Historical ecology of semi-enclosed coastal embayments: tools and techniques for discovering ecological events of the recent past
Yvette M. Pedretti A and Belinda J. Robson
A and Belinda J. Robson  A *
A *
A Environmental and Conservation Sciences, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
Marine and Freshwater Research - https://doi.org/10.1071/MF22005
Submitted: 5 January 2022 Accepted: 7 October 2022 Published online: 11 November 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The sheltered environments of coastal embayments have played a vital role for humans for millennia and their resources have underpinned modern industrial development globally. Their effective management and restoration remains an enormous challenge, owing, in part, to lack of recognition of the threshold changes that occurred in many bays prior to scientific study (i.e. >50 years ago). Advances in marine extraction technologies and increased clearing of catchments for agriculture and urbanisation in recent history (∼400 years) have resulted in profound physical, chemical and biological changes to these ecosystems. More recently, the integration of ecology, history, archaeology, economics and fisheries science have contributed to the emerging field of ‘marine historical ecology’ (MHE). The synthesis of information from these different disciplines can markedly improve knowledge of past ecosystem condition, thereby assisting managers to set realistic goals for environmental restoration to improve biodiversity and ecosystem function. This paper reviews historical knowledge of long-term environmental degradation processes in coastal embayments, summarising the wide range of methods and techniques used as evidence and providing examples from around the world, thereby illustrating the need for longer time-frames of reference for contemporary restoration ecology.
Keywords: coastal ecosystems, coastal management, ecosystem management, ecosystem services, large embayments, large marine ecosystems, marine historical ecology, marine conservation.
Introduction
Historical ecology focuses on describing past processes or patterns and integrating them into contemporary understanding of ecosystem function, so as to understand the dynamic nature of an ecosystem. Originally, the focus was on terrestrial ecosystems where human-induced impacts were reducing productivity or biodiversity, making ecosystem change conspicuous and easily recognisable (Swetnam et al. 1999; Lotze and Worm 2009; Szabó 2015). By contrast, marine ecosystems have been thought of as a cornucopia of abundant resources, as the last of the ‘commons’ where marine organisms were abundant and harvests were always plentiful, largely ignoring the environmental history of marine ecosystems (Hardin 1968; Bolster 2008; Pinnegar and Engelhard 2008). Although humans have exploited marine ecosystems for millennia, investigation of their historical ecology has taken place only recently (mostly in the 21st century: Jackson 2001; Jackson et al. 2001; Lotze and Worm 2009; Bolster 2012). The paucity of historical information on marine ecosystems may have occurred because land degradation is more visible (because humans are essentially terrestrial), together with past logistical limitations to studying marine environments (Pitcher 2001; Bolster 2012; Meadows et al. 2012). Until the introduction of new technologies in the late 20th century (Table 1), a lack of data had prevented reliable prediction of the distribution and condition of marine habitat, especially at large spatial scales (Auster et al. 2001; Lauer and Aswani 2010; Zu Ermgassen et al. 2012; Anderson et al. 2014). Even with new technologies such as remote sensing, suitable data are still available only for at most the last 50 years. Yet, to provide accurate historical baselines for appropriate management and conservation, investigations may need to reach back much farther in time to capture natural variability and historic anthropogenic influences, along with long-term and extreme climatic events (Bolster 2012).
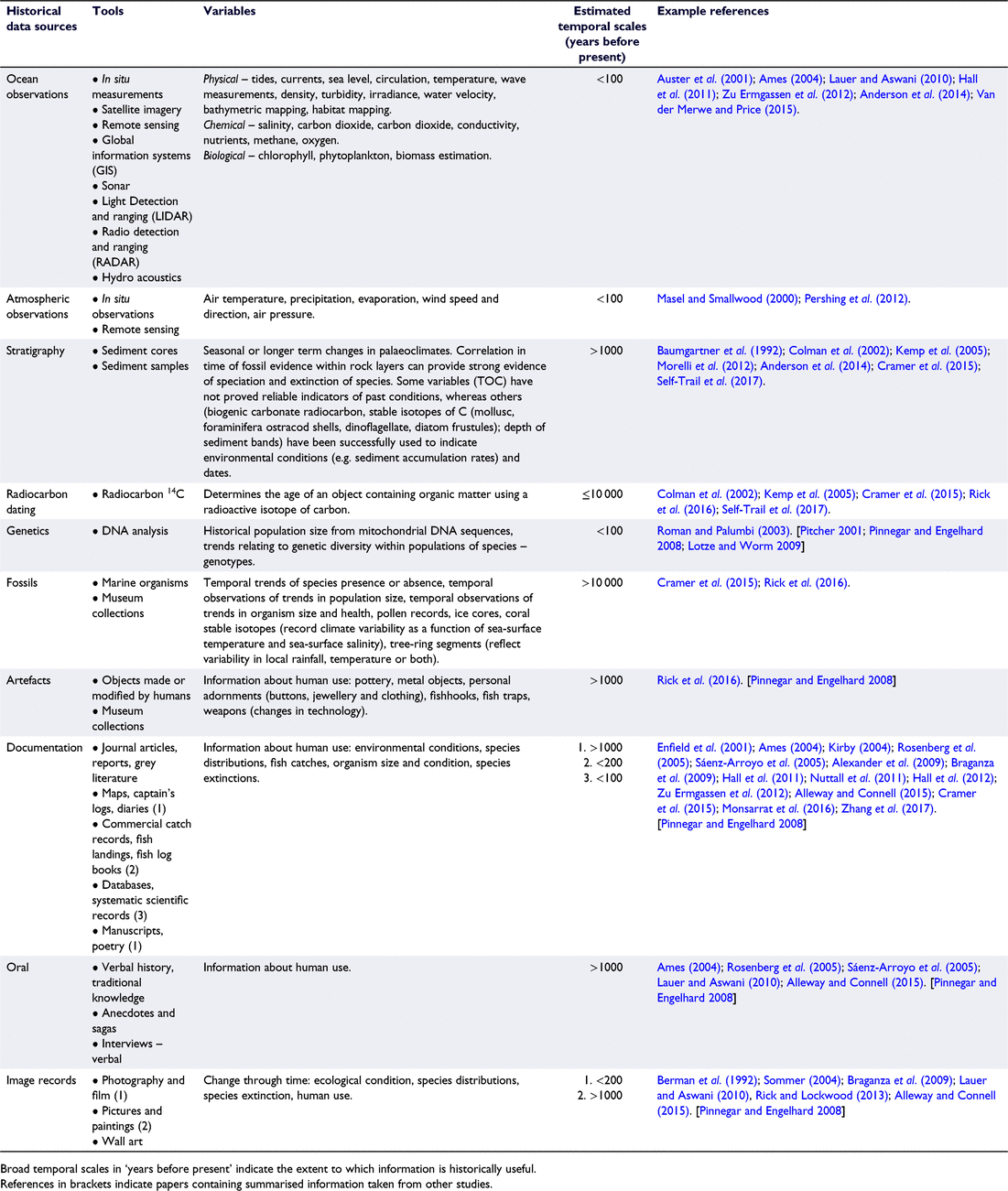
The aim of this review is to describe processes of historic degradation in semi-enclosed coastal embayments and the important consequences that have flowed from the omission of this historical information from environmental decision-making worldwide. A brief summary of major historical events during recent centuries (∼400 years) is provided. This is followed by a discussion focusing on extinction and extirpation of species, the consequences of shifting baselines in management decision-making and the interaction of natural v. anthropogenic impacts as drivers of change. Table 1 collates information on the wide range of tools available for use in marine historical studies, their associated variables and relevant temporal scales (years before present) indicating the historical extent of each tool. The next section examines whether processes of historic degradation over this time period have followed similar temporal sequences in different regions given their differing environmental histories (evidence summarised in Table 2). Consolidating information from a variety of different disciplines substantially improves knowledge of past ecosystem condition and assisting managers to set realistic goals for environmental restoration to improve biodiversity and ecosystem function.
Marine historical ecology (MHE) and the recent past (∼400 years)
A central tenet of conservation biology and restoration ecology is that appropriate reference conditions are needed to determine ecological goals for restoration programs (Swetnam et al. 1999; Lotze and Worm 2009). Several international organisations, (UN Millennium Ecosystem Assessment, the US Oceans Commission, the Pew Oceans Commission, and the European Council) have recommended that ocean policy use an ecosystem approach in the management of ocean resources (Alexander et al. 2009). Thus in recent decades, through the integration of ecology, history, archaeology, economics, and fishery science, marine scientists have contributed to the emerging field of ‘marine historical ecology’ (MHE). As a result, past changes in the ocean can be reconstructed beyond contemporary investigations to provide historical reference points for understanding ecosystem processes and conditions (Table 1: Lotze and Worm 2009; Engelhard et al. 2016). Table 1 provides a reference to investigate the type of tools, resultant variables and broad temporal scales applicable to the study of MHE. Example references associated with these tools (Table 1) are also provided as practical examples of the way in which the tools have been used.
This review is confined to information related to periods of global European colonisation and the present day (∼400 years). A time period that includes exploration (Age of Sail through to the Golden Age of Sail; late 16th century to early 19th century) and expansion (European Colonialism–Imperial Expansion; late 15th century to early 20th century; Rönnbäck 2012). The harnessing of steam power in the ‘First Industrial Revolution’ (middle of 18th century to middle of 19th century) resulted in the mechanisation of mercantile commodities such as textiles, tobacco and coffee (The British Museum 2017). Consequently, transportation times were dramatically reduced, increasing the volume of marine products extracted from the ocean and brought to market (Rodrique et al. 2017). Subsequently, a second period of industrialisation (late 19th to early 20th centuries) saw rapid advances in steel, electric and automobile industries. The latter phases led to advances in refrigeration that allowed perishable marine products to reach distant destinations, opening up additional markets to places far away from their coastal origin (Roberts 2007; Craig and Holt 2017). The development of new instruments and fishing techniques allowed ever-increasing volumes of marine coastal resources to be extracted. Although historical documents show concerns for impacts to these important environments, management and conservation measures often lagged behind (Roberts 2007). This often resulted in substantially changed marine environments that no longer represented the habitat described by the initial European explorers and settlers (Bolster 2008, 2012).
Degradation of marine ecosystems has a long history
Extirpation – a key marker of human impacts through time
Marine extirpations (loss of a taxon from a portion of its range, namely, area, region or habitat; US National Marine Fisheries Service 2006) and extinctions were greatest in the 19th and 20th centuries, mostly caused by overfishing and habitat loss (Jackson 2001; Reynolds et al. 2005; Srinivasan et al. 2012). Other, lesser impacts to coastal ecosystems occurred through pollution, eutrophication, species invasion and disease (Jackson et al. 2001; Lotze and Worm 2009). Currently, populations of many once abundant and commercially important marine species have been overexploited; their stocks are now in global decline (Jackson 2001; Reynolds et al. 2005; Alexander et al. 2009; Bolster 2012). For example, a major factor in the colonisation of North America by Europeans five centuries ago was the exceptional abundance of cod (Gadus morhua) in Newfoundland. Yet, overfishing and the introduction of trawlers in the middle of the 20th century depleted stocks to <3% of those in the 1500s−1800s. The collapse of the Newfoundland cod population dramatically altered marine ecosystems (because cod was an apex predator controlling trophic cascades) and its recovery is in doubt, even in the absence of fishing (Pitcher 2001; Myers and Worm 2005; Rosenburg et al. 2005; Lotze and Worm 2009). Similarly, large aggregations of Atlantic sturgeon (Acipenser sturio) were reported in the southern Baltic Sea until the 18th century, but by 1900, annual catches had dropped to <500 individuals and the fishery ceased in 1915. Atlantic sturgeon remains locally extinct in Europe and is critically endangered across the rest of its range (Debus 1996; Lotze et al. 2006).
Depending on their market value and ease of capture, the abundance of some whale species declined during the 1900s. Populations of southern (Eubalaena australis) and North Pacific (Eubalaena japonica) right whales, North Pacific grey whales (Eschrichtius robustus) and North Atlantic humpback whales (Megaptera novaeangliae) had their abundances reduced by 80–98% (Roman and Palumbi 2003; US National Marine Fisheries Service 2006). Additionally, there are only ∼400 North Atlantic right whales (Eubalaena glacialis) remaining, making it one of the most critically endangered large whales in the world (Monsarrat et al. 2016; NOAA Fisheries 2017; Bak 2020). These declines in whale numbers coincided with the introduction of factory ships with on-board facilities for processing and freezing whales that processed much higher tonnages than traditional methods (hand-thrown harpoons and rowboats; Roberts 2007; Newton 2013). The loss of large marine fauna was the first substantial anthropogenic impact to which many marine ecosystems were exposed.
Management responses to marine extirpations largely focused on restoring stocks of individual species rather than ecosystem processes (Jackson 2001; Jackson et al. 2001; Rosenberg et al. 2005; Goode 2006). As early as the 18th century, North America attempted to mitigate rapidly declining stocks of migratory fish through legislation, by prohibiting the construction of dams on public land and requiring the construction of fish passes around dams during spawning. Yet, these and future measures (severe restrictions on commercial and recreational fishing, implementation of hatcheries) were ineffective for a number of anadromous fish (e.g. Atlantic salmon, Salmo salar; striped bass, Morone saxatilis; American shad, Alosa sapidissima; and blue back herring, Alosa aestivalis) and, by the 19th century, the once seemingly inexhaustible supply of migratory fishes had collapsed or was in decline. Perhaps more than overfishing and industrialisation were involved in their irreversible decline (Buchsbaum et al. 2005; Roberts 2007)?
Investigating the chronology of historical events leading to the current status of species and their environments can be useful in determining why management actions have, or have not, been effective. For example, technologies such as fish ladders, lifts and hatcheries were ineffective because they were unable to overcome the impacts of multiple river dams on fish populations (Goode 2006; Thompson 2010; Hall et al. 2011). By the 1950s, less than 2% of the original spawning habitat for Atlantic salmon (and many other anadromous fish species) remained in New England (Buchsbaum et al. 2005). A disconnect between historical investigations into individual species and their once structurally complex habitats could lead to unachievable and costly conservation management goals (Airoldi et al. 2008). For example, the very high numbers of dugong (Dugong dugon) of 72 000 (1960s) to 4000 (mid-1990s) formerly reported could not be supported on the Great Barrier Reef today given the loss of seagrass area (Marsh et al. 2005). Understanding interactions between species and other parts of ecosystems, together with historical knowledge of key events in ecosystem degradation, should indicate how populations or ecosystem functions might be restored (or partially restored).
Until recently, conservation management and restoration have focused largely on data collected in the past 50 years of scientific monitoring, for which comprehensive numerical data may exist. Most ecological research is based on local, short-term (one to a few years) field studies used to determine baselines and environmental management objectives (Jackson 2001; Jackson et al. 2001; Rosenberg et al. 2005; Lotze and Worm 2009). Yet, the extent of human influence cannot be assessed solely on the basis of modern observations because many impacts commenced prior to the use of scientific monitoring (Al-Abdulrazzak et al. 2012; Nyström et al. 2012). Furthermore, the absence of historical data may lead to incorrect conclusions about species life-history characteristics such as size at reproductive maturity, life span, fecundity, migration patterns and times and locations used for spawning or spent as juveniles.
The consequences of shifting baselines
Without long-term data, temporal processes such as population decline, range contraction, thresholds and lag times (i.e. extinction debt) may be overlooked. Furthermore, where extirpation preceded ecological investigation, species absences have often been erroneously accepted as part of the natural baseline (Jackson 2001; Lotze and Worm 2009). Pauly (1995) hypothesised that each generation of marine scientists tends to accept as a baseline the abundance and composition of species occurring at the commencement of their career. Henceforth, they use this ‘baseline’ condition to evaluate change, often under the assumption that inadequate data exist for earlier periods. This results in a ‘shifting baseline’ that allows ecosystem condition to degrade through time (Pauly 1995), facilitates the creeping disappearance of species and the use of inappropriate reference points for measuring economic losses or identifying goals for management actions (Rosenberg et al. 2005; Pinnegar and Engelhard 2008; Lotze and Worm 2009). Therefore, one important role of historical ecology research is to provide improved knowledge of past ecosystem condition to provide more accurate baselines.
Case studies exist that show how historical data enable correct interpretation of the causes of contemporary environmental change. Initially, the ecological collapse of Caribbean coral reefs in the 1980s was attributed to the bottom-up effects of nutrient enrichment that facilitated macroalgal growth, which, eventually, outcompeted corals, covering the reefs (Burkepile and Hay 2006; Fitzpatrick and Keegan 2007). However, contemporaneous with the collapse of corals was the mass mortality, from an unknown pathogen, of the formerly ubiquitous herbivorous sea urchin Diadema antillaruma. Because this sea urchin had exerted top-down control on macroalgae, they were released from herbivory when the sea urchin was extirpated, covering the reef. Data from the 1950s indicated that assemblages of small fish species represented baseline community structure (Hughes 1994; Jackson and Sheldon 1994) that was subsequently altered by eutrophication and loss of Diadema. However, larger species of predatory and herbivorous fish had once formed part of the fish assemblage (prior to their disappearance during the 19th century as a result of overfishing), and they had exerted top-down control on macroalgae. Thus, environmental degradation of Caribbean coral reefs started with overfishing in the 19th century, leading to dependence on a single species of sea urchin to control macroalgal growth (Hay 1984; Jackson 2001; Pinnegar and Engelhard 2008). By using stratigraphy and radiocarbon isotopes (Table 1), Cramer et al. (2015) showed that, subsequently, land clearing and agriculture in the mid-20th century gradually changed marine-water quality, causing a shift from long-lived to short-lived corals. When epidemic disease then removed sea urchin grazing, macroalgae took advantage of changes in water quality to rapidly overgrow and replace the short-lived corals. The formation of macroalgal-dominated reefs then led to further changes to fish and invertebrate assemblages (Lessios 1988; Cramer et al. 2015). This sequence of anthropogenic impacts occurring over more than one century, starting with over-fishing and followed by a sequence of slow environmental change (water quality) and more rapid ecological change (epidemic), leading to ecosystem collapse, is not an isolated example (Table 2).
Interacting drivers of change – natural processes v. anthropogenic impacts
As the majority of changes to marine ecosystems take place through multiple interacting natural and anthropogenic drivers occurring at a range of different temporal and spatial scales, it is important to distinguish the impacts of natural drivers and natural variability from human impacts (Brierley and Kingsford 2009; Pershing et al. 2012; Schwerdtner Máñez et al. 2014). Natural variations in climate affect marine productivity, ranging from annual or seasonal to decadal cycles, but these patterns can be hard to attribute to natural causes. In some ecosystems, climatic forcing may be responsible for major population changes that conventional data fail to capture because of the short time (i.e. ∼50 years) these data have been available. For example, until recently, collapses in the Santa Barbara Basin anchovy and sardine populations had been attributed to anthropogenic impacts. Yet, examination of fish scales taken from sediment cores showed that nine major collapses and subsequent recoveries of anchovy and sardine populations had occurred over the past 1700 years (Table 1, Baumgartner et al. 1992). Together with more contemporary data, this suggests population cycles of anchovies and sardines occur every 50–70 years, determined by changing climatic conditions (Jackson 2001; Pinnegar and Engelhard 2008).
Regional climate can be heavily influenced by natural variability in large climatic systems over extended time scales. For example, Enfield et al. (2001) cross-referenced a variety of datasets, whereas Braganza et al. (2009) used dive logs, photographs, videotapes and collections as proxies for patterns of abundance to determine that the Atlantic Multidecadal Oscillation (AMO) affects sea-surface temperature in the North Atlantic Ocean by 0.4°C over a 65–80-year cycle (Table 1). Such effects are distinct from human impacts on climate, which commenced after the Industrial Revolution through the release of greenhouse gases (Steffen and Hughes 2013). Anthropogenic climate change now augments, amplifies and accelerates other human impacts (Jackson et al. 2001; Pinnegar and Engelhard 2008), but as contemporary data extend back only ∼50 years, it is often inadequate to capture long-term changes, leading to false assumptions about drivers of population dynamics. Methods that can extend records long enough through time to capture natural population variability provide a more accurate assessment of the role played by anthropogenic impacts (Table 1).
Recovery pathways and threshold effects
The idea that increasing biodiversity during ecosystem recovery increases ecosystem function underpins ecosystem restoration, but there is uncertainty regarding the recovery of important ecosystem processes such as decomposition and recycling of nutrients (Lake et al. 2007). Reference conditions used as goals in restoration may not always lead to success because it may be no longer possible to reach those conditions even when biodiversity increases. Recovery pathways will vary according to the losses in redundancy that occurred in each ecosystem over time. These losses affect ecosystem capacity to recover from both natural and anthropogenic impacts (Tett et al. 2007). Marine ecosystems may also encounter threshold effects that greatly change their stable states, to a point where even substantial reductions in disturbance have little or no effect on recovery. The resulting ecosystem becomes so different and has degenerated to the extent that it no longer provides the ecosystem processes and functions formerly present at the site (van de Koppel et al. 2009).
Information gathered on the Gulf of Maine (GoM, Table 2) in the United States of America indicates that a very different ecosystem existed during post-colonisation times (1600–1800s) from that of the present-day ecosystem. Large animals such as whales (e.g. North Atlantic right whales, E. glacialis; walrus, Odobenus rosmarus; seals, Arctocephalus spp.; and the great auk, Pinguinus impennis) were found there in large numbers. These and many other easily exploited species (e.g. oysters) were rapidly depleted, being conspicuous and easily accessed close to shore, with little fishing effort required to harvest thousands of individuals (Roberts 2007; Bolster 2012). This resulted in a loss of ecosystem redundancy that compromised its resilience to further changes. Later, during the industrial revolution (1800–1900s), advances in technologies (e.g. freezing and railway transport) resulted in declines in stocks of preferred species. Fishers moved on to the next most favourable species, from cod to haddock to ocean perch, until, finally, benthic species such as flounder, scallops and blue mussels were taken by trawlers (Pauly et al. 1998; Pauly and Palomares 2005). Contemporaneously, extensive industrial development and urbanisation occurred along New England rivers and catchment areas (Table 2, ‘Population increase over time’). Species lost at this time not only changed the appearance of the GoM marine ecosystem but negatively affected the processes and functions within it. This second loss of redundancy further compromised the capacity of the ecosystem to recover from further change (Buchsbaum et al. 2005; Anderson et al. 2014).
Chesapeake Bay, San Francisco Bay, Moreton Bay and the Wadden Sea (Table 2) follow a sequence of environmental change and loss of redundancy similar to those in GoM (Lake et al. 2007; Tett et al. 2007). Table 2 provides information on the exponential population growth that occurred in these coastal bays over time. The associated increase in urbanisation adds to the impacts and stresses on these environments, because natural shorelines were replaced with artificial surfaces. Management plans commonly focused on restoring stocks of individual species (e.g. oysters, seagrass or fish, see above) rather than looking at ways in which succession pathways could benefit the entire ecosystem (Jackson et al. 2001; Rosenberg et al. 2005; Goode 2006; Lake et al. 2007). Importantly, the resilience of many ecosystems has led to new recovery pathways that have resulted in the maintenance of important functions and processes (e.g. fishing, nurseries, bird habitat, nutrient uptake), even though they no longer resemble the coastal environments that existed prior to colonisation. Indeed, the global importance of these ecosystems, especially with regards to migratory species, is demonstrated by the number of international treaties and conventions they are party to (Table 2, ‘Importance’).
Sometimes, the ecosystem is degraded to a point where the original suite of species along with functions and processes are completely lost. Recovery pathways are unpredictable, and the target endpoint no longer resembles the original ecosystem even with management intervention (Lake et al. 2007; Tett et al. 2007). The example of Jiaozhou Bay (China, Table 2) illustrates how a coastal ecosystem becomes so degraded that it results in a simplified foodweb based on microalgae and microbes.
Consideration also needs to be given to recovery pathways because of impacts from global warming, because it augments, amplifies and accelerates other human impacts. Shark Bay (SB) in Western Australia is uniquely positioned to demonstrate impacts due to global warming because it has had minimal changes in population growth since 1911 (Table 2). An unprecedented marine heatwave occurred in SB between December 2010 and February 2011 (Pearce and Feng 2013). The temperate seagrass (Amphibolis antarctica) is at the northern edge of its geographic range in SB, where it covered ∼3700 km2 and comprised ∼85% of total seagrass cover (Walker et al. 1988). Following the heatwave, 36% of seagrass cover in SB was lost. Because SB contains one of the largest areas of seagrasses worldwide, this loss dramatically reduced habitat for a wide range of fauna, significantly reduced carbon sequestration capacity, and increased CO2 emissions from sediments through seagrass degradation (Arias-Ortiz et al. 2018). Such effects also have the potential to exacerbate climate-change impacts on ecosystems globally (Steffen and Hughes 2013; Thomson et al. 2015) and will be difficult to manage in more degraded ecosystems.
A predictable sequence of environmental change in coastal embayments
The following section examines human impacts in near-shore marine coastal embayments using both contemporary scientific data and novel information sources to determine whether historic degradation processes followed similar temporal sequences in different global regions. The coastal embayment examples in Table 2 comprise three oceans (Atlantic, Indian and Pacific) and three seas (North, Tasman and South Yellow) with many of their physical features ‘shape’, ‘climatic zones’, ‘barrier type’ and their ‘catchment and bay areas’ being very different (Table 2).
The history of environmental change
Anthropogenic impacts on marine ecosystems are often most intense in near-shore and coastal ecosystems because these are closest to where people live. Fossil records and shellfish middens show that people around the world extracted goods sustainably from coastal ecosystems for millennia (Erlandson and Rick 2010; Fitzpatrick et al. 2015). For example, even though the Aboriginal peoples of Australia hunted dugongs for millennia, populations remained large. European colonists reported seeing herds of dugongs comprising tens of thousands of large individuals as late as 1893 (in Moreton Bay Queensland, Jackson et al. 2001). Similarly, shell middens on the eastern coast of North America attest to a long history of sustainable extraction of oysters (shell size in middens commonly exceeded 30 cm; Jackson 2001). So, although most Indigenous coastal peoples used marine resources from embayments, there were indiscernible impacts on populations or ecosystems.
The greatest anthropogenic impacts to coastal marine ecosystems appear to have occurred during either times of exploration and colonisation or (later) times of rapid industrialisation (Worm et al. 2006; Poulsen 2010; Zu Ermgassen et al. 2012). One of the most expansive human migrations in recorded history occurred during ‘the age of sail’, in the 16th−19th centuries, when Europeans travelled around the world. In this early modern period of international trade, Europeans crossed vast oceans to seek their fortunes and acquire wealth, thereby acquiring influence, power and elevated social status (Rodrique et al. 2017). During this time, European colonisers relied on ocean products and services as never before. In doing so, they helped create empires of commerce, establishing both economic and social connections with the sea. Concepts of ecosystems and sustainability were not widespread in these cultures, so stocks of many economically important marine species (including large marine fauna such as whales, walrus, seals, cod) declined either to extirpation or extinction, changing many (perhaps most) marine ecosystems forever (Lotze and Worm 2009; Bolster 2012). Subsequently, as human populations have continued to grow, pressure on coastal areas has further degraded many marine ecosystems, exceeding their resistance and resilience to change.
Of near-shore ecosystems, shallow coastal embayments are some of those most intensely used by humans, both now and in the past (Lotze et al. 2006; Worm et al. 2006). Prior to anthropogenic impacts in the 19th or 20th century, the euphotic zone in shallow coastal marine embayments reached the seabed, permitting primary productivity throughout the water column. As a result, embayments have often contained very productive ecosystems supplying resources such as seafoods to humans, resulting in resident human populations. Interestingly, many embayments with a long history of human use are semi-enclosed by peninsulas or islands, facilitating human access and reducing flow-through by ocean currents. In following sections, we review historical data sources used to identify factors affecting the condition of shallow semi-enclosed marine embayments surrounded by industrial and urban land uses.
The dependence of human societies on coastal marine embayments
Over the past two centuries, human settlements along coastlines have become industrialised and urbanised to take advantage of convenient access to marine resources and shipping for transportation of goods and services (Lotze et al. 2006; Lotze and Worm 2009). In particular, semi-enclosed coastal marine embayments have characteristics that are highly desirable for both settlements and industry, namely, restricted water circulation, either by virtue of their shape or by obstructions to flow either above or below the water surface (e.g. sandbanks, submerged ocean banks, islands, bridges, causeways), and sheltered harbours that facilitate the loading of goods for transport (Table 2; Bolster 2012). Bays are also highly productive, usually containing habitat-forming organisms (ecosystem engineers, e.g. seagrass, kelps, oyster beds, mangroves or corals) that provide fish and shellfish nurseries and support a wide variety of other marine species that can be easily exploited because of their proximity to the shoreline (Jackson 2001; Jackson et al. 2001; Lotze et al. 2006). As a result, these ecosystems are often of local, regional or international importance (Table 2). Jackson (2001), designated habitat-forming marine species as ‘ecosystem engineers’ (sensu Jones et al. 1994) because they are able to moderate the availability of resources within their environment through creating, modifying and maintaining their habitat. They stabilise the physical environment and increase biodiversity and trophic complexity in embayments, resulting in more resilient ecosystems (Jackson 2001; Nyström et al. 2012). Consequently, impacts that remove ecosystem engineers from coastal embayments will have particularly large negative effects on ecosystems. For these reasons, coastal embayments provide ideal locations to show the role of historical ecology in understanding processes of ecosystem degradation and restoration.
The loss of ecosystem engineer species: the example of oysters
One nearly ubiquitous example of historical exploitation of resources from shallow marine environments is the harvesting of shellfish, which are both autogenic and allogenic ecosystem engineers (Jones et al. 1994; Meadows et al. 2012). Oysters and other filter-feeding shellfish were originally common and abundant in coastal embayments, and similar patterns of historical overexploitation of oyster reefs have occurred in semi-enclosed embayments worldwide (e.g. San Francisco Bay and Chesapeake Bay (USA), Botany Bay, Moreton Bay and Saint Vincent Gulf (Australia), Jackson et al. 2001; Kirby 2004, Table 2). Indeed, the long-time scales involved in the impacts of oyster exploitation and the lack of numerical data on oyster losses may have created a collective amnesia regarding the past distribution, abundance and extent of oyster reefs (Alleway and Connell 2015). For example, data from diaries and correspondence of fisheries inspectors, government reports, legislation, photographs, and maps (Table 1) were utilised to provide a baseline of a now extinct oyster fishery once present along the South Australian coastline. This extinct fishery once covered more than 1500 km of coastline, including large areas within Saint Vincent Gulf (Table 2); however, now the existence of this fishery, and its loss, are not well known. Dredging was the main driver for the loss of oyster reefs, commencing in the early days of European colonisation (c. 1836), until collapse 100 years later (Alleway and Connell 2015). As late as 1892, oysters were ‘plentiful’ and ‘thickly distributed’ (Inspector of Oyster Fisheries 1892, as cited in Alleway and Connell 2015). The degradation and decline of oysters in South Australia occurred prior to changes in adjacent catchments; so, they were driven solely by overexploitation.
Kirby (2004) reconstructed the history of oyster reef exploitation along three continental margins, namely, eastern North America, western North America, and eastern Australia. Multiple proxy variables (derived from fishery records, including the earliest laws to regulate oyster fisheries and a time-series of oyster landings, Table 1) were utilised, allowing information to be cross-referenced in space and time, greatly increasing the reliability of the historical information. These sets of information were used to compare the process and effects of shellfish exploitation among the coastlines. Along the North American coastline, oyster fishery exploitation progressed from north to south direction in a process termed ‘fishing down the coast’, whereby overharvesting first occurred in Massachusetts and New York and then spread southward to Chesapeake Bay and the Gulf of Mexico. Oyster landings from Chesapeake Bay on the eastern coast of North America were an order of magnitude greater than from any other oyster fishery in 1880 (Kirby 2004). Populations were so vast that they were used to restock northern fisheries and, by the late 1800s, some 400 000–600 000 tons (∼363 000–544 000 tonnes, Mg) of oysters were harvested from the Bay annually (Rick et al. 2016). Consequently, by the year 2000, oyster landings were only 2% of those in 1880 (Kirby 2004), clearly showing the loss in productivity. Oyster grounds are thus not only smaller, but also ∼10 times less productive than they were in the late 1800s (Zu Ermgassen et al. 2012).
Studies using fossils (Table 1), that go further back in time, show the effects of human harvesting of oysters even more profoundly. In Chesapeake Bay, average oyster size decreased from the Pleistocene (781 000 000–13 000 years ago, 87 mm) to the Holocene (3200–50 years ago, 79 mm), with the smallest average size of oysters being recorded in Modern (years 2000–2014, 72 mm) times (Rick et al. 2016). Very large size classes of oysters were absent from modern samples (cf. Pleistocene) probably as a result of overharvesting and the introduction and spread of oyster diseases (in 1949, Rick and Lockwood 2013). In the mere 400 years since European settlement of Chesapeake Bay, anthropogenic changes (widespread use of dredges since 1870, clearing of >50% of the forested catchment area for agriculture by the mid-1800s, fertilisers rapidly increasing sedimentation rates) accelerated the destruction of the oyster grounds to a point where they are now functionally extinct (Zu Ermgassen et al. 2012). Yet, for thousands of years, oysters had functioned as a key regulator of water quality, as a vital component of food webs, stabilised benthic and intertidal habitats and provided habitat for other species (Meadows et al. 2012; Nyström et al. 2012; Zu Ermgassen et al. 2012; Rick and Lockwood 2013).
Oyster reef degradation fundamentally changes ecosystem function through the loss of filtering capacity (Kirby 2004; Alleway and Connell 2015). For example, in Chesapeake Bay, oysters may have been able to filter the entire volume of the Bay every 3 days (Jackson et al. 2001; Rick et al. 2016). In both Chesapeake Bay and Saint Vincent Gulf, very significant ecosystem function was lost prior to later industrialisation and clearing for agriculture (Table 2, Colman et al. 2002; Alleway and Connell 2015). Loss of function of this magnitude would have profoundly changed these ecosystems, even without the concomitant loss of habitat for other species provided by shellfish beds and the biomass of oyster prey for predators. Although these losses occurred prior to modern marine science, they are likely to have significantly degraded embayment ecosystems.
Eutrophication and decreased water quality
Chesapeake Bay also suffers from eutrophication. Patterns of eutrophication were evident during early European colonisation in both the 17th and 18th centuries, demonstrated by increased sedimentation rates, total organic carbon and biogenic silica (Table 2, Kemp et al. 2005). However, the greatest changes to sediments have occurred within the past 50 years; intense and recurrent seasonal depletion of oxygen is unique to this time period (Allen et al. 2006; Zhang et al. 2017). Seasonal deoxygenation is associated with increased urbanisation and exponential population growth around Chesapeake Bay (Table 2), contributing increased nutrient loads (Kemp et al. 2005; Rick and Lockwood 2013). However, long-term evidence shows that when overfishing caused the loss of oysters and their filtering function, phytoplankton production replaced primary production by seagrasses and benthic diatoms. Later the ecosystem shifted again to be driven by microbial decomposition with episodes of hypoxia or anoxia (Jackson et al. 2001; deYoung et al. 2008). Evidently, lengthy time lags (decades to centuries) may occur between overfishing and population collapse. Time lags may occur because physiological tolerances of ecosystem engineers are not exceeded until a long time after overfishing occurs. Alternatively, density-dependent effects on population fertility that are not immediately obvious (i.e. extinction debt in long-lived species) can cause time lags (Jackson 2001; Scheffer and Carpenter 2003). Although many populations of shellfish and other ecosystem engineers in highly urbanised and industrialised marine embayments around the world are presently considered highly productive, they may in fact be on the brink of collapse because of overfishing (in preceding decades or centuries) combined with the delayed threshold effects associated with nutrient enrichment (Jackson 2001; Jackson et al. 2001).
Although the strong relationship between nutrient enrichment and harmful algal blooms (HABs) has long been established in freshwater ecosystems (Granéli and Turner 2006; Schindler and Vallentyne 2008), the link between anthropogenic eutrophication, HABs and nuisance macroalgal growth in coastal marine systems has gained global recognition only within recent decades (e.g. Lavery et al. 1991; Allen et al. 2006; Heisler et al. 2008). Nutrient enrichment of coastal waters results from agricultural and industrial activities supporting increased human population growth. Expansion of coastal settlements in the early 19th century significantly increased waste discharge, both directly and indirectly (through river discharge), into many coastal embayments around the world (e.g. Jiaozhou Bay (China), Wadden Sea (Netherlands), Saint Vincent Gulf (Australia), Chesapeake Bay and the Gulf of California (USA) (Table 2; Lotze 2005; Sáenz-Arroyo et al. 2005; Granéli and Turner 2006; Wang and Wang 2011). Nutrient enrichment directly stimulates algal growth, which may initially benefit ecosystems through increased primary production. However, algal blooms along coastlines have occurred with increasing frequency, lasted longer, occupied larger geographic extents and comprised more toxic species than previous decades, a pattern that is becoming accepted as typical of these environments (Heisler et al. 2008). In coastal waters, HABs may produce toxins that deter grazing, or their biomass can overwhelm grazing capacity. These toxins may kill fish and poison seafood, causing problems for human health. HABs may also cause nocturnally low dissolved oxygen concentrations, and decomposing blooms may reduce oxygen concentrations (through microbial activity) to such a degree that there is widespread mortality of marine life, regardless of whether toxins are produced (Allen et al. 2006; Heisler et al. 2008; Van der Merwe and Price 2015). HABs may also foul beaches, cause contact dermatitis in humans, and alter trophic interactions in food webs. The impacts of eutrophication may therefore proliferate through coastal ecosystems via direct and indirect pathways, causing alterations in food-web and ecosystem dynamics.
The economic impact of HABs can be substantial, including losses to fisheries, tourism, human amenity and through the costs of environmental monitoring and health care (Allen et al. 2006; Granéli and Turner 2006; Worm et al. 2006). They have prompted many governments to spend considerable resources to reduce nutrient loads and restore ecosystem health. Unfortunately, although the problem may simply appear to be caused by discharge of anthropogenic wastes into embayments, ceasing nutrient-rich discharge into coastal waters does not solve the HAB problem (Shen 2001; Dai et al. 2007). For example, despite recent steps taken to ameliorate heavy metal pollution and eutrophication in Jiaozhou Bay (China, Table 2), including restoration of adjacent rivers, coastline protection and moving industry out of the Bay area, water quality has continued to deteriorate (Zhang et al. 2017). Dissolved inorganic nitrogen (DIN) was the dominant pollutant (Shen 2001; Wang and Wang 2011), so management efforts were focussed on nutrient reduction in the catchment. Over 50 years, a synergistic effect of terrestrial nutrient inputs and changed hydrodynamics (caused by land reclamation) transformed DIN processes in the Bay. The role of factors such as altered hydrodynamics, were fully understood only when long-term data were used (Zhang et al. 2017). Historical investigations to understand processes leading to eutrophication are therefore extremely important. They may identify nutrient thresholds associated with significant ecosystem changes, which in turn may lead to improved management targets. For example, the loss of both significant filter-feeding functions and large grazing animals from embayments prior to the commencement of excessive nutrient discharge reduces the capacity of ecosystems to absorb the additional nutrients (and subsequent productivity), without significant changes to ecosystem composition, structure and function.
Summary of predictable processes of historic degradation in semi-enclosed coastal embayments
The literature shows a characteristic historical sequence of degradation in semi-enclosed coastal embayments during the modern era (∼400 years ago). It commenced with the sustainable use of ecosystem resources prior to European colonisation. During the early stage of exploration, there were plentiful species present that were easily caught, provided a substantial monetary gain, and were thus overfished leading to the loss of large marine fauna. Then, colonisation of coastal embayments led to the exploitation of species closer to the shore, such as shellfish and fish, either directly (shellfish) or indirectly (dredging, trawling) removing ecosystem engineers (e.g. seagrass, kelps, oyster beds, mangroves or corals). Simultaneously, habitat losses occurred in the surrounding catchment (building and farming practices), increasing run-off that delivered sediment and nutrients into bays. This resulted in profound change to ecosystem structure and function. Ecosystem change may then have stabilised until improved technology allowed vessels to travel greater distances and fish for longer periods of time. Fisheries that were once inaccessible provided new, plentiful fisheries resources (e.g. herring, salmon, shad), resulting in further species depletions and losses. In time, industries (especially those with overseas markets) were established along the coastline and urban areas grew to service industry and as part of a healthy coastal lifestyle (where boating, swimming and fishing were at your doorstep) with plentiful protein (fish, shellfish) free for the taking. Next, there may have been a time delay before further degradation occurred due to hydrological changes and seabed disturbance. In turn, those impacts cause further losses of ecosystem resilience. From this point onward, a comparatively smaller impact could lead to foodweb cascades and collapse, because the ecosystem has lost so much resilience through loss of species redundancy (e.g. Caribbean example). Thus, when managers use as a baseline data collected within the past 50 years, it may represent an ecosystem already heavily compromised. This limited resilience can then result in rapid degradation of an ecosystem once multiple new stressors (including global warming) are imposed.
Conclusions
-
Degrading anthropogenic impacts on semi-enclosed coastal embayments have been occurring over century scales, an exponential surge in exploitation and extraction is associated with European colonisation and industrial development (∼400 years). Researchers need to consider changes that occurred long before the age of scientific data to understand the degradation of coastal embayments.
-
The degradation of ecosystems in semi-enclosed coastal embayments by human overexploitation and pollution appears likely to have resulted in a consistent series of temporal changes, worldwide. This has commenced with the loss of large animals and ended with simplified and degraded ecosystems with low resilience to further impacts.
-
A wide range of potential sources and tools are listed in this Review (Table 1) but when researching a particular embayment, not all of these will be available or applicable because different sources or tools provide different types of information. Which types of information are sought by researchers will depend on the specific goals of the study, the individual characteristics of the embayment and its environmental history, and the records available for that location. Consequently, it is inappropriate for us to advocate any particular tools over others, as the most appropriate will be contingent on these factors.
-
The list of environmental problems given in Table 2 could be used to identify potential missing elements (e.g. marine megafauna, shellfish) or processes (e.g. water filtering by extensive shellfish beds) or damaging processes in any particular contemporary embayment to help define the historical extent of environmental change. In some cases, species key to ecosystem function in the past may have been completely lost, fundamentally changing the ecosystem, thereby potentially changing both restoration goals and what may be possible for restoration to achieve. Table 1 can then be used to identify potential sources of evidence of past ecosystem conditions that may be applicable to any particular coastal embayment and which may provide evidence of the past existence of potentially missing elements derived from Table 2.
-
A more complete understanding of environmental change enables managers and communities to understand contemporary resilience and integrity of coastal embayment ecosystems and then develop successful strategies to improve their biodiversity and ecosystem function.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This work was supported by Murdoch University and forms part of the PhD research of Y. M. Pedretti. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
Airoldi, L, Balata, D, and Beck, MW (2008). The gray zone: relationships between habitat loss and marine diversity and their applications in conservation. Journal of Experimental Marine Biology and Ecology 366, 8–15.| The gray zone: relationships between habitat loss and marine diversity and their applications in conservation.Crossref | GoogleScholarGoogle Scholar |
Al-Abdulrazzak, D, Naidoo, R, Palomares, MLD, and Pauly, D (2012). Gaining perspective on what we’ve lost: the reliability of encoded anecdotes in historical ecology. PLoS ONE 7, e43386.
| Gaining perspective on what we’ve lost: the reliability of encoded anecdotes in historical ecology.Crossref | GoogleScholarGoogle Scholar |
Alexander, KE, Leavenworth, WB, Cournane, J, Cooper, AB, Claesson, S, Brennan, S, Smith, G, Rains, L, Magness, K, Dunn, R, Law, TK, Gee, R, Bolster, WJ, and Rosenberg, AA (2009). Gulf of Maine cod in 1861: historical analysis of fishery logbooks, with ecosystem implications. Fish and Fisheries 10, 428–449.
| Gulf of Maine cod in 1861: historical analysis of fishery logbooks, with ecosystem implications.Crossref | GoogleScholarGoogle Scholar |
Allen JI, Anderson D, Burford M, Dyhrman S, Flynn K, Glibert PM, Granéli E, Heil C, Sellner K, Smayda T, Zhou M (2006) Global ecology & oceanography of harmful algal blooms: HABs in eutrophic systems. (Ed. PM Glibert) (SCOR & IOC) Available at https://www.whoi.edu/cms/files/GEOHAB_eutrophic_23003_23056.pdf
Alleway, HK, and Connell, SD (2015). Loss of an ecological baseline through the eradication of oyster reefs from coastal ecosystems and human memory. Conservation Biology 29, 795–804.
| Loss of an ecological baseline through the eradication of oyster reefs from coastal ecosystems and human memory.Crossref | GoogleScholarGoogle Scholar |
Alleway, HK, Connell, SD, Ward, TM, and Gillanders, BM (2014). Historical changes in mean trophic level of southern Australian fisheries. Marine and Freshwater Research 65, 884–893.
| Historical changes in mean trophic level of southern Australian fisheries.Crossref | GoogleScholarGoogle Scholar |
Ames, EP (2004). Atlantic cod stock structure in the Gulf of Maine. Fisheries 29, 10–28.
| Atlantic cod stock structure in the Gulf of Maine.Crossref | GoogleScholarGoogle Scholar |
Anderson, DM, Keafer, BA, Kleindinst, JL, McGillicuddy, DJ, Martin, JL, Norton, K, Pilskaln, CH, Smith, JL, Sherwood, CR, and Butman, B (2014). Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms. Deep-Sea Research – II. Topical Studies in Oceanography 103, 6–26.
| Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms.Crossref | GoogleScholarGoogle Scholar |
Arias-Ortiz, A, Serrano, O, Masqué, P, Lavery, PS, Mueller, U, Kendrick, GA, Rozaimi, M, Esteban, A, Fourqurean, JW, Marbà, N, Mateo, MA, Murray, K, Rule, MJ, and Duarte, CM (2018). A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nature Climate Change 8, 338–344.
| A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks.Crossref | GoogleScholarGoogle Scholar |
Auster, PJ, Joy, K, and Valentine, PC (2001). Fish species and community distributions as proxies for seafloor habitat distributions: the Stellwagen Bank National Marine Sanctuary example (Northwest Atlantic, Gulf of Maine). Environmental Biology of Fishes 60, 331–346.
| Fish species and community distributions as proxies for seafloor habitat distributions: the Stellwagen Bank National Marine Sanctuary example (Northwest Atlantic, Gulf of Maine).Crossref | GoogleScholarGoogle Scholar |
Bak CV (2020) ‘Threats to North Atlantic right whales and killer whales.’ (Nova Snova: New York, NY, USA). Available at https://go.exlibris.link/KPhkCLC0
Baumgartner, TR, Soutar, A, and Ferreira Bartrina, V (1992). Reconstruction of the history of Pacific sardine and northern anchovy populations over the past 2 millennia from sediments of the Santa-Barbara Basin, California. California Cooperative Oceanic Fisheries Investigations Reports 33, 24–40.
Bejder, M, Johnston, DW, Smith, J, Friedlaender, A, and Bejder, L (2016). Embracing conservation success of recovering humpback whale populations: evaluating the case for downlisting their conservation status in Australia. Marine Policy 66, 137–141.
| Embracing conservation success of recovering humpback whale populations: evaluating the case for downlisting their conservation status in Australia.Crossref | GoogleScholarGoogle Scholar |
Berman, J, Harris, L, Lambert, W, Buttrick, M, and Dufresne, M (1992). Recent invasions of the Gulf of Maine: three contrasting ecological histories. Conservation Biology 6, 435–441.
| Recent invasions of the Gulf of Maine: three contrasting ecological histories.Crossref | GoogleScholarGoogle Scholar |
Bolster, WJ (2008). Putting the ocean in Atlantic history: maritime communities and marine ecology in the Northwest Atlantic, 1500–1800. The American Historical Review 113, 19–47.
| Putting the ocean in Atlantic history: maritime communities and marine ecology in the Northwest Atlantic, 1500–1800.Crossref | GoogleScholarGoogle Scholar |
Bolster WJ (2012) ‘The mortal sea: fishing the Atlantic in the age of sail.’ (Harvard University Press: Cambridge, MA, USA)
| Crossref |
Braganza, K, Gergis, JL, Power, SB, Risbey, JS, and Fowler, AM (2009). A multiproxy index of the El Niño–Southern Oscillation, A.D. 1525–1982. Journal of Geophysical Research: Atmospheres 114, D05106.
| A multiproxy index of the El Niño–Southern Oscillation, A.D. 1525–1982.Crossref | GoogleScholarGoogle Scholar |
Brierley, AS, and Kingsford, MJ (2009). Impacts of climate change on marine organisms and ecosystems. Current Biology 19, R602–R614.
| Impacts of climate change on marine organisms and ecosystems.Crossref | GoogleScholarGoogle Scholar |
Buchsbaum R, Pederson J, Robinson WE (2005) ‘The decline of fisheries resources in New England: evaluating the impact of overfishing, contamination, and habitat degradation.’ (MIT Sea Grant College Program: Cambridge, MA, USA)
Burkepile, DE, and Hay, ME (2006). Herbivore vs. nutrient control of marine primary producers: context-dependent effects. Ecology 87, 3128–3139.
| Herbivore vs. nutrient control of marine primary producers: context-dependent effects.Crossref | GoogleScholarGoogle Scholar |
Christensen J (2008) Shark Bay 1616–1991: the spread of science and the emergence of ecology in a World Heritage Area. PhD thesis, University of Western Australia, Perth, WA, Australia. Available at https://research-repository.uwa.edu.au/en/publications/shark-bay-1616-1991-the-spread-of-science-and-the-emergence-of-ec
Colman, SM, Baucom, PC, Bratton, JF, Cronin, TM, McGeehin, JP, Willard, D, Zimmerman, AR, and Vogt, PR (2002). Radiocarbon dating, chronologic framework, and changes in accumulation rates of holocene estuarine sediments from Chesapeake Bay. Quaternary Research 57, 58–70.
| Radiocarbon dating, chronologic framework, and changes in accumulation rates of holocene estuarine sediments from Chesapeake Bay.Crossref | GoogleScholarGoogle Scholar |
Craig, LA, and Holt, MT (2017). The impact of mechanical refrigeration on market integration: the US egg market, 1890–1911. Explorations in Economic History 66, 85–105.
| The impact of mechanical refrigeration on market integration: the US egg market, 1890–1911.Crossref | GoogleScholarGoogle Scholar |
Cramer, KL, Leonard-Pingel, JS, Rodríguez, F, and Jackson, JBC (2015). Molluscan subfossil assemblages reveal the long-term deterioration of coral reef environments in Caribbean Panama. Marine Pollution Bulletin 96, 176–187.
| Molluscan subfossil assemblages reveal the long-term deterioration of coral reef environments in Caribbean Panama.Crossref | GoogleScholarGoogle Scholar |
Cuttriss, AK, Prince, JB, and Castley, JG (2013). Seagrass communities in southern Moreton Bay, Australia: coverage and fragmentation trends between 1987 and 2005. Aquatic Botany 108, 41–47.
| Seagrass communities in southern Moreton Bay, Australia: coverage and fragmentation trends between 1987 and 2005.Crossref | GoogleScholarGoogle Scholar |
Dai, J, Song, J, Li, X, Yuan, H, Li, N, and Zheng, G (2007). Environmental changes reflected by sedimentary geochemistry in recent hundred years of Jiaozhou Bay, North China. Environmental Pollution 145, 656–667.
| Environmental changes reflected by sedimentary geochemistry in recent hundred years of Jiaozhou Bay, North China.Crossref | GoogleScholarGoogle Scholar |
Debus L (1996) The decline of the European sturgeon Acipenser sturio in the Baltic and North Sea. In ‘Conservation of endangered freshwater fish in Europe’. (Eds A Kirchhofer, D Hefti) pp. 147–156. (Berkhäuser Verlag: Basel, Switzerland)
| Crossref |
deYoung, B, Barange, M, Beaugrand, G, Harris, R, Perry, RI, Scheffer, M, and Werner, F (2008). Regime shifts in marine ecosystems: detection, prediction and management. Trends in Ecology & Evolution 23, 402–409.
| Regime shifts in marine ecosystems: detection, prediction and management.Crossref | GoogleScholarGoogle Scholar |
Diggles, BK (2013). Historical epidemiology indicates water quality decline drives loss of oyster (Saccostrea glomerata) reefs in Moreton Bay, Australia. New Zealand Journal of Marine and Freshwater Research 47, 561–581.
| Historical epidemiology indicates water quality decline drives loss of oyster (Saccostrea glomerata) reefs in Moreton Bay, Australia.Crossref | GoogleScholarGoogle Scholar |
Early-Capistrán, M-M, Sáenz-Arroyo, A, Cardoso-Mohedano, J-G, Garibay-Melo, G, Peckham, SH, and Koch, V (2018). Reconstructing 290 years of a data-poor fishery through ethnographic and archival research: the East Pacific green turtle (Chelonia mydas) in Baja California, Mexico. Fish and Fisheries 19, 57–77.
| Reconstructing 290 years of a data-poor fishery through ethnographic and archival research: the East Pacific green turtle (Chelonia mydas) in Baja California, Mexico.Crossref | GoogleScholarGoogle Scholar |
Enfield, DB, Mestas-Nuñez, AM, and Trimble, PJ (2001). The Atlantic Multidecadal Oscillation and its relation to rainfall and river flows in the continental US. Geophysical Research Letters 28, 2077–2080.
| The Atlantic Multidecadal Oscillation and its relation to rainfall and river flows in the continental US.Crossref | GoogleScholarGoogle Scholar |
Engelhard, GH, Thurstan, RH, MacKenzie, BR, Alleway, HK, Bannister, RCA, Cardinale, M, Clarke, MW, Currie, JC, Fortibuoni, T, Holm, P, Holt, SJ, Mazzoldi, C, Pinnegar, JK, Raicevich, S, Volckaert, FAM, Klein, ES, and Lescrauwaet, A-K (2016). ICES meets marine historical ecology: placing the history of fish and fisheries in current policy context. ICES Journal of Marine Science 73, 1386–1403.
| ICES meets marine historical ecology: placing the history of fish and fisheries in current policy context.Crossref | GoogleScholarGoogle Scholar |
Eriksson, BK, van der Heide, T, van de Koppel, J, Piersma, T, van der Veer, HW, and Olff, H (2010). Major changes in the ecology of the Wadden Sea: human impacts, ecosystem engineering and sediment dynamics. Ecosystems 13, 752–764.
| Major changes in the ecology of the Wadden Sea: human impacts, ecosystem engineering and sediment dynamics.Crossref | GoogleScholarGoogle Scholar |
Erlandson, JM, and Rick, TC (2010). Archaeology meets marine ecology: the antiquity of maritime cultures and human impacts on marine fisheries and ecosystems. Annual Review of Marine Science 2, 231–251.
| Archaeology meets marine ecology: the antiquity of maritime cultures and human impacts on marine fisheries and ecosystems.Crossref | GoogleScholarGoogle Scholar |
Fitzpatrick, SM, and Keegan, WF (2007). Human impacts and adaptations in the Caribbean Islands: an historical ecology approach. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 98, 29–45.
| Human impacts and adaptations in the Caribbean Islands: an historical ecology approach.Crossref | GoogleScholarGoogle Scholar |
Fitzpatrick, SM, Rick, TC, and Erlandson, JM (2015). Recent progress, trends, and developments in island and coastal archaeology. The Journal of Island and Coastal Archaeology 10, 3–27.
| Recent progress, trends, and developments in island and coastal archaeology.Crossref | GoogleScholarGoogle Scholar |
Goode, A (2006). The plight and outlook for migratory fish in the Gulf of Maine. Journal of Contemporary Water Research & Education 134, 23–28.
| The plight and outlook for migratory fish in the Gulf of Maine.Crossref | GoogleScholarGoogle Scholar |
Granéli E, Turner JT (2006) ‘Ecology of harmful algae.’ (Springer: Berlin, Germany)
Hall, CJ, Jordaan, A, and Frisk, MG (2011). The historic influence of dams on diadromous fish habitat with a focus on river herring and hydrologic longitudinal connectivity. Landscape Ecology 26, 95–107.
| The historic influence of dams on diadromous fish habitat with a focus on river herring and hydrologic longitudinal connectivity.Crossref | GoogleScholarGoogle Scholar |
Hall, CJ, Jordaan, A, and Frisk, MG (2012). Centuries of anadromous forage fish loss: consequences for ecosystem connectivity and productivity. BioScience 62, 723–731.
| Centuries of anadromous forage fish loss: consequences for ecosystem connectivity and productivity.Crossref | GoogleScholarGoogle Scholar |
Hancock DA (1989) ‘A review of the shark bay pearling industry.’ (Fisheries Department of Western Australia: Perth, WA, Australia). Available at https://prospero.murdoch.edu.au/record=b1191597
Hardin, G (1968). The tragedy of the commons. Science 162, 1243–1248.
| The tragedy of the commons.Crossref | GoogleScholarGoogle Scholar |
Hay, ME (1984). Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical? Ecology 65, 446–454.
| Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical?Crossref | GoogleScholarGoogle Scholar |
Heisler, J, Glibert, PM, Burkholder, JM, Anderson, DM, Cochlan, W, Dennison, WC, Dortch, Q, Gobler, CJ, Heil, CA, Humphries, E, Lewitus, A, Magnien, R, Marshall, HG, Sellner, K, Stockwell, DA, Stoecker, DK, and Suddleson, M (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13.
| Eutrophication and harmful algal blooms: a scientific consensus.Crossref | GoogleScholarGoogle Scholar |
Hughes, TP (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551.
| Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef.Crossref | GoogleScholarGoogle Scholar |
Jackson, JBC (2001). What was natural in the coastal oceans? Proceedings of the National Academy of Sciences 98, 5411–5418.
| What was natural in the coastal oceans?Crossref | GoogleScholarGoogle Scholar |
Jackson, JBC, and Sheldon, PR (1994). Constancy and change of life in the sea. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 344, 55–60.
| Constancy and change of life in the sea.Crossref | GoogleScholarGoogle Scholar |
Jackson, JBC, Kirby, MX, Berger, WH, Bjorndal, KA, Botsford, LW, Bourque, BJ, Bradbury, RH, Cooke, R, Erlandson, J, Estes, JA, Hughes, TP, Kidwell, S, Lange, CB, Lenihan, HS, Pandolfi, JM, Peterson, CH, Steneck, RS, Tegner, MJ, and Warner, RR (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637.
| Historical overfishing and the recent collapse of coastal ecosystems.Crossref | GoogleScholarGoogle Scholar |
Jones, CG, Lawton, JH, and Shachak, M (1994). Organisms as ecosystem engineers. Oikos 69, 373–386.
| Organisms as ecosystem engineers.Crossref | GoogleScholarGoogle Scholar |
Kemp, WM, Boynton, WR, Adolf, JE, Boesch, DF, Boicourt, WC, Brush, G, Cornwell, JC, Fisher, TR, Glibert, PM, Hagy, JD, Harding, LW, Houde, ED, Kimmel, DG, Miller, WD, Newell, RIE, Roman, MR, Smith, EM, and Stevenson, JC (2005). Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecology Progress Series 303, 1–29.
| Eutrophication of Chesapeake Bay: historical trends and ecological interactions.Crossref | GoogleScholarGoogle Scholar |
Kirby, MX (2004). Fishing down the coast: historical expansion and collapse of oyster fisheries along continental margins. Proceedings of the National Academy of Sciences 101, 13096–13099.
| Fishing down the coast: historical expansion and collapse of oyster fisheries along continental margins.Crossref | GoogleScholarGoogle Scholar |
Kraeuter, JN, Buckner, S, and Powell, EN (2005). A note on a spawner–recruit relationship for a heavily exploited bivalve: the case of northern quahogs (hard clams), Mercenaria mercenaria in great south bay New York. Journal of Shellfish Research 24, 1043–1052.
| A note on a spawner–recruit relationship for a heavily exploited bivalve: the case of northern quahogs (hard clams), Mercenaria mercenaria in great south bay New York.Crossref | GoogleScholarGoogle Scholar |
Kraeuter, JN, Klinck, JM, Powell, EN, Hofmann, EE, Buckner, SC, Grizzle, RE, and Bricelj, VM (2008). Effects of the fishery on the Northern Quahog (=hard clam, Mercenaria mercenaria L.) population in Great South Bay, New York: a modeling study. Journal of Shellfish Research 27, 653–666.
| Effects of the fishery on the Northern Quahog (=hard clam, Mercenaria mercenaria L.) population in Great South Bay, New York: a modeling study.Crossref | GoogleScholarGoogle Scholar |
Lake, PS, Bond, N, and Reich, P (2007). Linking ecological theory with stream restoration. Freshwater Biology 52, 597–615.
| Linking ecological theory with stream restoration.Crossref | GoogleScholarGoogle Scholar |
Lauer, M, and Aswani, S (2009). Indigenous ecological knowledge as situated practices: understanding fishers’ knowledge in the Western Solomon Islands. American Anthropologist 111, 317–329.
| Indigenous ecological knowledge as situated practices: understanding fishers’ knowledge in the Western Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Lavery, PS, Lukatelich, RJ, and McComb, AJ (1991). Changes in the biomass and species composition of macroalgae in a eutrophic estuary. Estuarine, Coastal and Shelf Science 33, 1–22.
| Changes in the biomass and species composition of macroalgae in a eutrophic estuary.Crossref | GoogleScholarGoogle Scholar |
Lessios, HA (1988). Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annual Review of Ecology and Systematics 19, 371–393.
| Mass mortality of Diadema antillarum in the Caribbean: what have we learned?Crossref | GoogleScholarGoogle Scholar |
Lotze, HK (2005). Radical changes in the Wadden Sea fauna and flora over the last 2,000 years. Helgoland Marine Research 59, 71–83.
| Radical changes in the Wadden Sea fauna and flora over the last 2,000 years.Crossref | GoogleScholarGoogle Scholar |
Lotze, HK, and Worm, B (2009). Historical baselines for large marine animals. Trends in Ecology & Evolution 24, 254–262.
| Historical baselines for large marine animals.Crossref | GoogleScholarGoogle Scholar |
Lotze, HK, Reise, K, Worm, B, van Beusekom, J, Busch, M, Ehlers, A, Heinrich, D, Hoffmann, RC, Holm, P, Jensen, C, Knottnerus, OS, Langhanki, N, Prummel, W, Vollmer, M, and Wolff, WJ (2005). Human transformations of the Wadden Sea ecosystem through time: a synthesis. Helgoland Marine Research 59, 84–95.
| Human transformations of the Wadden Sea ecosystem through time: a synthesis.Crossref | GoogleScholarGoogle Scholar |
Lotze, HK, Lenihan, HS, Bourque, BJ, Bradbury, RH, Cooke, RG, Kay, MC, Kidwell, SM, Kirby, MX, Peterson, CH, and Jackson, JBC (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809.
| Depletion, degradation, and recovery potential of estuaries and coastal seas.Crossref | GoogleScholarGoogle Scholar |
Marsh, H, De’ath, G, Gribble, N, and Lane, B (2005). Historical marine population estimates: triggers or targets for conservation? The dugong case study. Ecological Applications 15, 481–492.
| Historical marine population estimates: triggers or targets for conservation? The dugong case study.Crossref | GoogleScholarGoogle Scholar |
Masel, J, and Smallwood, D (2000). Indications of long term changes in the species composition and catch rates of postlarval and juvenile prawns in Moreton Bay. Proceedings of the Royal Society of Queensland 109, 119–130.
Meadows, PS, Meadows, A, and Murray, JMH (2012). Biological modifiers of marine benthic seascapes: their role as ecosystem engineers. Geomorphology 157–158, 31–48.
| Biological modifiers of marine benthic seascapes: their role as ecosystem engineers.Crossref | GoogleScholarGoogle Scholar |
Monsarrat, S, Pennino, MG, Smith, TD, Reeves, RR, Meynard, CN, Kaplan, DM, and Rodrigues, ASL (2016). A spatially explicit estimate of the prewhaling abundance of the endangered North Atlantic right whale. Conservation Biology 30, 783–791.
| A spatially explicit estimate of the prewhaling abundance of the endangered North Atlantic right whale.Crossref | GoogleScholarGoogle Scholar |
Morelli, G, Gasparon, M, Fierro, D, Hu, W-P, and Zawadzki, A (2012). Historical trends in trace metal and sediment accumulation in intertidal sediments of Moreton Bay, southeast Queensland, Australia. Chemical Geology 300-301, 152–164.
| Historical trends in trace metal and sediment accumulation in intertidal sediments of Moreton Bay, southeast Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Myers, RA, and Worm, B (2005). Extinction, survival or recovery of large predatory fishes. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 360, 13–20.
| Extinction, survival or recovery of large predatory fishes.Crossref | GoogleScholarGoogle Scholar |
Newton JS (2013) ‘A savage history: whaling in the Pacific and Southern oceans.’ (NewSouth Publishing: Sydney, NSW, Australia)
NOAA Fisheries (2017) North Atlantic right whale (Eubalaena glacialis): western Atlantic stock. Marine Mammal Stock Assessment Reports by species/stock. (NOAA Fisheries) Available at https://media.fisheries.noaa.gov/dam-migration/ao2016whnr-w_508.pdf
Nuttall, MA, Jordaan, A, Cerrato, RM, and Frisk, MG (2011). Identifying 120 years of decline in ecosystem structure and maturity of Great South Bay, New York using the Ecopath modelling approach. Ecological Modelling 222, 3335–3345.
| Identifying 120 years of decline in ecosystem structure and maturity of Great South Bay, New York using the Ecopath modelling approach.Crossref | GoogleScholarGoogle Scholar |
Nyström, M, Norström, AV, Blenckner, T, de la Torre-Castro, M, Eklöf, JS, Folke, C, Österblom, H, Steneck, RS, Thyresson, M, and Troell, M (2012). Confronting feedbacks of degraded marine ecosystems. Ecosystems 15, 695–710.
| Confronting feedbacks of degraded marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pauly, D (1995). Anecdotes and the shifting baseline syndrome of fisheries. Trends in Ecology & Evolution 10, 430.
| Anecdotes and the shifting baseline syndrome of fisheries.Crossref | GoogleScholarGoogle Scholar |
Pauly, D, and Palomares, M-L (2005). Fishing down marine food web: it is far more pervasive than we thought. Bulletin of Marine Science 76, 197–211.
Pauly, D, Christensen, V, Dalsgaard, J, Froese, R, and Torres, F (1998). Fishing down marine food webs. Science 279, 860–863.
| Fishing down marine food webs.Crossref | GoogleScholarGoogle Scholar |
Pearce, AF, and Feng, M (2013). The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011. Journal of Marine Systems 111–112, 139–156.
| The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011.Crossref | GoogleScholarGoogle Scholar |
Pershing, AJ, Wahle, RA, Meyers, PC, and Lawton, P (2012). Large-scale coherence in New England lobster (Homarus americanus), settlement and associations with regional atmospheric conditions. Fisheries Oceanography 21, 348–362.
| Large-scale coherence in New England lobster (Homarus americanus), settlement and associations with regional atmospheric conditions.Crossref | GoogleScholarGoogle Scholar |
Pinnegar, JK, and Engelhard, GH (2008). The ‘shifting baseline’ phenomenon: a global perspective. Reviews in Fish Biology and Fisheries 18, 1–16.
| The ‘shifting baseline’ phenomenon: a global perspective.Crossref | GoogleScholarGoogle Scholar |
Pitcher, TJ (2001). Fisheries managed to rebuild ecosystems? Reconstructing the past to salvage the future. Ecological Applications 11, 601–617.
| Fisheries managed to rebuild ecosystems? Reconstructing the past to salvage the future.Crossref | GoogleScholarGoogle Scholar |
Poulsen, B (2010). The variability of fisheries and fish populations prior to industrialized fishing: an appraisal of the historical evidence. Journal of Marine Systems 79, 327–332.
| The variability of fisheries and fish populations prior to industrialized fishing: an appraisal of the historical evidence.Crossref | GoogleScholarGoogle Scholar |
Reynolds, JD, Dulvy, NK, Goodwin, NB, and Hutchings, JA (2005). Biology of extinction risk in marine fishes. Proceedings of the Royal Society of London – B. Biological Sciences 272, 2337–2344.
| Biology of extinction risk in marine fishes.Crossref | GoogleScholarGoogle Scholar |
Rick, TC, and Lockwood, R (2013). Integrating paleobiology, archeology, and history to inform biological conservation. Conservation Biology 27, 45–54.
| Integrating paleobiology, archeology, and history to inform biological conservation.Crossref | GoogleScholarGoogle Scholar |
Rick, TC, Reeder-Myers, LA, Hofman, CA, Breitburg, D, Lockwood, R, Henkes, G, Kellogg, L, Lowery, D, Luckenbach, MW, Mann, R, Ogburn, MB, Southworth, M, Wah, J, Wesson, J, and Hines, AH (2016). Millennial-scale sustainability of the Chesapeake Bay Native American oyster fishery. Proceedings of the National Academy of Sciences 113, 6568–6573.
| Millennial-scale sustainability of the Chesapeake Bay Native American oyster fishery.Crossref | GoogleScholarGoogle Scholar |
Roberts CM (2007) ‘The unnatural history of the sea.’ (Island Press/Shearwater Books: Washington, DC, USA)
| Crossref |
Rodrique JP, Comtois C, Slack B (2017) ‘The geography of transport systems.’ (Routledge: New York, NY, USA) Available at https://people.hofstra.edu/geotrans/index.html
Roman, J, and Palumbi, SR (2003). Whales before whaling in the North Atlantic. Science 301, 508–510.
| Whales before whaling in the North Atlantic.Crossref | GoogleScholarGoogle Scholar |
Rönnbäck, K (2012). The speed of ships and shipping productivity in the age of sail. European Review of Economic History 16, 469–489.
| The speed of ships and shipping productivity in the age of sail.Crossref | GoogleScholarGoogle Scholar |
Rosenberg, AA, Bolster, WJ, Alexander, KE, Leavenworth, WB, Cooper, AB, and McKenzie, MG (2005). The history of ocean resources: modeling cod biomass using historical records. Frontiers in Ecology and the Environment 3, 78–84.
| The history of ocean resources: modeling cod biomass using historical records.Crossref | GoogleScholarGoogle Scholar |
Sáenz-Arroyo, A, Roberts, CM, Torre, J, and Cariño-Olvera, M (2005). Using fishers’ anecdotes, naturalists’ observations and grey literature to reassess marine species at risk: the case of the Gulf grouper in the Gulf of California, Mexico. Fish and Fisheries 6, 121–133.
| Using fishers’ anecdotes, naturalists’ observations and grey literature to reassess marine species at risk: the case of the Gulf grouper in the Gulf of California, Mexico.Crossref | GoogleScholarGoogle Scholar |
Sáenz-Arroyo, A, Roberts, CM, Torre, J, Cariño-Olvera, M, and Hawkins, JP (2006). The value of evidence about past abundance: marine fauna of the Gulf of California through the eyes of 16th to 19th century travellers. Fish and Fisheries 7, 128–146.
| The value of evidence about past abundance: marine fauna of the Gulf of California through the eyes of 16th to 19th century travellers.Crossref | GoogleScholarGoogle Scholar |
Scheffer, M, and Carpenter, SR (2003). Catastrophic regime shifts in ecosystems: linking theory to observation. Trends in Ecology & Evolution 18, 648–656.
| Catastrophic regime shifts in ecosystems: linking theory to observation.Crossref | GoogleScholarGoogle Scholar |
Schindler DW, Vallentyne JR (2008) ‘The algal bowl: overfertilization of the world’s freshwaters and estuaries.’ (Earthscan: London, UK)
Schwerdtner Máñez, K, Holm, P, Blight, L, Coll, M, MacDiarmid, A, Ojaveer, H, Poulsen, B, and Tull, M (2014). The future of the oceans past: towards a global marine historical research initiative. PLoS ONE 9, e101466.
| The future of the oceans past: towards a global marine historical research initiative.Crossref | GoogleScholarGoogle Scholar |
Self-Trail, JM, Robinson, MM, Bralower, TJ, Sessa, JA, Hajek, EA, Kump, LR, Trampush, SM, Willard, DA, Edwards, LE, Powars, DS, and Wandless, GA (2017). Shallow marine response to global climate change during the Paleocene–Eocene Thermal Maximum, Salisbury Embayment, USA. Paleoceanography 32, 710–728.
| Shallow marine response to global climate change during the Paleocene–Eocene Thermal Maximum, Salisbury Embayment, USA.Crossref | GoogleScholarGoogle Scholar |
Shen, Z-L (2001). historical changes in nutrient structure and its influences on phytoplantkon composition in Jiaozhou Bay. Estuarine, Coastal and Shelf Science 52, 211–224.
| historical changes in nutrient structure and its influences on phytoplantkon composition in Jiaozhou Bay.Crossref | GoogleScholarGoogle Scholar |
Sommer R (2004) Historical change of wetland communities of Moreton Bay: an assessment of human and climatic influences. BMarSci(Hons) thesis, University of Queensland, Brisbane, Qld, Australia.
Srinivasan, UT, Watson, R, and Rashid Sumaila, U (2012). Global fisheries losses at the exclusive economic zone level, 1950 to present. Marine Policy 36, 544–549.
| Global fisheries losses at the exclusive economic zone level, 1950 to present.Crossref | GoogleScholarGoogle Scholar |
Steffen W, Hughes L (2013) The critical decade 2013: climate change science, risks and response. Climate Commission Secretariat, Department of Industry, Innovation, Climate Change, Science, Research and Tertiary Education, Australia.
Swetnam, TW, Allen, CD, and Betancourt, JL (1999). Applied historical ecology: using the past to manage for the future. Ecological Applications 9, 1189–1206.
| Applied historical ecology: using the past to manage for the future.Crossref | GoogleScholarGoogle Scholar |
Szabó, P (2015). Historical ecology: past, present and future. Biological Reviews 90, 997–1014.
| Historical ecology: past, present and future.Crossref | GoogleScholarGoogle Scholar |
Tett, P, Gowen, R, Mills, D, Fernandes, T, Gilpin, L, Huxham, M, Kennington, K, Read, P, Service, M, Wilkinson, M, and Malcolm, S (2007). Defining and detecting undesirable disturbance in the context of marine eutrophication. Marine Pollution Bulletin 55, 282–297.
| Defining and detecting undesirable disturbance in the context of marine eutrophication.Crossref | GoogleScholarGoogle Scholar |
The British Museum (2017) The industrial revolution and the changing face of Britain: shipbuilding and maritime trade. (The British Museum, UK) Available at https://www.britishmuseum.org/research/publications/online
Thompson C (2010) Gulf of Maine in Context: State of the Gulf of Maine Report, Gulf of Maine Council on the Marine Environment and Fisheries and Ocean Canada.
Thomson, JA, Burkholder, DA, Heithaus, MR, Fourqurean, JW, Fraser, MW, Statton, J, and Kendrick, GA (2015). Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Global Change Biology 21, 1463–1474.
| Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem.Crossref | GoogleScholarGoogle Scholar |
US National Marine Fisheries Service (2006) Review of the status of the right whales in the North Atlantic and North Pacific oceans. (NOAA, NMFS) Available at https://repository.library.noaa.gov/view/noaa/16284
van de Koppel J, Tett P, Naqvi SWA, Oguz T, Perillo GME, Rabalais NN, d’Alcalae MR, Su J, Zhang J (2009) Threshold effects in semi-enclosed marine systems. In ‘Watersheds, bays, and bounded seas: the science and management of semi-enclosed marine systems’. (Eds ER Urban, B Sundby, P Malanotte-Rizzoli, JM Melillo) pp. 31–47. (Island Press: Washington, DC, USA) Available at http://drs.nio.org/drs/handle/2264/3504
Van der Merwe, D, and Price, KP (2015). Harmful algal bloom characterization at ultra-high spatial and temporal resolution using small unmanned aircraft systems. Toxins 7, 1065–1078.
| Harmful algal bloom characterization at ultra-high spatial and temporal resolution using small unmanned aircraft systems.Crossref | GoogleScholarGoogle Scholar |
van der Veer, HW, Dapper, R, Henderson, PA, Jung, AS, Philippart, CJM, Witte, JIJ, and Zuur, AF (2015). Changes over 50 years in fish fauna of a temperate coastal sea: degradation of trophic structure and nursery function. Estuarine, Coastal and Shelf Science 155, 156–166.
| Changes over 50 years in fish fauna of a temperate coastal sea: degradation of trophic structure and nursery function.Crossref | GoogleScholarGoogle Scholar |
Walker, DI, Kendrick, GA, and McComb, AJ (1988). The distribution of seagrass species in Shark Bay, Western Australia, with notes on their ecology. Aquatic Botany 30, 305–317.
| The distribution of seagrass species in Shark Bay, Western Australia, with notes on their ecology.Crossref | GoogleScholarGoogle Scholar |
Wang, B, and Wang, Z (2011). Long-term variations in chlorophyll-a and primary productivity in Jiaozhou Bay, China. Journal of Marine Biology 2011, 1–7.
| Long-term variations in chlorophyll-a and primary productivity in Jiaozhou Bay, China.Crossref | GoogleScholarGoogle Scholar |
Watson, EB, and Byrne, R (2009). Abundance and diversity of tidal marsh plants along the salinity gradient of the San Francisco Estuary: implications for global change ecology. Plant Ecology 205, 113–128.
| Abundance and diversity of tidal marsh plants along the salinity gradient of the San Francisco Estuary: implications for global change ecology.Crossref | GoogleScholarGoogle Scholar |
Worm, B, Barbier, EB, Beaumont, N, Duffy, JE, Folke, C, Halpern, BS, Jackson, JBC, Lotze, HK, Micheli, F, Palumbi, SR, Sala, E, Selkoe, KA, Stachowicz, JJ, and Watson, R (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790.
| Impacts of biodiversity loss on ocean ecosystem services.Crossref | GoogleScholarGoogle Scholar |
Zhang, P, Su, Y, Liang, S-K, Li, K-Q, Li, Y-B, and Wang, X-L (2017). Assessment of long-term water quality variation affected by high-intensity land-based inputs and land reclamation in Jiaozhou Bay, China. Ecological Indicators 75, 210–219.
| Assessment of long-term water quality variation affected by high-intensity land-based inputs and land reclamation in Jiaozhou Bay, China.Crossref | GoogleScholarGoogle Scholar |
Zu Ermgassen, PSE, Spalding, MD, Blake, B, Coen, LD, Dumbauld, B, Geiger, S, Grabowski, JH, Grizzle, R, Luckenbach, M, McGraw, K, Rodney, W, Ruesink, JL, Powers, SP, and Brumbaugh, R (2012). Historical ecology with real numbers: past and present extent and biomass of an imperilled estuarine habitat. Proceedings of the Royal Society of London – B. Biological Sciences 279, 3393–3400.
| Historical ecology with real numbers: past and present extent and biomass of an imperilled estuarine habitat.Crossref | GoogleScholarGoogle Scholar |