Intermittent fasting restores fertility dysfunction caused by a high-fat diet in male rats: role of SIRT-1/NRF2/P38 MAPK/NLRP3
Dalia A. Hemead A , Nanees F. El-Malkey A , Mohamed Aref B * , Nievin Ahmed Mahran C , Esraa ElSheikh B , Mohamed A. Nassan D , Mohamed H. A. Gadelmawla E , Gamal A. Salem F , Amira F. A. Ahmed G , Sahar M. El-Sayed B , Eman H. Elsheikh H and Nehal I. Hendy A
B * , Nievin Ahmed Mahran C , Esraa ElSheikh B , Mohamed A. Nassan D , Mohamed H. A. Gadelmawla E , Gamal A. Salem F , Amira F. A. Ahmed G , Sahar M. El-Sayed B , Eman H. Elsheikh H and Nehal I. Hendy A
A
B
C
D
E
F
G
H
Abstract
Intermittent fasting (IF) is a dietary approach against obesity; however, investigations on its role in male fertility showed contradictory results.
As nuclear factor erythroid 2-related factor 2 (NRF2)/mitogen-activated protein kinase p38 (MAPK)/nucleotide-binding oligomerization domain-like receptor with a pyrin domain 3 (NLRP3) signaling pathways regulate inflammation and pyrotosis, this study aimed to elucidate whether these pathways are involved in the underlying molecular mechanisms and to investigate the prophylactic effects of IF on male reproduction dysfunction in obese rats.
Twenty-four adult rats were divided as follows: Control lean (CL), control positive (CP), which were fed standard diet for four non-consecutive days/week, with alternate fasting on the other 3 days (24 h fasting), high-fat diet group (HFD), and the HFD-fasting group (HFD-IF), which was fed a HFD, followed by fasting protocol as in CP group. Serum testosterone, inflammatory markers, semen analysis, testicular malondialdehyde (MDA) concentration, and superoxide dismutase (SOD) activity were measured. Also, testicular and epididymal histological study, immunohistochemical analysis of NLRP3 and NRF2 and reverse-transcription quantitative polymerase chain reaction (RT-qPCR) for mRNA expression of SIRT1, NRF2, p38AMPK and NLRP3 were performed.
Combining IF with HFD limited rats’ testicular spermatic and steroidogenesis impairment, histopathological alterations, by upregulating SIRT1/NRF2 and downregulating p38 MAPK/NLRP3 signaling pathways versus the HFD group. In the HFD-IF group, oxidative and inflammatory markers had a significant decrease versus in the HFD group.
IF has a beneficial effect on male reproductive health and emphasizes the significance of customized dietary strategies for addressing male fertility issues.
Further investigation is required to clarify more prophylactic mechanisms of IF.
Keywords: fertility, HFD, intermittent fasting, NLRP3, NRF2, P38 MAPK, SIRT-1, testis.
Introduction
Nearly 3 million adults die each year from the complications of obesity, which affects more than half a billion people (WHO Organization 2024). Overconsumption of fat causes obesity, which is generally linked to an increased risk of several diseases, such as insulin resistance, type 2 diabetes, dyslipidemia, hypertension, and reproductive disruption (Fernandez et al. 2011).
It is commonly recognized that the type and amount of dietary fat can affect the testicular metabolic processes, leading to increased oxidative stress (OXS), which in turn causes DNA damage, lipid peroxidation, and disruption of the antioxidant balance in the male reproductive system. Furthermore, two effects of OXS that are commonly connected to a lower semen quality and possibly male infertility are inflammation and apoptosis (Khalil et al. 2020).
Interestingly, OXS is essential for the start and progression of inflammation (Baazm et al. 2020). The NLRP3 inflammasome, which initiates innate immune responses by inducing the production of proinflammatory cytokines such as interleukin-1β (IL-1β), is largely activated by reactive oxygen species (ROS) (Baazm et al. 2020 as well as Čolak and Pap 2021). Male fertility issues associated with obesity have been connected to this cycle (Selvam et al. 2020). Notably, Sertoli cells are a major location for NLRP3 expression in both rodents and primates, and they are essential for testicular immune responses. Consequently, Sertoli cell function may be directly affected by NLRP3 expression, which could have an effect on male reproductive health (Arab et al. 2024).
Moreover, the transcription factor that embodies an adaptive cellular defence response to oxidative and metabolic stressors is nuclear factor erythroid 2-related factor 2 (NRF2) (Shaw and Chattopadhyay 2020). In situations of high ROS generation (Signorini et al. 2024) and in the case of exposure to environmental contaminants (Dong et al. 2024; Fu et al. 2024), and metabolic disorders (Falvo et al. 2023), it has the ability to protect spermatogenesis. This results in a decreased capacity of NRF2 to trigger the transcription of genes encoding antioxidant enzymes, which in turn stimulates testicular inflammation and pyroptosis, as evidenced by elevated NLRP3 levels (Venditti et al. 2024).
Besides NRF2, another important modulator of the inflammatory responses is Sirtuin 1 (SIRT1), a member of the histone deacetylase-Sirtuin family. SIRT1 suppresses immunological inflammation by reducing the expression of nuclear factor-κB (NF-κB) (Matsushita et al. 2013). Pavillard et al. (2017), offering proof that the NLRP3/SIRT1 pathways might interact by regulating NF-κB and/or changing the mitochondrial damage caused by OXS (Ammar et al. 2020).
A dietary approach known as intermittent fasting (IF) alternates cycles of calorie restriction with non-fasting, unrestricted eating periods. There is growing recognition of the impact of IF on the body’s metabolic processes. Studies have confirmed that IF has positive effects on obesity and its comorbidities (Zang et al. 2022; Tavakoli et al. 2025).
Interestingly, studies on the role of IF in male fertility have yielded contradictory results. Cienfuegos et al. (2022) demonstrated that in addition to improving metabolic issues, IF also had a positive effect on male reproductive health and sperm parameters such as sperm count and quality. However, other studies have shown that semen samples collected during the 6-week IF period, which is comparable to Ramadan, were associated with progressive motility and decreased semen volume, indicating that different fasting protocols and durations have different effects (Kumar and Kaur 2013; Engel et al. 2023).
The current study aims to address a major research gap concerning the effects of IF on OXS, inflammation, testicular tissue histological changes, and male reproductive function in obese rats. Numerous studies have demonstrated the link between IF and cellular dysfunction brought on by OXS (Ngo and Duennwald 2022; Rusetskaya et al. 2023). We postulate that by lowering OXS and inflammation, IF could enhance male reproductive function in obese rats. This would mitigate testicular dysfunction by controlling the SIRT1/NRF2/p38 MAPK/NLRP3 pathway.
Materials and methods
Animals
Twenty-four male Sprague Dawley rats, aged 8 weeks and weighing between 220 and 260 g, were imported from the animal house of the Faculty of Medicine at Zagazig University in Egypt. For 2 weeks, the animals were kept in sterile, conventional plastic cages (3/cage) at a temperature of 25 ± 2°C and a 12-h light/dark cycle. Rats had free access to food and water ad libitum.
Experimental animal design and procedures
Four equal groups (n = 6/each) were chosen at random from the experimental animals. Group of control lean (CL) (n = 6) was fed standard chow diet throughout the trial; the rats in the control positive group (CP) (n = 6) were fed freely standard chow diet for four non-consecutive days/week, that were alternating with 24-h fasting periods for the other three non-consecutive days/week (Abou-Bakr et al. 2024); in the HFD group (n = 6), the subjects were fed a high-fat diet (HFD) consisting of 58.3% fat, 20.2% protein, 21.5% carbohydrate, and 5.40 kcal/g for 8 weeks so as to induce obesity; and the HFD-Fasting group (HFD-IF) (n = 6) was fed a HFD for four non-consecutive days per week, alternating with 24-h fasting periods during the remaining three non-consecutive days per week for the whole duration of the study (8 weeks).
Every week, each animal’s body mass was measured with an electronic balance. Additionally, the body mass index (BMI) was computed at the conclusion of the study. At the conclusion of the trial, all animals (18 weeks-old) were starved overnight in preparation for their anesthesia-induced decapitation at nine in the morning by using ketamine/xylazine (40/6 mg/kg bodyweight i.p). The following parameters were measured using rat ELISA kits (Sigma-Aldrich Co., USA) after blood was collected in sterile centrifuge tubes, allowed to clot, and then centrifuged to extract serum: the chemical analysis of testosterone following the procedures of Chen et al. (1991), IL-1β and tumor necrosis factor-α (TNF-α) as described by Fernando et al. (1998).
Macro-morphological examination and tissue collection
After collecting blood samples, the animals were placed in a supine position. The scrotal skin was cleaned and disinfected using Betadine surgical scrub, followed by 70% alcohol. A sterile pair of scissors was used to carefully open the scrotum, exposing the testes and epididymis. The tunica vaginalis and spermatic cord were severed, and the right testis was gently separated from adhering tissues and washed with cold saline solution. The right testis and epididymis were then weighed, photographed, and prepared for quantitative real-time PCR (qRT-PCR), immunohistochemistry (IHC), and histological analysis. Meanwhile, the left epididymis was used to evaluate sperm parameters, and the left testis was homogenized for biochemical analysis. Hematoxylin and eosin (H&E) staining was used for the study of testicular and epididymal tissues.
Samples from rat tests from various groups were gathered, fixed in 10% buffered neutral formalin solution for a whole day, dehydrated in ethanol solutions of increasing concentration, cleaned in xylene, and then embedded in paraffin wax. A microtome (Leica RM 2155, England) was used to slice paraffin sections that were 5 μm thick. Following preparation, the slices were regularly stained with hematoxylin and eosin stains and subjected to microscopical examination (Suvarna et al. 2018). A Leica microscope and an Am Scope (Germany) digital camera were used to take pictures of every segment. Seminiferous tubules (ST) were counted in a ×10 microscopic field as part of the estimation of testicular morphometric analysis. Additionally, the mean diameters of ST and the heights of the germinal epithelium were measured in 10 seminiferous tubules per animal and were derived by using Image J, an open-source program (ver. 1.41, https://imagej.net/ij/download.html). According to Table 1, the score of lesions was also graded (Gibson-Corley et al. 2013).
| Organ | Main lesions | |||||
|---|---|---|---|---|---|---|
| Score | 0 | 1 | 2 | 3 | ||
| Testes | Atrophied seminiferous tubules | No detectable histopathological lesion | Rarely minimal or focal (very few and isolated lesions) | Multifocal (lesions distributed in multiple, distinct areas) | Patchy or diffuse (irregularly, widespread distributed lesion) | |
| Degenerated, necrotic germinal epithelium | ||||||
| Interstitial edema | ||||||
| Congested vasculatures | ||||||
| Epididymis | Vacuolated epithelial lining tubules | |||||
| Empty tubules from sperms | ||||||
| Interstitial edema | ||||||
| Dilated interstitial blood vessels | ||||||
| Lymphocytes infiltrates | ||||||
Epididymal spermatic parameters analysis
Each rat’s left cauda epididymis was carefully removed and minced in 1 mL of phosphate-buffered saline (pH 7.2) to create a suspension, from which the sperm count was determined using the Neubauer’s chamber standard procedure (Khaki et al. 2010). A portion of the suspension (up to mark 0.5) was removed using a special pipette by using a leukocyte hemocytometer. The sample was then diluted with phosphate-buffered saline until it reached mark 11. After carefully and thoroughly mixing the suspension, it was put into Neubauer’s counting chamber. The quantity of spermatozoa/mL under ×400 power light microscopy is equal to the total number of sperm in 8 squares × 5 × 104, the formula outlined by Murthy et al. (1988). The number of motile sperm cells divided by the total number of sperm cells (including motile and non-motile) was the formula used to calculate the sperm motility percentage. Sperm cells that moved were classified as motile, whereas those that did not, were classified as non-motile. Additionally, the aberrant forms of the sperm such as head defects (twin head, detached heads), mid-piece abnormalities (bent or swollen mid-piece), and tail defects (coiled tails around the head or the mid-piece) were examined utilizing the technique outlined by Oyeyemi et al. (2000).
Testicular antioxidant system evaluation
Using spectrophotometric assay kits (Bio diagnostic, Giza, Egypt), the left testes were homogenized in cold 50 mM phosphate buffer (pH 7.0) with 0.1 mM EDTA to create a 10% homogenate (w/v). The resulting supernatant was then centrifuged at 112g for 10 min at 4°C to estimate the antioxidant/oxidant enzymes by using rat ELISA Kit (Abcam, USA) for superoxide dismutase (SOD) activity, following the method described by Erel (2004), and malondialdehyde (MDA) concentration according to the methodology described by Chi et al. (2002).
Reverse-transcriptase quantitative PCR (RT-qPCR) mRNA expression assay
The Superscripts III System was used to reverse-transcribe whole RNA into cDNA (Invitrogen, Carlsbad, CA, USA). Using the DNA Engine with Chromo 4 Detector, quantitative PCR (qPCR) was performed using Super Mix (Platinum SYBR Green qPCR Kit; Invitrogen) (initial template denaturation at 95°C for 5 s, followed by 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s) (MJ Research, Waltham, MA, USA), as previously explained by Livak and Schmittgen (2001). The Q-Gene program (https://www.qgene.org/qgene/download.php) was utilized to determine the normalized expression of SIRT-1, Nrf2, P38 MAPK, and NLRP3 against β-actin on the basis of the cycle threshold values. Table 2 lists the primers utilized in this investigation.
| SIRT-1 | Forward, 5-TGTTTCCTGTGGGATACCTGA-3′ Reverse, 5-TGAAGAATGGTCTTGGGTCTT-3′ | |
| NRF2 | Forward, 5-AGCATAGAGCAGGACATGGAGCAAGT-3′ Reverse, 5′CTGGCTGGCATCATCAGTGGAGAGG-3′ | |
| P38 MAPK | Forward, 5-CGAAATGACCGGCTACGTGG-3′ Reverse, 5-CACTTCATCGTAGGTCAGGC-3′ | |
| NLRP3 | Forward, 5-TCTGTTCATTGGCTGCGGAT-3′ Reverse, 5-GCCTTTTTCGAACTTGCCGT-3′ | |
| β-actin | Forward 5′-TAGTTGCGTTACACCCTTTCTTG-3′ Reverse, 5′-TCACCTTCACCGTTCCAGTT-3′ |
Immunohistochemical (IHC) analysis of testicular and epididymal tissues
In accordance with Hsu et al. (1981) and according to manufacturer’s protocol, paraffin slices (5 μm thick) from various testicular and epididymal groups were stained by IHC by using Anti-NLRP3 and Anti-Nrf.2 antibodies (Abcam, Cambridge, UK). All experimental-group tissue sections were hydrated and dewaxed. The 3,3′-diaminobenzidine (DAB) chromogenic agent (Expose Mouse and Rabbit Specific HRP/DAB Detection Kit; Abcam, Ready-to-use, Cat. no. ab80436) was then used for staining. Hematoxylin counterstaining was performed. Every image of the IHC-stained tissue sections was taken with a Swift light microscope, connected to a Swift digital camera. Using ImageJ software, expression levels were examined for quantitative analysis so as to determine the percentage area of a positive reaction that manifested as brown staining under light microscopy (Tigrani and Weydert 2007). Each animal (N = 6 animals/group) had five immuno-labeled sections examined, at magnification of ×400, for analysis of each antigen.
Statistical analysis
The Statistical Package of Social Services (ver. 25, https://www.ibm.com/products/spss; SPSS) was used to analyze the data gathered for this study. The normality of the data distribution was assessed using the Shapiro–Wilk test. The mean and standard deviation were used to characterize normally distributed data. Significant pairs were identified using the l.s.d. post hoc test. Because the data were not normally distributed, the Kruskal–Wallis test was used to examine the differences in the primary injury grading system lesions. Dunn’s multiple comparison test was then employed to identify significant pairs.
Ethical approval
Zagazig University, Institutional Animal Care and Use Committee (ZU-IACUC), has reviewed and approved this study protocol in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals, (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2010), the UK Animals (Scientific Procedures), and the ARRIVE guidelines (Approval number ZU-IACUC/3/F/168/2024).
Results
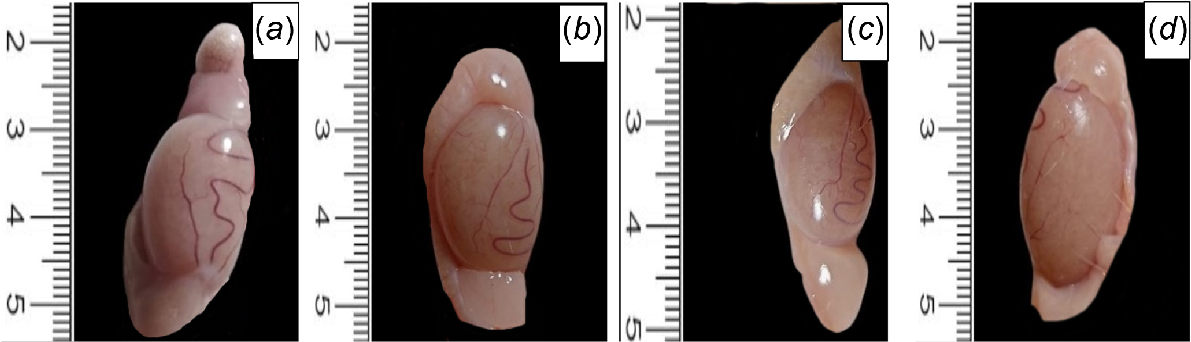
Effect of intermittent fasting (IF) on testicular and epididymal macro-morphology
Regarding the macro-anatomical analysis, the CL, CP, and HFD-IF groups had normal testicular and epididymal look, size, consistency, and contour, whereas in the HFD group, the epididymal was small, crowded, slightly cyanosed (Fig. 1).
Effect of intermittent fasting (IF) on rat anthropometric measures
Comparing the HFD group with the CL group, there was a statistically significant (P < 0.001) rise in the end BMI and a significant decrease in testicular weight (P < 0.01) and epididymal weight (P < 0.001). Furthermore, compared with the HFD group, the ultimate BMI of the HFD-IF group decreased statistically significantly (P < 0.001), whereas their testicular and epididymal weights increased statistically significantly (P < 0.05 and P < 0.001 respectively). When compared with the CL group, the end BMI of the CP group decreased, although not significantly, and their testicular and epididymal weights increased, also not significantly (P > 0.05), as shown in Table 3.
| Item | CL | CP | HFD | HFD-IF | |
|---|---|---|---|---|---|
| BMI (g/cm2) | 0.51 ± 0.076 | 0.48 ± 0.021 | 0.72 ± 0.099 A, B | 0.54 ± 0.051 C | |
| Testicular weight (g) | 2.19 ± 0.24 | 2.4 ± 0.41 | 1.49 ± 0.1 A, B | 1.89 ± 0.3 A, B, C | |
| Epididymal weight (g) | 0.201 ± 0.008 | 0.203 ± 0.01 | 0.140 ± 0.008 A, B | 0.164 ± 0.008 A, B, C | |
| Sperm count (%) | 69 ± 2.21 | 70 ± 4.19 | 17 ± 3.06 A, B | 36 ± 3.01 A, B, C | |
| Sperm motility (%) | 77 ± 4.94 | 78.17 ± 4.62 | 58.67 ± 3.14 A, B | 68 ± 5.36 A, B, C | |
| Sperm abnormal form (%) | 1.1 ± 0.23 | 1.08 ± 0.25 | 23.3 ± 4.2 A, B | 13.8 ± 2.15 A, B, C |
Effect of intermittent fasting (IF) on epididymal spermatic parameters
Table 3 indicates that the sperm count, motility, and abnormal morphology of the HFD group were all statistically significantly (P < 0.001) lower than those of the CL group. Additionally, compared with the HFD group, the HFD-IF group exhibited a statistically significant (P < 0.001) decrease in abnormal sperm morphology, a statistically significant (P < 0.001) rise in sperm count, and a statistically significant (P < 0.01) decrease in motility. However, when compared with the CL group, the former parameters showed a negligible change in the CP group (P > 0.05).
Effect of intermittent fasting (IF) on biochemical parameters
There was a statistically significant decrease in serum testosterone (P < 0.01) and FSH (P < 0.001) in HFD group when compared with the CL group, whereas there was a statistically significant increase in serum testosterone (P < 0.05) and FSH (P < 0.01) in the HFD-IF group compared with the HFD group. They showed a negligible increase in the CP group when compared with the CL group (P > 0.05) (Table 4).
| Item | CL | CP | HFD | HFD-IF | |
|---|---|---|---|---|---|
| Serum testosterone (mg/dL) | 5.81 ± 1.08 | 6.01 ± 1.24 | 3.9 ± 0.69 A, B | 5.14 ± 0.61 A, B, C | |
| Serum TNFα (pg/mL) | 58.16 ± 3.5 | 57.16 ± 4.1 | 77.1 ± 3.7 A, B | 67.5 ± 1.8 A, B, C | |
| Serum IL-1β (pg/mL) | 22.5 ± 1.23 | 21.8 ± 0.95 | 52.16 ± 5.72 A, B | 41.3 ± 5.01 A, B, C | |
| Testicular MDA (μg/g tissue) | 39.36 ± 1.08 | 38.7 ± 3.27 | 55.56 ± 7.33 A, B | 47.36 ± 3.17 A, B, C | |
| Testicular SOD (μg/g tissue) | 77.6 ± 8.06 | 82 ± 10.29 | 58.6 ± 5.64 A, B | 67.83 ± 2.78 A, B, C |
Values are expressed as means ± s.d. n = 6.
Abbreviations: IL-1β, interleukin 1β; TNFα, tumor necrosis factor-α; s.d., standard deviation; CL, control lean group; CP, control positive group; HFD, high-fat diet group; HFD-IF, HFD-fasting.
In terms of inflammatory markers, the serum concentrations of TNF-α and IL1b were significantly (P < 0.001) higher in the HFD group than in the CL group, whereas the serum concentrations in the HFD-IF group were significantly (P < 0.001) lower than those in the HFD group. When comparing the CP group with the CL group, there was no discernible drop in the former characteristics (P > 0.05) (Table 4).
In terms of oxidative and antioxidative markers, the testicular concentrations of MDA and SOD in the HFD group were considerably higher and lower respectively, than those in the CL group (P < 0.001). Additionally, compared with the HFD group, the HFD-IF group showed a statistically significant (P < 0.05) rise in testicular concentratons of SOD, and a statistically significant (P < 0.01) decrease in testicular concentrations of MDA. However, results indicated that there was no discernible difference in testicular concentrations of these markers between the CP and CL groups (P > 0.05) (Table 4).
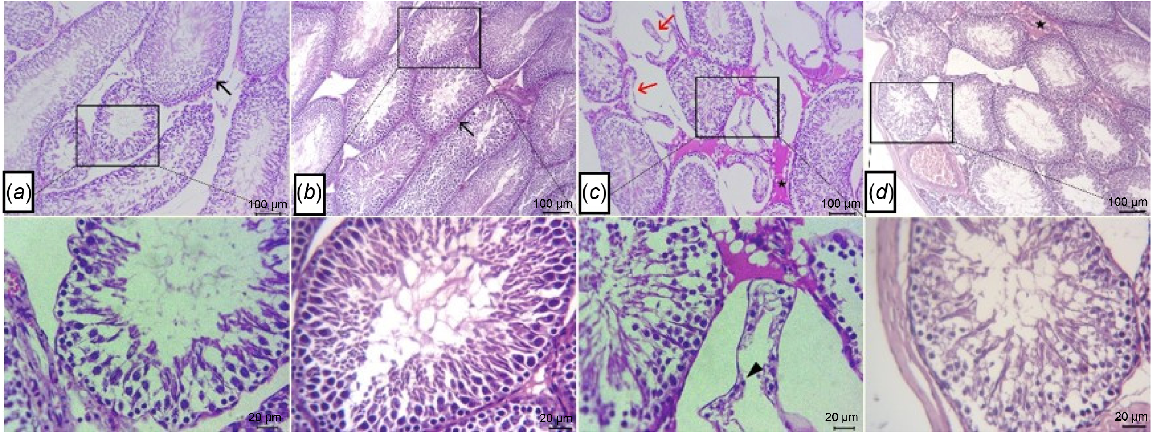
Effect of intermittent fasting (IF) on histopathological findings
Testes from both CL (Fig. 2a) and CP (Fig. 2b) showed normal histology of germinal epithelial lining seminiferous tubules and Leydig cells. The seminiferous tubules included spermatogonia, spermatocytes, spermatids, spermatozoa and Sertoli cells. The sections of HFD (Fig. 2c) showed a large number of atrophied seminiferous tubules, which rare represented by necrotic germinal cells with dark pyknotic nuclei beside interstitial edema between the tubules. In contrast, testes of HFD-IF group showed improvement in histopathological architectures of most seminiferous tubules and interstitial tissues, with mild interstitial edema and mild congestion of some blood vessels as presented in (Fig. 2d).
Photomicrographs of H&E-stained sections of tests, showing normal histology of germinal epithelial lining seminiferous tubules (arrows) and Leydig cells at both (a) control lean and (b) and control positive groups. (c) Necrotic germinal cells with pyknotic nuclei (arrowhead) within atrophied seminiferous tubules (arrows) beside interstitial edema (star) in HFD group. (d) Mild interstitial edema (star) and normal germinal epithelium (arrow) in HFD-IF group. Scale bars: 100 and 20 μm, as indicated in the figure.
Moreover, quantitative analysis of seminiferous tubules showed significant decrease in their number, diameter and height of epithelial lining in HFD groups when compare with those in the CL (P < 0.001) and CP (P < 0.001) groups. However, IF significantly reversed these findings, because there was a significant increase in previously mentioned parameters in HFD-IF group when compared with HFD group (P < 0.01, P < 0.05, and P < 0.01 respectively), as shown in Fig. 3a–c.
Representative bar chart, showing means ± s.d. of the (a) number, (b) diameter and (c) height of seminiferous tubules of all groups (n = 6). CL, control lean group; CP, control positive group; HFD, high-fat diet group; and HFD-IF, HFD-fasting. a, significant vs CL group; b, significant vs CP group; c, significant vs HFD group. *P < 0.05, **P < 0.01, ***P < 0.001.
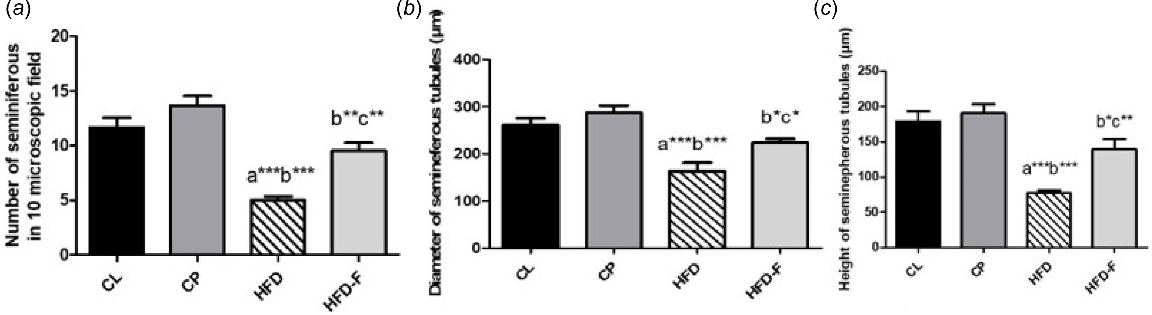
However, seminiferous tubule number, diameter and height of the epithelial lining were significantly lower in HFD-IF group than in the control positive group (P < 0.01, P < 0.05 and P < 0.05 respectively). Additionally, no significant (P > 0.05) differences were observed between the CL and CP groups in any of the previously mentioned morphometric parameters, as shown in Fig. 3a–c.
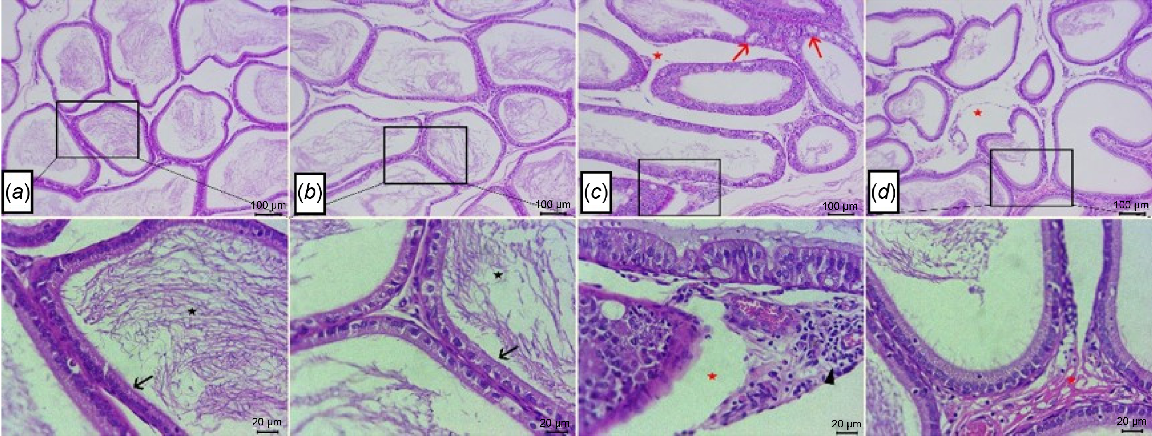
Regarding histological analysis of epididymal tissue, there were normal histological structures of epididymal tubules that formed from ciliated columnar to cuboidal lined epithelium, with stored mature sperms within epididymal lumina beside normal interstitial tissues seen at both CL group (Fig. 4a) and CP group (Fig. 4b). However, sections from HFD group (Fig. 4c) showed numerous vacuolated epithelial lining tubules, moderate number of epididymal lumina devoid of sperm, beside the interstitial edema with dilated blood vessels, and lymphocyte aggregates were also noticed. In contrast, epididymis from HFD-IF group (Fig. 4d) showed improvement in architecture of most epididymal tubules and interstitial tissues, with mild interstitial edema and appearance of low concentrations of mature spermatozoa within some tubular lumina.
Photomicrographs of H&E-stained sections of epididymis, showing normal histological structures of ciliated cuboidal lined epithelium (arrow) with stored mature sperms within epididymal lumina (stars) at both (a) control lean group and (b) control positive group. (c) Numerous vacuolated epithelial lining tubules (arrows), epididymal lumina devoid of sperms, interstitial edema (stars) with dilated blood vessels, and lymphocyte aggregates (arrow head) in HFD group. (d) Mild interstitial edema (stars) and low concentrations of spermatozoa within some tubular lumina in HFD-IF group. Scale bars: 100, 20 μm, as indicated in the figure.
In addition, the histological scoring system showed a significant increase in the score of testicular and epididymal injury in HFD groups in comparison to CL and CP groups. The score of atrophied seminiferous tubules, epididymal tubular vacuolated epithelial lining and epididymal congested dilated vessels were significantly (P < 0.001) decreased in HFD-IF group when compared with HFD group. No significant difference was found regarding any of the injury scoring items among CL, CP and HFD-IF groups, as shown in Table 5.
| Organ | Main lesion | Group | |||||
|---|---|---|---|---|---|---|---|
| CL | CP | HFD | HFD-IF | P-value (Kruskal–Wallis test) | |||
| Testes | Atrophied seminiferous tubules | 0 ± 0 | 0 ± 0 | 3 ± 0 A, B | 0 ± 0 C | <0.0001 | |
| Degenerated, necrotic germinal epithelium | 0 ± 0 | 0 ± 0 | 2 ± 0 A, B | 1 ± 0 | <0.0001 | ||
| Interstitial edema | 0 ± 0 | 0 ± 0 | 2 ± 0 A, B | 1 ± 0 | <0.0001 | ||
| Congested vasculatures | 0 ± 0 | 0 ± 0 | 2 ± 0 A, B | 1 ± 0 | <0.0001 | ||
| Epididymis | Vacuolated epithelial lining tubules | 0 ± 0 | 0 ± 0 | 3 ± 0 A, B | 0 ± 0 C | <0.0001 | |
| Empty tubules from sperms | 0 ± 0 | 0 ± 0 | 3 ± 0 A, B | 1 ± 0 | <0.0001 | ||
| Interstitial edema | 0 ± 0 | 0 ± 0 | 3 ± 0 A, B | 1 ± 0 | <0.0001 | ||
| Dilated interstitial blood vessels | 0 ± 0 | 0 ± 0 | 2 ± 0 A, B | 0 ± 0 C | <0.0001 | ||
| Lymphocytes infiltrates | 0 ± 0 | 0 ± 0 | 2 ± 0 A, B | 1 ± 0 | <0.0001 | ||
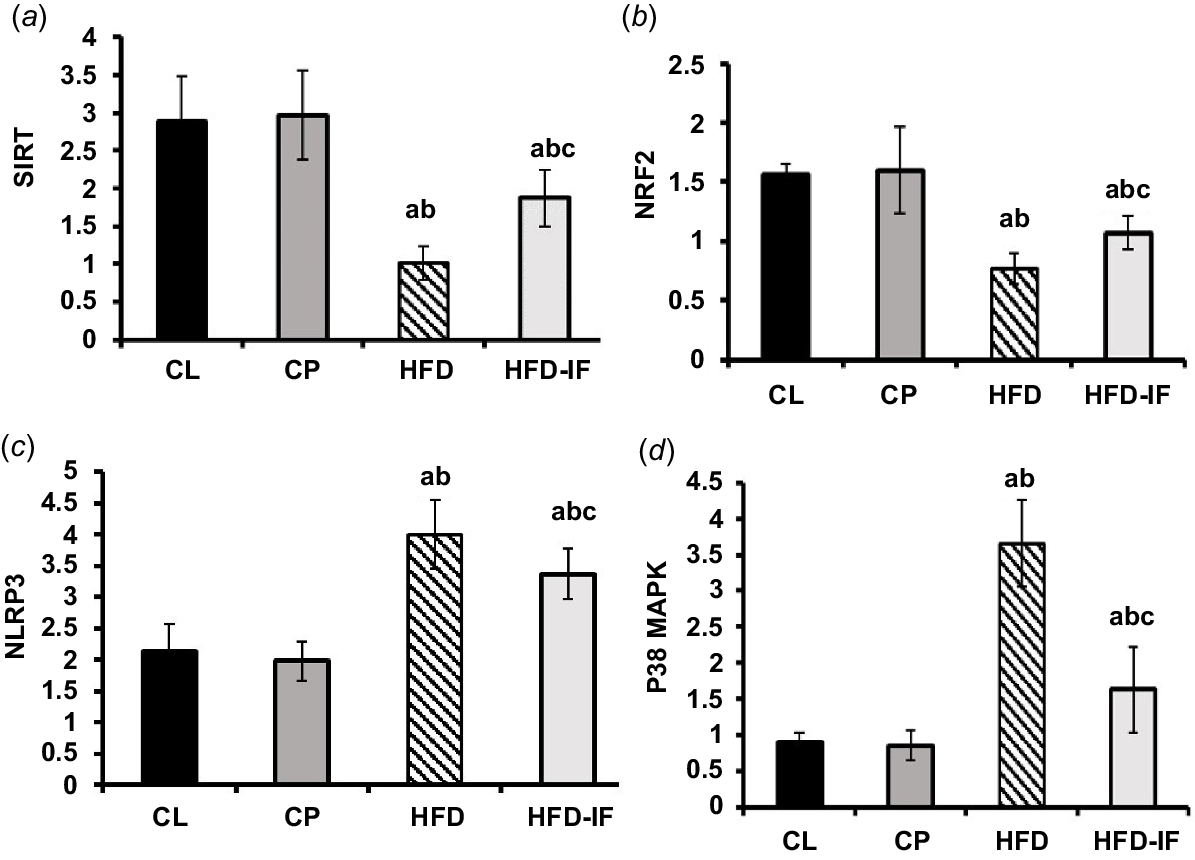
Effect of IF on SIRT1, NRF2, NLRP-3 and P38 MAPK mRNA expression
When compared with the CL group, the HFD group showed a statistically significant decrease in SIRT1 and NRF2 mRNA expression and a statistically significant increase in NLRP-3 and P38 MAPK expression (P < 0.001 for both). However, comparing the HFD-IF group with the HFD group showed a statistically significant drop in the expression of NLRP-3 (P < 0.05) and P38 MAPK (P < 0.001), as well as a statistically significant rise in SIRT1 (P < 0.01) and NRF2 (P < 0.05) mRNA expression. The former metrics showed a negligible difference between the CL group and the CP group (P > 0.05) (Fig. 5a–d).
Bar chart representing means ± s.d. of RT-qPCR analysis of (a) SIRT1, (b) NRF2, (c) NLRP-3, and (d) P38 MAPK mRNA expression in all groups. SIRT1, silent information regulator 1; NLRP-3, NLR family pyrin domain containing protein 3; p83MAPK, p38 mitogen-activated protein kinase; s.d., standard deviation; CL, control lean group; CP, control positive group; HFD, high-fat diet group; HFD-IF, HFD-fasting. a, significant vs CL group; b, significant vs CP group; c, significant vs HFD groups.
Effect of intermittent fasting (IF) on immuno-histochemical results
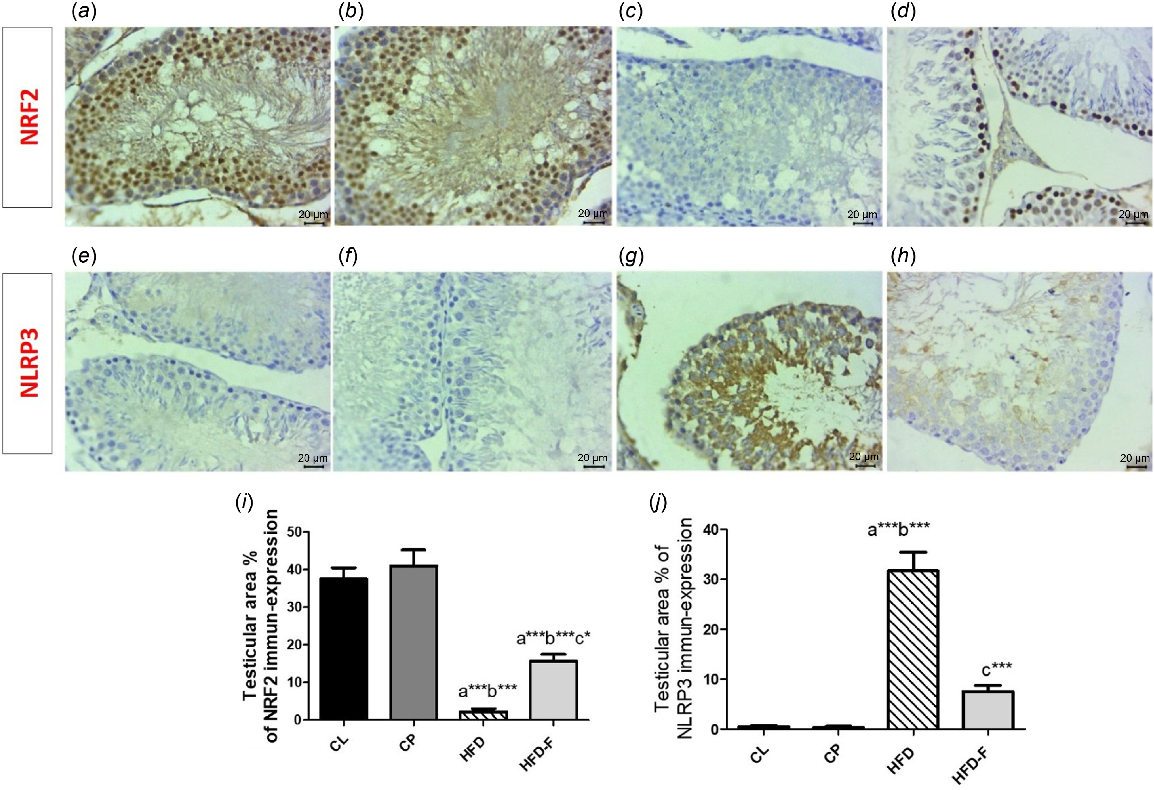
Immunohistochemistry expression of NRF2 in CL and CP groups exhibited strongly positive nuclear expression in a large number of germinal epithelial lining seminiferous tubules (Fig. 6a, b) and epithelial lining epididymal tubules (Fig. 7a, b), whereas in the HFD group, a negative expression of NRF2 in the epithelium lining seminiferous tubules (Fig. 6c) and in the epithelial linings epididymal tubules (Fig. 7c) was shown. In contrast, gradually re-established nuclear expressions were noticed in a low number of spermatogonia and spermatocytes (Fig. 6d) and cuboidal epithelial lining epididymal tubules (Fig. 7d) in the HFD-IF group. The comparison of percentage area of immuno-histochemical expression of NRF2 and NLRP3 in both testicular and epididymeal tissues was clearly illustrated at (Fig. 8a–d).
Photomicrographs of immune-expression of NRF2 and NLRP3 in rat test germinal epithelial of all groups, showing a high nuclear expression of NRF2 in a large number of germinal epithelial lining seminiferous tubules for both (a) CL and (b) CP groups, (c) a weak in the HFD group, and (d) a mild nuclear expression in the HFD-IF group. Weak NLRP3 immune-expression in both (e) CL and (h) CP groups, (g) high expression of NLRP3 in the HFD group, and (h) a weak NLRP3 immune-expression in the HFD-IF group. Arrowheads refers to positive stained cells (scale bar: 20 μm). (i–j) Histograms of the comparison of mean ± s.d. of percentage area of immuno-histochemical expression of (i) NRF2 and (j) NLRP3 in testicular tissues of all groups.
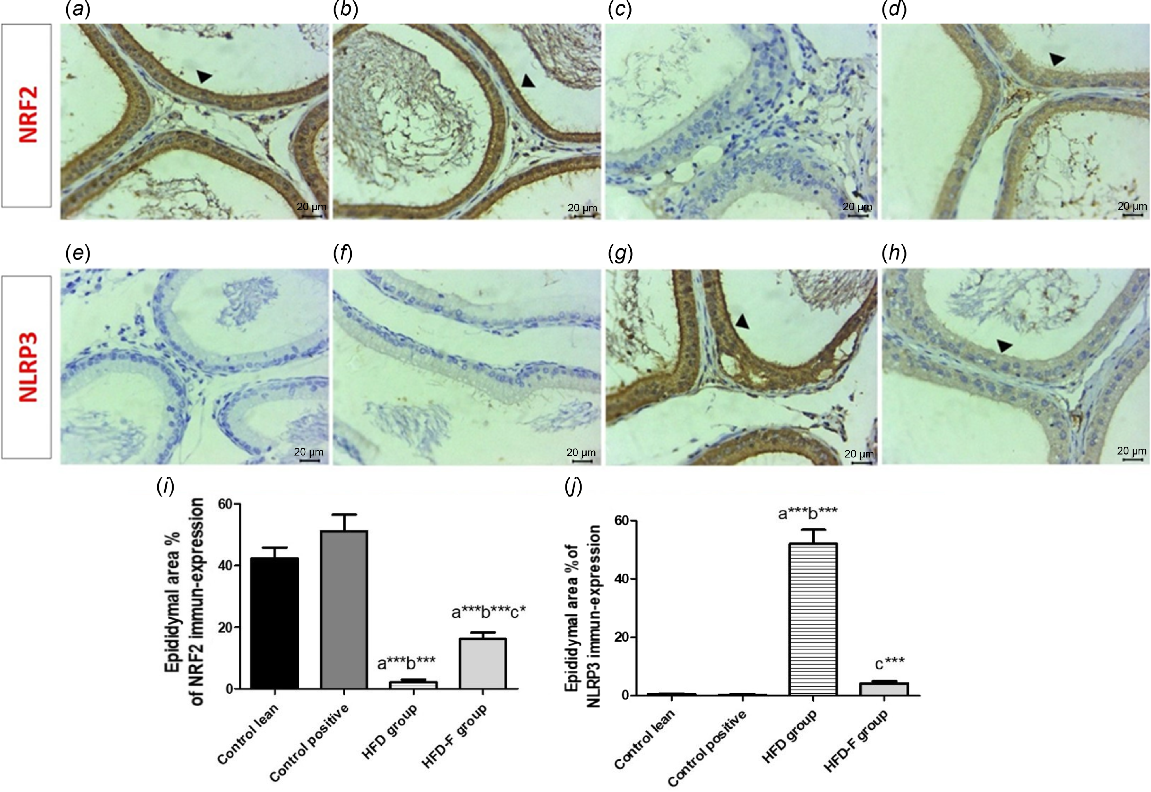
Photomicrographs of immune-expression of NRF2 and NLRP3 in rat epididymis of all groups, showing high nuclear expression of NRF2 in a large number of the cells in the tubular lining epithelium of both (a) CL and (b) CP groups, (c) a weak NRF2 expression in lining epithelial cells of the tubules in the HFD group, and (d) a mild nuclear expression of NRF2 in the tubular lining epithelium in HFD-IF group. Weak NLRP3 immune expression in both (e) CL and (f) CP groups. (g) High expression of NLRP3 in lining epithelial cells of epididymal tubules in the HFD group, and (h) a mild NLRP3 immune-expression in the HFD-IF group. Arrowheads refer to positive stained cells (scale bar: 20 μm). (i–j) Histograms of the comparison of mean ± s.d. of percentage area of immuno-histochemical expression of (i) NRF2 and (j) NLRP3 in epididymis tissues of all groups.
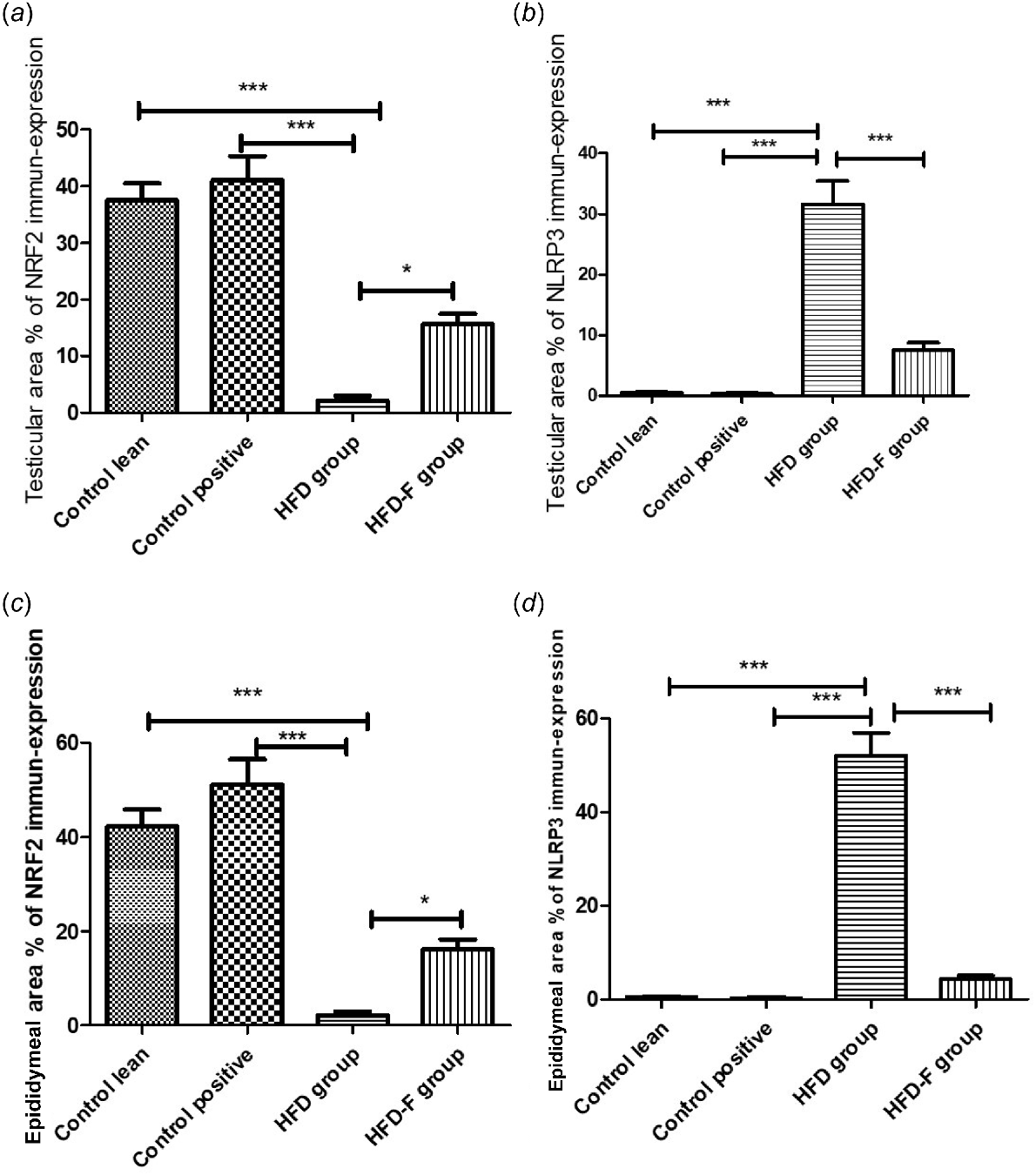
Histogram showing comparison of mean ± s.d. of pecentage area of immuno-histochemical expression of (a) NRF2 in testicular tissues, (b) NLRP3 in testicular tissues, (c) NRF2 in epididymeal tissues, and (d) NLRP3 in epididymeal tissues.
However, immuno-stained sections for NLRP3 exhibited non-observable immune-positive cells both in CL and CP groups within testicular (Fig. 6e, f respectively) or epididymal (Fig. 7e, f respectively) tissues. However, positive cytoplasmic stained cells were detected in HFD group in the epithelium lining seminiferous tubules (Fig. 6g) and within the epithelial lining epididymal tubules (Fig. 7g), whereas few positive cells were seen at HFD-IF in testicular and epididymal tissues (Figs 6h and 7h respectively).
Regarding area % of IHC expression of NRF, it showed a significant decrease in both testicular and epididymal tissues in HFD group when compared with CL (P < 0.001) and CP (P < 0.001) groups. However, its area % of IHC expression showed a significant increase in testicular (P < 0.05) and epididymal (P < 0.05) tissues in the HFD-IF group compared with the HFD group. However, area % of IHC expression of NRF2 in testicular (P < 0.05) and epididymal tissues was significantly lower in HFD-IF group than in the CL (P < 0.001) and CP (P < 0.001) groups. No significant (P > 0.05) change was detected between CL and CP groups (Figs 6i, 7i).
Regarding the area % of IHC expression of NLRP3, it showed a significant increase in the HFD group compared with the CL (P < 0.001) and CP (P < 0.001) groups. However, its area % of IHC expression showed a significant decrease in testicular (P < 0.001) and epididymal (P < 0.001) tissues in HFD-IF group when compared with the HFD group. No significant (P > 0.05) difference was detected among CL, CP and HFD-IF groups regarding this marker expression in both testicular and epididymal tissues (Figs 6j, 7j).
Discussion
The findings of the present study support the hypothesis that IF could enhance male reproductive function in obese rats by lowering inflammation and OXS. This would mitigate testicular dysfunction by regulating the SIRT1/NRF2/p38 MAPK/NLRP3 pathway. In comparison to rats fed a control diet, the rats fed an HFD 8 weeks in our study had greater bodyweights and BMIs. In accordance with Billah et al. (2022), we discovered that IF significantly decreased bodyweight and the BMI of the HFD-IF group in comparison to the HFD group and of the Control positive group in comparison to the Control negative group. Billah et al. (2022) found that the total bodyweight of rats fed a high-fat diet increased by 21%.
These results suggested that bodyweight increased with the conventional diet and HFD during the trial, but that this weight gain was reduced when IF was included in these diets. These findings, which were in line with those of Park et al. (2020), could be explained by the capacity of IF to promote browning of white adipose tissue and reduce fat cell size in HFD-fed mice (Park et al. 2020; Hamed et al. 2024).
Furthermore, on the basis of testicular morphology and function studies, we discovered that the HFD group had a higher proportion of aberrant forms and a significantly lower testicular weight, epididymal weight, sperm count, and motility than did the negative and positive control groups. This was supported by the results of the morphometric analysis and histopathological evaluation, which showed that the testicular sections of the HFD group had a significant increase in the histological injury score grading in both testicular and epididymal tissues, which is similar to what Crisóstomo et al. (2019) observed. The testicular sections also had a lot of atrophied seminiferous tubules and interstitial edema between them (2019), and these authors asserted that HFD decreased sperm quality, which did not improve after transferring to a regular diet, even if metabolic syndrome improved. Nevertheless, Billah et al. (2022), despite finding that the number of sperm in HFD-IF rats remained constant, did not rule out further detrimental effects of an energy-dense diet on sperm and testes.
Furthermore, our results demonstrated that the testosterone hormone concentrations in the HFD group were substantially lower than those in the control group. Even so, Falvo et al. (2023) and Migliaccio et al. (2019), who disagreed with us regarding the histological findings and claimed that the testicular histological organization in HFD-IF animals was identical to controls. Falvo et al. (2023) demonstrated a reduction in androgen receptors and blood testosterone hormone concentrations in the testes of rats given a high-fat diet for 6 weeks.
The augmentation of adipose tissue induced by a high-fat diet (HFD) may elucidate the previously mentioned deficits in spermatogenesis, steroidogenesis, and histopathological alterations. An excess of adipose tissue has the potential to elevate estrogen synthesis, which subsequently impairs the functionality of Leydig and Sertoli cells. This phenomenon may further compromise testosterone synthesis, disrupt the integrity of blood–testes barrier (BTB) proteins, and hinder the spermatogenetic process (Barbagallo et al. 2022). Additionally, it may alter sperm maturation, capacitation, and the ability of spermatozoa to adhere to and fertilize oocytes, although the underlying mechanisms remain to be elucidated (Bibi et al. 2022). Furthermore, the findings of the present study are consistent with those of Nasr et al. (2023), who documented that a high-fat diet downregulated the expression of steroidogenic genes StAR and CYP17A, correlating with diminished serum testosterone concentrations.
Interestingly, the results of this investigation demonstrated that IF dramatically reduced the HFD-induced deficits in spermatogenesis, steroidogenesis, and histological structure when compared with animals that were not fasting. The positive control group showed modest increases in these measures, but the changes were negligible when compared with the negative control group. Crucially, the histological characteristics of both control groups were normal. Sperm parameters (PH, viability, concentration, and motility) improved after 30 days of IF, but sperm morphology was not significantly affected, which was consistent with a prior study based on semen analyses of infertile men who received IF (Sayme et al. 2023).
Nevertheless, it was demonstrated that IF had complex effects on testosterone concentrations. The combination of the ketogenic diet and IF showed a middle-range hormone profile, falling between the ketogenic diet alone and the control diet with fasting. This suggests that the ketogenic diet and IF may have a synergistic effect on male hormonal regulation, even though Ustündağ et al. (2023) found that the ketogenic diet, which is a dietary regimen high in fat and low in protein, can increase testosterone and decrease estrogen.
In contrast, a prior clinical study by Moro et al. (2021) that used time-restricted eating (TRE) for 8 h, found that TRE decreased free testosterone, which implied that more testosterone is bound by carriers other than sex hormone-binding globulin such as albumin, because they noticed a normal circulating content of sex hormone-binding globulin. The limited sample size in that study might be the cause for this difference with our investigation. In addition, in the current study we measured total testosterone not the free type.
Unfortunately, a systemic OXS condition has also been identified after consuming a high-fat diet (Migliaccio et al. 2019). In the end, this damages cellular components and male germ cells, especially spermatozoa, whose DNA cannot be repaired once damaged because they lack the cytoplasmic enzymatic systems necessary for DNA repair and whose plasma membrane contains high amounts of polyunsaturated fatty acids (Pini et al. 2021; Minucci and Venditti 2022). That was consistent with our findings that showed that HFD-fed rats had much higher concentrations of MDA and significantly lower concentrations of SOD in their testes than did control rats.
Serum TNFα and IL1β were measured in the current study to assess the inflammatory state, because, in addition to OXS, inflammation has been demonstrated to alter testicular activity and sperm quality over an extended period of time in HFD feeding (Chang et al. 2021; Allam et al. 2022). Additionally, the H&E-stained sections showed lymphocyte aggregates in the epididymal tissue, and their concentrations were noticeably higher in the HFD group than in the control groups. It has been discovered that elevated TNFα and IL1β directly affect sperm motility and viability by causing DNA breakage and sperm mortality because sperm cells contain unique cytokine receptors, such as IL-1 R and TNF-αR (Paira et al. 2022).
However, Falvo et al. (2023) reported that short-term (st)-HFD-induced overweight did not induce testicular inflammation, despite the fact that it caused a significant testicular disfunction. The absence of inflammation may indicate that being overweight has a less severe effect on fertility than has obesity. However, in the current study, obesity was attained, as evidenced by the high BMI (0.96 ± 0.07) in this group exceeding the cutoff value of obesity (>0.68 gm/cm2) (Novelli et al. 2007). Additionally, De Moura e Dias et al. (2021) corroborated the results of the current investigation by demonstrating the time-dependent effects of HFD in causing obesity and determining that at least 3 weeks of HFD are necessary to achieve a notable impairment in testicular function.
When comparing the HFD-IF group to the HFD group, we found a large rise in SOD and a significant decrease in MDA, TNF-α, and IL-1β. These findings suggest that IF may help reduce OXS and inflammation in different animal models (Dai et al. 2022; He et al. 2023; Mousa et al. 2024), which could explain the improved testicular function in HFD-IF group in comparison to HFD group, because it has been demonstrated that sperm motility is improved by in vitro neutralization of TNF-α and IL-1β or their receptors (Brackett et al. 2007). This demonstrates the advantages of IF in the treatment of obesity and its associated comorbidities (Miyauchi et al. 2019; Touyz 2021).
Interestingly, so as to gain a deeper knowledge of the underlying mechanisms and the effects of IF on HFD-induced OXS, we investigated the SIRT1/NRF2/p38 MAPKs/NLRP3 pathways, which are involved in the cellular response to OXS (Pan et al. 2022; Suzuki et al. 2023). According to our findings, HFD significantly reduced SIRT1/NRF2 mRNA expression while increasing p38 MAPK and NLRP3 mRNA expression in testicular tissues. This was accompanied by a significant decrease in testicular and epididymal NRF2 IHC expression. In contrast, the testicular and epididymal NLRP3 inflammasome IHC expressions of the HFD group were meaningfully higher than subjects of the control groups.
In OXS circumstances, SIRT1 functions as a ROS ‘sensor.’ In addition to controlling energy metabolism and mitochondrial function (Abu Shelbayeh et al. 2023), it is crucial for controlling cellular death and autophagy following OXS (Tang et al. 2024). The effect of HFD on decreased spermatogenesis in mice with HFD-induced obesity may be mediated by testicular SIRT1 downregulation, which has been linked to OXS status (Xu et al. 2020; Yan et al. 2020; Zhang et al. 2021).
Moreover, overproduction of ROS causes NF-κB-p65 to become activated, which in turn increases the production of ROS and inflammatory cytokines such as IL-1β, IL-6, and TNF-α. This downregulates Nrf2 (Wardyn et al. 2015) and starts the transcription of a number of pro-inflammatory mediators, such as NLRP3 (Arab et al. 2024). Additionally, Zhou et al. (2020) and Akar et al. (2022) showed that downregulation of NRF2 and activation of p38 MAPK pathways respectively, exacerbated apoptosis within testicular tissues, resulting in damage to structural proteins that comprise the BTB in rats with type 1 diabetes and HFD-obese rats. In addition, Bo et al. (2020) noticed activation of NLRP3 inflammasome through the oxidative stress-mediated p38 MAPK signaling pathway in HepG2 cells.
Additionally, experiments on fat mice showed elevated NLRP3 expression (Fan et al. 2018). These results showed that NLRP3 might be involved in the pathological process of male infertility and Sertoli cell dysfunction brought on by obesity. Our findings showed that the HFDIF group considerably downregulated NLRP3 and significantly elevated SIRT1 and NRF2. These findings may provide additional explanations for the possible advantages of IF in treating testicular dysfunction in obese mice. By blocking the SIRT3/Nrf2/HO1 pathway, IF prevents intracerebral hemorrhage and suppresses neuroinflammatory responses (Dai et al. 2022). By initiating antistress responses such as the Nrf2/mTOR pathways, it also inhibits OXS and inflammation (Longo and Cortellino 2020; Vemuganti and Arumugam 2021). Moreover, IF increases NAD +/NADH through altering cellular metabolic pathways, which activates Sirt proteins (Stagg et al. 2021). Furthermore, it was believed that Nrf2 is a crucial target protein that regulates the activation of the NLRP3 inflammasome (Ramachandran et al. 2024).
Fasting has been demonstrated to help protect skeletal muscle from OXS by upregulating Nrf2-dependent genes, such as those controlling glutathione (GSH) metabolism. As a result, glutathione peroxidase 4, an antioxidant enzyme that depends on GSH, has increased, and MDA, a well-known by-product of lipid peroxidation, has been decreased (Lettieri-Barbato and Aquilano 2020). Additionally, a 24 h fast is known to decrease NLRP3 inflammasome activation, according to Traba et al. (2017). This effect might be mediated by SIRT3 activation, which controls ROS in the mitochondria. NLRP3 deficiency decreased obesity-induced Sertoli cell damage and BTB impairment, while increasing sperm quality and Leydig cell testosterone release. It also explains why the steroidogenesis of the HFDIF group improved somewhat when compared with HFD animals (Mu et al. 2022).
Conclusions
By upregulating SIRT1/NRF2 and downregulating p38MAPK/NLRP3 signaling pathways, IF appeared to lessen the oxidative stress and inflammation caused by the HFD, according to this study, which sheds important light on the intricate relationships between HFD and IF on male reproductive health indicators. This highlights the importance of tailored dietary approaches for managing male fertility concerns and raises the possibility that IF may improve male reproductive health. Future studies are recommended to assess how different durations, intensities, and timing of IF protocols influence the degree of SIRT-1/NRF2 activation and p38 MAPK/NLRP3 suppression in the reproductive system, and also to demonstrate effect of fasting on SIRT-1/NRF2, p38 MAPK/NLRP3 protein expression.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Experimental design: D. Hemead, N. El-Malkey, M. Aref, N. Mahran, E. ElSheikh, M. Nassan, M. Gadelmawla, A. F. A. Ahmed, S. M. El-Sayed and N. Hendy; experiment implementation: D. Hemead, N. Mahran, M. Aref, M. Gadelmawla, A. F. A. Ahmed, S. M. El-Sayed and N. Hendy; data analysis: N. El-Malkey, M. Aref, N. Mahran, E. ElSheikh, M. Nassan, G. Salem and N. Hendy; writing: D. Hemead, N. El-Malkey, M. Aref, N. Mahran, E. ElSheikh, M. Nassan, M. Gadelmawla, A. F. A. Ahmed, S. M. El-Sayed, G. Salem, and N. Hendy. All authors read and approved the final paper.
References
Abou-Bakr DS, El-Malah MM, El-Masry HG (2024) The effects of intermittent fasting as a diet regime on obese rats. Egyptian Journal of Chemistry 67(8), 375-385.
| Crossref | Google Scholar |
Abu Shelbayeh O, Arroum T, Morris S, Busch KB (2023) PGC-1α is a master regulator of mitochondrial lifecycle and ROS stress response. Antioxidants 12(5), 1075.
| Crossref | Google Scholar |
Akar F, Yildirim OG, Yucel Tenekeci G, Tunc AS, Demirel MA, Sadi G (2022) Dietary high-fructose reduces barrier proteins and activates mitogenic signalling in the testis of a rat model: regulatory effects of kefir supplementation. Andrologia 54(3), e14342.
| Crossref | Google Scholar |
Allam EA, Ibrahim HF, Abdulmalek SA, Abdelmeniem IM, Basta M (2022) Coenzyme Q10 alleviates testicular endocrine and spermatogenic dysfunction induced by high-fat diet in male Wistar rats: role of adipokines, oxidative stress and MAPK/ERK/JNK pathway. Andrologia 54(10), e14544.
| Crossref | Google Scholar |
Ammar A, Sissaoui H, Kermiche M, Oussama B (2020) Modelisation and numerical simulation of salt gradient solar pond: the alternating direction implicit method. Advances in Mathematics: Scientific Journal 11(5), 469-497.
| Crossref | Google Scholar |
Arab HH, Alsufyani SE, Ashour AM, Gad AM, Elhemiely AA, Gadelmawla MHA, Mahmoud MA, Khames A (2024) Targeting JAK2/STAT3, NLRP3/Caspase-1, and PK2/PKR2 pathways with arbutin ameliorates lead acetate-induced testicular injury in rats. Pharmaceuticals 17, 909.
| Crossref | Google Scholar |
Baazm M, Ghafarizadeh AA, Kamran ARN, Beyer C, Zendedel A (2020) Presence of the NLRP3 inflammasome components in semen of varicocele patients. International Journal of Fertility & Sterility 14(1), 46-50.
| Crossref | Google Scholar | PubMed |
Barbagallo F, La Vignera S, Cannarella R, Mongioì LM, Garofalo V, Leanza C, Marino M, Calogero AE, Condorelli RA (2022) Obesity and male reproduction: do sirtuins play a role? International Journal of Molecular Sciences 23(2), 973.
| Crossref | Google Scholar |
Bibi R, Jahan S, Afsar T, Almajwal A, Hammadeh ME, Alruwaili NW, Razak S, Amor H (2022) The influence of paternal overweight on sperm chromatin integrity, fertilization rate and pregnancy outcome among males attending fertility clinic for IVF/ICSI treatment. BMC Pregnancy and Childbirth 22, 620.
| Crossref | Google Scholar |
Billah MM, Khatiwada S, Lecomte V, Morris MJ, Maloney CA (2022) Ameliorating high-fat diet-induced sperm and testicular oxidative damage by micronutrient-based antioxidant intervention in rats. European Journal of Nutrition 61, 3741-3753.
| Crossref | Google Scholar | PubMed |
Bo N, Yilin H, Chaoyue Y, Lu L, Yuan Y (2020) Acrylamide induces NLRP3 inflammasome activation via oxidative stress- and endoplasmic reticulum stress-mediated MAPK pathway in HepG2 cells. Food and Chemical Toxicology 145, 111679.
| Crossref | Google Scholar |
Brackett NL, Cohen DR, Ibrahim E, Aballa TC, Lynne CM (2007) Neutralization of cytokine activity at the receptor level improves sperm motility in men with spinal cord injuries. Journal of Andrology 28(5), 717-721.
| Crossref | Google Scholar | PubMed |
Chang B, Song C, Gao H, Ma T, Li T, Ma Q, Yao T, Wang M, Li J, Yi X, Tang D, Cao S (2021) Leptin and inflammatory factors play a synergistic role in the regulation of reproduction in male mice through hypothalamic kisspeptin-mediated energy balance. Reproductive Biology and Endocrinology 19, 12.
| Crossref | Google Scholar |
Chen A, Bookstein JJ, Meldrum DR (1991) Diagnosis of a testosterone-secreting adrenal adenoma by selective venous catheterization. Fertility and Sterility 55(6), 1202-1203.
| Crossref | Google Scholar | PubMed |
Chi C-H, Shiesh S-C, Lin X-Z (2002) Total antioxidant capacity and malondialdehyde in acute abdominal pain. The American Journal of Emergency Medicine 20(2), 79-82.
| Crossref | Google Scholar | PubMed |
Cienfuegos S, Corapi S, Gabel K, Ezpeleta M, Kalam F, Lin S, Pavlou V, Varady KA (2022) Effect of intermittent fasting on reproductive hormone levels in females and males: a review of human trials. Nutrients 14(11), 2343.
| Crossref | Google Scholar |
Čolak E, Pap D (2021) The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. Journal of Medical Biochemistry 40(1), 1-9.
| Crossref | Google Scholar |
Crisóstomo L, Rato L, Jarak I, Silva BM, Raposo JF, Batterham RL, Oliveira PF, Alves MG (2019) A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction 158(4), 377-387.
| Crossref | Google Scholar | PubMed |
Dai S, Wei J, Zhang H, Luo P, Yang Y, Jiang X, Fei Z, Liang W, Jiang J, Li X (2022) Intermittent fasting reduces neuroinflammation in intracerebral hemorrhage through the Sirt3/Nrf2/HO-1 pathway. Journal of Neuroinflammation 19, 122.
| Crossref | Google Scholar |
De Moura e Dias M, Dos Reis SA, Da Conceição LL, Sediyama CMNdO, Pereira SS, De Oliveira LL, Gouveia Peluzio MdC, Martinez JA, Milagro FI (2021) Diet-induced obesity in animal models: points to consider and influence on metabolic markers. Diabetology & Metabolic Syndrome 13, 32.
| Crossref | Google Scholar |
Dong Z, Qin R, Zou P, Yao X, Cui P, Zhang F, Yang Y (2024) Occupational health risk assessment of PC production-caused pollution based on damage assessment and cyclic mitigation model. Engineering Construction & Architectural Management 32(7), 3679-3699.
| Crossref | Google Scholar |
Engel OJ, Doctory N, Sun B, Miller N, Levi M, Schreiber H, Samara N, Wiser A, Haikin Herzberger E (2023) The effect of month-long daily fasting on semen parameters: a retrospective cohort study. Gynecologic and Obstetric Investigation 88(6), 384-390.
| Crossref | Google Scholar | PubMed |
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clinical Biochemistry 37(4), 277-285.
| Crossref | Google Scholar | PubMed |
Falvo S, Latino D, Santillo A, Chieffi Baccari G, Senese R, Nuzzolillo F, Di Fiore MM (2023) Effects of a high-fat diet on rat epididymis. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 339, 535-544.
| Crossref | Google Scholar |
Fan W, Xu Y, Liu Y, Zhang Z, Lu L, Ding Z (2018) Obesity or overweight, a chronic inflammatory status in male reproductive system, leads to mice and human subfertility. Frontiers in Physiology 8, 1117.
| Crossref | Google Scholar |
Fernandez CDB, Bellentani FF, Fernandes GSA, Perobelli JE, Favareto APA, Nascimento AF, Cicogna AC, Kempinas WDG (2011) Diet-induced obesity in rats leads to a decrease in sperm motility. Reproductive Biology and Endocrinology 9, 32.
| Crossref | Google Scholar |
Fernando B, Marley R, Holt S, Anand R, Harry D, Sanderson P, Smith R, Hamilton G, Moore K (1998) N-acetylcysteine prevents development of the hyperdynamic circulation in the portal hypertensive rat. Hepatology 28(3), 689-694.
| Crossref | Google Scholar | PubMed |
Fu J, Lin Q, Ai B, Li M, Luo W, Huang S, Yu H, Yang Y, Lin H, Wei J, Su X, Zhang Z (2024) Associations between maternal exposure to air pollution during pregnancy and trajectories of infant growth: a birth cohort study. Ecotoxicology and Environmental Safety 269, 115792.
| Crossref | Google Scholar | PubMed |
Gibson-Corley KN, Olivier AK, Meyerholz DK (2013) Principles for valid histopathologic scoring in research. Veterinary Pathology 50(6), 1007-1015.
| Crossref | Google Scholar |
Hamed GM, Abou-Bakr DA, Saleh NKM, Elshishiny MIM, Morsy WE (2024) Metabolic consequences of polycystic ovary syndrome and their impact on hepatic function in high fat diet-fed rats: potential role of moderate intensity exercise. Journal of Evolutionary Biochemistry and Physiology 60, 1408-1427.
| Crossref | Google Scholar |
He Z, Xu H, Li C, Yang H, Mao Y (2023) Intermittent fasting and immunomodulatory effects: a systematic review. Frontiers in Nutrition 10, 1048230.
| Crossref | Google Scholar |
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. Journal of Histochemistry & Cytochemistry 29(4), 577-580.
| Crossref | Google Scholar | PubMed |
Khaki A, Fathiazad F, Nouri M, Khaki A, Maleki NA, Khamnei HJ, Ahmadi P (2010) Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytotherapy Research 24(9), 1285-1291.
| Crossref | Google Scholar |
Khalil SS, Aziz JA, Ismail KA, El-Malkey NF (2020) Comparative protective effects of N-acetylcysteine and melatonin against obesity-induced testicular dysfunction in rats. Canadian Journal of Physiology and Pharmacology 99(7), 708-719.
| Crossref | Google Scholar | PubMed |
Kumar S, Kaur G (2013) Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: a study of hypothalamo–hypophysial–gonadal axis. PLoS ONE 8(1), e52416.
| Crossref | Google Scholar |
Lettieri-Barbato D, Aquilano K (2020) Aging and immunometabolic adaptations to thermogenesis. Ageing Research Reviews 63, 101143.
| Crossref | Google Scholar | PubMed |
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4), 402-408.
| Crossref | Google Scholar | PubMed |
Longo VD, Cortellino S (2020) Fasting, dietary restriction, and immunosenescence. Journal of Allergy and Clinical Immunology 146(5), 1002-1004.
| Crossref | Google Scholar | PubMed |
Matsushita T, Sasaki H, Takayama K, Ishida K, Matsumoto T, Kubo S, Matsuzaki T, Nishida K, Kurosaka M, Kuroda R (2013) The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. Journal of Orthopaedic Research 31(4), 531-537.
| Crossref | Google Scholar |
Migliaccio V, Sica R, Scudiero R, Simoniello P, Putti R, Lionetti L (2019) Physiological adaptation to simultaneous chronic exposure to high-fat diet and dichlorodipheniletylhene (DDE) in Wistar rat testis. Cells 8(5), 443.
| Crossref | Google Scholar |
Minucci S, Venditti M (2022) New Insight on the in vitro effects of melatonin in preserving human sperm quality. International Journal of Molecular Sciences 23(9), 5128.
| Crossref | Google Scholar |
Miyauchi T, Uchida Y, Kadono K, Hirao H, Kawasoe J, Watanabe T, Ueda S, Okajima H, Terajima H, Uemoto S (2019) Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proceedings of the National Academy of Sciences 116(27), 13533-13542.
| Crossref | Google Scholar | PubMed |
Moro T, Tinsley G, Pacelli FQ, Marcolin G, Bianco A, Paoli A (2021) Twelve months of time-restricted eating and resistance training improves inflammatory markers and cardiometabolic risk factors. Medicine & Science in Sports & Exercise 53(12), 2577-2585.
| Crossref | Google Scholar | PubMed |
Mousa HSE, Qenawy NM, Abohashem AA (2024) The possible ameliorating effect of intermittent fasting on histological and biochemical changes induced by monosodium glutamate on renal cortex of adult male albino rat. Zagazig University Medical Journal 30, 276-288.
| Crossref | Google Scholar |
Mu Y, Yin T-L, Zhang Y, Yang J, Wu Y-T (2022) Diet-induced obesity impairs spermatogenesis: the critical role of NLRP3 in Sertoli cells. Inflammation and Regeneration 42, 24.
| Crossref | Google Scholar |
Murthy NVA, Wray SR, Melville GN, Wynter HH, Santha Ram NV, Hari Haran NV (1988) Testicular function in rats following immobilization stress. International Journal of Gynecology & Obstetrics 26(2), 297-299.
| Crossref | Google Scholar | PubMed |
Nasr N, Kahilo KA, Sadek KM, Abouzed TK, Shawky HA, Elsawy H, Shukry M, Dorghamm DA (2023) Assessment the relationship between high-fat diet feeding and male infertility in albino rats. Egyptian Journal of Veterinary Sciences 54(7), 139-148.
| Crossref | Google Scholar |
Ngo V, Duennwald ML (2022) Nrf2 and oxidative stress: a general overview of mechanisms and implications in human disease. Antioxidants 11(12), 2345.
| Crossref | Google Scholar |
Novelli ELB, Diniz YS, Galhardi CM, Ebaid GMX, Rodrigues HG, Mani F, Fernandes AAH, Cicogna AC, Novelli Filho JLVB (2007) Anthropometrical parameters and markers of obesity in rats. Laboratory Animals 41(1), 111-119.
| Crossref | Google Scholar | PubMed |
Oyeyemi MO, Akusu MO, Ola-Davies OE (2000) Effect of successive ejaculations on the spermiogram of West African dwarf goats (Capra hircus L.). Veterinarski Arhiv 70(4), 215-221.
| Google Scholar |
Paira DA, Silvera-Ruiz S, Tissera A, Molina RI, Olmedo JJ, Rivero VE, Motrich RD (2022) Interferon γ, IL-17, and IL-1β impair sperm motility and viability and induce sperm apoptosis. Cytokine 152, 155834.
| Crossref | Google Scholar |
Pan Z, Dong H, Huang N, Fang J (2022) Oxidative stress and inflammation regulation of sirtuins: new insights into common oral diseases. Frontiers in Physiology 13, 953078.
| Crossref | Google Scholar |
Park J, Seo Y-G, Paek Y-J, Song HJ, Park KH, Noh H-M (2020) Effect of alternate-day fasting on obesity and cardiometabolic risk: a systematic review and meta-analysis. Metabolism 111, 154336.
| Crossref | Google Scholar |
Pavillard LE, Cañadas-Lozano D, Alcocer-Gómez E, Marín-Aguilar F, Pereira S, Robertson AAB, Muntané J, Ryffel B, Cooper MA, Quiles JL, Bullón P, Ruiz-Cabello J, Cordero MD (2017) NLRP3-inflammasome inhibition prevents high fat and high sugar diets-induced heart damage through autophagy induction. Oncotarget 8, 99740-99756.
| Crossref | Google Scholar | PubMed |
Pini T, Haywood M, McCallie B, Lane SL, Schoolcraft WB, Katz-Jaffe M (2021) Liquid chromatography-tandem mass spectrometry reveals an active response to DNA damage in human spermatozoa. F&S Science 2(2), 153-163.
| Crossref | Google Scholar |
Ramachandran R, Manan A, Kim J, Choi S (2024) NLRP3 inflammasome: a key player in the pathogenesis of life-style disorders. Experimental & Molecular Medicine 56, 1488-1500.
| Crossref | Google Scholar | PubMed |
Rusetskaya NY, Loginova NY, Pokrovskaya EP, Chesovskikh YS, Titova LE (2023) Redox regulation of the NLRP3-mediated inflammation and pyroptosis. Biomeditsinskaya Khimiya 69(6), 333-352.
| Crossref | Google Scholar | PubMed |
Sayme N, Krebs T, Maas DHA, Kljajic M (2023) The impact of intermittent fasting on sperm parameters. Fertility and Sterility 120(4), e70.
| Crossref | Google Scholar |
Shaw P, Chattopadhyay A (2020) Nrf2–ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. Journal of Cellular Physiology 235(4), 3119-3130.
| Crossref | Google Scholar | PubMed |
Signorini C, Saso L, Ghareghomi S, Telkoparan-Akillilar P, Collodel G, Moretti E (2024) Redox homeostasis and nrf2-regulated mechanisms are relevant to male infertility. Antioxidants 13(2), 193.
| Crossref | Google Scholar |
Stagg DB, Gillingham JR, Nelson AB, Lengfeld JE, d’Avignon DA, Puchalska P, Crawford PA (2021) Diminished ketone interconversion, hepatic TCA cycle flux, and glucose production in D-β-hydroxybutyrate dehydrogenase hepatocyte-deficient mice. Molecular Metabolism 53, 101269.
| Crossref | Google Scholar |
Suzuki T, Takahashi J, Yamamoto M (2023) Molecular basis of the KEAP1-NRF2 signaling pathway. Molecules and Cells 46(3), 133-141.
| Crossref | Google Scholar | PubMed |
Tang Y, Ju W, Liu Y, Deng Q (2024) The role of SIRT1 in autophagy and drug resistance: unveiling new targets and potential biomarkers in cancer therapy. Frontiers in Pharmacology 15, 1469830.
| Crossref | Google Scholar |
Tavakoli A, Akhgarjand C, Ansar H, Houjaghani H, Khormani A, Djafarian K, Rostamian A, Ranjbar M, Farsani GM (2025) The effects of intermittent fasting on antioxidant and inflammatory markers and liver enzymes in postmenopausal, overweight and obese women with rheumatoid arthritis: a randomized controlled trial. Scientific Reports 15, 2357.
| Crossref | Google Scholar |
Tigrani D-Y, Weydert JA (2007) Immunohistochemical expression of osteopontin in epithelioid mesotheliomas and reactive mesothelial proliferations. American Journal of Clinical Pathology 127(4), 580-584.
| Crossref | Google Scholar | PubMed |
Touyz RM (2021) Gut dysbiosis–induced hypertension is ameliorated by intermittent fasting. Circulation Research 128(9), 1255-1257.
| Crossref | Google Scholar | PubMed |
Traba J, Geiger SS, Kwarteng-Siaw M, Han K, Ra OH, Siegel RM, Gius D, Sack MN (2017) Prolonged fasting suppresses mitochondrial NLRP3 inflammasome assembly and activation via SIRT3-mediated activation of superoxide dismutase 2. Journal of Biological Chemistry 292(29), 12153-12164.
| Crossref | Google Scholar |
Ustündağ H, Doğanay S, Öztürk B, Köse F, Kurt N, Aydemi ̇Celep N, Huyut MT, Betül Özgeriş F (2023) Exploring the impact of ketogenic diet and intermittent fasting on male rats’ testicular health: an analysis of hormonal regulation, oxidative stress, and spermatogenesis. Journal of Food Biochemistry 2023, 562120.
| Crossref | Google Scholar |
Vemuganti R, Arumugam TV (2021) Much ado about eating: intermittent fasting and post-stroke neuroprotection. Journal of Cerebral Blood Flow & Metabolism 41(7), 1791-1793.
| Crossref | Google Scholar | PubMed |
Venditti M, Romano MZ, Boccella S, Haddadi A, Biasi A, Maione S, Minucci S (2024) Type 1 diabetes impairs the activity of rat testicular somatic and germ cells through NRF2/NLRP3 pathway-mediated oxidative stress. Frontiers in Endocrinology 15, 1399256.
| Crossref | Google Scholar |
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochemical Society Transactions 43(4), 621-626.
| Crossref | Google Scholar | PubMed |
Xu D, Liu L, Zhao Y, Yang L, Cheng J, Hua R, Zhang Z, Li Q (2020) Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. Journal of Pineal Research 69(4), e12690.
| Crossref | Google Scholar |
Yan Q, Huang H, Lu S, Ou B, Feng J, Shan W, Li H, Wang Z, Hong A, Ma Y (2020) PACAP ameliorates fertility in obese male mice via PKA/CREB pathway-dependent Sirt1 activation and p53 deacetylation. Journal of Cellular Physiology 235(10), 7465-7483.
| Crossref | Google Scholar | PubMed |
Zang B-Y, He L-X, Xue L (2022) Intermittent fasting: potential bridge of obesity and diabetes to health? Nutrients 14(5), 981.
| Crossref | Google Scholar |
Zhang S, Zhang M, Sun S, Wei X, Chen Y, Zhou P, Zheng R, Chen G, Liu C (2021) Moderate calorie restriction ameliorates reproduction via attenuating oxidative stress-induced apoptosis through SIRT1 signaling in obese mice. Annals of Translational Medicine 9, 933.
| Crossref | Google Scholar | PubMed |
Zhou J, Xi Y, Zhang J, Tang J, Zhou X, Chen J, Nie C, Zhu Z, Ma B (2020) Protective effect of Dioscorea zingiberensis ethanol extract on the disruption of blood–testes barrier in high-fat diet/streptozotocin-induced diabetic mice by upregulating ZO-1 and Nrf2. Andrologia 52(3), e13508.
| Crossref | Google Scholar |