Gene and protein expression of connexins 37 and 43 in cumulus–oocytes complexes throughout the canine oestrous cycle
Monica De los Reyes A D , Jaime Palomino A , Carola Gallegos A , Roberto Espinoza A , Phillipe Dettleff B , Oscar A. Peralta
A D , Jaime Palomino A , Carola Gallegos A , Roberto Espinoza A , Phillipe Dettleff B , Oscar A. Peralta  A , Victor H. Parraguez
A , Victor H. Parraguez  C and George Ramirez A
C and George Ramirez A
A Laboratory of Animal Reproduction, Department of Animal Production Faculty of Veterinary Sciences, University of Chile, Casilla 2, Correo 15, La Granja, Santiago, Chile.
B Laboratory Inviogen, Department of Biological Sciences, Faculty of Veterinary Sciences, University of Chile, Casilla 2, Correo 15, La Granja, Santiago, Chile.
C Laboratory of Animal Physiology, Department of Biological Sciences, Faculty of Veterinary Sciences, University of Chile, Casilla 2, Correo 15, La Granja, Santiago, Chile.
D Corresponding author. Email: mdlreyes@uchile.cl
Reproduction, Fertility and Development 32(11) 976-987 https://doi.org/10.1071/RD20126
Submitted: 15 May 2020 Accepted: 30 May 2020 Published: 14 July 2020
Abstract
The aim of this study was to evaluate the expression of connexin (Cx) 37 and Cx43 in canine cumulus–oocyte complexes (COCs) during the oestrous cycle. Cx localisation was analysed by immunohistochemistry and immunofluorescence, whereas protein and gene expression was evaluated by western blotting and quantitative polymerase chain reaction respectively; comparisons were made using analysis of variance. Both Cx37 and Cx43 were expressed in all follicular stages; Cx43 was identified in cumulus cells and Cx37 was identified in cumulus cells, zonae pellucida and oocytes. Immunofluorescence analyses showed that Cx37 remained unchanged during the preovulatory stage but decreased after ovulation, whereas Cx43 remained unchanged before and after ovulation. Cx43 transcripts increased (P < 0.05) during anoestrus and dioestrus in medium-sized follicles but remained unaltered during the pro-oestrus and antral stages during oestrus, before and after ovulation. Cx37 mRNA levels decreased in ovulated COCs (P < 0.05). The highest levels of Cx37 protein (P < 0.05) were detected in the preantral stage during anoestrus. In contrast, strong Cx43 signals were detected in oestrus and in medium-sized antral follicles in dioestrus (P < 0.05). Overall, we demonstrated that Cx37 and Cx43 exhibit different expression patterns, suggesting specific roles throughout growth. Maintenance of Cx expression before ovulation indicates the involvement of Cx37 and Cx43 in the prolonged meiotic arrest.
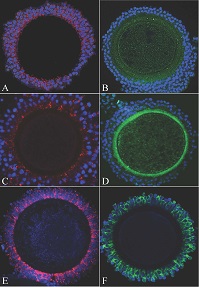
Additional keywords: follicle development, folliculogenesis, gap junction, meiotic resumption.
References
Ackert, C. L., Gittens, J. E., O’Brien, M. J., Eppig, J. J., and Kidder, G. M. (2001). Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 233, 258–270.| Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse.Crossref | GoogleScholarGoogle Scholar | 11336494PubMed |
Alexander, I. E., Clarke, C. L., Shine, J., and Sutherland, R. L. (1989). Progestin inhibition of progesterone receptor gene expression in human breast cancer cells. Mol. Endocrinol. 3, 1377–1386.
| Progestin inhibition of progesterone receptor gene expression in human breast cancer cells.Crossref | GoogleScholarGoogle Scholar | 2608065PubMed |
Andrade, G. M., del Collado, M., Meirelles, F. V., da Silveira, J. C., and Perecin, F. (2019). Intrafollicular barriers and cellular interactions during ovarian follicle development. Anim. Reprod. 16, 485–496.
| Intrafollicular barriers and cellular interactions during ovarian follicle development.Crossref | GoogleScholarGoogle Scholar | 32435292PubMed |
Barrett, S. L., and Albertini, D. F. (2010). Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J. Assist. Reprod. Genet. 27, 29–39.
| Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence.Crossref | GoogleScholarGoogle Scholar | 20039198PubMed |
Borowczyk, E., Johnson, M. L., Bilski, J. J., Borowicz, P. l., Redmer, D. A., Reynolds, L. P., and Grazul-Bilska, A. T. (2006). Gap junctional connexin 37 is expressed in sheep ovaries. Endocrine 30, 223–230.
| Gap junctional connexin 37 is expressed in sheep ovaries.Crossref | GoogleScholarGoogle Scholar | 17322584PubMed |
Bustin, S. A. (2002). Quantification of mRNA using real-time reverse transcription (RT-qPCR): trends and problems. J. Mol. Endocrinol. 29, 23–39.
| Quantification of mRNA using real-time reverse transcription (RT-qPCR): trends and problems.Crossref | GoogleScholarGoogle Scholar | 12200227PubMed |
Chang, H. M., Cheng, J. C., and Leung, P. C. (2013). Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells. J. Clin. Endocrinol. Metab. 98, E437–E445.
| Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells.Crossref | GoogleScholarGoogle Scholar | 23386650PubMed |
Chang, H. M., Cheng, J. C., Taylor, E., and Leung, P. C. (2014). Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells. Mol. Hum. Reprod. 20, 373–383.
| Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells.Crossref | GoogleScholarGoogle Scholar | 24413384PubMed |
Cheng, J. C., Chang, H. M., Fang, L., Sun, Y. P., and Leung, P. C. K. (2015). TGF-β1 up-regulates connective tissue growth factor expression in human granulosa cells through Smad and ERK1/2 signaling pathways. PLoS One 10, e0126532.
| TGF-β1 up-regulates connective tissue growth factor expression in human granulosa cells through Smad and ERK1/2 signaling pathways.Crossref | GoogleScholarGoogle Scholar | 25955392PubMed |
Clarke, H. J. (2018). History, origin, and function of transzonal projections: the bridges of communication between the oocyte and its environment. Anim. Reprod. 15, 215–223.
| History, origin, and function of transzonal projections: the bridges of communication between the oocyte and its environment.Crossref | GoogleScholarGoogle Scholar |
Concannon, P. W. (2011). Reproductive cycles of the domestic bitch. Anim. Reprod. Sci. 124, 200–210.
| Reproductive cycles of the domestic bitch.Crossref | GoogleScholarGoogle Scholar | 21055888PubMed |
Coticchio, G., Dal Canto, M., Renzini, M. M., Guglielmo, M. C., Brambillasca, F., Turchi, D., Novara, P. V., and Fadini, R. (2015). Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 21, 427–454.
| 25744083PubMed |
De los Reyes, M., Palomino, J., Parraguez, V. H., and Ramirez, F. (2017). Analysis of LH receptor in canine ovarian follicles throughout the estrous cycle. Theriogenology 93, 71–77.
| Analysis of LH receptor in canine ovarian follicles throughout the estrous cycle.Crossref | GoogleScholarGoogle Scholar | 28257870PubMed |
Enders, A. C., and Carter, A. M. (2012). Review: the evolving placenta: different developmental paths to a hemochorial relationship. Placenta 33, S92–S98.
| Review: the evolving placenta: different developmental paths to a hemochorial relationship.Crossref | GoogleScholarGoogle Scholar | 22061678PubMed |
Fernandez, T., Palomino, J., Parraguez, V. H., Peralta, O. A., and De los Reyes, M. (2016). Differential expression of GDF-9 and BMP-15 during follicular development in canine ovaries evaluated by flow cytometry. Anim. Reprod. Sci. 167, 59–67.
| Differential expression of GDF-9 and BMP-15 during follicular development in canine ovaries evaluated by flow cytometry.Crossref | GoogleScholarGoogle Scholar | 26876149PubMed |
Firestone, G. L., and Kapadia, B. J. (2012). Minireview: regulation of gap junction dynamics by nuclear hormone receptors and their ligands. Mol. Endocrinol. 26, 1798–1807.
| Minireview: regulation of gap junction dynamics by nuclear hormone receptors and their ligands.Crossref | GoogleScholarGoogle Scholar | 22935924PubMed |
Granot, I., and Dekel, N. (2002). The ovarian gap junction protein connexin43: regulation by gonadotropins. Trends Endocrinol. Metab. 13, 310–313.
| The ovarian gap junction protein connexin43: regulation by gonadotropins.Crossref | GoogleScholarGoogle Scholar | 12163234PubMed |
Itahana, K., Morikazu, Y., and Takizya, T. (1996). Differential expression of four connexin genes, Cx-26, Cx-30.3, Cx-32, and Cx-43, in the porcine ovarian follicle. Endocrinology 137, 5036–5044.
| Differential expression of four connexin genes, Cx-26, Cx-30.3, Cx-32, and Cx-43, in the porcine ovarian follicle.Crossref | GoogleScholarGoogle Scholar | 8895378PubMed |
Johnson, R. G., Meyer, R. A., Li, X. R., Preus, D. M., Tan, L., Grunenwald, H., Paulson, A. F., Laird, D. W., and Sheridan, J. D. (2002). Gap junctions assemble in the presence of cytoskeletal inhibitors, but enhanced assembly requires microtubules. Exp. Cell Res. 275, 67–80.
| Gap junctions assemble in the presence of cytoskeletal inhibitors, but enhanced assembly requires microtubules.Crossref | GoogleScholarGoogle Scholar | 11925106PubMed |
Johnson, M. L., Redmer, D. A., Reynolds, L. P., and Grazul-Bilska, A. T. (2017). Gap junctional connexin messenger RNA expression in the ovine uterus and placenta: effects of estradiol-17b-treatment, early pregnancy stages, and embryo origin. Domest. Anim. Endocrinol. 58, 104–112.
| Gap junctional connexin messenger RNA expression in the ovine uterus and placenta: effects of estradiol-17b-treatment, early pregnancy stages, and embryo origin.Crossref | GoogleScholarGoogle Scholar | 27835804PubMed |
Kalma, Y., Granot, I., Galiani, D., Barash, A., and Dekel, N. (2004). Luteinizing hormone-induced connexin 43 down-regulation: inhibition of translation. Endocrinology 145, 1617–1624.
| Luteinizing hormone-induced connexin 43 down-regulation: inhibition of translation.Crossref | GoogleScholarGoogle Scholar | 14684606PubMed |
Kidder, G. M., and Vanderhyden, B. C. (2010). Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 88, 399–413.
| Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar | 20555408PubMed |
Larsen, W. J., Wert, S. E., and Brunner, G. D. (1987). Differential modulation of rat follicle cell gap junction populations at ovulation. Dev. Biol. 122, 61–71.
| Differential modulation of rat follicle cell gap junction populations at ovulation.Crossref | GoogleScholarGoogle Scholar | 3596012PubMed |
Lei, R., Bai, X., Chang, Y., Li, J., Qin, Y., Chen, K., Gu, W., Xia, S., Zhang, J., Wang, Z., and Xing, G. (2018). Effects of fullerenol nanoparticles on rat oocyte meiosis resumption. Int. J. Mol. Sci. 19, 699.
| Effects of fullerenol nanoparticles on rat oocyte meiosis resumption.Crossref | GoogleScholarGoogle Scholar |
Lenhart, J. A., Downey, B. R., and Bagnell, C. A. (1998). Connexin43 gap junction protein expression during follicular development in the porcine ovary. Biol. Reprod. 58, 583–590.
| Connexin43 gap junction protein expression during follicular development in the porcine ovary.Crossref | GoogleScholarGoogle Scholar | 9475417PubMed |
Li, J., Mao, G., and Xia, G. (2012). FSH modulates PKAI and GPR3 activities in mouse oocyte of COC in a gap junctional communication (GJC)-dependent manner to initiate meiotic resumption. PLoS One 7, e37835.
| FSH modulates PKAI and GPR3 activities in mouse oocyte of COC in a gap junctional communication (GJC)-dependent manner to initiate meiotic resumption.Crossref | GoogleScholarGoogle Scholar | 23300804PubMed |
Luciano, A. M., Modina, S., Vassena, R., Milanesi, E., Lauria, A., and Gandolfi, F. (2004). Role of intracellular cyclic adenosine 39,59-monophosphate concentration and oocyte–cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte. Biol. Reprod. 70, 465–472.
| Role of intracellular cyclic adenosine 39,59-monophosphate concentration and oocyte–cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte.Crossref | GoogleScholarGoogle Scholar | 14568913PubMed |
Luciano, A. M., Franciosi, F., Modina, S. C., and Lodde, V. (2011). Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through camp-dependent mechanism. Biol. Reprod. 85, 1252–1259.
| Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through camp-dependent mechanism.Crossref | GoogleScholarGoogle Scholar | 21816847PubMed |
Norris, R. P., Baena, V., and Terasaki, M. (2017). Localization of phosphorylated connexin 43 using serial section immunogold electron microscopy. J. Cell Sci. 130, 1333–1340.
| Localization of phosphorylated connexin 43 using serial section immunogold electron microscopy.Crossref | GoogleScholarGoogle Scholar | 28202692PubMed |
Nuttinck, F., Humblot, P., Massip, A., Dessy, F., and Fleachon, J. E. (2000). Comparative immunohistochemical distribution of connexin 37 and connexin 43 throughout folliculogenesis in the bovine ovary. Mol. Reprod. Dev. 57, 60–66.
| Comparative immunohistochemical distribution of connexin 37 and connexin 43 throughout folliculogenesis in the bovine ovary.Crossref | GoogleScholarGoogle Scholar | 10954857PubMed |
Palomino, J., and De los Reyes, M. (2016). Temporal expression of GDF-9 and BMP-15 in canine ovarian follicles. Theriogenology 86, 1541–1549.
| Temporal expression of GDF-9 and BMP-15 in canine ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 27341772PubMed |
Pant, D., Reynolds, L. P., Luther, J. S., Borowicz, P. P., Stenbak, T. M., Bilski, J. J., Weigl, R. M., Lopes, F., Petry, K., Johnson, M. L., Redmer, D. A., and Grazul-Bilska, A. T. (2005). Expression of connexin 43 and gap junctional intercellular communication in the cumulus–oocyte complex in sheep. Reproduction 129, 191–200.
| Expression of connexin 43 and gap junctional intercellular communication in the cumulus–oocyte complex in sheep.Crossref | GoogleScholarGoogle Scholar | 15695613PubMed |
Praveen Chakravarthi, V., Kona, S. S. R., Siva Kumar, A. V. N., Bhaskar, M., and Rao, V. H. (2016). Quantitative patterns of expression of gap junction genes during in vivo or in vitro development of ovarian follicles in sheep. Small Rumin. Res. 143, 35–42.
| Quantitative patterns of expression of gap junction genes during in vivo or in vitro development of ovarian follicles in sheep.Crossref | GoogleScholarGoogle Scholar |
Renton, J. P., Boyd, J. S., Eckersall, P. D., Ferguson, J. M., Harvey, M. J., Mullaney, J., and Perry, B. (1991). Ovulation, fertilization and early embryonic development in the bitch (Canis familiaris). J. Reprod. Fertil. 93, 221–231.
| Ovulation, fertilization and early embryonic development in the bitch (Canis familiaris).Crossref | GoogleScholarGoogle Scholar | 1920293PubMed |
Russell, D. L., Gilchrist, R. B., Brown, H. M., and Thomsom, J. G. (2016). Bidirectional communication between cumulus cells and the oocyte: old hands and new players? Theriogenology 86, 62–68.
| Bidirectional communication between cumulus cells and the oocyte: old hands and new players?Crossref | GoogleScholarGoogle Scholar | 27160446PubMed |
Santiquet, N., Robert, C., and Richard, F. J. (2013). The dynamics of connexin expression, degradation and localization are regulated by gonadotropins during the early stages of in vitro maturation of swine oocytes. PLoS One 8, e68456.
| The dynamics of connexin expression, degradation and localization are regulated by gonadotropins during the early stages of in vitro maturation of swine oocytes.Crossref | GoogleScholarGoogle Scholar | 23861906PubMed |
Simon, A. M., Chen, H., and Jackson, C. L. (2006). Cx37 and Cx43 localize to zona pellucida in mouse ovarian follicles. Cell Commun. Adhes. 13, 61–77.
| Cx37 and Cx43 localize to zona pellucida in mouse ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 16613781PubMed |
Teilmann, S. C. (2005). Differential expression and localisation of connexin-37 and connexin-43 in follicles of different stages in the 4-week-old mouse ovary. Mol. Cell. Endocrinol. 234, 27–35.
| Differential expression and localisation of connexin-37 and connexin-43 in follicles of different stages in the 4-week-old mouse ovary.Crossref | GoogleScholarGoogle Scholar | 15836950PubMed |
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., and Speleman, F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034.1.
| Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes.Crossref | GoogleScholarGoogle Scholar | 12184808PubMed |
Wang, H. X., Tong, D., El-Gehani, F., Tekpetey, F., and Kidder, G. M. (2009). Connexin expression and gap junctional coupling in human cumulus cells: contribution to embryo quality. J. Cell. Mol. Med. 13, 972–984.
| Connexin expression and gap junctional coupling in human cumulus cells: contribution to embryo quality.Crossref | GoogleScholarGoogle Scholar | 18505471PubMed |
Wigglesworth, K., Lee, K. B., O’Brien, M. J., Peng, J., Matzuk, M. M., and Eppig, J. J. (2013). Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc. Natl. Acad. Sci. USA 110, E3723–E3729.
| Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes.Crossref | GoogleScholarGoogle Scholar | 23980176PubMed |
Yin, B. Y., Zhang, Y., Sun, J. H., Li, J. X., and Ma, Y. F. (2009). Connexin37 mRNA expression in in vivo and in vitro mouse oocyte. Zygote 17, 163–168.
| Connexin37 mRNA expression in in vivo and in vitro mouse oocyte.Crossref | GoogleScholarGoogle Scholar | 19222873PubMed |
Zhao, K., Kuperman, L., Geimonen, E., and Andersen, J. (1996). Progestin represses human connexin43 gene expression similarly in primary cultures of myometrial and uterine leiomyoma cells. Biol. Reprod. 54, 607–615.
| Progestin represses human connexin43 gene expression similarly in primary cultures of myometrial and uterine leiomyoma cells.Crossref | GoogleScholarGoogle Scholar | 8835382PubMed |
Zhou, C. J., Wu, S. N., Shen, J. P., Wang, D. H., Kong, X. W., Lu, A., Li, Y. J., Zhou, H. X., Zhao, Y. F., and Liang, C. G. (2016). The beneficial effects of cumulus cells and oocyte–cumulus cell gap junctions depends on oocyte maturation and fertilization methods in mice. PeerJ 4, e1761.
| The beneficial effects of cumulus cells and oocyte–cumulus cell gap junctions depends on oocyte maturation and fertilization methods in mice.Crossref | GoogleScholarGoogle Scholar | 26966678PubMed |


