU-infiltrated leaves, we kept the leaves after dark-chilling for 48 h at RT in the dark for up to 48 h (Fig. S3). Fv/Fm in DCMU-infiltrated leaves did not recover to the original level and was comparable to that measured immediately after the dark-chilling treatment for 48 h.
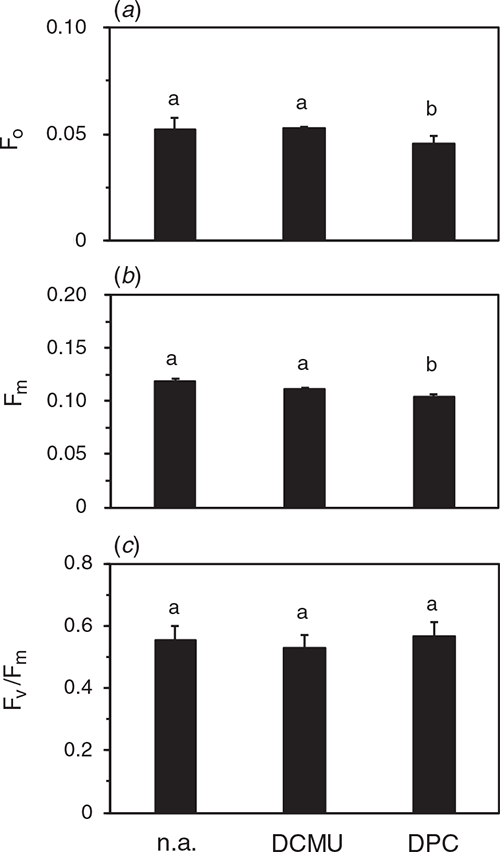
Fv/Fm measurements in isolated thylakoid samples
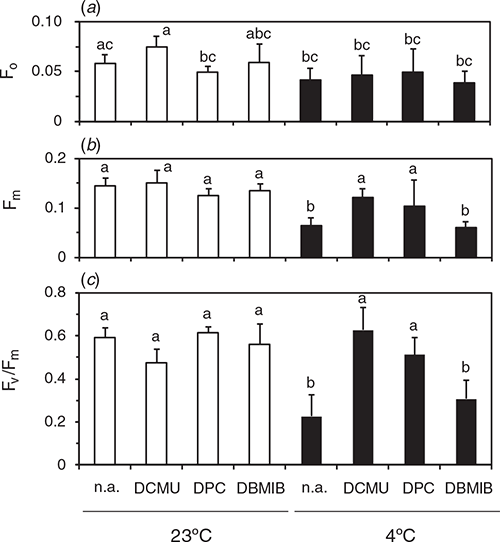
To understand causes of the differential effect of DPC and DCMU on Fv/Fm measured in leaf segments, we measured Fv/Fm in suspensions of thylakoids isolated from the leaves after the dark-chilling or dark-RT treatments for 48 h (Fig. 3). The chemicals were added in the suspension before the measurement. F0 measured in the presence of DCMU in the dark-RT samples did not differ significantly from the value with DBMIB or no addition (denoted as n.a. in figures). Fm levels after the dark-RT treatment did not differ irrespective of the chemicals. After the dark-chilling, Fm in the presence of DPC or DCMU was comparable to the level after the dark-RT. Fv/Fm after dark-RT was similar, irrespective of the chemicals. After the dark-chilling, Fv/Fm in the absence of chemicals was lower than that with DPC or DCMU. Although addition of DPC did not increase Fv/Fm in the leaf segments (Fig. 2), DPC at the same concentration markedly increased Fv/Fm in the thylakoid suspension. Fv/Fm with 10 µM DCMU did not differ from that with 1 mM DPC. We also examined effects of 5 µM DBMIB, an inhibitor of plastoquinone oxidation by the cytochrome b6f complex. Addition of DBMIB to the thylakoids isolated from the leaves after the dark-chilling of leaves did not increase Fm, and Fv/Fm did not differ significantly from no addition. These results indicate that, in the thylakoid suspension, 1 mM DPC was competent to reduce all QA by the SP: Fv/Fm measured in the presence of DPC reflected the plastoquinone-photoreducing activity of D1. DCMU was also effective in the thylakoid suspension.
|
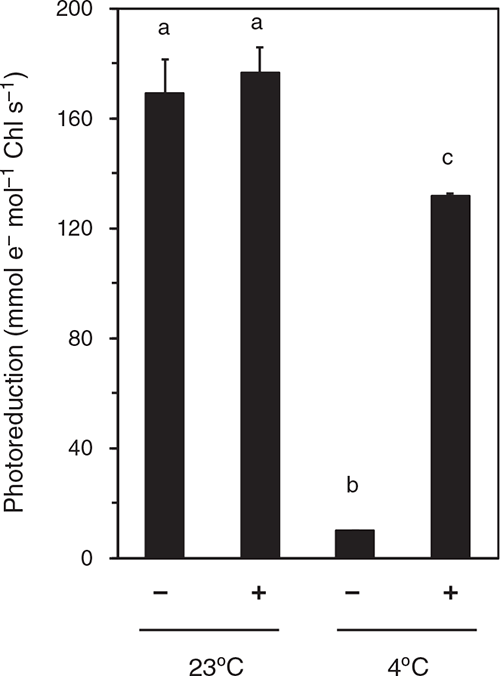
To examine the activity of OEC, we measured the rate of DCIP photoreduction in the thylakoid suspension (Fig. 4). At pH 6.5, DCIP accepts electrons from PSII (QB site) or plastoquinone pool rather than PSI (Izawa 1980). In thylakoids isolated from the leaves after the dark-RT treatment for 48 h, DCIP photoreduction rates attained ~170 mmol e– mol–1 Chl s–1 irrespective of the presence or absence of DPC. The dark-chilling treatment strongly suppressed the DCIP photoreduction rate in the absence of DPC (~10 mmol e– mol–1 Chl s–1), whereas addition of DPC largely recovered the rate (~130 mmol e– mol–1 Chl s–1). This would explain the increase in Fv/Fm by addition of DPC to the thylakoids isolated from leaves after dark-chilling treatment (Fig. 3). However, the DCIP photoreduction rate in the presence of DPC in the thylakoids isolated from the leaves after the dark-chilling treatment was lower than that in the thylakoids isolated from leaves after the dark-RT treatment.
|
Effect of UV-A irradiance on the OEC inactivation
In addition to the OEC inactivation by the dark-chilling treatment, we tried to photoinactivate OEC by exposing the leaves to UV-A light. The light source showed a peak at 365 nm (Fig. S1). Cucumber leaves were illuminated from the adaxial or abaxial side with UV-A at a photon flux density of 50 μmol m−2 s−1 for up to 6 h. After the dark treatment for 30 min, Fv/Fm was measured on both adaxial and abaxial sides. When the leaves were infiltrated with DCMU or H2O, the leaves exposed to UV-A were kept in the dark for 30 min and 100 μM DCMU or H2O was infiltrated in the dim light and then Fv/Fm were measured without further dark treatment. Fig. 5a shows Fv/Fm in the leaves illuminated from the adaxial side without solute-infiltration. Fv/Fm hardly declined irrespective of the adaxial or abaxial side measurement. Fv/Fm in DCMU-infiltrated leaves after UV-A exposure for 6 h did not differ from that in water-infiltrated leaves (Fig. 5c, e), indicating that OEC was not inactivated by exposure to UV-A irradiance from the adaxial side. In contrast, when the leaves were illuminated from the abaxial side, Fv/Fm measured without solute-infiltration decreased with time (Fig. 5b). The decline in the abaxial side was greater than that in the adaxial side (Fig. 5d, f). After UV-A illumination for 6 h, Fv/Fm in DCMU-infiltrated leaves were ~0.52 (abaxial data) and 0.69 (adaxial data), and greater than those in water-infiltrated leaves, ~0.46 and 0.63, respectively (Fig. 5b, d, f).
To analyse why Fv/Fm was high after exposure to UV irradiance from the adaxial side, we compared the excitation spectrum of PSII fluorescence measured at 690 nm in a leaf excited with monochromatic light from the adaxial side and that excited from the abaxial side (Fig. S7). Chlorophyll fluorescence intensity on the leaf adaxial surface was markedly lower for the excitation wavelength from 300 to 360 nm than that on the abaxial surface. When UV-A was illuminated from the leaf adaxial side, red chlorophyll fluorescence was hardly visible to the naked eye. In contrast, when UV-A was illuminated from the leaf abaxial side, the fluorescence could be clearly seen from both sides (data not shown). Transmittance of UV light in the adaxial epidermis peel was also significantly lower than that in the abaxial one, especially in a range of 300–380 nm (data not shown). These results indicate that the PSII tolerance to the UV-A illumination from the adaxial side was attributed to the presence of UV-absorbing compounds in the adaxial epidermal cells.
Effect of DCMU on Fv/Fm in PSII with inactive D1
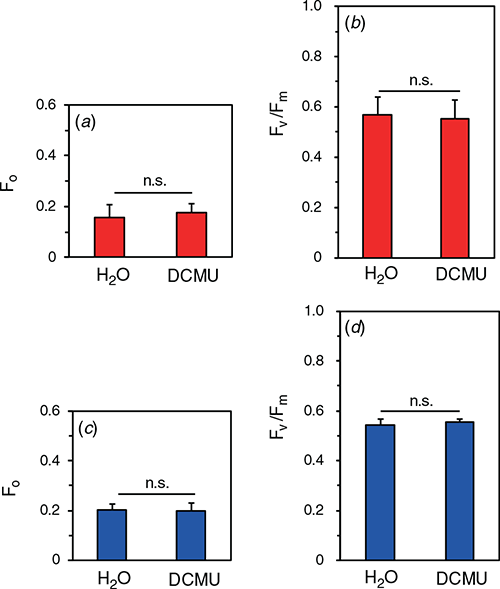
We examined effects of the damage to D1 on Fv/Fm. Detached leaves were vacuum-infiltrated with 1 mM lincomycin, an inhibitor of chloroplast-encoded protein synthesis. After the infiltration, the leaf segments were gently sandwiched with pieces of filter paper for 30 min to eliminate the lincomycin solution from the intercellular spaces. Then, blue- or red- actinic light at the PPFD level of 2000 μmol m−2 s−1 was illuminated for 30 min (for the spectra, see Fig. S1). After the dark treatment for 30 min, 100 μM DCMU or H2O were infiltrated just before the measurements. Neither F0 nor Fv/Fm after the exposure to red light was significantly different between DCMU- and water-infiltrated leaves (Fig. 6a, b). We expected that some effect of OEC inactivation on Fv/Fm might be observed with blue light, because blue light is well absorbed by the Mn4CaO5 cluster. However, neither F0 nor Fv/Fm in DCMU-infiltrated leaves after the exposure to the blue light differed from those in water-infiltrated leaves (Fig. 6c, d). The result of blue light suggests either that the damage to D1 occurred according to the excess hypothesis or that the damage to OEC was immediately followed by the damage to D1.
|
Inactivation of OEC and/or photo-damage of D1 by natural light
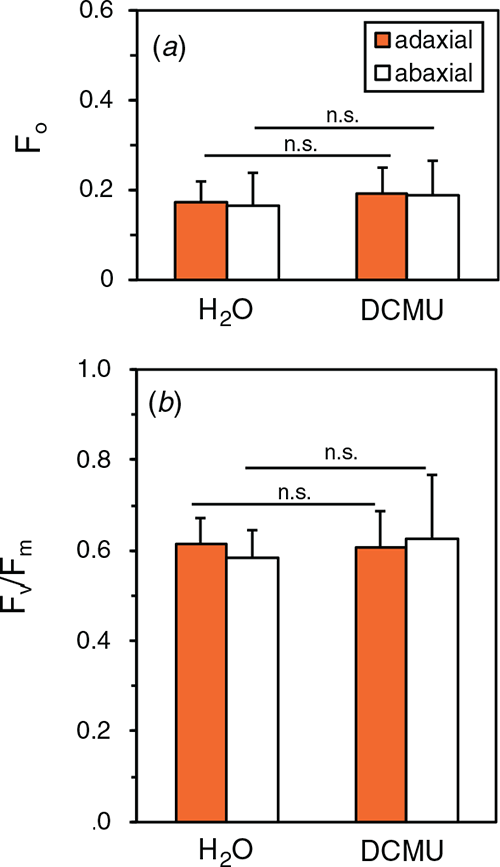
By using the DCMU-infiltration method, we examined the PSII photoinhibition in the natural solar radiation to examine whether PSII with inactive OEC and active D1 was present. Cucumber plants grown in the growth cabinet were transferred to the rooftop glasshouse and exposed to full sunlight for 3 h. PPFD levels ranged from 1050 ~1200 μmol m−2 s−1 (for the spectrum, see Fig. S1). After the dark treatment for 30 min at 23°C and subsequent infiltration of 100 μM DCMU or H2O, Fv/Fm was measured both on the adaxial and abaxial sides in the presence and absence of DCMU. Fv/Fm of both sides was comparable at ~0.60 not only in water-infiltrated leaves but also in DCMU-infiltrated leaves (Fig. 7). Further, we measured Fv/Fm and the DCIP photoreduction rate in the thylakoid suspension isolated from these leaves. Although F0 and Fm were slightly lower with DPC than those with DCMU or no addition, Fv/Fm levels showed no significant differences irrespective of the chemicals (Fig. 8). DCIP photoreduction rates did not differ between the presence and absence of DPC (Fig. S8). These results suggest that the leaves in the strong sunlight had no PSII with inactive OEC and active D1.
|
|
Examination of the DCMU-infiltration method of Fv/Fm with a direct excitation fluorometer
We used a direct excitation fluorometer (PAR-FluoPen FP110/S, Photon Systems) to examine the DCMU-infiltration method in cucumber leaves after the dark-chilling for 48 h (Fig. S9). F0 levels in DCMU-infiltrated leaves were higher irrespective of the dark-RT or dark-chilling treatment compared with the stable Fm levels, leading to lower Fv/Fm values. In this fluorometer, the fluorescence was induced by a strong light at 3000 μmol m−2 s−1 and F0 is estimated using a regression equation through the initial several data points recorded immediately after the onset of the strong light. Much weaker light would be needed to estimate a correct F0 in the presence of DCMU. Or Fv/Fm in DCMU-infiltrated leaves might be recalculated using F0 measured in water-infiltrated leaves (Fig. S10).
We also measured FJ, the fluorescence intensity at J-step at 2 ms in the OJIP-transient, and calculated another fluorescence parameter, (FJ – F0)/FJ from the same measurements for Fv/Fm with the PAR-FluoPen FP110/S. According to Osmond et al. (2017), (FJ – F0)/FJ reflects the QA-reducing activity, whereas Fv/Fm reflects the redox condition of plastoquinone pool. However, (FJ – F0)/FJ after the dark-chilling treatment did not differ statistically between water- and DCMU-infiltrated leaves, whereas Fv/Fm in DCMU-infiltrated leaves was greater than that in water-infiltrated leaves (Fig. S9). FJ and Fm in DCMU-infiltrated leaves were similar. Thus, similar (FJ – F0)/FJ between water- and DCMU-infiltrated leaves could be attributed to a high level of FJ in water-infiltrated leaves (Fig. S9).
(FJ – F0)/FJ and Fv/Fm for DCMU-infiltrated leaves were re-calculated using F0 obtained in water-infiltrated leaves (Fig. S10). Then, the difference in (FJ – F0)/FJ between DCMU- and water-infiltrated leaves after the dark-chilling treatment was statistically significant.
Discussion
In this study, we focussed on separate evaluation of the PSII with inactive OEC and active D1 from those with inactive D1 (with intact OEC or with OEC and D1 inactivated to the same extent), based on in vivo and in vitro Fv/Fm measurements with the PAM and direct excitation fluorometries, in the presence of chemicals such as DCMU and DPC. The present method is not new but a refined version of the pre-existing methods using DCMU and/or DPC (Izawa 1980; Strasser et al. 2004; Zavafer et al. 2017). Although D1 has several cofactors involved in PSII electron transport and thereby several functions, we focussed on the QA-reducing activity. Here, we simply call D1 activity to denote the QA-reducing activity.
The method is very simple. It is to just compare the Fv/Fm in DCMU-infiltrated leaves with that in water-infiltrated leaves (Fig. 2). In the thylakoid suspension, DPC at 1 mM also caused the increase in Fv/Fm (Fig. 3). However, due to its low solubility in water, it would be impossible to attain the effective concentration in the thylakoids by feeding it from the intercellular space: There was little effect of DPC infiltration on Fv/Fm in the dark-chilling treated leaves (Fig. 2c). Thus, we decided to use DCMU. The optimal concentration of DCMU would be different depending on the species or conditions, but it can be readily checked by monitoring the Kautsky transient (Fig. S4). When there are PSII with inactive OEC and active D1 in the leaf, Fv/Fm in the presence of DCMU would be greater than that in its absence, whereas Fv/Fm in the leaf having PSII with inactive D1 and active OEC and/or PSII with OEC and D1 inactivated to the same extent, would not increase by DCMU. Thus, the difference in Fv/Fm between the presence and absence of DCMU reflects the fraction of the PSII with inactive OEC and active D1.
Separate evaluation of differently damaged PSII by the DCMU-infiltration method with PAM
Our results with DCMU indicate that Fv/Fm measured in the conventional practice would underestimate the QA-reducing activity of D1. When the light source including UV is used for the photoinhibitory treatment or for the growth light, there could be some PSII that have inactivated OEC and functional D1. This might apply to the field studies especially those conducted at high elevations, because the solar radiation includes UV and the share of UV increases with the elevation (Sullivan et al. 1992; Wang et al. 2016). The electron transport rate through PSII is sensitive to UV, due to the sensitivity of OEC to UV. In this study, we demonstrated that the separate evaluation of PSII with inactive OEC and active D1 from PSII that have damaged D1 is possible (Figs 2, 3, 5). In cucumber leaves illuminated with UV-A light from the abaxial side, we detected considerable PSII with inactive OEC and active D1 (Fig. 5), indicating that the damage to PSII by the two-step mechanism had occurred.
In the present study, we hypothesised that, in the presence of DCMU, QA can be reduced even in the PSII with the inactive OEC. In the experiment with isolated thylakoids, we also used an inhibitor of binding of plastoquinone to the cytochrome b6/f complex, DBMIB, and found that DBMIB could not replace DCMU. These indicate that PSII with inactive OEC was able to transfer at least one electron to QA in response to a SP but was not able to reduce the whole plastoquinone pool. Thus, in the presence of DCMU, QA was reduced, whereas in its absence, QA was not fully reduced because the plastoquinone pool would be largely oxidised.
In the present study, Fv/Fm in DCMU-infiltrated leaves after the dark-RT treatment did not differ from that in water-infiltrated leaves (Fig. 2). Thus, we expected that Fv/Fm in DCMU-infiltrated leaves after the dark-chilling treatment would be comparable to that in the leaves after the dark-RT treatment. This was not the case (Fig. 2). The electron transport rate from DPC to DCIP in the thylakoids isolated from dark-chilled leaves was also significantly lower than that from H2O or DPC to DCIP in the thylakoids from dark-RT leaves (Fig. 4). Thus, the dark-chilling treatment might exert some negative effect on D1 as well. It is interesting to point out that Fv/Fm levels measured in thylakoids in the presence of DCMU or DPC did not differ between the dark-chilling and dark-RT treatment of the leaves, and that these values were both lower than that measured in the leaves after the dark-RT treatment (Fig. 3). However, after these dark treatments, the thylakoids were isolated and kept at 4°C. Thus, chilling would exert some effects on D1 functions (see also Fig. S6). The effect could be related to disorder in the lipids or thylakoid membranes. Concerning such membrane effects in cucumber at chilling temperature, there were some arguments (Peeler and Naylor 1988; Terashima et al. 1989, 1991a, 1991b).
Because of high sensitivity of PSII to environmental stresses, the Fv/Fm measurement is a routine practice to check physiological status of plants. Although our method is simple, some attention should be paid. DCMU is known to increase F0 due to the actinic effect of the measuring light (Tóth et al. 2005a; Lazár 2006). Thus, in this study, we used the fast acquisition mode in the PAM software, which allowed high resolution analyses of the fluorescence in the order of μs to detect the F0 level. With the systems that are unable to detect sub-ms data, we recommend the use of very weak measuring light, which is turned on just before the start of the SP. It is needed to manipulate samples in very dim safe light, particularly in the presence of DCMU. Otherwise, reduction of QA would occur.
F0 was not affected by the dark-chilling or UV-A illumination in the present study. However, the increases in F0 have been reported under some stress conditions. For example, high temperature stress increases F0 (Chen et al. 2009). The increase in F0 may be due to the release of the light-harvesting antenna complex II from PSII (Yamane et al. 1997) and dark reduction of QA via plastoquinone (Yamane et al. 2000). Thus, when the F0 changes after the exposure to severe stress conditions, especially heat stress, we need to carefully interpret the changes in Fv/Fm.
Fluorometers suitable for the DCMU-infiltration method
The OJIP analysis has been widely used (Strasser et al. 2000). The OJIP is the transient chlorophyll fluorescence rise induced by a dark-to-strong light transition or a SP, where O and P correspond to F0 and Fm, respectively, and J and I are inflections between O and P. A peak at around 200–300 µs in the OJIP transient has been assigned as K-peak and claimed to reflect the damage to OEC (Tóth et al. 2005b; Iermak et al. 2020). When leaves were treated at 40−50°C to give irreversible damage to OEC, the K-peak appeared in potato and pea (Guissé et al. 1995), rice and spinach (Yamane et al. 1997), and barley (Tóth et al. 2007). The occurrence of K-peak is explained as the faster outflow of electrons from P680 acceptors than the electron flow from PSII donor side due to the damage to OEC. An increase in the FK/FJ ratio, where FK and FJ are the fluorescence levels at K-step and J-step, respectively, has been also assigned to indicate inactivation of OEC (Srivastava and Strasser 1995; Tóth et al. 2005b; Iermak et al. 2020). In the present study, the fluorescence transients showed the peak around at 300 μs both in the water- and DCMU-infiltrated leaves after the inactivation of the OEC (Fig. S11). We recommend determination of Fv/Fm in DCMU-infiltrated leaves to quantify the fraction of PSII with inactive OEC and active D1, combined with the evaluation of the K-peak in the OJIP-transient.
Which instruments can be used for the DCMU-infiltration method? The fluorometer should have a high resolution data acquisition system. It also needs a stable flash for a few hundred ms. The DCMU infiltration-method can be made with the direct excitation fluorometers such as photosynthetic efficiency analysers (PEA) series (Hansatech Instruments Ltd) and portable battery-powered fluorometers (FluorPen series, Photon Systems Instruments, Czech Republic; Fig. S9) as well as the High-performance field and laboratory chlorophyll PAM fluorometers such as a PAM-2500 and a DUAL-PAM (Fig. 3) (Walz). Apart from these commercial fluorometers, a low-cost and highly customisable chlorophyll fluorometer is also available (Bates et al. 2019). Bates et al. (2019) have explained how to make this low-cost device with easy-to-acquire electrical components and an open-source microcontroller. It should be noted that F0 in DCMU-infiltrated leaves measured with the systems employing direct excitation in strong light tends to be overestimated. The devices used for the OJIP-analysis use strong light at 3000– 4000 μmol m−2 s−1 from the first time, whereas PAM fluorometers are equipped with low measuring light at 0.1 μmol m–2 s–1. Thus, for the use of F0, special care should be taken (Fig. S10). Further, as far as the sensitivity to OEC inactivation by the dark-chilling treatment, the use of Fv/Fm was better than (FJ – F0)/FJ (Fig. S9).
Damage to PSII with inactive OEC and active D1
The present study has given an answer to the question whether PSII with inactive OEC and active D1 exists. We expected that there could be some PSII with inactive OEC and active D1 after the photoinhibitory treatment with blue light. However, after the exposure to blue light at 2000 μmol m−2 s−1, Fv/Fm in DCMU-infiltrated leaves were not different from those in water-infiltrated leaves (Fig. 6), indicating that the D1 protein was first damaged according to the excess hypothesis or that the inactivation of the OEC by blue light was immediately followed by the damage to the D1 protein by excess light energy. There might be the third possibility that PSII was immediately degraded after OEC inactivation and therefore PSII with inactive OEC and active D1 did not accumulate. However, if much PSII were degraded in this way, Fm in DCMU-infiltrated leaves after dark-chilling treatment would be lower than that in the leaves treated in the dark at RT. In the glasshouse experiment as well, Fv/Fm in DCMU-infiltrated leaves did not differ from those in water-infiltrated leaves (Fig. 7). In the present study, we were able to detect PSII with inactive OEC and active D1 only in the experiment in which UV-A was illuminated from the abaxial side of the leaves (Fig. 5). Thus, in the full sunlight, PSII with inactive OEC and active D1 may be virtually absent.
Effects of UV tolerance in the adaxial surface of cucumber leaves
When the UV-A was applied from the adaxial side of the cucumber leaves, the leaves were tolerant to the UV-A irradiance (Fig. 5). The UV-induced fluorescence emission spectra suggest that the adaxial epidermis had the UV-absorbing compounds such as flavonoids to protect the mesophyll chloroplasts (Agati et al. 2013). Because UV-A has been argued as an important factor for the two-step theory, survey of the UV-A screening effects of the adaxial epidermis in various species should be conducted. For such surveys, the present excitation spectrum method would be very useful. Ecophysiological roles of the adaxial epidermis in protecting the OEC and/or D1 protein are needed to be clarified in the future.
Concluding remarks and future scopes
The DCMU-infiltration method would be a useful tool for the detailed analyses of PSII photoinibition in the field as well as PSII repair. Diurnal changes in PSII photoinhibitory status in nature should be examined with this method because light intensity and light quality including UV change along with the sunrise, daytime and sunset. Sensitivity of the OEC and D1 would also change. The PSII repair rate would markedly differ among PSII with inactive OEC and D1, PSII with inactive OEC and active D1, and PSII with active OEC and inactive D1. The repair rates for these PSII complexes should be separately examined. In such studies, PSII repair processes that proceed with or without light (Ono 2001; He and Chow 2003) should be carefully addressed. We have provided a perspective article in this Special Issue for Professor Wah Soon Chow. In this study, we could not separate PSII with inactive OEC and D1 from those with PSII with active OEC and inactive D1. We are planning to determine such PSII fractions by means of thermoluminescence (Higuchi et al. 2003), atomic absorption spectrometry of the PSII preparation (Shen et al. 1990), photoreduction assay (Terashima et al. 1989) and electron spin resonance spectroscopy (Kobayashi et al. 2016).
Availability of data
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by JSPS KAKENHI Grant Number (17H05718, 19K16162 and 19H04718).
Acknowledgements
We would like to thank Professor Hajime Wada (Department of Life Sciences, Graduate School of Arts and Sciences, The University of Tokyo) and Professor Tatsuru Masuda (Department of General Systems Studies, Graduate School of Arts and Sciences, The University of Tokyo) for valuable discussion. We thank Kyokko Trading Co. Ltd for a very helpful loan of a direct excitation fluorometer (PAR-FluoPen FP110/S). M. Kono is grateful to Shiho Nakano for her encouragement to do his best.
References
Agati G, Brunetti C, Di Ferdinando M, Ferrini F, Pollastri S, Tattini M (2013) Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiology and Biochemistry 72, 35–45.| Functional roles of flavonoids in photoprotection: new evidence, lessons from the past.Crossref | GoogleScholarGoogle Scholar | 23583204PubMed |
Baker NR (2008) Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annual Review of Plant Biology 59, 89–113.
| Chlorophyll fluorescence: A probe of photosynthesis in vivo.Crossref | GoogleScholarGoogle Scholar | 18444897PubMed |
Bates H, Zavafer A, Szabo M, Ralph PJ (2019) A guide to Open-JIP, a low-cost open-source chlorophyll fluorometer. Photosynthesis Research 142, 361–368.
| A guide to Open-JIP, a low-cost open-source chlorophyll fluorometer.Crossref | GoogleScholarGoogle Scholar | 31541419PubMed |
Butler WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Annual Review of Plant Physiology 29, 345–378.
| Energy distribution in the photochemical apparatus of photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Chen L-S, Li P, Cheng L (2009) Comparison of thermotolerance of sun-exposed peel and shaded peel of ‘Fuji’ apple. Environmental and Experimental Botany 66, 110–116.
| Comparison of thermotolerance of sun-exposed peel and shaded peel of ‘Fuji’ apple.Crossref | GoogleScholarGoogle Scholar |
Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology 43, 599–626.
| Photoprotection and other responses of plants to high light stress.Crossref | GoogleScholarGoogle Scholar |
Demmig-Adams B, Adams WW (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytologist 172, 11–21.
| Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation.Crossref | GoogleScholarGoogle Scholar |
Guisse B, Srivastava A, Strasser R (1995) The polyphasic rise of the chlorophyll a fluorescence (OKJIP) in heat-stressed leaves. Archives des Sciences 48, 147–160.
Hakala M, Tuominen I, Keranen M, Tyystjarvi T, Tyystjarvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochimica et Biophysica Acta 1706, 68–80.
| Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II.Crossref | GoogleScholarGoogle Scholar | 15620366PubMed |
He J, Chow WS (2003) The rate coefficient of repair of photosystem II after photoinactivation. Physiologia Plantarum 118, 297–304.
| The rate coefficient of repair of photosystem II after photoinactivation.Crossref | GoogleScholarGoogle Scholar |
Higuchi M, Noguchi T, Sonoike K (2003) Over-reduced states of the Mn-cluster in cucumber leaves induced by dark-chilling treatment. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1604, 151–158.
| Over-reduced states of the Mn-cluster in cucumber leaves induced by dark-chilling treatment.Crossref | GoogleScholarGoogle Scholar |
Iermak I, Szabo M, Zavafer A (2020) Analysis of OJIP transients during photoinactivation of photosystem II indicates the presence of multiple photosensitizers in vivo and in vitro. Photosynthetica 58, 497–506.
| Analysis of OJIP transients during photoinactivation of photosystem II indicates the presence of multiple photosensitizers in vivo and in vitro.Crossref | GoogleScholarGoogle Scholar |
Izawa S (1980) Acceptors and donors for chloroplast electron transport. Methods in Enzymology 69, 413–434.
| Acceptors and donors for chloroplast electron transport.Crossref | GoogleScholarGoogle Scholar |
Jones LW, Kok B (1966) Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiology 41, 1037–1043.
| Photoinhibition of chloroplast reactions. I. Kinetics and action spectra.Crossref | GoogleScholarGoogle Scholar | 16656345PubMed |
Kaniuga Z, Sochanowicz B, Zabek J, Krzystyniak K (1978) Photosynthetic apparatus in chilling-sensitive plants. I. Reactivation of hill reaction activity inhibited on the cold and dark storage of detached leaves and intact plants. Planta 140, 121–128.
| Photosynthetic apparatus in chilling-sensitive plants. I. Reactivation of hill reaction activity inhibited on the cold and dark storage of detached leaves and intact plants.Crossref | GoogleScholarGoogle Scholar | 24414467PubMed |
Kobayashi K, Endo K, Wada H (2016) Multiple impacts of loss of plastidic phosphatidylglycerol biosynthesis on photosynthesis during seedling growth of Arabidopsis. Frontiers in Plant Science 7, 336
| Multiple impacts of loss of plastidic phosphatidylglycerol biosynthesis on photosynthesis during seedling growth of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 27047516PubMed |
Kono M, Noguchi K, Terashima I (2014) Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant & Cell Physiology 55, 990–1004.
| Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Lazár D (2006) The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Functional Plant Biology 33, 9–30.
| The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light.Crossref | GoogleScholarGoogle Scholar | 32689211PubMed |
Li L, Aro EM, Millar AH (2018) Mechanisms of photodamage and protein turnover in photoinhibition. Trends in Plant Science 23, 667–676.
| Mechanisms of photodamage and protein turnover in photoinhibition.Crossref | GoogleScholarGoogle Scholar | 29887276PubMed |
Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annual Review of Plant Physiology and Plant Molecular Biology 45, 633–662.
| Photoinhibition of photosynthesis in nature.Crossref | GoogleScholarGoogle Scholar |
Lubitz W, Chrysina M, Cox N (2019) Water oxidation in photosystem II. Photosynthesis Research 142, 105–125.
| Water oxidation in photosystem II.Crossref | GoogleScholarGoogle Scholar | 31187340PubMed |
Margulies MM (1972) Effect of cold-storage of bean leaves on photosynthetic reactions of isolated chloroplasts. Inability to donate electrons to photosystem II and relation to manganese content. Biochimica et Biophysica Acta (BBA) – Bioenergetics 267, 96–103.
| Effect of cold-storage of bean leaves on photosynthetic reactions of isolated chloroplasts. Inability to donate electrons to photosystem II and relation to manganese content. Crossref | GoogleScholarGoogle Scholar |
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany 51, 659–668.
| Chlorophyll fluorescence – a practical guide.Crossref | GoogleScholarGoogle Scholar | 10938857PubMed |
Maxwell C, Griffiths H, Young AJ (1994) Photosynthetic acclimation to light regime and water-stress by the C3-CAM epiphyte Guzmania monostachia: gas-exchange characteristics, photochemical efficiency and the xanthophyll cycle. Functional Ecology 8, 746–754.
| Photosynthetic acclimation to light regime and water-stress by the C3-CAM epiphyte Guzmania monostachia: gas-exchange characteristics, photochemical efficiency and the xanthophyll cycle.Crossref | GoogleScholarGoogle Scholar |
Oguchi R, Terashima I, Chow WS (2009) The involvement of dual mechanisms of photoinactivation of Photosystem II in Capsicum annuum L. plants. Plant & Cell Physiology 50, 1815–1825.
| The involvement of dual mechanisms of photoinactivation of Photosystem II in Capsicum annuum L. plants.Crossref | GoogleScholarGoogle Scholar |
Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: Step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44, 8494–8499.
| Two-step mechanism of photodamage to photosystem II: Step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center.Crossref | GoogleScholarGoogle Scholar | 15938639PubMed |
Ono T (2001) Metallo-radical hypothesis for photoassembly of (Mn)(4)-cluster of photosynthetic oxygen evolving complex. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1503, 40–51.
| Metallo-radical hypothesis for photoassembly of (Mn)(4)-cluster of photosynthetic oxygen evolving complex.Crossref | GoogleScholarGoogle Scholar |
Öquist G, Greer DH, Ögren E (1987) Light stress at low temperature. In ‘Photoinhibition’. (Eds. DJ Kyle, CB Osmond, CJ Arntzen) pp. 67–87, (Elsevier: Amsterdam)
Osmond B, Chow WS, Wyber R, Zavafer A, Keller B, Pogson BJ, Robinson SA (2017) Relative functional and optical absorption cross-sections of PSII and other photosynthetic parameters monitored in situ, at a distance with a time resolution of a few seconds, using a prototype light induced fluorescence transient (LIFT) device. Functional Plant Biology 44, 985–1006.
| Relative functional and optical absorption cross-sections of PSII and other photosynthetic parameters monitored in situ, at a distance with a time resolution of a few seconds, using a prototype light induced fluorescence transient (LIFT) device.Crossref | GoogleScholarGoogle Scholar | 32480627PubMed |
Peeler TC, Naylor AW (1988) A comparison of the effects of chilling on thylakoid electron-transfer in pea (Pisum sativum L.) and cucumber (Cucumis sativus L.). Plant Physiology 86, 147–151.
| A comparison of the effects of chilling on thylakoid electron-transfer in pea (Pisum sativum L.) and cucumber (Cucumis sativus L.).Crossref | GoogleScholarGoogle Scholar | 16665857PubMed |
Roach T, Na CS, Stoggl W, Krieger-Liszkay A (2020) The non-photochemical quenching protein LHCSR3 prevents oxygen-dependent photoinhibition in Chlamydomonas reinhardtii. Journal of Experimental Botany 71, 2650–2660.
| The non-photochemical quenching protein LHCSR3 prevents oxygen-dependent photoinhibition in Chlamydomonas reinhardtii.Crossref | GoogleScholarGoogle Scholar | 31943079PubMed |
Schansker G, Toth SZ, Holzwarth AR, Garab G (2014) Chlorophyll a fluorescence: beyond the limits of the QA model. Photosynthesis Research 120, 43–58.
| Chlorophyll a fluorescence: beyond the limits of the QA model.Crossref | GoogleScholarGoogle Scholar | 23456268PubMed |
Shen JR, Terashima I, Katoh S (1990) Cause for dark, chilling-induced inactivation of photosynthetic oxygen-evolving system in cucumber leaves. Plant Physiology 93, 1354–1357.
| Cause for dark, chilling-induced inactivation of photosynthetic oxygen-evolving system in cucumber leaves.Crossref | GoogleScholarGoogle Scholar | 16667624PubMed |
Strasser RJ (2004) Analysis of the chlorophyll a fluorescence transient. In ‘Chlorophyll a fluorescence: a signature of photosynthesis, advances in photosynthesis and respiration’. (Eds G Papageorgiou, Govindjee) pp. 321–362. (Springer: Dordrecht)
Srivastava A, Strasser RJ (1995) How do land plants respond to stress temperature and stress light. Archives des Sciences 48, 135–146.
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In ‘Probing photosynthesis: mechanisms, regulation and adaptation’. (Eds M Yunus, U Pathre, P Mohanty) pp. 445–483. (Taylor & Francis: London)
Sullivan JH, Teramura AH, Ziska LH (1992) Variation in UV-B sensitivity in plants from a 3,000-M elevational gradient in Hawaii. American Journal of Botany 79, 737–743.
| Variation in UV-B sensitivity in plants from a 3,000-M elevational gradient in Hawaii.Crossref | GoogleScholarGoogle Scholar |
Suorsa M, Jarvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjarvi S, Paakkarinen V, Tikkanen M, Jansson S, Aro EM (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. The Plant Cell 24, 2934–2948.
| PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions.Crossref | GoogleScholarGoogle Scholar | 22822205PubMed |
Takahashi S, Milward SE, Yamori W, Evans JR, Hillier W, Badger MR (2010) The solar action spectrum of photosystem II damage. Plant Physiology 153, 988–993.
| The solar action spectrum of photosystem II damage.Crossref | GoogleScholarGoogle Scholar | 20460581PubMed |
Terashima I, Huang LK, Osmond CB (1989) Effects of leaf chilling on thylakoid functions, measured at room-temperature, in Cucumis sativus L. and Oryza sativa L. Plant & Cell Physiology 30, 841–850.
| Effects of leaf chilling on thylakoid functions, measured at room-temperature, in Cucumis sativus L. and Oryza sativa L.Crossref | GoogleScholarGoogle Scholar |
Terashima I, Kashino Y, Katoh S (1991a) Exposure of leaves of Cucumis sativus L. to low-temperatures in the light causes uncoupling of thylakoids. 1. Studies with isolated thylakoids. Plant & Cell Physiology 32, 1267–1274.
Terashima I, Sonoike K, Kawazu T, Katoh S (1991b) Exposure of leaves of Cucumis sativus L. to low-temperatures in the light causes uncoupling of thylakoids. 2. Nondestructive measurements with intact leaves. Plant & Cell Physiology 32, 1275–1283.
Tikhonov AN (2013) pH-dependent regulation of electron transport and ATP synthesis in chloroplasts. Photosynthesis Research 116, 511–534.
| pH-dependent regulation of electron transport and ATP synthesis in chloroplasts.Crossref | GoogleScholarGoogle Scholar | 23695653PubMed |
Tjus SE, Andersson B (1993) Loss of the trans-thylakoid proton gradient is an early event during photoinhibitory illumination of chloroplast preparations. Biochimica et Biophysica Acta 1183, 315–322.
| Loss of the trans-thylakoid proton gradient is an early event during photoinhibitory illumination of chloroplast preparations.Crossref | GoogleScholarGoogle Scholar |
Tóth SZ, Schansker G, Strasser RJ (2005a) In intact leaves, the maximum fluorescence level (FM) is independent of the redox state of the plastoquinone pool: a DCMU-inhibition study. Biochimica et Biophysica Acta 1708, 275–282.
| In intact leaves, the maximum fluorescence level (FM) is independent of the redox state of the plastoquinone pool: a DCMU-inhibition study.Crossref | GoogleScholarGoogle Scholar | 15869738PubMed |
Tóth SZ, Schansker G, Kissimon J, Kovacs L, Garab G, Strasser RJ (2005b) Biophysical studies of photosystem II-related recovery processes after a heat pulse in barley seedlings (Hordeum vulgare L.). Journal of Plant Physiology 162, 181–194.
| Biophysical studies of photosystem II-related recovery processes after a heat pulse in barley seedlings (Hordeum vulgare L.).Crossref | GoogleScholarGoogle Scholar | 15779828PubMed |
Tóth SZ, Schansker G, Garab G, Strasser RJ (2007) Photosynthetic electron transport activity in heat-treated barley leaves: the role of internal alternative electron donors to photosystem II. Biochimica et Biophysica Acta (BBA) – Bioenergetic 1767, 295–305.
| Photosynthetic electron transport activity in heat-treated barley leaves: the role of internal alternative electron donors to photosystem II. Crossref | GoogleScholarGoogle Scholar |
Wang QW, Kamiyama C, Hidema J, Hikosaka K (2016) Ultraviolet-B-induced DNA damage and ultraviolet-B tolerance mechanisms in species with different functional groups coexisting in subalpine moorlands. Oecologia 181, 1069–1082.
| Ultraviolet-B-induced DNA damage and ultraviolet-B tolerance mechanisms in species with different functional groups coexisting in subalpine moorlands.Crossref | GoogleScholarGoogle Scholar | 27139425PubMed |
Yamane Y, Kashino Y, Koike H, Satoh K (1997) Increases in the fluorescence Fo level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants. Photosynthesis Research 52, 57–64.
| Increases in the fluorescence Fo level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants.Crossref | GoogleScholarGoogle Scholar |
Yamane Y, Shikanai T, Kashino Y, Koike H, Satoh K (2000) Reduction of Q(A) in the dark: Another cause of fluorescence Fo increases by high temperatures in higher plants. Photosynthesis Research 63, 23–34.
| Reduction of Q(A) in the dark: Another cause of fluorescence Fo increases by high temperatures in higher plants.Crossref | GoogleScholarGoogle Scholar | 16252162PubMed |
Zavafer A, Cheah MH, Hillier W, Chow WS, Takahashi S (2015) Photodamage to the oxygen evolving complex of photosystem II by visible light. Scientific Reports 5, 16363
| Photodamage to the oxygen evolving complex of photosystem II by visible light.Crossref | GoogleScholarGoogle Scholar | 26560020PubMed |
Zavafer A, Koinuma W, Chow WS, Cheah MH, Mino H (2017) Mechanism of photodamage of the oxygen evolving Mn cluster of Photosystem II by excessive light energy. Scientific Reports 7, 7604
| Mechanism of photodamage of the oxygen evolving Mn cluster of Photosystem II by excessive light energy.Crossref | GoogleScholarGoogle Scholar | 28790352PubMed |
* These authors contributed equally to this work.