The discovery of mechano-bactericidal surfaces
Denver P. Linklater A and Elena P. Ivanova B C *A
B
C

Dr Denver Linklater is a research fellow at the Ian Holmes Imaging Centre, The University of Melbourne. She is an expert in electron microscopy. Her research interests are in the design and synthesis of nanomaterials for novel anti-microbial technologies, stem cell culture and tissue re-generation. |

Elena Ivanova is a distinguished professor of RMIT University. Her research interests are in design and fabrication of biomimetic antimicrobial micro- and nano-structured surfaces, materials biointerfaces and immobilisation of biomolecules and microorganisms in micro- and nano-environments. |
Abstract
There is a long evolutionary history of bacteria adapting to surface colonisation and biofilm formation and a similar timeline of evolutionary advances for insects to develop resistance towards bacterial colonisation and biofilm formation. Many nanostructured surfaces are superhydrophobic, meaning they repel water and therefore other contaminants. A decade ago, we discovered that the superhydrophobic nanopillared surfaces of insect wings (e.g. cicadas, dragonflies and damselflies) kill bacteria through physical disintegration rather than repelling their attachment. It is now well documented that the biomimetic nanostructures, such as pillars and spikes, can physically damage bacterial cells, leading to cell lysis and death. Research involving replication of these nanostructures on biomaterials and implantable devices could eliminate the need for antibiotics to kill bacteria on such surfaces, offering a promising alternative for preventing infections.
Keywords: antibiotics, bactericidal surfaces, biofilm prevention, biomimetic nano-structured surfaces, cicada and dragonflies wings, physical rupturing of bacterial cells, post-antibiotic era, synthetic analogues of mechano-bactericidal surfaces.
Introduction
Alexander Fleming, the father of modern antibiotics, predicted that microbes could develop resistance to antibiotics. In 1945, he was quoted saying ‘we are at the forefront of a new battle faced with dangerous “superbugs” that are impervious to our best antibiotics’. Seventy years on, this prediction has become reality and we face one of the greatest global health emergencies of the 21st Century. The emergence of ‘superbugs’ resistant to all available antibiotics is increasingly being reported. It is estimated that, out of 7.7 million deaths caused by bacterial infections each year, 1.27 million fatalities are likely due to antibiotic resistant bacteria.1 The economic cost of antibiotic resistance is expected to reach US$100 trillion by 2050 given current trends.2 With a greater chance of fatality due to infection, a major concern is that antimicrobial resistance could lead to a post-antibiotic era, a time where antibiotics no longer work.
Biofilms can form on a variety of surfaces, including medical devices, industrial equipment, and natural environments. They are known for their resilience, being highly resistant to antibiotics and disinfectants, making them a significant concern in healthcare and industry. This resistance is due to the protective extracellular polymeric substance (EPS) matrix and the altered state of the microorganisms within the biofilm. In medical settings, biofilms can form on medical devices like catheters, implants and prosthetics, leading to persistent and chronic infection.3 These infections are often hard to eradicate and can cause severe complications. In industrial environments, biofilms can form on equipment and pipelines, leading to corrosion, clogging and reduced efficiency.4 This can result in increased maintenance costs and downtime. Biofilms in natural and engineered water systems can affect water quality and contribute to the spread of pathogens. They can also affect the performance of water treatment processes. In the food industry, biofilms on processing equipment can harbour harmful bacteria, leading to contamination and foodborne illnesses.5 Overall, biofilm formation is a multifaceted problem that affects healthcare, industry and the environment, necessitating ongoing research and innovative solutions to manage and mitigate its impact.6
Surface topography is known to play a significant role in the bacterial cell-surface attachment process, particularly when the surface irregularities are comparable to the bacterial size and can provide shelter from unfavourable environmental factors (Fig. 1). The ‘Attachment Point’ theory, as proposed by Howell and Behrends7 and further explored by Scardino et al.,9 suggests that microorganisms smaller than the scale of the surface architecture will tend to attach in larger numbers within micrometre-scale shelters on textured surfaces.7–9 These microorganisms exhibit greater adhesion strength due to the multiple attachment points available on the surface. This theory highlights the importance of surface micro-texture in biofilm formation, indicating that even superhydrophobic surfaces might not be entirely effective in preventing biofilm formation if they provide micrometre-scale shelters for microorganisms (Fig. 2).
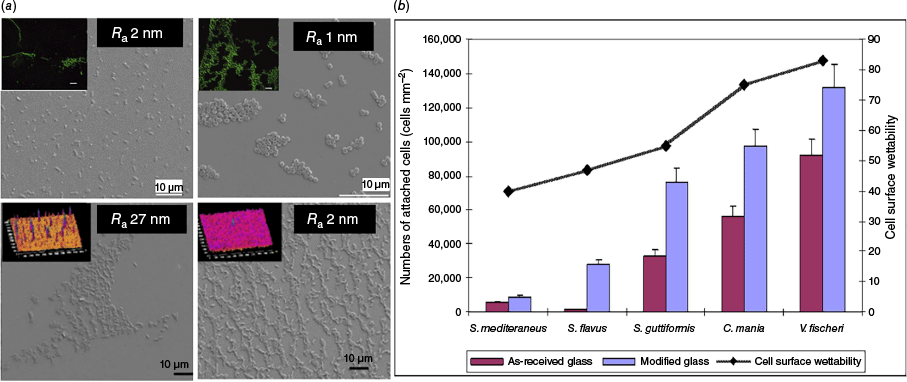
The impact of nanoscale surface roughness on bacterial attachment. (a) differential attachment patterns of two types of bacteria (C. marina, top, and Pseudomonalteromonas ischenkonii, bottom) on nanosmooth glass surfaces of different roughness. (b) Graph showing the attachment trend of five separate bacterial species to as-received or modified glass surfaces. Reproduced with permission from Mitik-Dineva et al.11
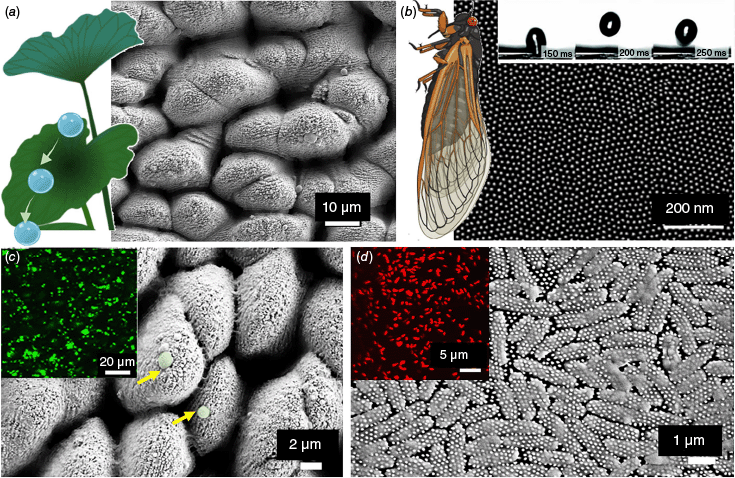
Superhydrophibic nanotopographies. (a) The superhydrophobic surface of the lotus leaf repels water droplets that clean contaminants from the leaf surface. The SEM image depicts a biomimetic titanium surface reminiscent of the lotus leaf surface topography. (b) The cicada wing nanotopography is also superhydrophobic (see bouncing water droplets in inset time series image). The SEM image shows the uniform nanopillar array. Superhydrophobic surfaces do not repel bacteria when submerged. (c) the SEM image and fluorescent image show the attachment of live bacteria (in green). By contrast, the bacteria attached to the cicada wing nanotopography in (d) are all dead (red in inset image). Reproduced with permission from Ivanova et al.24
Because the role of surface topography in bacterial cell adhesion remained poorly understood, primarily due to limited topographical characterisation in many studies, we performed intensive research to investigate the impact of nanoscale surface roughness on bacterial attachment.10 Our initial approach was to design mirror-smooth surfaces to understand what scale of nano-roughness could prevent bacterial attachment. First, we examined bacterial attachment propensity to nano-smooth chemically treated glass surfaces.11,12 The chemical treatment, which involved etching with a buffered solution of hydrofluoric acid, resulted in an average surface roughness of only a few nanometres. The surface modification led to a 70% reduction in nanoscale roughness without altering the chemical composition or surface charge of the glass. Despite this, there was a significant increase in the number of attached bacterial cells, along with morphological transformations and the production of EPS. Bacteria from three different taxa showed similar attachment tendencies, with the number of attached cells increasing by a factor of three on the modified surfaces. Additionally, we found that nanometrically and sub-nanometrically smooth thin silver (Ag) and titanium (Ti) films influenced bacterial attachment.13,14 Nano-smooth Ti surfaces were comprehensively characterised using atomic force microscopy to obtain nine parameters that describe the height, shape, and distribution of surface features. This analysis was correlated with the adhesion behaviour of Staphylococcus aureus and Pseudomonas aeruginosa. The latter was unable to adhere to any of the three sub-nanometrically smooth Ti surfaces. By contrast, S. aureus showed differential attachment with greater number of cells attached on surfaces with higher root mean square roughness, maximum roughness and higher surface areas. Cells preferred less ordered surfaces with evenly distributed peak heights and valley depths. The study demonstrated that traditional amplitudinal roughness parameters are not the sole determinants of bacterial adhesion. Spatial parameters also play a crucial role in predicting the extent of attachment.15–17
Plants and insects: bacteria free self-cleaning surfaces
Despite 3.5 billion years of bacterial evolution towards biofilm development, which resulted in the ability of bacteria to colonise practically any surface, several naturally existing surfaces such as insect wings and plant leaves are capable of maintaining sterility despite the abundance of potential contaminants in their surrounding environments.18 These self-cleaning properties arise from extreme degrees of hydrophobicity; water droplets repelled by the surface sweep away potential contaminating particle.19 Superhydrophobicity is defined as the property of maintaining high water contact angles (>150°) and small roll-off (tilt) angles (<10°) and is due to a combination of chemical composition of the material and nanostructured pattern.20 The superhydrophobic and antifouling properties of lotus leaves have fascinated scientists for years. These leaves exhibit a unique surface structure that repels water and prevents contamination by a combination of nanostructure, and wax coating. Lotus leaves have tiny bumps on their surface that measure less than 100 nm in size. These nanostructures create a rough surface that minimises the contact area between water droplets and the leaf. Furthermore, the surface of lotus leaves is covered with a layer of tiny wax crystals. This wax coating enhances the hydrophobicity of the leaf, causing water droplets to form nearly perfect spheres and roll off easily, taking dirt and microbes with them. These natural features inspired us to design an artificial superhydrophobic surfaces for creating bacteria-free surfaces to prevent colonisation and biofilm formation. We fabricated a lotus leaf-like superhydrophobic biomimetic surface on Ti using femtosecond laser oblation.21,22 Investigations of the interaction of S. aureus and P. aeruginosa with these superhydrophobic surfaces at the surface−liquid interface revealed a highly selective retention pattern for the two pathogenic bacteria. Although S. aureus cells were able to successfully colonise the superhydrophobic Ti surfaces, no P. aeruginosa cells were able to attach to the surface. After immersion for 50 min, nano-sized air bubbles covered 45% of the Ti surface. After 1 h, the number of cells of S. aureus cells attached to the lotus-like Ti increased. The ability for coccoid bacteria to successfully colonise superhydrophobic surfaces coincided with the replacement of trapped air by the incubation medium.
Inspired by the self-cleaning effect of insect wings, our further work was focused on cicada wings.23,24 Cicadas are fascinating insects famous for their loud songs. There are over 3000 species of cicadas globally, and over 200 species in Australia. Usually 17–20-year periodical cicadas emerge in large numbers to breed in summer.25 The wing surface of the Clanger cicada (Psaltoda claripennis) is superhydrophobic with water contact angles between 163 and 173°. The surfaces of their wings are covered with millions of tiny nanopillars of ~200 nm in height and 60 nm in diameter. Initially, we hypothesised that, since the wings are superhydrophobic, the bacteria will be cleaned from the surfaces by the rolling action of water droplets. Surprisingly, we found that the wing surface was not really effective at repelling the bacteria. By contrast, many cells were able to attach to the wing surface. However, altered cell morphology was clearly observed using optical and electron microscopy.23,24
Building on the underlying physical principle of bactericidal nanostructures, we explored other natural topologies with similar surface architectures. We observed that the dragonfly wing nanoarchitecture favours enhanced bactericidal activity with its increased deformation of the bacterial cell membrane and cell wall stress compared to cicada surfaces and led to excellent mechano-bactericidal efficacy toward Gram-positive and Gram-negative bacteria and endospores.26
The development of synthetic mechano-bactericidal surfaces involves creating nanostructures like nanopillars, spikes or needle-like protrusions that can physically rupture bacterial cell membranes upon contact. One notable example, and the first example of a synthetic mechano-bactericidal surface, is ‘black silicon’ created using a reactive-ion etching (RIE) technique. This process results in high aspect ratio a needle-like array of nano-protrusions, which exhibit bactericidal properties similar to those found on natural surfaces like dragonfly wings and capable of killing antibiotic-resistance bacterial strains.26 Advancements in nanofabrication techniques, such as nanoimprinting lithography, chemical etching, and hydrothermal treatments, have enabled the creation of these surfaces on various materials, including metals and polymers. Overall, mechanobactericidal surfaces represent a bioinspired approach to antimicrobial design, leveraging physical mechanisms to inactivate bacteria and reduce the reliance on chemical treatments. Our discovery began a new era in the design and fabrication of mechanobactericidal nanomaterials.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
E. P. Ivanova was supported by the Australian Research Council (ARC) Research Hub for Australian Steel Manufacturing under the Industrial Transformation Research Hubs scheme (grant number IH130100017), by the ARC Industrial Transformational Training Centre in Surface Engineering for Advanced Materials (grant number IC180100005), and by Discovery program (grant number DP230103188).
Acknowledgements
This research would not have been possible without the valuable support of excellent academics and students. E. P. Ivanova acknowledges the ARC for support.
References
2 Roope LSJ et al. (2019) The challenge of antimicrobial resistance: what economics can contribute. Science 364, eaau4679.
| Crossref | Google Scholar | PubMed |
3 Habib MB et al. (2025) Biofilm-mediated infections; novel therapeutic approaches and harnessing artificial intelligence for early detection and treatment of biofilm-associated infections. Microb Pathog 203, 107497.
| Crossref | Google Scholar | PubMed |
4 Sriyutha Murthy P, Venkatesan R (2008) Industrial biofilms and their control. In Marine and Industrial Biofouling (Flemming HC, Murthy PS, Venkatesan R, Cooksey K, eds). Springer Series on Biofilms, vol. 4, pp. 65–101. Springer. 10.1007/978-3-540-69796-1_4
5 Galié S et al. (2018) Biofilms in the food industry: health aspects and control methods. Front Microbiol 9, 898.
| Crossref | Google Scholar | PubMed |
6 Hasan J et al. (2013) Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol 31, 295-304.
| Crossref | Google Scholar | PubMed |
7 Howell D, Behrends B (2009) Consequences of antifouling coatings – the chemist’s perspective. In Biofouling (Dürr S, Thomason JC, eds). pp. c1–c8. Wiley. 10.1002/9781444315462.ch16
8 Schumacher JF et al. (2008) Engineered nanoforce gradients for inhibition of settlement (attachment) of swimming algal spores. Langmuir 24, 4931-4937.
| Crossref | Google Scholar | PubMed |
9 Scardino AJ et al. (2008) Attachment point theory revisited: the fouling response to a microtextured matrix. Biofouling 24, 45-53.
| Crossref | Google Scholar | PubMed |
10 Bazaka K et al. (2011) Do bacteria differentiate between degrees of nanoscale surface roughness? Biotechnol J 6, 1103-1114.
| Crossref | Google Scholar | PubMed |
11 Mitik-Dineva N et al. (2009) Differences in colonisation of five marine bacteria on two types of glass surfaces. Biofouling 25, 621-631.
| Crossref | Google Scholar | PubMed |
12 Mitik-Dineva N et al. (2008) Impact of nano‐topography on bacterial attachment. Biotechnol J 3, 536-544.
| Crossref | Google Scholar | PubMed |
13 Ivanova EP et al. (2010) Impact of nanoscale roughness of titanium thin film surfaces on bacterial retention. Langmuir 26, 1973-1982.
| Crossref | Google Scholar | PubMed |
14 Ivanova EP et al. (2011) The influence of nanoscopically thin silver films on bacterial viability and attachment. Appl Microbiol Biotechnol 91, 1149-1157.
| Crossref | Google Scholar | PubMed |
15 Webb HK et al. (2013) Bacterial attachment on sub-nanometrically smooth titanium substrata. Biofouling 29, 163-170.
| Crossref | Google Scholar | PubMed |
16 Truong VK et al. (2010) The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 31, 3674-3683.
| Crossref | Google Scholar | PubMed |
17 Ivanova EP et al. (2011) Differential attraction and repulsion of Staphylococcus aureus and Pseudomonas aeruginosa on molecularly smooth titanium films. Sci Rep 1, 165.
| Crossref | Google Scholar | PubMed |
18 Webb HK et al. (2014) Wettability of natural superhydrophobic surfaces. Adv Colloid Interface Sci 210, 58-64.
| Crossref | Google Scholar | PubMed |
19 Hasan J et al. (2012) Spatial variations and temporal metastability of the self-cleaning and superhydrophobic properties of damselfly wings. Langmuir 28, 17404-17409.
| Crossref | Google Scholar | PubMed |
20 Ivanova EP et al. (2013) Molecular organization of the nanoscale surface structures of the dragonfly Hemianax papuensis wing epicuticle. PLoS ONE 8, e67893.
| Crossref | Google Scholar | PubMed |
21 Fadeeva E et al. (2011) Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 27, 3012-3019.
| Crossref | Google Scholar | PubMed |
22 Truong VK et al. (2012) Air-directed attachment of coccoid bacteria to the surface of superhydrophobic lotus-like titanium. Biofouling 28, 539-550.
| Crossref | Google Scholar | PubMed |
23 Hasan J et al. (2013) Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl Microbiol Biotechnol 97, 9257-9262.
| Crossref | Google Scholar | PubMed |
24 Ivanova EP et al. (2012) Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 8, 2489-2494.
| Crossref | Google Scholar | PubMed |
25 Owen CL et al. (2017) How the aridification of Australia structured the biogeography and influenced the diversification of a large lineage of Australian Cicadas. Syst Biol 66, syw078.
| Crossref | Google Scholar | PubMed |
26 Ivanova EP et al. (2013) Bactericidal activity of black silicon. Nat Commun 4, 2838.
| Crossref | Google Scholar | PubMed |
 Dr Denver Linklater is a research fellow at the Ian Holmes Imaging Centre, The University of Melbourne. She is an expert in electron microscopy. Her research interests are in the design and synthesis of nanomaterials for novel anti-microbial technologies, stem cell culture and tissue re-generation. |
 Elena Ivanova is a distinguished professor of RMIT University. Her research interests are in design and fabrication of biomimetic antimicrobial micro- and nano-structured surfaces, materials biointerfaces and immobilisation of biomolecules and microorganisms in micro- and nano-environments. |


