Molecular dynamics simulations of cytochrome P450 aromatases reveal structural variances across the cat family
Rageshwari R. Marolikar A B C , Paul D. O’Leary A , Ajay Singh Panwar B * and Lisandra L. Martin A *
A *
A
B
C
Abstract
Aromatase (CYP19A1) is a key enzyme in steroidogenesis, converting androgens to oestrogens, essential for reproductive function in vertebrates. While human aromatase has been extensively studied, comparative analyses in mammals, particularly felids, remain limited.
This study investigates the structural and functional dynamics of aromatase in various cat species, including the extinct Homotherium latidens and extant species such as Panthera tigris, Puma concolor, Acinonyx jubatus, and Felis catus. The goal is to assess evolutionary differences affecting dimerisation and enzymatic activity.
Homology models of feline aromatase were built using the human aromatase crystal structure as a template. Molecular dynamics (MD) simulations were conducted in both solvent and membrane environments to evaluate dimer stability, electrostatic interactions, and haem cofactor retention.
Sequence analysis showed over 99% conservation within felids and ~86% identity with human aromatase, with 69 key residue differences. MD simulations revealed that substitutions at the dimerisation interface weakened electrostatic interactions, reducing dimer stability in felids compared to humans. Membrane embedding improved stability, particularly in human aromatase, due to strong hydrogen-bonding interactions.
Evolutionary divergence has altered dimerisation stability in feline aromatases, potentially influencing enzymatic function. Reduced dimer formation may impact substrate binding and catalytic efficiency.
These findings provide insights into aromatase evolution and function, offering a foundation for future research into species-specific steroid biosynthesis and potential drug design strategies.
Keywords: cat family, cytochrome P450 aromatase (CYP19A1), evolutionary adaptation, evolutionary divergence, homodimerisation, homology modelling, molecular dynamics simulations, structure – function.
Introduction
Aromatase (cytochrome P450 aromatase or CYP19A1) is a member of the cytochrome P450 superfamily, a diverse group of haem enzymes involved in the biosynthesis and metabolism of numerous endogenous and exogenous substrates across vertebrate species (Nebert and Russell 2002; Simpson et al. 2002; Fatima et al. 2020; Di Nardo et al. 2021; Hossack et al. 2023). As a key enzyme in steroidogenesis, aromatase catalyses the conversion of androgens into oestrogens, forming the rate-limiting step of oestrogen biosynthesis (Martin et al. 2015; Martin et al. 2017; Di Nardo et al. 2021). The process begins with cholesterol, which undergoes a series of enzymatic reactions to produce androgen precursors, such as androstenedione (ASD) and testosterone. These androgens are converted into aromatic oestrogens, including oestrone or oestradiol, through a three-step oxidation process catalysed by aromatase (Supplementary Fig. S1) (Ghosh et al. 2010; Martin et al. 2015; Gilardi and Di Nardo 2017; Fatima et al. 2020; Di Nardo et al. 2021). In mammals, aromatase is primarily expressed in the gonads, adipose tissue, placenta, and brain, where it is anchored to the membrane of the endoplasmic reticulum in oestrogen-producing cells (Hiller-Sturmhöfel and Bartke 1998; Stocco 2012), thus maintaining a tight local control over the synthesis and activity of oestrogens. The electrons necessary for its oxidative reactions are supplied by NADPH cytochrome P450 reductase (CPR), a membrane-bound flavoprotein (Santen et al. 2009; Yoshimoto and Guengerich 2014; Ghosh et al. 2018; Di Nardo et al. 2021; Zhang et al. 2022).
According to the National Center for Biotechnology Information (NCBI) database (NCBI 2025), aromatase has been isolated and sequenced from around 3725 animal species, out of which only a fraction have been characterised in terms of catalytic activity (Honma et al. 2005; Martin et al. 2015; Nair et al. 2016; Gilardi and Di Nardo 2017; Fatima et al. 2020). The complementary DNA specific to aromatase was first isolated and characterised by Simpson et al. in 1986 (Evans et al. 1986) although studied in tissue extracts earlier (Thompson and Siiteri 1974; Siiteri and Thompson 1976; Siiteri 1982; Evans et al. 1986). However, it was Ghosh and co-workers (Ghosh et al. 2010) who structurally characterised it by X-ray crystallography in 2009. Since then, several recombinantly modified human aromatases have been expressed and structurally characterised (Ghosh et al. 2012, 2018; Lo et al. 2013), with most structures differing based on the presence of various substrates, inhibitors, or agents to aid crystal growth, such as, polyethylene glycol (PEG) (Ghosh et al. 2018). Despite extensive sequencing of aromatases from various mammalian species (Harada et al. 1992; Conley et al. 2001; Hong et al. 2009), no structures suitable for crystallographic analysis from other mammals except for humans have been reported (Conley et al. 2001; Corbin et al. 2003; Martin et al. 2015). In a recent study by Fatima et al. (Martin et al. 2015; Fatima et al. 2020), 3D structural models of aromatase were generated for four diverse native Australian species, including a marsupial (tammar wallaby), monotreme (platypus), bird (emu), and reptile (bearded dragon), using homology modelling based on the human X-ray crystal structure and investigated using molecular dynamics simulations (Fatima et al. 2020). Despite the wide diversity in species, this study revealed a remarkable conservation of aromatase sequence and structure, likely driven by the evolutionary pressures essential for reproduction. Aromatase is expressed in various tissues beyond the gonads, including bone, breast, adipose tissue, and brain, thus conservation of aromatase sequence in mammals is vital for maintaining a tight local control over the synthesis and activity of oestrogens (Simpson and Dowsett 2002; Stocco 2012; Fatima et al. 2020). As oestrogens are crucial in reproduction, influencing the fitness and survival of species across evolutionary timelines, understanding evolution of aromatase offers insights into how this enzyme may have adapted to various environmental pressures (Martin et al. 2015; Di Nardo et al. 2021).
Although aromatase has been studied in a variety of species, direct comparative analyses of its structure and function across mammals and other vertebrates remain limited. Research has provided valuable insights into specific aspects of aromatase biology: for example, Martin et al. (2015) explored the structure and dimerisation of the human enzyme, while Conley and Hinshelwood (2001) reviewed its roles in humans and domestic animals. Other studies have broadened this perspective by examining aromatase in cetaceans (Wilson et al. 2005), rodents (MacLusky et al. 1987; Roselli and Resko 1993), and reptiles (Smith et al. 1995), shedding light on phylogenetic differences, brain-specific activity, and developmental expression (MacLusky et al. 1987; Roselli and Resko 1993; Smith et al. 1995; Conley and Hinshelwood 2001; Castro et al. 2005; Wilson et al. 2005; Li et al. 2015; Martin et al. 2015; Biegon 2016; Di Nardo et al. 2021). Despite this progress, most investigations remain species-specific or narrowly focused, underlining the need for broader comparative studies like the present work on Felidae aromatases. Additional rationale for our study, is that unlike humans and other well-characterised mammals, felids are induced ovulators, with ovulation in these species triggered by mating through a neuroendocrine cascade (Kauffman and Rissman 2006). This reproductive strategy contrasts with spontaneous ovulation observed in humans, monkeys, and rodents, where hormonal cycling and ovulation occur independently of mating stimuli (Saltzman et al. 2011). Such a fundamental physiological difference suggests the possibility that aromatase expression, regulation, or function may have diverged significantly in felids.
This study focuses on examining how aromatase has evolved structurally and functionally across members of the cat family (Felidae), which includes species that evolved over a ~5-million-year timescale (O’Brien and Johnson 2007; Barnett et al. 2020). The increasing availability of genomic data from UniProt and NCBI database (The UniProt Consortium 2019; Sayers et al. 2022), along with the human aromatase X-ray crystal structure (Ghosh et al. 2010), has enabled structural modelling using computational approaches. This work employs classical molecular dynamics simulations to differences in aromatases from multiple feline species, including the extinct Homotherium latidens (saber-toothed cat) (Barnett et al. 2020) and extant species, such as, lion, tiger, leopard cat, puma, cheetah, and domestic cat (see Table 1). Interestingly, domestic cats share 90% identity of their whole genome sequence with humans, making them an intriguing comparison for human studies (Pontius et al. 2007). Additionally, research has also shown that cats are prone to several diseases and genetic disorders similar to those observed in humans (O’Brien et al. 2002; Rijnberk et al. 2003; Menotti-Raymond et al. 2010; Burkholder et al. 2015). For example, the molecular mechanism of feline mammary carcinoma (FMC) (Gameiro et al. 2021) has benefitted from human breast cancer research, leading to biomarkers in common, establishing the potential of feline models for studying human health conditions. Thus, this study aims to highlight the structural changes observed across the cat family, utilising various bioinformatics and computational tools to better understand the evolutionary adaptations of aromatase.
| # | Species | Monomer simulations (ns)A | Dimer simulations (ns)A | |
|---|---|---|---|---|
| 1. | Homotherium latidens (saber-toothed cat) | 100 | 150 | |
| 2. | Panthera leo (lion)B | 0 | – | |
| 3. | Panthera tigris (tiger)B | 100 | – | |
| 4. | Prionailurus bengalensis (leopard cat)B | 0 | – | |
| 5. | Puma concolor (puma) | 100 | 150 | |
| 6. | Acinonyx jubatus (cheetah) | 100 | – | |
| 7. | Felis catus (cat) | 100 | – | |
| 8. | Homo sapiens (human) | 100 | 150 |
Materials and methods
Molecular dynamics (MD) simulation method
Fully-atomistic molecular dynamics simulations were conducted using Nanoscale Molecular Dynamics software (NAMD 2.14) (Phillips et al. 2005) with the CHARMM36 force field (Huang and MacKerell 2013), and the systems were solvated using the CHARMM-modified TIP3P water model. Visual Molecular Dynamics Software (VMD) was used for visualisation and analysis (Humphrey et al. 1996). Energy minimisation was performed using the conjugate gradient algorithm. Constraints applied to the protein backbone were gradually removed over a span of 2.04 ns. The temperature of the system was then increased gradually from 0 K to 310 K over 0.62 ns. This was followed by NPT equilibration for 1 ns, and then further pressure equilibration under the NPT ensemble at 310 K and 1 atm for 10 ns (NPT refers to the isothermal-isobaric ensemble, where the number of particles (N), pressure (P), and temperature (T) are held constant during the simulation). For membrane-embedded systems, standard CHARMM-GUI (Jo et al. 2008) protocols were followed for system construction and equilibration. After equilibration, production runs were performed without restraints: 100 ns for monomeric systems, 150 ns for dimeric systems and 350 ns for membrane systems. Further details on the methodology are provided in Supplementary Data S1.
Amino acid sequences and alignment
The seven cat family aromatase sequences in the study include: Homotherium latidens (homotherium) (Barnett et al. 2020), Panthera leo (lion) XP_042797965.1, Panthera tigris (tiger) XP_042844798.1, Prionailurus bengalensis (leopard cat) XP_043411365.1, Puma concolor (puma) XP_025775219.1, Acinonyx jubatus (cheetah) XP_053079996.1, and Felis catus (domestic cat) XP_044915259.1, which were compared to that of human aromatase. The amino acid sequence in the case of Homotherium latidens was computationally derived from the DNA sequence provided by Prof. Michael Westbury from the University of Copenhagen (Barnett et al. 2020; Westbury et al. 2021). Felis catus aromatase sequence (XM_006932525.3) was used as the reference genome to extract the approximate region of Homotherium latidens CYP19A1 gene and manually mapped for exon identification. The exons were spliced and translated using the Expert Protein Analysis System (ExPASy) translate tool (Artimo et al. 2012), which provided the final amino acid sequence of Homotherium latidens aromatase. The remaining six cat family aromatase sequences (listed in Table 1) were obtained from the NCBI database (Sayers et al. 2022).
Clustal Omega in default mode (Sievers et al. 2011) was used to perform sequence alignments on these seven cat family aromatase species and human aromatase (Protein Data Bank (PDB) ID: 3EQM) (Ghosh et al. 2010) and was visualised using the BioEdit Software (Hall 1999), as shown in Fig. 1. The aromatase sequences within the cat family exhibited approximately 99% conservation, whereas sequence identity decreased to around 86% if compared to human aromatase (Supplementary Information; Table S1). Position-specific conservation percentages were calculated, revealing high conservation (> 95%) of key functional regions, including the haem-binding site, cytochrome P450 oxidoreductase (CPR) binding residues, and six substrate recognition sites (SRSs) (Supplementary Information; Table S2 and Fig. S3).
Multiple sequence alignment (Edgar and Batzoglou 2006) of aromatase sequences from Homo sapiens (PDB: 3EQM), Homotherium latidens (Westbury et al. 2021), Panthera leo (NCBI: XP_042797965.1), Panthera tigris (NCBI: XP_042844798.1), Prionailurus bengalensis (NCBI: XP_043411365.1), Puma concolor (NCBI: XP_025775219.1), Acinonyx jubatus (NCBI: XP_053079996.1), Felis catus (NCBI: XP_044915259.1), aligned using Clustal Omega (Sievers et al. 2011) and compared in Bio-edit software (Hall 1999). The residues 1–54 belong to the transmembrane (TM) region, α helices are indicated and labelled in blue boxes from A–L, four β strands are labelled in red boxes, while K′–L loop and haem-binding regions are indicated by green boxes. The labelling of secondary structure elements is according to the X-ray crystal structure study by Ghosh et al. (2010) and visually represented in Fig. S2.
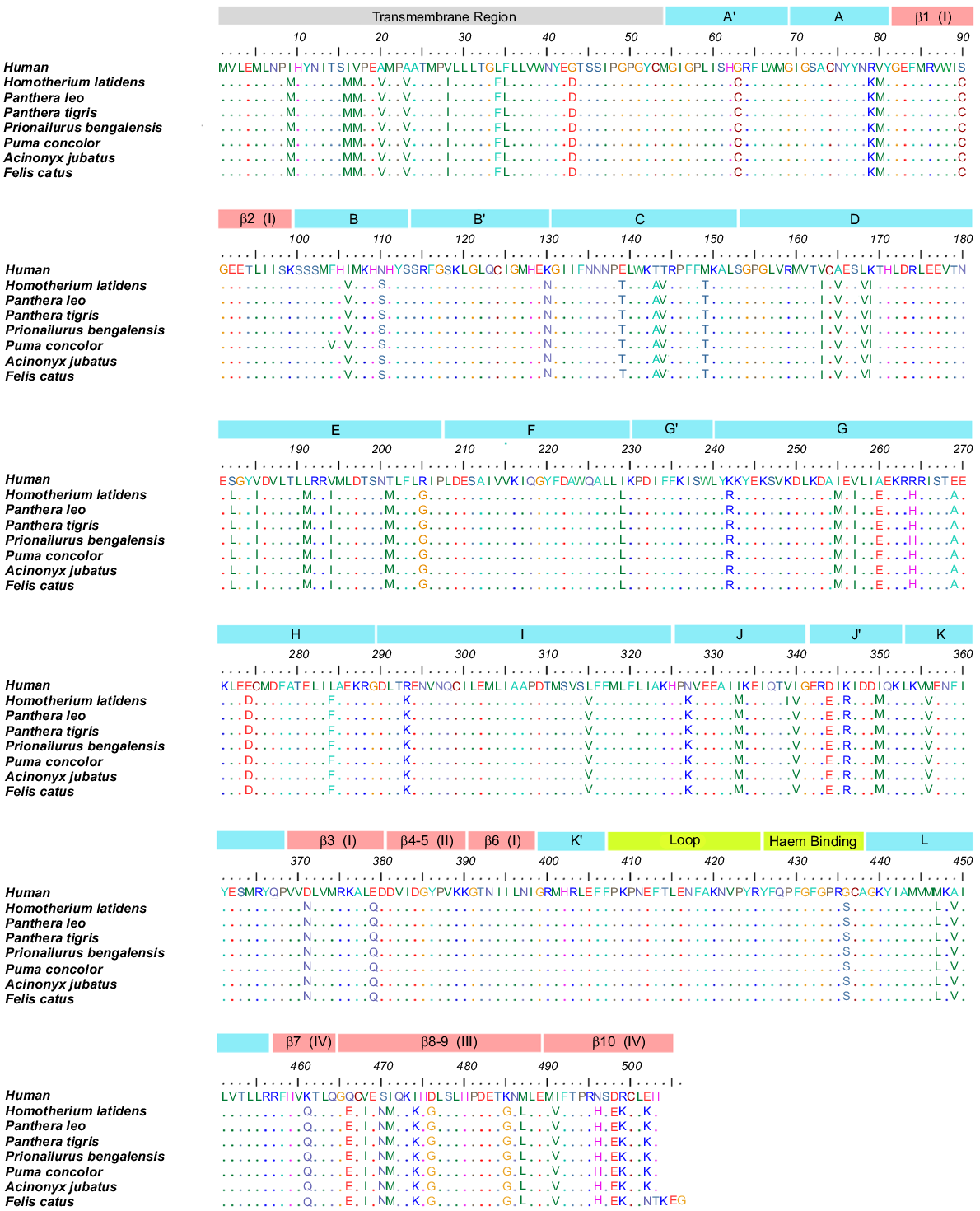
Homology modelling
Sequence alignments revealed that the aromatases of lion, tiger, and leopard cat were identical (Table S1); therefore, the tiger sequence was selected as representative structure for subsequent analysis (as mentioned in Table 1). The 3D structures of the remaining five cat family aromatases were constructed using the homology modelling tool SWISS-MODEL (Waterhouse et al. 2018), with the X-ray crystal structure of human aromatase (PDB ID: 3EQM (Ghosh et al. 2010), Fig. S2) serving as the template. The quality of the homology-modelled structures was evaluated using the Quality Assessment module of the SWISS-MODEL server (Waterhouse et al. 2018) (Table S3). Metrics such as QMEAN Z-scores (Benkert et al. 2008), low molprobity values (~2.12) (Chen et al. 2010) and > 94% of residues in favoured regions of the Ramachandran plot indicated that the modelled structures were of high quality. However, further refinement, particularly around proline and glycine residues, was anticipated to be resolved during the relaxation process of molecular dynamics simulations.
Molecular dynamics simulation systems
Aromatase monomers for five cat family members (Table 1) and human were constructed both with and without haem. A previously reported (Martin et al. 2015) dimeric structure of human aromatase was used as a template for the construction of Homotherium and puma aromatase dimers, representing the extinct and extant species of cat family. All simulations were conducted in the absence of androstenedione (ASD) and in the resting state, as this study focuses solely on observing dimeric interactions. Interactions in the presence of the substrate are beyond the scope of this work and are currently being investigated in our lab. Further details on the construction of monomeric and dimeric systems, both in solution and in the membrane, can be found in the Supplementary Data S1.
Results and discussion
Sequence alignment
The amino acid sequence alignments, shown in Fig. 1, for the human and cat family aromatases produced a sequence identity of > 99% within the cat family and ~86% identity between cats and the human sequence (Table S1). This high degree of conservation within the cat family was expected for an essential reproductive enzyme, further reinforcing that the structure and function of aromatases are preserved across all feline species included in this study. Specifically, positively charged residues on the enzyme’s surface, such as 108K, 142K, 145R, 150K, 352K, 354K, 420K, and 425R (Fatima et al. 2020) are completely conserved in both humans and within the cat family members investigated. In the human aromatase, these residues are implicated in binding to the redox partner, cytochrome P450 oxidoreductase (CPR), promoting electron transfer from NADPH to the haem centre (Table S2) (Ghosh et al. 2010; Gilardi and Di Nardo 2017; Di Nardo et al. 2021). Active site residues, including 306A, 309D, 310T, 478S, and 480H (Fig. 1) (Ghosh 2023), showed 100% conservation, indicating that the active site is similar for the cat family members and human aromatase (Ghosh et al. 2010; Di Nardo et al. 2021). Additionally, with over 95% conservation observed in the substrate-binding sites (Table S2), it is expected that the substrate, androstenedione (ASD), would participate in the catalytic cycle of cat family aromatase in a manner similar to its role in humans. This high level of conservation supports analogous CPR-aromatase and substrate-aromatase interactions in the cat family and in humans. A non-conserved non-polar to polar alteration of 436G (human) → 436S (cat family) was observed in the haem-binding domain, which being adjacent to the iron coordinating cysteine (CYS) residue, may slightly affect the binding affinity for the haem (Fatima et al. 2020). Within the cat family, sequence comparisons revealed that the extinct genus Homotherium shares over 99% sequence similarity with other feline members. Differences were identified between Homotherium and extant species, such as the puma, that includes 104F (Homotherium) → 104V (puma), 168V (Homotherium) → 168I (puma), and 339I (Homotherium) → 339V (puma). Key structural motifs, including the four-helix bundle (helices D, E, I, and L) and substrate recognition sites (SRSs), have been preserved, highlighting strong selective pressures to maintain enzymatic specificity and efficiency (Tiwary and Li 2009; Martin et al. 2015; Di Nardo et al. 2021; Zhang et al. 2022). These findings reflect the remarkable stability of aromatase sequences over 15 million years, while leaving room for ongoing evolutionary refinement that would likely require even longer evolutionary timescales.
Sequence comparisons also revealed that the 14% difference between the cat family and human aromatase sequences stems from 69 differing residues (Table 2). Of these, 45 amino acid residues represent conserved chemical properties (Table 2a), thus are likely to be important for maintaining aromatase structure, activity and/or function. The remaining 24 (Table 2b, c) are non-conserved changes. Most of these non-conserved residues were amino acid residues that were charged in humans which were replaced by either hydrophobic or polar residues in cats (Table 2c). To examine the structural orientation of these charged residues in humans that are not conserved in cats, the 3D structures of Homotherium, cheetah, tiger, and domestic cat were generated using the X-ray structure of the human aromatase (Ghosh et al. 2010) as a template for homology modelling, as described in the Materials and methods section. Further, molecular dynamics simulations were carried out to achieve equilibrated structures.
| (a) AA Type | n | Conserved amino acid residues (total = 45) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrophobic | 33 | 9I → M | 16I → M | 17V → M | 20A → V | 23A → V | 25A → V (Leopard cat) | 28V → I | 34L → F | 35F → L | 80V → M | |
| 10F → V | 106I → V | 163V → I | 165A → V | 168L → V | 191L → M | 194V → I | 229I → L | 255I → M | 257V → L | |||
| 284L → F | 315L → V | 333I → M | 340I → V | 350I → M | 356M → V (Human) | 339V → L | 447M → L | 449A → V | 468V → I | |||
| 471I → M | 487M → L | 491I → V | ||||||||||
| Positive | 6 | 76R → K | 242K → R | 264R → H | 293R → K | 346K → R | 499R → K | |||||
| Negative | 3 | 274E → D | 344D → E | 498D → E | ||||||||
| Polar | 2 | 110N → S | 470S → N | |||||||||
| Special case | 1 | 63G → C | ||||||||||
| (b) AA Type | n | Non-conserved amino acid residues (total = 13) | ||||
|---|---|---|---|---|---|---|
| Polar → +ve | 2 | 327N → K | 496N → H | |||
| Polar → −ve | 1 | 466Q → E | ||||
| Polar → special case | 1 | 90S → C | ||||
| Polar → hydrophobic | 4 | 143T → A | 144T → V | 182S → L | 201T → M | |
| Special case → −ve | 1 | 43G → D | ||||
| Special case → polar | 1 | 436G → S | ||||
| Hydrophobic → +ve | 1 | 474I → K | ||||
| Hydrophobic → −ve | 1 | 260A → E | ||||
| Hydrophobic → polar | 1 | 149M → T | ||||
| (c) AA Type | n | Non-conserved amino acid residues (total = 11) | |||
|---|---|---|---|---|---|
| −ve → +ve | 1 | 502E → K | |||
| −ve → special case | 1 | 476D → G | |||
| −ve → polar | 3 | 139E → T | 371E → N | 379E → Q | |
| −ve → hydrophobic | 1 | 269E → A | |||
| +ve → special case | 2 | 205R → G | 485K → G | ||
| +ve → polar | 2 | 130K → N | 461K → Q | ||
| +ve → hydrophobic | 1 | 169K → I | |||
MD simulations of aromatase monomer
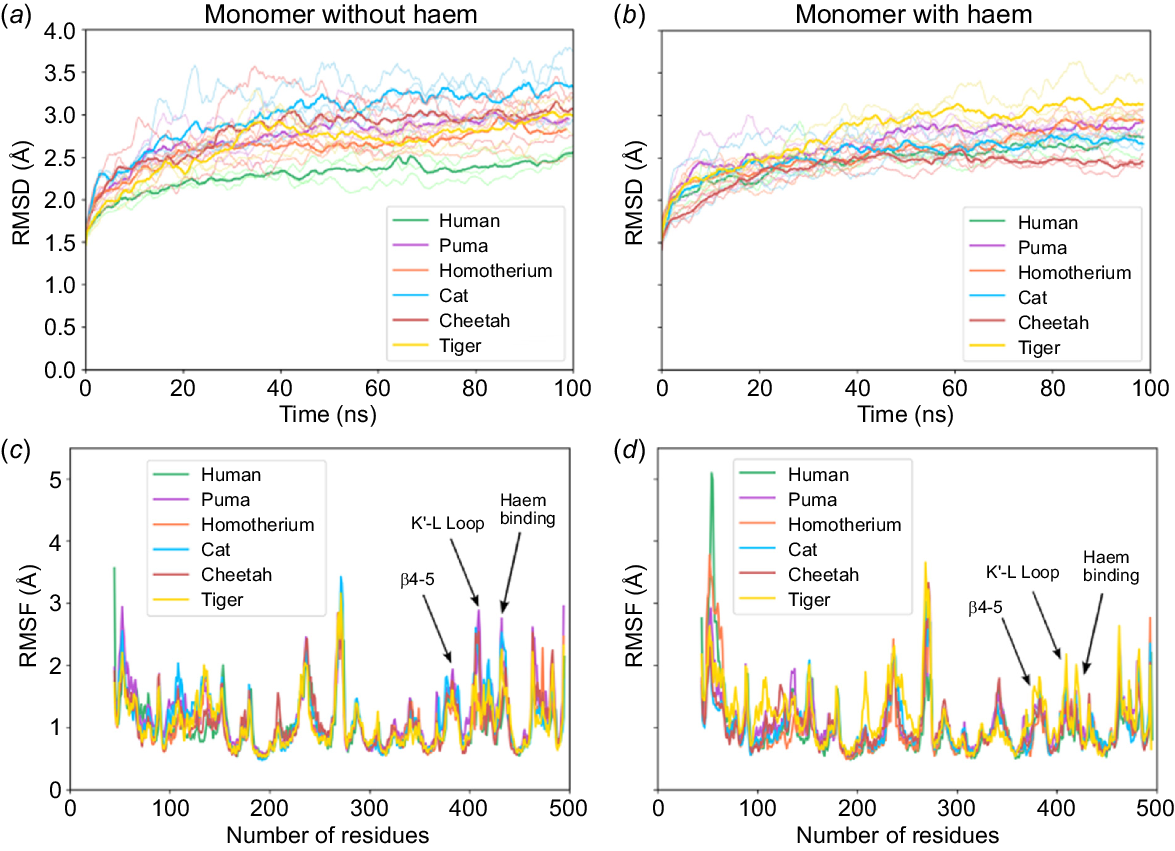
Fig. 2a, b plot the root mean square deviations (RMSD) of the protein backbone relative to the protein’s initial conformation, for all six aromatase species over a 100 ns simulation period. These simulations were conducted both without and with haem cofactor, respectively, to evaluate the stability of the equilibrated structures. Notably, the N-terminal region (residues 1–44) was excluded from these simulations, as the crystal structure of human aromatase lacks the N-terminal segment and includes only the globular domain (Ghosh et al. 2010). The 3D models incorporating haem were constructed with coordination bonds, where iron (Fe) was coordinated to both the axial water molecule and the cysteine residue (437C) to represent the resting state of haem (Fig. S4). All aromatase structures without haem exhibited higher RMSD values (~3 ångström (Å), Fig. 2a) compared to those with haem (RMSD ~2.5 Å, Fig. 2b). Additionally, RMSF (root mean square fluctuations) values (Fig. 2c, d) showed higher fluctuations in the β4–5 region, K′–L loop residues (400–460), and haem-binding residues in absence of haem. Hence, the RMSD and RMSF results suggest that haem plays a key role in stabilising the overall 3D structure. The lower RMSD values in the haem-bound systems are likely due to stabilising interactions between aromatase residues 115R, 141W, 375R, and 438A with the haem molecule (as shown in Fig. S7).
Panels (a, b) and (c, d) show the RMSD and RMSF plots, respectively, for six species: human (green), puma (magenta), Homotherium latidens (orange), cat (blue), cheetah (red), and tiger (yellow). Left panels correspond to simulations without haem, and right panels include haem. For each species, the bold lines represent the average across three replicate simulations, with lighter shades denoting individual replicates. These simulations were conducted in water as the solvent and ionised with 0.15 M NaCl forming a simulation box of 145 Å × 145 Å × 145 Å. The residues highlighted show a large difference in fluctuations in the absence/presence of haem, indicated as β4–5, K′–L loop and haem-binding regions.
Analysis of the equilibrated haem-containing aromatase structures revealed that the non-conserved charged residues in the cat family aromatases were located on the G-helix, B-helix, C-helix, and β-loop 6–7 regions (refer to Fig. S5). Of all the non-conserved residues, approximately 33% were situated in a region that was earlier proposed as a potential dimeric interface of the human aromatase structure by Fatima et al. (Martin et al. 2015; Fatima et al. 2020) (Fig. 3a–c). It was hypothesised that homodimerisation could potentially enhance the catalytic efficiency of androstenedione conversion to oestrone (Martin et al. 2015; Holien et al. 2017; Fatima et al. 2020). Their analysis suggested that dimerisation occurs through favourable electrostatic interactions (a distinct pattern of alternating positive and negative charges) at the proposed interface between the two monomers (Fatima et al. 2020). Therefore, the distribution of charged residues on the proposed dimerisation interface and the resulting surface electrostatic potential were analysed for human and cat family aromatases. Fig. 3a–c show that all non-conserved charged residues, namely 139Eh → Tc, 143Th → Ac, 144Th → Vc, 379Eh → Qc, 90Sh → Cc, 130Kh → Nc, 260Ah → Ec, and 269Eh → Ac, were located on the proposed dimeric interface of these enzymes (subscripts h and c denote human and cat family species, respectively, and superscripts indicate the positions of non-conserved amino acid residues). For ease of comparison, only the interfaces of human, puma, and Homotherium aromatases are shown in Fig. 3, as most cat family species share similar residues. Puma and Homotherium represent extant and extinct species, respectively, with human included as a reference. Electrostatic surfaces for the rest of the cat family species are provided in Fig. S8. The absence of 130Kh and presence of 260Ec reduces the overall electrostatic potential at the centre of the proposed dimerisation interface in cat family aromatases, which is positively charged in human aromatase (Fig. 3d–f). Thus, the absence of charged residues on the surface of cat family aromatases 379E, 139E, 130K and 269E revealed subtle differences between the surface electrostatic properties of cat family and human aromatase.
Panels (a), (b), and (c) illustrate the structural models of aromatases from human (green), puma (magenta), and Homotherium (yellow) highlighting the differences in the residues located at the dimerisation interface. The van der Waals (vdW) residues are colour-coded as: red for negative amino acids, blue for positive, ice-blue for polar, and grey for hydrophobic residues. Panels (d), (e), and (f) present the averaged electrostatic surfaces of human, puma and Homotherium aromatases, particularly focusing on the dimeric interface (Martin et al. 2015; Fatima et al. 2020), generated using Visual Molecular Dynamics (VMD). Additional details on the electrostatic surfaces of other cat family species are provided in Supplementary Information, Fig. S8.
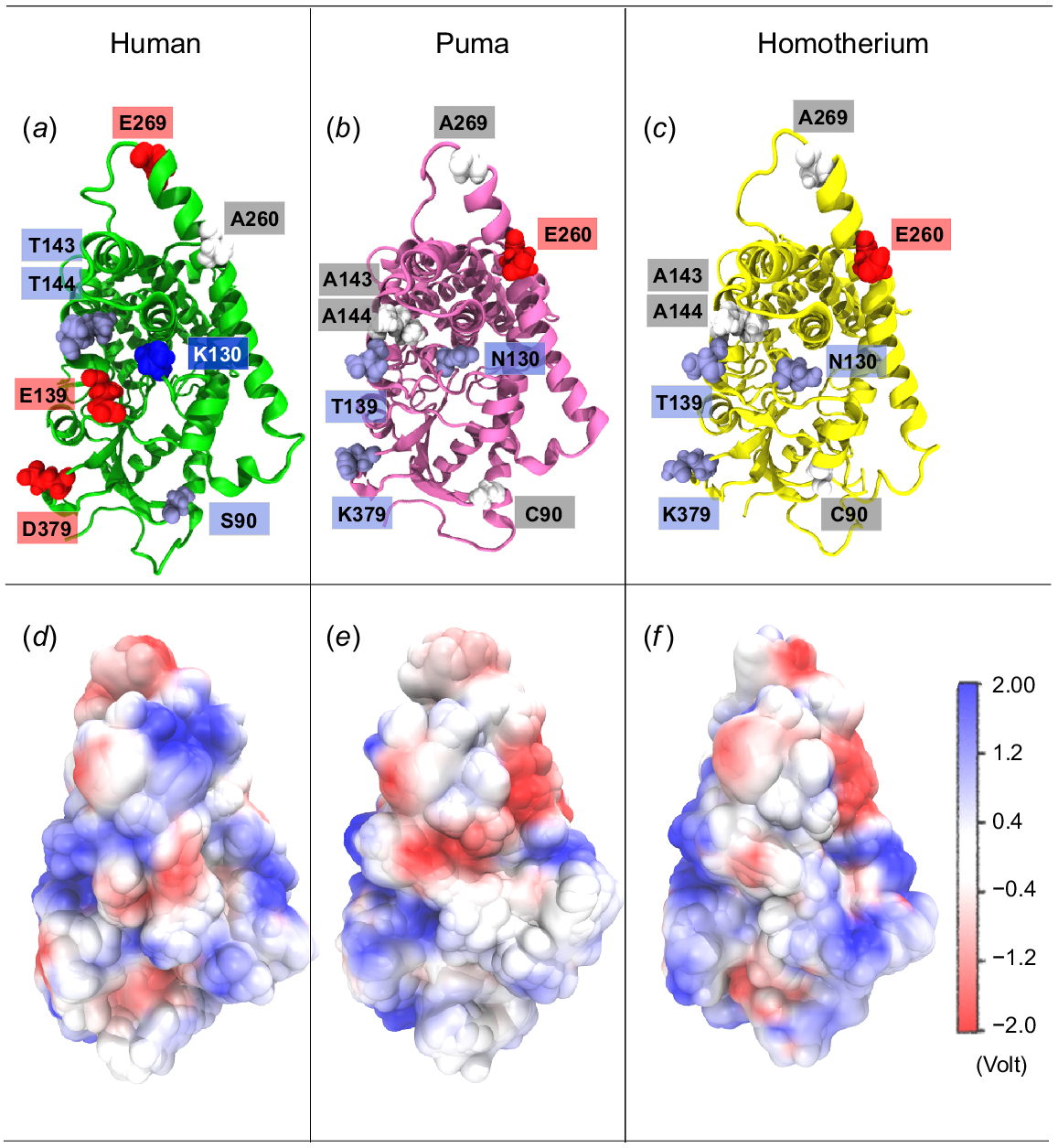
Although distinct stripes of opposite charges were observed in human aromatase (Fig. 3d), they appeared more diffused in cat family counterparts (Fig. 3e, f). This suggests reduced stability of cat family aromatase homodimers due to a potential weakening of monomer-monomer interactions at the proposed dimerisation interface. To investigate this further, homodimer structures of human, puma, and Homotherium aromatases were constructed (refer to Table 1), using the known human aromatase homodimer as a reference structure. Additional details on the construction of aromatase homodimers in solution and within membranes can be found in sections S1.2, S1.3 of the Supplementary Information.
MD simulations of aromatase dimers
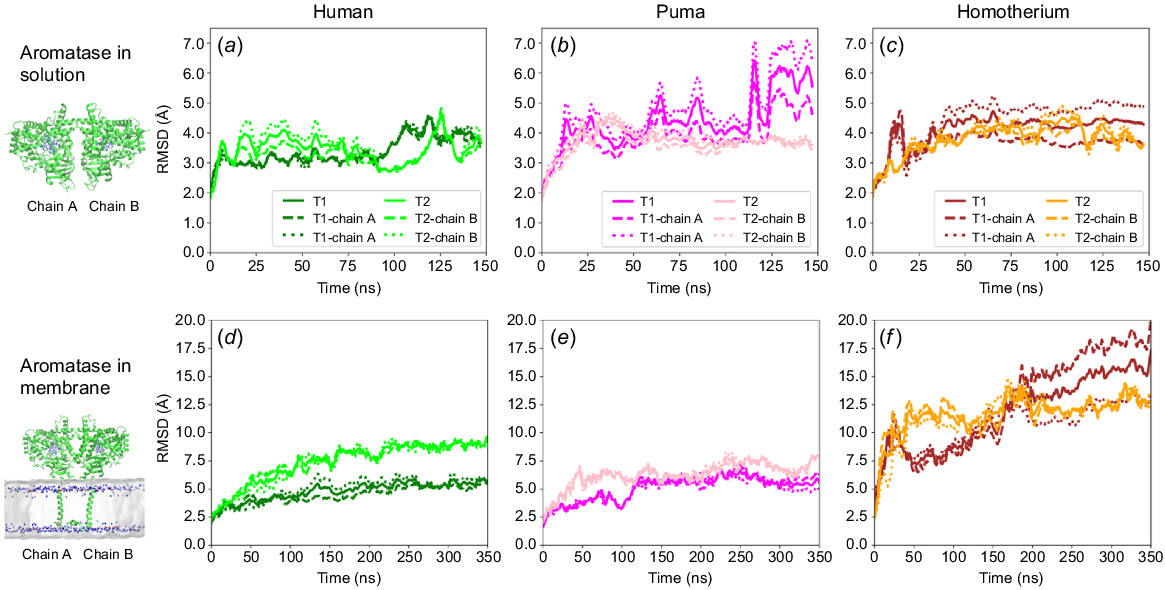
Fig. 4 compares the relative stability of human, puma and Homotherium homodimers in solution (Fig. 4a–c) and in membrane (Fig. 4d–f), respectively. The homodimer simulations in solution excluded the N-terminal regions (residues 1–44) to focus on the globular domains of the dimers in solution (Fig. S6b). The N-terminal region corresponds to the transmembrane domain of the aromatase when embedded in a membrane. Fig. 4a–c shows that the human dimer in solution exhibited lower RMSD values (~4 Å) compared to the cat family dimers in solution (~5 Å), suggesting stronger interfacial interactions in the case of the human homodimer. This is consistent with the proposed weakening of electrostatic interactions across the proposed dimerisation interface in the cat family aromatases (Fig. 3). Further, to mimic their natural state, membrane-embedded dimeric systems were also constructed for the human, puma, and Homotherium homodimers (Fig. S6c). Human aromatase homodimers exhibited significantly lower RMSD values (Fig. 4d) compared to both cat family homodimers (Fig. 4e, f), indicating greater stabilisation of the human homodimer in the membrane. Notably, the difference in stability between the human and cat family homodimers is more pronounced in membrane than in solution. The RMSF values (Fig. S10) also showed higher fluctuations in the I, J, and K helices and beta strands 1–2 and 4–5 of Homotherium and puma compared with the corresponding monomeric fluctuations (Fig. 2b). This suggests that dimer formation influences residue-residue interactions at the interface, which in turn affects fluctuations of the connected J and K helices on the outer end of interfaces. Since the I and K helices form the substrate-binding sites (SBS 4 and 5; Table S2) (Di Nardo et al. 2021), dimer formation could influence substrate interaction and activity towards aromatase. Further to understand the dimeric interactions, hydrogen bonding and salt bridge formation between interacting residues at the dimerisation interface were monitored throughout the simulation (Fig. S11–S14).
Panels (a–f) represent RMSD profiles of dimeric aromatase systems for human (light and dark green), puma (pink and magenta), and Homotherium latidens (orange and brown) simulated over 150 ns in solution and for 350 ns within a membrane. Bold lines indicate average RMSD values, whereas dotted lines represent individual chain RMSD values (a and b) for the trajectory sets T1 and T2.
Aromatase dimeric interactions
In solution, the human aromatase dimer initially formed numerous intermolecular hydrogen bonds and salt bridges (Figs S11, 12). However, towards the end of the simulations, only a few residue pairs interacted strongly: (a) ARG293A⋯GLY289B and oppositely charged residue pairs (b) GLU139A⋯LYS130B, (c) LYS130A⋯GLU139B, and (d) LYS390A⋯GLU92B (Fig. S11a, c). Here, subscripts A and B denote chain A and chain B of the homodimers. These residue pairs maintained ~3 Å distances, stabilising the dimer through hydrogen bonds and electrostatic interactions. In the puma dimer, LYS293A⋯GLY289B and LYS390A⋯GLU92B (Fig. S11a, b) formed hydrogen bonding and salt-bridge interactions, whereas THR129A⋯GLU139B (Fig. S11d) failed to interact due to repulsion from the negative centre, preventing the formation of stable salt bridges throughout the simulation. In Homotherium, the oppositely charged residue pair GLU92A⋯LYS390B (Fig. S11a, b, e) formed salt bridges and hydrogen bonds. Non-conserved mutations (130Kh → Nc and 260Ah → Ec in the cat family) resulted in a negative potential at the centre of the dimerisation interface, initially causing repulsion between residue pairs. Over time, the monomers adjusted, resulting in strong interactions between residue pairs, THR139A⋯GLU129B and LYS293A⋯GLY289B (H-bonding, Fig. S11a, b, e) for the remainder of the simulation. However, in some cases, snapshots (Fig. S9) revealed that the Homotherium homodimer in solution dissociated toward the end of the 150 ns simulation, increasing inter-dimer distance and reducing interactions. This suggests that a membrane could serve as a natural constraint at the N-terminal region, stabilising dimeric interactions. Therefore, these dimeric systems were embedded within a lipid bilayer to assess its effect on dimer stability.
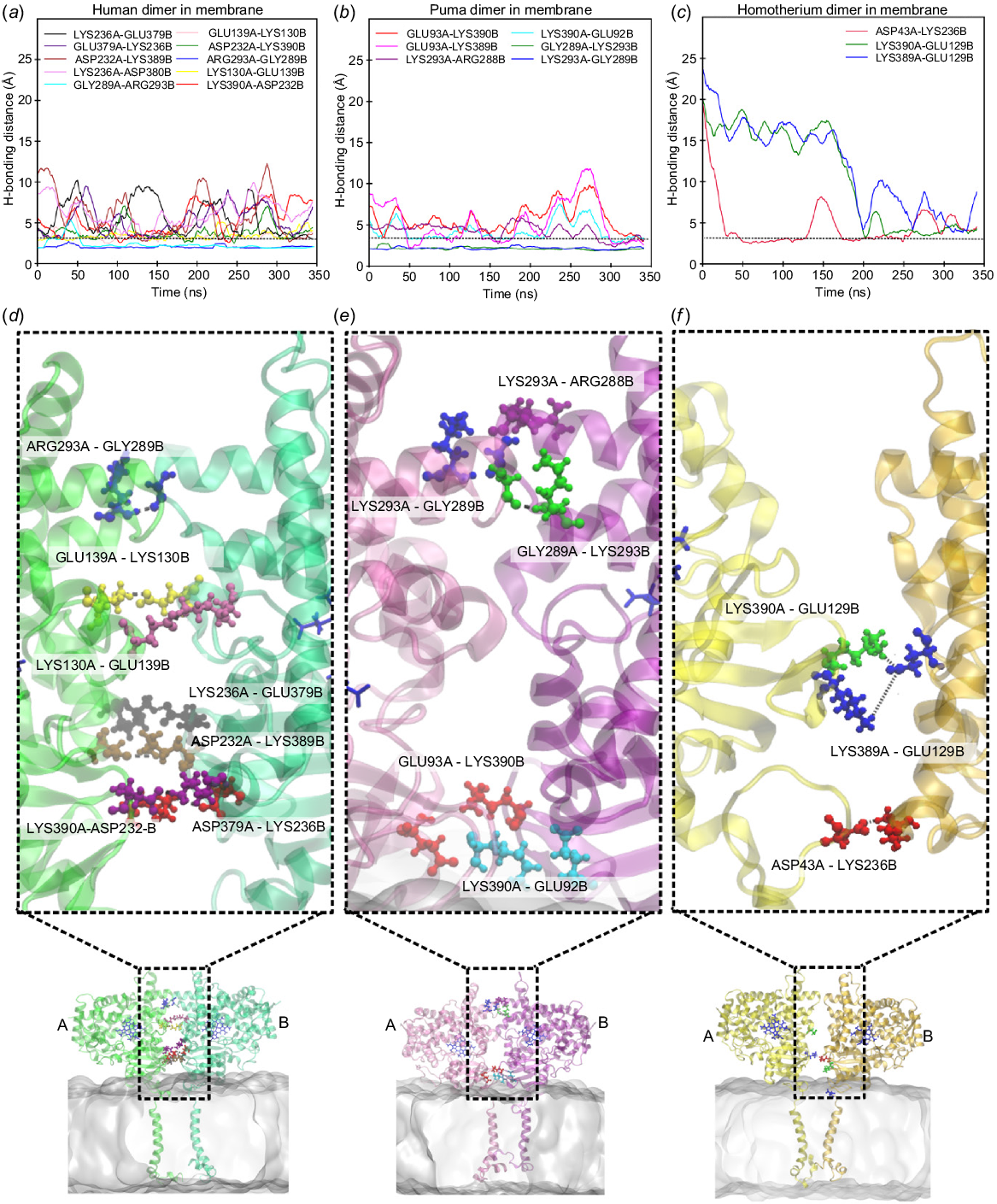
When embedded in the membrane, the human dimer exhibited greater stability, characterised by stronger hydrogen bonding and salt-bridge interactions (Fig. 5, Figs S13, S14). Specifically, residue pairs (1) ARG293A⋯GLY289B showed strong H-bonding interactions, while the rest of the oppositely charged residue pairs (2) GLU139A⋯LYS130B, (3) LYS389A⋯ASP232B, (4) LYS390A⋯ASP232B, and (5) LYS236A⋯GLU379B maintained ~3 Å distance between them, forming a constant hydrogen bond and salt bridges along with electrostatic interactions between them observed in both the trajectory sets T1 and T2 (Fig. 5a). In case of puma and Homotherium, the interacting residues varied between the two trajectory sets (Fig. 5b–c, Figs S13, S14) due to weaker interactions at the dimeric interface caused by the absence of key charged residues (379E, 139E, 130K, and 269E) in the cat family. Fig. 6 illustrates that human aromatase dimers form a significantly higher number of stabilising interactions, hydrogen bonds, salt bridges, and electrostatic forces compared to the cat family aromatases, both in solution and membrane environments. The diminished stability and fewer interacting pairs observed in puma and Homotherium suggest evolutionary differences in dimerisation stability and interaction patterns. These differences likely stem from the absence of key charged residues (379E, 139E, 130K, and 269E) on the surface of cat family aromatases, which, in humans, contribute to critical hydrogen bonding and electrostatic interactions. The reduced propensity for stable dimer formation in cat family aromatases highlight a potential evolutionary divergence, where structural adaptations may have influenced functional dynamics across species.
Panels (a–c) represent hydrogen bonding distances tracked throughout 350 ns simulation for dimer structures of human (green), puma (magenta), and Homotherium latidens (yellow) in membrane environments, with 3.5 Å as H-bonding cut-off distance. Structural representations in panels (d–f) highlights key amino acid residues hydrogen bonded in T1 trajectory simulations (detailed information on hydrogen bonding and salt-bridge interactions on trajectories T1 and T2 is provided in the Fig. S13–S15).
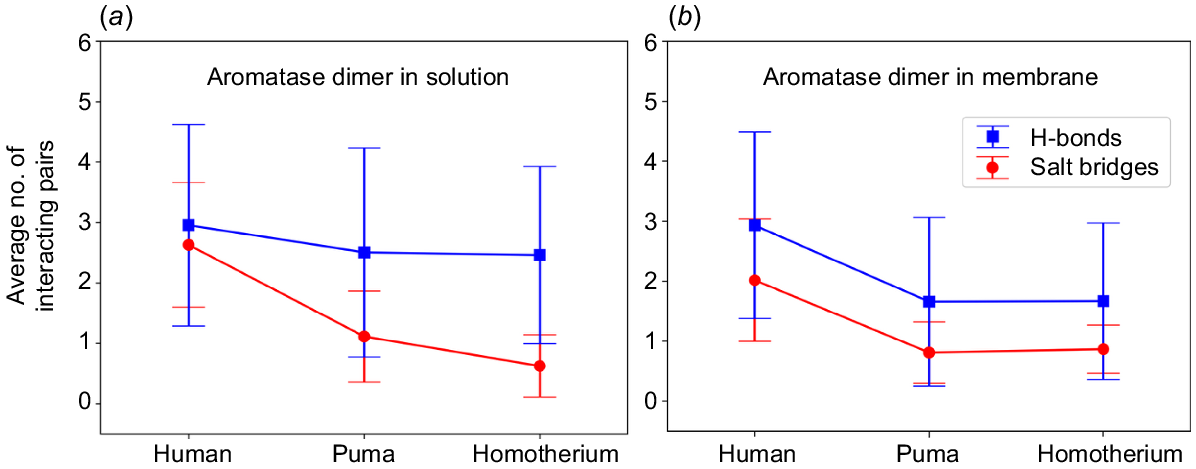
Panels (a) and (b), show the average number of hydrogen bonding (blue) and salt bridges (red) forming amino acid residue pairs between the human, puma and Homotherium homodimers when in solution (a) and when embedded in the membrane (b), tracked over 150 ns and 350 ns simulation time respectively.
Stability of haem
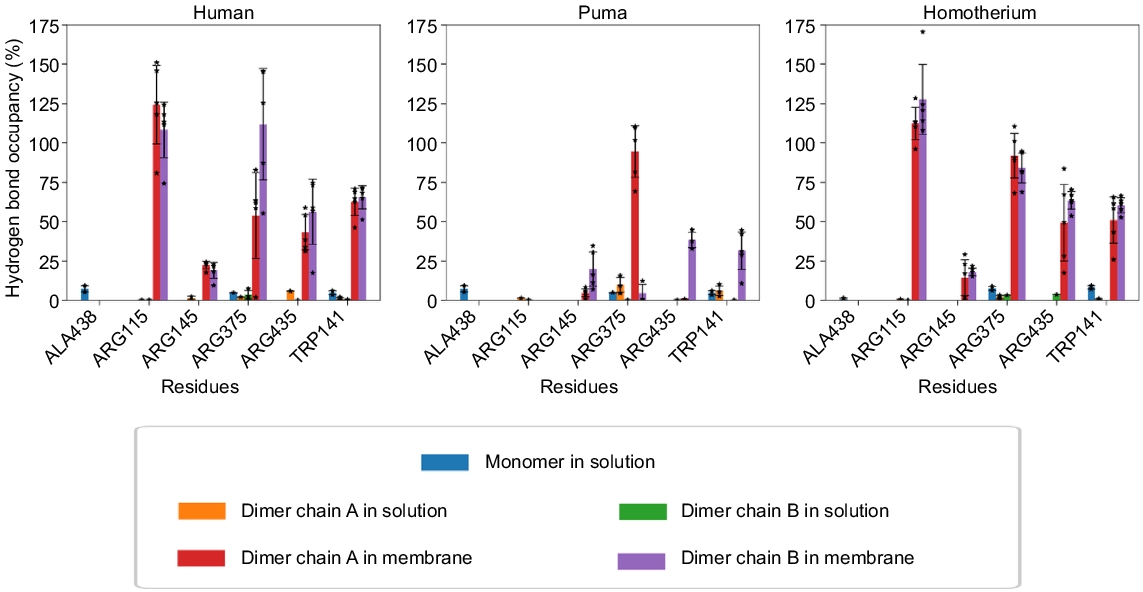
The stability of haem in aromatase is likely to have a direct impact on the enzyme’s ability to carry out its catalytic function (Gilardi and Di Nardo 2017). Haem, when bound to aromatase, undergoes a series of redox reactions that are essential for the conversion of androgens to oestrogens. Hence, when it becomes unstable, it affects the redox reactions, resulting in a decrease in enzymatic activity, and consequently, a decrease in oestrogen production (Hong et al. 2007). Comparative analysis (Fig. 7) showed that haem interactions were significantly stronger in dimeric, membrane-embedded systems than in monomers or dimers in solution, with a 10-fold increase in interaction occupancy. Cases where occupancy exceeded 100% reflect multiple hydrogen bonds involving the same haem atom. These findings suggest that the membrane environment enhances aromatase stability and function.
Percentage occupancy of hydrogen bonding interactions between aromatase residues and haem, tracked over the 150 ns of simulation time, for monomeric and dimeric structures in solution, and 350 ns in membrane-embedded dimeric systems (stars represent data points, and bars the standard deviation).
Stability of aromatase as a dimer
Cytochrome P450 enzymes have frequently demonstrated dimer formation in crystals through the interaction of the helices F and G regions (Ghosh et al. 2010; Reed and Backes 2017). In 2015, evolutionary studies suggested that dimerisation of human aromatase increased the enzymatic activity and efficiency (Martin et al. 2015; Fatima et al. 2020). Our simulations show that human aromatase dimers are significantly more stable in membranes, whereas cat family dimers exhibit lower stability. To simplify understanding, the key interactions observed at the dimeric interface are categorised as top, middle and bottom interactions (refer Fig. 5d–f). In human aromatase, H-bonding interaction between the residues ARG293(+ve) and GLY289 occurred at the top region of dimeric interfaces. In the cat family, ARG(+ve) is substituted by LYS293(+ve), which is a conserved substitution and maintains similar interaction patterns. In the middle region, electrostatic interactions, such as those between residues LYS130(+ve) and GLU139(−ve) in human aromatase, contribute to dimer stability. However, in cat family aromatases, LYS130 is replaced by ASN130 (polar) and GLU139(−ve) is replaced by THR139, a polar residue, which weakens the electrostatic interactions. At the lower dimer interface, interactions involving (+ve)LYS390⋯ASP232(−ve) and (+ve)LYS390⋯GLU92(−ve) were stabilised in human aromatase due to the constraints provided by the membrane and helped in the formation of strong electrostatics, H-bonding and salt-bridge interactions. On the other hand, these interactions were weaker in the case of cat family aromatase due to the absence of charge residues on the interface (Fig. 3). Additionally, haem structure stability was higher in homodimeric forms embedded in membrane compared to monomeric forms, as evidenced from Fig. 7. This comparative difference in stability likely contributes to higher catalytic efficiency observed in human aromatase, compared with some species. Overall, human homodimer aromatase appeared to be a stable aromatase system when embedded in the membrane that increased haem stability, as shown in Fig. 7, while also exhibiting strong dimeric interactions (Figs 5a and 6b). Puma aromatase, on the other hand, achieves an intermediate level of dimeric interactions (Figs 5b and 6b) but loses stability as an aromatase system, as observed with lower haem stability (Fig. 7). In contrast, Homotherium aromatase has the fewest dimeric interactions (Figs 5c and 6b), forming the least stable homodimer. These suggests human forms a stable homodimer, puma forms a homodimer which is less stable compared to human and Homotherium does not favour dimerisation interactions compared to rest of the species. To assess the stability and re-association of the dimeric interface, a 100 ns test simulation was performed starting with the two monomers placed 10 Å apart (Fig. S16). Over the course of the simulation, the monomers gradually moved closer, resulting in the decrease of the centre-of-mass distance and formation of interfacial contacts (Fig. S16). Thus, leading to an increase in the number of hydrogen bonds and salt bridges formed at the dimeric interface (Fig. S17). A comparison with the 350 ns membrane-embedded dimer simulation shows that the key interacting residues are largely restored, indicating reformation of the native interface (Table S6).
While comparative biochemical data on purified cat aromatase is limited, one study examining aromatase active in cat testes (Meyer et al. 2018) reported activity levels of approximately 0.45 pmol/min.mg protein, which is markedly lower than values commonly reported for human placental aromatase, which range from 3–4 pmol/min.mg protein (Okubo et al. 1996). While the assays were conducted in different tissue contexts and conditions, this difference provides in vitro experimental support for our computational observation that reduced dimer stability in the cat family may contribute to lower catalytic efficiency. Our simulations show that the human homodimer forms more stable interfacial contacts compared to both puma and Homotherium dimers, particularly in membrane environments. Given our earlier in vitro studies implicating oligomerisation in steroidogenic cytochrome P450 enzymes (Praporski et al. 2009; Ghosh et al. 2011), we are confident that homodimerisation influences catalytic behaviour, however, we acknowledge that further biochemical validation is desirable.
Conclusions
Aromatase plays a crucial role in steroidogenesis; however, only a few studies have investigated mammalian aromatases for their structure–function relationship. There is a paucity of comparative structural studies across species, especially among mammals or even vertebrates, due to the lack of structural data on aromatase, i.e. only the human aromatase has been studied by X-ray crystallography. However, we have previously reported comparisons for the porcine gonadal isoform (Martin et al. 2015) and also some for some Australian animals (Fatima et al. 2020). Hence, this study addresses this gap by conducting computational research on aromatases obtained from different cat family species like Homotherium, lion, tiger, leopard cat, puma, cheetah, and domestic cat. Human aromatase was used as the template for constructing 3D structures to explore the dynamic behaviour of cat family aromatases. The aromatase sequences from the cat family species exhibited approximately 86% sequence identity (Table S1) with human aromatase, with the most conserved regions located within the substrate-binding and cytochrome P450 reductase (CPR) binding sites. However, among the 69 non-conserved residues (Table 2), many charged residues in humans were replaced by uncharged residues in the cat family. To investigate the structural implications of these differences, molecular dynamics (MD) simulations were carried out, which revealed that haem-binding residues remained stable, particularly in the presence of haem, and that 33% of the non-conserved residues were located at the putative dimeric interface of human aromatase. The replacement of charged residues in human aromatase with uncharged residues in cat family aromatases led to altered electrostatic patterns at the dimeric interfaces, which when modelled in buffer resulted in fewer bonding interactions, and concomitant lower stability.
To further investigate these findings, simulations were conducted in a relatively more realistic environment where the dimers were embedded inside a membrane environment and again, homodimer interactions were monitored. The membrane constraints not only increased the interactions at the dimeric interface but also enhanced the stability of the haem cofactor in these systems. The equilibrated structures were studied to identify the significant structural changes in the dimeric system that could have a potential effect on interactions and overall stability when embedded inside the membrane. Notably, human aromatase dimers showed significantly greater stability than cat family dimers in the membrane, attributed to the presence of key charged residues such as LYS130 and GLU139, which facilitate strong electrostatic interactions at the dimer interface. In cat family aromatases, substitutions at these positions weakened these interactions, leading to decreased dimer stability. Our findings highlight the evolutionary differences that may impact substrate affinity, access pathways, and stability. This extensive study on mammalian aromatase lays a foundational understanding and underscores the need for further investigation through extended molecular dynamics simulations over larger timescales. Future research could incorporate diverse substrates to provide a deeper exploration of substrate binding affinities, access channel characteristics, and the impact of varying environments on catalytic activity. These continued efforts would enhance our understanding of how structural and environmental factors influence aromatase function, potentially informing more accurate models of its catalytic mechanisms across species.
Declaration of funding
This research did not receive any specific funding, other than support for a Ph.D. scholarship.
Acknowledgements
The authors acknowledge financial support from the Department of Biotechnology (DBT), Government of India (GOI), for Project IMURA0888 at IITB-Monash Research Academy, and for the award of a Ph.D. stipend to R.M.. We also thank Prof. Michael Westbury from the University of Copenhagen for providing the DNA sequence of Homotherium latidens, from which we computationally derived the amino acid sequence of aromatase. The authors also acknowledge support in the form of access to the SpaceTime supercomputing facility at IIT Bombay.
References
Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, De Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H (2012) ExPASy: SIB bioinformatics resource portal. Nucleic Acids Research 40, W597-W603.
| Crossref | Google Scholar | PubMed |
Barnett R, Westbury MV, Sandoval-Velasco M, Vieira FG, Jeon S, Zazula G, Martin MD, Ho SYW, Mather N, Gopalakrishnan S, Ramos-Madrigal J, de Manuel M, Zepeda-Mendoza ML, Antunes A, Baez AC, De Cahsan B, Larson G, O’Brien SJ, Eizirik E, Johnson WE, Koepfli KP, Wilting A, Fickel J, Dalén L, Lorenzen ED, Marques-Bonet T, Hansen AJ, Zhang G, Bhak J, Yamaguchi N, Gilbert MTP (2020) Genomic adaptations and evolutionary history of the extinct Scimitar-Toothed Cat, Homotherium latidens. Current Biology 30, 5018-5025.e5.
| Crossref | Google Scholar |
Benkert P, Tosatto SCE, Schomburg D (2008) QMEAN: A comprehensive scoring function for model quality assessment. Proteins: Structure, Function, and Bioinformatics 71, 261-277.
| Crossref | Google Scholar |
Biegon A (2016) In vivo visualization of aromatase in animals and humans. Frontiers in Neuroendocrinology 40, 42-51.
| Crossref | Google Scholar | PubMed |
Castro LFC, Santos MM, Reis-Henriques MA (2005) The genomic environment around the Aromatase gene: evolutionary insights. BMC Evolutionary Biology 5, 43.
| Crossref | Google Scholar |
Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallographica Section D: Biological Crystallography 66, 12-21.
| Crossref | Google Scholar | PubMed |
Conley A, Hinshelwood M (2001) Mammalian aromatases. Reproduction 121, 685-695.
| Crossref | Google Scholar | PubMed |
Conley A, Mapes S, Corbin CJ, Greger D, Walters K, Trant J, Graham S (2001) A comparative approach to structure–function studies of mammalian aromatases. Journal of Steroid Biochemistry and Molecular Biology 79, 289-297.
| Crossref | Google Scholar | PubMed |
Corbin CJ, Mapes SM, Lee YM, Conley AJ (2003) Structural and functional differences among purified recombinant mammalian aromatases: glycosylation, N-terminal sequence and kinetic analysis of human, bovine and the porcine placental and gonadal isozymes. Molecular and Cellular Endocrinology 206, 147-157.
| Crossref | Google Scholar |
Di Nardo G, Zhang C, Marcelli AG, Gilardi G (2021) Molecular and structural evolution of cytochrome p450 aromatase. International Journal of Molecular Sciences 22, 631-16.
| Crossref | Google Scholar | PubMed |
Edgar RC, Batzoglou S (2006) Multiple sequence alignment. Current Opinion in Structural Biology 16, 368-373.
| Crossref | Google Scholar | PubMed |
Evans CT, Ledesma DB, Schulz TZ, Simpson ER, Mendelson CR (1986) Isolation and characterization of a complementary DNA specific for human aromatase-system cytochrome P-450 mRNA. Proceedings of the National Academy of Sciences 83, 6387-6391.
| Google Scholar |
Fatima A, Holien JK, Tiwari C, Parker MW, Rodgers RJ, Martin LL (2020) Sequence comparisons of cytochrome P450 aromatases from Australian animals predict differences in enzymatic activity and/or efficiency. Biology of Reproduction 102, 1261-1269.
| Crossref | Google Scholar | PubMed |
Gameiro A, Urbano AC, Ferreira F (2021) Emerging biomarkers and targeted therapies in feline mammary carcinoma. Veterinary Sciences 8, 164.
| Crossref | Google Scholar | PubMed |
Ghosh D (2023) Structures and functions of human placental aromatase and steroid sulfatase, two key enzymes in estrogen biosynthesis. Steroids 196, 109249.
| Crossref | Google Scholar | PubMed |
Ghosh D, Griswold J, Erman M, Pangborn W (2010) X-ray structure of human aromatase reveals an androgen-specific active site. Journal of Steroid Biochemistry and Molecular Biology 118, 197-202.
| Crossref | Google Scholar | PubMed |
Ghosh D, Jiang W, Lo J, Egbuta C (2011) Higher order organization of human placental aromatase. Steroids 76, 753-758.
| Crossref | Google Scholar | PubMed |
Ghosh D, Lo J, Morton D, Valette D, Xi J, Griswold J, Hubbell S, Egbuta C, Jiang W, An J, Davies HM (2012) Novel aromatase inhibitors by structure-guided design. Journal of Medicinal Chemistry 55, 8464-8476.
| Crossref | Google Scholar | PubMed |
Ghosh D, Egbuta C, Lo J (2018) Testosterone complex and non-steroidal ligands of human aromatase. Journal of Steroid Biochemistry and Molecular Biology 181, 11-19.
| Crossref | Google Scholar | PubMed |
Gilardi G, Di Nardo G (2017) Heme iron centers in cytochrome P450: structure and catalytic activity. Rendiconti Lincei 28, 159-167.
| Crossref | Google Scholar |
Harada N, Ogawa H, Shozu M, Yamada K (1992) Genetic studies to characterize the origin of the mutation in placental aromatase deficiency. American Journal of Human Genetics 51, 666-672.
| Google Scholar | PubMed |
Hiller-Sturmhöfel S, Bartke A (1998) The endocrine system: an overview. Alcohol Health and Research World 22, 153.
| Google Scholar | PubMed |
Holien KJ, Parker WM, Conley JA, Corbin JC, Rodgers JR, Martin LL (2017) A homodimer model can resolve the conundrum as to how cytochrome P450 oxidoreductase and cytochrome b5 compete for the same binding site on cytochrome P450c17. Current Protein & Peptide Science 18, 515-521.
| Crossref | Google Scholar | PubMed |
Hong Y, Yu B, Sherman M, Yuan YC, Zhou D, Chen S (2007) Molecular basis for the aromatization reaction and exemestane-mediated irreversible inhibition of human aromatase. Molecular Endocrinology 21, 401-414.
| Crossref | Google Scholar | PubMed |
Hong Y, Li H, Yuan YC, Chen S (2009) Molecular characterization of aromatase. Annals of the New York Academy of Sciences 1155, 112-120.
| Crossref | Google Scholar | PubMed |
Honma W, Li W, Liu H, Scott EE, Halpert JR (2005) Functional role of residues in the helix B’ region of cytochrome P450 2B1. Archives of Biochemistry and Biophysics 435, 157-165.
| Crossref | Google Scholar | PubMed |
Hossack EJ, Hardy FJ, Green AP (2023) Building enzymes through design and evolution. ACS Catalysis 13, 12436-12444.
| Crossref | Google Scholar |
Huang J, MacKerell AD, Jr (2013) CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. Journal of Computational Chemistry 34, 2135-2145.
| Crossref | Google Scholar | PubMed |
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. Journal of Molecular Graphics 14, 33-38.
| Crossref | Google Scholar | PubMed |
Jo S, Kim T, Iyer VG, Im W (2008) CHARMM-GUI: a web-based graphical user interface for CHARMM. Journal of Computational Chemistry 29, 1859-1865.
| Crossref | Google Scholar | PubMed |
Kauffman AS, Rissman EF (2006) CHAPTER 42 – Neuroendocrine control of mating-induced ovulation. In ‘Knobil and Neill’s physiology of reproduction’. 3rd edn. (Ed. JD Neill) pp. 2283–2326. (Academic Press: St Louis) 10.1016/B978-012515400-0/50047-6
Li Q, Zhang F, Zhang S, Sheng X, Han Y, Weng Q, Yuan Z (2015) Seasonal expression of androgen receptor, aromatase, and estrogen receptor alpha and beta in the testis of the wild ground squirrel (Citellus dauricus Brandt). European Journal of Histochemistry 59(1), 2456.
| Crossref | Google Scholar |
Lo J, Di Nardo G, Griswold J, Egbuta C, Jiang W, Gilardi G, Ghosh D (2013) Structural basis for the functional roles of critical residues in human cytochrome p450 aromatase. Biochemistry 52, 5821-5829.
| Crossref | Google Scholar | PubMed |
MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS (1987) Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids 50, 459-474.
| Crossref | Google Scholar | PubMed |
Martin LL, Holien JK, Mizrachi D, Corbin CJ, Conley AJ, Parker MW, Rodgers RJ (2015) Evolutionary comparisons predict that dimerization of human cytochrome P450 aromatase increases its enzymatic activity and efficiency. Journal of Steroid Biochemistry and Molecular Biology 154, 294-301.
| Crossref | Google Scholar | PubMed |
Martin LL, Kubeil C, Simonov AN, Kuznetsov VL, Corbin CJ, Auchus RJ, Conley AJ, Bond AM, Rodgers RJ (2017) Electrochemistry of cytochrome P450 17α-hydroxylase/17,20-lyase (P450c17). Molecular and Cellular Endocrinology 441, 62-67.
| Crossref | Google Scholar | PubMed |
Menotti-Raymond M, Deckman KH, David V, Myrkalo J, O’Brien SJ, Narfström K (2010) Mutation discovered in a feline model of human congenital retinal blinding disease. Investigative Ophthalmology & Visual Science 51, 2852-2859.
| Crossref | Google Scholar | PubMed |
Meyer KB, Martino-Andrade AJ, Santos AS, Spercoski KM, Morais RN (2018) Domestic cat testicular aromatase activity as assessed by the tritiated water-release assay. Animal Reproduction 11, 549-556.
| Google Scholar |
Nair PC, McKinnon RA, Miners JO (2016) Cytochrome P450 structure-function: insights from molecular dynamics simulations. Drug Metabolism Reviews 48, 434-452.
| Crossref | Google Scholar | PubMed |
Nebert DW, Russell DW (2002) Clinical importance of the cytochromes P450. Lancet 360, 1155-1162.
| Crossref | Google Scholar | PubMed |
Okubo K, Jinbo M, Toma Y, Shimizu Y, Yanaihara T (1996) Aromatase and estrogen 2-hydroxylase activities of human placental microsomes in pregnancy-induced hypertension. Endocrine Journal 43, 363-368.
| Crossref | Google Scholar | PubMed |
O’Brien SJ, Johnson WE (2007) The evolution of cats. Scientific American 297, 68-75.
| Crossref | Google Scholar | PubMed |
O’Brien SJ, Menotti-Raymond M, Murphy WJ, Yuhki N (2002) The feline genome project. Annual Review of Genetics 36, 657-686.
| Crossref | Google Scholar | PubMed |
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K (2005) Scalable molecular dynamics with NAMD. Journal of Computational Chemistry 26, 1781-1802.
| Crossref | Google Scholar |
Pontius JU, Mullikin JC, Smith DR, Agencourt Sequencing Team, Lindblad-Toh K, Gnerre S, Clamp M, Chang J, Stephens R, Neelam B, Volfovsky N, Schäffer AA, Agarwala R, Narfström K, Murphy WJ, Giger U, Roca AL, Antunes A, Menotti-Raymond M, Yuhki N, Pecon-Slattery J, Johnson WE, Bourque G, Tesler G, NISC Comparative Sequencing Program, O’Brien SJ (2007) Initial sequence and comparative analysis of the cat genome. Genome Research 17, 1675-1689.
| Crossref | Google Scholar | PubMed |
Praporski S, Ng SM, Nguyen AD, Corbin CJ, Mechler A, Zheng J, Conley AJ, Martin LL (2009) Organization of cytochrome P450 enzymes involved in sex steroid synthesis. Journal of Biological Chemistry 284, 33224-33232.
| Crossref | Google Scholar | PubMed |
Reed JR, Backes WL (2017) Physical studies of P450–P450 interactions: predicting quaternary structures of P450 complexes in membranes from their X-ray crystal structures. Frontiers in Pharmacology 8, 28.
| Crossref | Google Scholar | PubMed |
Rijnberk A, Kooistra HS, Mol JA (2003) Endocrine diseases in dogs and cats: similarities and differences with endocrine diseases in humans. Growth Hormone & IGF Research 13, S158-S164.
| Crossref | Google Scholar | PubMed |
Roselli CE, Resko JA (1993) Aromatase activity in the rat brain: hormonal regulation and sex differences. Journal of Steroid Biochemistry and Molecular Biology 44, 499-508.
| Crossref | Google Scholar | PubMed |
Saltzman W, Tardif SD, Rutherford JN (2011) Chapter 15 - Hormones and reproductive cycles in primates. In ‘Hormones and reproduction of vertebrates’. 2nd edn (Eds DO Norris, KH Lopez) pp. 325–364. (Academic Press) 10.1016/B978-0-443-15986-2.00022-8
Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A (2009) History of aromatase: saga of an important biological mediator and therapeutic target. Endocrine Reviews 30, 343-375.
| Crossref | Google Scholar | PubMed |
Sayers EW, Bolton EE, Brister JR, Canese K, Chan J, Comeau DC, Connor R, Funk K, Kelly C, Kim S, Madej T, Marchler-Bauer A, Lanczycki C, Lathrop S, Lu Z, Thibaud-Nissen F, Murphy T, Phan L, Skripchenko Y, Tse T, Wang J, Williams R, Trawick BW, Pruitt KD, Sherry ST (2022) Database resources of the national center for biotechnology information. Nucleic Acids Research 50, D20-D26.
| Crossref | Google Scholar | PubMed |
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology 7, 539.
| Crossref | Google Scholar | PubMed |
Siiteri PK (1982) Review of studies on estrogen biosynthesis in the human. Cancer Research 42, 3269s-3273s.
| Google Scholar | PubMed |
Siiteri PK, Thompson EA (1976) Studies of human placental aromatase. In ‘Proceedings of the Fourth International Congress on Hormonal Steroids’. (Eds. VHT James, JR Pasqualini) pp. 317–322. (Pergamon) 10.1016/B978-0-08-019682-4.50030-5
Simpson ER, Dowsett M (2002) Aromatase and its inhibitors: significance for breast cancer therapy. Recent Progress in Hormone Research 57, 317-338.
| Crossref | Google Scholar | PubMed |
Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M (2002) Aromatase—a brief overview. Annual Review of Physiology 64, 93-127.
| Crossref | Google Scholar | PubMed |
Smith CA, Elf PK, Lang JW, Joss JMP (1995) Aromatase enzyme activity during gonadal sex differentiation in alligator embryos. Differentiation 58, 281-290.
| Crossref | Google Scholar |
Stocco C (2012) Tissue physiology and pathology of aromatase. Steroids 77, 27-35.
| Crossref | Google Scholar | PubMed |
The UniProt Consortium (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Research 47, D506-D515.
| Crossref | Google Scholar |
Thompson EA, Jr, Siiteri PK (1974) The involvement of human placental microsomal cytochrome P-450 in aromatization. Journal of Biological Chemistry 249, 5373-5378.
| Crossref | Google Scholar | PubMed |
Tiwary BK, Li W-H (2009) Parallel evolution between aromatase and androgen receptor in the animal kingdom. Molecular Biology and Evolution 26, 123-129.
| Crossref | Google Scholar | PubMed |
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Research 46, W296-W303.
| Crossref | Google Scholar | PubMed |
Westbury MV, Barnett R, Sandoval-Velasco M, Gower G, Vieira FG, de Manuel M, Hansen AJ, Yamaguchi N, Werdelin L, Marques-Bonet T, Gilbert MTP, Lorenzen ED (2021) A genomic exploration of the early evolution of extant cats and their sabre-toothed relatives. Open Research Europe 1, 25.
| Crossref | Google Scholar | PubMed |
Wilson JY, McArthur AG, Stegeman JJ (2005) Characterization of a cetacean aromatase (CYP19) and the phylogeny and functional conservation of vertebrate aromatase. General and Comparative Endocrinology 140, 74-83.
| Crossref | Google Scholar | PubMed |
Yoshimoto FK, Guengerich FP (2014) Mechanism of the third oxidative step in the conversion of androgens to estrogens by cytochrome P450 19A1 steroid aromatase. Journal of the American Chemical Society 136, 15016-15025.
| Crossref | Google Scholar | PubMed |
Zhang C, Gilardi G, Di Nardo G (2022) Depicting the proton relay network in human aromatase: New insights into the role of the alcohol-acid pair. Protein Science 31, e4389.
| Crossref | Google Scholar | PubMed |


