Fluorescence lifetime imaging microscopy (FLIM): a non-traditional approach to study host-microbial symbioses
Pranali Deore A , Iromi Wanigasuriya B , Sarah Jane Tsang Min Ching A , Douglas R. Brumley C , Madeleine J. H. van Oppen A D , Linda L. Blackall A * and Elizabeth Hinde BA School of BioSciences, The University of Melbourne, Vic., Australia.
B School of Physics, The University of Melbourne, Vic., Australia.
C School of Mathematics and Statistics, The University of Melbourne, Vic., Australia.
D Australian Institute of Marine Science, Townsville, Qld, Australia.

Dr Pranali Deore is an environmental microbiologist, and her current research focuses on developing advanced bio-optic tools for visualisation of microalgae-bacteria symbiosis. Her expertise is in microalgal photo-physiology and use of Fourier transform spectroscopy to study microbial interactions. She is currently a post-doctoral research fellow at the University of Melbourne. |

Dr Iromi Wanigasuriya is a molecular biologist and a research fellow at The University of Melbourne. She is currently using advanced microscopy techniques to understand dynamic epigenetic mechanisms. |

Sarah Jane Tsang Min Ching has a Master of Science and is a research associate at The University of Melbourne. She uses advanced molecular biology and microbiology techniques for isolation and identification of microbial symbionts of corals. Her past research involves understanding metabolomic changes in sea anemones (laboratory model of corals) transplanted with heat evolved Symbiodiniaceae strains. |

Dr Douglas Brumley BSc (Hons), PhD (Cantab) is a Senior Lecturer at The University of Melbourne. He leads an interdisciplinary research group which utilises mathematics, microfluidics and microscopy to study a range of dynamic processes in biology including bacterial motility, symbioses, nutrient cycling and flows around coral reefs. |

Professor Madeleine J. H. van Oppen is an ecological geneticist with an interest in microbial symbioses and climate change adaptation of reef corals. Her early career focused on evolutionary and population genetics of algae and fish, and subsequently corals. Currently, her team is using bioengineering approaches aimed at increasing coral climate resilience and the likelihood that coral reefs will survive this century. These interventions include coral host hybridisation and conditioning, directed evolution of microalgal symbionts and bacterial probiotics. |

Professor Linda L. Blackall is an environmental microbial ecologist, who has studied many different complex microbial communities ranging from host associated through to free living in numerous environments. One of her research fields is the microbiota of corals and sponges. The numerous methods she develops and employs in her research allow elucidation of microbial complexity and function in these diverse biomes. |

A/Professor Elizabeth Hinde is a cellular biophysicist with an expertise in fluorescence lifetime imaging microscopy and fluorescence fluctuation spectroscopy. She develops methods to spatially map live cell nuclear architecture and is using this technology to uncover the impact this dynamic structural framework has on DNA target search. |
Microbiology Australia 43(1) 22-27 https://doi.org/10.1071/MA22008
Submitted: 8 March 2022 Accepted: 17 March 2022 Published: 14 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Corals and their photosynthetic endosymbiotic algae (Symbiodiniaceae) produce a strong autofluorescent signal that spans the visible to near-infrared (NIR) spectrum. However, this broad-spectrum emission hinders the use of fluorescence in situ hybridisation (FISH) for the study of bacterial heterogeneity within the different niches of corals and Symbiodiniaceae, because FISH fluorophores also fluoresce within the visible to NIR spectrum. A solution to this impediment is to use fluorescence lifetime imaging microscopy (FLIM). The ‘lifetime’ property of fluorophores is a feature that enables sample (e.g. coral/Symbiodiniaceae) autofluorescence to be distinguished from FISH-labelled bacteria. In this manner, the location of bacteria around and within Symbiodiniaceae can be quantified along with their identity and spatial distribution. Furthermore, the ‘lifetime’ of the host and associated microbe cellular autofluorescence can be analysed in terms of endogenous fluorophore composition (e.g. metabolic co-factors, aromatic amino acids) and serves as information for symbiotic versus parasitic host-microbe association.
Keywords: autofluorescence, confocal microscopy, fluorescence lifetime imaging microscopy, label-free detection, microbial ecology, microbial symbiosis, phasor analysis, visualisation.
Introduction
Our understanding of microbes is largely based on classical phenotypic assays, advanced multi-omics, and optical microscopy methods. The discovery of genetically encoded fluorescent proteins (e.g. green fluorescent proteins), alongside advances in chemical fluorophore synthesis, has enabled exogenous incorporation of fluorescent molecules into a range of microbes, and development of fluorescence microscopy methods to visualise particular species within a complex biological system.1 However, fluorescence labelling and visualisation of multiple microbial species within a mixed community remains a challenge, because of difficulties in establishing pure cultures2 that are required for probe optimisation, and the presence of broad spectrum autofluorescence from different chemicals present in microbial consortia. In this review we explore how fluorescence in situ hybridisation (FISH) coupled with fluorescence lifetime imaging microscopy (FLIM) presents a unique opportunity to circumvent the latter technical hurdle in uncovering the in situ location and identity of bacteria in Symbiodiniaceae.
Fluorescence microscopy in microbial studies
FISH is a method that was first used to visualise bacteria in 19893 and it involves the hybridisation of fluorescently labelled oligonucleotides (~20 nucleotides) to the 16S rRNA or 23S rRNA in ribosomes, so that specific bacteria can be detected and their spatial location determined.1,4 The fluorophores commonly available for FISH exhibit excitation–emission properties that span the visible to near-infrared (NIR) spectrum. However, since this spectral range overlaps with the broad spectrum autofluorescence of samples such as cyanobacteria or algae,5 microbial biofilms,6 and coral or Symbiodiniaceae cells,7 selection of suitable FISH fluorophores to localise bacteria within these environments via conventional epifluorescence or confocal laser scanning microscopy (CLSM) has proven difficult. Nonetheless, there is a strong interest in the use of fluorescence microscopy methods that can distinguish FISH fluorophores from sample autofluorescence. In addition to FISH-based visualisation of microbes, fluorescent dyes have been used to directly count microbes in environmental samples,8 study microbial respiration activity using redox dyes,9 determine proteins involved in peptidoglycan synthesis using synthetic fluorescently labelled D-amino acids,10 and evaluate oxidative stress using enzymatically cleavable fluorophores.11
Fluorescence lifetime imaging microscopy
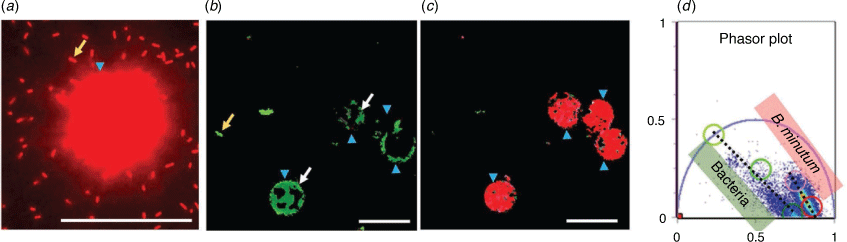
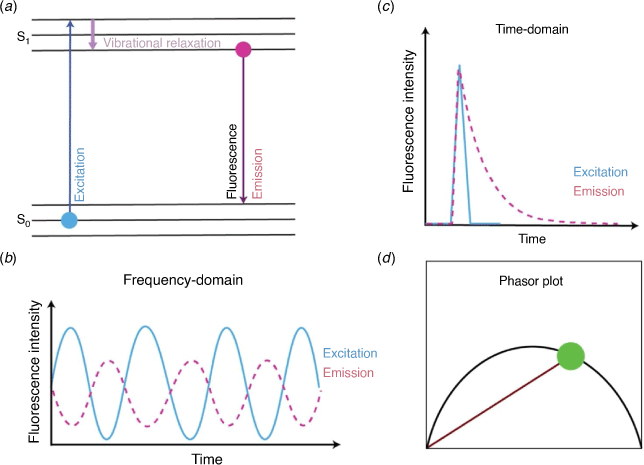
A fluorescence microscopy method that offers the opportunity to distinguish FISH fluorophore emission (and fluorescent dyes more broadly) from sample autofluorescence is fluorescence lifetime imaging microscopy (FLIM). FLIM is a method that measures the fluorescence lifetime of a fluorophore’s emission rather than its fluorescence intensity. The fluorescence lifetime of a fluorophore is defined as the time spent in the excited state upon absorption of a photon, before emitting the photon and returning to the ground state (Fig. 1a). FISH fluorophores typically exhibit a fluorescence lifetime on the order of nanoseconds, depending on their individual chemical structure and molecular environment,12 and in most cases this time is distinct from the fluorescence lifetimes of autofluorescent chemicals in a host organism, for example, Symbiodiniaceae (Fig. 2a, c). Thus, FLIM offers the capacity to readily detect FISH-labelled microbes within Symbiodiniaceae autofluorescence (Fig. 2b), despite their significant spectral overlap. Additionally, the fluorescence lifetime of holobiont (the collection of a host and its associated microbiome) autofluorescent endogenous chemicals (e.g. metabolic co-enzymes, structural proteins, vitamins, pigments and amino acids) can be determined and analysed to inform on the different molecular environments that host FISH-labelled bacteria. In particular, moieties of chlorophyll a, phycobiliproteins, nicotinamide adenine dinucleotide (phosphate) (NAD(P)H), flavins, aromatic amino acids, porphyrins13 all contribute to the fluorescence lifetime recorded throughout a holobiont, and these signals can be used as a label-free record of host and/or symbiont physiology.
|
FLIM imaging of FISH-labelled bacteria can be achieved using fluorescence (typically CLSM) microscopes and the time- or the frequency-domains (Fig. 1b, c). In the time-domain, the detection of fluorescence is coupled to the excitation source that is pulsed (one or two-photon) and a detection unit that can measure the arrival time of the emitted photons (e.g. via use of time-correlated single photon counting (TCSPC)). The successive photon arrival time within each FLIM image pixel is then represented in the form of a histogram and fitted to an exponential (or multi-exponential) that reports the characteristic fluorescence lifetime.12 In the frequency-domain, the excitation source is sinusoidally modulated and the detection unit measures the phase delay and change in amplitude that the fluorescent emission undergoes with respect to the excitation source (demodulation). Both time- and frequency-domain data can be transformed into a phasor representation (Fig. 1d) which is a fit free approach for lifetime analysis that facilitates multiple-component analysis within each pixel of a FLIM image.11 A representative phasor plot associated with Symbiodiniaceae and FISH labelled bacteria is shown in Fig. 2d.
Use of FLIM in microbial studies
So, what does this mean for microbiologists? Although FISH can enable taxonomic affiliation, quantification and localisation of probed microbial communities, distinguishing FISH probe fluorescence from host (e.g. Symbiodiniaceae) autofluorescence can be provided by FLIM (Fig. 2). The in situ determination of physiological and chemical processes requires metabolic probing of cells,4 which can be done by FLIM of endogenous metabolites. The lifetime property of fluorophores is influenced by the fluid viscosity, pH, temperature and ion concentration in the fluorophore vicinity, which provides information about fluorophore behaviour and function.12 This is vital for monitoring cellular heterogeneity and changes in the local microenvironment of microbes. For example, NAD(P)H is an important co-factor in numerous biosynthetic pathways and has a characteristic lifetime signature at 340 nm excitation, 470 nm emission. The autofluorescence lifetime of free (0.4 ns) and enzyme bound NAD(P) (1–5 ns) can be monitored using two-photon excitation in a FLIM setup providing information on the physiological variation among microbial cells.14
Monitoring the metabolic activity of NAD(P)H is a particularly promising method to study host invasion15 by beneficial (symbionts) and detrimental microbes (e.g. pathogens). For example, the acquisition of Symbiodiniaceae by coral larvae or early recruits is an important stage in the establishment of symbiosis. The variation in fluorescence lifetimes of endogenous compounds, including NAD(P)H and chlorophyll a, could help to determine physiological fitness of Symbiodiniaceae during its colonisation phase. In corals, climate change and other environmental disturbances are hypothesised to cause oxidative stress due to excessive build-up of reactive oxygen species (ROS), which leads to the separation of Symbiodiniaceae from the host,16 known as coral bleaching. The autofluorescence of endogenous NAD(P)H has been used for co-localisation and quantification of oxidative stress using two-photon-FLIM.17,18 Monitoring of enzyme-bound and free NAD(P)H dynamics is particularly important given the hypothesised role of NAD(P)H oxidase in ROS production in corals and Symbiodiniaceae.19 These approaches could be implemented for visualisation and tracking of spatiotemporal dynamics of ROS to better understand their role in coral bleaching. FLIM could also be used to link bacterial associations (intra-versus extracellular endosymbionts) with Symbiodiniaceae by measuring the in situ heterogeneity during varying physicochemical conditions such as of temperature, pH,12 and oxygen gradients20 at the single-cell level.21
Another procedure that can measure elemental distribution in a sample is nanoscale secondary ion mass spectrometry (NanoSIMS),22 which involves use of stable isotopes and FISH to visualise the incorporation of labelled substrates into single microbes in complex microbial communities. However, FLIM is a non-invasive procedure in comparison to the destructive technique of NanoSIMS.
The study of microbes in a spatially constrained environment using a fabricated microfluidic platform23,24 combined with FLIM can aid in understanding biogeochemical processes at the microscale level, even in mixed microbial scenarios.
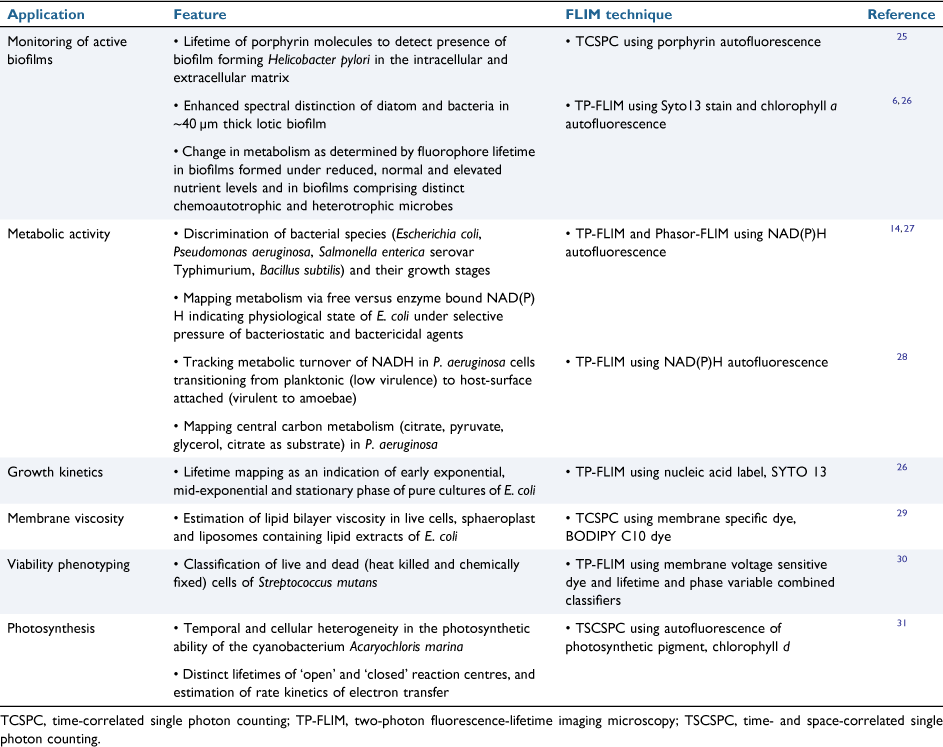
Several labelled and label-free FLIM detection approaches using specific (e.g. FISH) or non-specific fluorophores (e.g. Syto 13, DAPI, Hoechst 33342) and autofluorescent moieties (e.g. NAD(P)H and chlorophyll a) are outlined in Table 1.
|
Final remarks
Real time FLIM of labelled (via FISH) and label-free (via endogenous autofluoresence) microbes will provide a deeper understanding of the succession of endosymbionts within a host. The success of this prolonged live cell FLIM experiment will depend on the photostability of fluorescent probes and the stability of viable microbial cells. In recent years, advancement has been made in the use of photostable quantum dots (QDs), which are nanoparticles with long fluorescence lifetimes. QDs can be conjugated to FISH probes adding stability and flexibility to conventional FISH and FLIM. Additionally, the linear association between the QD’s fluorescence lifetime and pH could be exploited to monitor intracellular pH in response to cellular disturbances. The study of endogenous metabolic co-enzymes, structural proteins, vitamins, pigments, and amino acids by their autofluorescence via FLIM has been a major experimental approach in the eukaryotic biomedical field but has not yet been widely employed in prokaryotic fields. Endogenous autofluorescent chemicals permit label-free detection of microbial associations, including studying actively dividing cells, in environmental scenarios. We envisage that the quantitative information obtained by phasor-based FLIM analysis of these probes, combined with multivariate tools can help discriminate between symbiont enriched and/or depleted zones in natural ecosystems.
The additional modalities of FLIM such as Förster resonance energy transfer (FRET) that measures differences in the lifetime of a fluorophore arising from either donor and acceptor molecules, will pave the way to understanding physical interactions between symbiont and host cell or between two different microbial cells in consortia. In FRET, the fluorescence emission shift occurs in instances where donors transfer energy (due to physical proximity and interaction) to acceptor fluorophores. The FLIM-FRET technology is a promising means of studying environmental microbial associations (e.g. protein or metabolite translocation between symbiont and host or between two different microbes) and is yet to be explored.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflict of interest.
Declaration of funding
PD, STMJ; DRB, MvO, LLB and EH are supported by the Gordon Betty Moore foundation (project number – 9351); IW was supported by an Australian Research Council Discovery Project (DP180101387); DRB was supported by an Australian Research Council Discovery Early Career Researcher Award (DE180100911) and a Gordon & Betty Moore Foundation Symbiosis in Aquatic Systems grant; IW was supported by an Australian Research Council Discovery Project (DP180101387); EH is supported is supported by an Australian Research Council Future Fellowship (FT200100401) and MJHvO by an Australian Research Council Laureate Fellowship (FL180100036).
Author contributions
PD prepared the initial draft and all other co-authors shaped the final document.
References
[1] Amann, R and Fuchs, BM (2008) Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat Rev Microbiol 6, 339–348.| Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques.Crossref | GoogleScholarGoogle Scholar | 18414500PubMed |
[2] Wang, F et al.. (2021) Cultivation of uncultured marine microorganisms. J Mar Sci Technol 3, 117–120.
| Cultivation of uncultured marine microorganisms.Crossref | GoogleScholarGoogle Scholar |
[3] DeLong, EF et al.. (1989) Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science 243, 1360–1363.
| Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells.Crossref | GoogleScholarGoogle Scholar | 2466341PubMed |
[4] Pernthaler A (2010) Identification of environmental microorganisms by fluorescence in situ hybridization. In Handbook of Hydrocarbon and Lipid Microbiology (Timmis K, ed.). pp. 4127–4135. Springer.
[5] Cohen, AB et al.. (2021) Applying fluorescence in situ hybridization to aquatic systems with cyanobacteria blooms: autofluorescence suppression and high‐throughput image analysis. Limnol Oceanogr Methods 19, 457–475.
| Applying fluorescence in situ hybridization to aquatic systems with cyanobacteria blooms: autofluorescence suppression and high‐throughput image analysis.Crossref | GoogleScholarGoogle Scholar |
[6] Neu, TR et al.. (2004) Two-photon imaging for studying the microbial ecology of biofilm systems. Microbes Environ 19, 1–6.
| Two-photon imaging for studying the microbial ecology of biofilm systems.Crossref | GoogleScholarGoogle Scholar |
[7] Wangpraseurt, D et al.. (2014) Spectral effects on Symbiodinium photobiology studied with a programmable light engine. PLoS One 9, e112809. .
| Spectral effects on Symbiodinium photobiology studied with a programmable light engine.Crossref | GoogleScholarGoogle Scholar | 25389753PubMed |
[8] Kepner Jr, RL and Pratt, JR (1994) Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol Rev 58, 603–615.
| Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present.Crossref | GoogleScholarGoogle Scholar |
[9] Konopka, MC et al.. (2011) Respiration response imaging for real-time detection of microbial function at the single-cell level. Appl Environ Microbiol 77, 67–72.
| Respiration response imaging for real-time detection of microbial function at the single-cell level.Crossref | GoogleScholarGoogle Scholar | 21075887PubMed |
[10] Montón Silva, A et al.. (2018) The fluorescent D-amino acid NADA as a tool to study the conditional activity of transpeptidases in Escherichia coli. Front Microbiol 9, 2101.
| The fluorescent D-amino acid NADA as a tool to study the conditional activity of transpeptidases in Escherichia coli.Crossref | GoogleScholarGoogle Scholar | 30233559PubMed |
[11] Gardner, SG et al.. (2017) Reactive oxygen species (ROS) and dimethylated sulphur compounds in coral explants under acute thermal stress. J Exp Biol 220, 1787–1791.
| Reactive oxygen species (ROS) and dimethylated sulphur compounds in coral explants under acute thermal stress.Crossref | GoogleScholarGoogle Scholar | 28275004PubMed |
[12] Periasamy A, Clegg R (2009) FLIM microscopy in biology and medicine. CRC Press.
[13] Croce, AC and Bottiroli, G (2017) Autofluorescence spectroscopy for monitoring metabolism in animal cells and tissues. Methods Mol Biol 1560, 15–43.
| Autofluorescence spectroscopy for monitoring metabolism in animal cells and tissues.Crossref | GoogleScholarGoogle Scholar | 28155143PubMed |
[14] Bhattacharjee, A et al.. (2017) Metabolic fingerprinting of bacteria by fluorescence lifetime imaging microscopy. Sci Rep 7, 3743.
| Metabolic fingerprinting of bacteria by fluorescence lifetime imaging microscopy.Crossref | GoogleScholarGoogle Scholar | 28623341PubMed |
[15] Szaszák, M et al.. (2011) Fluorescence lifetime imaging unravels C. trachomatis metabolism and its crosstalk with the host cell. PLoS Path 7, e1002108.
| Fluorescence lifetime imaging unravels C. trachomatis metabolism and its crosstalk with the host cell.Crossref | GoogleScholarGoogle Scholar |
[16] Szabó M,et al. (2020) A review: the role of reactive oxygen species in mass coral bleaching. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms (Larkum A, et al., eds). pp. 459–488. Springer International Publishing.
[17] Datta, R et al.. (2015) Fluorescence lifetime imaging of endogenous biomarker of oxidative stress. Sci Rep 5, 9848.
| Fluorescence lifetime imaging of endogenous biomarker of oxidative stress.Crossref | GoogleScholarGoogle Scholar | 25993434PubMed |
[18] Wu, Z et al.. (2020) Real-time imaging and simultaneous quantification of mitochondrial H2O2 and ATP in neurons with a single two-photon fluorescence-lifetime-based probe. J Am Chem Soc 142, 7532–7541.
| Real-time imaging and simultaneous quantification of mitochondrial H2O2 and ATP in neurons with a single two-photon fluorescence-lifetime-based probe.Crossref | GoogleScholarGoogle Scholar | 32233469PubMed |
[19] Saragosti, E et al.. (2010) Extracellular production and degradation of superoxide in the coral Stylophora pistillata and cultured Symbiodinium. PLoS One 5, e12508.
| Extracellular production and degradation of superoxide in the coral Stylophora pistillata and cultured Symbiodinium.Crossref | GoogleScholarGoogle Scholar | 20856857PubMed |
[20] Wu, H-M et al.. (2019) Widefield frequency domain fluorescence lifetime imaging microscopy (FD-FLIM) for accurate measurement of oxygen gradients within microfluidic devices. Analyst 144, 3494–3504.
| Widefield frequency domain fluorescence lifetime imaging microscopy (FD-FLIM) for accurate measurement of oxygen gradients within microfluidic devices.Crossref | GoogleScholarGoogle Scholar | 31062784PubMed |
[21] Sebastián, M and Gasol, J (2019) Visualization is crucial for understanding microbial processes in the ocean. Phil Trans R Soc B 374, 20190083.
| Visualization is crucial for understanding microbial processes in the ocean.Crossref | GoogleScholarGoogle Scholar | 31587650PubMed |
[22] Herrmann, AM (2007) Nano-scale secondary ion mass spectrometry—a new analytical tool in biogeochemistry and soil ecology: a review article. Soil Biol Biochem 39, 1835–1850.
| Nano-scale secondary ion mass spectrometry—a new analytical tool in biogeochemistry and soil ecology: a review article.Crossref | GoogleScholarGoogle Scholar |
[23] Brumley, DR et al.. (2019) Bacteria push the limits of chemotactic precision to navigate dynamic chemical gradients. Proc Natl Acad Sci 116, 10792–10797.
| Bacteria push the limits of chemotactic precision to navigate dynamic chemical gradients.Crossref | GoogleScholarGoogle Scholar | 31097577PubMed |
[24] Son, K et al.. (2015) Live from under the lens: exploring microbial motility with dynamic imaging and microfluidics. Nat Rev Microbiol 13, 761–775.
| Live from under the lens: exploring microbial motility with dynamic imaging and microfluidics.Crossref | GoogleScholarGoogle Scholar | 26568072PubMed |
[25] Battisti, A et al.. (2021) Fluorescence lifetime imaging microscopy of porphyrins in Helicobacter pylori biofilms. Pharmaceutics 13, 1674.
| Fluorescence lifetime imaging microscopy of porphyrins in Helicobacter pylori biofilms.Crossref | GoogleScholarGoogle Scholar | 34683966PubMed |
[26] Walczysko, P (2008) In situ activity of suspended and immobilized microbial communities as measured by fluorescence lifetime imaging. Appl Environ Microbiol 74, 294–299.
| In situ activity of suspended and immobilized microbial communities as measured by fluorescence lifetime imaging.Crossref | GoogleScholarGoogle Scholar | 17981940PubMed |
[27] Torno, K et al.. (2013) Real-time analysis of metabolic activity within Lactobacillus acidophilus by phasor fluorescence lifetime imaging microscopy of NADH. Curr Microbiol 66, 365–367.
| Real-time analysis of metabolic activity within Lactobacillus acidophilus by phasor fluorescence lifetime imaging microscopy of NADH.Crossref | GoogleScholarGoogle Scholar | 23233088PubMed |
[28] Perinbam, K et al.. (2020) A shift in central metabolism accpmpanies virulence activation in Pseudomonas aeruginosa. mBio 11, e02730–e02718.
| A shift in central metabolism accpmpanies virulence activation in Pseudomonas aeruginosa.Crossref | GoogleScholarGoogle Scholar | 32156820PubMed |
[29] Mika, J et al.. (2016) Measuring the viscosity of the Escherichia coli plasma membrane using molecular rotors. Biophys J 111, 1528–1540.
| Measuring the viscosity of the Escherichia coli plasma membrane using molecular rotors.Crossref | GoogleScholarGoogle Scholar | 27705775PubMed |
[30] Dunkers, JP et al.. (2021) Toward absolute viability measurements for bacteria. J Biophotonics 14, e202100175.
| Toward absolute viability measurements for bacteria.Crossref | GoogleScholarGoogle Scholar | 34510771PubMed |
[31] Petrášek, Z et al.. (2009) Wide-field photon counting fluorescence lifetime imaging microscopy: application to photosynthesizing systems. Photosynth Res 102, 157–168.
| Wide-field photon counting fluorescence lifetime imaging microscopy: application to photosynthesizing systems.Crossref | GoogleScholarGoogle Scholar | 19533411PubMed |