Modelling menstruation in the common mouse: a narrative review
Laura M. Rogers A , Gendie E. Lash
A , Gendie E. Lash  B , Greg M. Anderson A and Jane E. Girling
B , Greg M. Anderson A and Jane E. Girling  A *
A *
A
B
Abstract
Despite occurring in up to 50% of the human population, menstruation is a fundamentally understudied process with limited treatment options when menstrual pathologies arise. Reasons for this deficit include the inherent ethical and technical constraints associated with researching menstruation. The multifactorial nature of many menstrual-related pathologies means in vivo research is necessary; however, this type of research is difficult in humans, and non-human species that menstruate naturally are often not suitable as research models. Consequently, most menstrual research relies on an artificially induced menstrual-like process in the non-menstruating laboratory mouse. This review investigates mouse models of menstruation and how specific technical variables are used to produce or modulate a menstrual-like process. The review describes two key categories of models, those that are ovariectomy-based versus those that are pseudopregnancy-based. The menstrual-like process occurring in these models varied slightly; the underlying reason for the variation is likely to be the method of progesterone withdrawal. Models that withdrew progesterone specifically had a far less rapid endometrial breakdown in comparison to those that withdrew all ovarian input. These outcomes suggest that a loss of ovarian factors other than progesterone is likely impacting the breakdown process. The review highlights the gaps in our understanding of the mechanisms of endometrial breakdown and repair in these proxies for menstruation and the subsequent impacts on any conclusions drawn from these models.
Keywords: decidualisation, induced menstruation, menstrual models, menstruation, mouse models, rodent models, uterine bleeding, uterine breakdown, uterine repair.
Introduction
Occurring in only a small percentage of mammalian species, menstruation is the process by which the endometrial lining of the uterus is shed in response to cyclic hormonal fluctuations (Kirkwood et al. 2021). It is estimated that an individual human can experience up to ~450 menstruation events across their reproductive lifespan, although this figure will be impacted by factors such as pregnancy, lactation, hormonal contraceptive use and health status (Chavez-MacGregor et al. 2008; Critchley et al. 2020). A variety of approaches have been used to study the mechanisms associated with both normal and abnormal menstruation. The use of mouse models of menstruation has proved central to this body of work and is the subject of this review.
Normal menstruation is defined by parameters set out by the International Federation of Gynaecology and Obstetrics (FIGO) including the frequency, duration, volume and regularity of menstrual bleeding (Jain et al. 2023). Bleeding frequency reflects the length of each menstrual cycle (normal = ≥24 and ≤38 days), while duration refers to the number of days over which bleeding occurs during menstruation (normal = ≤8 days). Regularity refers to the predictability of an individual’s cycle (variations in cycle length of ≤7–9 days), and lastly, volume refers to the volume of blood lost during menstruation and is patient determined. Menstrual cycles and bleeding patterns that fall outside of these defined ranges are classified as abnormal uterine bleeding (AUB) (Munro et al. 2011; Jain et al. 2023). Using more subjective criteria, heavy menstrual bleeding has been defined by the National Institute for Health and Care Excellence (NICE) as ‘menstrual blood loss which interferes with a woman’s physical, social, emotional and/or material quality of life. It can occur alone or in combination with other symptoms’ (NICE 2018).
AUB is estimated to occur in a third of menstruating individuals (Kocaoz et al. 2019). For those affected, menstrual irregularities are associated with pain, fertility issues, disrupted lifestyle, and a loss of productivity (Whitaker and Critchley 2016; Sacha and Souter 2017). Multiple factors can contribute to AUB, with nine main structural and non-structural classification categories summarised by the acronym PALM-COEIN (polyps, adenomyosis, leiomyoma, malignancy, hyperplasia, coagulopathic causes, ovulatory dysfunction, endometrial disorders, iatrogenic causes, and causes not otherwise specified) (Munro et al. 2011). AUB can be difficult to treat depending on its cause(s), though, treatments include modulating hormone levels and pain relief. In extreme cases, individuals may choose surgical intervention and undergo hysterectomy to relieve symptoms (Whitaker and Critchley 2016). The limited understanding about the mechanisms responsible for both normal and abnormal menstruation has limited development of medical interventions and treatments (Jain et al. 2023). Therefore, there is a need to further investigate the mechanisms of both abnormal and normal menstruation to ultimately provide better care and management options.
The mechanisms responsible for menstruation and AUB can be investigated using in vitro and ex vivo techniques with uterine tissue samples, blood samples and cell culture methods; the value of these applications has been summarised in a recent review (Tsolova et al. 2022). In vitro research allows researchers to work directly with human tissue. While invaluable data has been obtained using these approaches, there are associated limitations: in vitro and ex vitro experimental methodologies cannot fully reflect the complex systems and interactions that occur in the body during menstruation (Fitzgerald et al. 2020). Animal models are used in these cases, providing physiologically complete systems that can be actively modulated by researchers. Anatomically, primates are the best model due to their phylogenetic closeness to humans (Phillips et al. 2014); however, their use in research is restricted because of time constraints of working with long-lived species, ethical concerns and cost (Taylor 2001; Coleman 2011). Several bat species (Rasweiler and De Bonilla 1992; Zhang et al. 2007; Catalini and Fedder 2020), the elephant shrew (family Macroscelididae) (Van Der Horst 1954; Carter 2018; Catalini and Fedder 2020), and the spiny mouse (Acomys cahirinus) also undergo menstruation (Bellofiore et al. 2017; Bellofiore et al. 2018a, 2018b). These naturally menstruating species are largely unsuitable for menstrual research due, in part, to specialty housing requirements and a lack of common reagents for research use. While rodents such as laboratory mice (Mus musculus) and rats (Rattus rattus) are commonly used for reproductive research, these animals undergo oestrous cycles as opposed to menstrual cycles (Boyd et al. 2018). However, an artificial menstrual-like event can be induced in both mice and rats (Finn and Keen 1963; Finn and Hinchliffe 1964; Finn and Pope 1984).
In recent years, a number of reviews have been published that discuss new insights into the mechanisms of menstruation and AUB (Liu et al. 2020; Kirkwood et al. 2021; Tsolova et al. 2022; Holdsworth-Carson et al. 2023; Middelkoop et al. 2023). However, there is little discussion of the animal models from which these insights were created. This review seeks to document the protocols used to induce menstruation in rodents and whether any variation in technique between protocols has a notable effect on the measured outcomes. To accomplish this, our review was directed by the following questions: (1) what rodent models of induced menstruation are available, (2) what are the benefits and limitations of these models, and (3) how is menses assessed in these models?
Methods
Search design
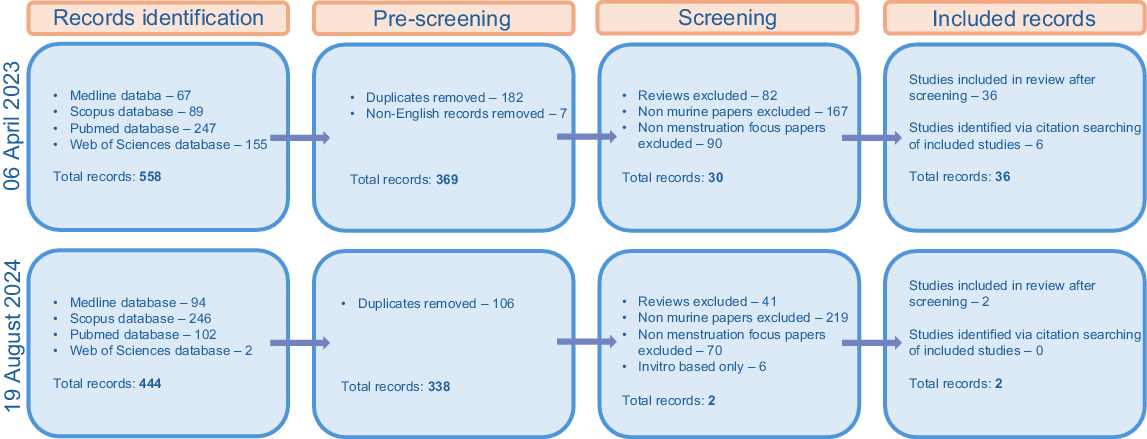
The strategy used for this review was based on an established methodological framework used within the field of biomedical research (Page et al. 2021). The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement provides a guideline for collecting and reviewing literature. The PRISMA flow chart was used to document the paper collection and exclusion process as follows. An initial search was performed on 06 April 2023. This was followed by a secondary search on 19 August 2024 to ensure that no recently published papers were missed (Fig. 1).
Search strategy
Articles were accessed via electronic searches on the databases PubMed, Scopus, Web of Science and Medline. Papers were assessed following the inclusion criteria outlined below. Records were limited to the English language, but no date limitations were set. Records identified via citation searching of included records were also collected if they met the inclusion criteria.
The search terms entered in the above databases were as follows:
‘menstrual’ OR menstruation OR ‘menses’
AND
‘mice’ OR ‘murine’ OR ‘mouse’ OR ‘rodent’ OR ‘rat’ OR ‘rats’
AND
‘decidual’ OR ‘decidualized’ OR ‘decidualised’
Web of science:
(ALL = (menstrual) OR ALL = (menstruation) OR ALL = (menses)) AND (ALL = (mice) OR ALL = (murine) OR ALL = (mouse) OR ALL = (rodent) OR ALL = (rat) OR ALL = (rats)) AND (ALL = (decidual) OR ALL = (decidualized) OR ALL = (decidualised))
The inclusion criteria for papers were as follows:
Published in English
Original research papers
Papers using mouse models of artificial menstruation
In vivo experiments
The breakdown and repair process was documented in the results
Papers were excluded for the following reasons:
Citation searching
Within the initial search result papers, any cited papers deemed relevant to the review were retrieved and assessed for inclusion eligibility.
Note: One paper identified via citation searching (Cao et al. 2010) was not available in English; however, it was thought to be of direct relevance as it was one of the first reported instances of a certain technique and was therefore translated from Chinese (simplified) to English (Google document translator) and included in the analysis.
Search results
The initial search for the key terms above resulted in 558 papers. Of these, 182 were duplicates and excluded. Seven papers were removed as they were not published in English. Titles and abstracts were read for the remaining 369 papers. In total, 339 papers were removed as they did not meet the inclusion/exclusion criteria, and 30 papers were selected for analysis. A further seven papers were sourced from citation searching, one of which was excluded, resulting in 36 papers. The selection process is outlined in Fig. 1.
The secondary search resulted in additional 444 results. Of these, 106 were duplicates and excluded. Titles and abstracts were read for the remaining 338 papers. After the removal of 336 papers that did not meet the inclusion/exclusion criteria, two additional papers were taken for analysis.
Rats were included in the original search terms to identify literature for this integrative review. However, due to the limited number of identified manuscripts (five papers), these were not included in the main body of the review which solely focused on mouse models. However, some of these rat models are of note and have been included in the discussion.
Commonly referenced models
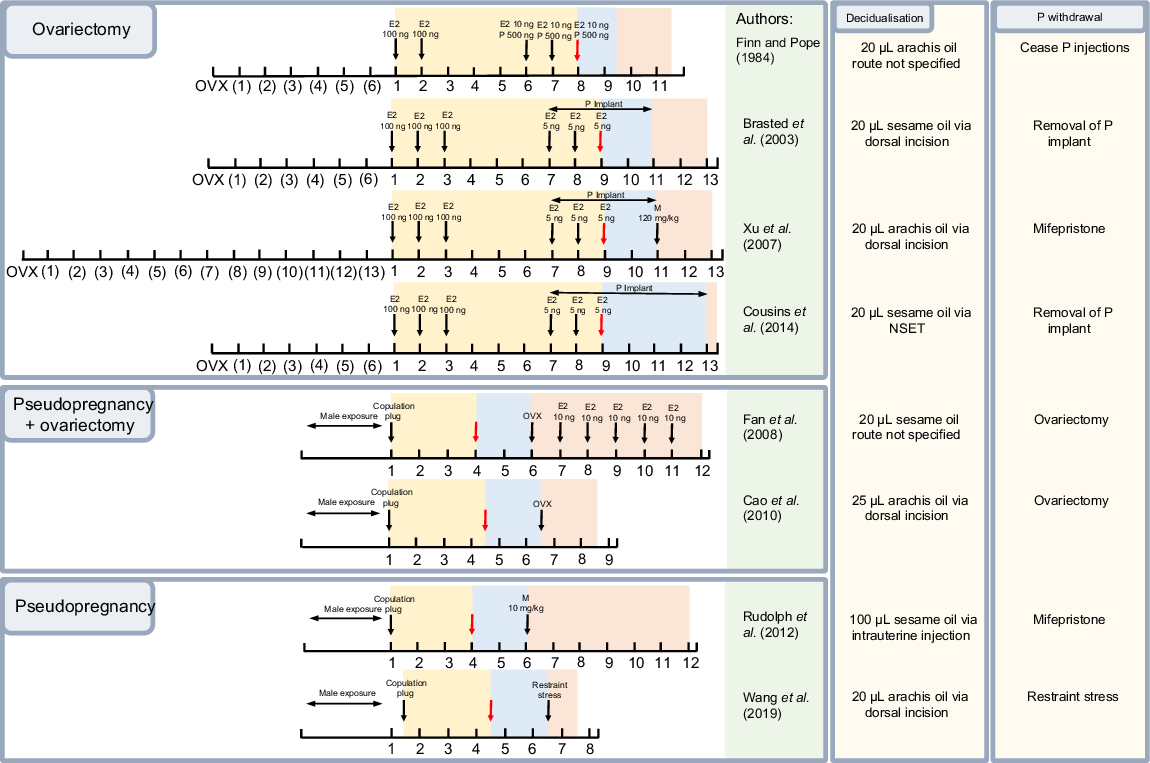
Numerous studies have used mice to model menstruation. The protocols used in these studies can be broadly grouped based on eight original models (Fig. 2). Several variables distinguished the models from each other: (1) whether the animals were ovariectomised or intact, (2) the length of the post ovariectomy rest period (if applicable), (3) the dose and timing of hormone administration for endometrial priming, (4) the stimuli and timing of decidualisation induction, (5) the timing and method of progesterone withdrawal, and (6) the timing and length of the tissue sampling period. A number of studies did not cite a reference model; however, their methods appeared influenced by at least one of the eight aforementioned models.
Representative timeline of original menstruation models. Yellow days indicate the endometrial priming period that mimics the proliferative phase of the menstrual cycle. Blue days indicate the decidualisation period mimicking the secretory phase. Orange days indicate the days post-progesterone withdrawal mimicking the menstrual phase of the cycle. Number of days indicated on axis. Black arrow = injection denoted on image, red arrow = decidualisation stimulus, E2 = oestradiol, P = progesterone, OVX = ovariectomy, M = mifepristone.
Priming the endometrium to undergo decidualisation is the first step in inducing a menstrual-like process (Finn and Pope 1984). In humans, changes in endometrial thickness are initiated by cyclic fluctuations in oestrogen and progesterone levels (Brasted et al. 2003). In mouse models, these fluctuations must be replicated. Firstly, an increase in oestrogen concentration, as occurs during the proliferative phase to encourage endometrial growth, is induced, and secondly, progesterone levels must increase to induce and maintain the decidualised endometrial tissue. This hormonal modulation can be achieved in mice using two different methods: ovariectomy and subsequent treatment with exogenous hormones, or induction of pseudopregnancy.
The first attempts to model menstruation in mice employed ovariectomy as the method of restricting endogenous hormones (Finn and Pope 1984). This method allows for unequivocal and simplified hormonal control as all endogenous ovarian input is removed. Variables for this procedure include incision location, anaesthesia, analgesia, and recovery period (not further discussed).
After the removal of the ovaries, administration of exogenous hormones is necessary to induce uterine function. In the absence of oestrogens, the uterus will regress, and the oestrogen-dependant proliferative phase will stall (De Clercq et al. 2017). Exogenous progesterone is then administered to mimic the secretory phase. The exact dose and timing of hormone administration can vary (Fig. 2). As progesterone is required for the maintenance of decidual tissue, a key tissue in menstruation, the timing of administration of these hormones, while nuanced, may be impactful. These models require the artificial induction of decidualisation as there is no implanting embryo to naturally induce this process.
While the timeline of hormone administration is similar across most of the ovariectomy-based models, the method of progesterone administration varies between the use of a subcutaneous implant versus repeated injections. The earliest model (Finn and Pope 1984) administered multiple progesterone injections over a period of 3 days. Later, Brasted et al. (2003) introduced subcutaneous steroid-filled silicone rubber tubing implants as the delivery route. Implants are beneficial as they reduce variability in serum progesterone levels and provide a way to withdraw progesterone in a rapid manner (Brasted et al. 2003). These benefits are reflected in the extensive use of subcutaneous implants in subsequent mouse models following their introduction.
Pseudopregnancy can also be used to achieve menstrual-like endometrial breakdown in mice (Rudolph et al. 2012). Mating female mice induces the release of both oestrogens and the formation of a functional (progesterone producing) corpus luteum as pregnancy is initiated (Cao et al. 2010). Mating with vasectomised males initiates these same changes, priming the uterus for decidualisation without initiating pregnancy. The interventions required in this model are limited to a decidualisation stimulus and a means of progesterone withdrawal, the timing of which can vary (Fig. 2). Decidual stimuli are required in these models also as, with pseudopregnancy, there is no fertilised embryo. A pseudopregnancy-based model achieves menstruation through largely intrinsic changes rather than relying on the exogenous intervention used with the ovariectomy model (Rudolph et al. 2012).
Both ovariectomy-based and intact (pseudopregnancy) models require artificially induced endometrial decidualisation. In the early twentieth century, it was found that a decidual reaction could be artificially induced in rodent endometrium (Loeb 1908). In this initial experiment, deciduomata (decidualised tissue) were created in pregnant guinea pigs by making cuts along the uterine wall. Following Loeb’s (1908) observations, it became apparent that decidualisation could be induced in a number of other species, such as rats (Long and Evans 1922; Evans 1928). Foreign bodies, such as silk thread piercing the endometrium, were found to be an effective decidual stimulus (Evans 1928), as well as oils, agar, carrageenan, and heparin (Finn and Keen 1963). Oil is now the most commonly used decidual stimulus (De Clercq et al. 2017). The oil, often peanut (arachis) or sesame oil, is injected into the uterine horn accessed by laparotomy (Finn and Pope 1984), laparoscopy (Peterse et al. 2018a) or via the vagina and cervix (Cousins et al. 2014). Laparotomy requires a large incision through which the uterine horn can be visualised and the stimulus injected. Injection via laparoscopy requires a small incision with an endoscope inserted through to visualise the area, and a secondary incision through which a needle is placed to inject into the uterine horn. Vaginal administration requires no incision and is instead performed by introducing oil into the uterus via the vagina and cervix. Vaginal administration can be done with a blunt catheter attached to a syringe (Peterse et al. 2018a), or a non-surgical embryo transfer device (NSET) (Cousins et al. 2014). The use of an NSET may be preferable to that of the blunt catheter due to potentially producing a reduced inflammatory response, though more research is needed in this area.
A limitation of artificial decidualisation is the inter-animal variability in decidual development. Various failure rates have been reported between the different methods (Peterse et al. 2018a). Reported failure rates vary between 11 and 30% (Table 1). Peterse et al. (2018a) proposed that the method by which the decidual stimulus is introduced to the uterus may influence this rate of failure. The authors found that vaginal decidualisation had the highest failure rate, while the surgical options produced a lower failure rate. While laparotomic and laparoscopic methods may result in less decidualisation failure, surgical options are more invasive, posing more risk of infection and stress to the animal. Environmental disruptions that can stress animals such as changes to light/dark cycles, loud noises, and movement may also impact decidualisation. These disruptions should be, and often are, minimised where possible in rodent research facilities, though further research into their impact on decidualisation success would be beneficial.
| Stimulus | Volume | Method | One/both uterine horns | Failure rate | Paper | |
|---|---|---|---|---|---|---|
| Arachis (peanut) oil | 15 μL | N/A | N/A | N/A | Li et al. (2012) | |
| N/A | Both | N/A | Zhang et al. (2016) | |||
| Dorsal incision | N/A | N/A | Chen et al. (2020) | |||
| N/A | Both | N/A | Zhou et al. (2024) | |||
| 18 μL | Intracervical using non-surgical transfer device | One | N/A | Armstrong et al. (2017) | ||
| 20 μL | N/A | Both | 17% | Finn and Pope (1984) | ||
| Laparotomic injection | Both | N/A | Cheng et al. (2007) | |||
| Dorsal incision | One (left) | N/A | Wang et al. (2013) | |||
| Micro syringe | Both | N/A | Xu et al. (2013a) | |||
| Dorsal incision | One (Left) | N/A | Xu et al. (2013b) | |||
| Dorsal incision | N/A | N/A | Chen et al. (2015) | |||
| Dorsal incision | Both | N/A | Wang et al. (2019) | |||
| Intracervical using non-surgical transfer device | One | 20% | Mao et al. (2023) | |||
| Dorsal incision | One (left) | N/A | Xu et al. (2007) | |||
| Intracervical using non-surgical transfer device | One | N/A | Maybin et al. (2018) | |||
| Intracervical using non-surgical transfer device | N/A | 16.6% | Reavey et al. (2021) | |||
| Dorsal incision | Both | N/A | Wang et al. (2023) | |||
| Intracervical using non-surgical transfer device | N/A | Approx. 10% | Martínez-Aguilar et al. (2024) | |||
| Sesame oil | 20 μL | Dorsal incision | One (Right) | 30% | Brasted et al. (2003) | |
| Dorsal incision | One (Right) | N/A | Kaitu’u-Lino et al. (2005) | |||
| Dorsal incision | One (Right) | N/A | Kaitu’u-Lino et al. (2007a) | |||
| Dorsal incision | One (Right) | N/A | Kaitu’u-Lino et al. (2007b) | |||
| N/A | One (Right) | N/A | Morison et al. (2007) | |||
| N/A | Both | N/A | Fan et al. (2008) | |||
| Dorsal incision | One (Right) | N/A | Kaitu’u-Lino et al. (2009) | |||
| N/A | One | N/A | Patterson et al. (2013) | |||
| Non-surgical transfer device | One | N/A | Cousins et al. (2014) | |||
| Non-surgical transfer device | One | N/A | Cousins et al. (2016b) | |||
| Non-surgical transfer device | One | N/A | Cousins et al. (2016c) | |||
| Non-surgical transfer device | One | N/A | Cousins et al. (2016a) | |||
| Non-surgical transfer device | Both | N/A | Cousins et al. (2019) | |||
| N/A | One (Right) | N/A | Evans et al. (2015) | |||
| Vaginal injection | Both | 25% | Peterse et al. (2018a) | |||
| Laparoscopic injection | Both | 17% | ||||
| Laparotomic injection | Both | 11% | ||||
| 50 μL | N/A | One | N/A | Menning et al. (2012) | ||
| 100 μL | Intrauterine injection | Both | N/A | Rudolph et al. (2012) | ||
| Intrauterine injection | Both | N/A | De Clercq et al. (2017) | |||
| Intrauterine injection | Both | N/A | Peterse et al. (2018b) |
Note: Failure rate = the percent of animals who did not display decidualised tissue following stimuli. N/A = authors did not specify this variable.
Using a larger volume of oil as the decidual stimulus both reduces failure rate and increases the size of the decidual reaction (Peterse et al. 2018a). In their pseudopregnancy model, Rudolph et al. (2012) suggest that the observed uniformly distributed decidualisation through both uterine horns was due to the use of a 5-fold larger stimulus than other models. While this increased volume of oil potentially reduces the decidual failure rate, it may not be the most appropriate option in studies of human physiology. In human females, the extent of decidualisation is naturally more restricted prior to menstruation relative to the deciduoma induced in mice. Artificially induced decidualisation in mice occurs rapidly and in an extreme manner, spreading through the entire uterine horn. In humans at the time of menstruation, decidualised tissue is limited to small areas surrounding spiral arterioles (Salamonsen and Evans 2018).
The final key event to be mimicked in a menstrual-like event is the withdrawal of progesterone. Maintenance of decidualised cells requires progesterone signalling, the withdrawal of which initiates the breakdown and shedding of endometrial tissue (Maybin and Critchley 2015). After this initial withdrawal, there is a rescue period (up to 12 h in mice) where progesterone can be returned and endometrial breakdown will not occur (Wang et al. 2013). Methods of inducing this withdrawal differ: progesterone receptor activation may be antagonised, or the ovarian activity may be inhibited completely.
As the first reported menstrual model administered progesterone via subcutaneous injection, withdrawal was done simply by ceasing these injections (Finn and Pope 1984). Similarly, the models that used progesterone implants require only the removal of the implant to stop its action (Brasted et al. 2003). Cousins et al. (2014) found that after implant removal, progesterone levels dropped rapidly, reducing by 50% within 4 h of the implant removal.
Progesterone activity can also be withdrawn pharmacologically using progesterone receptor antagonists such as mifepristone (RU486) (Xu et al. 2007). With this drug, progesterone remains physiologically present within the body; however, it is unable to act on progesterone receptors. Pharmacological intervention produces a more time-controlled, rapid and complete reduction of progesterone action in comparison to other withdrawal methods (Xu et al. 2007). However, mifepristone administration is associated with an increase in serum oestradiol levels, peaking at 16 h post mifepristone, a potentially undesirable artefact (Xu et al. 2007). The authors suggested this increase in oestradiol may be related to metabolism pathways of the drug; however, the mechanism is unclear. While being useful to block progesterone receptor, mifepristone also has a high affinity for the glucocorticoid receptor. The creators of the initial mifepristone protocol did not use it in a later paper (instead used implant/removal) due to the drug’s potential conflict with glucocorticoids, an area of focus in their particular research (Xu et al. 2013a).
Interestingly, of all pseudopregnancy-based papers, 67% use ovariectomy as a means of progesterone withdrawal. Following ovariectomy, Fan et al. (2008) found it necessary to administer 10 ng/100 μL of oestradiol (E2) to animals daily for the remainder of the experiment. The authors noted this exogenous oestradiol was administered to stop uterine regression in response to the complete loss of oestrogen following ovariectomy. Interestingly, other papers that used ovariectomy to withdraw progesterone did not administer oestradiol following the procedure (Cao et al. 2010). While these other papers had a tissue sampling period of up to 48 h, Fan et al. (2008) had a tissue sampling period of 6 days allowing more time for the uterus to be impacted by the loss of oestrogen. This long sampling period makes it unsurprising that the authors had to treat the uterus with oestradiol after ovariectomy. Rudolph et al. (2012) opted to use a single injection of mifepristone to withdraw progesterone activity. This use of a pharmacological antagonist as opposed to ovariectomy allowed for a longer tissue sampling timeframe without requiring exogenous hormone treatment.
Restraint stress can also trigger a reduction in progesterone levels and endometrial bleeding in artificially decidualised pseudopregnant mice (Wang et al. 2019). Wang et al. (2019) subjected mice to restraint stress by placing them into ventilated tubes for 3 h. Following restraint, mice had increased corticosterone levels, and a significant decline in serum progesterone levels with no change in oestradiol levels, followed by overt vaginal bleeding 24 h after the stress event.
Menstruation detection methods
Histological analysis of morphological changes occurring in the endometrium is the most common way to assess breakdown. Haematoxylin and eosin (H&E) staining of the uterine tissue aids assessment of general morphological changes such as tissue decidualisation, or the proliferation and breakdown of stromal endothelial cells (Xu et al. 2007; Chen et al. 2015). Brasted et al. (2003) graded tissue morphology following an arbitrary scoring system of zero to four with ‘0 = no, 1 = minimal, 2 = moderate, 3 = extensive, 4 = profound signs of destruction of the target cellular structure (including loss of adherence between cells, rounding of nuclei, large interstitial spaces)’. Following this, similar scoring systems with more detail have been introduced such as that by Kaitu’u-Lino et al. (2007a) where tissue was graded on a scale from one to five, indicating decidualised tissue through breakdown and to full repair with descriptions of the morphological features observed at each of these stages.
Histological analysis has demonstrated that the timeframe in which endometrial tissue breaks down and subsequently repairs varies between menstruation models. A representative schematic of histological images taken from papers following different protocols across the breakdown process is shown in Fig. 3a. The earliest model (ovariectomy-based) with published histological results indicated that breakdown was not apparent at 12 h post-progesterone withdrawal (Brasted et al. 2003). By 16 h post-progesterone withdrawal, changes in morphology were visible including a reduction in contact between decidualised cells. This progressed significantly by 24 h when large areas of necrotic decidualised tissue were observed, and the endometrium began to shed from the myometrium. Dissociation of the endometrium from the underlying uterine tissue appeared to occur as one large piece. By 36 h post-progesterone withdrawal, the sloughed endometrial debris was seen within the centre of the lumen. At this time, the remaining endometrium had begun re-epithelialisation. The luminal debris was largely gone by 48 h post-progesterone withdrawal, and the endometrium appeared similar to its pre-decidual state. This general 48-h progression is similar to those reported to occur in other ovariectomy-based models (Fig. 3b).
(a) Schematic of mouse uterus cross section during induced menstrual breakdown. (b) The progression of breakdown in the different models. Created in BioRender. Rogers, L. (2025) https://BioRender.com/e96i328.
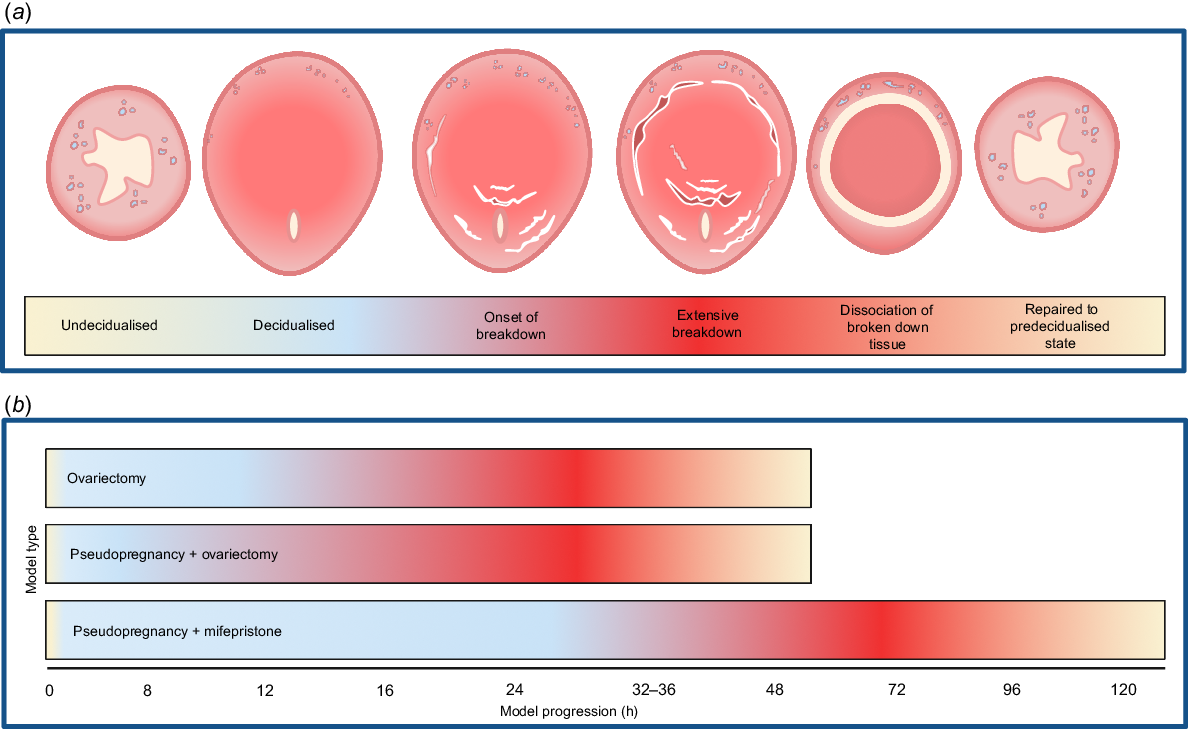
Many of the pseudopregnancy-based models follow a similar progression of breakdown and repair as the ovariectomy-based models. Cao et al. (2010) indicated that by 8 h post-progesterone withdrawal, there were signs of breakdown as decidualised cells underwent apoptosis. By 12 h, cell necrosis and blood vessel haemorrhaging were present. The decidualised cells throughout the functionalis had degenerated at 16 h with the tissue separated from the underlying tissue layer. Bleeding and necrotic tissue within the lumen was present at 24 h while simultaneously the remaining uterine tissue was beginning to repair. At 32 h, the shed epithelium was fully dissociated within the lumen. Repair appeared complete by 48 h after progesterone withdrawal; the endometrium was intact with no signs of breakdown. Similarly, Chen et al. (2015) reported that tissue necrosis and haemorrhage were present at 12 h after progesterone withdrawal. This tissue was separated from the underlying endometrium at 24 h. Rapid re-epithelialisation was observed by 32 h post withdrawal. By 48 h after progesterone withdrawal, the endometrium was fully restored (Fig. 3b). Despite administering oestradiol following ovariectomy to withdraw progesterone, Fan et al. (2008) also reported that the endometrium was restored by this 48-h timepoint.
One pseudopregnancy model was an outlier to this 48-h breakdown and repair process. The authors reported a far less rapid progression from breakdown to repair than all other models: breakdown was not yet visible at 24 h after progesterone withdrawal (Rudolph et al. 2012). By 48 h, signs of breakdown were apparent and endometrial tissue dissociation was present at 72 h post withdrawal. Re-epithelialisation was present at 120 h, in total taking 72 h longer than other models (Fig. 3b). The main point of difference between these pseudopregnancy models was that Rudolph et al. (2012) used mifepristone as a means of progesterone withdrawal, while the others ovariectomised their animals after decidualisation to completely remove progesterone (and other ovarian factors) from the system.
Endometrial breakdown is also evidenced by the presence of blood cells within the vaginal canal. This is detected by vaginal lavage in most cases, though blood cells can also be collected by vaginal swabbing. Rudolph et al. (2012) created a scoring system to characterise bleeding intensity by staining vaginal lavage smears with H&E. The categories ranged from 1 to 5 based on a small versus large number of erythrocytes in the lavage and included accompanying images. Bleeding can also be measured by alkaline haematin analysis of blood collected using cotton pellets placed in the mouse vagina (Menning et al. 2012; Mao et al. 2023; Martínez-Aguilar et al. 2024). Cotton pellets, however, can prove difficult as they can fall out or be removed by the animals. To prevent this, paper collars may be put on the mice (Menning et al. 2012) or the vagina can be sutured shut (Martínez-Aguilar et al. 2024). Suturing of the vagina, however, is invasive and can lead to animals reportedly gnawing on the sutures (Martínez-Aguilar et al. 2024).
While overt bleeding is commonplace in naturally menstruating animals, many early models did not formally document the occurrence of this event. Rudolph et al. (2012) were the first to characterise overt bleeding in mice; the authors noted overt bleeding was caused by a more prolonged endometrial bleeding period than seen in ovariectomy-based models. However, Menning et al. (2012) published research using an ovariectomy model that same year that also displayed overt bleeding. The onset of vaginal bleeding in ovariectomy-based models occurs between 4 and 16 h after progesterone withdrawal, shown in Table 2 (Xu et al. 2007; Menning et al. 2012; Cousins et al. 2014; Zhang et al. 2016). Peak bleeding was reported between 16 and 32 h. Cessation of bleeding was reported between 24 and 72 h post withdrawal. While other pseudopregnancy-based models displayed similar bleeding patterns (Cao et al. 2010), Rudolph et al. (2012) reported a later onset of bleeding at 48 h and cessation at 144 h after progesterone withdrawal. Wang et al. (2019) did not report bleeding peak or cessation times; however, the onset did not occur until 24 h which also indicates a more delayed bleeding period.
| Paper | Bleeding onset | Bleeding peak | Bleeding ceased | Method | |
|---|---|---|---|---|---|
| Xu et al. (2007) | 16 h | 32 h | 48 h (last check) | Vaginal smear | |
| Menning et al. (2012) | 8 h | 24 h | 72 h (last check) | Cotton ball | |
| Cousins et al. (2014) | 4 h | 12 h | 24 h (last check) | Overt vaginal bleeding | |
| Cousins et al. (2016a) | 4 h | 12 h | 24 h (last check 48 h) | Vaginal smear | |
| Zhang et al. (2016) | 8 h | 16 h | 24 h (last check) | Vaginal smear | |
| Cao et al. (2010) | 8 h | 32 h | 48 h (last check) | Vaginal smear | |
| Rudolph et al. (2012) | 48 h | 72 h | 144 h (last check) | Vaginal smear and overt vaginal bleeding | |
| Wang et al. (2019) | 24 h (only noted check) | N/A | N/A | Overt vaginal bleeding |
N/A = authors did not specify this variable.
A notable factor in the timing of bleeding cessation is an observer’s decision about the timeframe for checking bleeding. The bleeding cessation time point in all but one paper outlined in Table 2 is the last reported time point chosen by the authors. While a reduction in the number of animals bleeding or intensity is observed at this time point, in many cases there is still evidence of bleeding occurring in at least some animals. One paper included timepoints up to 48 h, after bleeding ceased in their animals, therefore confirming when bleeding was fully complete in their model (Cousins et al. 2016a). Having additional extended observation time points may be useful to clarify when bleeding has completely ceased.
Discussion
Eight main variations in mouse models of menstruation were identified in the literature, many of which stem back to an original ovariectomy-based model (Finn and Pope 1984). A small number of alternative models also exist that use pseudopregnancy to modulate hormonal activity. Between the models, there appear to be key differences in the timeline of tissue breakdown and repair, and in the extent of tissue change. The length of time over which endometrial breakdown occurs is the most notable difference between models. A potential factor in this variation is the used method of hormonal modulation. The withdrawal of all ovarian input via ovariectomy produces a far more rapid endometrial breakdown in comparison to the simple withdrawal of progesterone activity alone, implying that ovarian factors may play a larger role in the breakdown process than previously thought (Kaitu’u-Lino et al. 2007b; Fan et al. 2008; Cousins et al. 2016a).
Advantages and disadvantages of mouse modelling
As a means of researching menstruation, mouse models have multiple advantages. Anatomically, mice have both macro- and microscopic similarities to humans, which have recently been reviewed in detail (Ruberte et al. 2023). The glaring difference between the two species is that laboratory mice do not menstruate; however, artificially induced endometrial breakdown is a useful tool. These models have been used to investigate a wide range of processes involved in menstruation, including the role of inflammation (Kaitu’u-Lino et al. 2007a; Cousins et al. 2014, 2016b; Armstrong et al. 2017), mesenchymal-epithelial transition during endometrial repair (Patterson et al. 2013; Cousins et al. 2014; Kirkwood et al. 2019), and stem cell contribution (Cousins et al. 2019). Animal models are also useful for investigating how alterations in controlled variables impact the breakdown process. For instance, Maybin et al. (2018) used the model to assess whether local hypoxia responses are necessary for endometrial repair. While hypoxia was previously linked to endometrial function (Fan et al. 2008; Cousins et al. 2016c), the mechanisms by which this occurs were unknown, due, at least in part, to the technical and ethical difficulties in modulating hypoxic conditions in menstruating individuals. The authors noted a significant dysregulation in hypoxia inducible factor-1 (HIF-1) in individuals with heavy menstrual bleeding. Based on this, through testing of the mechanisms by which hypoxia works in mice — by blocking the action of HIF-1 — the authors were able to demonstrate that dysregulation in hypoxia response is a potential mechanism for heavy menstrual bleeding in humans. Other studies have used mouse modelling to investigate potential therapeutic targets for heavy menstrual bleeding by modulating factors such as HIF-2A and assessing differences in bleeding volume and repair extent in mice (Martínez-Aguilar et al. 2024). Furthermore, models of endometriosis have been developed using shed endometrial tissue collected from established menstruation models to investigate the pathophysiology of this condition (Greaves et al. 2014, 2015, 2017; Cousins et al. 2020).
Specificity in the terminology used when discussing models is crucial to ensure what is known about natural menstruation is not confounded by results obatined from studies of artificial endometrial breakdown and repair. Kaitu’u-Lino et al. (2007a) investigated the role of inflammatory responses in the endometrium using mouse models. The authors specifically referred to their outcomes in terms of endometrial breakdown and repair, as opposed to menstrual breakdown or menstruation. When discussing these outcomes, a specific set of terminology including explicitly saying endometrial breakdown and then likening to human menstruation in discussion, allows for clearer distinction between animal derived research and conclusions based on observations of human physiology.
Despite their lack of natural menstruation, laboratory mice are relatively small and therefore do not impose the same requirements for large facilities as more sizeable animals such as sheep or primates. Secondly, laboratory mice have less complicated upkeep requirements than other species, such as the naturally menstruating spiny mouse. The spiny mouse is highly impacted by both the environment and social interaction. These animals are sensitive to changes in diet and light/dark cycles, meaning these must be carefully selected and tightly controlled so as not to impact research outcomes (Haughton et al. 2016; Bellofiore and Evans 2019). The skin of the spiny mouse is very delicate due to possessing a skin degloving response that aids predator evasion in the wild (Bellofiore and Evans 2019; Bellofiore et al. 2020; Liu et al. 2020). Such delicate skin poses a risk of handling injuries and requires researchers to have specialised training for these animals. Therefore, the housing and upkeep of laboratory mice is often the least costly option. Events such as pseudopregnancy are easy to induce in mice, while this can be more difficult in larger species. There is also a long history of developing research technology specifically for use in laboratory mice. These tools are less developed for many other species — the current technology does not often translate across species. For example, the immunohistochemical markers CD45 and CD68 which are commonly used as markers of immune cells and macrophages, respectively, in laboratory mice, are not cell specific in spiny mouse tissue (Bellofiore et al. 2018b).
While naturally menstruating primates may be the best means of researching menstruation, as they are the physiologically closest to humans, there are deep rooted ethical considerations and concerns associated with the use of primates for biomedical research. Feelings of great discomfort are often associated with the idea of primate research models (Phillips et al. 2014). Primates are comparatively more expensive in terms of housing and maintenance, but also in terms of time input. Primates take longer than rodents to reach sexual maturity, with the rhesus macaque taking approximately 2.5–3.5 years (Mattison and Vaughan 2017). In comparison, mice can reach sexual maturity by 8 weeks of age (Dutta and Sengupta 2016). Cycle length also differs from laboratory rodents. The primate menstrual cycle varies between species; the rhesus macaque has an average cycle length of 25.5–29.5 days (Johnson and Phoenix 1978; Shimizu 2008). This provides a great comparative cycle timeframe to humans; however, it means that experiments take considerably longer than those using mouse models, especially pseudopregnancy models that do not require an ovariectomy recovery period. These factors result in a higher monetary and temporal cost associated with primate research. Due to these cost-limitations, ethical considerations, and general animal availability, it is likely that laboratory mice will remain the common animal used in the modelling of menstruation.
An unavoidable disadvantage of working with laboratory mice is the variability between animals which may impart variability on results. Inbreeding animals (to create specific strains) allows for a reduction in genetic variation. However, despite inbreeding, there is still the problem of developed variability between mice on an inter- and intra-strain level. Mouse strains are considered genetically distinct once removed from the original strain for 20 generations (Mekada et al. 2009). There are now several sub-strains of C57BL/6 mice available, many of which have been used in menstrual modelling, such as C57BL/6 J, C57BL/6JOlaHsd, C57BL/6N, and C57BL/6JRj. Between these sub-strains, there is both genetic and behavioural variability (Mekada et al. 2009; Matsuo et al. 2010; Chebib et al. 2021).
It is generally difficult to study repeat or cyclic events when they must be artificially induced. Ovariectomy-based models are difficult to undertake in a repeated manner due to the complete loss of ovarian input on the reproductive system. Pseudopregnancy-based models may be more advantageous in such cases, however, it would be hard to observe any effect on the uterus without its removal. Therefore, only research focused on observations of overt bleeding may be beneficial in this case. As the extent of vaginal bleeding is a difficult measure to accurately quantify and does not necessarily occur in all models, it is hard to determine whether useful data could be gained from repeat menstruation models even if the menstruation itself could be induced multiple times.
Ovariectomy vs pseudopregnancy models
Ovariectomy-based models provide tight control over timing and hormone level, however these models are invasive. A high degree of invasiveness produces both direct and indirect impacts on the animal and the results of the research. Directly, models that rely on surgery introduce the inherent risk of unfavourable surgical outcomes. For example, Mao et al. (2023) noted in their research that one of the animals died from incisional hernia following ovariectomy. Survival rates are not commonly reported; however, an investigation into methods to reduce mortality after ovariectomy in obese mice reported that their control animals (non-obese mice) had a 31% mortality rate after an ovariectomy procedure (Mattheis et al. 2016). Secondarily, surgery introduces increased stress to the animal, raising ethical issues and may also impact results. Increased corticosteroids in response to stress have been shown to impact the reproductive system of mice (Wei et al. 2019; Zheng et al. 2020). As Wang et al. (2019) showed, restraint stress can induce a drop in progesterone levels large enough to induce menstrual breakdown in pseudopregnant mice. Breakdown and repair happen rapidly in ovariectomy-based models, often within 48 h. Alongside the speed of this process is its severity. Breakdown of endometrial tissue spreads across the uterus resulting in the shedding of the endometrium in a large clump of tissue that can be seen held within the uterine lumen, separate from the wall. In humans, breakdown reportedly occurs in a piecemeal manner where patches break down alongside simultaneous repair in other patches. The shedding of the entire endometrium in one piece is possible in humans; however, this event, called a membranous dysmenorrhoea or a decidual cast, is uncommon and can require medical treatment (Jyoti et al. 2019).
Pseudopregnancy-based models provide less invasive means of inducing menstruation; however, control of hormone levels and timing is less precise as the model relies partly on intrinsic hormonal modulation. Interestingly, multiple pseudopregnancy-based models use ovariectomy later as a means of progesterone withdrawal (Fan et al. 2008; Cao et al. 2010). These models produce outcomes that are largely similar to ovariectomy-based models, while models that do not ovariectomise animals at any stage produce a different outcome (Rudolph et al. 2012). Breakdown and bleeding in these intact models are both later in onset and more extended. This extended menstrual outcome could be described as more physiologically like human cycling, as opposed to ovariectomy models that produce a 48-h rapid breakdown and repair cascade in the endometrium. While intact pseudopregnancy-based models may provide a more physiologically similar breakdown to humans, the extended breakdown and more piecemeal manner may make the model less suitable in certain studies. For example, mouse models of endometriosis utilise shed endometrial tissue from menstrual models to subsequently implant elsewhere, mimicking endometriosis lesions (Greaves et al. 2014, 2015, 2017; Cousins et al. 2020). These models require large amounts of endometrial tissue, meaning pseudopregnancy may not be appropriate in such cases. We have summarised pros and cons of ovariectomised and pseudopregnancy models in Table 3 which highlights that no model is ’one size fits all’ and every study should pick one that best aligns with their research focus.
| Ovariectomy | Intact pseudopregnancy | |||
|---|---|---|---|---|
| Pros | Cons | Pros | Cons | |
| • Used in multiple papers – well documented | • Invasive | • Less invasive | • Mating may not induce pseudopregnancy 100% of time | |
| • Highly time controlled | • Risk of surgical complications | • Relies on intrinsic hormonal changes | • Less time control | |
| • More ability to control hormone levels | • Requires multiple injections to maintain hormones | • Breakdown progression and morphology more similar to humans than ovariectomy | • Mifepristone may conflict with certain research focuses, e.g. cortisol | |
| • Produces large amount of shed endometrial tissue | • Breakdown rapid and extensive | • Easier to repeat if wanting to study multiple menstruation events before uterine collection | • Male mice need vasectomy | |
| • Currently less research using this model | ||||
Despite the process of breakdown and repair occurring in these models similarly to human menstruation, at the molecular level decidualised tissue differs between artificially induced and naturally produced decidualisation that occurs during pregnancy (Wang et al. 2020). RNA-seq assessment of artificially induced, natural and in vitro decidualised mouse tissue indicated that there were differences in gene expression between the modes of decidualisation induction. Artificial decidualisation was more similar to natural pregnancy decidualisation than in vitro decidualisation and provides a useful option to study decidual tissue without interference from embryonic factors. Despite in vitro decidualisation producing the most different gene expression, the method is advantageous for allowing manipulations in gene expression. A similar study compared artificially decidualised to naturally decidualised human tissue (Doi-Tanaka et al. 2024). This study also identified functional differences in gene expression between the modes of decidualisation induction. As dysfunction in the decidualisation process has been implicated in abnormal menstrual bleeding (Mao et al. 2023), the ability to model decidualisation in several ways is likely an important future avenue in menstruation focused research. To our knowledge, there is no research quantifying the differences in gene expression between mouse (whether artificial or natural) and human decidualised tissue.
Difference between hormone withdrawal methods: E&P withdrawal (OVX) vs P only (mifepristone)
There is a notable difference in the length of breakdown and repair between models; with breakdown and repair occurring over the course of 48 h in some and several days in others. The variable that differentiates these outcomes is the extent of hormonal withdrawal after decidualisation. Models that resulted in both the complete withdrawal of oestrogen and progesterone as well as other ovarian factors (due to ovariectomy) were faster than those that only withdrew progesterone activity (pseudopregnancy + mifepristone models). This suggests that when ovarian input is removed, the endometrium responds to induced changes in signalling and morphology differently. This outcome also suggests that the loss of other ovarian factors other than oestrogen and progesterone impact this endometrial breakdown and repair. For instance, the ovaries are a key producer of androgens which have been identified as a likely modulator of endometrial breakdown (Cousins et al. 2016b).
While Fan et al. (2008) continued to administer oestradiol after ovariectomy to avoid uterine regression, the endometrium still underwent complete repair in a similar timeframe to other ovariectomy models suggesting the oestrogen is not the determining factor for the onset and length of endometrial breakdown. It has previously been established that oestrogen is not required for endometrial repair (Kaitu’u-Lino et al. 2007b) suggesting that it is other ovarian related factors causing the extended breakdown period seen in the non-ovariectomised model (Rudolph et al. 2012).
It would be interesting to investigate how other ovarian factors impact menstrual progression to determine whether it is the point of difference in these models or if there is another mechanism at play. An increase in corticosterone induced by acute restraint stress appears to induce endometrial breakdown in artificially decidualised mice (Wang et al. 2019). The acute stress model of Wang et al. (2019) is an interesting experimental mechanism of inducing progesterone withdrawal without either ovariectomising or introducing exogenous drugs; however, an increase in glucocorticoid levels may produce unintended downstream effects. The physiological impacts that stress imparts on the body are well documented (Xiao et al. 1999). It would stand to reason that a surgical procedure vs a single subcutaneous injection would produce different stress responses with potentially downstream impacts on endometrial breakdown.
Rat menstruation models
It appears that early rat modelling was integral in the establishment of mouse menstruation models. Development of the current method of artificial decidualisation in mouse models traces back to early rat experiments (Long and Evans 1922; Evans 1928). A rat model of endometriosis used an ovariectomy-based model (Cousins et al. 2014) to induce menstruation to collect endometrial tissue for implantation into the uteri of other animals (Persoons et al. 2020). While the authors cite Cousins et al. (2014) for the model, Persoons et al. (2020) did not follow the modified method of non-surgical decidualisation induction and longer decidualisation period. The authors instead induced decidualisation via abdominal incision and retained a decidualisation period of 48 h, more similar to the model outlined originally by Brasted et al. (2003). As Cousins et al. (2014) described theirs as a modified version of the Brasted model, the changes made by Persoons et al. (2020) are not largely unexpected.
Two papers used early-pregnancy incomplete medical termination of pregnancy to model a menstrual-like breakdown of the endometrium (Li et al. 2023; Zhong et al. 2024). Rats were mated and miscarriage induced using mifepristone and misoprostol administered orally on day 7 of pregnancy. This produced both endometrial breakdown and subsequent vaginal bleeding, providing a useful rat model for investigating overt bleeding.
An interesting finding in rats is their ability to undergo spontaneous decidualisation during pseudopregnancy with vitamin E deficiency, requiring no external stimuli (Evans 1928; Lang et al. 2016). Following decidualisation, vaginal bleeding was also observed in these animals, making them a potential novel model of menstruation with minimal intervention. Evans (1928) observed what was described as the ‘placental sign’, the ‘appearance of blood in the vaginal canal at some time between the 13th and 15th days of (pseudo) pregnancy’. The authors noted this occurred in only a sub-group of their cohort. Similarly, Lang et al. (2016), reported that 18/34 pseudopregnant vitamin E deficient rats displayed blood in vaginal smears. While a potential method of modelling menstruation, attempts to increase the percent of animals bleeding would be necessary to make such a model viable. Running animals through multiple pseudopregnancy cycles may aid this as Lang et al. (2016) noted that the rate of bleeding increased with each successive cycle from 5.9% to 50% by the third (last attempted) cycle.
Conclusions
Over the last three decades, dozens of studies using mouse models of menstruation have been published. Among the models, there is a disconnect as to how the technical variables used produce their observed outcomes. What is also unclear from these studies is why a particular model was chosen for use. We have documented variations in induction of hormonal control, decidualisation, and hormonal withdrawal, and the links between these and how the endometrial breakdown process manifests. While the current models do have some notable factors that differentiate them from human menstruation, mice are the most convenient animals to investigate menstruation in vivo. This convenience makes it worthwhile to continue work to optimise mouse menstruation models in future.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
References
Armstrong GM, Maybin JA, Murray AA, Nicol M, Walker C, Saunders PTK, Rossi AG, Critchley HOD (2017) Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model. Scientific Reports 7(1), 17416.
| Crossref | Google Scholar | PubMed |
Bellofiore N, Evans J (2019) Monkeys, mice and menses: the bloody anomaly of the spiny mouse. Journal of Assisted Reproduction and Genetics 36(5), 811-817.
| Crossref | Google Scholar | PubMed |
Bellofiore N, Ellery SJ, Mamrot J, Walker DW, Temple-Smith P, Dickinson H (2017) First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus). American Journal of Obstetrics & Gynecology 216(1), 40.e41-40.e11.
| Crossref | Google Scholar |
Bellofiore N, Cousins F, Temple-Smith P, Dickinson H, Evans J (2018a) A missing piece: the spiny mouse and the puzzle of menstruating species. Journal of Molecular Endocrinology 61(1), R25-R41.
| Crossref | Google Scholar | PubMed |
Bellofiore N, Rana S, Dickinson H, Temple-Smith P, Evans J (2018b) Characterization of human-like menstruation in the spiny mouse: comparative studies with the human and induced mouse model. Human Reproduction 33(9), 1715-1726.
| Crossref | Google Scholar | PubMed |
Bellofiore N, Ellery SJ, Temple-Smith P, Evans J (2020) Pseudopregnancy and reproductive cycle synchronisation cannot be induced using conventional methods in the spiny mouse (Acomys cahirinus). Reproduction, Fertility and Development 32(4), 363-372.
| Crossref | Google Scholar | PubMed |
Boyd KL, Muehlenbachs A, Rendi MH, Garcia RL, Gibson-Corley KN (2018) Female reproductive system. In ‘Comparative anatomy and histology (Second Edition)’. (Eds PM Treuting, SM Dintzis, KS Montine) pp. 303–334. (Academic Press) 10.1016/B978-0-12-802900-8.00017-8
Brasted M, White CA, Kennedy TG, Salamonsen LA (2003) Mimicking the events of menstruation in the murine uterus. Biology of Reproduction 69(4), 1273-1280.
| Crossref | Google Scholar | PubMed |
Cao HZ, Xu X, Chen XH, Wang JD, He B (2010) Study on establishing menstruation model in mice using pseudo-pregnant mice. Journal of Reproductive Medicine 19(1), 35-39.
| Crossref | Google Scholar |
Carter AM (2018) Classics revisited: C. J. van der Horst on pregnancy and menstruation in elephant shrews. Placenta 67, 24-30.
| Crossref | Google Scholar | PubMed |
Catalini L, Fedder J (2020) Characteristics of the endometrium in menstruating species: lessons learned from the animal kingdom. Biology of Reproduction 102(6), 1160-1169.
| Crossref | Google Scholar | PubMed |
Chavez-MacGregor M, Van Gils CH, Van der Schouw YT, Monninkhof E, Van Noord PA, Peeters PH (2008) Lifetime cumulative number of menstrual cycles and serum sex hormone levels in postmenopausal women. Breast Cancer Research and Treatment 108(1), 101-112.
| Crossref | Google Scholar | PubMed |
Chebib J, Jackson BC, López-Cortegano E, Tautz D, Keightley PD (2021) Inbred lab mice are not isogenic: genetic variation within inbred strains used to infer the mutation rate per nucleotide site. Heredity 126(1), 107-116.
| Crossref | Google Scholar | PubMed |
Chen X, Liu J, He B, Li Y, Liu S, Wu B, Wang S, Zhang S, Xu X, Wang J (2015) Vascular endothelial growth factor (VEGF) regulation by hypoxia inducible factor-1 alpha (HIF1A) starts and peaks during endometrial breakdown, not repair, in a mouse menstrual-like model. Human Reproduction 30(9), 2160-2170.
| Crossref | Google Scholar | PubMed |
Chen X, Wu B, Wang S, Liu J, Gao H, Zhou F, Nan N, Zhang B, Wang J, Xu X, He B (2020) Hypoxia: involved but not essential for endometrial breakdown in mouse menstrual-like model. Reproduction 159(2), 133-144.
| Crossref | Google Scholar |
Cheng CW, Bielby H, Licence D, Smith SK, Print CG, Charnock-Jones DS (2007) Quantitative cellular and molecular analysis of the effect of progesterone withdrawal in a murine model of decidualization. Biology of Reproduction 76(5), 871-883.
| Crossref | Google Scholar |
Coleman K (2011) Caring for nonhuman primates in biomedical research facilities: scientific, moral and emotional considerations. American Journal of Primatology 73(3), 220-225.
| Crossref | Google Scholar | PubMed |
Cousins FL, Murray A, Esnal A, Gibson DA, Critchley HOD, Saunders PTK (2014) Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS ONE 9(1), e86378.
| Crossref | Google Scholar |
Cousins FL, Kirkwood PM, Murray AA, Collins F, Gibson DA, Saunders PTK (2016a) Androgens regulate scarless repair of the endometrial “wound” in a mouse model of menstruation. The FASEB Journal 30(8), 2802-2811.
| Crossref | Google Scholar |
Cousins FL, Kirkwood PM, Saunders PTK, Gibson DA (2016b) Evidence for a dynamic role for mononuclear phagocytes during endometrial repair and remodelling. Scientific Reports 6(1), 36748.
| Crossref | Google Scholar |
Cousins FL, Murray AA, Scanlon JP, Saunders PT (2016c) Hypoxyprobe™ reveals dynamic spatial and temporal changes in hypoxia in a mouse model of endometrial breakdown and repair. BMC Research Notes 9, 30.
| Crossref | Google Scholar |
Cousins FL, Dorien FO, Ong YR, Breault DT, Deane JA, Gargett CE (2019) Telomerase reverse transcriptase expression in mouse endometrium during reepithelialization and regeneration in a menses-like model. Stem Cells and Development 28(1), 1-12.
| Crossref | Google Scholar |
Cousins FL, Farley JK, Kerrigan R, Mukherjee S, Darzi S, Gargett CE, Deane JA (2020) The effects of hedgehog ligand neutralising antibody 5E1 in a mouse model of endometriosis. BMC Research Notes 1, 454.
| Crossref | Google Scholar |
Critchley HOD, Babayev E, Bulun SE, Clark S, Garcia-Grau I, Gregersen PK, Kilcoyne A, Kim J-JJ, Lavender M, Marsh EE, Matteson KA, Maybin JA, Metz CN, Moreno I, Silk K, Sommer M, Simon C, Tariyal R, Taylor HS, Wagner GP, Griffith LG (2020) Menstruation: science and society. American Journal of Obstetrics & Gynecology 223(5), 624-664.
| Crossref | Google Scholar | PubMed |
De Clercq K, Van den Eynde C, Hennes A, Van Bree R, Voets T, Vriens J (2017) The functional expression of transient receptor potential channels in the mouse endometrium. Human Reproduction 32(3), 615-630.
| Crossref | Google Scholar | PubMed |
Doi-Tanaka Y, Tamura I, Shiroshita A, Fujimura T, Shirafuta Y, Maekawa R, Taketani T, Sato S, Sugino N (2024) Differential gene expression in decidualized human endometrial stromal cells induced by different stimuli. Scientific Reports 14, 7726.
| Crossref | Google Scholar | PubMed |
Dutta S, Sengupta P (2016) Men and mice: relating their ages. Life Sciences 152, 244-248.
| Crossref | Google Scholar |
Evans HM (1928) Spontaneous deciduomata in pseudopregnancy with low vitamin E. American Journal of Physiology 85(1), 149-153.
| Crossref | Google Scholar |
Evans J, D’Sylva R, Volpert M, Jamsai D, Merriner DJ, Nie G, Salamonsen LA, O’Bryan MK (2015) Endometrial CRISP3 is regulated throughout the mouse estrous and human menstrual cycle and facilitates adhesion and proliferation of endometrial epithelial cells. Biology of Reproduction 92(4), 99.
| Crossref | Google Scholar |
Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR (2008) VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. The FASEB Journal 22(10), 3571-3580.
| Crossref | Google Scholar | PubMed |
Finn CA, Hinchliffe JR (1964) Reaction of the mouse uterus during implantation and deciduoma formation as demonstrated by changes in the distribution of alkaline phosphatase. Reproduction 8(3), 331-338.
| Crossref | Google Scholar |
Finn CA, Keen PM (1963) The induction of deciduomata in the rat. Development 11(4), 673-682.
| Crossref | Google Scholar |
Finn CA, Pope M (1984) Vascular and cellular changes in the decidualized endometrium of the ovariectomized mouse following cessation of hormone treatment: a possible model for menstruation. Journal of Endocrinology 100(3), 295-NP.
| Crossref | Google Scholar | PubMed |
Fitzgerald HC, Schust DJ, Spencer TE (2020) In vitro models of the human endometrium: evolution and application for women’s health. Biology of Reproduction 104(2), 282-293.
| Crossref | Google Scholar |
Greaves E, Cousins FL, Murray A, Esnal-Zufiaurre A, Fassbender A, Horne AW, Saunders PTK (2014) A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. The American Journal of Pathology 184(7), 1930-1939.
| Crossref | Google Scholar | PubMed |
Greaves E, Temp J, Esnal-Zufiurre A, Mechsner S, Horne AW, Saunders PTK (2015) Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. The American Journal of Pathology 185(8), 2286-2297.
| Crossref | Google Scholar | PubMed |
Greaves E, Horne AW, Jerina H, Mikolajczak M, Hilferty L, Mitchell R, Fleetwood-Walker SM, Saunders PTK (2017) EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Scientific Reports 7(1), 44169.
| Crossref | Google Scholar |
Haughton CL, Gawriluk TR, Seifert AW (2016) The biology and husbandry of the African spiny mouse (Acomys cahirinus) and the research uses of a laboratory colony. Journal of the American Association for Laboratory Animal Science 55(1), 9-17.
| Google Scholar | PubMed |
Holdsworth-Carson SJ, Menkhorst E, Maybin JA, King A, Girling JE (2023) Cyclic processes in the uterine tubes, endometrium, myometrium, and cervix: pathways and perturbations. Molecular Human Reproduction 29(5), gaad012.
| Crossref | Google Scholar |
Jain V, Munro MG, Critchley HOD (2023) Contemporary evaluation of women and girls with abnormal uterine bleeding: FIGO systems 1 and 2. International Journal of Gynecology & Obstetrics 162(S2), 29-42.
| Crossref | Google Scholar |
Johnson DF, Phoenix CH (1978) Sexual behavior and hormone levels during the menstrual cycles of rhesus monkeys. Hormones and Behavior 11(2), 160-174.
| Crossref | Google Scholar | PubMed |
Jyoti , Kumari S, Kumar D, Gupta P, Gupta N, Kumar A (2019) Recurrent decidual cast with membranous dysmenorrhea. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 8(2), 738-741.
| Crossref | Google Scholar |
Kaitu’u-Lino TJ, Shen J, Zhang J, Morison NB, Salamonsen LA (2005) Matrix metalloproteinases in endometrial breakdown and repair: functional significance in a mouse model. Biology of Reproduction 73(4), 672-680.
| Crossref | Google Scholar |
Kaitu’u-Lino TJ, Phillips DJ, Morison NB, Salamonsen LA (2009) A new role for activin in endometrial repair after menses. Endocrinology 150(4), 1904-1911.
| Crossref | Google Scholar |
Kaitu’u-Lino TJ, Morison NB, Salamonsen LA (2007a) Neutrophil depletion retards endometrial repair in a mouse model. Cell and Tissue Research 328(1), 197-206.
| Crossref | Google Scholar |
Kaitu’u-Lino TJ, Morison NB, Salamonsen LA (2007b) Estrogen is not essential for full endometrial restoration after breakdown: lessons from a mouse model. Endocrinology 148(10), 5105-5111.
| Crossref | Google Scholar |
Kirkwood PM, Gibson DA, Shaw I, Dobie R, Kelepouri O, Henderson NC, Saunders PTK (2019) Single-cell RNA sequencing and lineage tracing confirm mesenchyme to epithelial transformation (MET) contributes to repair of the endometrium at menstruation. Elife 16(11), e77663.
| Crossref | Google Scholar |
Kirkwood PM, Shaw IW, Saunders PTK (2021) Mechanisms of scarless repair at time of menstruation: insights from mouse models. Frontiers in Reproductive Health 3, 801843.
| Crossref | Google Scholar |
Kocaoz S, Cirpan R, Degirmencioglu AZ (2019) The prevalence and impacts heavy menstrual bleeding on anemia, fatigue and quality of life in women of reproductive age. Pakistan Journal of Medical Sciences 35(2), 365-370.
| Crossref | Google Scholar | PubMed |
Lang N, Wu B, He B, Wang L, Wang J (2016) Spontaneous decidualization in pseudopregnant rats with vitamin e deficiency. Biochemical and Biophysical Research Communications 473(4), 828-833.
| Crossref | Google Scholar | PubMed |
Li YF, Xu XB, Chen XH, Wei G, He B, Wang JD (2012) The nuclear factor-κB pathway is involved in matrix metalloproteinase-9 expression in RU486-induced endometrium breakdown in mice. Human Reproduction 27(7), 2096-2106.
| Crossref | Google Scholar |
Li Q, Ren J, Yang L, Sun H, Zhang X, Yan G, Han Y, Wang X (2023) Parsing the Q-markers of baoyin jian to treat abnormal uterine bleeding by high-throughput chinmedomics strategy. Pharmaceuticals 16(5), 719.
| Crossref | Google Scholar | PubMed |
Liu T, Shi F, Ying Y, Chen Q, Tang Z, Lin H (2020) Mouse model of menstruation: an indispensable tool to investigate the mechanisms of menstruation and gynaecological diseases. Molecular Medicine Reports 22(6), 4463-4474.
| Crossref | Google Scholar | PubMed |
Loeb L (1908) The production of deciduomata: and the relation between the ovaries and the formation of the decidua. Journal of the American Medical Association 50(23), 1897-1901.
| Crossref | Google Scholar |
Mao C, Liu X, Guo S-W (2023) Decreased glycolysis at menstruation is associated with increased menstrual blood loss. Reproductive Sciences 30(3), 928-951.
| Crossref | Google Scholar | PubMed |
Martínez-Aguilar R, Rowley BM, Walker C, Critchley HO, Carmeliet P, Maybin JA (2024) Limiting premenstrual endometrial hypoxia inducible factor 2 Alpha may fine-tune endometrial function at menstruation. The Journal of Clinical Endocrinology & Metabolism 110(4), 1135-1147.
| Crossref | Google Scholar |
Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T (2010) Behavioral profiles of three C57BL/6 substrains. Frontiers in Behavioral Neuroscience 4, 29.
| Crossref | Google Scholar |
Mattheis L, Jung J-S, Hiebl B, Garrels W, Kielstein H, Spielmann J (2016) Perioperative support reduces mortality of obese BALB/c mice after ovariectomy. Lab Animal 45(7), 262-267.
| Crossref | Google Scholar | PubMed |
Mattison JA, Vaughan KL (2017) An overview of nonhuman primates in aging research. Experimental Gerontology 94, 41-45.
| Crossref | Google Scholar |
Maybin JA, Critchley HOD (2015) Menstrual physiology: implications for endometrial pathology and beyond. Human Reproduction Update 21(6), 748-761.
| Crossref | Google Scholar | PubMed |
Maybin JA, Murray AA, Saunders PTK, Hirani N, Carmeliet P, Critchley HOD (2018) Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation. Nature Communications 9(1), 295.
| Crossref | Google Scholar | PubMed |
Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, Yoshiki A (2009) Genetic differences among C57BL/6 substrains. Experimental Animals 58(2), 141-149.
| Crossref | Google Scholar |
Menning A, Walter A, Rudolph M, Gashaw I, Fritzemeier K-H, Roese L (2012) Granulocytes and vascularization regulate uterine bleeding and tissue remodeling in a mouse menstruation model. PLoS ONE 7(8), e41800.
| Crossref | Google Scholar |
Middelkoop MA, Don EE, Hehenkamp WJK, Polman NJ, Griffioen AW, Huirne JAF (2023) Angiogenesis in abnormal uterine bleeding: a narrative review. Human Reproduction Update 29(4), 457-485.
| Crossref | Google Scholar | PubMed |
Morison NB, Zhang J, Tu’uhevaha J, Fraser IS, Salamonsen LA (2007) The long-term actions of etonogestrel and levonorgestrel on decidualized and non-decidualized endometrium in a mouse model mimic some effects of progestogen-only contraceptives in women. Reproduction 133(1), 309-321.
| Crossref | Google Scholar |
Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders (2011) FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynecology & Obstetrics 113(1), 3-13.
| Crossref | Google Scholar |
NICE (2018) Heavy menstrual bleeding: assessment and management. (National Institute for Health and Care Excellence ) Available at https://www.nice.org.uk/guidance/ng88
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71.
| Crossref | Google Scholar | PubMed |
Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK (2013) Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells and Development 22(6), 964-974.
| Crossref | Google Scholar | PubMed |
Persoons E, De Clercq K, Van den Eynde C, Pinto S, Luyten K, Van Bree R, Tomassetti C, Voets T, Vriens J (2020) Mimicking sampson’s retrograde menstrual theory in rats: a new rat model for ongoing endometriosis-associated pain. International Journal of Molecular Sciences 21(7), 2326.
| Crossref | Google Scholar |
Peterse D, De Clercq K, Goossens C, Binda MM, Dorien FO, Saunders P, Vriens J, Fassbender A, D’Hooghe TM (2018a) Optimization of endometrial decidualization in the menstruating mouse model for preclinical endometriosis research. Reproductive Sciences 25(11), 1577-1588.
| Crossref | Google Scholar |
Peterse D, Binda MM, Dorien FO, Vanhie A, Fassbender A, Vriens J, D’Hooghe TM (2018b) Of mice and women: a laparoscopic mouse model for endometriosis. Journal of Minimally Invasive Gynecology 25(4), 578-579.
| Crossref | Google Scholar |
Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, Hopkins WD, Hu S-L, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML (2014) Why primate models matter. American Journal of Primatology 76(9), 801-827.
| Crossref | Google Scholar |
Rasweiler JJ, De Bonilla H (1992) Menstruation in short-tailed fruit bats (Carollia spp.). Reproduction 95(1), 231-248.
| Crossref | Google Scholar |
Reavey JJ, Walker C, Murray AA, Brito-Mutunayagam S, Sweeney S, Nicol M, Cambursano A, Critchley HOD, Maybin JA (2021) Obesity is associated with heavy menstruation that may be due to delayed endometrial repair. Journal of Endocrinology 249(2), 71-82.
| Crossref | Google Scholar | PubMed |
Ruberte J, Schofield PN, Sundberg JP, Rodriguez-Baeza A, Carretero A, McKerlie C (2023) Bridging mouse and human anatomies; a knowledge-based approach to comparative anatomy for disease model phenotyping. Mammalian Genome 34(3), 389-407.
| Crossref | Google Scholar | PubMed |
Rudolph M, Döcke W-D, Müller A, Menning A, Röse L, Zollner TM, Gashaw I (2012) Induction of overt menstruation in intact mice. PLoS ONE 7(3), e32922.
| Crossref | Google Scholar | PubMed |
Sacha CR, Souter I (2017) Abnormal uterine bleeding in women with infertility. Current Obstetrics and Gynecology Reports 6(1), 42-50.
| Crossref | Google Scholar |
Salamonsen LA, Evans J (2018) Menstruation and endometrial repair. In ‘Encyclopedia of reproduction’. (Ed. MK Skinner) pp. 320–325. (Academic Press) 10.1016/B978-0-12-801238-3.64653-6
Shimizu K (2008) Reproductive hormones and the ovarian cycle in macaques. Journal of Mammalian Ova Research 25(3), 122-126.
| Crossref | Google Scholar |
Taylor R (2001) A step at a time: New Zealand’s progress toward hominid rights. Animal L 7, 35-43.
| Google Scholar |
Tsolova AO, Aguilar RM, Maybin JA, Critchley HOD (2022) Pre-clinical models to study abnormal uterine bleeding (AUB). eBioMedicine 84, 104238.
| Crossref | Google Scholar | PubMed |
Van Der Horst CJ (1954) Elephantulus going into anoestrus; menstruation and abortion. Philosophical Transactions of the Royal Society B: Biological Sciences 238(653), 27-61.
| Crossref | Google Scholar |
Wang Q, Xu X, He B, Li Y, Chen X, Wang J (2013) A critical period of progesterone withdrawal precedes endometrial breakdown and shedding in mouse menstrual-like model. Human Reproduction 28(6), 1670-1678.
| Crossref | Google Scholar | PubMed |
Wang S-F, Chen X-H, He B, Yin D-D, Gao H-J, Zhao H-Q, Nan N, Guo S-G, Liu J-B, Wu B, Xu X-B (2019) Acute restraint stress triggers progesterone withdrawal and endometrial breakdown and shedding through corticosterone stimulation in mouse menstrual-like model. Reproduction 157(2), 149-161.
| Crossref | Google Scholar | PubMed |
Wang C, Zhao M, Zhang W-Q, Huang M-Y, Zhu C, He J-P, Liu J-L (2020) Comparative analysis of mouse decidualization models at the molecular level. Genes 11(8), 935.
| Crossref | Google Scholar | PubMed |
Wang S, Chen X, Guo S, Zhou F, Zhang X, Lu C, Yang X, Wang Q, He B, Wang J, Wang H, Xu X (2023) CXCR4, regulated by HIF1A, promotes endometrial breakdown via CD45+ leukocyte recruitment in a mouse model of menstruation. Reproductive Biology 23(3), 100785.
| Crossref | Google Scholar | PubMed |
Wei Y, Li W, Meng X, Zhang L, Shen M, Liu H (2019) Corticosterone injection impairs follicular development, ovulation and steroidogenesis capacity in mice ovary. Animals 9(12), 1047.
| Crossref | Google Scholar |
Whitaker L, Critchley HOD (2016) Abnormal uterine bleeding. Best Practice & Research Clinical Obstetrics & Gynaecology 34, 54-65.
| Crossref | Google Scholar | PubMed |
Xiao E, Xia-Zhang L, Ferin M (1999) Stress and the menstrual cycle: short- and long-term response to a five-day endotoxin challenge during the luteal phase in the rhesus monkey. The Journal of Clinical Endocrinology & Metabolism 84(2), 623-626.
| Crossref | Google Scholar | PubMed |
Xu XB, He B, Wang JD (2007) Menstrual-like changes in mice are provoked through the pharmacologic withdrawal of progesterone using mifepristone following induction of decidualization. Human Reproduction 22(12), 3184-3191.
| Crossref | Google Scholar | PubMed |
Xu X, Chen X, Li Y, Cao H, Shi C, Guan S, Zhang S, He B, Wang J (2013a) Cyclooxygenase-2 regulated by the nuclear factor-κB pathway plays an important role in endometrial breakdown in a female mouse menstrual-like model. Endocrinology 154(8), 2900-2911.
| Crossref | Google Scholar |
Xu X, Chen X, Wang J, He B (2013b) Matrix metalloproteinase expression in a mouse menstrual-like model by pharmacologic progesterone withdrawal. Journal of Animal and Veterinary Advances 12(13), 1139-1146.
| Crossref | Google Scholar |
Zhang X, Zhu C, Lin H, Yang Q, Ou Q, Li Y, Chen Z, Racey P, Zhang S, Wang H (2007) Wild fulvous fruit bats (rousettus leschenaulti) exhibit human-like menstrual cycle. Biology of Reproduction 77(2), 358-364.
| Crossref | Google Scholar | PubMed |
Zhang G-H, Cui L-J, Li A-Y, Zhang J-P, Liu Y, Zhao J-S, Xu X-B, He B, Wang J-D, Chu L, Li Y-F (2016) Endometrial breakdown with sustained progesterone release involves NF-κB-mediated functional progesterone withdrawal in a mouse implant model. Molecular Reproduction and Development 83(9), 780-791.
| Crossref | Google Scholar | PubMed |
Zheng H-T, Zhang H-Y, Chen S-T, Li M-Y, Fu T, Yang Z-M (2020) The detrimental effects of stress-induced glucocorticoid exposure on mouse uterine receptivity and decidualization. The FASEB Journal 34(11), 14200-14216.
| Crossref | Google Scholar | PubMed |
Zhong S, Qian L, Tan Y (2024) Xue ping tablets treat abnormal uterine bleeding via VEGF-ERK1/2 pathway. Heliyon 10(9), e30079.
| Crossref | Google Scholar |
Zhou F, Wang S, Lu W, Chen X, Guo S, Lu C, Zhang X, Wu J, Wang S, Long Z, He B, Zhuang T, Xu X (2024) The essential role of PGF2α/PTGFR in Molding endometrial breakdown and vascular dynamics, regulated by HIF-1α in a mouse menstrual-like model. Reproductive Sciences 31(9), 2718-2730.
| Crossref | Google Scholar |