Physiological and cannabinoid responses of hemp (Cannabis sativa) to rock phosphate dust under tropical conditions
Luca De Prato A B * , Omid Ansari
A B * , Omid Ansari  C , Giles E. S. J. Hardy D E , John Howieson A , Graham O’Hara A and Katinka X. Ruthrof D F
C , Giles E. S. J. Hardy D E , John Howieson A , Graham O’Hara A and Katinka X. Ruthrof D F
A Murdoch University, Food Futures Institute, Murdoch, WA, Australia.
B Medicann Health Aust Pty Ltd, Osborne Park, WA, Australia.
C HempGenTech Pty Ltd, Kenmore, Qld, Australia.
D Murdoch University, Harry Butler Institute, Murdoch, WA, Australia.
E ArborCarbon, Murdoch University, Murdoch, WA, Australia.
F Department of Biodiversity, Conservation and Attractions, Kensington, WA 6151, Australia.
Functional Plant Biology 50(5) 378-389 https://doi.org/10.1071/FP22264
Submitted: 1 February 2022 Accepted: 27 February 2023 Published: 28 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Growing a high-value crop such as industrial hemp (Cannabis sativa L.) in post-mining environments is economically and environmentally attractive but faces a range of biotic and abiotic challenges. An opportunity to investigate the cultivation of C. sativa presented itself as part of post-mining activities on Christmas Island (Australia) to profitably utilise disused phosphate (PS) quarries. Challenges to plant growth and cadmium (Cd) uptake were addressed in this study using potted plants under fully controlled conditions in a growth chamber. A complete nutritional spectrum, slow-release fertiliser was applied to all plants as a control treatment, and two levels of rock PS dust, a waste product of PS mining that contains 35% phosphorus (P) and 40 ppm of naturally occurring Cd, were applied at 54 and 162 g L−1. After 12 weeks, control plants (no PS dust) significantly differed in phenological development, with no flower production, lower aboveground biomass and reduced photosynthesis efficiency than those with P applied as rock dust. Compared with the controls, the 54 g L−1 level of P dust increased shoot biomass by 38%, while 162 g L−1 increased shoot biomass by 85%. The concentration of Δ9-tetrahydrocannabinol also increased with the higher P levels. Cd uptake from PS dust by C. sativa was substantial and warrants further investigation. However, there was no increase in Cd content between the 54 and 162 g L−1 application rates in seed and leaf. Results indicate that hemp could become a high-value crop on Christmas Island, with the readily available rock PS dust providing a source of P.
Keywords: agriculture post-mining, cadmium, Cannabis sativa, flowering, heavy metal, industrial hemp, nutrition, photosynthesis.
Introduction
A range of biotic and abiotic challenges face agricultural activities in post-mining soils, including compromised soil fertility (Howieson et al. 2017) and the presence of heavy metals (Ruthrof et al. 2018a, 2018b). However, there are increasing needs to utilise such land given rising worldwide demands for food, and the desire to provide opportunities for communities around depleted mines. Further research and understanding of plant growth and physiology in the presence of degraded soils are needed (Godfray et al. 2010; Böhm et al. 2011).
Phosphorous (P) is essential for plant physiology and growth and is required for reproductive processes (López-Arredondo et al. 2014). Phosphorus plays a key role in energy generation, photosynthesis, glycolysis, membrane synthesis and stability, carbohydrate metabolism, symbiotic nitrogen fixation and colonisation of mycorrhizal fungi (Vance et al. 2003). When P is limiting, plants develop dark-green or purple shoots due to anthocyanin accumulation (Vance et al. 2003). Also, they tend to produce a higher number of lateral roots to explore the superficial soil patches where P is usually located (López-Arredondo et al. 2014), to increase in P transporters (Chiou et al. 2001; Liu et al. 2001), and to promote higher root exudation of organic acids and acid phosphatases (Liu et al. 2001; Ryan et al. 2001; Vance et al. 2003; López-Arredondo et al. 2014). For example, P deficient white lupin (Lupinus albus L.), pigeon pea (Cajanus cajan L.) and barrel medic (Medicago truncatula L.) increased the production of acid root exudates to release the inorganic P bound to cations (Liu et al. 2001).
In industrial hemp (Cannabis sativa L.), Vera et al. (2004) reported an increase in plant height and a reduction in seed yield with P fertilisation while, in subsequent studies, Vera et al. (2010) and Aubin et al. (2015) found a limited response of seed yield to P nutrition. Two medicinal C. sativa genotypes under controlled environment conditions showed reduced growth parameters and plant development with P rates lower than 30 mg L−1, while P excess did not affect the plant (Shiponi and Bernstein 2021a). The same study also showed that P deficiency affected the uptake and translocation of other nutrients, such as N, Mg, Zn and Ca, with an added rate of P of 30 mg L−1 (Shiponi and Bernstein 2021a). In another study on the identical medicinal C. sativa genotypes, P deficiency reduced net photosynthesis and stomatal conductance while decreasing the intercellular CO2 leaf concentration (Shiponi and Bernstein 2021b). However, further information is required regarding the role of P in C. sativa as the effects of P and other nutrients on growth and morpho-physiology were shown to be genotype dependant (Saloner et al. 2019; Shiponi and Bernstein 2021a).
A diverse range of plant physiological processes can be affected by the presence of heavy metals including inhibition of growth, photosynthesis, ion and water uptake, nitrate assimilation (Prasad and Strzalka 2013) and ultrastructural modifications of plant tissues and cells can also result (Gamalero et al. 2009). Morphologically, effects include inhibition of root elongation and premature senescence (Deikman 1997) and effects on fruit ripening, abscission, and senescence (Deikman 1997; Gamalero et al. 2009). Copper toxicity in hemp caused the inhibition of protein expression and regulation of the protein profile (Elisa et al. 2007). The overall health of plants is important in determining plant responses to heavy metals. For example, on a post-mining phosphate (PS) soil on Christmas Island, Australia, heavy metal leaf concentrations were lower when potassium was added to legume crops (Ruthrof et al. 2018a).
Following mining operations, soils can contain significant levels of heavy metals from the mining process, or via disturbance of naturally occurring heavy metals in the substrate (Allan 1995). In Nauru and Christmas Island, for example, heavy metals such as cadmium (Cd) occur naturally (Ruthrof et al. 2018a; Diarra and Prasad 2021). Hemp can grow on disturbed substrates (Petrová et al. 2012) and in both hemp and flax, for instance, concentrations of heavy metals, such as lead (Pb), nickel (Ni), Cd, zinc (Zn) and chromium (Cr), can differ between plant organs with levels in roots > stems > leaves > seeds (Angelova et al. 2004). However, although aboveground biomass has lower levels of heavy metals, hemp could be suited for phytoextraction of some heavy metals (Ni, Pb, and Cd) and could potentially remove approximately 126 g ha−1 of Cd per vegetation period without affecting fibre quality (Bhargava et al. 2012). In a glasshouse trial, hemp plants accumulated 832 μg of Cd/plant on average (Linger et al. 2005), while another study reported that hemp accumulated 66 μg g−1 (Cd) in the shoots (Citterio et al. 2003) (considering an average biomass of 10 t ha−1, it would be approximately 830 g ha−1 in the former study, and 660 g ha−1 in the latter study). However, accumulation in aboveground organs is a vital consideration when food or medicines are the final products. Genotype was shown to be the most important factor affecting heavy metal uptake (Angelova et al. 2004). In terms of plant physiology, Linger et al. (2005) showed that levels of Cd over 50 μg g−1 in the leaves of hemp strongly affected plant viability and vitality. In Cd polluted soils, hemp had reduced chlorophyll synthesis with a decrease in photosynthetic capabilities and energy distribution of PSII (Linger et al. 2005).
To explore the impacts of growing hemp on post-mining land and to understand its P requirements and responses to Cd, a tropical/subtropical hemp variety was grown under fully controlled growth room conditions. Exposure to two levels of Christmas Island rock PS dust was tested to assess the responses of hemp to phenology, photosynthesis, growth, Cd uptake, and cannabinoid production. Given the various challenges outlined above, the following questions were asked: (1) what are the effects of the addition of P dust on hemp photosynthesis, phenology, growth, biomass, seed and cannabinoid production; and (2) what is the uptake and deposition of Cd in leaves and seeds?
Materials and methods
Study location and design
A potting trial was conducted in December 2018–March 2019 in a fully controlled growth room in secure premises at the Department of Primary Industries and Regional Development (DPIRD), South Perth, Western Australia (licence number: 028). The room measured 2.5 m (length) by 2 m (width) by 2.3 m (height). The light source was composed of 24 600 W metal halide lamps, which provided an average intensity of 350–400 PPFD (photosynthetic photon flux density) μmol m−2 s−1 at the canopy level, a sufficient intensity for hemp growth (Magagnini et al. 2018). Daylength was set at 12 h and temperature at an average temperature of 25–27°C to mimic the environmental conditions of Christmas Island (10°29′06″S, 105°37′38″E).
An Australian tropical/subtropical variety of industrial hemp (Cannabis sativa L.), ‘ECO-MC16’, with low photoperiod sensitivity, was used for the experiment. Ecofibre Ltd. (Brisbane, Qld, Australia) provided and bred the variety for crop production at lower latitudes and was previously grown on Christmas Island in preliminary field trials (De Prato 2021) and glasshouse trials (De Prato et al. 2022a).
On 19 December 2018, 275 seeds were placed in 11 covered plastic containers (175 mm × 120 mm × 55 mm) for germination; 25 seeds were placed in each container on a paper towel, soaked with distilled water, and then covered with a second paper towel. Containers were wrapped in aluminium foil to ensure no light penetration (Sera et al. 2017) and placed in the dark at 25°C for 72 h at which time there were on average 20 germinated seeds in each container.
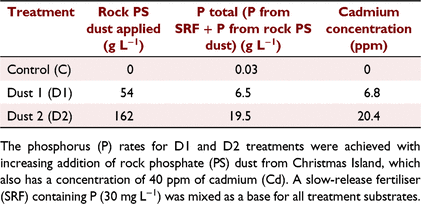
On 23 December 2018, seedlings were transplanted singly into 200 mm (3.2 L), free-draining standard pots (Premium Plastic, Wangara, Western Australia) containing a base medium of cocopeat and perlite in a ratio of 2:1 (v:v). Three grams of a six-month wax based slow-release fertiliser (SRF) 18:1:10 (N:P:K) with trace elements (Troforte Innovations, Wangara, Western Australia) were mixed for each litre of the substrate with a base P equal to 30 mg L−1 of P, considered a sufficient P amount by Shiponi and Bernstein (2021a). Following that, three rates of rock dust from Christmas Island (containing 30% P2O5) were set up: (1) a zero control (C); (2) a treatment with 54 g L−1 rock PS dust added (D1); and (3) a treatment with 162 g L−1 added (D2) of the substrate. The dust is a P-rich waste product from PS rock mining and contains Cd (Tables 1 and 2).

|
|
There were 22 pots in each treatment, and pots were arranged in a complete randomised design. Each pot was connected to an automatic irrigation dripper (4 L h−1 per pot), starting at 60 mL daily/pot and increasing according to plant size until harvest, reaching 540 mL/pot daily. Pots were re-randomised every fortnight.
Plant development was assessed at Day 10 after transplanting, then weekly, using a coding method to record development data and flowering (Mediavilla et al. 1998). This also included weekly height (cm) and general plant observations. Further physiological data were collected from only the female plants (code 2201–2202). On Day 66, data were collected from female plants for net photosynthesis (PN) with increasing photosynthetic photon flux density (PPFD) using a LcPro+ (ADC Bioscientific, UK) on the youngest, fully expanded leaf on five randomly selected plants per treatment. Each step between changing conditions lasted at least 3 min to allow the leaf to stabilise to the changing environment (Mengistu et al. 2012; Tang et al. 2017). On the following day (Day 67 from emergence), the PN rate under increasing CO2 concentration at constant PPFD (1600 μmol m−2 s−1) and temperature (27°C) were measured with the same instrument and process. At the same time (Day 67 from emergence), chlorophyll α-fluorescence and other parameters of PSII were also collected with a chlorophyll fluorometer efficiency analyser (Handy-PEA Fluorometer, Hansatech Instruments Ltd, King’s Lynn, Norfolk, UK) with the Handy-PEA dark leaf clips placed on the youngest, fully expanded leaves on five female plants for each treatment after 30 min of dark adaptation (Maxwell and Johnson 2000; Malceva et al. 2011). The maximum yield of primary photochemistry (ΨPo = 1−(Fo/Fm) = Fv/Fm) is the main indicator for plant stress measurement on the dark fluorescence (OJIP), while the photochemical activity of the PSII is an indicator of the number and the size of active photosynthetic reaction centres (Fv/Fo) (Cen et al. 2017). Performance index (PIabs) was also recorded, which is the expression of the product of three terms representing the divergences of energy in PS II (Maxwell and Johnson 2000; Strasser et al. 2000; Stirbet and Govindjee 2011).
Final height (cm) and stalk diameter (measured at the soil level, mm) were measured for all plants before harvesting. On Day 70, male plants were harvested at the senescence stage (2103/4) (Mediavilla et al. 1998), while female plants were grown until Day 90 when plants had reached seed ripening stage (2204) (Mediavilla et al. 1998). Each plant shoot was cut at soil level and placed in a labelled paper bag for drying in a ventilated oven at 40°C for 48 h or until a constant dry weight was achieved (Calzolari et al. 2017). Dry weight was then recorded; flowers and bracts were separated for cannabinoid analysis and tested via GC-MS (De Prato et al. 2022b), and seed weight was assessed for harvest index (HI). The soil was gently separated from roots, washed with tap water to eliminate all the residual potting mix material and placed into labelled paper bags and dried at 40°C for 48 h or until a constant dry weight was achieved (Calzolari et al. 2017).
Leaf samples from five random female plants for each treatment were collected after being fully dried and ground with an electric spice grinder (model BCG200BSS, Breville). Between samples, the grinder was wiped with a 70% ethanol solution to avoid contamination. Each sample was then stored in a 25 mL vial and analysed for inorganic minerals through an ICP-AES analysis methodology at the Marine and Freshwater Research Laboratory at Murdoch University (Perth, Western Australia), a NATA accredited laboratory. Total P and Cd concentrations were obtained and analysed.
The cannabinoid profile was measured in dried female flowers collected from three separate plants for each treatment, following the methodology described by De Prato et al. (2022b). Cannabinoids included the following: Δ9-tetrahydrocannabinol (Δ9-THC or THC), tetrahydrocannabinolic acid (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabinol (CBN), cannabigerol (CBG), cannabigerolic acid (CBGA), cannabichromene (CBC), cannabidivarin (CBDV) and Δ8-tetrahydrocannabinol (Δ8-THC).
Statistical analysis
All statistical analyses were performed in R studio software (RStudio Team 2020). For time to flowering data, a generalised linear model was used (glm function) to test a logistic regression; residuals were analysed to verify the fitness of the model. A repeated measure ANOVA was performed for height and dust treatments vs time after assumptions for fitness were tested. Gas exchange data were analysed with the package ‘plantecophys’ for non-linear data according to the Farquhar-von Caemmerer-Berry (FvCB) model of leaf photosynthesis (Duursma 2015). For dry shoot and root weight data, a linear mixed model (lmer function) was used with treatment as a fixed effect and replicate as a random variable (Zuur et al. 2009; Bates et al. 2014). Assumption of normality and homoscedasticity were tested for all model fitness. The graphs were then created with the ggplot2 package (Wickham 2011). For the cannabinoid results, statistical metabolomics analysis was run through Metaboanalyst ver. 4.0 (Chong et al. 2019). The data were log10 transformed and normalised, a one-way ANOVA (P < 0.05) was run on the single cannabinoid.
Results
Time to flowering
The weekly flowering data were used to create a simulated model of the time to flowering in response to P addition (Fig. 1). Time to flowering (considered as full when 50% of stems flowers on individual plants (Tang et al. 2016)) differed significantly (P < 0.001) between male and female plants, with female plants reaching full flowering around 16 days later than male plants (Fig. 1). Time to flowering for the control treatment (0 g L−1 rock PS dust) showed a significant (P < 0.001) delay compared to plants with added rock PS dust. This delay was approximately 15 and 10 days for males and females, respectively. Female plants in the control treatment did not reach 100% plant flowering.
Plant photosynthesis
Plant PN with increasing PPFD (μmol m−2 s−1) (Fig. 2) was higher with the increasing addition of P rock dust (P < 0.001). The addition of 162 g L−1 (D2) showed the highest PN values, followed by the lower application of rock PS dust (D1) and then the control (0 g L−1 rock PS dust) treatment (P < 0.001).
Responses of PN with increasing CO2 were also significant and positive (P < 0.001) (Fig. 3). However, PN with increasing CO2 was not affected by increasing rock PS dust rate (P = 0.9820), and the model regression was 66% (R2 = 0.66).
Chlorophyll α-fluorescence analysis (Table 3) did not reveal significant (P > 0.05) differences between the treatments for the Fv/Fm, PIabs, and Fv/Fo. However, Fv/Fm and Fv/Fo were lower in the lower dust level (D1) treatment, indicating a possible increase in plant stress level.

|
Growth responses
Female plants grew taller (P < 0.001) with the addition of rock PS dust. The fitted model showed a linear regression of R2 = 0.91 for height on days from emergence (Fig. 4).
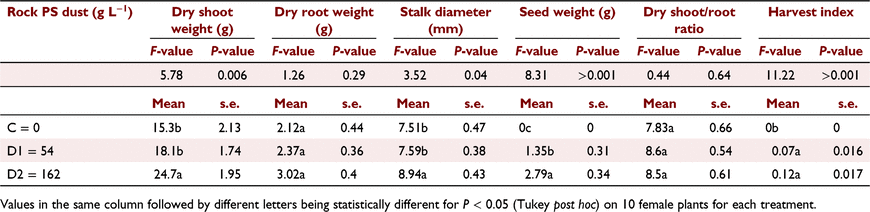
The addition of the rock PS dust resulted in complete female flowering development and a final shoot biomass increase of 69%, and 109% for 54 g L−1 (D1) and 162 g L−1 (D2) rock PS dust, respectively, compared to the zero control (Table 4). The increase was displayed only for shoot dry weight due to bigger inflorescences and thicker stems. Final stalk diameter for female plants was greater with increased rock PS dust application, with plants in the higher rate (D2, 162 g L−1) having the thickest stalks (Table 4). Root dry weight and shoot/root ratio showed a similar increasing trend, even though it was not significantly (P > 0.05) different (Table 4).
Seed weight was higher with increasing rock PS dust (Table 4). The highest rate (D2) doubled seed yield compared to the lower rate (D1), while the control treatment did not produce any seeds as female plants either did not flower or reach the seeding stage. The HI values were higher (Table 4) with rock PS dust. On average, every mg L−1 of P added by the dust treatments, increased the seed weight by 0.05 g.
Cannabinoids, nutrient and cadmium analysis
Cannabinoid concentrations showed that rock PS dust affected the plant chemical profile differently (Fig. 5). Four cannabinoids (CBDV, CBN, Δ8-THC and Δ9-THC) of the 10 compounds analysed were affected (P < 0.01) by rock PS dust addition treatments. For CBN, Δ9-THC and Δ8-THC, the highest application rock PS dust rate (D2) produced (P < 0.01) higher concentrations than the lower level of dust or the control treatments. In contrast, CBDV, CBG and CBD decreased with the addition of rock PS dust rates, as the concentrations were higher on the control treatment (Fig. 5).
The concentration of P in the leaves increased with rock PS dust addition rate, with the difference being significantly (P < 0.05) higher than the control in the plants receiving the highest rate (D2) (Fig. 6a). Cd concentrations in leaves were higher (P < 0.001) for D1 and D2 than in the control treatment (Fig. 6b). No seeds were produced in the control (C) plants; therefore, Cd concentration analysis could not be performed. Phosphorus at the two levels applied did not result in a difference in Cd concentration in the seeds, which contained 0.55 (±0.09 s.e.) mg kg−1 and 0.68 (±0.05 s.e.) mg kg−1 Cd in plants at the low (D1) and high (D2) rates, respectively.
Discussion
In this study, hemp plants grown without rock PS dust had significantly delayed and limited flowering development in female and male plants. Female plants grown in the zero P treatment were stunted and produced no seeds, while plants treated with rock PS dust developed well and completed their life cycle. Increased availability of P was critical for flowering and seed formation. Soils deficient in P are well known to limit plant development and growth (Plénet et al. 2000a; Vance et al. 2003; Jin and Hasegawa 2008; López-Arredondo et al. 2014). Delayed flowering has also been observed in P-deficient thale cress (Arabidopsis thaliana L.) plants, which showed limited starch synthesis resulting from a low activity of phosphor-isomerases (Plaxton and Tran 2011). These responses show that carbohydrate metabolism plays a role in floral initiation (Yu et al. 2000; Xiong et al. 2009). In Arabidopsis, P starvation and stress reaction signalling also cause post-translational processes implicated in plant regulation for floral initiation (Jin and Hasegawa 2008). Similarly, in the present study, P deficient hemp plants might have processed a limited quantity of carbohydrates in response to P deficiency, thereby limiting, and for some treatments eliminating, flowering. However, a few studies on other genotypes of C. sativa showed that a P rate similar to our control (30 mg L−1) was optimal for flower development, and P rates lower than 7.5 and 15 mg L−1, respectively, reduced flowering and plant growth (Cockson et al. 2020; Shiponi and Bernstein 2021b).
At the cellular level, P deprivation affects the rate of photosynthesis, respiration and photosynthate partitioning in leaves (Thorsteinsson and Tillberg 1987; Lauer et al. 1989). In the present study, in response to the increasing P concentration in leaves, the PN rate at comparable PPFD showed a significant increase with the addition of rock PS dust for both levels (D1 and D2) vs control plants. This indicates that hemp plants grown at 30 mg L−1 had functional limitations due to P deficiency, which may hamper growth, as suggested by Shiponi and Bernstein (2021a). Increasing CO2 concentration at a set PPFD at leaf level showed an increase in PN; plants treated with the highest P concentration showed a lower PN rate under increasing CO2 concentration than those given less or no P dust. This could be linked to lower stomatal conductance and, therefore, higher regulation of gas exchange (De Prato et al. 2022c). These results differ from Shiponi and Bernstein (2021b) and Cockson et al. (2020) estimates of medical C. sativa genotypes morpho-physiology, which showed a satisfactory functional plant development at P rates at and above 30 mg L−1 for PN, stomatal conductance and transpiration rates. However, the findings from the present study are broadly consistent with earlier studies on P deficiency. In other species, such as Hordeum vulgare L., Spinacia oleracea L. and Glycine max L., P deficiency also caused a decline in the PN rate, along with a decrease in the sucrose starch ratio (Foyer and Spencer 1986). The variation between the previous C. sativa studies could be related to genotypical differences in mineral responses, as indicated previously by Saloner et al. (2019).
The importance of P for reproductive development and seed filling was shown in G. max, where large percentages of P were remobilised from plant tissue to seeds despite the impact on metabolic efficiency (Lauer et al. 1989). In the present study, under control treatment with base P availability, there was less P in the foliage of hemp and the PN rate was reduced, resulting in delayed plant development/flowering and reduced biomass. These responses in hemp mirrored those in G. max (Lauer et al. 1989) and other genotypes of C. sativa (Shiponi and Bernstein 2021a). In the latter study on medicinal genotypes of C. sativa, Shiponi and Bernstein (2021a) showed that P deficiency (under 15 mg L−1) affected the uptake and translocation of other mineral nutrients, such as Mg, Zn and Ca, with the retention of said elements in root tissues.
Plant growth and biomass accumulation are affected by P deficiency due to inorganic PS being necessary for carbon export from the chloroplast (Lauer et al. 1989). Furthermore, plants grown under P starvation have been found to modify the shoot/root ratio with an increased proliferation of root hairs and an increase in the secretion of organic acids in root exudates to mobilise and extract P from the soil (Ryan et al. 2001). In another study, the growth of eight annual pasture species was compared in a P deficient soil, revealing that the requirement for P changed during the life cycle, although final biomass was negatively affected by P deficiency for all species (Ozanne et al. 1969). In industrial hemp field experiments, no effect of additional P has been recorded (Aubin et al. 2015), while in a P deficient soil, P fertilisation increased height, but biomass and seed yields were reduced (Vera et al. 2004). In a controlled environment, effects of P under 15 mg L−1 on C. sativa genotypes showed limitations on photosynthesis, stomatal conductance, intercellular CO2 and other nutrients uptake and translocation on shoots, such as Mg, Zn and Ca, resulting in decreased aboveground biomass, inflorescence development and cannabinoid content (Cockson et al. 2020; Shiponi and Bernstein 2021a, 2021b). Similarly, in the present study, the tropical/subtropical hemp variety responded to increasing P fertilisation rates with increasing height, biomass, and seed yields. However, plant flower development and growth were highly decreased by P rate at 30 mg L−1 compared to the studies mentioned above. This response is partly associated with the increased PN rate and P availability at leaf level seen in plants treated with either level of P dust and it could be related to genotype response variability (Saloner et al. 2019). Higher PN rates could be related to a higher production of carbohydrates and sugars that would increase biomass production. Similarly, a decrease in hemp shoot biomass by P deficiency could be linked to previous findings on Zea mays L. cultivated in P deficient soil where shoot biomass and seed yield were reduced significantly by lower light absorption due to reduced leaf growth (Plénet et al. 2000a, 2000b).
In the present study, root biomass was not increased by P addition which may have been due to the size of the pot limiting root growth, and the effect must be investigated under field conditions. Seed production in the control treatment was significantly reduced due to the marked effects of P deficiency on flowering, as shown by the increased seed weight with P dust supply. This indicates an effect of P nutrition on reproductive efficiency in hemp similar to that seen in the pasture legumes such as clover (Trifolium subterraneum L.) and serradella (Ornithopus compressus L.), which displayed an increase in seed yields in the field by roughly 20 kg ha−1 for each kg ha−1 of P applied (Bolland 1985). Further investigation is needed to understand seed production dynamics in large scale hemp field trials, at sites where P supply is marginal.
Changes in the hemp cannabinoid profile have been associated with environmental effects and nutritional deficiencies (Valle et al. 1978; Bócsa et al. 1997; Small et al. 2003; Bernstein et al. 2019). An extra hour of daylength increased Δ9-THC, CBN and Δ8-THC while reducing CBD and CBDV concentrations in some tropical/subtropical hemp varieties (De Prato et al. 2022a). In particular, nitrogen (Bernstein et al. 2019; Saloner and Bernstein 2021; De Prato et al. 2022a) and slow-release potassium (De Prato et al. 2022c) can affect cannabinoid concentrations. In the present trial, the cannabinoids significantly increased by P fertilisation were CBN (the degraded form of THCs), Δ8-THC and the psychoactive Δ9-THC. At the same time, CBDV and CBD were higher in the control plants, a similar trend to the previous hemp findings under different potassium applications (De Prato et al. 2022c). The trend could be related to plant limitation on nutrient uptake, such as nitrogen, as indicated by Saloner and Bernstein (2021) and Shiponi and Bernstein (2021a). Thus, although these metabolic changes are likely related to higher amino acid and sugar synthesis due to an increased PN rate with increased K or P, the results are different for the various cannabinoids and explained by the C. sativa genotypic variability responses (Saloner et al. 2019).
The Cd concentration in leaves and seeds was similar in the present study and increasing levels of dust application (resulting in increased Cd in the soil) did not cause a parallel increase in Cd levels in leaves and seeds. This is in contrast to the results of Linger et al. (2002), who reported hemp Cd concentrations to be higher in leaves than seeds. In the present study, the fact that Cd concentration in leaves and seeds did not increase with increasing P levels indicates the plant excluded Cd from uptake or Cd content was diluted due to the increased shoot growth and seed production. Linger et al. (2005) and Citterio et al. (2003) observed no difference in the growth of hemp plants with soil concentrations of 17 and 27 ppm Cd. However, with 72 ppm of Cd, photosynthesis and growth were reduced (Linger et al. 2005). We found that the maximum soil concentration of Cd was comparatively lower at 20 ppm, and thus is not unexpected that there was no difference in the growth of our hemp plants. Given that the hemp plants in this study accumulated Cd when P rock dust was added, it is essential to identify the end use for the crop before applying fertilisers or P dust containing heavy metals, such as Cd.
Conclusion
The present study investigated the growth of a tropical/subtropical variety of C. sativa in a P deficient substrate ameliorated with a byproduct of PS processing, a dust containing 30% of P2O5. When P was deficient, PN rate and growth were reduced, flowering initiation prevented or delayed, and final shoot biomass reduced. Cannabinoids, such as Δ9-THC and Δ8-THC, increased in concentration with P addition, and this could be linked with the increased PN level and other nutrient uptake. Rock dust rich in P is a valuable source of this element, and readily available after PS mining; however, the Cd contained may accumulate in leaves and seeds and will require verification under larger-scale field trials.
Data availability
The data that support this study are available in the article.
Conflicts of interest
The entire experiment was conducted by Murdoch University, independent of Ecofibre Ltd. Dr Omid Ansari was an employee of Ecofibre Ltd. when the research was conducted. All other authors declare no conflicts of interest with respect to the work described in this manuscript. Fertilisers were provided by Sunpalm Australia, rock PS dust was received from Phosphate Resources Ltd and industrial hemp seeds were obtained by Ecofibre Ltd. However, those companies had no role in the study design, data collection or writing of the report.
Declaration of funding
Phosphate Resource Limited (PRL) and the Australian Research Council (ARC) (Project LP140100690) provided project funding.
Acknowledgements
We thank Dr Ron Yates and the DPIRD for support and the growth space availability; Emeritus Professor Jen McComb at Murdoch University for suggestions; and the Post-Harvest and Food Biosecurity Laboratory (Murdoch University) for the use of laboratory instruments for cannabinoid testing.
References
Allan RJ (1995) Impact of mining activities on the terrestrial and aquatic environment with emphasis on mitigation and remedial measures. In ‘Heavy metals’. (Ed. U Förstner, W Salomons, P Mader) pp. 119–140. (Springer: Berlin, Heidelberg)Angelova V, Ivanova R, Delibaltova V, Ivanov K (2004) Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Industrial Crops and Products 19, 197–205.
| Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp).Crossref | GoogleScholarGoogle Scholar |
Aubin M-P, Seguin P, Vanasse A, Tremblay GF, Mustafa AF, Charron J-B (2015) Industrial hemp response to nitrogen, phosphorus, and potassium fertilization. Crop, Forage & Turfgrass Management 1, 1–10.
| Industrial hemp response to nitrogen, phosphorus, and potassium fertilization.Crossref | GoogleScholarGoogle Scholar |
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 48
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bernstein N, Gorelick J, Zerahia R, Koch S (2019) Impact of N, P, K, and humic acid supplementation on the chemical profile of medical Cannabis (Cannabis sativa L). Frontiers in Plant Science 10, 736
| Impact of N, P, K, and humic acid supplementation on the chemical profile of medical Cannabis (Cannabis sativa L).Crossref | GoogleScholarGoogle Scholar |
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. Journal of Environmental Management 105, 103–120.
| Approaches for enhanced phytoextraction of heavy metals.Crossref | GoogleScholarGoogle Scholar |
Bolland MDA (1985) Effects of phosphorus on seed yields of subterranean clover, serradella and annual medics. Australian Journal of Experimental Agriculture 25, 595–602.
| Effects of phosphorus on seed yields of subterranean clover, serradella and annual medics.Crossref | GoogleScholarGoogle Scholar |
Bócsa I, Máthé P, Hangyel L (1997) Effect of nitrogen on tetrahydrocannabinol (THC) content in hemp (Cannabis sativa L.) leaves at different positions. Journal of the International Hemp Association 4, 78–79.
Böhm C, Quinkenstein A, Freese D, Hüttl RF (2011) Assessing the short rotation woody biomass production on marginal post-mining areas. Journal of Forest Science 57, 303–311.
| Assessing the short rotation woody biomass production on marginal post-mining areas.Crossref | GoogleScholarGoogle Scholar |
Calzolari D, Magagnini G, Lucini L, Grassi G, Appendino GB, Amaducci S (2017) High added-value compounds from Cannabis threshing residues. Industrial Crops and Products 108, 558–563.
| High added-value compounds from Cannabis threshing residues.Crossref | GoogleScholarGoogle Scholar |
Cen H, Weng H, Yao J, He M, Lv J, Hua S, Li H, He Y (2017) Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus Huanglongbing. Frontiers in Plant Science 8, 1509
| Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus Huanglongbing.Crossref | GoogleScholarGoogle Scholar |
Chiou T-J, Liu H, Harrison MJ (2001) The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. The Plant Journal 25, 281–293.
| The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface.Crossref | GoogleScholarGoogle Scholar |
Chong J, Wishart DS, Xia J (2019) Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Current Protocols in Bioinformatics 68, e86
| Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis.Crossref | GoogleScholarGoogle Scholar |
Citterio S, Santagostino A, Fumagalli P, Prato N, Ranalli P, Sgorbati S (2003) Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant and Soil 256, 243–252.
| Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L.Crossref | GoogleScholarGoogle Scholar |
Cockson P, Schroeder-Moreno M, Veazie P, Barajas G, Logan D, Davis M, Whipker BE (2020) Impact of phosphorus on Cannabis sativa reproduction, cannabinoids, and terpenes. Applied Sciences 10, 7875
| Impact of phosphorus on Cannabis sativa reproduction, cannabinoids, and terpenes.Crossref | GoogleScholarGoogle Scholar |
De Prato L (2021) Genotype by environment interactions of industrial hemp (Cannabis sativa L.) varieties under tropical conditions. PhD thesis, Murdoch University.
De Prato L, Ansari O, Hardy GESJ, Howieson J, O’Hara G, Ruthrof KX (2022a) The cannabinoid profile and growth of hemp (Cannabis sativa L.) is influenced by tropical daylengths and temperatures, genotype and nitrogen nutrition. Industrial Crops and Products 178, 114605
| The cannabinoid profile and growth of hemp (Cannabis sativa L.) is influenced by tropical daylengths and temperatures, genotype and nitrogen nutrition.Crossref | GoogleScholarGoogle Scholar |
De Prato L, Timmins M, Ansari O, Ruthrof KX, Hardy GESJ, Howieson J, O’Hara G (2022b) Semi-quantitative analysis of cannabinoids in hemp (Cannabis sativa L.) using gas chromatography coupled to mass spectrometry. Journal of Cannabis Research 4, 51
| Semi-quantitative analysis of cannabinoids in hemp (Cannabis sativa L.) using gas chromatography coupled to mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
De Prato L, Ansari O, Hardy GESJ, Howieson J, O’Hara G, Ruthrof KX (2022c) Morpho-physiology and cannabinoid concentrations of hemp (Cannabis sativa L.) are affected by potassium fertilisers and microbes under tropical conditions. Industrial Crops and Products 182, 114907
| Morpho-physiology and cannabinoid concentrations of hemp (Cannabis sativa L.) are affected by potassium fertilisers and microbes under tropical conditions.Crossref | GoogleScholarGoogle Scholar |
Deikman J (1997) Molecular mechanisms of ethylene regulation of gene transcription. Physiologia Plantarum 100, 561–566.
| Molecular mechanisms of ethylene regulation of gene transcription.Crossref | GoogleScholarGoogle Scholar |
Diarra I, Prasad S (2021) The current state of heavy metal pollution in Pacific Island Countries: a review. Applied Spectroscopy Reviews 56, 27–51.
| The current state of heavy metal pollution in Pacific Island Countries: a review.Crossref | GoogleScholarGoogle Scholar |
Duursma RA (2015) Plantecophys – an R package for analysing and modelling leaf gas exchange data. PLoS ONE 10, e0143346
| Plantecophys – an R package for analysing and modelling leaf gas exchange data.Crossref | GoogleScholarGoogle Scholar |
Elisa B, Marsano F, Cavaletto M, Berta G (2007) Copper stress in Cannabis sativa roots: morphological and proteomic analysis. Caryologia 60, 96–101.
| Copper stress in Cannabis sativa roots: morphological and proteomic analysis.Crossref | GoogleScholarGoogle Scholar |
Foyer C, Spencer C (1986) The relationship between phosphate status and photosynthesis in leaves. Planta 167, 369–375.
| The relationship between phosphate status and photosynthesis in leaves.Crossref | GoogleScholarGoogle Scholar |
Gamalero E, Lingua G, Berta G, Glick BR (2009) Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress. Canadian Journal of Microbiology 55, 501–514.
| Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress.Crossref | GoogleScholarGoogle Scholar |
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327, 812–818.
| Food security: the challenge of feeding 9 billion people.Crossref | GoogleScholarGoogle Scholar |
Howieson J, Calmy H, Ballard N, Skinner P, WO’Hara G, Skinner L, Ruthrof KX, Swift R, Ballard V, St Hardy GE, McHenry MP (2017) Bread from stones: post-mining land use change from phosphate mining to farmland. The Extractive Industries and Society 4, 290–299.
| Bread from stones: post-mining land use change from phosphate mining to farmland.Crossref | GoogleScholarGoogle Scholar |
Jin JB, Hasegawa PM (2008) Flowering time regulation by the SUMO E3 ligase SIZ1. Plant Signaling & Behavior 3, 891–892.
| Flowering time regulation by the SUMO E3 ligase SIZ1.Crossref | GoogleScholarGoogle Scholar |
Lauer MJ, Blevins DG, Sierzputowska-Gracz H (1989) 31P-nuclear magnetic resonance determination of phosphate compartmentation in leaves of reproductive soybeans (Glycine max L.) as affected by phosphate nutrition. Plant Physiology 89, 1331–1336.
| 31P-nuclear magnetic resonance determination of phosphate compartmentation in leaves of reproductive soybeans (Glycine max L.) as affected by phosphate nutrition.Crossref | GoogleScholarGoogle Scholar |
Linger P, Müssig J, Fischer H, Kobert J (2002) Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential. Industrial Crops and Products 16, 33–42.
| Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential.Crossref | GoogleScholarGoogle Scholar |
Linger P, Ostwald A, Haensler J (2005) Cannabis sativa L. growing on heavy metal contaminated soil: growth, cadmium uptake and photosynthesis. Biologia Plantarum 49, 567–576.
| Cannabis sativa L. growing on heavy metal contaminated soil: growth, cadmium uptake and photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Liu J, Uhde-Stone C, Li A, Vance C, Allan D (2001) A phosphate transporter with enhanced expression in proteoid roots of white lupin (Lupinus albus L.). Plant and Soil 237, 257–266.
| A phosphate transporter with enhanced expression in proteoid roots of white lupin (Lupinus albus L.).Crossref | GoogleScholarGoogle Scholar |
López-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L (2014) Phosphate nutrition: improving low-phosphate tolerance in crops. Annual Review of Plant Biology 65, 95–123.
| Phosphate nutrition: improving low-phosphate tolerance in crops.Crossref | GoogleScholarGoogle Scholar |
Magagnini G, Grassi G, Kotiranta S (2018) The effect of light spectrum on the morphology and cannabinoid content of Cannabis sativa L. Medical Cannabis and Cannabinoids 1, 19–27.
| The effect of light spectrum on the morphology and cannabinoid content of Cannabis sativa L.Crossref | GoogleScholarGoogle Scholar |
Malceva M, Vikmane M, Stramkale V (2011) Changes of photosynthesis-related parameters and productivity of Cannabis sativa under different nitrogen supply. Environmental and Experimental Biology 9, 61–69.
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany 51, 659–668.
| Chlorophyll fluorescence – a practical guide.Crossref | GoogleScholarGoogle Scholar |
Mediavilla V, Jonquera M, Schmid-Slembrouck I, Soldati A (1998) Decimal code for growth stages of hemp (Cannabis sativa L.). Journal of the International Hemp Association 5, 68–74.
Mengistu T, Sterck FJ, Anten NPR, Bongers F (2012) Frankincense tapping reduced photosynthetic carbon gain in Boswellia papyrifera (Burseraceae) trees. Forest Ecology and Management 278, 1–8.
| Frankincense tapping reduced photosynthetic carbon gain in Boswellia papyrifera (Burseraceae) trees.Crossref | GoogleScholarGoogle Scholar |
Ozanne PG, Keay J, Biddiscombe EF (1969) The comparative applied phosphate requirements of eight annual pasture species. Australian Journal of Agricultural Research 20, 809–818.
| The comparative applied phosphate requirements of eight annual pasture species.Crossref | GoogleScholarGoogle Scholar |
Petrová S, Benešová D, Soudek P, Vaněk T (2012) Enhancement of metal(loid)s phytoextraction by Cannabis sativa L. Journal of Food, Agriculture and Environment 10, 631–641.
Plaxton WC, Tran HT (2011) Metabolic adaptations of phosphate-starved plants. Plant Physiology 156, 1006–1015.
| Metabolic adaptations of phosphate-starved plants.Crossref | GoogleScholarGoogle Scholar |
Plénet D, Etchebest S, Mollier A, Pellerin S (2000a) Growth analysis of maize field crops under phosphorus deficiency. Plant and Soil 223, 119–132.
| Growth analysis of maize field crops under phosphorus deficiency.Crossref | GoogleScholarGoogle Scholar |
Plénet D, Mollier A, Pellerin S (2000b) Growth analysis of maize field crops under phosphorus deficiency. II. Radiation-use efficiency, biomass accumulation and yield components. Plant and Soil 224, 259–272.
| Growth analysis of maize field crops under phosphorus deficiency. II. Radiation-use efficiency, biomass accumulation and yield components.Crossref | GoogleScholarGoogle Scholar |
Prasad MNV, Strzalka K (2013) ‘Physiology and biochemistry of metal toxicity and tolerance in plants.’ pp. 432. (Springer Netherlands: Dordrecht, NL)
RStudio Team (2020) RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. Available at http://www.rstudio.com/
Ruthrof KX, Fontaine JB, Hopkins AJM, McHenry MP, O’Hara G, McComb J, Hardy GESJ, Howieson J (2018a) Potassium amendment increases biomass and reduces heavy metal concentrations in Lablab purpureus after phosphate mining. Land Degradation & Development 29, 398–407.
| Potassium amendment increases biomass and reduces heavy metal concentrations in Lablab purpureus after phosphate mining.Crossref | GoogleScholarGoogle Scholar |
Ruthrof KX, Steel E, Misra S, McComb J, O’Hara G, Hardy GESJ, Howieson J (2018b) Transitioning from phosphate mining to agriculture: responses to urea and slow release fertilizers for Sorghum bicolor. Science of The Total Environment 625, 1–7.
| Transitioning from phosphate mining to agriculture: responses to urea and slow release fertilizers for Sorghum bicolor.Crossref | GoogleScholarGoogle Scholar |
Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Physiology and Plant Molecular Biology 52, 527–560.
| Function and mechanism of organic anion exudation from plant roots.Crossref | GoogleScholarGoogle Scholar |
Saloner A, Bernstein N (2021) Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Industrial Crops and Products 167, 113516
| Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.).Crossref | GoogleScholarGoogle Scholar |
Saloner A, Sacks MM, Bernstein N (2019) Response of medical cannabis (Cannabis sativa L.) genotypes to K supply under long photoperiod. Frontiers in Plant Science 10, 1369
| Response of medical cannabis (Cannabis sativa L.) genotypes to K supply under long photoperiod.Crossref | GoogleScholarGoogle Scholar |
Sera B, Sery M, Gavril B, Gajdova I (2017) Seed germination and early growth responses to seed pre-treatment by non-thermal plasma in hemp cultivars (Cannabis sativa L.). Plasma Chemistry and Plasma Processing 37, 207–221.
| Seed germination and early growth responses to seed pre-treatment by non-thermal plasma in hemp cultivars (Cannabis sativa L.).Crossref | GoogleScholarGoogle Scholar |
Shiponi S, Bernstein N (2021a) Response of medical cannabis (Cannabis sativa L.) genotypes to P supply under long photoperiod: functional phenotyping and the ionome. Industrial Crops and Products 161, 113154
| Response of medical cannabis (Cannabis sativa L.) genotypes to P supply under long photoperiod: functional phenotyping and the ionome.Crossref | GoogleScholarGoogle Scholar |
Shiponi S, Bernstein N (2021b) The highs and lows of P supply in medical Cannabis: effects on cannabinoids, the ionome, and morpho-physiology. Frontiers in Plant Science 12, 657323
| The highs and lows of P supply in medical Cannabis: effects on cannabinoids, the ionome, and morpho-physiology.Crossref | GoogleScholarGoogle Scholar |
Small E, Pocock T, Cavers PB (2003) The biology of Canadian weeds. 119. Cannabis sativa L. Canadian Journal of Plant Science 83, 217–237.
| The biology of Canadian weeds. 119. Cannabis sativa L.Crossref | GoogleScholarGoogle Scholar |
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. Journal of Photochemistry and Photobiology B: Biology 104, 236–257.
| On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient.Crossref | GoogleScholarGoogle Scholar |
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In ‘Probing photosynthesis: mechanisms, regulation and adaptation’. (Eds M Yunus, U Pathre, P Mohanty) pp. 445–483. (Taylor & Francis Group: Boca Raton, FL, USA)
Tang K, Struik PC, Yin X, Thouminot C, Bjelková M, Stramkale V, Amaducci S (2016) Comparing hemp (Cannabis sativa L.) cultivars for dual-purpose production under contrasting environments. Industrial Crops and Products 87, 33–44.
| Comparing hemp (Cannabis sativa L.) cultivars for dual-purpose production under contrasting environments.Crossref | GoogleScholarGoogle Scholar |
Tang K, Struik PC, Amaducci S, Stomph T-J, Yin X (2017) Hemp (Cannabis sativa L.) leaf photosynthesis in relation to nitrogen content and temperature: implications for hemp as a bio-economically sustainable crop. GCB Bioenergy 9, 1573–1587.
| Hemp (Cannabis sativa L.) leaf photosynthesis in relation to nitrogen content and temperature: implications for hemp as a bio-economically sustainable crop.Crossref | GoogleScholarGoogle Scholar |
Thorsteinsson B, Tillberg J-E (1987) Carbohydrate partitioning, photosynthesis and growth in Lemna gibba G3. II. Effects of phosphorus limitation. Physiologia Plantarum 71, 271–276.
| Carbohydrate partitioning, photosynthesis and growth in Lemna gibba G3. II. Effects of phosphorus limitation.Crossref | GoogleScholarGoogle Scholar |
Valle JR, Vieira JE, Aucélio JG, Valio IF (1978) Influence of photoperiodism on cannabinoid content of Cannabis sativa L. Bulletin on Narcotics 30, 67–68.
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157, 423–447.
| Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource.Crossref | GoogleScholarGoogle Scholar |
Vera CL, Malhi SS, Raney JP, Wang ZH (2004) The effect of N and P fertilization on growth, seed yield and quality of industrial hemp in the Parkland region of Saskatchewan. Canadian Journal of Plant Science 84, 939–947.
| The effect of N and P fertilization on growth, seed yield and quality of industrial hemp in the Parkland region of Saskatchewan.Crossref | GoogleScholarGoogle Scholar |
Vera CL, Malhi SS, Phelps SM, May WE, Johnson EN (2010) N, P, and S fertilization effects on industrial hemp in Saskatchewan. Canadian Journal of Plant Science 90, 179–184.
| N, P, and S fertilization effects on industrial hemp in Saskatchewan.Crossref | GoogleScholarGoogle Scholar |
Wickham H (2011) ggplot2. Wiley Interdisciplinary Reviews: Computational Statistics 3, 180–185.
| ggplot2.Crossref | GoogleScholarGoogle Scholar |
Xiong Y, DeFraia C, Williams D, Zhang X, Mou Z (2009) Deficiency in a cytosolic ribose-5-phosphate isomerase causes chloroplast dysfunction, late flowering and premature cell death in Arabidopsis. Physiologia Plantarum 137, 249–263.
| Deficiency in a cytosolic ribose-5-phosphate isomerase causes chloroplast dysfunction, late flowering and premature cell death in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Yu T-S, Lue W-L, Wang S-M, Chen J (2000) Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiology 123, 319–326.
| Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation.Crossref | GoogleScholarGoogle Scholar |
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) ‘Mixed effects models and extensions in ecology with R.’ (Eds M Gail, K Krickeberg, JM Samet, A Tsiatis, W Wong) (Springer: New York, NY, USA)