Live biotherapies for improved respiratory health in children
Tamlyn Fairall A , Yi Bin Koh A , Anastasia Basuki A , Mark P. Nicol A B and Ritika Kar Bahal A *A
B

Tamlyn Fairall is a research scientist at the Marshall Centre for Infectious Disease, The University of Western Australia (UWA). Tamlyn has research interests in infectious disease and microbiology and how this can contribute to advancements in public health. |

Yi Bin Koh is a PhD student at The University of Western Australia. Her research focuses on interbacterial relationships within the nasopharynx, aiming to address childhood pneumonia. |

Anastasia Basuki is an Honours student at The University of Western Australia. Currently, Anastasia is researching the mechanisms of interactions between nasopharyngeal bacteria, aiming to contribute to future research in this area. |

Mark Nicol is professor of microbiology at The University of Western Australia. He studied medicine and medical microbiology at the University of the Witwatersrand and completed his PhD in childhood tuberculosis in Cape Town. His research explores the role of the early life microbiome in acute and chronic respiratory illnesses in children. He also has an active research programme involved in the development and evaluation of better diagnostic tests for tuberculosis and pneumonia. He is Chair of the WHO Advisory Group on Tuberculosis Diagnostics and Laboratory Strengthening. |

Dr Ritika Kar Bahal is a BrightSpark Early Career Child Health Research fellow at The University of Western Australia. She completed her PhD in tuberculosis research from University of Delhi, India, and relocated to Perth in 2018. Her current research explores the role of the early life microbiome and bacterial–bacterial interactions in respiratory illnesses in children. Dr Bahal has received several prestigious scholarships and awards, such as the Nature Microbiome Accelerator award 2024 and the FEMS ECR talk award at ASM National Meeting 2024. She works closely with community members in co-designing translational goals for her research. |
Abstract
Pneumonia remains the leading cause of death in children under five outside of the neonatal period. Antibiotic treatment for bacterial pneumonia is usually effective; however, the rise of antimicrobial resistance threatens treatment outcomes and the adverse health outcomes of administrating antibiotics in early childhood can be lifelong. Children with pneumonia also place immense pressure on health systems, therefore, the prevention of pneumonia in children must be prioritised. An alternative novel potential prevention strategy for respiratory infections, is the use of live biotherapeutics. This is based on recent research showing that the composition of the nasopharyngeal microbiome follows succession patterns in early childhood, with certain bacterial genera, being associated with healthy respiratory outcomes whereas others are associated with poor respiratory outcomes. Our research uses nasopharyngeal samples from the Drakenstein Child Health Study (DCHS) to create a biobank of isolates for researching bacterial interactions within the nasopharyngeal microbiome. We aim to identify candidate bacterial strains that may be used in the development of live biotherapies targeting potentially pathogenic bacteria and therefore, improve respiratory health outcomes in children.
Keywords: biobank, commensal bacteria, DCHS, Drakenstein Child Health Study, live biotherapeutics, microbial interactions, nasopharyngeal microbiome, pathogens, pneumonia, respiratory tract.
The burden of pneumonia
Childhood pneumonia or lower respiratory tract infection (LRTI) is a significant global health burden, causing over 500,000 deaths in children under 5 years of age in 2021, making it the leading infectious cause of mortality outside of the neonatal period.1 Childhood pneumonia increases the risk of premature death from respiratory diseases later in life, including lung function deficits and higher asthma risk.2 Despite advances in prevention and treatment strategies, the global burden of childhood pneumonia remains substantial, and public healthcare systems, particularly in low- and middle-income countries, are most heavily affected, with a high demand for treatment and hospitalisations associated with childhood pneumonia.3 In Australia, pneumonia persists as a significant contributor to hospitalisation, responsible for 2–8 hospitalisations per 1000 person-years.4 In Western Australia, Indigenous children have 14 times the risk of hospitalisation for pneumonia compared to non-Indigenous children.4
Whereas most pneumonia cases in children are caused by viruses (~60%), bacterial causes are responsible for one-quarter of cases,2 and bacterial co-infection may increase the severity of viral pneumonia. Severe pneumonia is usually bacterial in aetiology and requires empirical antibiotic treatment.2,5 The Australian Report on Antimicrobial Use and Resistance identified the highest rate of antibiotic dispensing was for children under 4 years old, with pneumonia accounting for 4.1% of hospital prescriptions in the 2021 National Antimicrobial Prescribing Survey.6 Globally, childhood pneumonia is a major driver of antibiotic use, contributing to the rise of antimicrobial resistance. In 2019, antimicrobial resistance directly caused ~1.27 million deaths, with LRTIs being the most common underlying infection.7 Although antibiotics are generally effective for bacterial pneumonia, resistance threatens treatment outcomes, and early childhood antibiotic use has been linked to long-term health risks such as asthma and obesity.8–10 This underscores the urgent need for novel preventive measures.
The nasopharyngeal microbiome as a gatekeeper
The nasopharyngeal microbiome houses a community of various bacteria, viruses and fungi that interact with one another. The nasopharyngeal microbiome is closely linked to pneumonia, as the microbial balance in the respiratory tract influences risk of pneumonia by affecting pathogen overgrowth and host immune responses.11
Studies have identified distinct differences in the microbial composition of healthy individuals and those with pneumonia, indicating that certain microbial profiles are associated with pneumonia.12,13 The nasopharyngeal microbiome serves as a reservoir for pneumonia-associated bacterial species such as Streptococcus pneumoniae and Haemophilus influenzae that can translocate to the lower respiratory tract and cause infection.12,14,15 By contrast, in healthy infants, the respiratory tract establishes a protective environment that modulates inflammation and provides colonisation resistance, a process mediated by commensal bacteria that inhibit the overgrowth of pathogenic species.7 Commensal bacteria are non-pathogenic residents of the nasopharynx that may promote respiratory health by stimulating the host’s immune system to induce protective responses, by directly inhibiting colonisation by pathogenic bacteria or by improving epithelial barrier integrity.16,17 Common commensal bacterial genera of the nasopharynx include Corynebacterium, Dolosigranulum and some Streptococcus species.18
Findings from lower respiratory tract infection case–control studies
Observational studies conducted within a South African birth cohort of infants enrolled in the Drakenstein Child Health Study (DCHS) have identified associations between specific bacteria and LRTI.19 The DCHS consisted of 1000 mother–child pairs. Mothers and children were enrolled and followed from the second trimester to at least 5 years old, with children having 6-monthly nasopharyngeal swabs. The nasopharyngeal swabs were used to characterise the children’s nasopharyngeal microbiome through targeted and untargeted approaches.19 A nested case–control study within the DCHS cohort used 16S rRNA gene amplicon sequencing of nasopharyngeal swabs to compare microbiome composition between LRTI cases and healthy controls.20 Findings showed that LRTI was associated with increased relative abundance of Haemophilus (q = 0.0003) and decreased relative abundance of Dolosigranulum (q = 0.001), Corynebacterium (q = 0.091) and Neisseria (q = 0.004).20
Findings from a Dutch child cohort revealed that children who had experienced more than two respiratory tract infections (RTIs) had a prolonged absence of Corynebacterium and Dolosigranulum.17 Similarly, a West Australian infant cohort12 showed that succession patterns in the nasopharyngeal microbiome differed between samples from healthy children and infants with acute respiratory illness. In those with acute respiratory illness, microbiome profiles were dominated by Moraxella, Streptococcus and Haemophilus whereas healthy samples had profiles enriched in Staphylococcus and Corynebacterium.12
These findings highlight that certain bacterial taxa, such as Corynebacterium and Dolosigranulum, are associated with less frequent RTIs.10 Certain species, such as C. propinquum, C. pseudodiphtheriticum, C. accolens and D. pigrum, are rarely isolated outside of the nasopharynx or nasal cavity, which further supports the niche-specific roles they may have in respiratory health.7
Interactions of respiratory commensals with respiratory pathogens
In a recent scoping review, we have identified the key bacterial–bacterial interactions reported between nasopharyngeal bacterial species.21 The review highlights that Corynebacterium species were associated with a reduced risk of colonisation with the respiratory pathogen Streptococcus pneumoniae.22 A longitudinal study also identified that Corynebacterium and Dolosigranulum were negatively associated with the co-occurrence of Moraxella and Staphylococcus.23 Health-associated bacteria may also play a role in the severity of viral infections, as a decreased abundance of Corynebacterium and Dolosigranulum has been correlated with more severe cases of respiratory syncytial virus (RSV) disease and poorer clinical outcomes.12 Conversely, microbiota profiles dominant in bacterial genera such as Streptococcus and Haemophilus are associated with higher susceptibility to respiratory tract infections and more severe RSV disease.13,24
Species-specific interactions between Corynebacterium spp. and respiratory pathogens have been documented in in vitro assays, with certain species of Corynebacterium exhibiting differential interactions. Notably, C. propinquum, C. pseudodiphtheriticum, C. accolens, C. tuberculostearicum, C. coyleae and C. striatum inhibited S. pneumoniae in vitro.22,25 In one study, C. pseudodiptheriticum did not independently inhibit S. pneumoniae but exhibited a inhibitory effect when combined with with D. pigrum.16 Additionally, C. propinquum has shown indirect inhibition of competing coagulase-negative Staphylococcus species by sequestering iron, whereas C. pseudodiphtheriticum can directly inhibit S. aureus in vitro.26
Despite these findings, the underlying mechanisms of the interactions among nasopharyngeal bacteria remain largely unexplored.21 Further investigation is required to elucidate these mechanisms and to determine whether enhancing colonisation with health-associated bacteria can improve respiratory health outcomes, through the development of novel preventative strategies such as live biotherapies that reinforce a healthy microbiome in the nasopharynx.
Building a biobank of bacterial isolates as a resource of strains for live biotherapy development
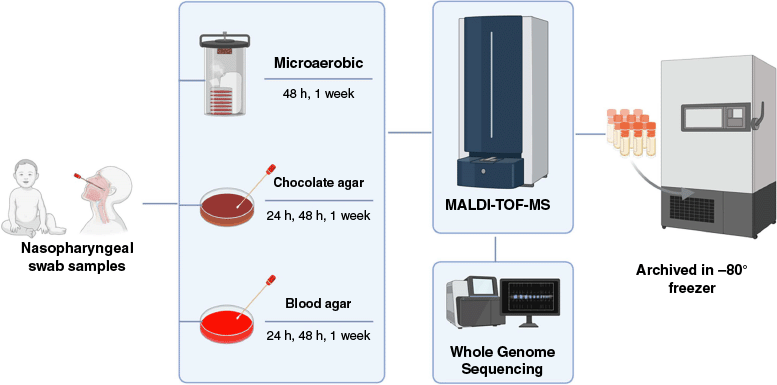
At The University of Western Australia (UWA), we established a biobank of commensal bacterial isolates cultured from the nasopharyngeal swab samples from the DCHS cohort. The biobank was established to support further discovery work on bacterial interactions, understand strain level diversity and assist in the development of live biotherapy against respiratory pathogens. The DCHS nasopharyngeal swab samples stored in STGG (skim milk, tryptone, glucose and glycerol) were cultured on non-selective nutritive media, Columbia blood agar supplemented with 5% sheep blood and chocolate agar under aerobic and microaerobic conditions to capture a broad range of nasopharyngeal commensals. The agar plates were incubated at 37°C for 24, 48 h and 1 week. Distinct colony morphotypes were isolated, purified, barcoded and archived in glycerol stocks at −80°C for long-term preservation. The isolates are identified by matrix-assisted laser desorption ionisation–time of flight–mass spectrometry (MALDI-TOF-MS) using the Bruker Biotyper. The MALDI-TOF-MS identification was further confirmed by whole genome sequencing (Fig. 1).
Flowchart illustrating the stepwise process for establishing a biobank of bacterial isolates recovered from nasopharyngeal swabs in STGG. MALDI-TOF-MS, matrix assisted laser desorption ionisation–time of flight–mass spectrometry.
The collection of isolates in our biobank currently include nasopharyngeal strains of a broad range of Streptococcus, Corynebacterium, Staphylococcus, Moraxella species, Candida, Rothia, Dolosigranulum, Eikenella, Enterococcus, Gemella, Bordetella, Kocuria, Dermacoccus, Agromyces, Peribacillus and Stenotrophomonas species. Each isolate in the biobank is linked to detailed metadata of the participant, including participant age, health status, antibiotic exposure, as well as microbiome data such as co-colonising taxa, enabling context-specific functional studies. We envisage this biobank as a valuable resource not only for ongoing research within our laboratory but also as a platform for collaborative investigations into the nasopharyngeal microbiome.
Live biotherapies for respiratory health
As antimicrobial resistance continues to rise, live biotherapeutics may offer targeted, sustainable modulation of the respiratory microbiome, potentially restoring microbial balance and enhancing mucosal immunity without disrupting the existing flora, particularly in early life when microbial colonisation shapes long-term health trajectories. Success in developing microbiome-based live biotherapies or probiotics has been achieved with a novel vaginal synbiotic called VS-01. VS-01 comprises three Lactobacillus crispatus strains that work to restore an optimal vaginal microbiome, preventing vaginal dysbiosis, alleviating discomfort and reducing the need for antibiotic treatment.27 Research programs such as ours are focused on translating microbiome science into live biotherapies for respiratory infections in children.
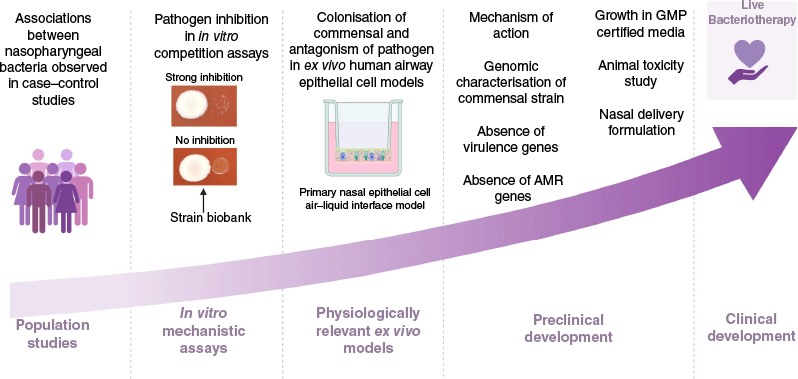
Central to our approach is the development of targeted live biotherapy based on beneficial nasopharyngeal bacteria. We leverage the curated collection of clinical nasopharyngeal bacterial isolates from DCHS cohort described above, enabling meaningful interpretation of host-microbe and microbe–microbe interactions (Fig. 2).
Illustration of the process of identifying interactions between nasopharyngeal isolates from the Drakenstein Child Health Study (DCHS) and validating the interactions through in vitro assays.
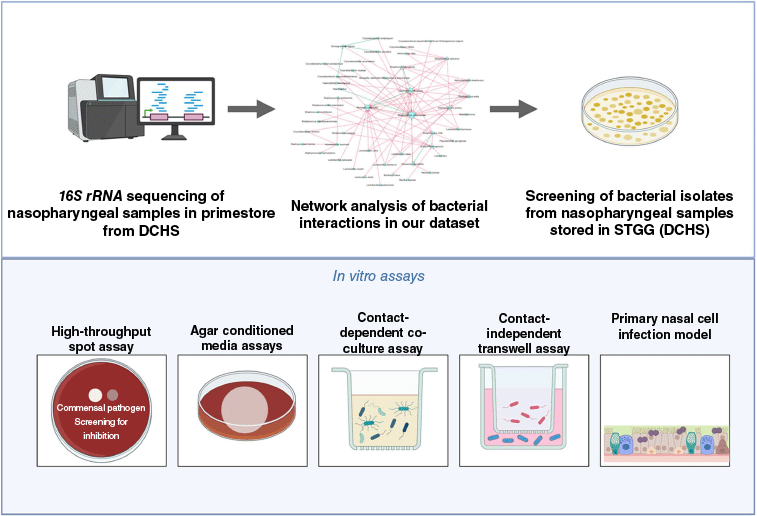
Using network analysis tools such as NetCoMi (Network Construction and Comparison for Microbiome Data, see https://netcomi.de), we identify key bacterial interactions and health-associated microbes from longitudinal microbiome data. These in silico associations are then experimentally validated using isolates from the biobank. Through a suite of physiologically relevant screening assays (Fig. 2), including co-culture inhibition assays and airway cell culture models, we evaluate the capacity of candidate strains to inhibit common respiratory pathogens such as S. pneumoniae. Candidate commensal strains that demonstrate robust inhibitory potential are then prioritised for mechanistic and immunomodulatory studies (Fig. 3). Promising candidate strains exhibiting robust inhibitory or immunomodulatory effects undergo genomic screening to confirm the absence of known virulence factors, mobile genetic elements and antimicrobial resistance genes, ensuring safety for potential clinical use. Strains meeting these criteria then enter the translational phase. This translational phase includes (i) adaptation for growth in plant-based or chemically defined (non-animal product) based media or Good Manufacturing Practice (GMP)-certified media, (ii) preclinical safety evaluation through animal toxicity studies, and (iii) development of nasal spray formulations tailored for effective upper respiratory tract delivery. These steps are critical to ensure both the safety and feasibility of clinical application. By integrating microbiome data with functional and translational validation, our goal is to deepen our understanding of the bacterial interactions within the nasopharynx that underpin respiratory health and disease. By identifying and validating protective strains, we aim to develop novel, evidence-based targeted live biotherapies that reduce colonisation by respiratory pathogens, modulate immune response or enhance barrier integrity and ultimately lower the burden of lower respiratory tract infections in children.
Data availability
Data that support this study can be made available upon request to the corresponding author.
Declaration of funding
The Drakenstein Child Health Study is funded by Bill and Melinda Gates Foundation. This project was funded by the Western Australian State Government and Channel 7 Telethon Trust through the WA Child Research Fund and separately through the Channel 7 Telethon Trust and Wesfarmers Centre for Vaccine and Infectious Diseases. Mark P. Nicol was supported by the Stan Perron Charitable Foundation, an Australian National Health and Medical Research Council Investigator Grant (APP1174455) and Western Australian Future Health Research and Innovation Fund, which is an initiative of the WA State Government. Ritika K. Bahal is supported by the Western Australian Early-Career Child Health Researcher Fellowships Program, which is a co-funded partnership programme of the Future Health Research and Innovation Fund, the Stan Perron Charitable Foundation and the BrightSpark Foundation. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Acknowledgements
We are grateful to all the investigators involved in this work, especially Robbie Haines, Vanessa Tenaglia and Erwin Paz for their contributions to the biobank.
References
1 Bender RG et al. (2024) Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect Dis 24, 974-1002.
| Crossref | Google Scholar |
2 O’Brien KL et al. (2019) Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case–control study. Lancet 394, 757-779.
| Crossref | Google Scholar |
3 Zar HJ, Bush A (2023) Early childhood lower respiratory tract infection: a key determinant of premature adult respiratory mortality. Lancet 401, 1135-1137.
| Crossref | Google Scholar | PubMed |
4 Mejbah Uddin B et al. (2018) Role of viral and bacterial pathogens in causing pneumonia among Western Australian children: a case–control study protocol. BMJ Open 8, e020646.
| Crossref | Google Scholar | PubMed |
5 Bradley JS et al. (2011) Executive summary: The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 53, 617-630.
| Crossref | Google Scholar | PubMed |
6 Royal Melbourne Hospital and the National Centre for Antimicrobial Stewardship (2024) Antimicrobial prescribing practice in Australian hospitals. Results of the 2021 Hospital National Antimicrobial Prescribing Survey. Australian Government Department of Health and Aged Care, Canberra, ACT, Australia. https://www.amr.gov.au/sites/default/files/2024-01/antimicrobial-prescribing-practice-in-australian-hospitals-results-of-the-2021-hospital-national-antimicrobial-prescribing-survey.pdf
7 Man WH et al. (2019) Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: a matched case–control study. Lancet Respir Med 7, 417-426.
| Crossref | Google Scholar | PubMed |
8 Lu Y et al. (2023) Early-life antibiotic exposure and childhood asthma trajectories: a national population-based birth cohort. Antibiotics 12, 314.
| Crossref | Google Scholar | PubMed |
9 Murray CJL et al. (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399, 629-655.
| Crossref | Google Scholar |
10 Claassen-Weitz S et al. (2023) Succession and determinants of the early life nasopharyngeal microbiota in a South African birth cohort. Microbiome 11, 127.
| Crossref | Google Scholar | PubMed |
11 Flynn M, Dooley J (2021) The microbiome of the nasopharynx. J Med Microbiol 70, 001368.
| Crossref | Google Scholar | PubMed |
12 Teo SM et al. (2015) The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17, 704-715.
| Crossref | Google Scholar |
13 Reyman M et al. (2021) Microbial community networks across body sites are associated with susceptibility to respiratory infections in infants. Commun Biol 4, 1233.
| Crossref | Google Scholar | PubMed |
14 Horn KJ et al. (2021) Corynebacterium species inhibit Streptococcus pneumoniae colonization and infection of the mouse airway. Front Microbiol 12, 804935.
| Crossref | Google Scholar | PubMed |
15 Claassen-Weitz S et al. (2021) The association between bacteria colonizing the upper respiratory tract and lower respiratory tract infection in young children: a systematic review and meta-analysis. Clin Microbiol Infect 27, 1262-1270.
| Crossref | Google Scholar | PubMed |
16 Brugger SD et al. (2020) Dolosigranulum pigrum cooperation and competition in human nasal microbiota. mSphere 5, e00852-20.
| Crossref | Google Scholar | PubMed |
17 Bosch A et al. (2017) Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. A prospective cohort study. Am J Respir Crit Care Med 196, 1582-1590.
| Crossref | Google Scholar | PubMed |
18 de Steenhuijsen Piters WAA et al. (2019) Interaction between the nasal microbiota and S. pneumoniae in the context of live–attenuated influenza vaccine. Nat Commun 10, 2981.
| Crossref | Google Scholar | PubMed |
19 Zar HJ et al. (2015) Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax 70, 592-594.
| Crossref | Google Scholar | PubMed |
20 Claassen-Weitz S et al. (2025) Nasopharyngeal microbiota in South African infants with lower respiratory tract infection: a nested case–control study of the Drakenstein Child Health Study. Clin Infect Dis ciaf184 [Published online early 17 April 2025].
| Crossref | Google Scholar | PubMed |
21 Yu K et al. (2025) Interactions between bacteria in the human nasopharynx: ascoping review. Lancet Microbe 101062.
| Crossref | Google Scholar | PubMed |
22 Xu L et al. (2021) Nasopharyngeal microbiome composition associated with Streptococcus pneumoniae colonization suggests a protective role of Corynebacterium in young children. PLoS ONE 16, e0257207.
| Crossref | Google Scholar | PubMed |
23 Biesbroek G et al. (2014) The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am J Respir Crit Care Med 190, 298-308.
| Crossref | Google Scholar | PubMed |
24 de Steenhuijsen Piters WA et al. (2016) Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med 194, 1104-1115.
| Crossref | Google Scholar | PubMed |
25 Kelly MS et al. (2022) Non-diphtheriae Corynebacterium species are associated with decreased risk of pneumococcal colonization during infancy. ISME J 16, 655-665.
| Crossref | Google Scholar | PubMed |
26 Hardy BL, Merrell DS (2021) Friend or foe: interbacterial competition in the nasal cavity. J Bacteriol 203, e00480-20.
| Crossref | Google Scholar | PubMed |
27 Ravel J et al. (2024) A novel multi-strain vaginal synbiotic is effective in optimizing the vaginal microbiome: results from a randomized, placebo-controlled clinical trial. American Journal of Obstetrics & Gynecology 231(6), S1303.
| Crossref | Google Scholar |
 Tamlyn Fairall is a research scientist at the Marshall Centre for Infectious Disease, The University of Western Australia (UWA). Tamlyn has research interests in infectious disease and microbiology and how this can contribute to advancements in public health. |
 Yi Bin Koh is a PhD student at The University of Western Australia. Her research focuses on interbacterial relationships within the nasopharynx, aiming to address childhood pneumonia. |
 Anastasia Basuki is an Honours student at The University of Western Australia. Currently, Anastasia is researching the mechanisms of interactions between nasopharyngeal bacteria, aiming to contribute to future research in this area. |
 Mark Nicol is professor of microbiology at The University of Western Australia. He studied medicine and medical microbiology at the University of the Witwatersrand and completed his PhD in childhood tuberculosis in Cape Town. His research explores the role of the early life microbiome in acute and chronic respiratory illnesses in children. He also has an active research programme involved in the development and evaluation of better diagnostic tests for tuberculosis and pneumonia. He is Chair of the WHO Advisory Group on Tuberculosis Diagnostics and Laboratory Strengthening. |
 Dr Ritika Kar Bahal is a BrightSpark Early Career Child Health Research fellow at The University of Western Australia. She completed her PhD in tuberculosis research from University of Delhi, India, and relocated to Perth in 2018. Her current research explores the role of the early life microbiome and bacterial–bacterial interactions in respiratory illnesses in children. Dr Bahal has received several prestigious scholarships and awards, such as the Nature Microbiome Accelerator award 2024 and the FEMS ECR talk award at ASM National Meeting 2024. She works closely with community members in co-designing translational goals for her research. |