Modulation of ion transporter genes of salt-stressed sorghum (Sorghum bicolor L. Moench) by foliar application of digitoxin
Hanan Shaaban A * , Alyaa S. Abdel Halim B , Hemmat I. Khattab A and Rabab A. Abdulhai C
A * , Alyaa S. Abdel Halim B , Hemmat I. Khattab A and Rabab A. Abdulhai C
A
B
C
Abstract
Salinity poses a major threat to cereal crops such as sorghum. The foliar application of digitoxin at concentrations of 50, 100, and 200 ppm was tested for its potential to alleviate salt stress in sorghum (Sorghum bicolor) exposed to 200 mM NaCl. Various growth parameters were analyzed, such as relative water content, malondialdehyde (MDA), osmoregulatory compunds (soluble carbohydrates and proline), ionic markers (Na+ and K+ levels in shoots and roots), and the expression of specific ion transporter genes including NHX, SOS1, AKT1, PPV, and PHA1 during the seedling stage. Digitoxin treatment significantly enhanced biochemical and ionic characteristics in salt-stressed plants by enhancing the membrane stability index and reducing MDA levels while boosting soluble carbohydrates, free amino acids, and proline. Real-time PCR showed that digitoxin application triggered the upregulation of genes promoting Na+ and K+ balance and reducing ion toxicity. This study underscores the potential role of digitoxin in improving salt tolerance through its influence on the regulation of ion transporter gene expression specific for K+ and Na+ ion transport and homeostasis. The effect of digitoxin on the ion transporters seems to be dose-dependent. The mechanism of digitoxin’s effect on ion transporter gene expression of salt-stressed plants is discussed.
Keywords: abiotic stress, digitoxin, foliar application, ion homeostasis, ion transporter genes, salinity, secondary metabolites, sorghum.
Introduction
Salinity poses a significant threat to global agriculture, severely affecting the growth and yield of essential crops. It was reported that salinization has degraded around 2000–4000 hectares of irrigated land worldwide (Zaman et al. 2016). Sorghum (Sorghum bicolor L.), ranked as the fifth most important cereal crop, is significantly impacted by soil salinization across the globe (Quan et al. 2021). In general, most cereal crops are sensitive to salt during both the vegetative and early reproductive stages (Gerona et al. 2019). The exposure of sorghum to excessive salinity ultimately leads to negative impacts on the growth and productivity of crops (Kenneth and Neeltje 2002). Notably, soil salinization induces ionic, osmotic, and oxidative stresses, which can have severe consequences for plant vitality and, in extreme cases, may lead to plant death. The ability of sorghum to tolerate salinity is closely related to its capability to maintain ion homeostasis within the cytoplasm under stressful conditions (Fu and Yang 2023; Mahmood et al. 2024). Sorghum develops various adaptive mechanisms in response to salt stress, including the exclusion of sodium ions (Na+), which is achieved by minimizing Na+ transport from the roots to the shoots through unloading from the xylem into the roots (Almodares et al. 2014; Shakeri and Emam 2017). Furthermore, plants can compartmentalize Na+ ions into the vacuoles to maintain the osmotic balance (De Lacerda et al. 2003). Some plants enhance the selective uptake and transport of potassium (K+) and calcium (Ca2+) over Na+ as a strategy for improving salt tolerance (Shakeri et al. 2020). Meanwhile, the accumulation of Na+ at concentrations exceeding a certain threshold in leaves can induce toxic levels and negatively affect the absorption and distribution of essential nutrients such as K+, Ca2+, and Mg2+ (Zhao et al. 2021; Khattab et al. 2024a, 2024b). The resulting imbalance in ion concentrations can significantly impair photosynthetic efficiency and overall growth (Netondo et al. 2004; Joardar et al. 2018).
Consequently, developing strategies to augment the resilience of sorghum in saline conditions is of paramount importance. Ion transporters such as AKT, SOS1, HAK, and HKT play crucial roles in maintaining Na+/K+ homeostasis by facilitating the removal of Na+ ions from the cell, thereby sustaining a low cytosolic Na+ concentration and a high cytosolic K+ concentration, which helps reduce the Na+/K+ ratio under salt stress (Qin et al. 2019; Imtiaz et al. 2023). Environmental stressors such as salinity, alkalinity, nutrient deficiencies, and low pH can induce the expression and activity of plasma membrane H+-ATPase (Chang et al. 2009; Zhu et al. 2009; Cao et al. 2020). Among these, SOS1 is the key determinant for Na+ transport from the cytoplasm to the apoplast, a process facilitated by a proton gradient generated by plasma membrane H+-ATPase (Schachtman and Liu 1999; Shi et al. 2000). Effective management of cytoplasmic Na+ levels, preservation of the cytosolic K+/Na+ ratio, and regulation of vacuolar osmotic potential during salt stress are associated with vacuolar Na+ sequestration (Maathuis and Amtmann 1999). In addition, the significance of the TaHKT1 gene to enhance salt tolerance in durum wheat by preventing Na+ influx has been emphasized by Roy et al. (2013). Na+/H+ antiporters, also known as NHXs, play a vital role in sequestering Na+ ions into vacuoles, contributing further to salt tolerance mechanisms (Al-Harrasi et al. 2020). Nowadays, it is essential to explore natural stress tolerance mechanisms alongside exogenous alleviators to formulate effective crop-breeding strategies (Haque et al. 2021). Various approaches are being employed to mitigate the adverse effects of salinity, including the search for natural alleviators such as secondary metabolites. These compounds protect plants against both biotic and abiotic stresses (Ramakrishna and Ravishankar 2011). Secondary metabolites also have significant applications in many fields such as medicine, nutrition, and cosmetics (Ramakrishna and Ravishankar 2011). Among the most frequently used natural products are cardiac glycosides, synthesized by various plant species as a defense mechanism against pests (Zalucki et al. 2001; Dobler et al. 2015). The Digitalis genus, known for producing digoxin and digitoxin, serves as the primary source of these compounds, which are well-recognized treatments for heart failure and cardiac arrhythmia (Goldberger and Goldberger 2012). Cardiac glycosides are a diverse group of natural products characterized by their ability to inhibit Na+/K+-ATPase activity, a vital membrane ion transporter in nearly all animal cells. They consist of three main components: a steroid core, an unsaturated lactone ring, and a carbohydrate moiety, which is primarily composed of D-glucose, d-digitoxose, d-mannose, or d-galactose (Cornelius et al. 2013). The efficacy of cardiac glycosides can vary based on the type of attached sugar (Kumavath et al. 2021). Ouabain, a cardiac glycoside, is thought to exert its protective effects against heart failure by binding to and inhibiting the Na+/K+-ATPase enzyme, leading to the modulation of Ca2+ levels and the production of reactive oxygen species (ROS) (Patel et al. 2019). The inhibition of the Na+/K+-ATPase α-subunit can activate signaling pathways involving mitogen-activated protein kinases (MAPKs), ultimately resulting in increased intracellular Ca2+ levels (Rhee et al. 2019). Moreover, the activation of MAPKs can promote mitochondrial ROS production (Xie et al. 1999; Tian et al. 2003).
Beyond their cardiac applications, cardiac glycosides are being explored as anticancer agents, for regulating blood pressure, and they exhibit antiviral properties (Bejček et al. 2021; Shandell et al. 2022). Reports showed that ouabain displayed a double role in regulating Na+/K+-ATPase activity, particularly in human skeletal muscle cells (Kravtsova et al. 2021). Although ouabain is typically known as a specific Na+/K+-ATPase inhibitor, studies have shown that at low (nanomolar) concentrations, it can activate Na+/K+-ATPase signaling (Ketchem et al. 2016). Further analysis revealed that chronic ouabain treatment increases MAPK phosphorylation and activates the MAPK signaling pathway, as evidenced by elevated mRNA levels. Meanwhile, chronic ouabain treatment doubled MAPK phosphorylation levels and thereby stimulated Na+/K+-ATPase activity (Pirkmajer et al. 2021). This suggests a regulatory feedback loop where ouabain can help in protection against heat stress (HS)-induced changes in Na+/K+-ATPase function and membrane stability in animals (Kravtsova et al. 2021). In light of these challenges, the research focused on developing strategies to enhance the resilience of sorghum grown in saline environments. By understanding the physiological and biochemical mechanisms that underlie salt tolerance, researchers can identify potential solutions to mitigate the negative impacts of salinity on crop productivity. Searching for novel alleviators is an important issue.
Digitoxin, a cardiac glycoside, is a novel agent that may improve sorghum salt tolerance. The current study investigates digitoxin’s effects on growth parameters and the regulation of ion transporter genes (AKT, SOS1, HAK, and HKT), providing insights into the mechanisms by which digitoxin aids in maintaining Na+/K+ homeostasis under saline conditions. This research not only advances the understanding of the physiological responses of sorghum to salt stress but also underscores the potential of secondary metabolites such as digitoxin as sustainable strategies for improving crop resilience in saline environments.
Materials and methods
Experimental design
A greenhouse experiment was conducted during the growth seasons at the botanical garden of the Faculty of Science, Ain Shams University. The study aimed to investigate the impact of different concentrations of digitoxin (50, 100, and 200 mg/mL) on the growth and ion transporter genes of sorghum plants exposed to 200 mM NaCl. Uniformly sized and colored pure sorghum grains were surface sterilized with a 1% sodium hypochlorite solution for 10 min, followed by rinsing for 5 min with distilled water (Abid et al. 2017). The sterilized grains were then sown in pots (30 cm wide and 18 cm deep) containing a homogeneous mixture of 5 kg of clay and sandy soil (1:1, w/w). After 20 days from seedling emergence, thinning was performed, leaving five uniform seedlings per pot. The pots were divided into five sets: the first set was irrigated with water as a control, whereas the second, third, fourth, and fifth sets were irrigated with 200 mM NaCl. In addition to the irrigation with NaCl, the third, fourth, and fifth sets were treated with 50, 100, and 200 mg/mL digitoxin, respectively, as a foliar spray one day before the exposure to NaCl treatment. All pots were arranged in a randomized complete block design, with relative humidity ranging from 60–70% and temperatures recorded as 24°C (maximum) and 17°C (minimum). Finally, seedlings were harvested 45 days after sowing to measure various growth parameters and conduct physiological and biochemical analyses.
Physiological and biochemical measurements
Shoot length, as well as fresh and dry weights, were measured to identify morphological differences between control samples and those treated with various concentrations of digitoxin under salt stress.
The relative water content (RWC) was determined using flag leaves harvested in the morning and preserved in nylon bags. Healthy leaf pieces were selected, weighed, and placed in Petri dishes with distilled water for 4 h at 23°C. After removing excess moisture, the leaves were weighed again to determine their turgid weight. The leaves were then dried at 70°C for 48 h, and their dry weight (DW) was measured. RWC was calculated using the following equation (Smart and Bingham 1974):
Fresh leaf tissue (0.5 g) from various treatments was homogenized in 10 mL of chilled 80% acetone. The absorbance of the filtrates was recorded at 645 nm and 663 nm for chlorophyll a and b (Chl a and b), and at 480 nm for carotenoids using a UV-visible + T80 spectrophotometer (Jakhar and Mukherjee 2014).
The proline content was estimated according to the method of Bates et al. (1973). Frozen samples (0.5 g) were homogenized in 10 mL of 3% sulfosalicylic acid at 4°C. The extract was then filtered using the Whatman No. 2 filter paper. A mixture of 2 mL each of acid-ninhydrin and glacial acetic acid was added to 2 mL of the resultant filtrate, and the mixture was incubated for 1 h at 100°C. The reaction was stopped by placing the mixture on ice. The mixture was then extracted with 4 mL of toluene, and the absorbance of the solution was measured at 520 nm. Pure proline was used to create a calibration curve, and the proline level was measured in micromol per gram of the fresh weight (FW).
The total soluble carbohydrates were extracted according to the method described by Prud’homme et al. (1992). The total soluble reducing sugars were assayed by the method of Somogyi-Nelson (Smogyi 1952). The reduced sugar content was expressed as milligrams per gram FW.
The leaf membrane stability index (MSI) was determined using the protocols of Sairam (1994) and Rady (2011). For this, 100 mg of leaf tissue was divided into two sets and immersed in 10 mL of distilled water. One set was incubated at 40°C for 30 min, and its electrical conductivity (C1) was measured. The other set was boiled at 100°C for 10 min to record its conductivity (C2). MSI was calculated using the formula: MSI = [1 − (C1/C2)] × 100. This formula quantifies MSI as a percentage, indicating the stability of leaf cell membranes under stress.
The total free amino acid content was quantified using the ninhydrin reagent method, following Misra et al. (1975) with some modifications. Fresh leaf tissue (0.5 g) was homogenized in 10 mL of 80% ethanol and filtered. A 0.1 mL extract aliquot was mixed with 2 mL of ninhydrin reagent (0.2 g ninhydrin in 100 mL ethanol) and heated in a boiling water bath for 20 min. After cooling, the resulting purple color was measured at 570 nm using a colorimeter, with a reagent blank as the control. Glycine was used to prepare a standard curve, and free amino acid content was expressed as milligrams per gram FW.
The plant tissue (0.1 g) was homogenized with 0.5 mL of 0.1% trichloroacetic acid (TCA), centrifuged, and combined with 20% TCA and 0.5% thiobarbituric acid (TBA) following the method of Heath and Packer (1968). The mixture was heated at 95°C for 30 min, cooled in an ice bath, and centrifuged at 10,000g for 10 min. The supernatant’s absorbance was measured at 532 and 600 nm. MDA concentration was calculated using an extinction coefficient of 155 mM−1 cm−1.
Molecular analyses
The gene expression levels of NHX, SOS1, AKT1, PHA1, and VPPase in sorghum tissues were determined using quantitative real-time polymerase chain reaction (qPCR). The primer sequences used for qPCR are provided in Table 1. The total RNA extraction from each sample was performed according to the manufacturer’s guidelines, using the Thermo Scientific Gene JET Plant RNA Purification Mini Kit (Cat. #: K0801, Thermo Scientific, MA, USA). The RNA samples underwent DNase I treatment to eradicate any genomic DNA contamination. The Revert Aid First Strand cDNA Synthesis Kit (Cat#: K1622, Thermo Scientific, MA, USA) was used to create the first strand cDNA. A total of 1 μg of total RNA was used for this purpose. The QuantiNova SYBR Green PCR kit (Cat. #: 208252, Qiagen, CA, USA) was used according to the manufacturer’s instructions to measure gene expression levels quantitatively. The Quant Studio 3 Real-Time PCR System (Thermo Scientific, Massachusetts, USA) was utilized for quantitative real-time analysis. The comparative Ct approach (2−ΔΔCt) (Livak and Schmittgen 2001) was used to evaluate relative gene expression. Actin-1 was used as the internal reference.
| Gene name | Accession number | Primer sequence | Amplicon size (bp) | |
|---|---|---|---|---|
| SOS1 | XM_002443629.2 | F: 5′-GGTGTAGTAAAGTGGACGAGTC-3′ R: 5′-ATCAGCACCTCATACAGTCCC-3′ | 98 | |
| PHA1 | XM_002466601.1 | F: 5′-CGTATTGTGCTAGGATTTATGCTA-3′ R: 5′-CTGCCAAGTATCCACCAAGT-3′ | 202 | |
| AKT1 | XM_002458189.2 | F: 5′-GCACAGGCTACCAGAGACAGA-3′ R: 5′-GCGACGCGATAAACCCACC – 3′ | 90 | |
| VPPase | XM_021448945.1 | F 5′-GCTACGGCGACTACCTCATC −3′ R: 5′-CCTTCGGAGATAGCGTTCTG −3′ | 108 | |
| NHX1 | XM_002461123.1 | F: 5′-GGAGTGTTTTCTGGATTGCTC-3′ R: 5′-CATCACAATACCGCAGAAGAAT-3′ | 174 | |
| Actin-1 | NM_001426217.1 | F: 5′-GGCTGAGTACGATGAGTCCG-3′ R: 5′-GCCGATAGACCGGCAGAAAA-3′ | 176 |
Statistical analysis
The data are presented as mean ± standard error (s.e.), based on 10 replicates for growth measurements and three replicates for chemical analyses. Statistical analysis was performed using one-way ANOVA, followed by Duncan’s Multiple Comparison Test, with IBM SPSS Statistics. Differences were considered statistically significant at a P-value of <0.05, as determined by the least significant difference (l.s.d.) at the 0.05 level (Snedecor and Cochram 1980).
Results
Alteration in morphological parameter and RWC of salt-stressed sorghum seedlings treated with digitoxin
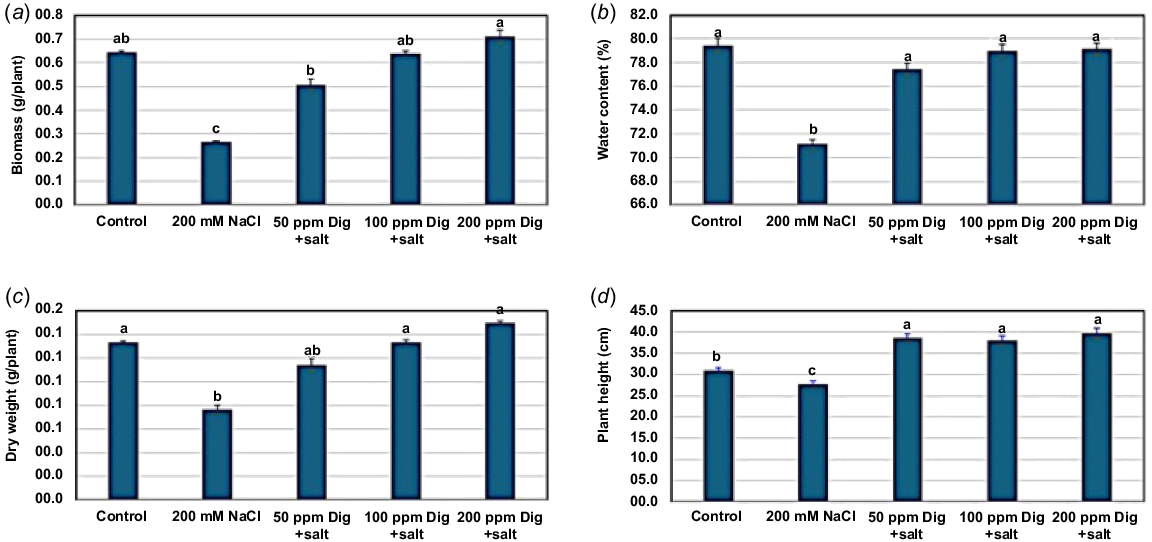
The results obtained in the present investigation indicated that NaCl (200 mM) had an inhibitory effect on the measured growth parameters of sorghum seedlings including shoot and root lengths, and FWs and DWs of shoot or root (Figs 1, 2) as compared with those of control plants. Meanwhile, foliar application of different concentrations of digitoxin (50, 100, and 200 ppm) markedly reversed the inhibitory effect of salinity (Figs 1, 2) as compared with the salt-stressed sorghum plants. Such a stimulatory effect of digitoxin on the growth parameters of salt-stressed sorghum shoots and roots was positively related to digitoxin concentrations. The greatest increases in the measured growth parameters were displayed with 200 ppm digitoxin. In addition, the reduction in growth parameters of salt-stressed sorghum seedlings was associated with a reduction in the RWC (Fig. 2). Notably, the foliar application of digitoxin improved the RWC of salt-stressed sorghum seedlings, particularly at 100 and 200 ppm, as compared with salt-stressed plants.
Sorghum seedlings grown under unstressed normal conditions (1) control and salt-stressed seedlings untreated (2) and treated with 50 ppm (3), 100 ppm (4), and 200 ppm (5) of digitoxin, respectively.

Impact of digitoxin treatment on the growth parameters of salt-stressed Sorghum. Digitoxin treatment (50, 100, and 200 ppm) showed significant mitigation of the salt-stress effects on different growth parameters including biomass (a), water content (b), dry weight (c), and plant height (d), in comparison with the untreated salt-stressed (200 mM NaCl) plants. Each value is the mean of 10 replicates ± s.e. with P value <0.05. The bars with the same letter in the same graph are non-significant and the values with different letters are significant.
Alteration in pigment levels in salt-stressed sorghum leaves treated with digitoxin
Simultaneously, improvements in morphological parameters were associated with a significant rise in pigment levels, particularly Chl a and b and carotenoids, in the salt-stressed sorghum leaves treated with different concentrations of digitoxin (Table 2). These increases showed a positive relation with the applied concentration of digitoxin. The most substantial increases in the photosynthetic pigment levels were observed in stressed sorghum leaves treated with a high dose of digitoxin (200 ppm). However, the Chl a/b ratio significantly increased in salt-stressed sorghum leaves (Table 2) compared with that in the unstressed control leaves. Meanwhile, foliar application of digitoxin significantly decreased the Chl a/b ratio in stressed sorghum leaves as compared with salt-stressed ones. The reduction in these ratios showed a non-significant relation to the applied digitoxin concentrations and the unstressed control leaves.
| Treatments | Photosynthetic pigments (μg/g FW) | ||||
|---|---|---|---|---|---|
| Chl a | Chl b | Carotenoids | Chl a/b | ||
| Control | 531 ± 22b | 465 ± 14c | 188 ± 6d | 1.14 ± 0.03b | |
| 200 mM NaCl | 446 ± 16c | 357 ± 12d | 174 ± 5d | 1.24 ± 0.05a | |
| 50 ppm digitoxin + salt | 521 ± 24b | 473 ± 14c | 212 ± 7c | 1.10 ± 0.03bc | |
| 100 ppm digitoxin + salt | 593 ± 29a | 507 ± 16b | 241 ± 8b | 1.06 ± 0.02c | |
| 200 ppm Digitoxin + salt | 615 ± 32a | 552 ± 18a | 260 ± 8a | 1.11 ± 0.02c | |
| F-value | 458.96** | 1625.34*** | 1325.22*** | 24.62** | |
Each value is a mean of three different replicates ±s.e.
**, *** significance at P < 0.01, 0.001, respectively.
Alteration in membrane peroxidation product (MDA) and MSI of salt-stressed sorghum plants treated with digitoxin
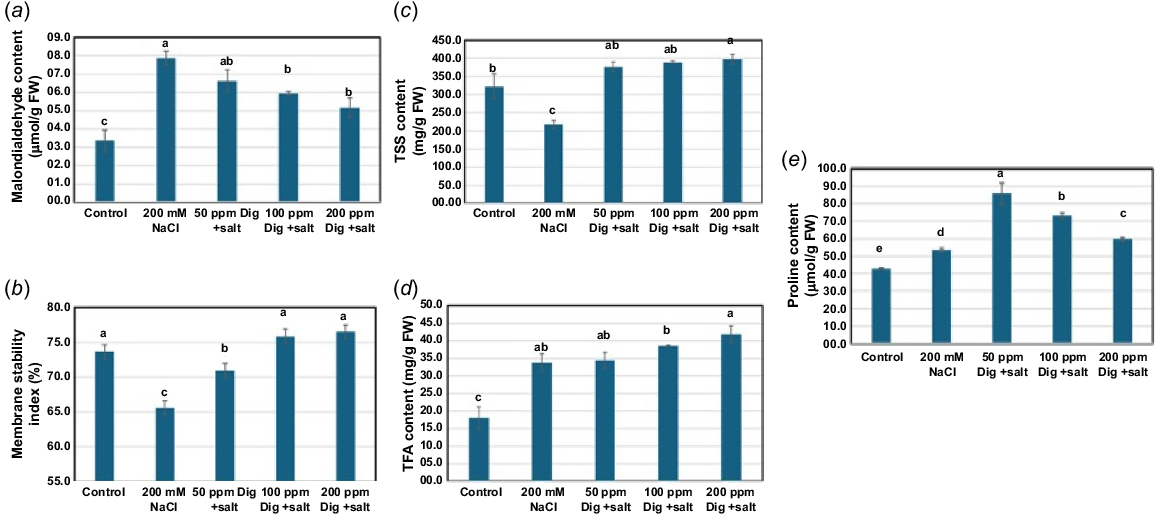
The data of the current study showed that the exposure of sorghum seedlings to salinity (200 mM) significantly reduced the percentage of MSI, however it caused a significant increase in the MDA level as compared with those of unstressed control seedlings (Fig. 3a, b). Notably, foliar application of different concentrations of digitoxin significantly reduced the accumulation of MDA in parallel with increments in the percentage of MSI reduction of salt-stressed sorghum leaves as compared with those of the untreated salt-stressed reference control. The maximum increments in the percentage MSI were displayed in salt-stressed sorghum leaves treated with 200 ppm followed by 100 ppm digitoxin.
Impact of digitoxin treatment on oxidative damage induced in salt-stressed sorghum. Digitoxin treatment (50, 100, and 200 ppm) showed significant mitigation of the oxidative stress induced in salt-stressed Sorghum plant, as shown for MDA content (a), membrane stability (b), total soluble sugar (c), total free amino acids (d), and proline content (e), in comparison with the untreated salt-stressed (200 mM NaCl) plants. Each value is the mean of 10 replicates ± s.e. with P value <0.05. The values with the same letter in the same graph are non-significant and the values with different letters are significant.
Alteration in some osmoprotectants in salt-stressed sorghum treated with digitoxin
Salinity stress induced the accumulation of both total amino acids and proline and was associated with a marked decrease in the total soluble sugars in stressed sorghum seedlings as compared with the control unstressed plants (Fig. 3c–e). Meanwhile, foliar application of different concentrations of digitoxin significantly enhanced the accumulation of total soluble sugars, total free amino acids, and proline (Fig. 3c–e). Notably, the maximum increment in total soluble sugars and total free amino acids was attained in stressed seedlings treated with 200 ppm digitoxin followed by 100 ppm. However, the greatest increase in proline level was measured in stressed seedlings treated with 50 ppm digitoxin followed by 100 ppm digitoxin.
Alteration in Na+ and K+ uptake and transporter genes in salt-stressed Sorghum seedlings
The current data showed that the levels of Na+ were higher in roots compared to shoots. Sodium ion (Na+) content and Na+/K+ ratios were higher in salt-stressed sorghum shoots and roots as compared with those of the unstressed control (Table 3). Meanwhile, the K+ level was markedly decreased (Table 3). Notably, K+ content was greater in shoots compared to root systems. However, foliar application of 100 and 200 ppm digitoxin markedly decreased the accumulation of Na+ and the Na+/K+ ratio in salt-stressed sorghum roots and shoots as compared with that of untreated stressed seedlings. Meanwhile, the greatest increments in Na+ accumulation were measured in stressed seedlings treated with 50 ppm digitoxin followed by stressed untreated seedling’s roots and shoots. Contrarywise, the K+ content markedly increased in stressed shoots and roots of various digitoxin-treated sorghum seedlings compared to salt-stressed ones (Table 3). The increments in K+ levels and as well K+/Na+ ratios were digitoxin concentration-dependent. Notably, the uptake, transport, and accumulation of Na+ and K+ in the plant cytosol and vacuoles were displayed via transporters produced by transporter genes.
| Treatments | Shoot system (mg/g DW) | Root system (mg/g DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Na+/K+ | K+/Na+ | Na+ | K+ | Na+/K+ | K+/Na+ | ||
| Control | 0.42 | 30.40 | 0.014 | 72.381 | 1.99 | 8.43 | 0.236 | 4.236 | |
| 200 mM NaCl | 2.72 | 26.20 | 0.146 | 6.859 | 4.66 | 5.09 | 0.916 | 1.092 | |
| 50 ppm digitoxin + salt | 2.73 | 26.54 | 0.103 | 9.722 | 6.23 | 7.27 | 0.857 | 1.167 | |
| 100 ppm digitoxin + salt | 1.09 | 28.56 | 0.038 | 26.200 | 5.15 | 7.39 | 0.697 | 1.435 | |
| 200 ppm digitoxin + salt | 1.05 | 35.72 | 0.029 | 34.019 | 5.13 | 8.18 | 0.627 | 1.595 | |
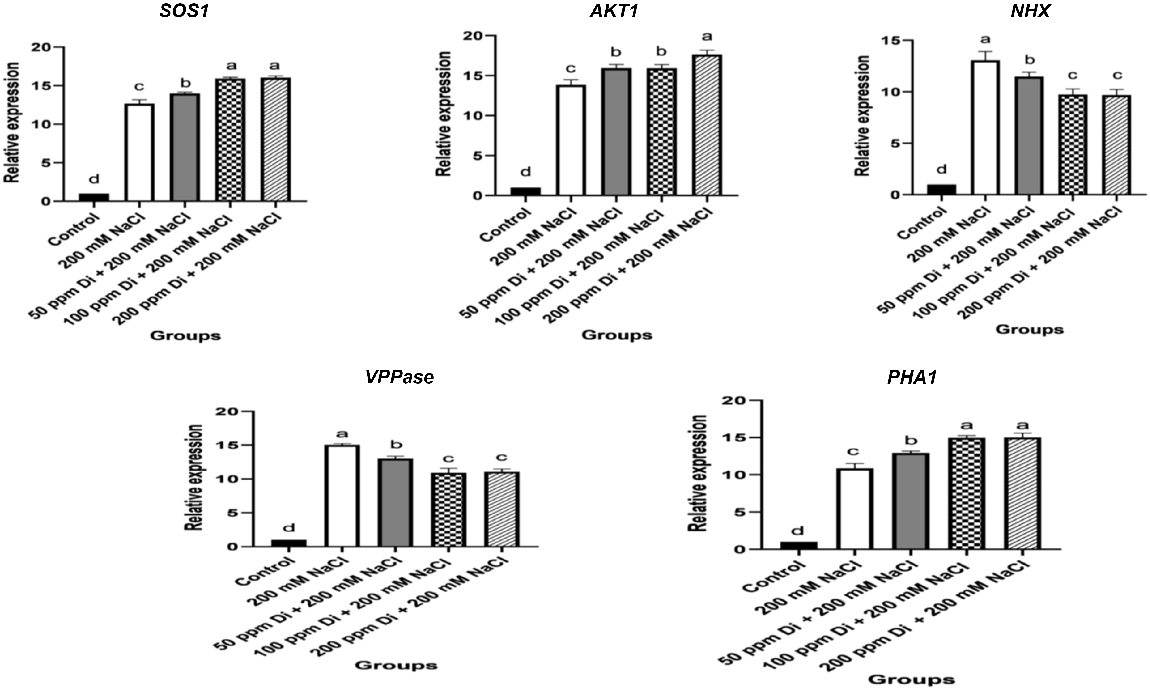
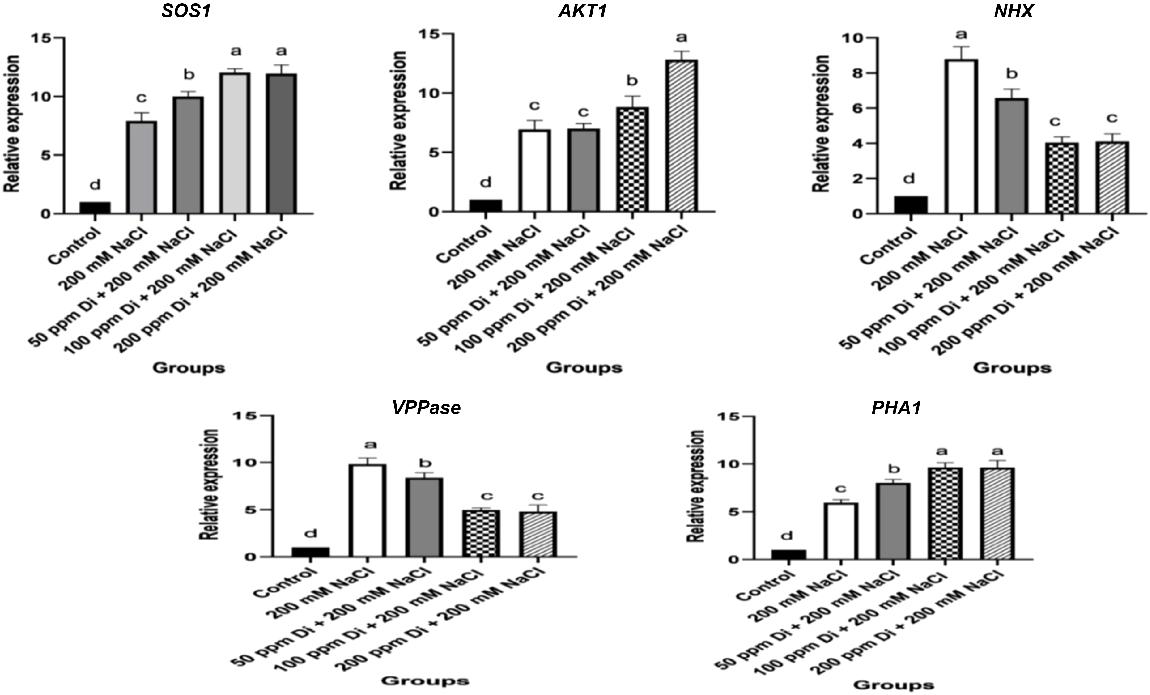
The expression of ion channel transporter genes was significantly modulated in salt-stressed sorghum shoots and roots (Figs 4, 5). The data of qPCR analysis revealed that the expression of Na+/H+ antiporter genes (SOS1 and NHX1), K+ transporter gene AKT1, plasma membrane H+-ATPase-1 (PHA1), and pyrophosphate-energized vacuolar membrane proton pump 1 (VPPase) were significantly increased in salt-stressed sorghum roots and shoots compared to those of the control unstressed ones (Figs 4, 5). Meanwhile, foliar application of different concentrations of digitoxin induced further increments in the expression of Na+/K+ transporter genes including PHA1, AKT1, and SOS1, however, the expression of VPPase, and NHX1 in roots and shoots of digitoxin-treated salt-stressed seedlings were markedly lower than those of the untreated salt-stressed ones (Figs 4, 5). Meanwhile, the expression of the investigated ion transporter genes was more significant in treated and untreated stressed roots compared to their corresponding shoots.
Effect of digitoxin on the expression of ion transport genes: SOS1, AKT1, NHX, PHA, and VPPase of salt-stressed sorghum roots. Data are expressed as mean ± s.d. Different letters indicate a significant difference between the mean values of measured parameters. A one-way analysis of variance (ANOVA) was followed by Duncan’s Multiple Comparison Test.
Effect of digitoxin on the expression of ion transport genes: SOS1, AKT1, NHX, PHA, and VPPase of salt-stressed sorghum shoots. Data are expressed as mean ± s.d. Different letters indicate a significant difference between the mean values of measured parameters. A one-way analysis of variance (ANOVA) was followed by Duncan’s Multiple Comparison Test.
In summary, the foliar application of digitoxin improved different aspects of salt-stressed sorghum seedlings. This improvement was particularly high with digitoxin application at 100 and 200 ppm concentration.
Discussion
Salinity stress has a significant detrimental impact on plants, primarily due to the accumulation of Na+ and Cl− ions in plant tissues exposed to high NaCl levels. Data from the current investigation indicated that NaCl (200 mM) had an inhibitory effect on the measured growth parameters of sorghum seedlings including plant height, biomass, and plant dry weight (Figs 1,2) as compared with those of control plants. The reduction in growth parameters under salt stress is primarily attributed to ion toxicity and nutrient imbalances (Banakar et al. 2022). The increase in osmotic pressure induced by salt stress lowers soil water potential, reducing water uptake by roots and consequently inhibiting the growth of salt-stressed plants (Karimi et al. 2020; Sjöman et al. 2021; Khattab et al. 2024a). Sodium ion (Na+) accumulation suppresses the key regulatory genes involved in cell cycle progression, reducing meristem cell numbers and impairing growth, nutrient acquisition, and water uptake (Balasubramaniam et al. 2023). Salt stress exacerbates plant damage through a combination of ion toxicity, osmotic stress, nutrient imbalance, water deficits, and soil structure deterioration (Zhao et al. 2016; Sjöman et al. 2021; Balasubramaniam et al. 2023). The effects of salinity on plant growth have been extensively studied across a range of species, including Eruca sativa Mill (Petretto et al. 2019), Fragaria × ananassa Duch (Denaxa et al. 2022), and flax and sorghum (Khattab et al. 2024a, 2024b). Notably, the reduction in the investigated growth parameters was concomitant with a decline in the percentage of water content in salt-stressed sorghum (Fig. 2). Such an effect was attributed to the rapid uptake and accumulation of Na+ which negatively affected plant–water relations. It has been demonstrated that the RWC, water uptake, and transpiration markedly decrease in different salt-stressed plant species (Elhakem 2019; Khan et al. 2021; Lu and Fricke 2023). The extent of decline in leaf water potential depends on the salinity stress and osmotic conditions in the rooting medium (Petretto et al. 2019; Balasubramaniam et al. 2023). Foliar application of different concentrations of digitoxin mitigated the injurious effect of salt. The increases in the estimated growth parameters of treated salt-stressed sorghum seedlings were positively related to the concentration of the applied digitoxin (Figs 1,2). Such an effect of digitoxin may be due to its impact on the activation of the Ca2+ exchanger which consequently increases Ca2+ content and thereby increases the MAPK activity, which consequently activates the MAPK cascade under salt stress (Majeed et al. 2023) and thereby nullifies the salt-stress injuries. Alternately, it may be due to the carbohydrate moiety of digitoxin, which may contribute to lowering the osmotic potential, thereby increasing RWC and increasing plant tolerance (Khattab et al. 2024b).
Chlorophyll is an important indicator reflecting the strength of photosynthesis (Cruz and Avenson 2021). The current results showed that levels of Chl a and b, as well as carotenoids, are significantly decreased in the salt-stressed sorghum leaves (Table 2). The decline in chlorophyll content has been reported under salt-stress conditions due to increased oxidation and degradation of chlorophyll stimulated by the accumulation of ROS (Balasubramaniam et al. 2023; Khattab et al. 2024a; Rajabi Dehnavi et al. 2024). Similar studies performed on various plant species have shown that salt stress leads to reduced photosynthetic rates at low salt concentrations and severely damages chloroplast structures and photosynthetic machinery at moderate to high salt concentrations (Hnilickova et al. 2021; Hannachi et al. 2022; Balasubramaniam et al. 2023). Meanwhile, the pigment content, in terms of Chl a and b as well as carotenoids, was markedly increased in salt-stressed sorghum plants treated with different concentrations of digitoxin (Table 2). Digitoxin application increases chlorophyll and carotenoid content in salt-stressed sorghum, possibly by enhancing pigment synthesis and inhibiting ROS damage through antioxidant activity (Khattab et al. 2024b).
MDA levels serve as key indicators of plant stress. Elevated MDA levels reflect the extent of lipid peroxidation and cellular damage under adverse conditions (Sheldon et al. 2017; Petretto et al. 2019; Karimi et al. 2020; Balasubramaniam et al. 2023). In this study, salt-stressed sorghum seedlings exhibited a substantial increase in MDA content alongside a decline in the MSI, highlighting stress-induced cellular injury (Fig. 3a, b). The damaging effects of salinity are partly due to the overproduction of ROS, which leads to membrane lipid peroxidation and reduced MSI, causing enhanced electrolyte leakage (Kumar et al. 2018). Elevated MDA accumulation under salinity has been documented in various salt-stressed plant species (Ibrahim et al. 2021; Youssef et al. 2021; Khattab et al. 2024a). Treating salt-stressed sorghum seedlings with digitoxin significantly decreased MDA levels and improved MSI ( Fig. 3a, b). Such effects suggest reduced cellular damage compared to those of the untreated stressed plants. Such an effect of digitoxin application might be due to the mitigation of ROS accumulation concomitant with the increments in cytoplasmic Ca2+, which reduce plasma membrane permeability and maintain its integrity under salt stress (Guo et al. 2021). Similar findings were reported in digitoxin-treated flax plants subjected to salt stress (Khattab et al. 2024b).
Osmoregulation plays a critical role in sorghum’s adaptation to salinity stress by maintaining water balance and cell integrity. The key compounds involved include free amino acids, proline, and carbohydrates, which help plants tolerate salt stress (Zhang et al. 2020; Mohammadi Alagoz et al. 2023; Rajabi Dehnavi et al. 2024). The current results indicated that salt-stressed sorghum seedlings exhibited an increment in the accumulation of proline and free amino acids as compared to those of unstressed control (Fig. 3d, e). These results are consistent with the findings of Xie et al. (2020) and Khattab et al. (2024a, 2024b). The increments in proline accumulation might be attributed to the augmentation in the activity of proline synthesis enzymes and to the decrease in proline degradation (Zulfiqar and Ashraf 2023). Some salt-tolerant plants can synthesize and accumulate excess amounts of proline (Abid et al. 2020). Additionally, digitoxin significantly stimulates the accumulation of total free amino acids as well as proline in stressed sorghum seedlings (Fig. 3d, e). The increments in total free amino acids were positively related to the digitoxin dose. Meanwhile, the proline accumulation was negatively related to the digitoxin dose. Proline as well as free amino acids serve as osmoprotectants, membrane stabilizers, source of nitrogen and carbon, and ROS-neutralizing buffers (Zouari et al. 2019; Hussein and Alshammari 2022).
The exposure of sorghum seedlings to salt stress (200 mM) significantly reduced the level of total soluble sugar accumulation as compared with that of the unstressed control plants (Fig. 3c). The observed decline in soluble carbohydrate content is likely a result of disturbances in their synthesis, transport, and utilization pathways (Amombo et al. 2022; Varshney et al. 2023). This finding is probably similar to Ji et al. (2022) who stated that prolonged and relatively high NaCl stress stimulates a decline in total sugar content in stressed Juglans microcarpa L. seedlings due to the greater decomposition level compared to the level of sugar synthesis (Ji et al. 2022). Recent studies have shown that soluble sugars display a crucial role in osmotic regulation by maintaining cell turgor, water uptake, and ion transport under stressful conditions (Wang et al. 2021). Digitoxin-treated seedlings, however, showed increased soluble sugar accumulation, particularly at the high applied dose (Fig. 3c). The accumulation of sugar enhances osmotic regulation and membrane stability (Colak et al. 2020). It was stated that soluble sugars are vital for cell turgor and water uptake and play a protective role by aiding osmotic balance and mitigating NaCl’s effects (Singh et al. 2015; Abid et al. 2020).
Plants adapt to high-sodium salt conditions by maintaining cell turgor and water uptake through increased osmolyte accumulation in the cytoplasm. This is achieved either by synthesizing solutes internally or by absorbing them from the soil solution. (Chinnusamy et al. 2005; Shabala et al. 2010). Data from the present work showed increments in the accumulation of Na+ and the Na+/K+ ratio in stressed sorghum roots and shoots concomitant with a decline in their K+ levels as compared with those of the unstressed seedlings (Table 3). The accumulation of Na+ was more pronounced in salt-stressed roots compared to shoots. Similarly, Na+ was accumulated most sharply in sorghum and flax plants exposed to salinity (Calone et al. 2020; Khattab et al. 2024a, 2024b). Na+ accumulation in roots is one of the tolerance strategies that contribute to the consequent reduction in root osmotic potential thereby sustaining water uptake. This Na⁺ buildup may promote metabolic tolerance or compartmentalization within different cell or plant components (Calone et al. 2020). In addition, foliar application of digitoxin at concentrations of 100 and 200 ppm significantly reduced the accumulation of Na+ and increased K+ levels in salt-stressed sorghum shoots and roots thereby increasing the ratios of K+/Na+ compared with salt stressed values (Table 3). Meanwhile, the application of a low dose of digitoxin (50 ppm) increased the levels of Na+ in salt-stressed sorghum root and shoot. Such an effect may be attributed to the impact of digitoxin on Na+/K+-ATPase which regulates the influx and efflux of both ions. Similarly, a low dose of ouabain inhibits the Na+/K+-ATPase activity inducing an increment in Na+ level and enhancing the rat’s cardiac force. However, this effect seems to be dose-dependent (Meira et al. 2015). A higher K+/Na+ ratio is effective in ion management, reducing the influx of harmful ions while promoting the retention of essential nutrients. This phenomenon aligns with salt-tolerant plants, which naturally possess an enhanced ability to maintain an elevated K+/Na+ ratio, limiting Na+ absorption and enhancing K+ assimilation (Saqib et al. 2011; Amombo et al. 2022; Balasubramaniam et al. 2023).
Digitoxin treatment may enhance the salt tolerance of sorghum by promoting Na⁺ sequestration into vacuoles, where it functions as an osmolyte to maintain cellular osmotic balance. Shabala et al. (2010) highlighted the critical role of vacuolar Na⁺ compartmentalization in barley salt tolerance. Sorghum exhibits a similar strategy by regulating Na⁺ accumulation through restricted Na⁺ transport to shoots, xylem unloading, and vacuolar sequestration (Shakeri and Emam 2017; Yang et al. 2018). Additionally, cardiac glycosides, including digitoxin, inhibit Na⁺/K⁺-ATPase activity (Katz 1985), increasing intracellular Ca2⁺ levels (Arispe et al. 2008), which can enhance salt stress resilience (Feng et al. 2023). Guo et al. (2021) demonstrated that exogenous Ca2+ improves K+ channel activity, facilitates K+ uptake across root plasma membranes, and reduces Na+ influx by decreasing plasma membrane permeability to Na+. Similarly, digitoxin has been shown to promote the accumulation of Ca2+, K+, and Mg2+ in flax, mitigating nutrient imbalances caused by salt stress (Khattab et al. 2024b).
Plant cells maintain high K+ and low Na+ cytoplasmic levels through specialized transport mechanisms involving ion channels and transporter proteins encoded by specific genes. Na+ uptake occurs via channels such as the high-affinity K+ transporter (HKT), while Na+ efflux is managed by the SOS1 Na+/H+ antiporter, which is essential for Na+ exclusion and salt tolerance. Additionally, Na+ toxicity is mitigated by its sequestration in vacuoles through Na+/H+ antiporters such as NHX1, NHX5, and others (Apse et al. 1999; Rahnama et al. 2011). In salt-stressed sorghum, the expression of transporter genes such as SOS1, AKT1, NHX, PHA, and VPPase is significantly upregulated in both roots and shoots under 200 mM NaCl exposure (Figs 4,5). Specifically, SOS1 facilitates Na⁺ exclusion, AKT1 regulates K+ uptake, and NHX proteins manage vacuolar Na+ sequestration. Foliar digitoxin application modulates these gene expressions, enhancing SOS1, AKT1, and PHA expression in stressed roots and shoots, with upregulation correlating positively with digitoxin concentrations. Conversely, NHX and VPPase expression levels were higher in untreated stressed plants compared to digitoxin-treated ones, with maximal expression observed in untreated stressed roots and shoots.
The enhanced AKT1 expression in digitoxin-treated plants corresponds to increased K+ levels, underscoring its critical role in K+ uptake and Na+/K+ homeostasis during salt stress, which mitigates its adverse effects (Feng et al. 2019, 2023). Furthermore, SOS1 was upregulated in digitoxin-treated shoots and roots exposed to salt stress. SOS1 also participates in the long-distance transport of Na+ from roots to shoots, oxidative stress, and intracellular pH balance (Oh et al. 2010; Quintero et al. 2011; Feki et al. 2014). Notably, the further upregulation of SOS1 in stressed digitoxin-treated roots and shoots might be due to the effect of generated ROS and NADPH oxidase which further augments Na⁺ efflux, protecting cells from ionic stress (Chung et al. 2008). The expression of SOS1 from different plants can improve tolerance of the salt-sensitive Arabidopsis mutant phenotype (Shi et al. 2000; Martínez-Atienza et al. 2007). Notably, the expression of wheat SOS1 promoted the growth of transgenic tobacco (Nicotiana tabacum) under NaCl treatment (Zhou et al. 2016). Such results suggest the crucial role of SOS1 in maintaining cytoplasmic Na+ levels, thereby enhancing the plant’s salt tolerance.
Furthermore, NHX1 is the most commonly known transporter playing a crucial role in vacuolar Na+ sequestration. The greatest increase in NHX1 expression was measured in untreated salt-stressed roots and shoots followed by those of the lower digitoxin treatment (50 ppm). The overexpression of NHX1 improves salt tolerance in many species including tomato (Zhang and Blumwald 2001), rice (Chen et al. 2007), tobacco (Gouiaa et al. 2012), barley (Adem et al. 2014), and alfalfa (Sandhu et al. 2017). However, a marked reduction in the NHX1 transcript level was observed in salt-stressed wheat roots, whereas almost no change in the NHX1 transcript level was detected in leaves (Mullan et al. 2007). These results showed the importance of tissue specificity in plant salt-stress tolerance. The decline in NHX1 expression in stressed digitoxin-treated organs was concomitant with the upregulation of PHA1 and downregulation of VPPase, compared with untreated salt-stressed plants. Notably, PHA1 and VPPase are overexpressed in stressed sorghum roots and shoots (Figs 4 and 5). Stritzler et al. (2017) showed that the PHA1 gene from potatoes encodes an ATPase with proton-pumping activity, which supports sucrose–starch metabolism in potato stolons to maintain apoplastic sucrose levels. Additionally, the proton pump interactor (PPI), has been found to enhance ATPase activity in Arabidopsis thaliana (PPI1; Morandini et al. 2002) and Solanum tuberosum (StPPI1; Muñiz García et al. 2011). Salt stress affects the expression of plasma membrane H+-ATPase (Sahu and Shaw 2009). PM H+-ATPases provide the driving force for the transport of ions and metabolites through transporters and channels (Palmgren 2001). Stritzler et al. (2017) demonstrated that the PHA1 gene cloned from potato has ATPase and proton pumping activity. PHA1 is involved in the sucrose–starch metabolism in potato stolons, to maintain the apoplastic sucrose (Stritzler et al. 2017). PPI is an interaction partner of PM H+-ATPase characterized in A. thaliana (PPI1; Morandini et al. 2002) and Solanum tuberosum (StPPI1; Muñiz García et al. 2011); this protein increases the activity of the proton pump in vitro. PM H+-ATPase transcript levels are differentially regulated by environmental and hormonal signals, such as salt stress (Sahu and Shaw 2009; Stritzler et al. 2017). Salt stress significantly increases PHA1, PHA2, and PHA3 expression suggesting a role for these PM H+-ATPases in salinity tolerance, (Janicka-Russak and Klobus 2007; Sahu and Shaw 2009). Such an effect proves that NHX1 is fuelled by a H+ gradient across the tonoplast that is maintained by VPPase (Silva and Gerós 2009). The overexpression of VPPase and PHA1 improved salt tolerance in salt-stressed sorghum. Notably, the expression of both H+-ATPase (PHA1) and VPPase was significantly upregulated in salt-stressed sorghum roots and shoots and may help in the compartmentation of Na+ ions and thus enhance salt tolerance (Li et al. 2011). Notably, SbVPPase helps transgenic plants in the accumulation of Na+ and K+ for osmotic adjustment. It has been observed that transgenic tobacco plants overexpressing Thellungiella halophila VPPase, cotton plants overexpressing Arabidopsis vacuolar proton pyrophosphatase AVP1, and finger millet expressing SbVPPase accumulated Na+ and displayed enhanced salt tolerance (Gao et al. 2006; Pasapula et al. 2011; Anjaneyulu et al. 2014). In animal cells, a cardio glycoside inhibits both Na+ efflux and ATPase activity, suggesting a link between the Na+ pump and ATPase (Glynn and Karlish 1975). Likewise, the inhibition of the Na+ efflux pump by a low concentration of ouabain-treated corn and barley roots displayed the same relation (Nassert and Baker 1972). Meanwhile, Leonard and Hodges (1973), reported no effect on oat root ATPase.
Digitoxin treatments enhance the expression of AKT1 in salt-stressed sorghum shoots and roots as compared to stressed untreated ones (Figs 4,5). The overexpression of AKT1 was concomitant with the increments of the K+/Na+ ratio in stressed digitoxin-treated shoots and roots. Kobayashi et al. (2017) stated that the AtHKT1 gene was involved in Na+ recirculation from shoots to roots probably by mediating Na+ loading into phloem sap in the shoots and unloading it in roots thereby protecting young rice leaf blades exposed to salt stress. The overexpression of SOS1 and PHA genes as well as AKT1, concomitant with the reduction in Na+ accumulation and increase in K+/Na+ ratios in digitoxin-treated plants (100 and 200 ppm), indicated the role of digitoxin in the regulation of ion homeostasis and improvement of salt-stress tolerance of sorghum seedlings.
In conclusion, the overexpression of Na+ and K+ transporters genes such as HKT1, NHX, SOS1, PHA1, and VPPase enables plants to counteract salinity stress through complex regulation of Na⁺ exclusion, K⁺ retention, and intracellular compartmentalization. This regulation not only maintains ionic balance but also maintains a favorable K+/Na+ ratio, sustains optimal photosynthesis, improves membrane integrity, and ensures sufficient levels of photosynthetic pigments. Thus, the maintenance of photosynthetic capacity improves seedling growth and supports plant growth and development under adverse salt-stress conditions. Meanwhile, the application of digitoxin in particularly high doses (100 and 200 ppm) mitigated the salt-stress injuries by the overexpression of SOS1, HKT1, and PHA1 which perhaps collectively maintained the cell ionic homeostasis, supporting growth and development under challenging saline conditions. The highlighted defense mechanisms of digitoxin enabled sorghum seedlings to effectively counteract ionic toxicity, Additionally, plants adjust their sap composition by accumulating Na+ to modulate tissue water potential and sustain growth. At low digitoxin concentrations, increased Na⁺ content alongside proline accumulation aided in water potential adjustment. However, the precise mechanisms underlying digitoxin’s effects on plant tolerance remain complex and not fully understood, necessitating further advanced molecular studies for clarification.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
References
Abid G, Hessini K, Aouida M, Aroua I, Baudoin J-P, Muhovski Y, Mergeai G, Sassi K, Machraoui M, Souissi F, Jebara M (2017) Agro-physiological and biochemical responses of Faba Bean (Vicia faba L. var. ‘minor’) genotypes to water deficit stress. Biotechnology, Agronomy, Society and Environment 21, 146-159.
| Crossref | Google Scholar |
Abid M, Zhang YJ, Li Z, Bai DF, Zhong YP, Fang JB (2020) Effect of salt stress on growth, physiological and biochemical characters of four kiwifruit genotypes. Scientia Horticulturae 271, 109473.
| Crossref | Google Scholar |
Adem GD, Roy SJ, Zhou M, Bowman JP, Shabala S (2014) Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biology 14, 113.
| Crossref | Google Scholar | PubMed |
Al-Harrasi I, Jana GA, Patankar HV, Al-Yahyai R, Rajappa S, Kumar PP, Yaish MW (2020) A novel tonoplast Na+/H+ antiporter gene from date palm (PdNHX6) confers enhanced salt tolerance response in Arabidopsis. Plant Cell Reports 39(8), 1079-1093.
| Crossref | Google Scholar | PubMed |
Almodares A, Hadi MR, Kholdebarin B, Samedani B, Akhavan Kharazian Z (2014) The response of sweet Sorghum cultivars to salt stress and accumulation of Na+, Cl− and K+ ions in relation to salinity. Journal of Environmental Biology 35(4), 733-739.
| Google Scholar | PubMed |
Amombo E, Ashilenje D, Hirich A, Kouisni L, Oukarroum A, Ghoulam C, El Gharous M, Nilahyane A (2022) Exploring the correlation between salt tolerance and yield: research advances and perspectives for salt-tolerant forage sorghum selection and genetic improvement. Planta 255(3), 71.
| Crossref | Google Scholar | PubMed |
Anjaneyulu E, Reddy PS, Sunita MS, Kishor PBK, Meriga B (2014) Salt tolerance and activity of antioxidative enzymes of transgenic finger millet overexpressing a vacuolar H(+)-pyrophosphatase gene (SbVPPase) from Sorghum bicolor. Journal of Plant Physiology 171(10), 789-798.
| Crossref | Google Scholar | PubMed |
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285(5431), 1256-1258.
| Crossref | Google Scholar | PubMed |
Arispe N, Diaz JC, Simakova O, Pollard HB (2008) Heart failure drug digitoxin induces calcium uptake into cells by forming transmembrane calcium channels. Proceedings of the National Academy of Sciences 105(7), 2610-2615.
| Crossref | Google Scholar |
Balasubramaniam T, Shen G, Esmaeili N, Zhang H (2023) Plants’ response mechanisms to salinity stress. Plants 12(12), 2253.
| Crossref | Google Scholar | PubMed |
Banakar MH, Amiri H, Sarafraz Ardakani MR, Ranjbar GH (2022) Susceptibility and tolerance of fenugreek (Trigonella Foenum-Graceum L.) to salt stress: physiological and biochemical inspections. Environmental and Experimental Botany 194, 104748.
| Crossref | Google Scholar |
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant and Soil 39(1), 205-207.
| Crossref | Google Scholar |
Bejček J, Jurášek M, Spiwok V, Rimpelová S (2021) Quo vadis cardiac glycoside research? Toxins 13(5), 344.
| Crossref | Google Scholar | PubMed |
Calone R, Sanoubar R, Lambertini C, Speranza M, Antisari LV, Vianello G, Barbanti L (2020) Salt tolerance and Na allocation in Sorghum bicolor under variable soil and water salinity. Plants 9(5), 561.
| Crossref | Google Scholar | PubMed |
Cao Y, Zhang M, Liang X, Li F, Shi Y, Yang X, Jiang C (2020) Natural variation of an EF-hand Ca2+-binding-protein coding gene confers saline-alkaline tolerance in maize. Nature Communications 11(1), 186.
| Crossref | Google Scholar | PubMed |
Chang C, Hu Y, Sun S, Zhu Y, Ma G, Xu G (2009) Proton pump OsA8 is linked to phosphorus uptake and translocation in rice. Journal of Experimental Botany 60(2), 557-565.
| Crossref | Google Scholar | PubMed |
Chen H, An R, Tang J-H, Cui X-H, Hao F-S, Chen J, Wang X-C (2007) Over-expression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Molecular Breeding 19(3), 215-225.
| Crossref | Google Scholar |
Chinnusamy V, Jagendorf A, Zhu J-K (2005) Understanding and improving salt tolerance in plants. Crop Science 45(2), 437-448.
| Crossref | Google Scholar |
Chung J-S, Zhu J-K, Bressan RA, Hasegawa PM, Shi H (2008) Reactive oxygen species mediate Na+-induced SOS1 MRNA stability in Arabidopsis. The Plant Journal 53(3), 554-565.
| Crossref | Google Scholar | PubMed |
Colak N, Tarkowski P, Ayaz FA (2020) Effect of N-Acetyl-L-Cysteine (NAC) on soluble sugar and polyamine content in wheat seedlings exposed to heavy metal stress (Cd, Hg and Pb). Botanica Serbica 44(2), 191-201.
| Crossref | Google Scholar |
Cornelius F, Kanai R, Toyoshima C (2013) A structural view on the functional importance of the sugar moiety and steroid hydroxyls of cardiotonic steroids in binding to Na,K-ATPase. The Journal of Biological Chemistry 288(9), 6602-6616.
| Crossref | Google Scholar | PubMed |
Cruz JA, Avenson TJ (2021) Photosynthesis: a multiscopic view. Journal of Plant Research 134(4), 665-682.
| Crossref | Google Scholar | PubMed |
De Lacerda CF, Cambraia J, Oliva MA, Ruiz HA, Prisco JTN (2003) Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environmental and Experimental Botany 49(2), 107-120.
| Crossref | Google Scholar |
Denaxa N-K, Nomikou A, Malamos N, Liveri E, Roussos PA, Papasotiropoulos V (2022) Salinity effect on plant growth parameters and fruit bioactive compounds of two strawberry cultivars, coupled with environmental conditions monitoring. Agronomy 12(10), 2279.
| Crossref | Google Scholar |
Dobler S, Petschenka G, Wagschal V, Flacht L (2015) Convergent adaptive evolution – how insects master the challenge of cardiac glycoside-containing host plants. Entomologia Experimentalis et Applicata 157(1), 30-39.
| Crossref | Google Scholar |
Elhakem AH (2019) Mitigation of the salinity influences on maize (Zea Mays L.) productivity by exogenous applications of glycine betaine. Journal of Stress Physiology & Biochemistry 15, 21-28.
| Google Scholar |
Feki K, Quintero FJ, Khoudi H, Leidi EO, Masmoudi K, Pardo JM, Brini F (2014) A constitutively active form of a durum wheat Na+/H+ Antiporter SOS1 confers high salt tolerance to transgenic arabidopsis. Plant Cell Reports 33(2), 277-288.
| Crossref | Google Scholar | PubMed |
Feng H, Tang Q, Cai J, Xu B, Xu G, Yu L (2019) Rice OsHAK16 functions in potassium uptake and translocation in shoot, maintaining potassium homeostasis and salt tolerance. Planta 250(2), 549-561.
| Crossref | Google Scholar | PubMed |
Feng C, Gao H, Zhou Y, Jing Y, Li S, Yan Z, Xu K, Zhou F, Zhang W, Yang X, Hussain MA, Li H (2023) Unfolding molecular switches for salt stress resilience in soybean: recent advances and prospects for salt-tolerant smart plant production. Frontiers in Plant Science 14, 1162014.
| Crossref | Google Scholar | PubMed |
Fu H, Yang Y (2023) How plants tolerate salt stress. Current Issues in Molecular Biology 45(7), 5914-5934.
| Crossref | Google Scholar | PubMed |
Gao F, Gao Q, Duan XG, Yue GD, Yang AF, Zhang JR (2006) Cloning of an H+-PPase gene from Thellungiella Halophila and its heterologous expression to improve tobacco salt tolerance. Journal of Experimental Botany 57(12), 3259-3270.
| Crossref | Google Scholar | PubMed |
Gerona MEB, Deocampo P, Egdane JA, Ismail AM, Dionisio-Sese ML (2019) Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Science 26(4), 207-219.
| Crossref | Google Scholar |
Glynn IM, Karlish SJ (1975) The sodium pump. Annual Review of Physiology 37, 13-55.
| Crossref | Google Scholar | PubMed |
Goldberger ZD, Goldberger AL (2012) Therapeutic ranges of serum digoxin concentrations in patients with heart failure. The American Journal of Cardiology 109(12), 1818-1821.
| Crossref | Google Scholar | PubMed |
Gouiaa S, Khoudi H, Leidi EO, Pardo JM, Masmoudi K (2012) Expression of wheat Na+/H+ antiporter TNHXS1 and H+- pyrophosphatase TVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Molecular Biology 79(1–2)), 137-155.
| Crossref | Google Scholar | PubMed |
Guo Y, Liu Y, Zhang Y, Liu J, Gul Z, Guo X-R, Abozeid A, Tang Z-H (2021) Effects of exogenous calcium on adaptive growth, photosynthesis, ion homeostasis and phenolics of Gleditsia Sinensis Lam. plants under salt stress. Agriculture 11(10), 978.
| Crossref | Google Scholar |
Hannachi S, Steppe K, Eloudi M, Mechi L, Bahrini I, Van Labeke M-C (2022) Salt stress induced changes in photosynthesis and metabolic profiles of one tolerant (‘Bonica’) and one sensitive (‘Black Beauty’) eggplant cultivars (Solanum Melongena L.). Plants 11(5), 590.
| Crossref | Google Scholar | PubMed |
Haque MA, Rafii MY, Yusoff MM, Ali NS, Yusuff O, Datta DR, Anisuzzaman M, Ikbal MF (2021) Advanced breeding strategies and future perspectives of salinity tolerance in rice. Agronomy 11(8), 1631.
| Crossref | Google Scholar |
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125(1), 189-198.
| Crossref | Google Scholar | PubMed |
Hnilickova H, Kraus K, Vachova P, Hnilicka F (2021) Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca Oleracea L. Plants 10(5), 845.
| Crossref | Google Scholar | PubMed |
Hussein H-AA, Alshammari SO (2022) Cysteine mitigates the effect of NaCl salt toxicity in flax (Linum Usitatissimum L) plants by modulating antioxidant systems. Scientific Reports 12(1), 11359.
| Crossref | Google Scholar | PubMed |
Ibrahim MFM, Ibrahim HA, Abd El-Gawad HG (2021) Folic acid as a protective agent in snap bean plants under water deficit conditions. The Journal of Horticultural Science and Biotechnology 96(1), 94-109.
| Crossref | Google Scholar |
Imtiaz K, Ahmed M, Annum N, Tester M, Saeed NA (2023) AtCIPK16, a CBL-interacting protein kinase gene, confers salinity tolerance in transgenic wheat. Frontiers in Plant Science 14, 1127311.
| Crossref | Google Scholar | PubMed |
Jakhar S, Mukherjee D (2014) Chloroplast pigments, proteins, lipid peroxidation and activities of antioxidative enzymes during maturation and senescence of leaves and reproductive organs of Cajanus Cajan L. Physiology and Molecular Biology of Plants 20(2), 171-180.
| Crossref | Google Scholar | PubMed |
Janicka-Russak M, Kłobus G (2007) Modification of plasma membrane and vacuolar H+-ATPases in response to NaCL and ABA. Journal of Plant Physiology 164(3), 295-302.
| Crossref | Google Scholar | PubMed |
Ji X, Tang J, Zhang J (2022) Effects of salt stress on the morphology, growth and physiological parameters of Juglansmicrocarpa L. Seedlings. Plants 11(18), 2381.
| Crossref | Google Scholar | PubMed |
Joardar JC, Razir SAA, Islam M, Kobir MH (2018) Salinity impacts on experimental fodder sorghum production. SAARC Journal of Agriculture 16(1), 145-155.
| Crossref | Google Scholar |
Karimi S, Karami H, Vahdati K, Mokhtassi-Bidgoli A (2020) Antioxidative responses to short-term salinity stress induce drought tolerance in walnut. Scientia Horticulturae 267, 109322.
| Crossref | Google Scholar |
Katz AM (1985) Effects of digitalis on cell biochemistry: sodium pump inhibition. Journal of the American College of Cardiology 5(5), 16A-21A.
| Crossref | Google Scholar |
Ketchem CJ, Conner CD, Murray RD, DuPlessis M, Lederer ED, Wilkey D, Merchant M, Khundmiri SJ (2016) Low dose ouabain stimulates NaK ATPase α1 subunit association with angiotensin II type 1 receptor in renal proximal tubule cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1863(11), 2624-2636.
| Crossref | Google Scholar | PubMed |
Khan AA, Wang T, Hussain T, Amna, Ali F, Shi F, Latef AAHA, Ali OM, Hayat K, Mehmood S, Zainab N, Muneer MA, Munis MFH, Soliman MH, Chaudhary HJ (2021) Halotolerant-Koccuria Rhizophila (14asp)-induced amendment of salt stress in pea plants by limiting Na+ uptake and elevating production of antioxidants. Agronomy 11(10), 1907.
| Crossref | Google Scholar |
Khattab HI, Abdel Halim AS, Helal NM (2024a) Enhancement of salt stress tolerance of Sorghum bicolor grown in soil remediated by Thidium gratum fern via upregulation of Na+/K+ transporter genes. Plant Physiology Reports 29(3), 678-695.
| Crossref | Google Scholar |
Khattab HI, Sadak MS, Dawood MG, Elkady FMA, Helal NM (2024b) Foliar application of esculin and digitoxin improve the yield quality of salt-stressed flax by improving the antioxidant defense system. BMC Plant Biology 24(1), 963.
| Crossref | Google Scholar |
Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T, Nayef MA, Shabala S, An G, Ma JF, Horie T (2017) OsHKT1;5 Mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. The Plant Journal 91(4), 657-670.
| Crossref | Google Scholar | PubMed |
Kravtsova VV, Paramonova II, Vilchinskaya NA, Tishkova MV, Matchkov VV, Shenkman BS, Krivoi II (2021) Chronic ouabain prevents Na,K-ATPase dysfunction and targets AMPK and IL-6 in disused rat soleus muscle. International Journal of Molecular Sciences 22(8), 3920.
| Crossref | Google Scholar | PubMed |
Kumar M, Kumar R, Jain V, Jain S (2018) Differential behavior of the antioxidant system in response to salinity induced oxidative stress in salt-tolerant and salt-sensitive cultivars of Brassica Juncea L. Biocatalysis and Agricultural Biotechnology 13, 12-19.
| Crossref | Google Scholar |
Kumavath R, Paul S, Pavithran H, Paul MK, Ghosh P, Barh D, Azevedo V (2021) Emergence of cardiac glycosides as potential drugs: current and future scope for cancer therapeutics. Biomolecules 11(9), 1275.
| Crossref | Google Scholar | PubMed |
Leonard RT, Hodges TK (1973) Characterization of plasma membrane-associated adenosine triphosphase activity of oat roots. Plant Physiology 52(1), 6-12.
| Crossref | Google Scholar | PubMed |
Li Z, Zhang Z, Xie JX, Li X, Tian J, Cai T, Cui H, Ding H, Shapiro JI, Xie Z (2011) Na/K-ATPase mimetic PNaKtide peptide inhibits the growth of human cancer cells. Journal of Biological Chemistry 286(37), 32394-32403.
| Crossref | Google Scholar | PubMed |
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4), 402-408.
| Crossref | Google Scholar | PubMed |
Lu Y, Fricke W (2023) Salt stress—regulation of root water uptake in a whole-plant and diurnal context. International Journal of Molecular Sciences 24(9), 8070.
| Crossref | Google Scholar | PubMed |
Maathuis FJM, Amtmann A (1999) K+ Nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany 84(2), 123-133.
| Crossref | Google Scholar |
Mahmood MZ, Odeibat HA, Ahmad R, Gatasheh MK, Shahzad M, Abbasi AM (2024) Low apoplastic Na+ and intracellular ionic homeostasis confer salinity tolerance upon Ca2SiO4 Chemigation in Zea Mays L. under salt stress. Frontiers in Plant Science 14, 1268750.
| Crossref | Google Scholar | PubMed |
Majeed Y, Zhu X, Zhang N, ul-Ain N, Raza A, Haider FU, Si H (2023) Harnessing the role of mitogen-activated protein kinases against abiotic stresses in plants. Frontiers in Plant Science 14, 932923.
| Crossref | Google Scholar | PubMed |
Martínez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu J-K, Pardo JM, Quintero FJ (2007) Conservation of the salt overly sensitive pathway in rice. Plant Physiology 143(2), 1001-1012.
| Crossref | Google Scholar | PubMed |
Meira EF, Siman FDM, Faria TO, Júnior RFR, de Batista PR, Stefanon I, Vassallo DV, Padilha AS (2015) Low-dose ouabain administration increases Na+,K+-ATPase activity and reduces cardiac force development in rats. Pharmacological Reports 67(2), 253-259.
| Crossref | Google Scholar | PubMed |
Misra PS, Mertz ET, Glover DV (1975) Studies on corn proteins. VIII. Free amino acid content of Opaque-2 double mutants. Cereal Chemistry 52(6), 844-848.
| Google Scholar |
Morandini P, Valera M, Albumi C, Bonza MC, Giacometti S, Ravera G, Murgia I, Soave C, de Michelis MI (2002) A novel interaction partner for the C-terminus of Arabidopsis thaliana plasma membrane H+-ATPase (AHA1 Isoform): site and mechanism of action on H+-ATPase activity differ from those of 14-3-3 proteins. The Plant Journal 31(4), 487-497.
| Crossref | Google Scholar | PubMed |
Mullan DJ, Colmer TD, Francki MG (2007) Arabidopsis–Rice–Wheat gene orthologues for Na+ transport and transcript analysis in wheat-L. elongatum aneuploids under salt stress. Molecular Genetics and Genomics 277(2), 199-212.
| Crossref | Google Scholar | PubMed |
Muñiz García MN, País SM, Téllez-Iñón MT, Capiati DA (2011) Characterization of StPPI1, a proton pump interactor from Solanum Tuberosum L. that is up-regulated during tuber development and by abiotic stress. Planta 233(4), 661-674.
| Crossref | Google Scholar | PubMed |
Nassert H, Baker DA (1972) Extrusion of sodium ions by barley roots I. Characteristics of the extrusion mechanism. Annals of Botany 36(5), 881-887.
| Crossref | Google Scholar |
Netondo GW, Onyango JC, Beck E (2004) Sorghum and salinity: I. Response of growth, water relations, and ion accumulation to NaCl salinity. Crop Science 44(3), 797-805.
| Crossref | Google Scholar |
Oh DH, Lee SY, Bressan RA, Yun DJ, Bohnert HJ (2010) Intracellular consequences of SOS1 deficiency during salt stress. Journal of Experimental Botany 61(4), 1205-1213.
| Crossref | Google Scholar | PubMed |
Palmgren MG (2001) PLANT PLASMA MEMBRANE H+-ATPases: powerhouses for nutrient uptake. Annual Review of Plant Physiology and Plant Molecular Biology 52, 817-845.
| Crossref | Google Scholar | PubMed |
Pasapula V, Shen G, Kuppu S, Paez-Valencia J, Mendoza M, Hou P, Chen J, Qiu X, Zhu L, Zhang X, Auld D, Blumwald E, Zhang H, Gaxiola R, Payton P (2011) Expression of an Arabidopsis Vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought- and salt tolerance and increases fibre yield in the field conditions. Plant Biotechnology Journal 9(1), 88-99.
| Crossref | Google Scholar | PubMed |
Patel CN, Kumar SP, Modi KM, Soni MN, Modi NR, Pandya HA (2019) Cardiotonic steroids as potential Na+/K+-ATPase inhibitors – a computational study. Journal of Receptors and Signal Transduction 39(3), 226-234.
| Crossref | Google Scholar | PubMed |
Petretto GL, Urgeghe PP, Massa D, Melito S (2019) Effect of salinity (NaCl) on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiology and Biochemistry 141, 30-39.
| Crossref | Google Scholar | PubMed |
Pirkmajer S, Petrič M, Chibalin AV (2021) The role of AMPK in regulation of Na+,K+-ATPase in skeletal muscle: does the gauge always plug the sink? Journal of Muscle Research and Cell Motility 42(1), 77-97.
| Crossref | Google Scholar | PubMed |
Prud’homme PM, Gonzalez B, Billard JP, Boucaud J (1992) Carbohydrate content, frutane and sucrose enzyme activities in roots, stubble and leaves of rye grass (Lolium perenne L.) as affected by source/sink modification after cutting. Journal of Plant Physiology 140, 282-291.
| Crossref | Google Scholar |
Qin H, Wang J, Chen X, Wang F, Peng P, Zhou Y, Miao Y, Zhang Y, Gao Y, Qi Y, Zhou J, Huang R (2019) Rice Os DOF 15 contributes to ethylene-inhibited primary root elongation under salt stress. New Phytologist 223(2), 798-813.
| Crossref | Google Scholar | PubMed |
Quan X, Liang X, Li H, Xie C, He W, Qin Y (2021) Identification and characterization of wheat germplasm for salt tolerance. Plants 10(2), 268.
| Crossref | Google Scholar | PubMed |
Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim W-Y, Ali Z, Fujii H, Mendoza I, Yun D-J, Zhu J-K, Pardo JM (2011) Activation of the plasma membrane Na/H antiporter salt-overly-sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proceedings of the National Academy of Sciences of the United States of America 108(6), 2611-2616.
| Crossref | Google Scholar | PubMed |
Rady MM (2011) Effect of 24-Epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Scientia Horticulturae 129(2), 232-237.
| Crossref | Google Scholar |
Rahnama A, Poustini K, Tavakkol-Afshari R, Ahmadi A, Alizadeh H (2011) Growth properties and ion distribution in different tissues of bread wheat genotypes (Triticum Aestivum L.) differing in salt tolerance. Journal of Agronomy and Crop Science 197(1), 21-30.
| Crossref | Google Scholar |
Rajabi Dehnavi A, Zahedi M, Piernik A (2024) Understanding salinity stress responses in sorghum: exploring genotype variability and salt tolerance mechanisms. Frontiers in Plant Science 14, 1296286.
| Crossref | Google Scholar | PubMed |
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signaling & Behavior 6(11), 1720-1731.
| Crossref | Google Scholar | PubMed |
Rhee Y-H, Moon JH, Jung JY, Oh C, Ahn J-C, Chung P-S (2019) Effect of photobiomodulation therapy on neuronal injuries by ouabain: the regulation of Na, K-ATPase; Src; and Mitogen-activated protein kinase signaling pathway. BMC Neuroscience 20(1), 19.
| Crossref | Google Scholar | PubMed |
Roy SJ, Huang W, Wang XJ, Evrard A, Schmöckel SM, Zafar ZU, Tester M (2013) A novel protein kinase involved in Na(+) exclusion revealed from positional cloning. Plant, Cell & Environment 36(3), 553-568.
| Crossref | Google Scholar | PubMed |
Sahu BB, Shaw BP (2009) Salt-inducible isoform of plasma membrane H+ATPase gene in rice remains constitutively expressed in natural halophyte, Suaeda Maritima. Journal of Plant Physiology 166(10), 1077-1089.
| Crossref | Google Scholar | PubMed |
Sairam RK (1994) Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian Journal of Experimental Biology 32, 584-593.
| Google Scholar |
Sandhu D, Cornacchione MV, Ferreira JFS, Suarez DL (2017) Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Scientific Reports 7, 42958.
| Crossref | Google Scholar | PubMed |
Saqib ZA, Akhtar J, Saqib M, Ahmad R (2011) Contrasting leaf Na+ uptake and transport rates conferred differences in salt tolerance of wheat genotypes. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science 61(2), 129-135.
| Crossref | Google Scholar |
Schachtman D, Liu W (1999) Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends in Plant Science 4(7), 281-287.
| Crossref | Google Scholar | PubMed |
Shabala S, Shabala S, Cuin TA, Pang J, Percey W, Chen Z, Conn S, Eing C, Wegner LH (2010) Xylem ionic relations and salinity tolerance in barley. The Plant Journal 61(5), 839-853.
| Crossref | Google Scholar | PubMed |
Shakeri E, Emam Y (2017) Selectable traits in sorghum genotypes for tolerance to salinity stress. Journal of Agriculture, Science and Technology 19, 1319-1332.
| Google Scholar |
Shakeri E, Emam Y, Pessarakli M, Tabatabaei SA (2020) Biochemical traits associated with growing sorghum genotypes with saline water in the field. Journal of Plant Nutrition 43, 1136-1153.
| Crossref | Google Scholar |
Shandell MA, Capatina AL, Lawrence SM, Brackenbury WJ, Lagos D (2022) Inhibition of the Na+/K+-ATPase by cardiac glycosides suppresses expression of the IDO1 immune checkpoint in cancer cells by reducing STAT1 activation. Journal of Biological Chemistry 298(3), 101707.
| Crossref | Google Scholar | PubMed |
Sheldon AR, Dalal RC, Kirchhof G, Kopittke PM, Menzies NW (2017) The effect of salinity on plant-available water. Plant and Soil 418, 477-491.
| Crossref | Google Scholar |
Shi H, Ishitani M, Kim C, Zhu J-K (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences 97(12), 6896-6901.
| Crossref | Google Scholar |
Silva P, Gerós H (2009) Regulation by salt of vacuolar H+-ATPase and H+-pyrophosphatase activities and Na+/H+ exchange. Plant Signaling & Behavior 4(8), 718-726.
| Crossref | Google Scholar | PubMed |
Singh V, Nguyen CT, van Oosterom EJ, Chapman SC, Jordan DR, Hammer GL (2015) Sorghum genotypes differ in high temperature responses for seed set. Field Crops Research 171, 32-40.
| Crossref | Google Scholar |
Sjöman H, Levinsson A, Emilsson T, Ibrahimova A, Alizade V, Douglas P, Wiström B (2021) Evaluation of Alnus Subcordata for urban environments through assessment of drought and flooding tolerance. Dendrobiology 85, 39-50.
| Crossref | Google Scholar |
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiology 53(2), 258-260.
| Crossref | Google Scholar | PubMed |
Smogyi M (1952) Notes on sugar determination. The Journal of Biological Chemistry 195(1), 19-23.
| Google Scholar | PubMed |
Stritzler M, Muñiz García MN, Schlesinger M, Cortelezzi JI, Capiati DA (2017) The plasma membrane H+-ATPase gene family in Solanum Tuberosum L. role of PHA1 in tuberization. Journal of Experimental Botany 68(17)), 4821-4837.
| Crossref | Google Scholar | PubMed |
Tian J, Liu J, Garlid KD, Shapiro JI, Xie Z (2003) Involvement of mitogen-activated protein kinases and reactive oxygen species in the inotropic action of ouabain on cardiac myocytes. A potential role for mitochondrial K(ATP) Channels. Molecular and Cellular Biochemistry 242(1–2), 181-187.
| Google Scholar | PubMed |
Wang S, Huang D-Y, Zhu Q-H, Li B-Z, Xu C, Zhu H-H, Zhang Q (2021) Agronomic traits and ionomics influence on Cd accumulation in various Sorghum (Sorghum bicolor (L.) Moench) genotypes. Ecotoxicology and Environmental Safety 214, 112019.
| Crossref | Google Scholar | PubMed |
Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A (1999) Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. Journal of Biological Chemistry 274(27), 19323-19328.
| Crossref | Google Scholar | PubMed |
Xie E, Wei X, Ding A, Zheng L, Wu X, Anderson B (2020) Short-term effects of salt stress on the amino acids of Phragmites australis root exudates in constructed wetlands. Water 12(2), 569.
| Crossref | Google Scholar |
Yang Z, Zheng H, Wei X, Song J, Wang B, Sui N (2018) Transcriptome analysis of sweet Sorghum inbred lines differing in salt tolerance provides novel insights into salt exclusion by roots. Plant and Soil 430(1–2), 423-439.
| Crossref | Google Scholar |
Youssef MHM, Raafat A, El-Yazied AA, Selim S, Azab E, Khojah E, El Nahhas N, Ibrahim MFM (2021) Exogenous application of alpha-lipoic acid mitigates salt-induced oxidative damage in sorghum plants through regulation growth, leaf pigments, ionic homeostasis, antioxidant enzymes, and expression of salt stress responsive genes. Plants 10(11), 2519.
| Crossref | Google Scholar | PubMed |
Zalucki MP, Brower LP, Alonso-M A (2001) Detrimental effects of latex and cardiac glycosides on survival and growth of first-instar monarch Butterfly Larvae Danaus Plexippus Feeding on the Sandhill Milkweed Asclepias Humistrata. Ecological Entomology 26(2), 212-224.
| Crossref | Google Scholar |
Zaman M, Shahid SA, Pharis RP. (2016) Salinity a serious threat to food security—where do we stand? International Atomic Energy Agency Bulletin 39, 9-10.
| Google Scholar |
Zhang H-X, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nature Biotechnology 19, 765-768.
| Crossref | Google Scholar |
Zhang H, Zhao Y, Zhu J-K (2020) Thriving under stress: how plants balance growth and the stress response. Developmental Cell 55(5), 529-543.
| Crossref | Google Scholar | PubMed |
Zhao X-h, Yu H-q, Wen J, Wang X-g, DU Q, Wang J, Wang Q (2016) Response of root morphology, physiology and endogenous hormones in maize (Zea Mays L.) to potassium deficiency. Journal of Integrative Agriculture 15(4), 785-794.
| Crossref | Google Scholar |
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P (2021) Regulation of plant responses to salt stress. International Journal of Molecular Sciences 22, 4609.
| Crossref | Google Scholar | PubMed |
Zhou Y, Lai Z, Yin X, Yu S, Xu Y, Wang X, Cong X, Luo Y, Xu H, Jiang X (2016) Hyperactive mutant of a wheat plasma membrane Na+/H+ antiporter improves the growth and salt tolerance of transgenic tobacco. Plant Science 253, 176-186.
| Crossref | Google Scholar | PubMed |
Zhu Y, DI T, Xu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H(+)-ATPase of rice roots to low PH as related to ammonium nutrition. Plant, Cell & Environment 32(10), 1428-1440.
| Crossref | Google Scholar | PubMed |
Zouari M, Hassena AB, Trabelsi L, Rouina BB, Decou R, Labrousse P (2019) Exogenous proline-mediated abiotic stress tolerance in plants: possible mechanisms. In ‘Osmoprotectant-mediated abiotic stress tolerance in plants’. (Eds M Hossain, V Kumar, D Burritt, M Fujita, P Mäkelä) pp. 99–121. (Springer International Publishing: Cham)
Zulfiqar F, Ashraf M (2023) Proline alleviates abiotic stress induced oxidative stress in plants. Journal of Plant Growth Regulation 42(8)), 4629-4651.
| Crossref | Google Scholar |


