43 Factors affecting vitrification of canine epididymal spermatozoa
S. Stoddard A , J. Linn A , A. Lemma A and G. Wirtu AA Tuskegee University College of Veterinary Medicine, Tuskegee, AL, USA
Reproduction, Fertility and Development 34(2) 256-257 https://doi.org/10.1071/RDv34n2Ab43
Published: 7 December 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS
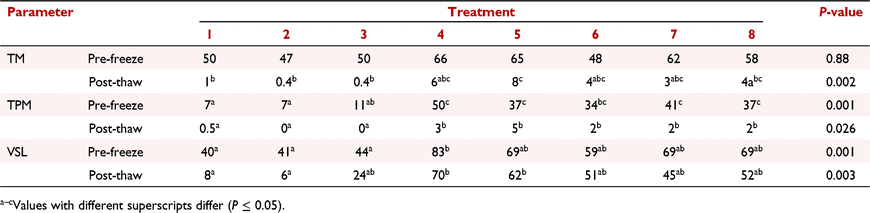
There is a growing interest in understanding factors affecting sperm cryosurvival after vitrification, which provides some practical advantages over slow freezing. Most studies reported low post-thaw motility (<10%) after warming (Caturla-Sánchez et al. 2017 Cryobiology 80, 126); however, Pipan et al. (2020 Animals 10, 653) reported ∼50% post-thaw motility after supplementing the vitrification media with soy lecithin in place of egg yolk. Myoinositol has antioxidant effects and improved canine sperm cryosurvival (Qamar et al. 2019 Animals 9, 1038). The present study was designed to evaluate the effects of myoinositol and low concentration of glycerol on the cooling tolerance and cryosurvival of canine epididymal spermatozoa after vitrification. Testes (n = 5) were collected after routine orchiectomies from the Alabama Animal Alliance and Tuskegee University College of Veterinary Medicine. Epididymal samples were extracted from the cauda epididymis, centrifuged, and resuspended in Tris-citrate-sucrose (TCS) extender (Pipan et al. 2020 Animals 10, 653). Subsequently, samples with at least 70% motility were divided at 200 million spermatozoa per mL into the following eight treatments: (1) TCS, (2) TCS + 1 mg mL−1 myoinositol, (3) TCS + 1% poly-vinyl alcohol, (4) TCS + 20% egg yolk, (5) Treatment 4 + 1 mg mL−1 myoinositol, (6) Treatment 4 + 0.5% glycerol, (7) Treatment 4 + 1.5% glycerol, and (8) Treatment 4 + 2.5% glycerol. Samples were cooled to 4°C for 30 min, and pre-freeze motility was determined using IVOSII computer-assisted sperm analysis (CASA). Samples were then vitrified by dropping 50 mL into liquid nitrogen from 11 cm, and pellets were stored for at least 1 day before thawing. Pellets were thawed at 42°C in 200 µL of TCS. Pre-freeze and post-thaw treatment effects on sperm parameters [% total motility™, % total progressive motility (TPM), and straight linear velocity (VSL)) were analysed using a one-way ANOVA in SPSS with Tukey’s post-hoc test. Treatments affected (P ≤ 0.05) pre-freeze TPM, pre-freeze VSL, post-thaw TM, post-thaw TPM, and post-thaw VSL, but not pre-freeze TM (Table 1). Supplementation of media with egg yolk, but not a low concentration of glycerol, had a beneficial effect on the cryosurvival of canine epididymal spermatozoa. Treatment effects were apparent starting from the cooling period.
|


