Complement in chronic wound infections: complementary or a cascade of chaos?
Michelle N. Chamoun A * and Gabriela Gonzalez Matheus A BA
B

Dr Michelle Chamoun is a postdoctoral research fellow at the Frazer Institute, The University of Queensland. Her current research, in the laboratory of Dr Timothy Wells, focuses on antibody-mediated serum resistance of Gram-negative bacterial infections. Her expertise is in Immune dysregulation in Gram-negative bacterial infections and has a special interest in chronic skin and wound infections. |

Dr Gabriela Gonzalez Matheus is a medical doctor working in the dermatology field with a specific interest in chronic wounds and skin healing. She recently completed a Master of Philosophy on immune-mediated chronicity of wounds colonised by Pseudomonas aeruginosa. |
Abstract
Chronic wounds are non-healing ulcers and are frequently accompanied with bacterial infection. These wounds bypass the normal healing process due to impaired cellular responses and excessive inflammation, likely triggered by bacterial infection and biofilm formation. Complement dysregulation, particularly the overproduction of anaphylatoxin C5a, is a pathogenic variable in many inflammatory diseases and other infectious diseases. There is some evidence that overproduction of C5a may contribute to poor healing outcomes; however, more research is required to clarify its role and determine how this influences host–pathogen interactions within the wound environment. Studying the complement system in a research environment is challenging due to factors such as sample composition, timing of collection, temperature and freeze–thaw cycles, all potentially affecting data reliability. Furthermore, various methods are available to study complement activity, including assays for bacterial opsonisation, serum bactericidal killing, and detection or quantification of individual complement components. This article explores the important role of complement dysregulation in chronic wound healing and discusses important considerations for advancing research in this area, including optimisation of laboratory processes to ensure accurate data.
Keywords: biofilm, chronic wounds, complement, host–pathogen interactions, inflammation, Pseudomonas aeruginosa, sample processing, Staphylococcus aureus.
Introduction
Chronic wounds are defined as wounds that fail to heal after 3 months of treatment. In Australia, each year it is estimated that 450,000 people suffer from chronic wounds, costing the health care system well over A$6.6 billion.1 These wounds also have a profound negative effect on the patient’s quality of life and psychosocial wellbeing. Underlying conditions such as venous insufficiency, atherosclerosis, and type-2 diabetes influence the development of non-healing wounds.2 These wounds are often colonised by numerous bacterial species, which complicate healing, delay wound closure and negatively affect health outcomes.3 The precise relationship between the wound microbiome, biofilm production and dysregulated immune response in chronic wounds remains poorly understood.4
Normal v. chronic wound healing
Wound healing is a complex physiological process that occurs over four main phases including haemostasis, inflammation, proliferation and remodelling.5 Acute wounds typically progress through these phases in an orderly and timely manner, as illustrated in Fig. 1a.6,7 The early inflammatory responses are important to drive initial wound healing, remove foreign debris and prevent infection. By contrast, chronic wound healing often stalls somewhere in the late inflammatory phase before wound closure can occur. This leads to hypergranulation at the wound edges, decreased fibroblast function, excessive and persistent inflammation, and ultimately immune dysfunction.5,7–9 These pathological changes contribute to fibrosis and the development of non-healing ulcers, impairing proliferation and preventing tissue repair (Fig. 1b).
Acute v. chronic wound healing with host–pathogen interactions. (a) Typical progression of acute wound healing is depicted through the four main phases. (b) By contrast, chronic wound healing stalls in the late inflammatory stage. (c) A comparison of the immune responses and microbial clearance mechanisms in acute (left) and chronic (right) wounds. Acute wounds exhibit adequate inflammation and cell infiltration facilitating pathogen clearance utilising neutrophil extracellular traps (NETs) and phagocytosis. Additionally, commensal skin bacteria such as Staphylococcus may inhabit the wound. Chronic wounds, however, are often infected with biofilm-forming Gram-negative bacteria such as P. aeruginosa, which penetrate deeper tissue layers. These wounds are characterised by excessive inflammation, immune dysregulation, and increased antimicrobial resistance. Created in BioRender (see https://BioRender.com/0wu586v).
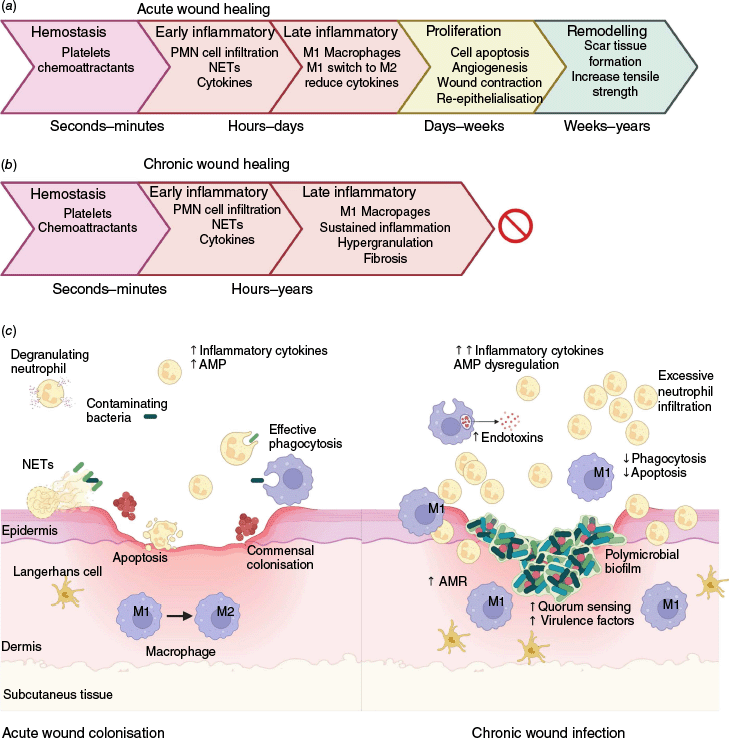
Host–pathogen interactions and immune dysregulation in chronic wounds
Bacteria in wounds can significantly affect both local and systemic immune responses; however, the relationship between bacterial burden and the extent of these immune alterations are unclear.4 Following skin injury, the innate immune system is activated in acute wounds, leading to the recruitment of neutrophils and macrophages, and the induction of pro-inflammatory cytokines including Intereukin-6 (IL-6), Interleukin 1β (IL-1β), and tumour necrosis factor (TNF).7 Antimicrobial peptides (AMPs) are also produced and include α- and β-defensins, cathelicidins and histatins8,10 (Fig. 1c left). Acute wound colonisation by Staphylococcus aureus is common and increases immune cell recruitment compared to aseptic wounds.11
By contrast, in chronic wounds there is often increased bacterial abundance and diversity, particularly of Gram-negative species.4 Pseudomonas aeruginosa is a common wound pathogen that can impair wound healing by dysregulation of immune responses, such as suppression of vascular endothelial growth factor (VEGF) and S100A8/A9 AMP production, with increased neutrophil infiltration.3,4 In the chronic wound stage, some AMPs such as human β-defensins hBD2 and hBD3 can be further upregulated.4,8 In fact, Histadin-1-3 and P113 have progressed to clinical trials that target chronic P. aeruginosa wound infections.10
Bacterial biofilms refer to polymicrobial aggregates that are embedded within a protective matrix of extracellular polymeric substances and are present in up to 90% of chronic wounds.3 Biofilms produced by Gram-negative bacteria can release significant levels of endotoxins into the wound environment, promoting antimicrobial resistance, impairing tissue repair, and perpetuating inflammation4,8 (Fig. 1c right). Quorum sensing within biofilms facilitates bacterial communication and upregulation of virulence factors, enhancing the bacteria’s ability to trigger an inflammatory response and delay wound healing.3 Persistent inflammation may further contribute to the dysfunctional cellular and pro-inflammatory cytokine responses characteristic of chronic wounds. These include elevated numbers of inflammatory cells (including neutrophils, macrophages and Langerhans cells), excessive production of Interleukin 1α and TNF, as well as defects in phagocytosis and apoptosis, and impaired phenotypic switching of pro-inflammatory macrophages (M1) to an anti-inflammatory phenotype (M2)5,8,9 (Fig. 1c right). Biofilm mechanisms aside, polymicrobial infections with pathogenic organisms are important for chronic wound formation.4 The use of probiotics in wounds can potentially assist in the reduction of microbial load by species antagonism and immunomodulatory mechanisms; however, the heterogeneity of results thus far limits this as a viable treatment option.12
Complement in wound healing and bacterial interactions
The complement cascade is a key component of the innate immune system, consisting of a proteolytic cascade involving up to 50 molecules that defend against invading pathogens. This system is activated by three pathways that converge at the cleavage of complement component C3, cleaving C3 into C3a and C3b. C3b subsequently initiates cleavage of C5 into C5a and C5b. C5b in combination with several other complement proteins forms the membrane attack complex (MAC), which creates pores in the bacterial membranes, leading to pathogen lysis.13 C3a and C5a are important anaphylatoxins and drive inflammatory responses for vasodilation and inflammatory cell infiltration, with C5a being the most potent.14,15 Complement is produced systemically and locally in the tissues, where it contributes to infection control and wound healing.14 However, as with all inflammatory processes, balance is essential. Whereas complement helps control infection, dysregulation can perpetuate tissue damage.
C5a is implicated in the pathogenesis of many inflammatory diseases and some infectious diseases.15 In a study of pneumonia patients, elevated serum C5a was associated with increased inflammatory IL-6 and C reactive protein compared to healthy controls. Additionally, Pneumococcus-infected mice treated with anti-C5a antibodies experienced less severe disease.16 Similarly, C5a receptor knock-out (KO) mice infected with Escherichia coli in a pyelonephritis model had a lower bacterial load and reduced renal lesions when compared to wild-type (WT) mice.17 It appears that targeting C5a or its receptor may lead to reduced tissue damage. Elevated levels of C5a were detected in wound fluid of a diabetic murine model compared to WT controls, therefore modulation of C5a may also protect against excessive wound damage.18
Mice lacking C3 or C5 showed reduced inflammation, neutrophil infiltration and increased rate of healing 48 h post-wounding. Only the C3 KO group had increased mast cells, which they hypothesised led to increased vascularity and accelerated healing.19 Conversely, topical application of C3 and C5 in a collagen carrier in rats was associated with improved wound healing and strength compared to collagen carrier alone.14,20 These contrasting findings may reflect the differences between local and systemic complement activity. For instance, in a porcine burn wound model, prolonged elevation of serum C3 levels were observed up to 60days post-injury, likely leading to complement dysregulation, while local C3 levels in the wound remained unchanged.21 Importantly, complement activity may differ between animal species so direct comparison should be avoided. Few studies have investigated complement in human chronic wounds, with one reporting elevated serum C3 in patients with chronic leg ulcers.22
Knowledge of bacterial–complement interactions in wounds is also lacking. Cystic fibrosis lung P. aeruginosa isolates were reported to produce proteases (AprA and LasB) that degrade C5a, helping modulate host immune responses. These proteases were more active in acute isolates, suggesting that reduced C5a-cleavage by chronic isolates may contribute to complement dysregulation in persistent infections, including in P. aeruginosa-infected chronic wounds.23 Elastase-producing P. aeruginosa strains were shown to partially degrade C3 and components of wound fluid when introduced into plasma.24 In another study, enzymatic treatment targeting P. aeruginosa exopolysaccharides (Pel and Psl), critical for biofilm formation, increased C3 deposition in pooled serum, and attenuated wound infection in a murine model.25 These studies demonstrate that P. aeruginosa can target C3, likely interfering with complement effector functions such as MAC, to inhibit bacterial clearance and wound healing. Yet overall, the role of complement dysregulation in chronic wound infections, particularly in relation bacterial interactions with C3, C5 and their split products, remains poorly understood and should be a key focus of future research.
Future perspectives on pathogen–complement interaction research
Studying complement–bacterial interactions presents significant challenges due to the complexity and instability of the complement system. Complement proteins are heat labile and inherently unstable, making the appropriate selection and handling of specimens crucially important. Serum is generally preferred for functional complement analysis, whereas EDTA plasma is more suitable for stabilisation of complement split products, due to the chelation of calcium and magnesium to inhibit further complement activity.26 Citrate and heparin plasma are considered less suitable due to inadequate chelating ability, and negative complement component interactions respectively26,27 (Fig. 2). Despite these general recommendations, sample-specific validation is important. For example, one study found similar C5a levels across serum, EDTA plasma and citrate plasma, but noted increased C4d and C3a in serum. If study constraints limit access to multiple sample types, properly handled serum may serve as a versatile alternative for broader complement studies.
Recommended workflow for collection, storage and analysis of specimens for wound complement research. This schematic outlines optimal steps for studying complement activity in wounds with considerations of specimen type, timing of collection and storage, and analysis methods for functional studies or detection of individual components. Created in BioRender (see https://BioRender.com/7mmt8yc).
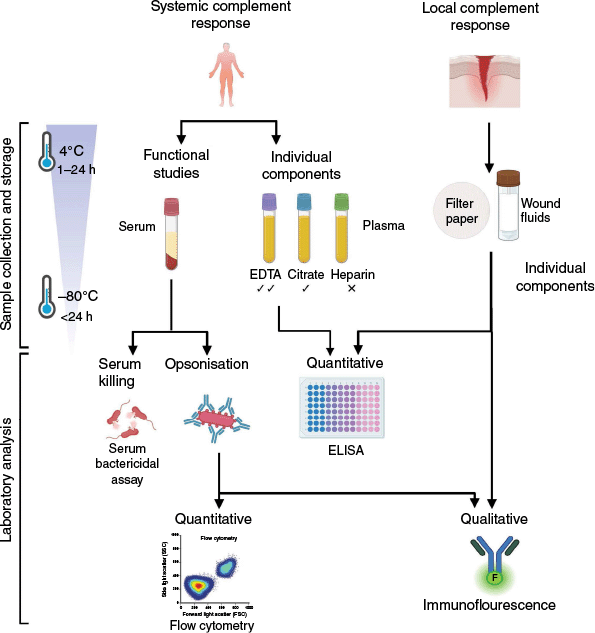
Optimal preservation of complement activity requires rapid freezing at −80°C with minimal freeze–thaws cycles, ideally on ice,27 yet this may be unpractical in a clinical or research setting. Complement biomarkers in citrate and EDTA plasma can stay stable within 24 and 48 h respectively when stored promptly at 4°C.28 Prolonged storage beyond these timeframes, however, has been associated with increased levels of C4d and C3a. Less is known about the optimal handling of complement-containing wound fluids. Nevertheless, C5a has been successfully detected from filter paper secured in place within the wound for various timepoints.18 In theory, fluid collected during wound irrigation or debridement with saline could be used for complement analysis if processed in the presence of protease inhibitors to stabilise complement components.
Irrespective of sample type, enzyme-linked immunosorbent assays (ELISA) remains the most reliable method for quantifying individual complement proteins and cleaved products.26,27 In the context of bacterial infection, complement deposition can be assessed qualitatively using immunofluorescent staining of tissue sections or qualitatively by flow cytometry with gating for bacterial cells.21,29 Additionally, serum bactericidal assays are a common method for assessing bacterial sensitivity to complement and are widely used in our laboratory.30 An optimised workflow to guide complement sample handling and testing is shown in Fig. 2. For a more detailed guide describing complement activation assessment methods, readers are referred to the following review.27
Conclusion
This article highlights how the persistent inflammation in chronic wounds predisposes tissues to ongoing infection and perpetuates a cycle of inflammation and impaired healing. Although the microbial contribution to chronic wound pathology remains a ‘chicken-and-egg’ scenario, current evidence suggests a positive feedback loop exists between bacterial persistence and immune dysregulation. The role of complement dysregulation in wounds deserves further investigation, particularly in relation to localised wound responses. To advance our understanding, future complement-focused research should prioritise rigorous sampling protocols, appropriate specimen processing and storage, and robust laboratory analyses.
Data availability
No original data were used in this publication. All data are previously published in cited sources.
References
1 Wood F (2024) 5 Point Plan to Solve Chronic Wounds. Wounds Australia. https://woundsaustralia.org/int/woundsaus/uploads/Publications/5%20point%20plan%202024/wounds%20australia%205%20point%20plan%202024.pdf (accessed 30 April 2025)
2 Frykberg RG, Banks J (2015) Challenges in the treatment of chronic wounds. Adv Wound Care 4, 560-582.
| Crossref | Google Scholar | PubMed |
3 Matheus GG et al. (2025) Understanding the pathophysiology of Pseudomonas aeruginosa colonization as a guide for future treatment for chronic leg ulcers. Burns Trauma 13, tkae083.
| Crossref | Google Scholar | PubMed |
4 Versey Z et al. (2021) Biofilm-innate immune interface: contribution to chronic wound formation. Front Immunol 12, 648554.
| Crossref | Google Scholar | PubMed |
5 Wilkinson HN, Hardman MJ (2020) Wound healing: cellular mechanisms and pathological outcomes. Open Biol 10, 200223.
| Crossref | Google Scholar | PubMed |
6 Velnar T et al. (2009) The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 37, 1528-1542.
| Crossref | Google Scholar | PubMed |
7 Rodríguez-Rodríguez N et al. (2022) Wound chronicity, impaired immunity and infection in diabetic patients. MEDICC Rev 24, 44-58.
| Crossref | Google Scholar | PubMed |
8 MacLeod AS, Mansbridge JN (2016) The innate immune system in acute and chronic wounds. Adv Wound Care 5, 65-78.
| Crossref | Google Scholar | PubMed |
9 Martin P, Nunan R (2015) Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 173, 370-378.
| Crossref | Google Scholar | PubMed |
10 Haidari H et al. (2023) Therapeutic potential of antimicrobial peptides for treatment of wound infection. Am J Physiol Cell Physiol 324, C29-C38.
| Crossref | Google Scholar | PubMed |
11 Van Der Laan N et al. (2001) Immunohistopathological appearance of three different types of injury in human skin. Inflamm Res 50, 350-356.
| Crossref | Google Scholar | PubMed |
12 Knackstedt R et al. (2020) The role of topical probiotics on wound healing: a review of animal and human studies. Int Wound J 17, 1687-1694.
| Crossref | Google Scholar | PubMed |
13 Panelius J, Meri S (2015) Complement system in dermatological diseases – fire under the skin. Front Med 2, 3.
| Crossref | Google Scholar | PubMed |
14 Wang Z et al. (2022) Inflammatory microenvironment of skin wounds. Front Immunol 13, 789274.
| Crossref | Google Scholar | PubMed |
15 Ghosh M, Rana S (2023) The anaphylatoxin C5a: structure, function, signaling, physiology, disease, and therapeutics. Int Immunopharmacol 118, 110081.
| Crossref | Google Scholar | PubMed |
16 Müller-Redetzky H et al. (2020) Neutralizing complement C5a protects mice with pneumococcal pulmonary sepsis. Anesthesiology 132, 795-807.
| Crossref | Google Scholar | PubMed |
17 Li K et al. (2017) C5aR1 promotes acute pyelonephritis induced by uropathogenic E. coli. JCI Insight 2, e97626.
| Crossref | Google Scholar | PubMed |
18 Cunnion KM et al. (2017) Complement activation and STAT4 expression are associated with early inflammation in diabetic wounds. PLoS ONE 12, e0170500.
| Crossref | Google Scholar | PubMed |
19 Rafail S et al. (2015) Complement deficiency promotes cutaneous wound healing in mice. J Immunol 194, 1285-1291.
| Crossref | Google Scholar | PubMed |
20 Sinno H et al. (2013) Complements C3 and C5 individually and in combination increase early wound strength in a rat model of experimental wound healing. Plast Surg Int 2013, 243853.
| Crossref | Google Scholar | PubMed |
21 Korkmaz HI et al. (2017) The local and systemic inflammatory response in a pig burn wound model with a pivotal role for complement. J Burn Care Res 38, e796-e806.
| Crossref | Google Scholar | PubMed |
22 Cazander G et al. (2012) Complement activation and inhibition in wound healing. Clin Dev Immunol 2012, 534291.
| Crossref | Google Scholar | PubMed |
23 Mateu-Borrás M et al. (2022) Pseudomonas aeruginosa adaptation in cystic fibrosis patients increases C5a levels and promotes neutrophil recruitment. Virulence 13, 215-224.
| Crossref | Google Scholar | PubMed |
24 Schmidtchen A et al. (2003) Elastase-producing Pseudomonas aeruginosa degrade plasma proteins and extracellular products of human skin and fibroblasts, and inhibit fibroblast growth. Microb Pathog 34, 47-55.
| Crossref | Google Scholar | PubMed |
25 Pestrak MJ et al. (2019) Treatment with the Pseudomonas aeruginosa glycoside hydrolase PslG combats wound infection by improving antibiotic efficacy and host innate immune activity. Antimicrob Agents Chemother 63, e00234-19.
| Crossref | Google Scholar | PubMed |
26 Willrich M et al. (2021) Complement testing in the clinical laboratory. Crit Rev Clin Lab Sci 58, 447-478.
| Crossref | Google Scholar | PubMed |
27 Brandwijk R et al. (2022) Pitfalls in complement analysis: a systematic literature review of assessing complement activation. Front Immunol 13, 1007102.
| Crossref | Google Scholar | PubMed |
28 Yang S et al. (2015) Effect of blood sampling, processing, and storage on the measurement of complement activation biomarkers. Am J Clin Pathol 143, 558-565.
| Crossref | Google Scholar | PubMed |
29 Wonfor T et al. (2022) Novel method for detecting complement C3 deposition on Staphylococcus aureus. Sci Rep 12, 15766.
| Crossref | Google Scholar | PubMed |
30 Hickson SM et al. (2024) Antibody-mediated serum resistance protects Pseudomonas aeruginosa during bloodstream infections. J Infect Dis 230, e221-e229.
| Crossref | Google Scholar | PubMed |
 Dr Michelle Chamoun is a postdoctoral research fellow at the Frazer Institute, The University of Queensland. Her current research, in the laboratory of Dr Timothy Wells, focuses on antibody-mediated serum resistance of Gram-negative bacterial infections. Her expertise is in Immune dysregulation in Gram-negative bacterial infections and has a special interest in chronic skin and wound infections. |
 Dr Gabriela Gonzalez Matheus is a medical doctor working in the dermatology field with a specific interest in chronic wounds and skin healing. She recently completed a Master of Philosophy on immune-mediated chronicity of wounds colonised by Pseudomonas aeruginosa. |


