Bacteriophages as biocontrol agents of bacterial contaminants of bivalves and their microalgal feeds
Tuan Son Le A B and İpek Kurtböke A *A
B

Tuan Son Le is currently employed at Tréidlia Biovet, Sydney, and at the Research Institute for Marine Fisheries (Vietnam). He obtained his Bachelor of Environmental Science degree in 2009 from the Vietnam National University in Hanoi, and Master of Fisheries Sciences degree in 2012 at the Pukyong National University in the Republic of Korea. He received a PhD in microbiology in 2019 from the University of the Sunshine Coast, Qld, where he investigated the use of bacteriophages to control bacterial diseases in aquaculture. |

Assoc. Prof. İpek Kurtböke has been working in the field of biodiscovery and has been an active member of the international actinomycete research community since 1982. She currently conducts research and teaches in the field of applied microbiology and biotechnology at the University of the Sunshine Coast, Qld. She has also been an active member of the World Federation of Culture Collections (WFCC) and currently is the President of the Federation. She was also the Editorial Board member of Microbiology Australia for 21 years (2004–24). |
Abstract
A FAO 2024 report1 noted that aquaculture production worldwide has excelled, and aquaculture produced surpassed captured fish resulting in an increase of 4.4% in 2022 compared to 2020. Production comprised 185.4 × 106 tonnes of aquatic animals and 37.8 × 106 tonnes of algae, which is also used as feed in the industry. These figures indicate that aquaculture production will continue to expand and will be one of the fastest growing sectors for food-production into coming decades. Bivalve molluscs will likely remain a major component of this production. However, the microbial infestation of bivalves and their microalgal feeds and subsequent disease occurrences are of significant concern. This article highlights the use of bacteriophages to eliminate pathogenic bacterial species in environments where bivalves are cultivated so they can be protected from bacterial diseases.
Keywords: antibiotic resistance, aquaculture diseases, bacteriophage, biocontrol, bivalves, sustainable aquaculture, Vibrio infections of molluscs.
Bivalves and associated diseases
Compared to many other cultured sea products bivalves feed primarily on natural phytoplankton and require minimum husbandry as a result they are perceived to be well-suited for aquaculture.1–4 A recent case study5 related to regenerative and restorative aquaculture noted that seaweed and bivalve mollusc production in aquaculture are major sectors globally, each product contributing more than 97 and 89% respectively of the total production from marine aquaculture and marine wild harvest combined.5 In 2020, global cultivation of algae dominated by marine macroalgae, known as seaweed, grew by 0.5 × 106 tonnes within 1 year.3 Simultaneously, total global aquaculture production of molluscs, mostly bivalves, was 17.7 × 106 tonnes equalling A$46.7 billion in value.3 Co-culture-farming of these species side by side or co-located nearby at the same site has also gained importance.4–6 Bivalve hatcheries, however, have been constantly threatened by bacterial pathogens that caused high economic losses to the industry.7,8 The agents that cause diseases in marine bivalves include viruses, bacteria, fungi, protozoans, helminths and parasitic crustaceans.8,9 Vibrio species, however, are one of the major bacterial groups identified as agents that caused the outbreaks8–15 (Table 1). Particularly in hatcheries, Vibrio infections have occurred during almost all phases of the bivalve life cycles.15 For example, Vibrio aestuarianus caused 30% mortality among Pacific oyster (Crassostrea gigas) mortality on an oyster farm in France14 as well as V. splendidus, V. brasiliensis, V. harveyi and V. alginolyticus causing nearly 37% mortality rate in adult Pacific oysters (C. gigas) in experimental challenge trials in China.12 Vibrio strains were linked to mortality in oyster hatcheries in Tasmania (Australia) as well.16 Moreover, these pathogenic bacteria have become resistant to antibiotics that are used to control microbial diseases of bivalves in aquaculture. Antibiotic resistant Vibrio species frequently are isolated from oysters. Examples include research of Kang et al.,17 where 100% V. alginolyticus isolated (n = 15) from shellfish farming areas in South Korea showed resistance to ampicillin, vancomycin and erythromycin. In their review, Albini et al.18 highlighted the existence of antibiotic-resistant bacteria in marine oysters referring to data collected from studies all over the world (Asia, n = 54; Europe, n = 27; America, n = 16; Africa, n = 2; Oceania, n = 1; and intercontinental and multicentre, n = 3).
| Host | Disease/bacterial species | Life stage | Location of mortality | Oyster morality | References | |
|---|---|---|---|---|---|---|
| Chilean scallop (Argopecten purpuratus) | V. chagasii | Larvae | Inglesa Bay and Tongoy Bay (Chile) | Larval mortalities were up to 65.51 ± 4.40%. | 8 | |
| Pacific oysters (Crassostrea gigas) | Vibrio aestuarianus and V. harveyi | Adult | Oyster farms in Baynes Sound, British Columbia (BC), Canada | Mortalities were up to 50%. | 9 | |
| Pacific oysters (Crassostrea (Magallana) gigas) | Vibrio aestuarianus | Adult | Scottish oyster farm | Causing up to 100% mortality in 2 days at 25°C, 3 days at 22°C, and in 6 days at 15°C with an infection dose of 106 CFU | 10 | |
| Pacific oysters (C. gigas) | V. splendidus | Oyster spat | France | In the laboratory, up to 36% oyster mortality rate by intrapallial injection | 11 | |
| Pacific oysters (C. gigas) | V. splendidus, V. brasiliensis, V. harveyi and V. alginolyticus | Adults | Sanggou bay, Rongcheng, Shandong, China | In the laboratory, after 7-day exposure, mortality rate was up to 36.7% | 12 | |
| Pacific oysters (C. gigas) | V. crassostreae | Juvenile oyster | France | In the laboratory, after a 5-day injection, the cumulative mortality rate was up to 50% | 13 | |
| Pacific oysters (C. gigas) | V. aestuarianus | Adult | Oregon and Washington coasts (USA), France, Ireland and Scotland | Cumulative oyster mortality rate was ~30% in farm | 14 | |
| In the laboratory the mortality rate was up to 75% after a 15-day trial |
CFU, colony-forming units.
The major potential sources of Vibrio infections in oyster hatcheries are broodstock (parent) oysters, microalgae used as food for oyster larvae (which is usually cultured on-site), and the seawater used within the hatchery for larval rearing despite prior treatment by filtration or often bactericidal UV exposure.19 Lewis et al.20 indicated that high bacterial concentration (2.0 × 106 colony-forming units mL–1) in microalgae fed to oyster larvae could cause disease. Accordingly, considerable effort had to be directed toward reducing or eliminating bacterial contamination of microalgae cultures before they are used as a larval food source.
Bacteriophage treatments
Bacteriophages have been used as biocontrol agents to eliminate pathogenic bacteria in both medical and environmental settings. Their use has been shown to be an environmentally friendly solution to control pathogenic Vibrio species including aquaculture settings. Examples of such uses are listed in Table 2.
| Host | Pathogens | Phage name | Source of isolation | Administration style | Dosage | Results | References | |
|---|---|---|---|---|---|---|---|---|
| Shrimp larvae (Penaeus monodon) | V. harveyi | VHM1, VHM2 and VHS1 | Water and sediments in Palk Strait | Directly add phage suspension | MOI of 104 | The survival rates of treatment and control were 60–88.3 and 26.6–35% after 96 h of an experiment. | 21 | |
| Greenlip abalone (Haliotis laevigata) | V. harveyi | vB_VhaS-tm and vB_VhaS-a | Hatchery water and oyster tissues | Bath exposure | Bacteria of 106 CFU mL–1 and bacteriophage of 102 PFU mL–1 | The survival rates of treatment and control were 70 and 0% after 7 days of experiment. | 22 | |
| Live feeds (Artemia salina) | V. alginolyticus | ΦGrn1 and ΦSt2 | Water samples on the north coastline of Crete, Greece | Directly add phage suspension | MOI of 100 and Vibrio load (105 CFU mL–1) | Vibrio concentration decreased 93% after 4 h phage treatment | 23 | |
| Oyster (Ostrea plicatula) | V. parahaemolyticus | VPp1 | Wastewater | Phage suspension (depuration) | MOI of 0.1. | Vibrio concentration decreased 2.35–2.76 log CFU g–1 after 36 h | 24 |
MOI, multiplicity of infection; CFU, colony-forming units; PFU, plaque-forming unit.
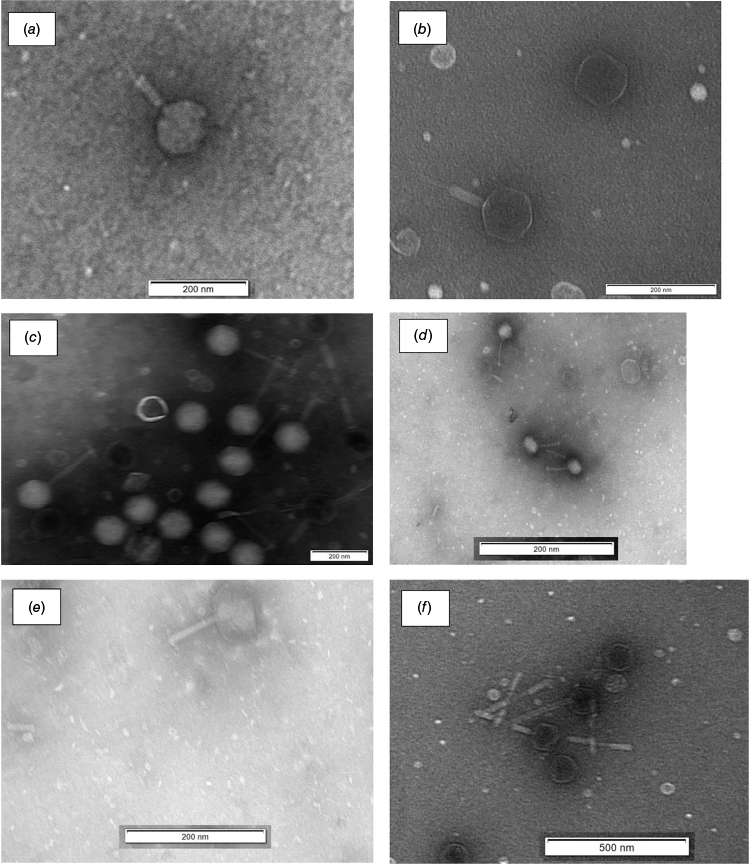
In a recent study conducted at the University of the Sunshine Coast (UniSC) bacteriophages (Fig. 1) successfully reduced cell numbers of the Vibrio species on oyster larvae as well as on the microalgae (Shellfish Diet 1800 and Pavlova 1800) used to feed these oysters,25–28 thus significantly reducing pathogenic bacterial numbers both on the product and its feed (Table 3). These phages were isolated using a local Vibrio species (USC-26005) but were also active against V. harveyi (ATCC 14126) and V. alginolyticus (ATCC 17749), as well as against another local Vibrio isolate (USC-26004) from Sunshine Coast, Qld, showing cross infectivity that provided superior power toward elimination of the targeted bacteria.
Transmission electron micrographs of the Vibrio bacteriophages isolated against Vibrio spp. (a) Φ-1, (b) Φ-2, (c) Φ-3, (d) Φ-4, (e) Φ-5, (f) Φ-6.26
| Species and disease | Pathogens | Phage name | Source of isolation | Administration style | Dosage | Results | References | |
|---|---|---|---|---|---|---|---|---|
| Microalgae (feed products Shellfish Diet 1800 and Pavlova 1800) | V. alginolyticus | Φ-1, Φ-2 and Φ-3 | Coastal areas in the Sunshine Coast, Qld, Australia | Directly add phage suspension | MOI of 100 | After 2 h of treatment, a 10-times reduction of Vibrio concentration was observed | 25,26 | |
| Oyster (Saccostrea glomerata) larvae | V. alginolyticus USC-26004 | Φ-5, Φ-6, and Φ-7 | Coastal areas in the Sunshine Coast, Qld, Australia | Directly add phage suspension | MOI of 1000 | The mortality rate of larvae in phage treatment was 28.2% whereas in the control treatments 77.9% after 24 h of incubation | 26,27 |
Conclusions
The increasing prevalence of antibiotic resistance of bacteria, and the problems of antibiotic residues ending up in pristine environments where aquaculture farming happen indicate the need for alternative approaches to control bacterial diseases of bivalves. Bacteriophage therapy may be a viable alternative for controlling bacterial pathogens associated with hatcheries or farms for both bivalves and their algal feeds.25–28 However, to date, only a few studies on actionable phage-based therapies have been reported in the literature that targeted phage application in laboratories, and even fewer have been successfully translated from laboratory settings to farm environments. The feasibility of the phage therapy approach on an industrial scale has yet to be established and is not widely approved for bacterial treatment due to unknowns related to the behaviour and safety of bacteriophages in natural environments.25–28
Bivalves are filter-feeding aquatic species, and thus considered a bacterial reservoir, and high levels of bacteria can reach production sites, resulting in contamination of the surrounding water.15 The UniSC study clearly indicates that phages are effective prevention agents against bacteria and might be easily applied at farm sites by routine applications.25–28
The effectiveness of the phage therapy approach to control pathogenic agents depends on different factors such as phage/bacteria ratio, environmental conditions (temperature, pH, UV, salinity), neutralisation of their antibacterial activity due to antibodies, dose and phage administration and bacterial resistance to phages. Thus, before initiating commercial level application, trials in hatcheries must be conducted to determine the safety of the phage approach. Risks of phage-mediated gene transfer and the development of phage-resistant bacteria also need to be investigated in ‘real-life’ situations.25–28
References
1 Food and Agriculture Organization of the United Nations (2024) In Brief to The State of World Fisheries and Aquaculture 2024. Blue transformation in action. FAO, Rome, Italy. 10.4060/cd0690en
2 Food and Agriculture Organization of the United Nations (2022) FAO report: global fisheries and aquaculture production reaches a new record high. https://www.fao.org/newsroom/detail/fao-report-global-fisheries-and-aquaculture-production-reaches-a-new-record-high/en
3 Wijsman J et al. (2019) Global production of marine bivalves. Trends and challenges. In Goods and Services of Marine Bivalves (Smaal A et al., eds). pp. 7–26. Springer International Publishing, Cham, Switzerland. 10.1007/978-3-319-96776-9_2
4 Duthie I (2012) Shellfish production aquaculture technology: global perspective of bivalve hatchery processes. A Report for Nuffield Australia farming scholars. Nuffield Australia Project Number 1017. Nuffield Australia, Moama, NSW, Australia. https://www.nuffieldscholar.org/sites/default/files/reports/2010_AU_Ian-Duthie_Shellfish-Production-Aquaculture-Technology-Global-Perspective-Of-Bivalve-Hatchery-Processes.pdf
5 The Nature Conservancy (2024) A case study for regenerative and restorative aquaculture: co-culturing seaweed with bivalve molluscs. https://www.aquaculturescience.org/content/dam/tnc/nature/en/documents/CoCulture_TNC_web1.pdf
8 Urtubia R et al. (2023) First report, characterization and pathogenicity of Vibrio chagasii isolated from diseased reared larvae of Chilean scallop, Argopecten purpuratus (Lamarck, 1819). Pathogens 12, 183.
| Crossref | Google Scholar | PubMed |
9 Cowan M et al. (2023) Role of the Vibrio community, reproductive effort, and environmental parameters in intertidal Pacific oyster summer mortality in British Columbia, Canada. Aquaculture 565, 739094.
| Crossref | Google Scholar |
10 Bean TP et al. (2024) Scottish oyster mortality event and association with Vibrio aestuarianus. Aquac Rep 39, 102480.
| Crossref | Google Scholar |
11 Gay M et al. (2004) Two Vibrio splendidus related strains collaborate to kill Crassostrea gigas: taxonomy and host alterations. Dis Aquat Organ 62, 65-74.
| Crossref | Google Scholar | PubMed |
12 Wang H et al. (2021) Screening of bacterial pathogens associated with mass summer mortality of the Pacific oyster, Crassostrea gigas, in China. Aquac Rep 20, 100672.
| Crossref | Google Scholar |
13 Bruto M et al. (2017) Vibrio crassostreae, a benign oyster colonizer turned into a pathogen after plasmid acquisition. ISME J 11, 1043-1052.
| Crossref | Google Scholar | PubMed |
14 Lupo C et al. (2019) Modeling the transmission of Vibrio aestuarianus in Pacific oysters using experimental infection data. Front Vet Sci 6, 142.
| Crossref | Google Scholar | PubMed |
15 Travers M-A et al. (2015) Bacterial diseases in marine bivalves. J Invertebr Pathol 131, 11-31.
| Crossref | Google Scholar | PubMed |
16 Lewis T et al. (2002) Pathogenic Vibrio parahaemolyticus in Australian oysters. FRDC Project 2002-409. Fisheries Research and Development Corporation. https://www.frdc.com.au/project/2002-409
17 Kang C-H et al. (2016) Antimicrobial susceptibility of Vibrio alginolyticus isolated from oyster in Korea. Environ Sci Pollut Res Int 23, 21106-21112.
| Crossref | Google Scholar | PubMed |
18 Albini E et al. (2022) A systematic review and meta-analysis on antimicrobial resistance in marine bivalves. Front Microbiol 13, 1040568.
| Crossref | Google Scholar | PubMed |
19 Helm MM et al. (2004) Hatchery culture of bivalves: a practical manual. FAO Fisheries Technical Paper 471. Food and Agriculture Organization of the United Nations, Rome, Italy. https://openknowledge.fao.org/handle/20.500.14283/y5720e
20 Lewis T et al. (1988) The use of 0.2-μm membrane-filtered seawater for improved control of bacterial levels in microalgal cultures fed to larval Pacific oysters (Crassostrea gigas). Aquaculture 69, 241-251.
| Crossref | Google Scholar |
21 Stalin N, Srinivasan P (2017) Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India. Vet Microbiol 207, 83-96.
| Crossref | Google Scholar | PubMed |
22 Wang Y et al. (2017) Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata). Aquaculture 473, 251-258.
| Crossref | Google Scholar |
23 Kalatzis PG et al. (2016) Isolation and characterization of two lytic bacteriophages, φSt2 and φGrn1; phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS ONE 11, e0151101.
| Crossref | Google Scholar | PubMed |
24 Rong R et al. (2014) Reductions of Vibrio parahaemolyticus in oysters after bacteriophage application during depuration. Aquaculture 418, 171-176.
| Crossref | Google Scholar |
25 Le TS et al. (2020) Use of bacteriophages to control Vibrio contamination of microalgae used as a food source for oyster larvae during hatchery culture. Curr Microbiol 77, 1811-1820.
| Crossref | Google Scholar | PubMed |
27 Le TS et al. (2020) Application of bacteriophages to control Vibrio alginolyticus contamination in oyster (Saccostrea glomerata) larvae. Antibiotics 9, 415.
| Crossref | Google Scholar | PubMed |
28 Le TS, Kurtböke Dİ (2019) Bacteriophages as biocontrol agents in aquaculture. Microbiol Aust 40, 37-41.
| Crossref | Google Scholar |
 Tuan Son Le is currently employed at Tréidlia Biovet, Sydney, and at the Research Institute for Marine Fisheries (Vietnam). He obtained his Bachelor of Environmental Science degree in 2009 from the Vietnam National University in Hanoi, and Master of Fisheries Sciences degree in 2012 at the Pukyong National University in the Republic of Korea. He received a PhD in microbiology in 2019 from the University of the Sunshine Coast, Qld, where he investigated the use of bacteriophages to control bacterial diseases in aquaculture. |
 Assoc. Prof. İpek Kurtböke has been working in the field of biodiscovery and has been an active member of the international actinomycete research community since 1982. She currently conducts research and teaches in the field of applied microbiology and biotechnology at the University of the Sunshine Coast, Qld. She has also been an active member of the World Federation of Culture Collections (WFCC) and currently is the President of the Federation. She was also the Editorial Board member of Microbiology Australia for 21 years (2004–24). |


