Distribution and size of lipid droplets in oocytes recovered from young lamb and adult ovine ovaries
Amirhossein Abazarikia A B D , Federica Ariu B , Mahsa Rasekhi C , Mahdi Zhandi
A B D , Federica Ariu B , Mahsa Rasekhi C , Mahdi Zhandi  A and Sergio Ledda B
A and Sergio Ledda B
A Department of Animal Science, College of Agriculture and Natural Resources, University of Tehran, Karaj 31587-77871, Iran.
B Department of Veterinary Medicine, Section of Obstetrics and Gynecology, University of Sassari, Sassari, Italy.
C Department of Animal and Marine Biotechnology, National Institute of Genetic Engineering and Biotechnology, Tehran 1497716316, Iran.
D Corresponding author. Email: abazarikia1984@gmail.com
Reproduction, Fertility and Development 32(11) 1022-1026 https://doi.org/10.1071/RD20035
Submitted: 2 February 2020 Accepted: 2 June 2020 Published: 7 July 2020
Abstract
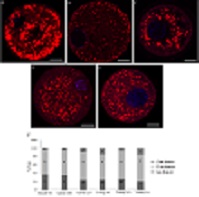
This study evaluated the distribution and size of lipid droplets (LDs) in oocytes recovered from young and adult ovine ovaries. Collected oocytes were categorised on the basis of their major diameter (small (SO), 70–90 µm; medium (MO), >90–110 µm; large (LO), >110–130 µm) and were stained with Nile red to detect LDs. In adult and young oocytes, a diffuse pattern distribution of LDs was dominant in all classes except adult LO and young SO and LO. Larger LDs (i.e. >3 µm) were mostly present in young SO and LO, whereas smaller LDs (1–3 µm) were detected in the other adult and young oocyte categories.
Additional keywords: growth, livestock.
References
Annes, K., Müller, D. B., Vilela, J. A., Valente, R. S., Caetano, D. P., Cibin, F. W., Milazzotto, M. P., Mesquita, F. S., Belaz, K. R., and Eberlin, M. N. (2019). Influence of follicle size on bovine oocyte lipid composition, follicular metabolic and stress markers, embryo development and blastocyst lipid content. Reprod. Fertil. Dev. 31, 462–472.| Influence of follicle size on bovine oocyte lipid composition, follicular metabolic and stress markers, embryo development and blastocyst lipid content.Crossref | GoogleScholarGoogle Scholar | 30282571PubMed |
Ariu, F., Strina, A., Murrone, O., Falchi, L., Bebbere, D., Ledda, S., Zedda, M. T., Pau, S., and Bogliolo, L. (2016). Lipid droplet distribution of immature canine oocytes in relation to their size and the reproductive stage. Anim. Sci. J. 87, 147–150.
| Lipid droplet distribution of immature canine oocytes in relation to their size and the reproductive stage.Crossref | GoogleScholarGoogle Scholar | 26419408PubMed |
Brusentsev, E. Y., Mokrousova, V., Igonina, T., Rozhkova, I., and Amstislavsky, S. Y. (2019). Role of lipid droplets in the development of oocytes and preimplantation embryos in mammals. Russ. J. Dev. Biol. 50, 230–237.
| Role of lipid droplets in the development of oocytes and preimplantation embryos in mammals.Crossref | GoogleScholarGoogle Scholar |
Crosier, A. E., Farin, P. W., Dykstra, M. J., Alexander, J. E., and Farin, C. E. (2000). Ultrastructural morphometry of bovine compact morulae produced in vivo or in vitro. Biol. Reprod. 62, 1459–1465.
| Ultrastructural morphometry of bovine compact morulae produced in vivo or in vitro.Crossref | GoogleScholarGoogle Scholar | 10775201PubMed |
Dadarwal, D., Adams, G., Hyttel, P., Brogliatti, G., Caldwell, S., and Singh, J. (2015a). Organelle reorganization in bovine oocytes during dominant follicle growth and regression. Reprod. Biol. Endocrinol. 13, 124.
| Organelle reorganization in bovine oocytes during dominant follicle growth and regression.Crossref | GoogleScholarGoogle Scholar | 26577904PubMed |
Dadarwal, D., Honparkhe, M., Dias, F., Alce, T., Lessard, C., and Singh, J. (2015b). Effect of superstimulation protocols on nuclear maturation and distribution of lipid droplets in bovine oocytes. Reprod. Fertil. Dev. 27, 1137–1146.
| Effect of superstimulation protocols on nuclear maturation and distribution of lipid droplets in bovine oocytes.Crossref | GoogleScholarGoogle Scholar | 24942058PubMed |
Dunning, K. R., Russell, D. L., and Robker, R. L. (2014). Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction 148, R15–R27.
| Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation.Crossref | GoogleScholarGoogle Scholar | 24760880PubMed |
Fair, T., Hulshof, S., Hyttel, P., Greve, T., and Boland, M. (1997). Oocyte ultrastructure in bovine primordial to early tertiary follicles. Anat. Embryol. (Berl.) 195, 327–336.
| Oocyte ultrastructure in bovine primordial to early tertiary follicles.Crossref | GoogleScholarGoogle Scholar | 9108198PubMed |
Genicot, G., Leroy, J., Van Soom, A., and Donnay, I. (2005). The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology 63, 1181–1194.
| The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes.Crossref | GoogleScholarGoogle Scholar | 15710202PubMed |
González-Serrano, A. F., Ferreira, C. R., Pirro, V., Lucas-Hahn, A., Heinzmann, J., Hadeler, K.-G., Baulain, U., Aldag, P., Meyer, U., Piechotta, M., Jahreis, G., Dänicke, S., Cooks, R. G., and Niemann, H. (2016). Effects of long-term dietary supplementation with conjugated linoleic acid on bovine oocyte lipid profile. Reprod. Fertil. Dev. 28, 1326–1339.
| Effects of long-term dietary supplementation with conjugated linoleic acid on bovine oocyte lipid profile.Crossref | GoogleScholarGoogle Scholar |
Gu, L., Liu, H., Gu, X., Boots, C., Moley, K. H., and Wang, Q. (2015). Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell. Mol. Life Sci. 72, 251–271.
| Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes.Crossref | GoogleScholarGoogle Scholar | 25280482PubMed |
He, B., Yin, C., Gong, Y., Liu, J., Guo, H., and Zhao, R. (2018). Melatonin-induced increase of lipid droplets accumulation and in vitro maturation in porcine oocytes is mediated by mitochondrial quiescence. J. Cell. Physiol. 233, 302–312.
| Melatonin-induced increase of lipid droplets accumulation and in vitro maturation in porcine oocytes is mediated by mitochondrial quiescence.Crossref | GoogleScholarGoogle Scholar | 28240360PubMed |
Homa, S. T., Racowsky, C., and McGaughey, R. W. (1986). Lipid analysis of immature pig oocytes. J. Reprod. Fertil. 77, 425–434.
| Lipid analysis of immature pig oocytes.Crossref | GoogleScholarGoogle Scholar | 3735242PubMed |
Kim, J. Y., Kinoshita, M., Ohnishi, M., and Fukui, Y. (2001). Lipid and fatty acid analysis of fresh and frozen–thawed immature and in vitro matured bovine oocytes. Reproduction 122, 131–138.
| Lipid and fatty acid analysis of fresh and frozen–thawed immature and in vitro matured bovine oocytes.Crossref | GoogleScholarGoogle Scholar | 11425337PubMed |
Ledda, S., Bogliolo, L., Calvia, P., Leoni, G., and Naitana, S. (1997). Meiotic progression and developmental competence of oocytes collected from juvenile and adult ewes. J. Reprod. Fertil. 109, 73–78.
| Meiotic progression and developmental competence of oocytes collected from juvenile and adult ewes.Crossref | GoogleScholarGoogle Scholar | 9068416PubMed |
Ledda, S., Bogliolo, L., Leoni, G., and Naitana, S. (2001). Cell coupling and maturation-promoting factor activity in in vitro-matured prepubertal and adult sheep oocytes. Biol. Reprod. 65, 247–252.
| Cell coupling and maturation-promoting factor activity in in vitro-matured prepubertal and adult sheep oocytes.Crossref | GoogleScholarGoogle Scholar | 11420246PubMed |
Leoni, G. G., Palmerini, M. G., Satta, V., Succu, S., Pasciu, V., Zinellu, A., Carru, C., Macchiarelli, G., Nottola, S. A., and Naitana, S. (2015). Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS One 10, e0124911.
| Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model.Crossref | GoogleScholarGoogle Scholar | 26636977PubMed |
Masala, L., Burrai, G. P., Bellu, E., Ariu, F., Bogliolo, L., Ledda, S., and Bebbere, D. (2017). Methylation dynamics during folliculogenesis and early embryo development in sheep. Reproduction 153, 605–619.
| Methylation dynamics during folliculogenesis and early embryo development in sheep.Crossref | GoogleScholarGoogle Scholar | 28250235PubMed |
McEvoy, T. G., Coull, G. D., Broadbent, P. J., Hutchinson, J. S. M., and Speake, B. K. (2000). Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 118, 163–170.
| Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida.Crossref | GoogleScholarGoogle Scholar | 10793638PubMed |
Murphy, D. J., and Vance, J. (1999). Mechanisms of lipid-body formation. Trends Biochem. Sci. 24, 109–115.
| Mechanisms of lipid-body formation.Crossref | GoogleScholarGoogle Scholar | 10203758PubMed |
Ordoñez-Leon, E. A., Merchant, H., Medrano, A., Kjelland, M., and Romo, S. (2014). Lipid droplet analysis using in vitro bovine oocytes and embryos. Reprod. Domest. Anim. 49, 306–314.
| Lipid droplet analysis using in vitro bovine oocytes and embryos.Crossref | GoogleScholarGoogle Scholar | 24467659PubMed |
Sturmey, R. G., O’Toole, P. J., and Leese, H. J. (2006). Fluorescence resonance energy transfer analysis of mitochondrial: lipid association in the porcine oocyte. Reproduction 132, 829–837.
| Fluorescence resonance energy transfer analysis of mitochondrial: lipid association in the porcine oocyte.Crossref | GoogleScholarGoogle Scholar | 17127743PubMed |
Sudano, M. J., Rascado, T. D., Tata, A., Belaz, K. R., Santos, V. G., Valente, R. S., Mesquita, F. S., Ferreira, C. R., Araújo, J. P., and Eberlin, M. N. (2016). Lipidome signatures in early bovine embryo development. Theriogenology 86, 472–484.e1.
| Lipidome signatures in early bovine embryo development.Crossref | GoogleScholarGoogle Scholar | 27107972PubMed |
Thiam, A. R., and Beller, M. (2017). The why, when and how of lipid droplet diversity. J. Cell Sci. 130, 315–324.
| The why, when and how of lipid droplet diversity.Crossref | GoogleScholarGoogle Scholar | 28049719PubMed |


